Abstract
Identification of new disease-associated biomarkers; specific targeting of such markers by monoclonal antibodies (mAbs); and application of advances in recombinant technology, including the production of humanized and fully human antibodies, has enabled many improved treatment outcomes and successful new biological treatments of some diseases previously neglected or with poor prognoses. Of the 110 mAbs preparations currently approved by the FDA and/or EMA, 46 (including 13 antibody–drug conjugates) recognizing 29 different targets are indicated for the treatment of cancers, and 66, recognizing 48 different targets, are indicated for non-cancer disorders. Despite their specific targeting with the expected accompanying reduced collateral damage for normal healthy non-involved cells, mAbs, may cause types I (anaphylaxis, urticaria), II (e.g., hemolytic anemia, possibly early-onset neutropenia), III (serum sickness, pneumonitis), and IV (Stevens–Johnson syndrome, toxic epidermal necrolysis) hypersensitivities as well as other cutaneous, pulmonary, cardiac, and liver adverse events. MAbs can provoke severe infusion reactions that resemble anaphylaxis and induce a number of systemic, potentially life-threatening syndromes with low frequency. A common feature of most of these syndromes is the release of a cascade of cytokines associated with inflammatory and immunological processes. Epidermal growth factor receptor-targeted antibodies may provoke papulopustular and mucocutaneous eruptions that are not immune-mediated.
1. Introduction
In the last decade, along with the continuing development of the disciplines of ge-nomics, proteomics, and bioinformatics and the application of molecular biological approaches to elucidate the functions of single genes, advances have led to insights into the complexities and multifaceted nature of diseases such as cancer, immune and inflammatory-based diseases, metabolic disorders, neurological diseases, transplantation, and some poorly understood dermatologic toxicities [1,2,3,4,5,6]. Specific, targeted approaches now employed in many monoclonal antibody (mAb), fusion protein, and cytokine therapies have been enabled by advances in recombinant DNA technology, the preparation of human recombinant antibody libraries, today’s sequencing methods, parallel proteome analyses employing techniques such as mass spectroscopy, and single B cell technologies [5,6,7]. The U.S. Food and Drug Authority (FDA) Office of Orphan Products Development and its European equivalent have provided extra stimulus for the development of therapies for “orphan diseases”, that is, diseases with less than 200,000 patients [8]. This stimulus has led to the introduction of effective approved mAb therapies for some diseases with low patient numbers previously neglected because of the lack of pathogenetic and pathophysiological insights into rare disorders where the potentially small market often precluded investigations [9].
Expanding understanding of ligand–receptor interactions; downstream signaling; and the delineation of immunological and inflammatory interplay between cells, anti-bodies, cytokines, and chemokines has contributed to the identification and selection of new disease biomarker targets. This, in turn, has created the opportunity to specifically target implicated cells, largely without inflicting collateral damage on normal healthy non-involved cells [10]. However, in addition to true hypersensitivities and infusion reactions, the expanding list of disease indications has sometimes brought with it adverse effects on the lungs, heart, liver, immune system, and skin in a variety of poorly, or partially understood, complex adverse responses [3]. A number of systemic potentially life-threatening syndromes most associated with inflammatory and immunological processes, often with cytokine involvement, also occur with low frequency during or following mAb therapy [3].
Although there are many hundreds of mAbs intended for therapeutic use at various stages of development, here we restrict examination to the 110 antibodies currently registered and approved by the U.S. Food and Drug Administration (FDA) and/or European Medicines Agency (EMA). Note, however, that some of these mAbs were first approved by other agencies while some others are already approved by other agencies but not the FDA and EMA.
Here, focus is directed to the classification of the 110 mAbs, their antibody targets, approved disease indications, and the adverse events associated with their use.
2. Evolution of Monoclonal Antibodies to Avoid Immunogenicity
Early realization that the murine composition of the first mAbs provoked a high incidence of adverse events including anaphylaxis and cytokine release syndrome, together with their poor pharmacokinetics, led to an ongoing iterative program to reduce, and ultimately eliminate, these undesirable features [3,11,12]. The mouse mAbs ibritumomab tiuxetan and tositumomab were soon followed by chimeric antibodies such as abciximab, cetuximab, infliximab, and others in which variable (antigen binding) regions were inserted into the constant regions of human immunoglobulins (Figure 1). Occasional serious hypersensitivities occurring after chimeric antibody infusions led to production of so-called humanized antibodies in which only approx. 5–10% of murine proteins remained after substituting mouse complementarity-determining (hypervariable) regions in place of human sequences (Figure 1). It became apparent, however, that even single amino acid changes could result in changes in antibody binding and affinity, and posttranslational glycosylation sometimes produced reductions in specificity, potency, and solubility without a reduction in immunogenicity. Development of the powerful technologies of phage display and transgenic mice finally enabled the production of fully human mAbs; however, immunogenicty can still be an occasional problem [3] due to the presence of anti-idiotype antibodies and antibodies to some mAbs (anti-glycan, anti-hinge, anti-allotype, rheumatoid factors) occurring in normal sera and sera of pretreated patients.
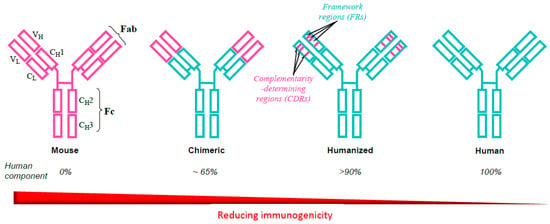
Figure 1.
Evolution of the development of therapeutic monoclonal antibodies from murine to fully human proteins to avoid unwanted immunogenicity. The iterative process proceeded stepwise through chimeric constructs incorporating mouse immunoglobulin variable regions into constant regions of human immunoglobulins and via humanized antibodies by substituting mouse complementarity determining regions (CDRs) in place of human sequences. Fully human antibodies have been developed with the application of phage display and transgenic mice technologies. Reproduced with permission from Baldo BA. Safety of biologics therapy. Monoclonal antibodies, cytokines, fusion proteins, hormones, enzymes, coagulation proteins, vaccines, botulinum toxins. Cham, Switzerland: Springer Nature; 2016 [3].
3. Monoclonal Antibody Targets and Indications
Of the 110 currently approved and registered mAbs (Table 1 and Table 2), two, alemtuzumab and denosumab, are each marketed as two separately approved products with different indications for each. Alemtuzumab, under trade names of Lemtrada® and Campath®/MabCampath® [13,14], is indicated for multiple sclerosis and B cell chronic lymphocytic leukemia, respectively, while denosumab as Prolia® is indicated for bone loss and, as Xgeva®, for bone metastases from solid tumors and giant cell tumor of bone [15,16]. Therefore, while the total number of approved mAbs shown in Table 1 and Table 2 is 112 (66 for non-cancer and 46 for cancer therapies), alemtuzumab and denosumab each appear in both lists under different trade names.

Table 1.
Therapeutic monoclonal antibodies for non-cancer therapy currently marketed with regulatory approval from the U.S. FDA or EMA or both (as at December 2021).

Table 2.
Therapeutic monoclonal antibodies for cancer therapy currently marketed with regulatory approval from the U.S. FDA or EMA or both (as at December 2021).
With the steady increase in the identification and association of biomarker targets [3,17] for an expanding range of diseases, a total of 77 different targets have thus far been utilized in the preparation of the 110 currently approved mAbs with some targets complementary to more than one mAb (Table 3). In particular, there are 29 targets for the 46 different mAb cancer therapies (Table 2) and a collective of 48 targets for a diverse range of 66 mAbs for non-cancer disorders, including 27 inflammatory and/or immune disorders and 39 other diseases/applications (Table 1). For the mAbs used for non-cancer therapies, 14 different targets have been employed two or more times (Table 3). For example, TNF as target has been utilized for four mAbs—adalimumab, certolizumab pegol, golimumab, and infliximab—each used in the treatments of inflammatory diseases including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, plaque psoriasis, and Crohn’s disease. IL-6R serves as target for three different mAbs—sarilumab and tocilizumab, each used to treat rheumatoid arthritis, and satralizumab-mwge, indicated for a quite different condition, neuromyelitis optica spectrum disorder (Table 1 and Table 3). For the treatment of cancers, eight different targets are utilized for more than one mAb. The targets HER2, EGFR, programmed cell death protein 1 PD-1, and its ligand PD-L1 have been used as complementary targets for, respectively, five, four, three, and four different mAbs (Table 2 and Table 3).

Table 3.
Targets with more than one complementary approved therapeutic monoclonal antibody.
4. Adverse Events to Monoclonal Antibody Therapy
Despite their target specificity, their low tendency for drug–drug interactions, and their generally better patient tolerance than small molecule drugs, mAbs are, unsurprisingly, not free of adverse effects, which may manifest as immune, non-immune, or direct cytotoxic reactions. Table 4 and Table 5 summarize adverse events associated with mAbs used for non-cancer and cancer therapies, respectively. For all mAbs, there is the possibility of injection site reactions, infusion reactions, hypersensitivity, and immunogenicity, although these effects are more likely with some mAbs than others. Many of the approved mAbs are subject to warnings for “hypersensitivity”, often without further qualification, which is generally unhelpful given the loose usage of this term and the fact that it often has a different meaning to clinicians and investigators in different branches of medicine [18,19]. Immunogenicity is always a concern even with fully human antibodies since anti-idiotype responses can occur [3,20].

Table 4.
Adverse events associated with approved 1 monoclonal antibodies used for non-cancer therapies (as at December 2021).

Table 5.
Adverse events associated with approved 1 monoclonal antibodies used for cancer therapy (as at December 2021).
Adverse events, divided into immune, that is true hypersensitivities, and non-immune, are herein considered.
4.1. Immune-Mediated Adverse Responses (Hypersensitivities) to Approved Monoclonal Antibodies
Collectively, patient responses to mAbs cover the full range of hypersensitivities from types I to IV (Box 1) [19] with the type I IgE-antibody-mediated hypersensitivity responses—anaphylaxis; urticaria (e.g., to ofatumumab and alemtuzumab); and, rarely, angioedema (e.g., with trastuzumab) occasionally seen. Chimeric mAbs with mouse and/or rat sequences (abciximab, basiliximab, blinatumomab, brentuximab vedotin, catumaxomab, cetuximab, dinutuximab, infliximab, obiltoxaximab, rituximab, and siltuximab) are considered to be the highest risk for type I reactions. Overall, however, reports of type I hypersensitivities are relatively rare, and perhaps less than expected, with only two FDA black box warnings issued thus far (for the humanized mAbs reslizumab and obiltoxaximab) and two FDA warning/precaution for palivizumab and brentuximab vedotin. Table 6 lists 19 mAbs with warnings for, and reports of, anaphylaxis, with 5 employed in cancer therapy (Table 5) and 14 for other disorders (Table 4). Severe infusion reactions that occur with some mAbs and which show some similar symptoms to anaphylaxis (see Section 4.2) can sometimes make distinguishing the two difficult and lead to doubts about the true incidence of anaphylaxis.

Table 6.
Individual approved monoclonal antibodies associated with adverse events affecting different organs and tissues.
Box 1. Hypersensitivity reactions, known and some suspected, to approved monoclonal antibodies used for therapy.
- TypeI hypersensitivity: Warnings for, and reports of, anaphylaxis account for ≈18% of mAbs, 14 used for non-cancer indications and 5 for cancer indications. Reslizumab and obiltoxaximab are covered by a black box warning for anaphylaxis. Urticaria occurs more often with the non-cancer mAbs.
- Serious infusion reactions with signs and symptoms resembling, and sometimes confused with anaphylaxis, occur with some mAbs, for example, alemtuzumab, cetuximab, dinutuximab, ibritumomab tiuxetan, naxitamab-gqgk, panitumumab, rituximab, trastazumab, and vedolizumab. Cytokine release appears to be involved.
- There is as yet no good evidence that many cytopenias are type II hypersensitivities, but these may occur with, for example, abciximab, alemtuzumab for multiple sclerosis and rituximab. Autoimmune hemolytic anemia may be induced by alemtuzumab and rituximab and rituximab-induced early- and late-onset neutropenia may be immune-mediated.
- Type III hypersensitivities, serum sickness-like reactions, cutaneous vasculitis, and hypersensitivity pneumonitis (may be a combined type III and IV hypersensitivity) occur with, for example, infliximab, adalimumab, and alirocumab. Checkpoint inhibitors including ipilimumab, nivolumab, and avelumab (Table 5) may also induce hypersensitivity pneumonitis. Chimeric mAbs (e.g., rituximab) and the humanized mAb omalizumab may cause a serum sickness-like reaction.
- Precise mechanisms for immune-mediated colitis, hepatitis, nephritis, hypothyroidism, and endocrinopathies induced by mAbs targeted to PD-1 and PD-L1 checkpoint inhibitors are not yet established.
- Type IV hypersensitivities: Rare Stevens–Johnson syndrome reactions have been reported to adalimumab, brentuximab vedotin, infliximab, and rituximab; toxic epidermal necrolysis has been induced by ibritumomab tiuxetan and rituximab. Adalimumab, ibritumomab tiuxetan, infliximab, and naxitamab-gqgk have been implicated in cases of erythema multiforme (EM). Paraneoplastic pemphigus, lichenoid dermatitis, and vesiculobullous dermatitis have occurred after rituximab. Dermatitis may occur after some mAbs, e.g., bevacizumab, catumaxomab, denosumab, and panitumumab. Immune-mediated cutaneous reactions induced by, e.g., cemiplimab-rwlc and durvalumab may be type IV hypersensitivities but mechanisms are not yet unequivocally established. Skin manifestations of rash and pruritus, often seen after many mAbs (Table 4 and Table 5), are generally not true hypersensitivity reactions.
There are a number of reports of mAb-induced cytopenias suggesting an underlying immune mechanism [19], but because of the lack of proper investigations, there are few convincing reports of the involvement of mAbs in type II hypersensitivity responses (Box 1). Thrombocytopenia after abciximab treatment [24,25] and cases of alemtuzumab-induced immune thrombocytopenia [26,27], neutropenia [27], autoimmune hemolytic anemia [28,29], and pure red cell aplasia [27] provide perhaps the best examples of immune-mediated true hypersensitivity responses. Apart from abciximab and alemtuzumab, rituximab has been implicated in thrombocytopenia [30], anemia [30], severe autoimmune hemolytic anemia [31], and early-onset and late-onset forms of neutropenia [30,32,33]. Although early- and late-onset neutropenia are well-known side effects of rituximab, the mechanisms have yet to be firmly established. Both forms are suspected examples of a mAb-induced type II hypersensitivity, although late-onset neutropenia may involve autoantibodies and appears to be due to a different mechanism than the early-onset form. Involvement of trastuzumab in severe thrombocytopenia has been reported [34]. See also the section on cytopenias below and Table 6.
Hypersensitivity (cutaneous) vasculitis (Figure 2), serum sickness, and hypersensitivity pneumonitis are examples of type III hypersensitivities induced by mAbs (Box 1, Table 5). Apart from the fully human mAbs adalimumab and alirocumab (the latter subject to a warning), for possible hypersensitivity vasculitis, again, the chimeric antibodies, such as rituximab and infliximab, are the biggest cause of reactions. For example, cutaneous vasculitis associated with infliximab in the treatment of rheumatoid arthritis is known [35], and there are a number of reports of rituximab-induced vasculitis [36,37] and serum sickness [38,39,40]. In fact, rituximab-induced serum sickness is said to occur in up to 20% of treated patients [41]. Checkpoint inhibitors ipilimumab, nivolumab, pembrolizumab, cemiplimab-rwlc, atezolizumab, avelumab, and durvalumab (Table 5) may cause hypersensitivity pneumonitis, generally thought to be a combined type III and IV hypersensitivity in a Th1/Th17 response [42,43,44]. As well as the adverse pulmonary reactions (Table 5 and Table 6), the checkpoint inhibitors may also provoke immune-mediated colitis, endocrinopathies, hepatitis, nephritis, and thyroiditis, reactions that might involve a type III hypersensitivity mechanism (Table 6).
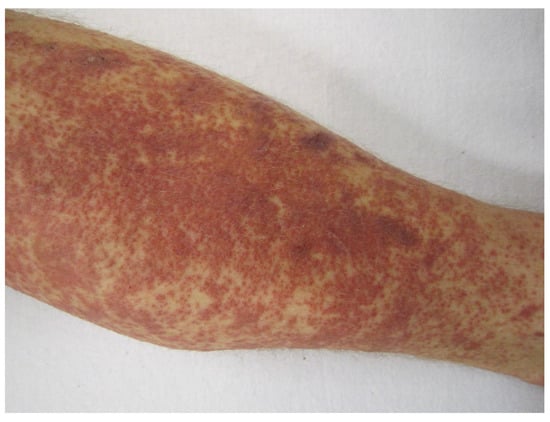
Figure 2.
Cutaneous (hypersensitivity) vasculitis, also known as cutaneous small-vessel vasculitis and cutaneous leukocytoclastic vasculitis. Author James Heilman MD. CC BY-SA 3.0 <https://creativecommons.org/licenses/by-sa/3.0>, via Wikimedia Commons (accessed on 14 December 2021).
Almost 40% of the 110 approved mAbs are associated with some sorts of adverse cutaneous effects, including type IV hypersensitivities [19] with rare cases of life-threatening cutaneous toxidermias (Table 4, Table 5 and Table 6, Box 1). Ibritumomab has an FDA boxed warning for severe cutaneous and mucocutaneous reactions, which includes Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), erythema multiforme (EM) (Figure 3), exfoliative dermatitis, and bullous dermatitis. Warnings and precautions apply to brentuximab vedotin for SJS; rituximab has been involved in cases of SJS, TEN, paraneoplastic pemphigus, lichenoid dermatitis, and vesiculobullous dermatitis; and EM has occurred with naxitamab-gqgk therapy. EM, SJS, and psoriasis have been reported for adalimumab. EGFR-targeted mAbs are known for so-called dermatologic acneiform toxicities that are not immune-mediated (see below, Section 4.2, Cutaneous reactions).
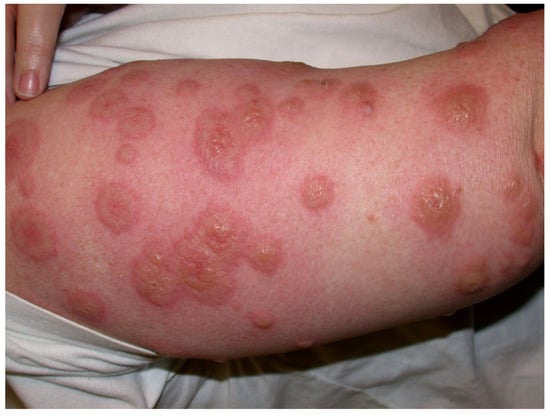
Figure 3.
Erythema multiforme with circumscribed bullous lesions. Image courtesy of Dr Adrian Mar.
4.2. Non-Immune-Mediated Adverse Responses to Approved Monoclonal Antibodies
As mentioned above, despite the specific targeting of mAbs to a particular disease-/disorder-associated tissue(s), the range and number of adverse events during or following therapy can sometimes be large and diverse. As summarized and discussed in Table 4, Table 5 and Table 6 and Box 2, many of these events do not have an immune basis or such a basis has yet to be convincingly demonstrated, either because sufficient investigation has yet to be undertaken or because of the clinical and laboratory difficulties involved in defining a precise mechanism(s). The list of recorded mAb-induced non-immune events is extensive and includes injection site reactions, infusion reactions, cytopenias, lung and liver injuries, heart effects, dermatologic toxicities, embryo and fetal toxicities, and a number of potentially life-threatening syndromes occurring with low frequency (Table 6, Box 2). It should be pointed out, however, that within some of these categories, it might be argued that there is, or may be, an immunological component with the involvement of cells and/or cytokines normally present in many inflammatory and immunological reactions.
Injection site reactions are very common, and when the preferred terms used in the Federal Adverse Event (FAERS) reporting system to describe such reactions are considered, namely, irritation, erythema, rash, bruising, swelling, induration, extravasation, reactions, pruritus, urticaria, hemorrhage, hematoma, and pain, it becomes apparent as to why such patient reactions are seen so regularly. In any injected population, it is to be expected that at least some individuals will respond with at least one of the above adverse effects. In the post-marketing period, the larger the population injected, the wider the collective list of adverse effects seen. The preferred terms listed are from the Medical Dictionary for Regulatory Activities (MedDRA; http://www.meddra.org/ accessed on 14 December 2021). Of the 66 approved mAbs for non-cancer therapy surveyed here, the FDA in its warnings, precautions, and lists of adverse reactions mentions injection site reactions as an adverse event for 24 (≈37%).
Box 2. Non-immune-mediated adverse events to monoclonal antibodies (mAbs).
- Infusion reactions. Usually mild–moderate or controllable by premedication. Fatal reactions can occur. Reactions have been recorded for almost 50% of approved mAbs. FDA boxed warnings for infusion reactions apply to 8 mAbs used for cancer therapy and 1 mAb used for other therapies.
- Cytopenia: Mechanisms of mAb-induced thrombocytopenia, neutropenia, lymphopenia, and hemolytic anemia are often not investigated/established. Cytopenia seen in more than 30% of the mAbs, especially those used in cancer therapy. Some may be immune-mediated.
- mAb-induced lung disease: Pathogenesis and pathophysiology are generally not known. At least 21 mAbs implicated. Some reactions are known, or suspected, to be immune-mediated.
- Cardiac events: Mechanisms mostly obscure. At least 20 mAbs implicated.
- Liver events: At least 22 mAbs implicated. Immune-mediated hepatitis is seen but other mechanisms often not well understood.
- Dermatologic toxicities: 39 (≈36%) of the mAbs elicited adverse cutaneous reactions of different severity from mild to severe. Rash and/or pruritus are common and were not included in the assessments. Apart from severe toxidermias (see text), papulopustular (acneiform) skin eruptions occur in response to EGFR-targeted antibodies, in particular, cetuximab, necitumumab, and panitumumab. Adverse reactions were seen in ≈29% of the non-cancer group and 50% of the mAbs used for cancer therapy.
- Embryo-fetal toxicity is recognized for 27 (≈25%) of the mAbs, including eight antibody–drug conjugates.
- Cytokine release syndrome (CRS): The distinguishing features between CRS and infusion reactions are often not clear. mAbs implicated include blinatumomab and catumaxomab.
- Tumor lysis syndrome (TLS): Anti-cancer mAbs may destroy large numbers of cells in a short period of time. Seen with brentuximab vedotin, blinatumomab, rituximab, and polatuzumab vedotin-piiq.
- Progressive multifocal leukoencephalopathy (PML): Rare but occasionally seen after mAbs directed to B cells, e.g., brentuximab vedotin, rituximab, obinutuzumab, vedolizumab, polatuzumab vedotin-piiq, and natalizumab.
- Other syndromes of poorly understood pathogenesis: Reversible posterior leukoencephalopathy syndrome (RPLS) 1 (cases reported after, e.g., bevacizumb and ramucirumab); immune reconstitution inflammatory syndrome (IRIS) (natalizumab); systemic inflammatory response syndrome (SIRS) (catumaxomab, eculizumab); capillary leak syndrome (CLS) (bevacizumab, dinutuximab); macrophage activation syndrome (MAS) (canakinumab).
1 Also known as posterior reversible encephalopathy syndrome (PRES)
Infusion reactions [3,19] to mAbs are common, usually with mild to moderate ‘flu’-like symptoms, but serious, potentially fatal reactions can occur. Table 6 shows that infusion reactions are known for 53 of the 110 approved mAbs (Table 4 and Table 5). Reactions may resemble anaphylaxis, and hypotension, cardiac arrest, urticaria, rash and pruritus may occur, usually after the first or second infusion, but IgE antibody reactions generally have a faster onset (often within minutes) and effects are more severe. The cytokines tumor necrosis factor (TNF) and IL-6, as well as high counts of circulating lymphocytes (e.g., >50 × 109/L) are thought to be involved [45]. The highest incidence of reactions occurs with human–rodent chimeric antibodies, e.g., rituximab and infliximab, and some humanized mAbs such as alemtuzumab, ocrelizumab, and trastuzumab. Table 6 lists the 53 mAbs shown to provoke infusion reactions. Rituximab and trastuzumab show the highest incidence of reactions with incidences for first infusion reactions of ≈77% and ≈40%, respectively. Premedication may be necessary in order to avoid or lessen reactions, for example, as sometimes found necessary with elotuzumab infused for multiple myeloma [46]. Overall, mAbs involved show a two to one infusion reaction ratio of mAbs for cancer compared to those for other indications. Eight mAbs for cancer indications carry a black box warning for infusion reactions, while 22 are subject to a warnings and precautions notice. The corresponding warnings for mAbs used for non-cancer therapies are one and nine, respectively.
Cytopenias commonly occur during and/or following mAb therapy, especially as a result of anti-cancer therapies. Of 34 mAbs implicated in the induction of cytopenias, 24 (≈71%) are anti-cancer agents and 10 (≈29%) relate to other indications (Table 4, Table 5 and Table 6). FDA boxed warnings have been issued for three mAbs, namely, for sacituzumab govetican-hziy-induced severe neutropenia and for cytopenia following ibritumomab tiuxetan and alemtuzumab, while FDA general warnings and precautions apply to 21 other mAbs listed in Table 7. In addition, other warnings of adverse events apply to brodalumab for neutropenia; to tocilizumab for neutropenia and thrombocytopenia; and to different cytopenias, namely, lymphocytopenia, for a high proportion of anti-neoplastic mAbs (Table 4 and Table 5). Note that because mechanisms of mAb-induced thrombocytopenia, neutropenia, lymphocytopenia, anemia, and what is often simply termed ‘cytopenia’ are often not investigated, some events may, in fact, be immune-mediated.

Table 7.
Approved monoclonal antibodies subject to FDA warnings and precautions for cytopenias.
Mab-induced pulmonary adverse events comprise a heterogeneous group of disorders, many of which remain poorly understood mechanistically. Of the 21 mAbs (counting alemtuzumab as Lemtrada® and Campath® as one mAb) listed in Table 6 and Box 3 (see also Table 4 and Table 5), immune-mediated or hypersensitivity pneumonitis is recognized as an important adverse event for an increasing number of mAbs, particularly checkpoint inhibitors [42,43,44,47]. This condition is now considered to be a combined type III and IV hypersensitivity in a Th1/Th17 response. Pneumonitis associated with checkpoint inhibitors is a rare, potentially fatal immune disease with an incidence of 2–5% [48]. Interestingly, the incidence is higher in non-small cell lung cancer than in melanoma [49]. For rituximab, while early-onset organizing pneumonia may be a hypersensitivity reaction, its prognosis is poorer than the late-onset form [50], which may be either a toxicity or due to immune restoration. Acute respiratory distress syndrome (ARDS) [51], seen for example with rituximab, trastuzumab, and ado-trastuzumab, may result from the release of pro-inflammatory cytokines such as IL-1β, TNF-α, IL-6, and IL-8, which are elevated both in bronchoalveolar lavage fluid and circulating plasma in ARDS patients [52]. Rituximab, alemtuzumab, trastuzumab, and panitumumab are responsible for the most severe and widest range of adverse lung events (Box 3).
Adverse cardiac events have occurred with at least 20 of the 110 approved mAbs (Table 4, Table 5 and Table 6 and Table 8) in a range of effects, including cardiomyopathy, myocardial infarction, cardiac arrhythmias, cardiopulmonary arrest, congestive heart failure, left ventricular dysfunction (LVD), decreased left ventricular ejection fraction (LVED), and QT interval prolongation (Table 8). FDA black box warnings apply to necitumumab for cardiopulmonary arrest; romosozumab-aqqg for the risk of myocardial infarction, cardiac events, stroke, and cardiovascular death; aldo-trastuzumab emtansine for cardiac toxicity; margetuximab-cmkb for LVD; and pertuzumab and trastuzumab, each for cardiomyopathy. Patients given mAbs targeted to HER2, namely, trastuzumab, ado-trastuzumab emtansine, and pertuzumab, show an increased risk of decreased LVED, especially if also given anthracyclines. Trastuzumab increases the risk of myocardial infarction 4–6 times, and again, the risk is highest when anthracyclines are also administered. Fam-trastuzumab deruxtecan-nxki carries a warning for LVD. Kounis and coworkers [53] believe that it is likely that many of the cardiac toxicities associated with mAbs used in cancer therapy share the same pathophysiology with Kounis syndrome. Suggested possible mAb involvements include -ximabs (e.g., rituximab, cetuximab, brentuximab); -zumabs (alemtuzumab, bevacizumab, trastuzumab, pertuzumab); -umabs (ipilimumab, panitumumab); and -omabs (catumaxomab, ibritumomab).

Table 8.
Cardiac adverse events caused by approved monoclonal antibodies used for therapy.
Box 3. Pulmonary adverse events caused by approved monoclonal antibodies.
- Mouse antibodies
- ߋ
- Ibrutumomab tiuxetan: hypersensitivity bronchospasm
- Human-mouse chimeric antibodies
- ߋ
- Cetuximab: interstitial pneumonitis
- ߋ
- Infliximab: interstitial lung disease
- ߋ
- Rituximab: ARDS, BOOP, bronchospasm, diffuse alveolar hemorrhage, immune-mediated (hypersensitivity) pneumonitis
- Humanised antibodies
- ߋ
- Ado-trastuzumab: interstitial lung disease, pneumonitis, ARDS, dyspnea, pulmonary infiltrates, radiation pneumonitis
- ߋ
- Alemtuzumab: pneumonitis, bronchospasm, diffuse alveolar hemorrhage, pulmonary infection
- ߋ
- Amivantamab-vmjw: interstitial lung disease, pneumonitis
- ߋ
- Atezolizumab: immune-mediated pneumonitis, dyspnea
- ߋ
- Bevacizumab: anaphylaxis/bronchospasm, pulmonary hemorrhage from tumor site
- ߋ
- Dostarlimab-gxly: immune-mediated pneumonitis
- ߋ
- Fam-trastuzumab deruxtecan-nxki: interstitial lung disease, pneumonitis
- ߋ
- Pembrolizumab: immune-mediated pneumonitis, dyspnea
- ߋ
- Trastuzumab: ARDS, BOOP, dyspnea, interstitial pneumonitis, pleural effusions, pulmonary infiltrates/fibrosis/edema
- Fully human antibodies
- ߋ
- Adalimumab: interstitial lung disease
- ߋ
- Avelumab: immune-mediated pneumonitis
- ߋ
- Cemiplimab-rwlc: immune-mediated pneumonitis
- ߋ
- Durvalumab: immune-mediated pneumonitis, dyspnea
- ߋ
- Golimumab: interstitial lung disease
- ߋ
- Ipilimumab: immune-mediated pneumonitis
- ߋ
- Nivolumab: immune-mediated pneumonitis, dyspnea
- ߋ
- Panitumumab: interstitial lung disease, lung infiltrates, pneumonitis, pulmonary fibrosis
- ߋ
- Tisotumab vedotin-tftv: pneumonitis
ARDS—acute respiratory distress syndrome; BOOP—bronchiolitis obliterans organizing pneumonia; ‘pneumonitis’ is used when the mechanism remains uncertain.
As occurs with mAb-induced pulmonary adverse events, checkpoint inhibitors, both PD-L1- and PD-l-targeted mAbs, may elicit immune adverse reactions in the liver in the form of immune-mediated hepatitis. Another immune-based adverse effect may occur with the CD25 (IL-2R α-chain)-targeted mAb daclizumab, which is subject to an FDA box warning for hepatic injury including via an autoimmune mechanism. Other mAb-provoked adverse liver injuries include direct toxicities and reactivation of hepatitis (Table 4, Table 5 and Table 6 and Table 9). FDA warnings and precautions for non-immune mAb-induced liver injury apply to adalimumab, certolizumab pegol, evolocumab, golimumab, infliximab, natalizumab, vedolizumab, brentuximab vedotin, catumaxomab, cemiplimab-rwlc, elotuzumab, ofatumumab, polatuzumab vedotin-piiq, and rituximab. Four mAbs are subject to boxed warnings, gemtuzumab ozogamicin and inotuzumab ozogamicin for hepatotoxicity, including severe or fatal hepatic veno-occlusive disease; ado-trastuzumab emtansine for hepatotoxicity; and obinutuzumab for hepatitis B reactivation (Table 9). Three of these four mAbs are antibody–drug conjugates, suggesting involvement of the attached toxin in the severe hepatotoxicities. A warning applies to satralizumab-mwge for elevated liver enzymes ALT and AST.

Table 9.
Liver adverse events induced by approved monoclonal antibodies used for therapy.
Cutaneous reactions have been associated with at least 39 of the 110 different mAbs (≈36%; counting alemtuzumab and denosumab each only once) (Table 6). As discussed above, some of these reactions are true type IV hypersensitivities, and there are a few recorded examples of type I reactions such as urticaria (e.g., alirocumab), but mechanisms remain to be established for many of the other adverse events (Table 4 and Table 5, Box 2). FDA warnings and precautions for dermatologic toxicity/reactions have been issued for cemiplimab-rwlc, cetuximab, denosumab, dostarlimab-gxly, durvalumab, enfortumab vedotin-ejfv, and mogamulizumab-kpkc. It is not clear whether or not at least some of the skin reactions following checkpoint inhibitors (cemiplimab-rwlc, dostarlimab-gxly, durvalumab) are immune-mediated. Skin reactions to loncastumab tesirine-lpyl may also demonstrate photosensitivity. Antibodies targeted to EGFR, namely, amivantamab-vmjw, cetuximab, necitumumab, and panitumumab, are known to produce, and are subject to, FDA warnings for papulopustular (acneiform) skin eruptions (Figure 4) [54] and mucocutaneous reactions (mucositis, xerosis, paronychia, fissures, palmar-plantar rash, skin hyperpigmentation, and others), both of which are not immune-mediated. In addition, panitumumab carries an FDA black box warning for dermatologic toxicity and has been implicated in cases of erythema, exfoliation, paronychia, skin fissures, photosensitivity, xerosis, and pruritus.
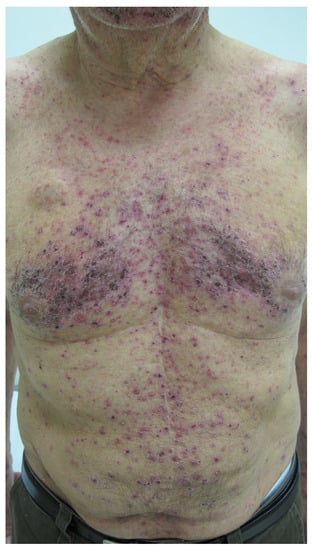
Figure 4.
Papulopustular (acneiform) eruption on a patient during treatment with panitumumab, targeted to epidermal growth factor receptor (EGFR). From Fabbrocini, G.; Cameli, N.; Romano, M.C.; et al. Chemotherapy and skin reactions. J. Exp. Clin. Cancer Res. 2012, 31, 50. DOI: 10.1186/1756-9966-31-50 [54], an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0) (accessed on 14 December 2021).
4.3. Rare Syndromes Associated with Monoclonal Antibody Therapy
Some rare, potentially life-threatening syndromes (Box 4) may occur with low frequency following the administration of some mAbs. Cytokine release syndrome (CRS) [55] shows similarities to severe infusion reactions in that both are related to a high lymphocyte count; counts greater than 50 × 109/L are associated with CRS and the release of TNF and IL-6. Symptoms include fever, chills, hypotension, nausea, vomiting, dyspnea, and an increase in liver enzymes. Rituximab is a well-known cause of CRS; other implicated mAbs are alemtuzumab, blinatumomab, and catumaxomab. Hemophagocytic lymphohistiocytosis (HLH) [56] is a rare, highly inflammatory disorder resembling cytokine storm involving proliferation of activated T cells and macrophages with the release of large amounts of cytokines, particularly IFN gamma, TNF, and GM-CSF. IL-1 and IL-6 released from activated macrophages are responsible for the inflammatory response, tissue damage, and symptoms of HLH. Two forms of HLH are known, primary, or familial, HLH and secondary, or acquired, HLH that occurs after malignancy, infection, or immunodeficiency. Blinatumomab is well known to be a rare cause and, more recently, immune checkpoint inhibitors avelumab, ipilimumab, and nivolumab have been rarely implicated. In the immune reconstitution inflammatory syndrome (IRIS) [57], also called immune recovery syndrome, restoration of immunity is, paradoxically, accompanied by deterioration of a known or new condition. Examples of the syndrome are seen in AIDS and tuberculosis. The pathogenesis of the condition is poorly understood. MAbs implicated in IRIS are adalimumab, ibalizumab-uiyk, infliximab, and natalizumab. Macrophage activation syndrome (MAS) [58] resembles HLH, but the name is traditionally reserved for the HLH-like inflammatory reaction seen in at least 10% of patients with rheumatologic diseases, in particular systemic juvenile idiopathic arthritis (SJIA). MAS, which can be rapidly fatal, is mediated by an uncontrolled proliferation of T cells and macrophages exhibiting hemophagocytic activity [59]. MAbs known to precipitate the syndrome include alemtuzumab, canakinumab, and tocilizumab. Progressive multifocal leukoencephalopathy (PML) [60] is a rare, usually fatal demyelinating disease characterized by inflammation and progressive brain damage. It is caused by infection with the normally harmless JC virus that becomes lethally active in immunosuppressed patients, in some autoimmune diseases, and in patients receiving chemotherapy, including some biologics. MAbs involved include belimumab, brentuximab vedotin, infliximab, eculizumab, natalizumab, ofatumumab, polatuzumab vedotin-piiq, rituximab, and vedolizumab. In reversible posterior encephalopathy syndrome (RPLS), also called posterior reversible encephalopathy syndrome (PRES [61]), edematous changes occur in the brain perhaps as a result of systemic hypertension leading to hypoxia and vasogenic edema. However, some cases of RPLS appear to occur in the absence of hypertension and others in the absence of inflammation. MAbs associated with RPLS include bevacizumab, certolizumab pegol, infliximab, dinutuximab, naxitamab-gqgk, ramucirumab, rituximab, and ustekinumab. Systemic capillary leak syndrome (SCLS) [62], also known simply as capillary leak syndrome, vascular leak syndrome, and Clarkson’s disease, has symptoms of body weight increase, malaise, weakness, pyrexia, myalgia, abdominal pain/vomiting, and diarrhea. An increase in vascular permeability and extravasation of fluids leads to peripheral and interstitial edema and, in severe form, pulmonary and cardiovascular failure. MAbs reported to be associated with CLS include alemtuzumab, basiliximab, bevacizumab, catumaxomab, dinutuximab, the immune checkpoint inhibitor nivolumab, and rituximab. Systemic inflammatory response syndrome (SIRS) [63], related to sepsis, can cause organ dysfunction and failure. It may be caused by infection or have a noninfectious basis such as trauma, pancreatitis, ischemia, anaphylaxis, or treatment with a biologic agent. The condition proceeds via activation of an inflammatory cascade of cytokines including TNF; IFN gamma; and IL-1, -6, and -8. SIRS has been reported following catumaxomab and eculizumab. Tumor lysis syndrome (TLS) [64] occurs most often in patients with leukemia and high-grade lymphomas where there are large numbers of cancer cells. Death of the cells results in marked ionic imbalance due to hypercalcemia, hyperkalemia, hyperphosphatemia, and hyperuricemia. This can lead to renal failure, cardiac arrhythmias, seizures, and death. The mAbs most often associated with TLS are alemtuzumab, blinatumomab, brentuximab vedotin, ipilimumab, obinutuzumab, polatuzumab vedotin-piiq, and rituximab (Box 4).
Box 4. Monoclonal antibodies associated with rare syndromes.
- Cytokine release syndrome (CRS)Alemtuzumab; blinatumomab; catumaxomab; rituximab
- Hemophagocytic lymphohistiocytosis (HLH)Alemtuzumab; avelumab; blinatumomab; ipilimumab; nivolumab
- Immune reconstitution inflammatory syndrome (IRIS)Adalimumab; ibalizumab-uiyk; infliximab; natalizumab
- Macrophage activation syndrome (MAS)Alemtuzumab; canakinumab; tocilizumab
- Progressive multifocal leukoencephalopathy (PML)Belimumab; brentuximab vedotin; infliximab; eculizumab; natalizumab; ocrelizumab; ofatumumab; polatuzumab vedotin-piiq; rituximab; vedolizumab
- Reversible posterior encephalopathy syndrome (RPLS)Bevacizumab; certolizumab pegol; infliximab; dinutuximab; naxitamab-gqgk; ramucirumab; rituximab; ustekinumab
- Systemic capillary leak syndrome (SCLS)Alemtuzumab; basiliximab; bevacizumab; catumaxomab; dinutuximab; nivolumab; rituximab
- Systemic inflammatory response syndrome (SIRS)Catumaxomab; eculizumab
- Tumor lysis syndrome (TLS)Alemtuzumab; blinatumomab; brentuximab vedotin; ipilimumab; obinutuzumab; polatuzumab vedotin-piiq; rituximab
5. Concluding Remarks
At the beginning of 2022, the catalog of mAbs approved for therapy by the FDA and/or EMA consisted of 66 approved for non-cancer indications and 46 for cancer therapy. Unsurprisingly because of their clinical success, the number of approved mAbs continues to expand, for example, in the 17 year period 1997–2013, 34 mAbs were approved, whereas in the 7 years of 2014–2020, the approved total was 61 (Figure 5) [65]. From 1997 until the present time (December 2021), 110 mAbs have received approval from the FDA and/or EMA (Figure 5). In 2021, 14 products were approved: aducanumab, amivantamab, anifrolumab, bimekizumab, casirivimab + imdevimab; dostarlimab-gxly, efgartigimod-alfa-fcab, evinacumab, loncastuximab teserine-lpyl, regdanvimab, sotrovimab, tezepelumab-ekko, tisotumab vedotin, and tralokinumab. Approved by the EMA, casirivimab + imdevimab, regdanvimab, and sotrovimab are the first three preparations for the treatment of COVID-19, each targeted to the spike protein receptor-binding domain of SARS-CoV-2 (Table 1). It is clear that from information on the numbers of mAbs already undergoing clinical assessment, as well as some already marketed for other indications with the view of repurposing for the treatment of COVID-19, further approvals of mAb preparations to treat this disease are imminent [65]. In late December 2021, efgartigimod-alfa-fcab indicated for myasthenia gravis and tezepelumab-ekko for severe asthma were approved by the FDA.
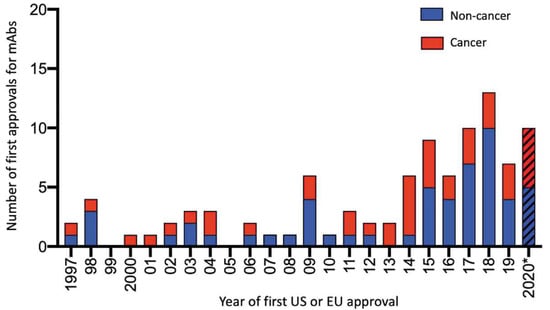
Figure 5.
Numbers of mAbs approved by the FDA and/or EMA during the 24 year period 1997–2020. Biosimilar and Fc fusion proteins are not included. Note that in 2021, 14 mAb products were approved. *: Data publicly available as of 25 November 2020. From Kaplon, H.; Reichert, J.M. Antibodies to watch in 2021. Mabs 2021, 13, e1860476, doi.org/10.1080/19420862.2020.1860476 [65], an Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial License (http://creativecommons.org/licenses/by-nc/4.0) (accessed on 14 December 2021).
In the next few years, research and clinical progress in disease pathogenesis and the identification of new disease biomarker targets, together with ongoing orphan drug development programs, will continue an inevitable expansion of the list of approved mAbs. Aspects of this expansion of great interest include a growing list of new indications; further mechanistic insights into the interplay between antibodies, cells, cytokines, chemokines, receptor interactions and downstream signaling; the appearance of new, and some unexpected, adverse events; and progress in understanding and treating such events.
Funding
No funding was received in relation to the preparation of this work.
Institutional Review Board Statement
Not relevant to this review.
Informed Consent Statement
Not relevant to this review.
Data Availability Statement
Any relevant data is available from author on request.
Conflicts of Interest
The author declares no conflict of interest.
References
- Ossipow, V.; Fischer, N. (Eds.) Methods and protocols. In Method in Molecular Biology, 2nd ed.; Humana Press: New York, NY, USA, 2014; Volume 1131. [Google Scholar]
- Weiner, G.J. Building better monoclonal antibody-based therapeutics. Nat. Rev. Cancer 2015, 15, 361–370. [Google Scholar] [CrossRef]
- Baldo, B.A. Safety of Biologics Therapy: Monoclonal Antibodies, Cytokines, Fusion Proteins, Hormones, Enzymes, Coagulation Proteins, Vaccines, Botulinum Toxins; Springer Nature: Cham, Switzerland, 2016; pp. 1–215. [Google Scholar]
- Kumar, R.; Parray, H.A.; Shrivastava, T.; Sinha, S.; Luthra, K. Phage display antibody libraries: A robust approach for generation of recombinant human monoclonal antibodies. Int. J. Biol. Macromol. 2019, 135, 907–918. [Google Scholar] [CrossRef]
- Kaur, H.; Reusch, D. (Eds.) Monoclonal antibodies. In Physicochemical Analysis; Academic Press: London, UK, 2021. [Google Scholar]
- Pedrioli, A.; Oxenius, A. Single B cell technologies for monoclonal antibody discovery. Trends Immunol. 2021, 42, 1143–1158. [Google Scholar] [CrossRef]
- Mondon, P.; Dubreuil, O.; Bouayadi, K.; Kharrat, H. Human antibodies: A race to engineer and explore a larger diversity. Front. Biosci. 2008, 13, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Rare Diseases: Common Issues in Drug Development Guidance for Industry. February 2019. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/rare-diseases-common-issues-drug-development-guidance-industry (accessed on 14 December 2021).
- Mulberg, A.E.; Bucci-Rechtweg, C.; Giuliano, J.; Jacoby, D.; Johnson, F.K.; Liu, Q.; Marsden, D.; McGoohan, S.; Nelson, R.; Patel, N.; et al. Regulatory strategies for rare diseases under current global regulatory statutes: A discussion with stakeholders. Orphanet J. Rare Dis. 2019, 14, 36. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.A.; Pagani, M. Adverse events to nontargeted and targeted chemotherapeutic agents. Immunol. Allergy Clin. N. Am. 2014, 34, 565–596. [Google Scholar] [CrossRef] [PubMed]
- Almagro, J.C.; Fransson, J. Humanization of antibodies. Front. Biosci. 2008, 13, 1619–1633. [Google Scholar] [CrossRef]
- Parray, H.A.; Shukla, S.; Samal, S.; Shrivastava, T.; Ahmed, S.; Sharma, C.; Kumar, R. Hybridoma technology a versatile method for isolation of monoclonal antibodies, its applicability across species, limitations, advancement and future perspectives. Int. Immunopharmacol. 2020, 85, 106639. [Google Scholar] [CrossRef]
- Lemtrada (alemtuzumab). Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/103948s5158lbl.pdf (accessed on 14 December 2021).
- Campath (alemtuzumab). Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/103948s5070lbl.pdf (accessed on 14 December 2021).
- Prolia (denosumab). Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125320s205lbl.pdf (accessed on 14 December 2021).
- Xgeva (denosumab). Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf (accessed on 14 December 2021).
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer. 2012, 12, 278–287. [Google Scholar] [CrossRef]
- Baldo, B.A.; Pham, N.H. Drug Allergy: Clinical Aspects, Diagnosis, Mechanisms, Structure-Activity Relationships, 2nd ed.; Springer Nature: Cham, Switzerland, 2021; pp. 6–8. [Google Scholar]
- Baldo, B.A. Adverse events to monoclonal antibodies used for cancer therapy. Focus on hypersensitivity responses. OncoImmunology 2013, 2, e26333. [Google Scholar] [CrossRef]
- Stephens, S.; Emtage, S.; Vetterlein, O.; Chaplin, L.; Bebbington, C.; Nesbitt, A.; Sopwith, M.; Athwal, D.; Novak, C.; Bodmer, M. Comprehensive pharmacokinetics of a humanized antibody and analysis of residual anti-idiotypic responses. Immunology 1995, 85, 668–674. [Google Scholar]
- Howard, J.F.; Bril, V.; Vu, T.; Karam, C.; Peric, S.; Margania, T.; Murai, H.; Bilinska, M.; Shakarishvili, R.; Smilowski, M.; et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): A multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021, 20, 526–536. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Colice, G.; Griffiths, J.M.; Almqvist, G.; Ponnarambil, S.; Kaur, P.; Ruberto, G.; Bowen, K.; Hellqvist, Å.; Mo, M.; et al. NAVIGATOR: A phase 3 multicentre, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate Nthe efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma. Respir. Res. 2020, 13, 266. [Google Scholar] [CrossRef]
- Menzies-Gow, A.; Corren, J.; Bourdin, A.; Chupp, G.; Israel, E.; Wechsler, M.E.; Brightling, C.E.; Griffiths, J.M.; Hellqvist, Å.; Bowen, K.; et al. Tezepelumab in Adults and Adolescents with Severe, Uncontrolled Asthma. N. Engl. J. Med. 2021, 384, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Curtis, B.R.; Swyers, J.; Divgi, A.; McFarland, J.G.; Aster, R.H. Thrombocytopenia after second exposure to abciximab-coated platelets. Blood 2002, 9, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhambi, B.; Nyitray, W.; Sharma, G.; Shambaugh, S.; Antonescu, A.; Shukla, P.; Denny, E. Delayed profound thrombocytopenia presenting 7 days after use of abciximab (ReoPro). J. Cardiovasc. Pharmacol. Ther. 2002, 7, 21–24. [Google Scholar] [CrossRef]
- Cuker, A.; Bass, A.D.; Nadj, C.; Agius, M.A.; Steingo, B.; Selmaj, K.W.; Thoits, T.; Guerreiro, A.; Van Wijmeersch, B.; Ziemssen, T.; et al. Immune thrombocytopenia in alemtuzumab-treated MS patients: Incidence, detection, and management. Mult. Scler. J. 2020, 26, 48–56. [Google Scholar] [CrossRef]
- Aitken, L.; Patel, R.; D’Rozario, J.; Choi, P. Alemtuzumab induced red cell aplasia and other immune cytopenias—Not so ‘pure’. Immunotherapy 2021, 14, 95–99. [Google Scholar] [CrossRef]
- Reickmann, P.; Lenz, A.; Hoffmann, M. Fatal autoimmune hemolytic anemia associated with alemtuzumab in a MS patient with severe relapsing remitting disease course and prior immune therapies (P2.103). Neurology 2016, 86 (Suppl. 16), P2.103. [Google Scholar]
- Desai, P.A.; Romere, C.M.; Nguyen, L.; Saksena, A.; Abdullah, S.J.; Diaz, A.E. Severe Coombs positive autoimmune hemolytic anemia after alemtuzumab infusion for relapsing remitting multiple sclerosis. What can we learn? Blood 2018, 132 (Suppl. 1), 2331. [Google Scholar] [CrossRef]
- Cattaneo, C.; Spedini, P.; Casari, S.; Re, A.; Tucci, A.; Borlenghi, E.; Ungari, M.; Ruggeri, G.; Rossi, G. Delayed-onset peripheral blood cytopenia after rituximab: Frequency and risk factor assessment in a consecutive series of 77 treatments. Leuk. Lymphoma 2006, 47, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, E.; Topart, D.; Richard, B.; Jourdan, J.; Sotto, A. Severe autoimmune hemolytic anemia following rituximab therapy in a patient with a lymphoproliferative disorder. Leuk. Lymphoma 2003, 44, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Dunleavy, K.; Tay, K.; Wilson, W.H. Rituximab-associated neutropenia. Semin. Hematol. 2010, 47, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Weissmann-Brenner, A.; Brenner, B.; Belyaeva, I.; Lahav, M.; Rabizadeh, E. Rituximab-associated neutropenia: Description of three cases and an insight into the underlying pathogenesis. Med. Sci. Monit. 2011, 17, CS133–CS137. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, M.; Gogas, H.; Katsandris, A.; Meletis, J. Severe thrombocytopenia related to trastuzumab infusion. Med. Sci. Monit. 2011, 17, CS85–CS87. [Google Scholar] [CrossRef][Green Version]
- Anandacoomarasamy, A.; Kannangara, S.; Barnsley, L. Cutaneous vasculitis associated with infliximab in the treatment of rheumatoid arthritis. Intern. Med. J. 2005, 35, 638–640. [Google Scholar] [CrossRef]
- Kandula, P.; Kouides, P.A. Rituximab-induced leukocytoclastic vasculitis: A case report. Arch. Dermatol. 2006, 142, 243–253. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, H.O.; Kim, H.Y.; Park, Y.M. Rituximab-induced vasculitis: A case report and review of the medical published work. J. Dermatol. 2009, 36, 284–287. [Google Scholar] [CrossRef]
- D’Arcy, C.A.; Mannik, M. Serum sickness secondary to treatment with the murine-human chimeric antibody IDEC-C2B8 (rituximab). Arthritis Rheum. 2001, 44, 1717–1718. [Google Scholar] [CrossRef]
- Hellerstedt, B.; Ahmed, A. Delayed-type hypersensitivity reaction or serum sickness after rituximab treatment. Ann. Oncol. 2003, 14, 1792. [Google Scholar] [CrossRef]
- Finger, E.; Scheinberg, M. Development of serum sickness-like symptoms after rituximab infusion in two patients with severe hypergammaglobulinemia. J. Clin.Rheumatol. 2007, 13, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Fong, L.; Small, E.J. Anti-cytotoxic T-lymphocyte antigen-4 antibody: The first in an emerging class of immunomodulatory antibodies for cancer treatment. J. Clin. Oncol. 2008, 26, 5275–5283. [Google Scholar] [CrossRef] [PubMed]
- Mir, T.; Al-Masalmeh, N.; Ambreen, S. Acute pneumonitis due to nivolumab and ipilimumab combination. Am. J. Ther. 2022, 29, e126–e128. [Google Scholar] [CrossRef]
- Zhu, S.; Fu, Y.; Zhu, B.; Zhang, B.; Wang, J. Pneumonitis induced by immune checkpoint inhibitors: From clinical data to translational investigation. Front. Oncol. 2020, 10, 1785. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Zhu, E.C.; Wu, J.-B.; Li, T.; Hou, Y.-L.; Wang, D.-Y.; Gao, Z.-H. Risk of pneumonitis and pneumonia associated with immune checkpoint inhibitors for solid tumors: A systematic review and meta-analysis. Front. Immunol. 2019, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Winkler, U.; Jensen, M.; Manzke, O.; Schulz, H.; Diehl, V.; Engert, A. Cytokine-release syndrome in patients with B-cell chronic lymphocytic leukemia and high lymphocyte counts after treatment with an anti-CD20 monoclonal antibody (rituximab, IDEC-C2B8). Blood 1999, 94, 2217–2224. [Google Scholar] [CrossRef]
- Nooka, A.K.; Gleason, C.; Ollivierre Sargeant, M.; Walker, M.; Watson, M.; Panjic, E.H.; Lonial, S. Managing infusion reactions to new monoclonal antibodies in multiple myeloma: Daratumumab and elotuzumab. J. Oncol. Pract. 2018, 14, 414–422. [Google Scholar] [CrossRef]
- Huang, A.; Xu, Y.; Zang, X.; Wu, C.; Gao, J.; Sun, X.; Xie, M.; Ma, X.; Deng, H.; Song, J.; et al. Beigelman-Aubry, C. Radiographic features and prognosis of early- and late-onset non-small cell lung cancer immune checkpoint inhibitor-related pneumonitis. BMC Cancer 2021, 21, 634. [Google Scholar] [CrossRef]
- Pozzessere, C.; Bouchaab, H.; Jumeau, R.; Letovanec, I.; Daccord, C.; Bourhis, J.; Prior, J.O.; Peters, S.; Lazor, R.; Beigelman-Aubry, C. Relationship between pneumonitis induced by immune checkpoint inhibitors and the underlying parenchymal status: A retrospective study. ERJ Open Res. 2020, 6, 00165–02019. [Google Scholar] [CrossRef]
- Nishino, M.; Giobbie-Hurder, A.; Hatabu, H.; Ramaiya, N.H.; Hodi, F.S. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: A systematic review and meta-analysis. JAMA Oncol. 2016, 2, 1607–1616. [Google Scholar] [CrossRef]
- Lioté, H.; Lioté, F.; Séroussi, B.; Mayaud, C.; Cadranel, J. Rituximab-induced lung disease: A systematic literature review. Eur. Respir. J. 2010, 35, 681–687. [Google Scholar] [CrossRef]
- Han, S.; Rama, K.; Mallampalli, R.K. The acute respiratory distress syndrome: From mechanism to translation. J. Immunol. 2015, 194, 855–860. [Google Scholar] [CrossRef]
- Meduri, G.U.; Annane, D.; Chrousos, G.P.; Marik, P.E.; Sinclair, S.E. Activation and regulation of systemic inflammation in ARDS: Rationale for prolonged glucocorticoid therapy. Chest 2009, 136, 1631–1643. [Google Scholar] [CrossRef]
- Kounis, N.G.; Soufras, G.D.; Tsigkas, G.; Hahalis, G. Adverse cardiac events to monoclonal antibodies used for cancer therapy. Oncoimmunology 2014, 3, e27987. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Cameli, N.; Romano, M.C.; Mariano, M.; Panariello, L.; Bianca, D.; Monfrecola, G. Chemotherapy and skin reactions. J. Exp. Clin. Cancer Res. 2012, 31, 50. [Google Scholar] [CrossRef]
- Maude, S.L.; Barrett, D.; Teachey, D.; Grupp, S.A. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer J. 2014, 20, 119–122. [Google Scholar] [CrossRef]
- Daver, N.; McClain, K.; Allen, C.E.; Parikh, S.A.; Otrock, Z.; Rojas-Hernandez, C.; Blechacz, B.; Wang, S.; Minkov, M.; Jordan, M.B.; et al. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer 2017, 123, 3229–3240. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Shrestha, U. Immune Reconstitution Inflammatory Syndrome. Available online: https://www.ncbi.nlm.nih.gov/books/NBK567803/ (accessed on 14 December 2021).
- Malissen, N.; Lacotte, J.; Du-Thanh, A.; Gaudy-Marqueste, C.; Guillot, B.; Grob, J.-J. Macrophage activation syndrome: A new complication of checkpoint inhibitors. Eur. J. Cancer 2017, 77, 88–89. [Google Scholar] [CrossRef]
- Crayne, C.B.; Albeituni, S.; Nichols, K.E.; Cron, R.Q. The immunology of macrophage activation syndrome. Front. Immunol. 2019, 10, 119. [Google Scholar] [CrossRef]
- Cortese, I.; Reich, D.S.; Nath, A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat. Rev. Neurol. 2021, 17, 37–51. [Google Scholar] [CrossRef]
- Feske, S.K. Posterior reversible encephalopathy syndrome: A review. Semin. Neurol. 2011, 31, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Druey, K.M.; Greipp, P.R. Narrative review: The systemic capillary leak syndrome. Annals Intern. Med. 2010, 153, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Kaukonen, K.-M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.C.; Jones, D.P.; Pui, C.-H. The tumor lysis syndrome. N. Engl. J. Med. 2002, 364, 1844–1854. [Google Scholar] [CrossRef]
- Kaplon, H.; Reichert, J.M. Antibodies to watch in 2021. MABS 2021, 13, e1860476. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).