Abstract
Land abandonment is a multifaceted, nonlinear, worldwide phenomenon that is influenced by a variety of factors and opinions. The goal of this study was to understand the significance of land abandonment for true bugs and syrphids in three grassland management regimes that includes abandoned, intensive, and extensive alpine organic grasslands. In 2021 and 2022, we sampled true bugs and syrphids by applying observation plot and sweep netting sampling methods. Extensive grasslands had significantly higher true bug and syrphid abundance compared to abandoned grasslands. However, no difference of species richness was found in studied grassland regimes. Large numbers of unique species (25.5% true bugs and 21.5% syrphids) only occurred in the abandoned grasslands but not in intensive and extensive grasslands. Similarly, true bug assemblages in abandoned grasslands differed significantly from assemblages in intensive and extensive grasslands. We found that extensive grassland can manage to increase true bugs and syrphid abundance. Likewise, undisturbed abandoned grassland is not a threat to insect diversity, and supports the survival of more unique true bug and syrphid species. A mosaic landscape consisting of abandoned grassland along with grassland having different, mainly extensive, management intensity could be an ideal arrangement for alpine biodiversity conservation.
1. Introduction
In recent decades, agricultural systems and land usage in Europe have seen a tremendous transition due to interdependent changes in the social, financial, technical, and cultural context [1,2]. Such a transformation in agricultural areas caused landscape and biological alterations that resulted in land abandonment [3,4], which is associated with a loss of insect and plant biodiversity [5,6]. However, the literature also showed that land abandonment could enhance overall heterogeneity and biodiversity at different scales [7]. Therefore, two distinct land abandonment scenarios are possible: on the one hand, land abandonment may assist with passive landscape restoration [8], which will help to support biodiversity while minimizing the direct human effect on the environment. On the other hand, farmland biodiversity may be threatened by the land abandonment of agricultural areas [9]. The space allocated for agricultural areas has been decreasing especially in the European traditional alpine grassland “EUROSTAT, http://www.europa.eu/ (accessed on 2 February 2023)”. Among the various habitats, permanent alpine grassland provides a substantial contribution to the insect biodiversity of alpine agroecosystems [10,11]. However, between 1980 and 2000, 40% of grassland areas in the Alps were abandoned, which resulted in a reduction of 17% in livestock units [12]. Alpine grassland contributes greatly to the insect biodiversity of mountain agro-ecosystems while also providing a broad range of ecosystem services with socioeconomic value to human society [5]. Due to intensification, insect diversity in remaining agricultural areas, particularly in alpine grassland of high conservation value, is coming under more and more pressure. There is a consensus that intensive grassland management practices like frequent mowing or intense grazing have a negative impact on the diversity of insects [13,14]. To avoid such insect decline, a change in grassland management (reduced mown frequency, i.e., extensive management) was recommended to possibly halt insect decline [15,16,17]. Even though several studies have concentrated on how grassland management regimes affect insect biodiversity at the field scale [18,19,20], detailed studies considering three important grassland management regimes (intensive, extensive, and abandoned grassland) are still lacking.
In this study, we contribute to filling this gap by quantifying the impact of grassland management regimes on insect biodiversity in an Austrian alpine region. As important biotic elements of grassland ecosystems, we considered true bugs and syrphids as contrasting ecological groups inhabiting the grasslands. True bugs are a relatively sedentary insect group with a low level of food specialization [21], while syrphids are more mobile and display higher habitat specialization [22]. We selected three grassland types representing different management regimes including intensive grassland (three or more cuts per year), extensive grassland (one or two cuts per year), and abandoned grassland (not mown for more than 10 to 20 years), all of which are certified organic grassland.
In this study, we address the following questions: (a) Do true bug and syrphid abundance and species richness in abandoned grassland differ from low and high-intensity farming practices? (b) How do true bugs and syrphid species coexist among studied grassland management regimes? We hypothesized that (1) extensive grassland promotes a higher abundance of true bugs and syrphids compared to abandoned grassland; (2) there are more unique true bugs and syrphid species in abandoned grassland compared to extensive and intensive grassland because abandoned grassland is undisturbed, thus providing stable conditions, and further has woody vegetation offering additional structural complexity [10,11]; and (3) true bugs species assemblages in abandoned grassland are different from those in intensive and extensive grassland. Our findings might help policymakers to understand the influence of the present organic grassland structural shift from low- to high-intensity farming practices on insect biodiversity, as well as evaluate the impacts of abandonment in alpine agricultural systems.
2. Materials and Methods
2.1. Study Area and Site Characteristics
The study area is between 47°97′ N and 48°14′ N in the province of Lower Austria covering a total area of 19,186 km2. The yearly temperature is 10.36 °C (50.65 F) and it is 1.54% higher than Austria’s average temperature. The study area has 128.92 rainy days (35.32% of the time) and typically receives about 51.55 mm of precipitation annually. The study area is prevalently alpine with an average height from 633 m to 1345 m, and it is divided into several valleys with a dense hydrographical network [23]. Around 60% of the land is used for livestock farming, which dominates the agriculture sector.
The field experiment was carried out from May to August 2021 and 2022. Two distinct certified organic grassland types (intensive and extensive) were selected, which were close to each other and nearby abandoned grassland. In total, we surveyed 10 replicates of each extensive, intensive, and abandoned grassland (n = 30) located on south-facing slopes. Extensive grasslands did not receive inorganic fertilizers and were mown once or twice (two late mowings) per year. Intensive grasslands were mown a maximum four times per year. Both grassland types had similar topography and long-term history (>20 years) of grassland management and were used for hay production. At least 10–20 years have passed since management of the abandoned grasslands was discontinued. Extensive and intensive grasslands ranged in size from 550 to 2150 m2, whereas those that were abandoned ranged in size from 300 to 2300 m2. Grasses and legumes (i.e., Bromus erectus, Lotus corniculatus) dominated plant communities of the extensive grasslands, whereas few species of legumes and grasses (i.e., Lolium perenne, Trifolium repens) were dominant in intensive grasslands, and abandoned grasslands were dominated by Ajuga reptans and Cruciata laevipes. The soils of the study areas were all loamy humus Cambisols “http://www.unserboden.at/ (accessed on 3 February 2023).
2.2. Syrphids (Syrphidae) Sampling
Syrphids (Diptera: Syrphidae) sampling was achieved using two different methods: observation plot survey and sweep netting [24]. In observation plot surveys, we established five 4 m2 plots each at a distance of 10 m from a predetermined starting point. Each plot was observed for 10 min, and fast moving syrphid individuals were caught with an entomological hand net. Syrphids were also sampled with standardized sweep-netting. A total of 90 sweeps (three transects of 30 sweeps each, separated by 20 m) were performed from May to August 2021 and 2022 in each study site. All study sites were sampled in a randomized sequence to reduce sampling bias and considered most syrphid activity times between 9 am and 6 pm. Since the weather is known to have a significant impact on syrphid activity, syrphid sampling was only conducted while it was sunny weather [25]. No attempts were made to observe syrphid larvae. Syrphids within observation surveys and sweep netting were either identified on the study site or caught and preserved in 70% ethanol for later identification in the laboratory [26,27].
2.3. True Bugs (Hemiptera) Sampling
Similar to syrphid, we applied a sweep netting method with a 40 cm diameter sweep net to sample true bugs. In the middle of each study site, we applied three parallel 30 m long sweep net samplings each separated by 10 m distance. The sweep net was emptied after a few sweeps to avoid damage to the specimens. The sampling was repeated four times (May to August) in both 2021 and 2022 and completed under favorable weather i.e., sunny and dry days with temperatures ≥25 °C and little wind. The individuals captured were preserved in 70% ethanol and identified to species in the laboratory (adult true bugs were identified to species level while larvae were indeterminable and thus only counted). We identified true bugs using a variety of entomological handbooks of Wagner [28,29,30] and Strauss [31].
2.4. Vegetation Parameters
We estimated overall flower cover and plant height as a predictor of studied insect diversity. We recorded plant height (height from the ground to the top of the plant) and flower cover (abundance of insect-pollinated flowers) in five randomly selected plots per study site. The distance between each plot was 10 m. We placed a wooden frame of 1 × 1 m size, divided into 25 squares, on the ground to estimate overall flower cover [32]. The number of squares containing at least one nectar-producing flower was counted. Similar to the flower cover, plant height was measured five times per study site using a measuring tape, vertically inserted up to the soil level.
2.5. Statistical Analysis
We applied generalized linear mixed models (GLMMs) from the R package lme4 to evaluate abundance and species richness of true bugs and syrphids in the studied grassland management regimes [33]. We considered only true bugs adults for species richness because nymphs of many species cannot be identified up to species level, while adults and nymphs both were used in abundance analysis. We pooled all plots (five observation plots) and sweep nettings (three sweep nettings) per study site and added replications as a group level variable. This tells the lmer to fit GLMM models with a varying-intercept group effect using the variable replication. We used the Poisson family in GLMMs models since the response variables in our dataset (abundance and species richness) were count data. In all models we applied the dispersiontest function from the package AER [34] and the dispersion_glmer from the blmeco package [35] to counter overdispersion. To correct for overdispersion in a model, an extra observation-level random effect was added [36]. If a significant difference between studied grassland management regimes was identified, the glht function from package multcomp was used to perform Tukey’s post-hoc pairwise comparisons [37]. The multicollinearity in regression analysis among predictor variables (flower cover and plant height) was checked by using variance inflation factor (VIF) from package car that measures the correlation and strength of correlation between the predictor variables [38].
We generated Venn diagrams to understand true bugs and syrphids species overlapping attributes between studied grassland management regimes. Further, the species assemblage patterns were assessed by using Non-metric Multidimensional Scaling (NMDS) [39] after Hellinger transformation [40]. We used adonis from package vegan to calculate PERMANOVA (Bray-Curtis dissimilarities, 999 permutations) as performed in Hussain [32]. PERMANOVA assumption “homogeneity of variances” was fulfilled by using the function betadisper. Afterward, a multilevel pairwise comparison based on adonis, i.e., pairwise.adonis, was used to generate test results of species assemblages. The R software version 3.5.1 [41] was used for analysis, and model results were reported using criteria following Zuur and Ieno [42].
3. Results
In total, we found 6641 individuals (5119 adults and 1522 nymphs) and 98 species of true bugs, and 2315 individuals and 95 species of syrphids. Trigonotylus caelestialium (Kirkaldy, 1902), Leptopterna dolabrata (Linnaeus, 1758), Stenotus binotatus (Fabricius, 1794) and Megaloceroea recticornis (Geoffroy, 1785) comprised 51.7% of true bugs abundance, and Melanostoma mellinum (Linnaeus, 1758), Sphaerophoria scripta (Linnaeus, 1758) and Myathropa florea (Linnaeus, 1758) comprised 43% of syrphids abundance. Total abundance and species richness of true bugs and syrphids for each grassland management regime are shown in Appendix Table A1.
Extensive grasslands exhibited significantly higher true bug and syrphid abundance compared to abandoned grasslands. However, true bugs and syrphid species richness had a varied response among grassland management regimes, i.e., extensive grasslands had higher true bugs species richness compared to intensive grassland but was similar to abandoned grassland, and syrphids species richness was similar among the grassland management regimes studied (Table 1, Figure 1). True bugs and syrphids abundance increased with plant height, while flower cover caused a significant increase in syrphid abundance and species richness only. True bugs species richness was unaffected by plant height and flower cover. A VIF value of about 1 indicates that there is no correlation between a studied predictor variable, and that the coefficient estimates and p-values in the regression output are likely reliable (Appendix Table A2).

Table 1.
Generalized linear mixed effects models (GLMM) and Tukey’s post-hoc pairwise tests for differences in true bugs and syrphids abundance and richness amongst grassland management regimes (abandoned as intercept, extensive, and intensive) are summarized. The estimate of the corresponding variable in the final model (estimate), z-value (Z), standard errors (Std. error), and significant effects at p < 0.05 are marked in bold. CI = 95% confidence intervals; R2m = marginal R2; R2c = conditional R2; dispersion: 0.75 to 1.4.
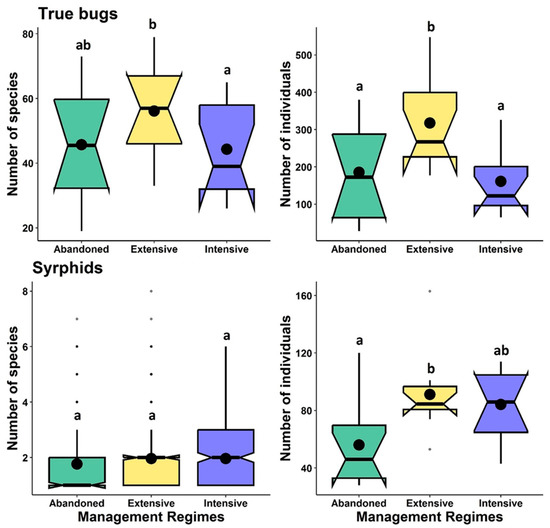
Figure 1.
Effects of studied grassland management regimes on the abundance and species richness of true bugs and syrphids. Box–Whisker plots display the medians (—), mean (●), notches, 25% and 75% percentiles, and outlier values (•). Box–Whisker plots with distinct letters differ significantly from one another (p < 0.05) using Tukey’s post-hoc pairwise tests.
The variation in species between the studied grassland management regimes was characterized using Venn diagrams (Figure 2). Overall, 30 true bugs and 28 syrphids were shared between grassland management regimes. Further, 8 true bugs and 11 syrphids species occurred exclusively in intensive grassland, 11 true bugs and 16 syrphid species occurred exclusively in extensive grassland, and 25 true bugs and 20 syrphid species occurred exclusively in abandoned grassland. Large numbers of species (25.5% true bugs and 21.5% syrphids) were only observed in abandoned grassland (Figure 2). True bugs assemblages in abandoned grassland differed significantly from those in intensive and extensive grassland (Table 2; Figure 3). Syrphids assemblages appeared to be similar in all three studied grassland management regimes. As syrphids assemblage data did not fulfil homogeneity of variance assumption, we did not calculate PERMANOVA.
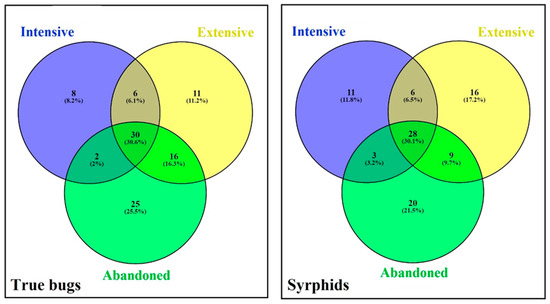
Figure 2.
Venn diagrams showing the main overlaps and differences in true bugs and syrphids species among grassland management regimes. Numbers and percentages are calculated using the total data set.

Table 2.
PERMANOVA (Pairwise-adonis) for true bugs in grassland management regimes i.e., abandoned, extensive, and intensive grassland. Significant p-values (<0.05) are shown in bold.
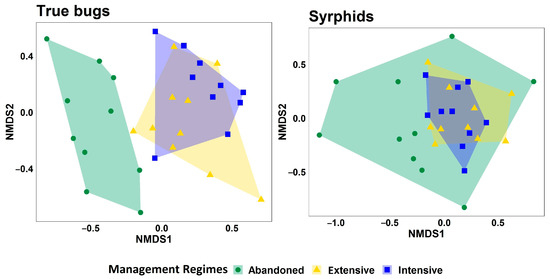
Figure 3.
Non-metric Multidimensional Scaling (NMDS) depending on species assemblages of true bugs and syrphids in grassland management regimes.
4. Discussion
It is well established that extensive grassland makes a considerable contribution to the insect biodiversity in agro-ecosystems [43]. There is a relatively large agreement that intense grassland management practices like frequent cutting or heavy grazing have a negative impact on insect biodiversity [13]. Of the three grassland management regimes, extensive grassland had a significant effect on true bug and syrphid abundance when compared to abandoned grassland. Surprisingly, abandoned and extensive grassland had a similar true bug and syrphid species richness. It is known that farming methods have a significant impact on true bugs [44]. As previously stated by Moir [45], it appears that the abundance of true bugs is a more sensitive indicator of the stability of grassy ecosystems than species richness. Woody vegetation, like in the abandoned grassland, is also known to influence insect assemblages [46]. Even in the alpine region, where semi-natural grasslands still support a rich invertebrate fauna [47], abandoned grassland probably serves as a secondary habitat for true bugs. Syrphids are reported to require pollen and nectar as a food source [48], and a variety of floral resources in extensive grassland might have a favorable impact on the abundance of syrphids.
Certain management practices, like mowing, directly affect insects by harming or eliminating individuals or by altering the habitat [49]. This is substantiated in our results due to the presence of more unique true bugs and syrphids species in abandoned grassland, which proves that the abandonment of organic grassland is not a threat to alpine insect diversity. According to published research, grassland management changes the vegetation’s structure and plant species composition [50], and it could have an impact on the microclimate and other elements of the microhabitat [51]. Due to the high sensitivity of many insects to microclimatic conditions, such indirect effects may change true bugs species compositions for two main reasons. Firstly, true bugs are known to have close relationships with plant species and achieve their highest richness in extensive grasslands [15]. Rapid changes in the vegetation structure of the plant community due to management in intensive and extensive grassland seems to have direct effects on true bugs at a species level and might force true bugs towards more stable abandoned grassland. Secondly, true bugs have both larval and adult stages that coexist in the same habitat and are sensitive to environmental changes [52]. There are several behaviors similar for observed syrphid species (i.e., some species from genus Pipizella or Cheilosia) that were only seen in abandoned grasslands, i.e., they prefer dense vegetation and fly close to the ground cover and bushes [53]. These species may require the period of transitional abandonment, which serves as a suitable habitat that offers resources for survival, reproduction, and mobility for true bugs and syrphids at species level.
The results presented here demonstrated that management effects are important for the species assemblages of true bugs and syrphids. Non-metric Multidimensional Scaling (NMDS) indicates that abandoned grassland has different true bugs assemblages compared to intensive and extensive grassland. It is probable that true bugs used stable and undisturbed abandoned grassland as a refuge because intensive and extensive grasslands are generally more disturbed by agricultural activities [54]. Contrary to true bugs, syrphids are mobile and spread across various grassland management regimes.
Interestingly, true bugs and syrphids increased with plant height. It is probable that many abundant true bugs were zoophagous or polyphagous herbivores and thus more responsive to the greater structural complexity of vegetation [15]. This may be because undisturbed and stable vegetation may provide more resources for overwintering, feeding, oviposition, and resting, thereby providing a larger potential area for colonization [55]. However, syrphids are known to depend on pollen and nectar for nutrition [48], and a greater abundance of floral resources within extensive grassland was associated with a higher syrphid abundance [56]. Future studies should also consider other predictor variables, i.e., duration of the abandonment and the structure of the surrounding landscape, for a more elaborative explanation of insect response to grassland abandonment.
Our results demonstrate the relevance of grassland management regimes for insect biodiversity in alpine landscapes where a large proportion of conventionally maintained grassland is present. This appears to be important considering the current high land use pressure [1], which has resulted in a large proportion of agricultural landscapes being abandoned especially in the alpine region. In support of hypothesis 1, extensive grassland promotes a high abundance of true bugs and syrphids compared to abandoned grassland. Abandoned grassland seems to be vital for true bugs and syrphids biodiversity in providing habitat for more unique species compared to intensive and extensive grassland, thus confirming hypothesis 2. In addition, true bugs assemblages were found to be different in abandoned grassland, thus being partly in line with hypothesis 3, indicating that other factors such as habitat disturbance [15] might drive variation in assemblages apart from grassland management.
5. Conclusions
It is challenging to explain the divergent effects of a complex and spatially diverse process of grassland management regimes such as land abandonment. In the literature, grassland abandonment is frequently viewed as a barrier to biodiversity conservation, an impression generally widespread among plant ecologists [57], and extensive grassland considered as a biodiversity rich habitat [5,58]. We found that extensive grassland can manage to increase true bugs and syrphid abundance. However, extensive and intensive grasslands are more disturbed by agricultural activities, and therefore drive more true bugs and syrphids species towards natural, undisturbed ecosystems of abandoned grassland. Our findings indicate that there are no “one-size-fits-all” policy solutions for managing biodiversity conservation upon land abandonment in alpine grassland. A mosaic landscape consisting of abandoned grassland along with grassland having different, mainly extensive, management intensity could be an ideal arrangement for alpine biodiversity conservation.
Author Contributions
Conceptualization, R.I.H. and T.F.; methodology, R.I.H.; study site selection, D.A. and W.S.; formal analysis, R.I.H.; resources, J.K.F. and T.F.; writing—original draft preparation, R.I.H.; writing—review and editing, R.I.H., T.F., D.A., W.S. and J.K.F.; supervision, T.F.; project administration, T.F. and J.K.F.; funding acquisition, J.K.F. and T.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study is part of the research project “Farmer Clusters for Realising Agrobiodiversity Management across Europe (FRAMEwork)” which was funded by the European Union, grant number 862731.
Data Availability Statement
The data presented in this study are available in figures and tables provided in the manuscript.
Acknowledgments
Special thanks to all the farmers and landowners for their permission to conduct investigations on their organic grassland farms. Thanks to Norbert Schuller for providing working materials and help in field sampling.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
List of true bugs and syrphids abundance and species richness in studied grassland management regimes. Abundance is given in numbers, while empty space indicates absence.
Table A1.
List of true bugs and syrphids abundance and species richness in studied grassland management regimes. Abundance is given in numbers, while empty space indicates absence.
| Species | Abandoned | Extensive | Intensive | |
|---|---|---|---|---|
| True bugs | ||||
| 1 | Adelphocoris lineolatus | 8 | 105 | 88 |
| 2 | Adelphocoris quadripunctatus | 10 | 16 | 12 |
| 3 | Adelphocoris seticornis | 37 | 26 | |
| 4 | Aelia acuminata | 1 | 2 | 2 |
| 5 | Alydus calcaratus | 1 | ||
| 6 | Berytinus clavipes | 2 | ||
| 7 | Berytinus crassipes | 1 | ||
| 8 | Berytinus minor | 5 | 11 | |
| 9 | Calocoris affinis | 10 | 6 | |
| 10 | Calocoris roseomaculatus | 1 | 3 | |
| 11 | Campyloneura virgula | 1 | ||
| 12 | Capsodes gothicus | 3 | ||
| 13 | Capsus ater | 6 | 13 | 15 |
| 14 | Carpocoris fuscispinus | 2 | 13 | 5 |
| 15 | Carpocoris purpureipennis | 2 | 7 | 1 |
| 16 | Charagochilus gyllenhalii | 1 | ||
| 17 | Chlamydatus pulicarius | 3 | 2 | |
| 18 | Chlamydatus pullus | 1 | ||
| 19 | Closterotomus biclavatus | 3 | ||
| 20 | Closterotomus norwegicus | 6 | 4 | |
| 21 | Coreus marginatus | 4 | 4 | |
| 22 | Coriomeris denticulatus | 1 | 2 | |
| 23 | Corizus hyoscyami | 6 | 3 | |
| 24 | Criocoris crassicornis | 14 | 1 | 2 |
| 25 | Cymus claviculus | 15 | 3 | 1 |
| 26 | Cymus glandicolor | 30 | 6 | |
| 27 | Deraeocoris ruber | 12 | 8 | 5 |
| 28 | Dicranocephalus medius | 1 | ||
| 29 | Dicyphus errans | 8 | ||
| 30 | Dicyphus hyalinipennis | 2 | ||
| 31 | Dolycoris baccarum | 2 | 12 | 6 |
| 32 | Dryophilocoris flavoquadrimaculatus | 1 | ||
| 33 | Europiella alpina | 3 | ||
| 34 | Eurygaster maura | 1 | 2 | 5 |
| 35 | Eysarcoris aeneus | 2 | 1 | |
| 36 | Eysarcoris venustissimus | 1 | ||
| 37 | Gastrodes abietum | 2 | ||
| 38 | Globiceps sphaegiformis | 2 | ||
| 39 | Hadrodemus m-flavum | 2 | ||
| 40 | Halticus apterus | 2 | 34 | 1 |
| 41 | Halticus sp. | 2 | 1 | |
| 42 | Himacerus mirmicoides | 57 | 25 | 2 |
| 43 | Kalama tricornis | 2 | 19 | 11 |
| 44 | Kleidocerys resedae | 3 | 2 | |
| 45 | Legnotus limbosus | 1 | ||
| 46 | Leptopterna dolabrata | 77 | 791 | 65 |
| 47 | Liocoris tripustulatus | 34 | ||
| 48 | Liorhyssus hyalinus | 2 | ||
| 49 | Lygocoris pabulinus | 3 | 15 | |
| 50 | Lygus pratensis | 30 | 98 | 107 |
| 51 | Lygus rugulipennis | 2 | 20 | 27 |
| 52 | Lygus sp. | 1 | ||
| 53 | Lygus wagneri | 1 | ||
| 54 | Megaloceroea recticornis | 159 | 194 | 36 |
| 55 | Megalonotus sabulicola | 1 | ||
| 56 | Mermitelocerus schmidtii | 11 | ||
| 57 | Metatropis rufescens | 6 | ||
| 58 | Metopoplax fuscinervis | 1 | ||
| 59 | Metopoplax origani | 1 | ||
| 60 | Myrmus miriformis | 4 | 4 | |
| 61 | Nabis ferus | 13 | 25 | 10 |
| 62 | Nabis flavomarginatus | 1 | ||
| 63 | Nabis rugosus | 67 | 29 | 6 |
| 64 | Notostira elongata | 33 | 99 | 94 |
| 65 | Nysius senecionis | 1 | 1 | |
| 66 | Orthocephalus saltator | 2 | ||
| 67 | Orthops basalis | 2 | 3 | 3 |
| 68 | Orthops kalmii | 5 | 11 | 4 |
| 69 | Oxycarenus pallens | 1 | ||
| 70 | Palomena prasina | 1 | ||
| 71 | Panaorus adspersus | 1 | ||
| 72 | Pentatoma rufipes | 1 | ||
| 73 | Peribalus strictus | 5 | 4 | 1 |
| 74 | Peritrechus geniculatus | 1 | ||
| 75 | Piezodorus lituratus | 1 | ||
| 76 | Pilophorus sp. | 1 | ||
| 77 | Pithanus maerkelii | 1 | 2 | |
| 78 | Plagiognathus arbustorum | 36 | ||
| 79 | Plagiognathus chrysanthemi | 15 | 206 | 36 |
| 80 | Polymerus holosericeus | 5 | ||
| 81 | Polymerus unifasciatus | 1 | 10 | |
| 82 | Rhopalus parumpunctatus | 22 | 10 | 6 |
| 83 | Rhopalus subrufus | 5 | ||
| 84 | Rhyparochromus pini | 3 | 1 | |
| 85 | Rhyparochromus vulgaris | 1 | 2 | |
| 86 | Rubiconia intermedia | 1 | ||
| 87 | Saldula pallipes | 2 | ||
| 88 | Scolopostethus affinis | 1 | ||
| 89 | Scolopostethus thomsoni | 2 | ||
| 90 | Spilostethus saxatilis | 2 | 13 | |
| 91 | Stenodema calcarata | 53 | 95 | 38 |
| 92 | Stenodema laevigata | 237 | 51 | 37 |
| 93 | Stenotus binotatus | 291 | 194 | 50 |
| 94 | Stictopleurus abutilon | 2 | 3 | 1 |
| 95 | Stictopleurus crassicornis | 1 | 1 | |
| 96 | Stictopleurus punctatonervosus | 4 | 2 | |
| 97 | Trigonotylus caelestialium | 24 | 211 | 557 |
| 98 | Tupiocoris rhododendri | 1 | ||
| Syrphids | ||||
| 1 | Baccha elongata | 6 | ||
| 2 | Brachyopa scutellaris | 1 | ||
| 3 | Cheilosia albitarsis | 3 | 4 | 1 |
| 4 | Cheilosia antiqua | 1 | 1 | |
| 5 | Cheilosia bergenstammi | 1 | ||
| 6 | Cheilosia chrysocoma | 1 | ||
| 7 | Cheilosia fraterna | 3 | ||
| 8 | Cheilosia grossa | 1 | ||
| 9 | Cheilosia impressa | 1 | 1 | |
| 10 | Cheilosia lasiopa | 1 | ||
| 11 | Cheilosia latifrons | 2 | ||
| 12 | Cheilosia nasutula | 1 | ||
| 13 | Cheilosia pagana | 2 | ||
| 14 | Cheilosia soror | 1 | ||
| 15 | Cheilosia spp. | 3 | 3 | 3 |
| 16 | Cheilosia variabilis | 1 | ||
| 17 | Cheilosia vernalis | 5 | 1 | |
| 18 | Chrysogaster solstitialis | 10 | 4 | |
| 19 | Chrysogaster virescens | 1 | 2 | |
| 20 | Chrysotoxum bicinctum | 3 | 2 | 5 |
| 21 | Chrysotoxum cautum | 2 | ||
| 22 | Chrysotoxum elegans | 1 | 1 | |
| 23 | Chrysotoxum festivum | 2 | 3 | 5 |
| 24 | Chrysotoxum octomaculatum | 1 | ||
| 25 | Dasysyrphus albostriatus | 1 | ||
| 26 | Didea alneti | 1 | ||
| 27 | Epistrophe eligans | 1 | ||
| 28 | Episyrphus balteatus | 40 | 67 | 41 |
| 29 | Eristalinus aeneus | 5 | ||
| 30 | Eristalinus sepulchralis | 1 | ||
| 31 | Eristalis arbustorum | 6 | 14 | 1 |
| 32 | Eristalis nemorum | 2 | ||
| 33 | Eristalis pertinax | 2 | ||
| 34 | Eristalis tenax | 48 | 61 | 58 |
| 35 | Eumerus ornatus | 1 | ||
| 36 | Eumerus sp. | 1 | ||
| 37 | Eumerus strigatus | 1 | ||
| 38 | Eumerus tuberculatus | 1 | ||
| 39 | Eupeodes bucculatus | 1 | 1 | 3 |
| 40 | Eupeodes corollae | 4 | 9 | 11 |
| 41 | Eupeodes lapponicus | 7 | 5 | 13 |
| 42 | Eupeodes latifasciatus | 3 | 2 | |
| 43 | Eupeodes luniger | 3 | 2 | 5 |
| 44 | Eupeodes nitens | 1 | ||
| 45 | Eupeodes lapponicus | 3 | 3 | 2 |
| 46 | Helophilus hybridus | 1 | ||
| 47 | Helophilus pendulus | 1 | ||
| 48 | Helophilus trivittatus | 2 | 2 | |
| 49 | Leucozona laternaria | 1 | ||
| 50 | Melanostoma mellinum | 60 | 143 | 188 |
| 51 | Melanostoma scalare | 4 | 4 | 8 |
| 52 | Meligramma cincta | 1 | 1 | |
| 53 | Merodon aberrans | 1 | ||
| 54 | Myathropa florea | 110 | 103 | 66 |
| 55 | Neoascia obliqua | 1 | 2 | |
| 56 | Neoascia podagrica | 2 | 1 | 8 |
| 57 | Orthonevra nobilis | 1 | ||
| 58 | Paragus haemorrhous | 1 | 1 | |
| 59 | Paragus spp. | 6 | 2 | |
| 60 | Parasyrphus annulatus | 1 | ||
| 61 | Parasyrphus lineolus | 1 | ||
| 62 | Parasyrphus lineolus | 1 | ||
| 63 | Philhelius pedissequus | 1 | ||
| 64 | Pipiza noctiluca | 4 | 1 | 2 |
| 65 | Pipizella spp. | 9 | ||
| 66 | Pipizella viduata | 35 | 77 | 51 |
| 67 | Pipizella viduata | 2 | 3 | 6 |
| 68 | Pipizella virens | 30 | 61 | 41 |
| 69 | Platycheirus albimanus | 10 | 10 | 18 |
| 70 | Platycheirus angustatus | 4 | ||
| 71 | Platycheirus clypeatus | 1 | 1 | |
| 72 | Platycheirus podagratus | 1 | ||
| 73 | Platycheirus scutatus | 1 | ||
| 74 | Platycheirus spp. | 1 | ||
| 75 | Rhingia campestris | 12 | 2 | 2 |
| 76 | Scaeva pyrastri | 1 | 3 | 4 |
| 77 | Scaeva selenitica | 8 | 1 | 7 |
| 78 | Sphaerophoria batava | 2 | 2 | |
| 79 | Sphaerophoria interrupta | 6 | 6 | 16 |
| 80 | Sphaerophoria scripta | 43 | 158 | 128 |
| 81 | Sphaerophoria taeniata | 6 | 43 | 58 |
| 82 | Sphegina clunipes | 1 | ||
| 83 | Syritta pipiens | 18 | 13 | 7 |
| 84 | Syrphus ribesii | 4 | 9 | 2 |
| 85 | Syrphus torvus | 11 | 35 | 40 |
| 86 | Syrphus vitripennis | 6 | 8 | 10 |
| 87 | Volucella inanis | 1 | ||
| 88 | Volucella pellucens | 2 | ||
| 89 | Xanthandrus comtus | 1 | ||
| 90 | Xanthogramma laetum | 1 | ||
| 91 | Xanthogramma pedissequum | 2 | 1 | 1 |
| 92 | Xylota segnis | 6 | 4 | 4 |
| 93 | Xylota tarda | 2 | ||
| 94 | Xylota xanthocnema | 2 | ||
| 95 | Xylota sylvarum | 1 |

Table A2.
Generalized linear mixed models (GLMMs) showing the effects of flower cover and plant height on true bugs and syrphids abundance and species richness. Significant p-values are shown in bold and variance inflation factor (VIF).
Table A2.
Generalized linear mixed models (GLMMs) showing the effects of flower cover and plant height on true bugs and syrphids abundance and species richness. Significant p-values are shown in bold and variance inflation factor (VIF).
| True Bugs | Estimate | Std. Error | Z | p | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| abundance | (Intercept) | 1.5423 | 0.3697 | 4.172 | <0.001 | 2.27–9.65 | ||
| Flower cover | −0.001 | 0.0073 | −0.18 | 0.8575 | 0.98–1.01 | |||
| Plant height | 0.0045 | 0.0020 | 2.199 | 0.0278 | 1.00–1.01 | |||
| R2m | 0.007 | R2c | 0.289 | Dispersion | 0.963 | VIF | 1.001 | |
| richness | (Intercept) | 0.9409 | 0.1828 | 5.147 | <0.001 | 1.79–3.67 | ||
| Flower cover | 0.0023 | 0.0037 | 0.618 | 0.537 | 0.99–1.01 | |||
| Plant height | −0.0008 | 0.0011 | −0.706 | 0.48 | 1.00–1.00 | |||
| R2m | 0.001 | R2c | 0.265 | Dispersion | 1.002 | VIF | 1.004 | |
| Syrphids | ||||||||
| abundance | (Intercept) | 0.6237 | 0.0734 | 8.489 | <0.001 | 1.62–2.15 | ||
| Flower cover | 0.0128 | 0.0035 | 3.671 | <0.001 | 1.01–1.02 | |||
| Plant height | 0.0026 | 0.0009 | 2.702 | 0.007 | 1.00–1.00 | |||
| R2m | 0.025 | R2c | 0.038 | Dispersion | 0.906 | VIF | 1.004 | |
| richness | (Intercept) | 0.4589 | 0.0664 | 6.906 | <0.001 | 1.39–1.80 | ||
| Flower cover | 0.0111 | 0.0034 | 3.269 | 0.001 | 1.00–1.02 | |||
| Plant height | 0.0018 | 0.0009 | 1.983 | 0.047 | 1.00–1.00 | |||
| R2m | 0.020 | R2c | 0.029 | Dispersion | 0.767 | VIF | 1.003 |
References
- Allen, D.C.; Bateman, H.L.; Warren, P.S.; de Albuquerque, F.S.; Arnett-Romero, S.; Harding, B. Long-term effects of land-use change on bird communities depend on spatial scale and land-use type. Ecosphere 2019, 10, e02952. [Google Scholar] [CrossRef]
- Martínez-Núñez, C.; Martínez-Prentice, R.; García-Navas, V. Land-use diversity predicts regional bird taxonomic and functional richness worldwide. Nat. Commun. 2023, 14, 1320. [Google Scholar] [CrossRef] [PubMed]
- Winkler, K.; Fuchs, R.; Rounsevell, M.; Herold, M. Global land use changes are four times greater than previously estimated. Nat. Commun. 2021, 12, 2501. [Google Scholar] [CrossRef] [PubMed]
- Baur, B.; Cremene, C.; Groza, G.; Rakosy, L.; Schileyko, A.A.; Baur, A.; Stoll, P.; Erhardt, A. Effects of abandonment of subalpine hay meadows on plant and invertebrate diversity in Transylvania, Romania. Biol. Conserv. 2006, 132, 261–273. [Google Scholar] [CrossRef]
- Marini, L.; Fontana, P.; Klimek, S.; Battisti, A.; Gaston, K.J. Impact of farm size and topography on plant and insect diversity of managed grasslands in the Alps. Biol. Conserv. 2009, 142, 394–403. [Google Scholar] [CrossRef]
- Bonelli, S.; Cerrato, C.; Barbero, F.; Boiani, M.V.; Buffa, G.; Casacci, L.P.; Fracastoro, L.; Provenzale, A.; Rivella, E.; Zaccagno, M.; et al. Changes in alpine butterfly communities during the last 40 years. Insects 2022, 13, 43. [Google Scholar] [CrossRef]
- Marini, L.; Scotton, M.; Klimek, S.; Isselstein, J.; Pecile, A. Effects of local factors on plant species richness and composition of Alpine meadows. Agric. Ecosyst. Environ. 2007, 119, 281–288. [Google Scholar] [CrossRef]
- Bowen, M.E.; McAlpine, C.A.; House, A.P.; Smith, G.C. Regrowth forests on abandoned agricultural land: A review of their habitat values for recovering forest fauna. Biol. Conserv. 2007, 140, 273–296. [Google Scholar]
- Uchida, K.; Ushimaru, A. Biodiversity declines due to abandonment and intensification of agricultural lands: Patterns and mechanisms. Ecol. Monogr. 2014, 84, 637–658. [Google Scholar] [CrossRef]
- Hussain, R.I.; Walcher, R.; Brandl, D.; Jernej, I.; Arnberger, A.; Zaller, J.G.; Frank, T. Influence of abandonment on syrphid assemblages in mountainous meadows. J. Appl. Entomol. 2018, 142, 450–456. [Google Scholar] [CrossRef]
- Walcher, R.; Hussain, R.I.; Sachslehner, L.; Bohner, A.; Jernej, I.; Zaller, J.G.; Arnberger, A.; Frank, T. Long-term abandonment of mountain meadows affects bumblebees, true bugs and grasshoppers: A case study in the Austrian Alps. Appl. Ecol. Environ. Res. 2019, 17, 5887–5908. [Google Scholar] [CrossRef]
- Streifeneder, T.; Tappeiner, U.; Ruffini, F.V.; Tappeiner, G.; Hoffmann, C. Perspective on the transformation of agricultural structures in the Alps. Comparison of agro-structural indicators synchronized with a local scale. Rev. Geogr. Alp. 2007, 95, 27–40. [Google Scholar] [CrossRef]
- Raven, P.H.; Wagner, D.L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl. Acad. Sci. USA 2021, 118, e2002548117. [Google Scholar] [CrossRef] [PubMed]
- Baur, B.; Cremene, C.; Groza, G.; Schileyko, A.; Baur, A.; Erhardt, A. Intensified grazing affects endemic plant and gastropod diversity in alpine grasslands of the Southern Carpathian mountains (Romania). Biologia 2007, 62, 438–445. [Google Scholar] [CrossRef]
- Di Giulio, M.; Edwards, P.J.; Meister, E. Enhancing insect diversity in agricultural grasslands: The roles of management and landscape structure. J. Appl. Ecol. 2001, 38, 310–319. [Google Scholar] [CrossRef]
- Noordijk, J.; Schaffers, A.P.; Heijerman, T.; Boer, P.; Gleichman, M.; Sýkora, K.V. Effects of vegetation management by mowing on ground-dwelling arthropods. Ecol. Eng. 2010, 36, 740–750. [Google Scholar] [CrossRef]
- Hussain, R.I.; Walcher, R.; Vogel, N.; Krautzer, B.; Rasran, L.; Frank, T. Effectiveness of flowers strips on insect’s restoration in intensive grassland. Agric. Ecosyst. Environ. 2023, 348, 108436. [Google Scholar] [CrossRef]
- Brandl, M.; Hussain, R.I.; Maas, B.; Rabl, D.; Pachinger, B.; Holzinger, W.; Krautzer, B.; Moser, D.; Frank, T. Improving insect conservation values of agri-environment schemes through diversified seed mixtures. Biol. Conserv. 2022, 269, 109530. [Google Scholar] [CrossRef]
- Hussain, R.I.; Brandl, M.; Maas, B.; Krautzer, B.; Frank, T.; Moser, D. Establishing new grasslands on crop fields: Short-term development of plant and arthropod communities. Restor. Ecol. 2022, 30, e13641. [Google Scholar] [CrossRef]
- Sohlström, E.H.; Brose, U.; van Klink, R.; Rall, B.C.; Rosenbaum, B.; Schädler, M.; Barnes, A.D. Future climate and land-use intensification modify arthropod community structure. Agric. Ecosyst. Environ. 2022, 327, 107830. [Google Scholar] [CrossRef]
- Torma, A.; Bozsó, M.; Gallé, R. Secondary habitats are important in biodiversity conservation: A case study on orthopterans along ditch banks. Anim. Biodivers. Conserv. 2018, 41, 97–108. [Google Scholar] [CrossRef]
- Moquet, L.; Laurent, E.; Bacchetta, R.; Jacquemart, A.L. Conservation of hoverflies (Diptera, Syrphidae) requires complementary resources at the landscape and local scales. Insect Conserv. Divers. 2018, 11, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Central Institute for Meteorology and Geodynamics. Zentral-Anstalt für Meteorologie und Geodynamik. Available online: https://www.zamg.ac.at/cms/en/ (accessed on 25 February 2023).
- Hussain, R.I.; Walcher, R.; Brandl, D.; Arnberger, A.; Zaller, J.G.; Frank, T. Efficiency of two methods of sampling used to assess the abundance and species diversity of adult Syrphidae (Diptera) in mountainous meadows in the Austrian and Swiss Alps. Eur. J. Entomol. 2018, 115, 150–156. [Google Scholar] [CrossRef]
- Mudri-Stojnić, S.; Andrić, A.; Jozan, Z.; Vujić, A. Pollinator diversity (Hymenoptera and Diptera) in semi-natural habitats in Serbia during summer. Arch. Biol. Sci. 2012, 64, 777–786. [Google Scholar] [CrossRef]
- Stubbs, A. British Hoverflies: An Illustrated Identification Guide; British Entomological & Natural History Society: Wokingham, UK, 1983. [Google Scholar]
- Van Veen, M.P. Hoverflies of Northwest Europe: Identification Keys to the Syrphidae; Brill: Leiden, The Netherlands, 2010. [Google Scholar]
- Wagner, E. Blindwanzen und Miriden. In Die TierweltDeutschlands und der Angrenzenden Meeresteile 41; Gustav Fischer: Jena, Germany, 1952. [Google Scholar]
- Wagner, E. Die Tierwelt Deutschlands und der angren-zenden Meeresteile nach ihren Merkmalen und nach ihrerLebensweise. In Wanzen Oder Heteroptera I. Pentatomorpha; Fischer: Jena, Germany, 1966; Volume 54. [Google Scholar]
- Wagner, E. Die Tierwelt Deutschlands und der angren-zenden Meeresteile nach ihren Merkmalen und nach ihrerLebensweise. In Wanzen Oder Heteroptera II. Cimi-Comorpha; Fischer: Jena, Germany, 1967; Volume 55. [Google Scholar]
- Strauss, G. CORISA Wanzenabbildungen. Biberach. 2010. Available online: www.corisa.de (accessed on 2 February 2023).
- Hussain, R.I.; Brandl, M.; Maas, B.; Rabl, D.; Walcher, R.; Krautzer, B.; Entling, M.H.; Moser, D.; Frank, T. Re-established grasslands on farmland promote pollinators more than predators. Agric. Ecosyst. Environ. 2021, 319, 107543. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kleiber, C.; Zeileis, A. Applied Econometrics with R; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Korner-Nievergelt, F.; Roth, T.; Von Felten, S.; Gu’elat, J.; Almasi, B.; KornerNievergelt, P. Bayesian Data Analysis in Ecology Using Linear Models with R, BUGS, and Stan; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Harrison, X.A. Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2014, 2, e616. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; He, D.; Wang, X.; Bai, M. The modeling and forecasting of carabid beetle distribution in northwestern China. Insects 2021, 12, 168. [Google Scholar] [CrossRef]
- Leyer, I.; Wesche, K. Multivariate Statistik in der Ökologie: Eine Einführung; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Sławska, M.; Bruckner, A.; Sławski, M. Edaphic Collembola assemblages of European temperate primeval forests gradually change along a forest-type gradient. Eur. J. Soil Biol. 2017, 80, 92–101. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing (Ver. v3.1.1). 2018. Available online: http://www.R-project.org (accessed on 2 February 2023).
- Zuur, A.F.; Ieno, E.N. A protocol for conducting and presenting results of regression-type analyses. Methods Ecol. Evol. 2016, 7, 636–645. [Google Scholar] [CrossRef]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands—More important for ecosystem services than you might think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Kőrösi, Á.; Batary, P.; Orosz, A.; Redei, D.; Baldi, A. Effects of grazing, vegetation structure and landscape complexity on grassland leafhoppers (Hemiptera: Auchenorrhyncha) and true bugs (Hemiptera: Heteroptera) in Hungary. Insect Conserv. Divers 2012, 5, 57–66. [Google Scholar] [CrossRef]
- Moir, M.L.; Brennan, K.E.C. Using bugs (Hemiptera) as ecological and environmental indicators in forest ecosystems. In Ecology Research Progress; Nova Science Publishers, Inc.: New York, NY, USA, 2007; pp. 79–115. [Google Scholar]
- Torma, A.; Gallé, R. Fine scale pattern of true bug assemblages (Heteroptera) across two natural edges. Acta Zool. Acad. Sci. Hung. 2011, 57, 367–383. [Google Scholar]
- Fischer, M.; Rudmann-Maurer, K.; Weyand, A.; Stöcklin, J. Agricultural land use and biodiversity in the Alps. Mt. Res. Dev. 2008, 28, 148–155. [Google Scholar] [CrossRef]
- Almohamad, R.; Verheggen, F.; Haubruge, É. Searching and oviposition behavior of aphidophagous hoverflies (Diptera: Syrhidae): A review. Biotechnol. Agron. Soc. Environ. 2009, 13, 467–481. [Google Scholar]
- Proske, A.; Lokatis, S.; Rolff, J. Impact of mowing frequency on arthropod abundance and diversity in urban habitats: A meta-analysis. Urban For. Urban Green. 2022, 76, 127714. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, J.; An, Y.; Xin, X.; Xu, D.; Yan, R.; Xu, L.; Shen, B.; Hou, L. Grassland Ecosystem Progress: A Review and Bibliometric Analysis Based on Research Publication over the Last Three Decades. Agronomy 2023, 13, 614. [Google Scholar] [CrossRef]
- Reid, A.M.; Hochuli, D.F. Grassland invertebrate assemblages in managed landscapes: Effect of host plant and microhabitat architecture. Austral Ecol. 2007, 32, 708–718. [Google Scholar] [CrossRef]
- Musolin, D.L. Insects in a warmer world: Ecological, physiological and life-history responses of true bugs (Heteroptera) to climate change. Glob. Chang. Biol. 2007, 13, 1565–1585. [Google Scholar] [CrossRef]
- Speight, M.C.D. Species accounts of European Syrphidae (Diptera). In Syrph the Net, the Database of European Syrphidae; Syrph the Net Publications: Dublin, Ireland, 2014; Volume 78, p. 321. [Google Scholar]
- Yuan, Z.Y.; Jiao, F.; Li, Y.H.; Kallenbach, R.L. Anthropogenic disturbances are key to maintaining the biodiversity of grasslands. Sci. Rep. 2016, 6, 22132. [Google Scholar] [CrossRef] [PubMed]
- Dennis, R.L.; Shreeve, T.G.; Van Dyck, H. Towards a functional resource-based concept for habitat: A butterfly biology viewpoint. Oikos 2003, 102, 417–426. [Google Scholar]
- Meyer, B.; Jauker, F.; Steffan-Dewenter, I. Contrasting resource-dependent responses of hoverfly richness and density to landscape structure. Basic Appl. Ecol. 2009, 10, 178–186. [Google Scholar] [CrossRef]
- Öckinger, E.; Eriksson, A.K.; Smith, H.G. Effects of grassland abandonment, restoration and management on butterflies and vascular plants. Biol. Conserv. 2006, 133, 291–300. [Google Scholar]
- Ravetto Enri, S.; Nucera, E.; Lonati, M.; Alberto, P.F.; Probo, M. The Biodiversity Promotion Areas: Effectiveness of agricultural direct payments on plant diversity conservation in the semi-natural grasslands of the Southern Swiss Alps. Biol. Conserv. 2020, 29, 4155–4172. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).