Abstract
CC chemokine receptor 6 (CCR6) is one of the members of the G-protein-coupled receptor (GPCR) family that is upregulated in many immune-related cells, such as B lymphocytes, effector and memory T cells, regulatory T cells, and immature dendritic cells. The coordination between CCR6 and its ligand CC motif chemokine ligand 20 (CCL20) is deeply involved in the pathogenesis of various diseases, such as cancer, psoriasis, and autoimmune diseases. Thus, CCR6 is an attractive target for therapy and is being investigated as a diagnostic marker for various diseases. In a previous study, we developed an anti-mouse CCR6 (mCCR6) monoclonal antibody (mAb), C6Mab-13 (rat IgG1, kappa), that was applicable for flow cytometry by immunizing a rat with the N-terminal peptide of mCCR6. In this study, we investigated the binding epitope of C6Mab-13 using an enzyme-linked immunosorbent assay (ELISA) and the surface plasmon resonance (SPR) method, which were conducted with respect to the synthesized point-mutated-peptides within the 1–20 amino acid region of mCCR6. In the ELISA results, C6Mab-13 lost its ability to react to the alanine-substituted peptide of mCCR6 at Asp11, thereby identifying Asp11 as the epitope of C6Mab-13. In our SPR analysis, the dissociation constants (KD) could not be calculated for the G9A and D11A mutants due to the lack of binding. The SPR analysis demonstrated that the C6Mab-13 epitope comprises Gly9 and Asp11. Taken together, the key binding epitope of C6Mab-13 was determined to be located around Asp11 on mCCR6. Based on the epitope information, C6Mab-13 could be useful for further functional analysis of mCCR6 in future studies.
1. Introduction
The CC chemokine receptor 6 (CCR6) is a seven-transmembrane chemokine receptor belonging to the G-protein-coupled receptor (GPCR) family [1,2,3,4,5]. CCR6 was identified as a specific receptor for the CC motif chemokine ligand 20 (CCL20) in 1997 [6]. It is reportedly associated with various diseases, such as cancer [7,8,9], autoimmune diseases [10,11,12,13], psoriasis [14,15,16,17], and inflammatory bowel disease (IBD) [18,19,20,21,22]. The expression of CCR6 is found in B cells or T cells [23], such as effector memory T cells [24], immature dendritic cells [25], Th17 cells [26], and regulatory T (Treg) cells [27], and thus affects the activity and directionality of immune cells [23,24,26]. Mice lacking CCR6 exhibited impaired leukocyte homeostasis, which results in severe contact hypersensitivity and defects in delayed-type hypersensitivity responses. These results suggested that CCR6 plays a critical role in the regulation of leukocyte homeostasis [28].
The chemokine ligand CCL20, also known as macrophage inflammatory protein-3α (MIP-3α) [29], liver- and activation-regulated chemokine (LARC) [30], or Exodus-1 [31], is a crucial CCR6 ligand. The binding of CCL20 to CCR6 can activate a variety of intracellular signaling pathways, including the calcium signaling, PI3K-Akt, MEK-ERK, STAT3, and NF-κB pathways [32]. These pathways play essential roles in the differentiation, migration, and plasticity of CD4+ T lymphcytes [33,34,35,36]. These findings suggest that CCR6-CCL20 signaling could provoke cross-talk with the signalling of T-cell receptors and cytokines to regulate the mitigation of CD4+ T lymphcytes in the inflammatory microenvironment.
CCL20 is secreted by various immune-related cells, such as B cells [23], Th17 cells [37], dendritic cells [38], and natural killer cells [39]. Although various CC chemokine receptor–ligand pairs exist, the CCR6/CCL20-regulated immune response has currently become a focus of immunological research with respect to disease development [10,38,40,41]. The expression of CCR6 and CCL20 has been found to be dysregulated in the colonic mucosa and serum from IBD patients [20,22]. CCR6+ T lymphcytes are involved in an imiquimod-induced psoriasis model [42]. Furthermore, the tumor-promoting effects of CCR6/CCL20 within the tumor microenvironment have been reported in many cancer types, such as renal cell carcinoma [43], gastric cancer [44], cervical cancer [45], and lung cancer [46,47]. Treg cells in peripheral blood (~60%) express CCR6, presenting increased suppressive activity and higher FOXP3 expression in patients with oral squamous cell carcinoma [48]. These findings have made the CCL20/CCR6 axis an attractive therapeutic target for various diseases., and inhibitors targeting the CCR6/CCL20 axis are also being actively developed [10].
Previously, we developed various monoclonal antibodies (mAbs) against chemokine receptors, including mouse CCR2 [49], mouse CCR3 [50], mouse CCR4 [51], mouse CCR6 (mCCR6) [52], mouse CCR9 [53], and mouse CXCR6 [54]. The N-terminus of GPCRs, including CCR6, CCR9, and CXCR6, has been identified as a ligand-binding domain [55,56,57,58]. Interestingly, the binding between CCL20 and CCR6 has been elucidated [59]. CCR6 and CCL20 have shallow binding modes on the receptor surface, which induce allosteric conformational changes and are considered to trigger binding to intracellular G-proteins [59]. Analysis of the ligand-binding mode and the characterization of antibody epitopes are important for predicting neutralizing activity and assessing efficacy against antigens.
In this study, we performed an epitope identification of a rat anti-mCCR6 mAb (C6Mab-13; IgG1, kappa) using enzyme-linked immunosorbent assay (ELISA) and surface plasmon resonance (SPR) analysis against the alanine-substituted N-terminal peptides of mCCR6.
2. Materials and Methods
2.1. Antibodies
The rat anti-mCCR6 mAb (clone C6Mab-13) used herein was previously developed [52]. In summary, one rat was intraperitoneally immunized with a keyhole-limpet-hemocyanin (KLH)-conjugated N-terminal peptide of mCCR6 (1–19 amino acids (aa) + C-terminal cysteine). Subsequently, the hybridoma supernatants were screened with the mCCR6p1-19C peptide using ELISA following flow cytometry using mCCR6-overexpressed CHO-K1 cells and endogenously mCCR6-expressed P388 (mouse lymphoid neoplasma) and J774-1 (mouse macrophage-like) cells [52].
We purchased secondary peroxidase-conjugated anti-rat immunoglobulins from Sigma-Aldrich Corp. (St. Louis, MO, USA).
2.2. Peptides
The mCCR6 (Accession No.: NM_001190333.1) peptide (1-MNSTESYFGTDDYDNTEYYS-20) and 1× alanine residue-substituted peptides (Table 1) were synthesized utilizing PEPscreen (Sigma-Aldrich Corp.).

Table 1.
Identification of C6Mab-13 epitope using point mutant peptides of mCCR6 via enzyme-linked immunosorbent assay.
2.3. ELISA
Synthesized mCCR6 peptides were immobilized on Nunc Maxisorp 96-well immunoplates (Thermo Fisher Scientific Inc., Waltham, MA, USA) at 10 µg/mL for 30 min at 37 °C. After being washed with phosphate-buffered saline (PBS) containing 0.05% Tween20 (PBST; Nacalai Tesque, Inc., Kyoto, Japan), the wells were blocked with 1% bovine serum albumin (BSA)-containing PBST for 30 min at 37 °C. The plates were incubated with 10 µg/mL of C6Mab-13 for 30 min at 37 °C followed by peroxidase-conjugated anti-rat immunoglobulins (1:20,000 diluted; Sigma-Aldrich Corp.) for 30 min at 37 °C. Enzymatic reactions were conducted at room temperature using the ELISA POD Substrate TMB Kit (Nacalai Tesque, Inc.). Optical density was measured at 655 nm using an iMark microplate reader (Bio-Rad Laboratories, Inc., Berkeley, CA, USA).
2.4. Measurement of Dissociation Constants Using Surface Plasmon Resonance (SPR)
The dissociation constants (KD) between C6Mab-13 and the epitope region peptides were measured using SPR. C6Mab-13 was immobilized on the CM5 sensor chip according to the manufacturer’s protocol (Cytiva, Marlborough, MA, USA). In summary, C6Mab-13 was diluted to 10 μg/mL by an acetate buffer (pH 4.0; Cytiva) and immobilized using an amine-coupling reaction. The surface of flow cell 2 of the CM5 sensor chip was treated with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide and N-hydroxysuccinimide (NHS), followed by an injection of C6Mab-13. The unreacted NHS-ester was blocked with ethanolamine after C6Mab-13 immobilization. The KD between C6Mab-13 and mCCR6 peptides (50, 25, 12.5, 6.25, and 3.13 µM) were measured using Biacore X100 (Cytiva) at 25 °C. The buffer was filtrated with PBS containing 0.05% (v/v) Tween 20 and 0.24% (v/v) dimethyl sulfoxide (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan). The single-cycle kinetics method was used to measure the binding signals. The data were analyzed using 1:1 binding kinetics to determine the association rate constant (ka), dissociation rate constant (kd), and KD using BIAevaluation software (Cytiva).
3. Results
3.1. Epitope Identification of C6Mab-13 by ELISA Using 1× Alanine-Substituted mCCR6 Peptides
We previously developed an anti-mCCR6 mAb, C6Mab-13 (rat IgG1, kappa), by immunizing a rat with a KLH-conjugated mCCR6 N-terminal peptide [52]. C6Mab-13 is applicable to ELISA and is useful for detecting mCCR6-expressing cells via flow cytometry [52]. To characterize the binding epitope of C6Mab-13, we synthesized 20 different 1× alanine-substituted mCCR6 peptides between Met1 to Ser20. The sequences are listed in Table 1. The results of ELISA using alanine-substituted peptides and C6Mab-13 demonstrated that C6Mab-13 bound to point mutants, such as M1A, N2A, S3A, T4A, E5A, S6A, Y7A, F8A, G9A, T10A, D12A, Y13A, D14A, N15A, T16A, E17A, Y18A, Y19A, and S20A, as well as the 1–20 aa wild-type (WT) sequence (positive control) (Figure 1A). In contrast, C6Mab-13 did not react with the D11A peptide (Figure 1A). These results indicate that Asp11 was the critical aa, which is included in the C6Mab-13 epitope. The results are summarized schematically in Figure 1B.
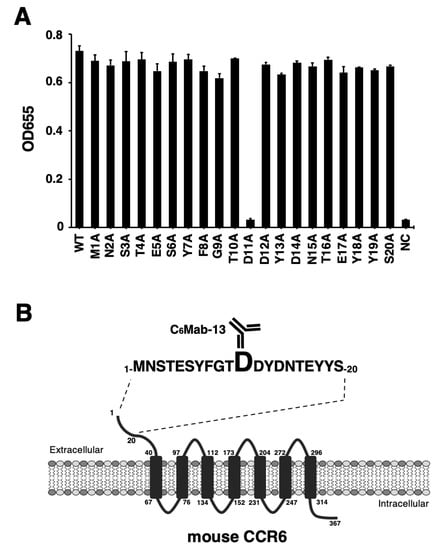
Figure 1.
Determination of the C6Mab-13 epitope by ELISA using alanine-substituted peptides of mCCR6. (A) Synthesized peptides of mCCR6 (10 µg/mL) were immobilized on immunoplates for 30 min at 37 °C. The plates were incubated with 10 µg/mL of C6Mab-13, followed by treatment with peroxidase-conjugated anti-rat immunoglobulins. Optical density was measured at 655 nm (OD655) using a microplate reader. (B) The schematic illustration of mCCR6 and the C6Mab-13 epitope. The C6Mab-13 epitope of mCCR6 comprises Asp11 from ELISA experiments.
3.2. Epitope Identification of C6Mab-13 by SPR Using 1× Alanine-Substituted mCCR6 Peptides
To confirm the C6Mab-13 epitope, we measured the binding affinity between C6Mab-13 and the synthesized peptides, including 20 point mutants and the WT of mCCR6, using Biacore X100. The peptides’ sequences are presented in Table 1, and the measured values are summarized in Table 2. The ka, kd, and KD of G9A and D11A were not determined. These results demonstrated that Gly9 and Asp11 were the critical amino acids of the C6Mab-13 epitope.

Table 2.
The KD between C6Mab-13 and 1× alanine-substituted peptides determined by surface plasmon resonance.
Mutant peptides of F8A, T10A, Y13A, and D14A increased the KD values by 15.5-, 4.4-, 16.5-, and 2.8-fold, respectively (Table 2), indicating that Phe8, Thr10, Tyr13, and Asp14 may contribute to the binding of C6Mab-13 to mCCR6.
4. Discussion
This study examined the binding epitope of C6Mab-13 through a 1× alanine-substituted-peptide-scanning method using ELISA and SPR. We concluded that Asp11 is a pivotal epitope aa in ELISA, while Gly9 and Asp11 are critical in SPR. This epitope is located outside the region of all three extracellular domains of CCR6 and N-terminal residues from Tyr27 to Leu38, to which the chemokine ligand CCL20 binds [59,60]. There is a possibility that structural changes might occur upon C6Mab-13′s binding to CCR6, which leads to allosteric effects on CCL20 binding. Therefore, we will investigate the neutralizing activity of C6Mab-13 between CCL20 and CCR6 in the future study.
A recent report showed that low rather than high affinity of mAb to a target provokes elevated activity through inducing the clustering of receptors. These findings provide new insights for antibody-mediated receptor signaling [61]. Since CCR6 is involved in intracellular signaling [62], the relationship between antibody affinity and the effect of cellular signaling should be investigated in future studies.
The epitope-mapping results obtained using ELISA (Figure 1) and SPR (Table 2) indicated a similar region of mCCR6 as the binding epitope. However, Gly9 was only identified as the critical aa by via SPR analysis (Table 2). The experimental system differs between both experiments, as follows: (i) for ELISA, the synthesized peptides were immobilized on immunoplates, while C6Mab-13 was immobilized on a CM5 sensor chip in the SPR analysis; (ii) the reaction times between the antigen and the antibody were different; and (iii) the secondary antibody was only used for ELISA. These different conditions may have precipitated the inconsistent results of both experiments in this study.
In the SPR analysis, mutant peptides of F8A, T10A, Y13A, and D14A increased the KD values by 15.5-, 4.4-, 16.5-, and 2.8-fold, respectively (Table 2). These results indicate that Phe8, Thr10, Tyr13, and Asp14 may contribute to C6Mab-13′s binding to mCCR6. In the future, we will adopt the cell-based alanine- or 2× alanine-scanning methods for a detailed epitope analysis of C6Mab-13, as we have clarified the epitope of mAb [63].
When CCL20 is secreted in tumor tissues [64], it attracts CCR6-expressing Treg cells [65], which are involved in tumor progression and poor prognosis [66,67]. Therefore, novel cancer treatment strategies using CCR6-expressing chimeric antigen receptor-T (CAR-T) cells have been designed [68,69]. Furthermore, removing immunosuppressive cells, such as CCR6+ Treg cells, may enhance antitumor efficacy [70]. In this study, we demonstrated that C6Mab-13 possesses high binding affinity against mCCR6, which was expressed in Chinese hamster ovary-K1 cells (KD: 2.8 × 10−9 M according to flow cytometric analysis) [52]. Therefore, C6Mab-13 is expected to be useful for antitumor evaluations considering the depletion of CCR6-expressing Treg cells in mouse models.
Author Contributions
T.T. and M.T. performed the experiments. M.K.K. and Y.K. designed the experiments. T.T. and H.S. analyzed the data. T.T., H.S. and Y.K. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by Japan Agency for Medical Research and Development (AMED) under Grant Numbers: JP22ama121008 (to Y.K.), JP22am0401013 (to Y.K.), JP22bm1004001 (to Y.K.), JP22ck0106730 (to Y.K.), and JP21am0101078 (to Y.K.), and by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI) grant nos. 21K20789 (to T.T.), 22K06995 (to H.S.), 21K07168 (to M.K.K.), and 22K07224 (to Y.K.).
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper. Additional inquiries should be addressed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morein, D.; Erlichman, N.; Ben-Baruch, A. Beyond Cell Motility: The Expanding Roles of Chemokines and Their Receptors in Malignancy. Front. Immunol. 2020, 11, 952. [Google Scholar] [CrossRef]
- Mollica Poeta, V.; Massara, M.; Capucetti, A.; Bonecchi, R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front. Immunol. 2019, 10, 379. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. Febs. J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. The chemokine superfamily revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, E.; Struyf, S.; Van Damme, J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor. Rev. 2003, 14, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Imai, T.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Hieshima, K.; Nomiyama, H.; Yoshie, O. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J. Biol. Chem. 1997, 272, 14893–14898. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Grochans, S.; Gutowska, I.; Barczak, K.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands. Int. J. Mol. Sci. 2020, 21, 7619. [Google Scholar] [CrossRef]
- Kadomoto, S.; Izumi, K.; Mizokami, A. The CCL20-CCR6 Axis in Cancer Progression. Int. J. Mol. Sci. 2020, 21, 5186. [Google Scholar] [CrossRef]
- Chen, W.; Qin, Y.; Liu, S. CCL20 Signaling in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1231, 53–65. [Google Scholar] [CrossRef]
- Gómez-Melero, S.; Caballero-Villarraso, J. CCR6 as a Potential Target for Therapeutic Antibodies for the Treatment of Inflammatory Diseases. Antibodies 2023, 12, 30. [Google Scholar] [CrossRef]
- Misra, D.P.; Agarwal, V. Th17.1 lymphocytes: Emerging players in the orchestra of immune-mediated inflammatory diseases. Clin. Rheumatol. 2022, 41, 2297–2308. [Google Scholar] [CrossRef] [PubMed]
- Meitei, H.T.; Jadhav, N.; Lal, G. CCR6-CCL20 axis as a therapeutic target for autoimmune diseases. Autoimmun. Rev. 2021, 20, 102846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yang, G.; Xiao, F.; Xie, J.; Wang, S.; Lu, L.; Cui, D. Role of Th22 Cells in the Pathogenesis of Autoimmune Diseases. Front. Immunol. 2021, 12, 688066. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Xing, M.; Hong, S.; Liu, L.; Ding, X.J.; Sun, X.Y.; Luo, Y.; Wang, C.X.; Zhang, M.; et al. Current evidence on the role of lipid lowering drugs in the treatment of psoriasis. Front. Med. 2022, 9, 900916. [Google Scholar] [CrossRef]
- Furue, M.; Furue, K.; Tsuji, G.; Nakahara, T. Interleukin-17A and Keratinocytes in Psoriasis. Int. J. Mol. Sci. 2020, 21, 1275. [Google Scholar] [CrossRef] [PubMed]
- Furue, K.; Ito, T.; Tsuji, G.; Nakahara, T.; Furue, M. The CCL20 and CCR6 axis in psoriasis. Scand. J. Immunol. 2020, 91, e12846. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, M.N.; Lonsdorf, A.S.; Hwang, S.T.; Farber, J.M. CCR6 as a possible therapeutic target in psoriasis. Expert. Opin. Ther. Targets 2010, 14, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.G.; Giorgio, C.; Allodi, M.; Palese, S.; Barocelli, E.; Ballabeni, V.; Szpakowska, M.; Chevigné, A.; Piet van Hamburg, J.; Davelaar, N.; et al. Discovery of small-molecules targeting the CCL20/CCR6 axis as first-in-class inhibitors for inflammatory bowel diseases. Eur. J. Med. Chem. 2022, 243, 114703. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Adams, D.H. Chemokines and Chemokine Receptors as Therapeutic Targets in Inflammatory Bowel Disease; Pitfalls and Promise. J. Crohns. Colitis. 2018, 12, S641–S652. [Google Scholar] [CrossRef]
- Skovdahl, H.K.; Granlund, A.; Østvik, A.E.; Bruland, T.; Bakke, I.; Torp, S.H.; Damås, J.K.; Sandvik, A.K. Expression of CCL20 and Its Corresponding Receptor CCR6 Is Enhanced in Active Inflammatory Bowel Disease, and TLR3 Mediates CCL20 Expression in Colonic Epithelial Cells. PLoS ONE 2015, 10, e0141710. [Google Scholar] [CrossRef] [PubMed]
- Cheluvappa, R. Experimental appendicitis and appendectomy modulate the CCL20-CCR6 axis to limit inflammatory colitis pathology. Int. J. Colorectal. Dis. 2014, 29, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Kaser, A.; Ludwiczek, O.; Holzmann, S.; Moschen, A.R.; Weiss, G.; Enrich, B.; Graziadei, I.; Dunzendorfer, S.; Wiedermann, C.J.; Mürzl, E.; et al. Increased expression of CCL20 in human inflammatory bowel disease. J. Clin. Immunol. 2004, 24, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.Y.S.; Körner, H. The CCR6-CCL20 axis in humoral immunity and T-B cell immunobiology. Immunobiology 2019, 224, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Takata, H.; Takiguchi, M. Functional expression of chemokine receptor CCR6 on human effector memory CD8+ T cells. Eur. J. Immunol. 2007, 37, 54–65. [Google Scholar] [CrossRef]
- Rescigno, M. CCR6(+) dendritic cells: The gut tactical-response unit. Immunity 2006, 24, 508–510. [Google Scholar] [CrossRef]
- Annunziato, F.; Cosmi, L.; Santarlasci, V.; Maggi, L.; Liotta, F.; Mazzinghi, B.; Parente, E.; Filì, L.; Ferri, S.; Frosali, F.; et al. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 2007, 204, 1849–1861. [Google Scholar] [CrossRef]
- Essien, K.I.; Katz, E.L.; Strassner, J.P.; Harris, J.E. Regulatory T Cells Require CCR6 for Skin Migration and Local Suppression of Vitiligo. J. Invest. Dermatol. 2022, 142, 3158–3166.e3157. [Google Scholar] [CrossRef]
- Varona, R.; Villares, R.; Carramolino, L.; Goya, I.; Zaballos, A.; Gutiérrez, J.; Torres, M.; Martínez, A.C.; Márquez, G. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J. Clin. Invest. 2001, 107, R37–R45. [Google Scholar] [CrossRef]
- Power, C.A.; Church, D.J.; Meyer, A.; Alouani, S.; Proudfoot, A.E.; Clark-Lewis, I.; Sozzani, S.; Mantovani, A.; Wells, T.N. Cloning and characterization of a specific receptor for the novel CC chemokine MIP-3alpha from lung dendritic cells. J. Exp. Med. 1997, 186, 825–835. [Google Scholar] [CrossRef]
- Hieshima, K.; Imai, T.; Opdenakker, G.; Van Damme, J.; Kusuda, J.; Tei, H.; Sakaki, Y.; Takatsuki, K.; Miura, R.; Yoshie, O.; et al. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J. Biol. Chem. 1997, 272, 5846–5853. [Google Scholar] [CrossRef]
- Hromas, R.; Gray, P.W.; Chantry, D.; Godiska, R.; Krathwohl, M.; Fife, K.; Bell, G.I.; Takeda, J.; Aronica, S.; Gordon, M.; et al. Cloning and characterization of exodus, a novel beta-chemokine. Blood 1997, 89, 3315–3322. [Google Scholar] [PubMed]
- Kulkarni, N.; Meitei, H.T.; Sonar, S.A.; Sharma, P.K.; Mujeeb, V.R.; Srivastava, S.; Boppana, R.; Lal, G. CCR6 signaling inhibits suppressor function of induced-Treg during gut inflammation. J. Autoimmun. 2018, 88, 121–130. [Google Scholar] [CrossRef]
- Oh, H.; Ghosh, S. NF-κB: Roles and regulation in different CD4(+) T-cell subsets. Immunol. Rev. 2013, 252, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Noubade, R.; Krementsov, D.N.; Del Rio, R.; Thornton, T.; Nagaleekar, V.; Saligrama, N.; Spitzack, A.; Spach, K.; Sabio, G.; Davis, R.J.; et al. Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood 2011, 118, 3290–3300. [Google Scholar] [CrossRef] [PubMed]
- Haxhinasto, S.; Mathis, D.; Benoist, C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 2008, 205, 565–574. [Google Scholar] [CrossRef]
- Yang, X.O.; Panopoulos, A.D.; Nurieva, R.; Chang, S.H.; Wang, D.; Watowich, S.S.; Dong, C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007, 282, 9358–9363. [Google Scholar] [CrossRef]
- Harper, E.G.; Guo, C.; Rizzo, H.; Lillis, J.V.; Kurtz, S.E.; Skorcheva, I.; Purdy, D.; Fitch, E.; Iordanov, M.; Blauvelt, A. Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: Implications for psoriasis pathogenesis. J. Invest. Dermatol. 2009, 129, 2175–2183. [Google Scholar] [CrossRef]
- Ito, T.; Carson, W.F.T.; Cavassani, K.A.; Connett, J.M.; Kunkel, S.L. CCR6 as a mediator of immunity in the lung and gut. Exp. Cell. Res. 2011, 317, 613–619. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Eri, R. Modulation of the CCR6-CCL20 Axis: A Potential Therapeutic Target in Inflammation and Cancer. Medicina (Kaunas) 2018, 54, 88. [Google Scholar] [CrossRef]
- Lee, A.Y.S.; Körner, H. CCR6/CCL20 chemokine axis in human immunodeficiency virus immunity and pathogenesis. J. Gen. Virol. 2017, 98, 338–344. [Google Scholar] [CrossRef]
- Lee, A.Y.; Körner, H. CCR6 and CCL20: Emerging players in the pathogenesis of rheumatoid arthritis. Immunol. Cell. Biol. 2014, 92, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R.; Ichimura, Y.; Kubota, N.; Konishi, R.; Nakamura, Y.; Mizuno, S.; Takahashi, S.; Fujimoto, M.; Nomura, T.; Okiyama, N. The Role of PD-L1 on Langerhans Cells in the Regulation of Psoriasis. J. Invest. Dermatol. 2022, 142, 3167–3174.e3169. [Google Scholar] [CrossRef]
- Kadomoto, S.; Izumi, K.; Hiratsuka, K.; Nakano, T.; Naito, R.; Makino, T.; Iwamoto, H.; Yaegashi, H.; Shigehara, K.; Kadono, Y.; et al. Tumor-Associated Macrophages Induce Migration of Renal Cell Carcinoma Cells via Activation of the CCL20-CCR6 Axis. Cancers 2019, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Wu, D.; Yang, Y.; Li, Z.; Zhang, J.; Li, C. CrkL meditates CCL20/CCR6-induced EMT in gastric cancer. Cytokine 2015, 76, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Lou, X.M.; He, Y. Preferential recruitment of Th17 cells to cervical cancer via CCR6-CCL20 pathway. PLoS ONE 2015, 10, e0120855. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhao, X.; Zhu, J.; Zhang, L.; Chen, Y.; Zhang, B.; Li, Y.; Wang, M.; Zhang, Z.; Wang, C. lncRNA-u50535 promotes the progression of lung cancer by activating CCL20/ERK signaling. Oncol. Rep. 2019, 42, 1946–1956. [Google Scholar] [CrossRef]
- Zhang, X.P.; Hu, Z.J.; Meng, A.H.; Duan, G.C.; Zhao, Q.T.; Yang, J. Role of CCL20/CCR6 and the ERK signaling pathway in lung adenocarcinoma. Oncol. Lett. 2017, 14, 8183–8189. [Google Scholar] [CrossRef]
- Lee, J.J.; Kao, K.C.; Chiu, Y.L.; Jung, C.J.; Liu, C.J.; Cheng, S.J.; Chang, Y.L.; Ko, J.Y.; Chia, J.S. Enrichment of Human CCR6(+) Regulatory T Cells with Superior Suppressive Activity in Oral Cancer. J. Immunol. 2017, 199, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Li, G.; Asano, T.; Saito, M.; Kaneko, M.K.; Suzuki, H.; Kato, Y. Development of a Novel Anti-Mouse CCR2 Monoclonal Antibody (C(2)Mab-6) by N-Terminal Peptide Immunization. Monoclon. Antib. Immunodiagn. Immunother. 2022, 41, 80–86. [Google Scholar] [CrossRef]
- Asano, T.; Suzuki, H.; Tanaka, T.; Saito, M.; Li, G.; Goto, N.; Nanamiya, R.; Kaneko, M.K.; Kato, Y. C(3)Mab-3: A Monoclonal Antibody for Mouse CC Chemokine Receptor 3 for Flow Cytometry. Monoclon. Antib. Immunodiagn. Immunother. 2022, 41, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Takei, J.; Suzuki, H.; Asano, T.; Tanaka, T.; Kaneko, M.K.; Kato, Y. Development of a Novel Anti-Mouse CCR4 Monoclonal Antibody (C(4)Mab-1) by N-Terminal Peptide Immunization. Monoclon. Antib. Immunodiagn. Immunother. 2022, 41, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Tanaka, T.; Suzuki, H.; Li, G.; Nanamiya, R.; Tateyama, N.; Isoda, Y.; Okada, Y.; Kobayashi, H.; Yoshikawa, T.; et al. Development of a Novel Anti-Mouse CCR6 Monoclonal Antibody (C(6)Mab-13) by N-Terminal Peptide Immunization. Monoclon. Antib. Immunodiagn. Immunother. 2022, 41, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Asano, T.; Suzuki, H.; Tanaka, T.; Yoshikawa, T.; Kaneko, M.K.; Kato, Y. Establishment of a Sensitive Monoclonal Antibody Against Mouse CCR9 (C(9)Mab-24) for Flow Cytometry. Monoclon. Antib. Immunodiagn. Immunother. 2023, 42, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Suzuki, H.; Kaneko, M.K.; Kato, Y. Cx(6)Mab-1: A Novel Anti-Mouse CXCR6 Monoclonal Antibody Established by N-Terminal Peptide Immunization. Monoclon. Antib. Immunodiagn. Immunother. 2022, 41, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, S.; Vela, M.; Franco-Villanueva, A.; Carramolino, L.; Gutiérrez, J.; Gómez, L.; Lozano, M.; Salvador, B.; García-Gallo, M.; Martínez, A.C.; et al. Antitumor effects of a monoclonal antibody to human CCR9 in leukemia cell xenografts. MAbs 2014, 6, 1000–1012. [Google Scholar] [CrossRef] [PubMed]
- Chain, B.; Arnold, J.; Akthar, S.; Brandt, M.; Davis, D.; Noursadeghi, M.; Lapp, T.; Ji, C.; Sankuratri, S.; Zhang, Y.; et al. A Linear Epitope in the N-Terminal Domain of CCR5 and Its Interaction with Antibody. PLoS ONE 2015, 10, e0128381. [Google Scholar] [CrossRef]
- Gómez-Melero, S.; García-Maceira, F.I.; García-Maceira, T.; Luna-Guerrero, V.; Montero-Peñalvo, G.; Túnez-Fiñana, I.; Paz-Rojas, E. Amino terminal recognition by a CCR6 chemokine receptor antibody blocks CCL20 signaling and IL-17 expression via β-arrestin. BMC Biotechnol. 2021, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Durán, G.; Romo-Mancillas, A. Behavior of Chemokine Receptor 6 (CXCR6) in Complex with CXCL16 Soluble form Chemokine by Molecular Dynamic Simulations: General Protein—Ligand Interaction Model and 3D-QSAR Studies of Synthetic Antagonists. Life 2021, 11, 346. [Google Scholar] [CrossRef]
- Wasilko, D.J.; Johnson, Z.L.; Ammirati, M.; Che, Y.; Griffor, M.C.; Han, S.; Wu, H. Structural basis for chemokine receptor CCR6 activation by the endogenous protein ligand CCL20. Nat. Commun. 2020, 11, 3031. [Google Scholar] [CrossRef]
- Dragan, P.; Merski, M.; Wiśniewski, S.; Sanmukh, S.G.; Latek, D. Chemokine Receptors-Structure-Based Virtual Screening Assisted by Machine Learning. Pharmaceutics 2023, 15, 516. [Google Scholar] [CrossRef]
- Yu, X.; Orr, C.M.; Chan, H.T.C.; James, S.; Penfold, C.A.; Kim, J.; Inzhelevskaya, T.; Mockridge, C.I.; Cox, K.L.; Essex, J.W.; et al. Reducing affinity as a strategy to boost immunomodulatory antibody agonism. Nature 2023, 614, 539–547. [Google Scholar] [CrossRef]
- Frick, V.O.; Rubie, C.; Keilholz, U.; Ghadjar, P. Chemokine/chemokine receptor pair CCL20/CCR6 in human colorectal malignancy: An overview. World J. Gastroenterol. 2016, 22, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Isoda, Y.; Tanaka, T.; Suzuki, H.; Asano, T.; Yoshikawa, T.; Kitamura, K.; Kudo, Y.; Ejima, R.; Ozawa, K.; Kaneko, M.K.; et al. Epitope Mapping Using the Cell-Based 2 × Alanine Substitution Method About the Anti-mouse CXCR6 Monoclonal Antibody, Cx(6)Mab-1. Monoclon. Antib. Immunodiagn. Immunother. 2023, 42, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Kojder, K.; Simińska, D.; Bohatyrewicz, R.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4. Int. J. Mol. Sci. 2020, 21, 8412. [Google Scholar] [CrossRef] [PubMed]
- Rutihinda, C.; Haroun, R.; Saidi, N.E.; Ordoñez, J.P.; Naasri, S.; Lévesque, D.; Boisvert, F.M.; Fortier, P.H.; Belzile, M.; Fradet, L.; et al. Inhibition of the CCR6-CCL20 axis prevents regulatory T cell recruitment and sensitizes head and neck squamous cell carcinoma to radiation therapy. Cancer Immunol. Immunother. 2023, 72, 1089–1102. [Google Scholar] [CrossRef]
- Jia, S.N.; Han, Y.B.; Yang, R.; Yang, Z.C. Chemokines in colon cancer progression. Semin. Cancer Biol. 2022, 86, 400–407. [Google Scholar] [CrossRef]
- Chen, K.J.; Lin, S.Z.; Zhou, L.; Xie, H.Y.; Zhou, W.H.; Taki-Eldin, A.; Zheng, S.S. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS ONE 2011, 6, e24671. [Google Scholar] [CrossRef]
- Jin, L.; Cao, L.; Zhu, Y.; Cao, J.; Li, X.; Zhou, J.; Liu, B.; Zhao, T. Enhance anti-lung tumor efficacy of chimeric antigen receptor-T cells by ectopic expression of C-C motif chemokine receptor 6. Sci. Bull. 2021, 66, 803–812. [Google Scholar] [CrossRef]
- Zhong, C.; Chen, J. CAR-T cell engineering with CCR6 exhibits superior anti-solid tumor efficacy. Sci. Bull. 2021, 66, 755–756. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kang, M.; Jeon, M.; Chung, Y.; Kim, A.R.; Lee, Y.J.; Kim, E.S.; Nam, H.; Park, J.; Lee, J.Y.; et al. CEACAM1 marks highly suppressive intratumoral regulatory T cells for targeted depletion therapy. Clin. Cancer Res. 2023, in press. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).