Abstract
Biodiversity is threatened due to land-use change, overexploitation, pollution, and anthropogenic climate change, altering ecosystem functioning around the globe. Protecting areas rich in biodiversity is often difficult without fully understanding and mapping species’ ecological niche requirements. As a result, the umbrella species concept is often applied, whereby conservation of a surrogate species is used to indirectly protect species that occupy similar ecological communities. One such species is the greater sage-grouse (Centrocercus urophasianus), which has been used as an umbrella to conserve other species within the sagebrush (Artemisia spp.) ecosystem. Sagebrush-steppe ecosystems within the United States have experienced drastic loss, fragmentation, and degradation of remaining habitat, threatening sagebrush-dependent fauna, resulting in west-wide conservation efforts to protect sage-grouse habitats, and presumably other sagebrush wildlife. We evaluated the effectiveness of the greater sage-grouse umbrella to conserve biodiversity using data-driven spatial occupancy and abundance models for seven sagebrush-dependent (obligate or associated) species across the greater Wyoming Basins Ecoregional Assessment (WBEA) area (345,300 km2) and assessed overlap with predicted sage-grouse occurrence. Predicted sage-grouse habitat from empirical models only partially (39–58%) captured habitats identified by predicted occurrence models for three sagebrush-obligate songbirds and 60% of biodiversity hotspots (richness of 4–6 species). Sage-grouse priority areas for conservation only captured 59% of model-predicted sage-grouse habitat, and only slightly fewer (56%) biodiversity hotspots. We suggest that the greater sage-grouse habitats may be partially effective as an umbrella for the conservation of sagebrush-dependent species within the sagebrush biome, and management actions aiming to conserve biodiversity should directly consider the explicit mapping of resource requirements for other taxonomic groups.
1. Introduction
Ecosystems across the globe are losing biodiversity at an alarming rate, with extinction estimated at 8–1000 times the background rate [1,2], and more than one quarter of described species are classified as threatened with extinction [3]. Major drivers of biodiversity decline include land-use change, overexploitation, pollution, and anthropogenic climate change [4,5,6,7]. Loss of biodiversity can have important consequences for ecosystem function and services [7,8,9]. Trends in biodiversity among biomes, while variable, continue to decline at increasing rates worldwide [10]. These concerns have resulted in national and international initiatives to protect 30% of the most biodiverse lands and waters by 2030 [11]. Although targeted conservation through protected areas and legislation has shown some success in reducing biodiversity loss [12], ecological representation and coverage of threatened species by protected areas remain inadequate [13].
As ecosystems are modified and animal populations decline, there are fewer locations and opportunities to conserve areas with functioning ecosystems that support associated species. Delaying actions to conserve landscapes risks further loss of lands to anthropogenic activities and species extirpation. Hotspots of biological diversity have been targeted as an approach to conserving habitat for multiple species. Such approaches identify areas where several target species may coincide with areas at risk of habitat loss [14]. These analyses are often conducted at broad spatial extents, including global [14], continental, and other coarse spatial scales [15,16]. Identifying biodiversity hotspots minimally requires a basic understanding of the ecological niche, or the range of environmental factors that allow each species to meet life history requirements and persist [17]. In the simplest form, a basic understanding of non-interactive scenopoetic conditions is required to map each species’ fundamental niche requirements [17,18,19]. Generic species range maps or species distribution models should capture fundamental niche conditions, or those conditions that broadly encompass the range of conditions where a species should be able to occur. Factors such as competition (i.e., competitive exclusion) and fitness variation (i.e., source–sink habitats; [20]) reduce the ecological (and thus geographical) space used by a species, whereby the realized niche is then a reduced subset of resources (and places) where the species persists. These realized niche conditions are likely not captured by such broad range or distribution maps [21].
However, when assessing biodiversity across broad scales, such as globally or ecosystem-wide, coarse inputs, including range or distribution maps, are often used [22]. At finer resolution, hotspots based on species richness (frequently still from range maps) are often applied, but this approach is often ineffective at capturing hotspots of unrelated taxa [15,23]. However, when mapping hotspots of biodiversity using specific niche requirements there may be a disconnect in scale, as these typically are considered at more local extents (e.g., [24]) due to a paucity of data that would allow quantification across larger (i.e., national or global) extents.
In lieu of mapping niche requirements for all (or many) species that occupy similar ecological communities, surrogate or umbrella species are often identified and used to indirectly protect the entire assemblage of species [25]. As originally conceived, an umbrella species should have large area requirements [26], with sufficient habitat protection for that species to persist, and other species should fall under that umbrella of protection [27], particularly those of conservation concern [26,28]. Ideal umbrella species may be habitat specialists [29,30], particularly if their realized niche requirements have been assessed and mapped. Some consider umbrella species to be most effective when there are co-occurring and taxonomically related species that need conservation actions [26]. Conceptually, using an umbrella species approach should therefore provide benefits to other species and ultimately increase the effectiveness of limited conservation resources. However, given that species within an ecosystem have different habitat requirements and life histories, it seems unlikely that a single species could represent the needs of all other species [31], and in practice the umbrella approach may be flawed [32], particularly when considering multiple taxonomic groups [23] or varying (smaller) scales [33]. Nevertheless, the umbrella approach may be a useful proxy for some species and in some ecological contexts with thorough evaluation to determine appropriate uses in conservation planning [34].
We evaluate the efficacy of using the greater sage-grouse (Centrocercus urophasianus, hereafter “sage-grouse”) as an umbrella species for the conservation of other sagebrush-(Artemisia spp.) dependent (obligate or associated) species that inhabit the ecologically and culturally important sagebrush ecosystem of the Western United States [35]. While other assessments on the effectiveness of sage-grouse as an umbrella species have occurred within the eastern range of the sage-grouse [32,34,35], none have occurred across large spatial extents covering multiple states. The sage-grouse is considered a sagebrush-obligate species because of its complete dependence on the sagebrush plant community through all life stages. In addition, large, contiguous habitat patches are needed to meet all seasonal habitat requirements [36,37,38,39]. Over the past two decades, sage-grouse have received significant attention from conservation and management communities due to large-scale reductions in range extent [40] and declining population numbers throughout the remaining portion of their range [41]. Since the late 1990s, sage-grouse were the subject of multiple petitions for listing under the Endangered Species Act of 1973 (ESA; 16 U.S.C. §1531 et seq.); these successive reviews led to dramatic increases in the level of investment into maintaining and improving sagebrush habitats on public and private lands (>$1 billion by federal, state, and local agencies, private landowners, and non-governmental organizations; [42]). Planning efforts by federal and state agencies to improve the effectiveness of sage-grouse conservation actions led to the development of a spatial strategy known as Priority Areas for Conservation (PACs), where conservation efforts are emphasized for the 39 sage-grouse populations across their range [43].
Although management actions and conservation strategies are being developed explicitly for the protection of sage-grouse, there is hope that these will also indirectly benefit some of the >735 species of plants, vertebrates, or invertebrates inhabiting the sagebrush ecosystem [44,45,46,47,48,49], providing a conceptual and logistical shortcut to reaching conservation goals [50,51]. Sage-grouse meet many of the criteria for an effective umbrella species, needing large contiguous habitat patches, having a well-described life history, and legal protection [48,52,53,54]. However, the level of coverage provided by a sage-grouse umbrella likely depends on how individual species respond to management and conservation actions and the realized overlap in resource requirements.
A previous ecoregional assessment effort developed spatial models predicting the occurrence and abundance of 15 different vertebrate species across the greater Wyoming Basins Ecoregion (Figure 1; [55]). For that effort, field data on the occurrence and abundance of vertebrates were collected over two years (2005–2006) across an area of 345,300 km2 [56] and spatial models were developed predicting the occurrence (logistic regression) and abundance (generalized linear models fit with Poisson or negative binomial distributions to counts) of individual species. All models were evaluated with independent data not used in model development, such as sage-grouse lek count data or point count data from breeding bird surveys, to ensure accurate predictive capacity [55]. We use range maps and these predictive models derived for seven sagebrush-dependent species (ScienceBase [57]) to evaluate the potential effectiveness of sage-grouse as a management target [58,59] for conserving biodiversity [60] within the sagebrush ecosystem.
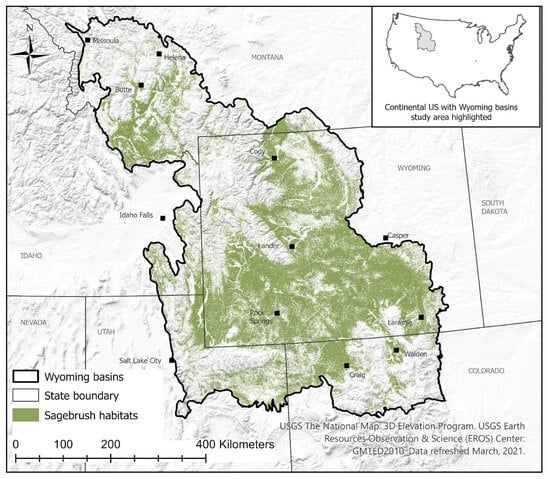
Figure 1.
The location of the Wyoming Basins ecoregional area in the western continental United States. Sagebrush habitats include all sagebrush land cover types [61] mapped as sagebrush (see [55]).
Here, we ask if (i) sage-grouse are an effective umbrella for other sagebrush-dependent (obligate or associated) vertebrate species of conservation concern at coarse (range maps) scales, finer (occurrence models) scales, and management units (PACs); and (ii) whether the most biodiverse habitats coincide with sage-grouse habitats at these same scales.
2. Materials and Methods
2.1. Study Area
The Wyoming Basins Ecoregional Assessment study area (WBEA) falls within five western states: Montana, Idaho, Wyoming, Utah, and Colorado, with over 50% of the WBEA located in Wyoming (Figure 1). Land management within WBEA primarily falls to federal agencies (61%; U.S. Forest Service, Bureau of Land Management, National Park Service, Bureau of Indian Affairs; [62]). Private landowners manage much of the remaining land (33%), state agencies control 5%, and The Nature Conservancy manages the remaining 1%. Although this area encompasses a portion of the Rocky Mountains, the dominant vegetation type is sagebrush (38%), and two-thirds of the WBEA study area is in Wyoming [63]. Less than 2% of sagebrush habitat in WBEA is protected from conversion to other land cover types. Although multiple threats exist (climate change, fragmentation, anthropogenic development, altered fire regimes, etc.; [42]), the presence of large contiguous patches of sagebrush in this region continues to support one of the most secure populations of sage-grouse in the West [41]. In addition, WBEA supports numerous other sagebrush-dependent species as well as those associated with mountainous and forested habitats (see [62] for a thorough description).
2.2. Background Species
When considering the usefulness of sage-grouse (see [64]) as an umbrella species within WBEA, we focused on six background species that have strong affiliations with sagebrush habitats, including four sagebrush-obligate species and two sagebrush-associated species. The three sagebrush-obligate bird species [65], Brewer’s sparrow (Spizella breweri), sagebrush sparrow (Artemisiospiza nevadensis), and sage thrasher (Oreoscoptes montanus), are considered to be the species of greatest conservation need in the sagebrush shrubland ecoregion in Wyoming [66]. We also considered the pronghorn (Antilocapra Americana; [67]), a big-game species that, while not considered a species of concern in Wyoming, relies on sagebrush habitats throughout the year for food and cover [68]. In addition, pronghorn are an economically important species, with almost half (47.1%) of the total population found in Wyoming [69]. Outdoor recreation, including big-game hunting, contributes $5.6 billion in spending annually to the state economy [70], and over 50,000 hunters participated in the pronghorn harvest in Wyoming in 2019 [71]. All four obligate species are tightly tied to sagebrush habitats and have some measure of vulnerability because of this association [66]. Sagebrush steppe is one of the most imperiled ecosystems in North America [72], and habitat loss from anthropogenic disturbances in sagebrush landscapes has been shown to have negative impacts on sage-grouse [73,74,75] and sagebrush sparrow [76,77,78]. Although Brewer’s sparrow, sagebrush sparrow, and sage thrasher coexist with sage-grouse in this region, these species may not necessarily benefit from the sage-grouse umbrella. Habitat manipulations undertaken to benefit sage-grouse can negatively affect other sagebrush-obligate vertebrates, as these species clearly partition their niches [79].
Sagebrush-associated species with weaker, but still significant, associations to sagebrush may also benefit from management under a sage-grouse umbrella. The sagebrush-associated species that we evaluated were the green-tailed towhee (Pipilo chlorurus; [65]) and the greater short-horned lizard (Phrynosoma hernandesi, hereafter “short-horned lizard”; [80]). While neither of these species are currently thought to be at risk in the region [66], both have characteristics that make them of management interest. The green-tailed towhee is a shrubland species that favors the ecotone between sagebrush and other shrub species [81]. The species’ foraging behaviors are adapted for open woodlands with a shrub understory, precisely the types of habitats targeted for conifer removal under sage-grouse management plans [82]. Short-horned lizards occur in a wide array of habitats. Though limited in distribution, the species is a conservation concern primarily because little is known about their habitat requirements and ecology [59,66]. We could have considered non-sagebrush-dependent species, which would alter our assessment and results, but also take our focus away from specifically evaluating sagebrush-dependent species under the sage-grouse umbrella.
All six background species have geographic ranges that substantially overlap that of sage-grouse within our study area (Figure 2). Ranges for bird species were developed by NatureServe [83] while that for the short-horned lizard was hand-digitized from maps in Stebbins (2003; [84]). To produce the range map for pronghorn across the WBEA study area, we combined the range maps for this species provided by the managing resource agency in each of the five states which overlap the study area (see [85]). All range maps should be considered the maximum extent of occurrence of the species within WBEA rather than an area of occupancy [86]. This definition typically results in overestimates of the true ranges [87,88], yet are regularly used in conservation planning because of the need to be inclusive rather than exclusive.
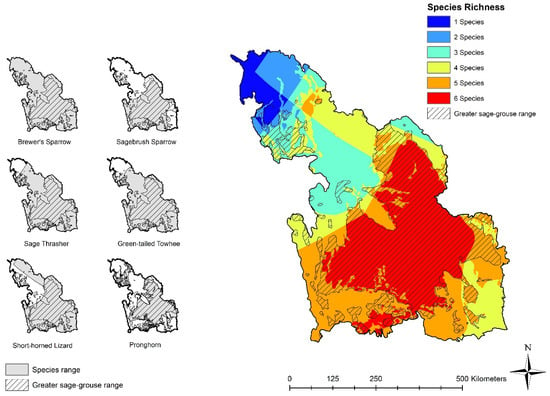
Figure 2.
Species distribution range maps of individual sagebrush-dependent species (Brewer’s sparrow (Spizella breweri), sagebrush sparrow (Artemisiospiza nevadensis), sage thrasher (Oreoscoptes montanus), pronghorn (Antilocapra americana), green-tailed towhee (Pipilo chlorurus), and greater short-horned lizard (Phrynosoma hernandesi)) within the Wyoming Basins (left panels) with a course spatial estimate of biodiversity (richness, right panel) based on summing the range map overlaps for all six species. Original species range distributions are adapted from Hanser et al. [55]. Greater sage-grouse (Centrocercus urophasianus) range shown in hatching.
2.3. Data Analyses
We conducted a hierarchical assessment of species overlap with and without sage-grouse, beginning with a simple comparison of range maps, followed by an assessment of spatial overlap from predicted occurrence models, which spatially identify ‘habitat’ for each species. First, we determined the amount of range overlap between sage-grouse and the background species within WBEA using overlays in ArcMAP 10.2 (Redlands, CA, USA) with existing range maps or predicted models for each species [55]. We calculated the percentage of WBEA that was designated as a given species range and then assessed how much of a species range within WBEA was captured by the sage-grouse range. This afforded some insight into the potential of sage-grouse as an umbrella for each sagebrush-dependent (obligate/associated) species across vertebrate taxonomic groups. We then assessed biodiversity (species richness) as the cumulative number of sagebrush-dependent species whose range overlapped a given pixel. Richness values range from 1–6 and we compared these with the range overlap of greater sage-grouse.
To highlight the importance of considering data resolution (i.e., coarse range maps versus model-predicted occurrence relationships) when initiating management actions, we carried out the same assessment outlined above using the model-predicted occurrence layers developed from field data and statistical models [55]. Models of occurrence or abundance were based on environmental (i.e., sagebrush cover), anthropogenic (i.e., distance to roads), and abiotic (i.e., terrain indices) covariates [55] for all species, including sage-grouse, and only predicted within the extent of each species’ known range [55]. We thresholded abundance models (Poisson or negative binomial) developed for Brewer’s sparrow, sage thrasher, and sagebrush sparrow from point counts, to define the occurrence of suitable habitat that was predicted to support at least one breeding songbird pair [65]. Sage-grouse occurrence was previously modeled using an ordered logistic regression model to predict the absence, low abundance, and high abundance of sage-grouse pellets from transect surveys [64]. For our application, we considered both low and high abundance to define sage-grouse occurrence and therefore identified habitats used by sage-grouse. Pronghorn and short-horned lizard logistic regression models predicted occurrence based on presence and absence from transect surveys [67]. Thus, surfaces considered in our analyses represented predicted occurrences of each species at 90 m resolution across the WBEA study area [64,65,67,80], where predictions were restricted to roughly 289,120 km2 of sagebrush habitat within the WBEA [56]. We similarly developed biodiversity richness assessments based on predicted occurrence for each species across the WBEA. We evaluated predicted species distributions and richness throughout the WBEA and the overlapping sage-grouse distributional range within the basin.
To further evaluate the effectiveness of conservation efforts, we assessed the ability of PACs to capture species richness within the WBEA. PACs were identified by the Western Association of Fish and Wildlife Agencies (WAFWA) as sage-grouse management areas, based upon the high density of sage-grouse attending leks (breeding display sites), essentially buffering those leks based on the distances females move from a lek to nest. These PACs are thought to capture 75% of breeding populations within 25% of the species’ range [89], although explicit occupancy and abundance are not mapped with this process. Since the location of PACs was restricted to known sage-grouse range, we assessed the effectiveness of PACs at capturing 1) sage-grouse predicted occurrence, and 2) areas of biodiversity. Background species were not assessed individually in relation to PACs.
3. Results
3.1. Sage-Grouse and Species Distribution
3.1.1. Range Maps and Sage-Grouse Overlap
Brewer’s sparrow range was the most ubiquitous, covering the entire WBEA study area, while pronghorn range was the most limited within the Wyoming Basins (46%; Table 1). When considering species ranges within the WBEA captured by the delineated sage-grouse range (47% of WBEA; Table 1), pronghorn had the highest proportion of their range captured (86%) while Brewer’s sparrow had the least (47%; Table 1). The extent of each species’ distribution is delineated by range maps shown in Figure 2.

Table 1.
The percentage of the Wyoming Basins (WBEA) study area that is considered habitat for sagebrush-dependent species (Brewer’s sparrow (Spizella breweri), sagebrush sparrow (Artemisiospiza nevadensis), sage thrasher (Oreoscoptes montanus), pronghorn (Antilocapra americana), green-tailed towhee (Pipilo chlorurus), greater short-horned lizard (Phrynosoma hernandesi), and the greater sage-grouse (Centrocercus urophasianus; sage-grouse)) based on range maps and species occurrence models. Also included is the percentage of each species’ range within the WBEA that overlaps with the sage-grouse range and predicted occurrence. Reported percentages are relative to the WBEA area (345,300 km2; range maps) and the predicted space within the WBEA (289,000 km2; species occurrence models). Original species range distributions and predicted occurrence models are adapted from Hanser et al. [55].
3.1.2. Species Occurrence Models and Sage-Grouse Overlap
Occurrence for each background species was predicted over less of the WBEA, when compared to range maps, for all species except pronghorn (Table 1; Figure 3). Sage-grouse were predicted to occur across 24% of the entire WBEA (Table 1; compared to 47% based on the range map). Brewer’s sparrow was again the most widespread of the species and the greater short-horned lizard and sage thrasher had the most restrictive predicted habitat occurrence of the background species. Still, these more restricted species had at least 90% of their predicted habitat within the WBEA captured by the sage-grouse range, while the widespread Brewer’s sparrow had much less of their predicted habitat fall within the sage-grouse range (Table 1; Figure 3).
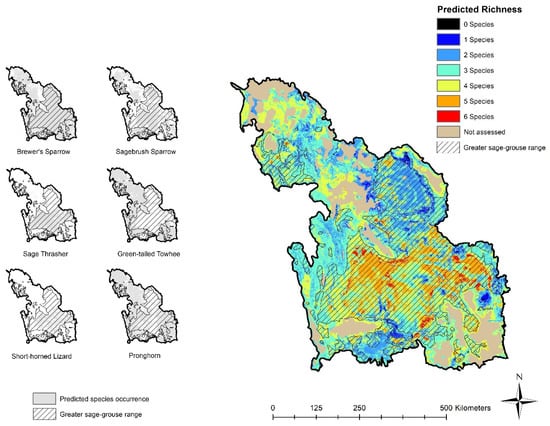
Figure 3.
The predicted occurrence of sagebrush-dependent species (Brewer’s sparrow (Spizella breweri), sagebrush sparrow (Artemisiospiza nevadensis), sage thrasher (Oreoscoptes montanus), pronghorn (Antilocapra americana), green-tailed towhee (Pipilo chlorurus) and greater short-horned lizard (Phrynosoma hernandesi)) within the Wyoming Basins based on predicted species abundance or occurrence models (left panels) with combined predictions estimating biodiversity (species richness, right panel) from summing predicted occurrence for all six species. Models are predicted at 90 m pixels and are adapted from Hanser et al. [55].
Limiting the evaluation to areas where sage-grouse were predicted to occur was much more restrictive, with only 39% of the sage-grouse range within the WBEA (Table 2; Figure 4) predicted to support sage-grouse occurrence. Background species habitat, based on predicted occurrence models, varied in their overlap with predicted sage-grouse occurrence (Table 2). Although Brewer’s sparrow was predicted to occur across virtually all sage-grouse range within the WBEA (Figure 4), predicted sage-grouse occurrence (24% of WBEA; Table 1) captured 39% of Brewer’s sparrow occurrence (Table 2). Across the entire WBEA, 64% of Brewer’s sparrow occurrence was captured by sage-grouse occurrence (64%; Table 1; Figure 3). Green-tailed towhee was predicted to occur across 42% of the sage-grouse range; 27% occurrence coincided with predicted sage-grouse occurrence (Table 2). Across the entire WBEA, 10% of predicted green-tailed towhee occurrence was captured by sage-grouse occurrence (Table 1; Figure 3). Pronghorn had 60% predicted occurrence within sage-grouse range, and 44% of pronghorn habitats aligned with predicted sage-grouse occurrence (Table 2; Figure 4). That percentage was much smaller (21%) when considering the predicted occurrence for pronghorn across the entire WBEA (Table 1; Figure 3). The short-horned lizard had the lowest predicted occurrence (28%) within the sage-grouse range; 68% of that aligned with predicted sage-grouse occurrence (Table 2), and nearly all of that overlap was captured (67%) when considering the predicted occurrence for lizards across the entire WBEA (Table 1; Figure 3). Sagebrush sparrow and sage thrasher both had greater than 55% predicted occurrence within the sage-grouse range, but only about half of those habitats coincided with predicted sage-grouse habitat (Table 2; Figure 4). Of the total predicted occurrence of Sagebrush sparrow and Sage thrasher across the WBEA, 32% and 52% coincided with predicted sage-grouse occurrence (Table 1; Figure 3).
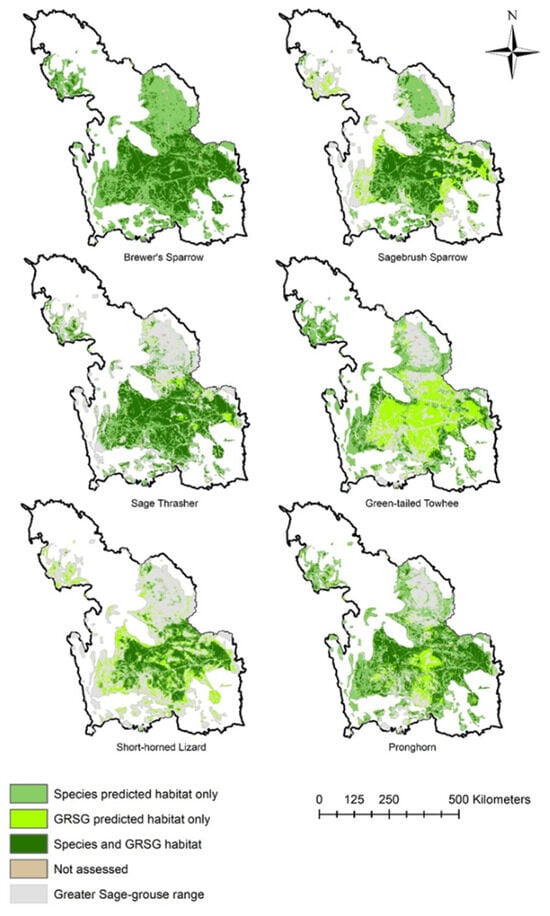
Figure 4.
Model-predicted habitat occurrence of sagebrush-dependent species (Brewer’s sparrow (Spizella breweri), sagebrush sparrow (Artemisiospiza nevadensis), sage thrasher (Oreoscoptes montanus], pronghorn (Antilocapra americana), green-tailed towhee (Pipilo chlorurus) and greater short-horned lizard (Phrynosoma hernandesi)) in comparison with predicted greater sage-grouse (GRSG; Centrocercus urophasianus; sage-grouse) habitat occurrence restricted to the sage-grouse range within the Wyoming Basin Ecoregional Assessment study area. Each panel shows the concordance (overlap) of each species’ predicted habitat (occurrence) with predicted sage-grouse habitat (occurrence). All models are based on 90 m predictions and are adapted from those developed by Hanser et al. [55].
3.1.3. Management Areas and Sage-Grouse Overlap
Within the WBEA sage-grouse range, 39% was predicted sage-grouse occurrence (Table 2), 46% of which was captured by the Priority Areas for Conservation (PACs).

Table 2.
Comparison of species’ predicted occurrence within the Wyoming Basins (WBEA) restricted to the greater sage-grouse (Centrocercus urophasianus; sage-grouse) range. Species included Brewer’s sparrow (Spizella breweri), sagebrush sparrow (Artemisiospiza nevadensis), sage thrasher (Oreoscoptes montanus), pronghorn (Antilocapra americana), green-tailed towhee (Pipilo chlorurus), and greater short-horned lizard (Phrynosoma hernandesi). Occurrence percentages are relative to the predicted occurrence within WBEA greater sage-grouse range (162,780 km2). Models are predicted at 90 m pixels and are adapted from Hanser et al. [55].
Table 2.
Comparison of species’ predicted occurrence within the Wyoming Basins (WBEA) restricted to the greater sage-grouse (Centrocercus urophasianus; sage-grouse) range. Species included Brewer’s sparrow (Spizella breweri), sagebrush sparrow (Artemisiospiza nevadensis), sage thrasher (Oreoscoptes montanus), pronghorn (Antilocapra americana), green-tailed towhee (Pipilo chlorurus), and greater short-horned lizard (Phrynosoma hernandesi). Occurrence percentages are relative to the predicted occurrence within WBEA greater sage-grouse range (162,780 km2). Models are predicted at 90 m pixels and are adapted from Hanser et al. [55].
| Species | Area within Sage-Grouse Range (km2) | Percent of Sage-Grouse Range (%) | Percent Overlap with Sage-Grouse Predicted Occurrence (%) |
|---|---|---|---|
| Brewer’s sparrow | 161,253 | 99 | 39 |
| sagebrush sparrow | 90,827 | 56 | 48 |
| sage thrasher | 95,512 | 59 | 58 |
| green-tailed towhee | 68,532 | 42 | 27 |
| short-horned lizard | 46,031 | 28 | 68 |
| pronghorn | 98,501 | 60 | 44 |
| greater sage-grouse | 63,486 | 39 |
3.2. Sage-Grouse and Species Richness
3.2.1. Range Maps and Richness
Using coarsely mapped range boundaries as surrogates for suitable habitat, roughly 33% of the WBEA had mapped ranges overlapping for all six background species, 96% of which occurred within the delineated sage-grouse range (Table 3; Figure 2). Seventy-seven percent of the WBEA had a richness of four or more background species based on range maps (Table 3; Figure 2). While richness areas containing five or six species were well captured by the sage-grouse range map, lower richness areas (containing 1–4 species) were less well represented, with 12% or less of these areas captured (Table 3; Figure 2).

Table 3.
The percentage of the Wyoming Basins (WBEA) study area that is estimated to support varying degrees of species richness based on range maps and occurrence models of background species. Also included is the percentage of each richness level within the WBEA that overlaps with the greater-sage grouse (Centrocercus urophasianus; sage-grouse) range and predicted occurrence. Reported percentages are relative to the WBEA area (345,300 km2; range maps) and the predicted space within the WBEA (289,000 km2; species occurrence models). Original species range distributions and predicted occurrence models are adapted from Hanser et al. [55].
3.2.2. Species Occurrence Models and Richness
When using predicted species occurrence models to define habitat, the proportion of the landscape that supported the most biodiverse areas was limited, with only 1% of the WBEA projected to have co-occurrence of all six background species; however, virtually all of those highest richness areas (99%) occurred within the delineated sage-grouse range and 65% of these areas also overlapped with predicted sage-grouse occurrence (Table 3; Figure 3). Similarly, while only 10% of WBEA was predicted to have five of the background species co-occur, 95% and 73% of that habitat was contained within the sage-grouse range or coincided with predicted sage-grouse occurrence, respectively. Approximately 38% of WBEA was predicted to support high-richness areas based on predicted occurrence maps (co-occurrence of 4–6 background species), and roughly two-thirds (67%) of those areas also occurred within the sage-grouse range (Table 3; Figure 3). Less biodiverse areas were not as well captured (Table 3; Figure 3).
3.2.3. Management Areas within Sage-Grouse Range and Richness from Predicted Occurrence
Fifty-nine percent of predicted sage-grouse occurrence fell within PACs. Biodiverse areas identified by the high richness of predicted background species occurrence were limited to a small portion of the sage-grouse range (i.e., 2% for co-occurrence of all six species; Table 4; Figure 3 and Figure 5). Areas supporting four or more species (i.e., high richness ≥ 4) cumulatively covered 47% of the sage-grouse range (Table 4; Figure 5A). Within the WBEA sage-grouse range, high richness habitats collectively covered 75,457 km2 (Table 4). Sixty percent of these high richness habitats were captured by model-predicted sage-grouse occurrence (Table 4; Figure 5B). Comparatively, the PACs coincided with 56% of high richness habitats, by area, across the sage-grouse range (Table 4; Figure 5C). However, the PACs encompassed a larger proportion of areas identified as having lower richness (1–3 species) than did predicted sage-grouse occurrence (Table 4; Figure 5C).

Table 4.
Richness within the Wyoming Basins (WBEA) greater sage-grouse (Centrocercus urophasianus; sage-grouse) range, model-predicted sage-grouse occurrence and priority areas of conservation. Richness was calculated based on the sum of predicted occurrence for sagebrush-dependent species (Brewer’s sparrow (Spizella breweri), sagebrush sparrow (Artemisiospiza nevadensis), sage thrasher (Oreoscoptes montanus), pronghorn (Antilocapra americana), green-tailed towhee (Pipilo chlorurus) and greater short-horned lizard (Phrynosoma hernandesi)). Reported percentages are relative to the richness within the background species’ combined model space within WBEA sage-grouse range (162,790 km2). All models are based on 90 m predictions and are adapted from those developed by Hanser et al. [55].
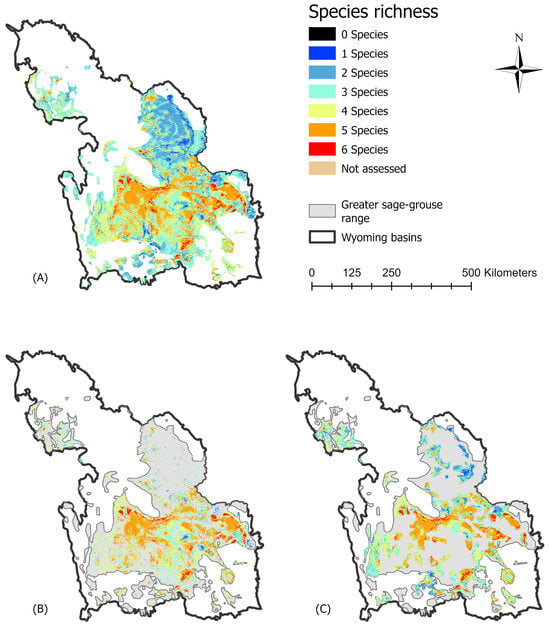
Figure 5.
Predicted biodiversity (species richness) based on the sum of predicted occurrence for sagebrush-dependent species in the Wyoming Basins Ecoregional Assessment (WBEA) study area (Brewer’s sparrow (Spizella breweri), sagebrush sparrow (Artemisiospiza nevadensis), sage thrasher (Oreoscoptes montanus), green-tailed towhee (Pipilo chlorurus), greater short-horned lizard (Phrynosoma hernandesi), and pronghorn (Antilocapra americana)). (A) Richness across the greater sage-grouse (Centrocercus urophasianus; sage-grouse) range in the WBEA; (B) Richness overlap within sage-grouse model-predicted occurrence of greater sage-grouse (non-gray; predicted absences in gray); and (C) Richness overlap within priority areas for conservation (non-gray) is shown for evaluation of a single species umbrella or identified conservation areas to capture biodiverse habitat for sagebrush vertebrates. All models are based on 90 m predictions and are adapted from those developed by Hanser et al. [55].
4. Discussion
Broad-scale range maps can be effective at capturing fundamental niche conditions [22], and in the absence of empirically modeled habitat relationships that more discretely predict occurrence across space, such maps can work well to predict biodiversity hotspots [23]. Not all habitat within a species’ range is necessarily used, nor contributes the resources required to sustain populations (realized niche concept; [18,19]). In the sagebrush ecosystem, we found that range maps suggested 33% of all habitats effectively supported all six of the sagebrush-dependent background species of conservation concern across our study area (Table 3; Figure 2). However, predicted occurrence models suggested that only 1% of the WBEA contained realized niche conditions that could support all six species (Table 3; Figure 3). While these biodiverse areas were largely contained within the sage-grouse range (five species: 95%; all six species: 99%; Table 3), these areas represent a relatively small portion of the sage-grouse range (19% combined; Table 4). Although hotspots of biodiversity are limited, the sage-grouse occurrence model appears to do less well capturing hotspots on the landscape (75% and 64% of five and six species classes, respectively; Table 4). However, the occurrence model is much more discriminatory, only identifying 24% of the WBEA as predicted sage-grouse habitat (Table 1), while the range map classifies 47% as being sage-grouse habitat (Table 1). Scale of inference clearly plays a role here, but caution should be exercised when applying broad-scale range maps to identify biodiversity hotspots for conservation. Conservation actions can be expensive, and the increased resolution of the occurrence models should help target actions to benefit multiple species.
An effective umbrella species occupies and uses a diversity of habitats over a broad landscape [25], such that any habitat protections for that species should effectively capture the niche requirements of many species falling under that umbrella [27]. Conceptually, sage-grouse should make an effective umbrella to conserve other species and the entire sagebrush ecosystem [35], as they are a sagebrush-obligate species [37], have diverse resource requirements across life stages [90], and occupy large home ranges [91]. In addition, unprecedented conservation and management efforts for the species have been undertaken since the turn of the century [43]. In practice, the umbrella concept can be difficult to achieve. Taxonomic differences [23,33], variation in scales of resource requirements [92], and simple niche segregation/partitioning [19] could make it difficult for one species to capture many other species, resulting in holes in the sage-grouse umbrella [32]. Indeed, our analyses suggest that at the most basic level, as an umbrella for other sagebrush-obligate birds (Brewer’s sparrow, sagebrush sparrow, and sage thrasher), sage-grouse may be only partially effective, as has been found by others [32,93]. Even when explicitly considering model-predicted annual sage-grouse habitat requirements from pellet occurrence [64], sage-grouse only partially (39–58%, Table 2) captured all habitats identified for sagebrush-obligate songbirds from count-based models [65]. It is possible that our occurrence models may not have suitably captured niche conditions for each species by not directly considering population processes. However, the sagebrush-obligate songbird models, which were successfully [65] validated using independent Breeding Bird Survey data [94], did track the density of bird species, which may be a better indicator by means of which to capture the true demography [95] and better represent the umbrella [60], and thus, umbrella effectiveness. It may be useful to further assess whether the sage-grouse umbrella captures resource conditions that support stable or increasing songbird populations (sources based on demography) better than the overlap with individual species occurrence. However, population trajectories of sage-grouse and these obligate songbirds are not necessarily correlated [96]. Also, more than one sagebrush ecosystem umbrella species might need to be considered, if species with different niche requirements than sage-grouse are to be protected [59,93,97]. Similarly, umbrella species from other systems may need to be considered, so species at ecotone boundaries do not fall through the umbrella [98]. This is a concern with the pinyon jay (Gymnorhinus cyanocephalus), occurring at the shrub-forest ecotone, where jay abundance has been shown to increase with greater cover of both pinyon–juniper (juniper—Juniperus spp., and pinyon pines—Pinus edulis and Pinus monophyla) and sagebrush [93,99].
Priority Areas for Conservation (PACs) were previously identified for 39 sage-grouse populations across the species’ range, where conservation efforts would be emphasized to help maintain sage-grouse populations and associated species [43]. PACs have been shown to capture areas supporting higher sage-grouse population trends [96]. However, despite PACs reportedly capturing 75% of the breeding sage-grouse populations within 25% of the species range [89], only 59% of model-predicted sage-grouse habitat in the WBEA was ‘protected’ by these conservation areas (Figure 5). Although our results support the application of the PACs to conserve and protect sage-grouse habitat and sagebrush-dependent species, it may be that more explicit mapping of sage-grouse resource requirements [90,100,101] is required to better identify and manage habitats for the persistence of sage-grouse populations [102]. While the sage-grouse occurrence model only marginally improved the capture of biodiverse (richness ≥ 4 species) habitats within WBEA sage-grouse range compared to capture by PACs (60% versus 56%, by area; Table 4), the buffering approach used to define the PACs effectively includes more of the landscape than the more restrictive sage-grouse occurrence model (75,276 km2 versus 44,976 km2; Figure 5). These additional landscapes may include other habitats not directly used by sage-grouse. Thus, PACs encompass more habitats with lower species richness (1–3 species; Table 4), which tended to capture the diversity of niche requirements for other species, particularly those of species not as closely aligned to sage-grouse. This may be useful, but invokes management or protection across nearly twice the landscape area.
Spatially explicit predictive models enabled the assessment of realized distributions rather than solely focusing on the broad extent provided by coarse-scale range or distribution maps [19]. Predictive models for all species used in our analyses were developed using a standardized suite of habitat and anthropogenic covariates [55], enabling us to gain insight into why some species are better captured than others by the sage-grouse umbrella. Sage-grouse had a strong positive relationship with sagebrush habitat within a 1 km radius [64]. This strong affinity to sagebrush as a specialist is one of the attributes for defining a good candidate for an umbrella species [29,30]. The three sagebrush-obligate bird species (Brewer’s sparrow, sagebrush sparrow, and sage thrasher) also shared this affinity for sagebrush landscapes and all three had reasonable overlap with sage-grouse occurrence. In general, the passerine birds had more extensive distributions than the realized distribution of sage-grouse (see Table 1), which reduced their potential to be fully covered under the umbrella. However, the specific types of sagebrush habitats and scale of the response in the occurrence models we based our analysis on led to different distributions within the study area. For example, the sage thrasher was dependent on big sagebrush (A. tridentata) at smaller spatial scales (270 m vs. 1 km) while the sagebrush sparrow responded to all sagebrush types within an 18 km radius [65], which likely led to a higher level of concordance for sage thrasher because its occurrence was nested within areas predicted to support sage-grouse.
A further examination of covariates included in the predictive models we used to define habitat for sagebrush-associated species provides additional explanation for the differences we observed. The green-tailed towhee was predicted to occur in mountain foothill sagebrush communities that have higher productivity and are in proximity to forests or woodlands [65]. These habitat conditions are generally avoided by sage-grouse during most of the year, except for some brood-rearing habitats in the late-summer or fall [37], helping to explain the lack of concordance between green-tailed towhee and sage-grouse occurrence models (Table 2). Pronghorn had a weak affinity for sagebrush at a local scale [67], but additional similarities to sage-grouse in responses to environmental and anthropogenic features (riparian, elevation, powerlines and roads) led to a similar response with sage-grouse [64] and a fairly moderate degree of habitat overlap (44%, Table 2; Figure 4) within the sage-grouse range. The distribution of the greater short-horned lizard is limited to the eastern portion of the sagebrush biome [59], but 98% of the range was contained within the sage-grouse range in our study area (Table 1). For such species with small ranges that are largely contained within the range of greater sage-grouse, broad management actions across the sage-grouse range may be less likely to directly capture and benefit these species [59]. In this case, 68% of the predicted lizard occurrence overlapped with predicted sage-grouse habitat (Table 2; Figure 4), the highest proportion of habitats captured by the sage-grouse umbrella. However, it is important to consider explicit habitat associations. Short-horned lizards were predicted to occur within large landscapes containing expanses of sagebrush (5 km; [80]), similar to sage-grouse. While rare species are often not captured by umbrella species [15] or biodiversity hotspots [103], the short-horned lizard’s affinity for sagebrush habitats in that occurrence model [80] led to a strong predicted overlap under the sage-grouse umbrella, which has been observed range-wide as well [59,104]. Notably, short-horned lizards also occur in habitats with lower productivity, restrained to relatively flat terrain and low moisture [80]. Thus, management actions targeted at enhancing sagebrush and perennial herbaceous cover for sage-grouse may not benefit lizards.
We focused on six sagebrush-dependent vertebrate species with sufficient sample sizes from our study area to support previous modeling efforts [55], but numerous other members of this ecosystem may occur within the sage-grouse range, and the usefulness of this umbrella is relatively unknown (but see [32]) until these species are directly evaluated [105]. Indeed, even slight differences in habitat associations can fail to produce the desired conservation outcomes [106]. Statistical advances from hierarchical community models permit studying the entire community, including sparse records from rare species [107,108], but these also implicitly assume rare species respond similarly to the mean community response, informed by more common species [109]. We did not consider non-sagebrush-dependent species, and these are expected to be less well conserved by the sage-grouse umbrella [32,98,110]. Additionally, invertebrates such as arthropods and insects are relatively understudied compared with vertebrates and are seldom considered when evaluating the conservation potential of focal species [111]. Aside from sagebrush cover, forbs are important components of sage-grouse brood-rearing habitat [112] and increasing forb cover may benefit invertebrate communities [113]; sage-grouse abundance has been shown to correspond with harvester ant abundance [97]. We only studied one reptile species, but analyses of the broader reptile community may support the sage-grouse umbrella concept for many species in this group, albeit with variability [59]. Other data types could be incorporated in our approach to represent species distribution and habitat use, including migration corridors for ungulates [114].
While we relied on coarse distribution maps and predicted occurrence to evaluate overlap with greater sage-grouse habitat and protected areas, additional demographic and population data are needed to adequately assess conservation value [95,115]. For example, population trends of songbirds did not correspond with the location of PACs across Wyoming [96]. The relevance of sage-grouse to the abundance of sagebrush-obligate species also has been inconsistent [110,116]. Furthermore, the overlap of species in the broader landscape does not indicate how species may respond to management aimed at benefiting sage-grouse. Reductions in sagebrush cover to promote brood-rearing habitat may be detrimental to other sagebrush-obligate species [79,117,118]. Finally, correlations in distribution also depend on the scale at which these are examined [119], and concordance at broader scales may contrast with disparities at finer scales [110,116]. Assessing occurrence across multiple scales [92] may be required to better understand overlapping niche conditions, as richness indices tend not to capture diverse taxonomic groups across large spatial extents (i.e., continental USA; [23]).
Opportunities to conserve important habitats under the sage-grouse umbrella are increasingly limited by anthropogenic activities that reduce the availability and ecosystem function of sagebrush, potentially limiting its ability to support species. Cultivation and energy development continue to fragment, degrade, and reduce habitat cover, particularly in the eastern sage-grouse range [120,121]. Wildfires and the subsequent invasion of annual grasses are driving sagebrush loss across the western range [122,123,124]. Climate change is expected to further reduce sagebrush cover [125], increase drought severity, and influence conifer encroachment. Identifying and protecting local hotspots that provide habitat for multiple species can be an effective strategy to buffer against further species declines that result from landscape change. Such preventative actions are more efficient than restoring habitats and recovering populations, particularly when a high degree of intervention is required to avert species extinction. However, as the sagebrush ecosystem continues to change, the spatial coverage of the sage-grouse umbrella will not always stand up to the ‘weather’ and new holes in the umbrella will develop as species’ habitats are altered. Yet, umbrella analyses can be important conservation planning tools that can inform near-term conservation investments.
5. Conclusions
We assessed the realized niche conditions of multiple sagebrush-dependent species under the umbrella of the greater sage-grouse, and the umbrella did account for the needs of multiple sagebrush species of concern in key hotspots (e.g., sagebrush-dependent vertebrates); however, the umbrella was not a catch-all. Species needs are diverse and scale-dependent, influencing the degree to which umbrella species are suitable surrogates for habitat protection. Focal species can provide an effective umbrella that includes hotspots of species richness, but holes in coverage should also be expected [32]. Conservation planning aimed at multiple species will need to consider what might be missed by not explicitly identifying species’ specific needs. Although the umbrella concept and related analyses have limitations, they provide a simple approach that can facilitate conservation actions for multiple species. The sage-grouse umbrella in our assessment was able to capture the most biodiverse hot spots where all six species were predicted to occur. However, other biodiverse areas with 4–5 species were not as well captured, and for some species, only a small portion (<10%) of predicted habitat was captured by sage-grouse, despite only including other sagebrush-dependent species. Management actions could use a dual conservation approach, considering measures that protect biodiverse habitats for sagebrush-dependent species, while also focusing on high richness values from our predicted occurrence models that fall outside of the sage-grouse umbrella.
Realistic conservation planning requires consideration of funding resources and the socio-ecological climate in which management and resource decisions are made. Using charismatic species that attract conservation resources to serve as an umbrella species can be a practical means of mobilizing interest and resources to achieve broader conservation objectives. In the sagebrush ecosystem, greater sage-grouse represent a reasonable umbrella not only due to their diverse habitat requirements and broad range, but because of the financial investments directed towards the management of this species [114,126]. However, understanding how well an umbrella might work, and which species are not well captured, could help to better target conservation and management efforts. While this requires good spatial data and the development of detailed spatial models that predict species resource needs across the landscape, such models are becoming more common (e.g., [93,104]). The billions of dollars that have or will be used to support sage-grouse conservation initiatives could be targeted to specific locations that jointly maximize the conservation of sage-grouse and other species, while also avoiding potential negative consequences to non-target species [93,99]. Rather than assuming an umbrella species adequately covers the needs of several species, analyses such as those summarized here provide a data-driven means to identify locations with high species richness and likelihood of occurrence. Prioritizing these landscapes for habitat protection and restoration can help to meet national and international goals to conserve 30 percent of land and water by 2030 [11].
Author Contributions
Conceptualization: C.L.A., D.J.S. and S.E.H.; Methodology: C.L.A. and D.J.S.; Formal Analysis: D.J.S. and C.L.A.; Funding Acquisition: C.L.A., D.J.S., J.A.H., A.P.M., M.L. and S.E.H.; Writing—Original Draft Preparation: C.L.A., D.J.S., J.A.H. and A.P.M.; Writing—Review & Editing: C.L.A., D.J.S., J.A.H., A.P.M., M.L. and S.E.H. All authors have read and agreed to the published version of the manuscript.
Funding
Funding for this project came from the U.S. Geological Survey with partial funding support from the Western Association of Fish and Wildlife Agencies through a grant from the U.S. Department of the Interior, Fish and Wildlife Service to Colorado State University in the amount of $25,492.
Data Availability Statement
The data presented in this study are available in Hanser et al. (2011a) at [55]. These data were derived from the following resources available in the public domain: https://www.sciencebase.gov/catalog/item/54417620e4b0b0a643c73d2d.
Acknowledgments
We are grateful for financial and logistical support from the U.S. Geological Survey and thank all of the original partners that supported data collection and analyses for the initial work on the Wyoming Basins Ecoregional Assessment Project. We thank D.D. Pilliod and three anonymous reviewers for helpful comments on our manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- International Union for Conservation of Nature and Natural Resources [IUCN]. The IUCN Red List of Threatened Species. Version 2020-2. Available online: https://www.iucnredlist.org (accessed on 13 November 2020).
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlo, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Dirzo, R.; Raven, P.H. Global state of biodiversity and loss. Annu. Rev. Environ. Resour. 2003, 28, 137–167. [Google Scholar] [CrossRef]
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global consequences of land use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Young, H.S.; McCauley, D.M.; Galetti, M.; Dirzo, R. Patterns, causes, and consequences of Anthropocene defaunation. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 333–358. [Google Scholar] [CrossRef]
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.K.; Hungate, B.A.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; O’Connor, M.I. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 2012, 486, 105–108. [Google Scholar] [CrossRef]
- Dirzo, R.; Young, H.; Galetti, M.; Ceballow, G.; Isaac, N.J.B.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef]
- America the Beautiful. Conserving and Restoring America the Beautiful. 2021. Available online: https://www.doi.gov/sites/doi.gov/files/report-conserving-and-restoring-america-the-beautiful-2021.pdf (accessed on 15 January 2022).
- Hoffmann, M.; Hilton-Taylor, C.; Angulo, A.; Böhm, M.; Brooks, T.M.; Butchart, S.H.M.; Carpenter, K.E.; Chanson, J.; Collen, B.; Cox, N.A.; et al. The impact of conservation on the status of the world’s vertebrates. Science 2010, 330, 1503–1509. [Google Scholar] [CrossRef]
- Maxwell, S.L.; Cazalis, V.; Dudley, N.; Hoffmann, M.; Rodrigues, A.S.L.; Stolton, S.; Visconti, P.; Woodley, S.; Kingston, N.; Lewis, E.; et al. Area-based conservation in the twenty-first century. Nature 2020, 586, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fenseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Reid, W.V. Biodiversity hotspots. Trends Ecol. Evol. 1998, 13, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.H. Population dynamic principles. Philos. Trans. R. Soc. Lond. Biol. Sci. 1994, 334, 61–68. [Google Scholar] [CrossRef]
- Grinnell, J. The niche-relationships of the California Thrasher. Auk 1917, 34, 427–433. [Google Scholar] [CrossRef]
- Hutchinson, G.E. Concluding remarks. In Cold Spring Symposium on Quantitative Biology; Yale University: New Haven, CT, USA, 1957; pp. 415–427. [Google Scholar]
- Soberon, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef]
- Pulliam, H.R. Sources, Sinks, and Population Regulation. Am. Nat. 1988, 132, 652–661. [Google Scholar] [CrossRef]
- Knick, S.T.; Hanser, S.E.; Preston, K.L. Modeling ecological minimum requirements for distribution of greater sage-grouse leks: Implications for population connectivity across their western range, USA. Ecol. Evol. 2013, 3, 1539–1551. [Google Scholar] [CrossRef]
- Scott, J.M.; Davis, F.; Csuti, B.; Noss, R.; Butterfield, B.; Groves, C.; Anderson, H.; Caicco, S.; D’Erchia, F.; Edwards, T.C., Jr.; et al. Gap analysis: A geographic approach to protection of biological diversity. Wildl. Monogr. 1993, 123, 1–41. [Google Scholar]
- Flather, C.H.; Knowles, M.S.; Kendall, I.A. Threatened and endangered species geography: Characteristics of hot spots in the conterminous United States. BioScience 1998, 48, 365–376. [Google Scholar] [CrossRef]
- Keinath, D.A.; Andersen, M.D.; Beauvais, G.P. Range and Modeled Distribution of Wyoming’s Species of Greatest Conservation Need; The Wyoming Natural Diversity Database, Laramie, Wyoming for the Wyoming Game and Fish Department, Cheyenne, Wyoming and the U.S. Geological Survey: Fort Collins, CO, USA, 2010; Available online: https://www.uwyo.edu/wyndd/_files/docs/reports/wynddreports/u10kei01wyus.pdf (accessed on 19 November 2020).
- Berger, J. Population constraints associated with the use of black rhinos as an umbrella species for desert herbivores. Conserv. Biol. 1997, 11, 69–78. [Google Scholar] [CrossRef]
- Branton, M.; Richardson, J.S. Assessing the value of the umbrella-species concept for conservation planning with meta-analysis. Conserv. Biol. 2011, 25, 9–20. [Google Scholar] [CrossRef]
- Noss, R.F. Indicators for monitoring biodiversity: A hierarchical approach. Conserv. Biol. 1990, 4, 355–364. [Google Scholar] [CrossRef]
- Smith, I.T.; Knetter, S.J.; Svancara, L.K.; Karl, J.W.; Johnson, T.R.; Rachlow, J.L. Overlap Between Sagebrush Habitat Specialists Differs Among Seasons: Implications for Umbrella Species Conservation. Rangel. Ecol. Manag. 2021, 78, 142–154. [Google Scholar] [CrossRef]
- Ozaki, K.; Isono, M.; Kawahara, T.; Iida, S.; Kudo, T.; Fukuyama, K. A mechanistic approach to evaluation of umbrella species as conservation surrogates. Conserv. Biol. 2006, 20, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Roberge, J.M.; Mikusinski, G.; Svensson, S. The white-backed woodpecker: Umbrella species for forest conservation planning? Biodivers. Conserv. 2008, 17, 2479–2494. [Google Scholar] [CrossRef]
- Lambeck, R.J. Focal species: A multi-species umbrella for nature conservation. Conserv. Biol. 1997, 11, 849–856. [Google Scholar] [CrossRef]
- Carlisle, J.D.; Keinath, D.A.; Albeke, S.A.; Chalfoun, A.D. Identifying holes in the greater sage-grouse conservation umbrella. J. Wildl. Manag. 2018, 82, 948–957. [Google Scholar] [CrossRef]
- Pendergast, J.R.; Quinn, R.M.; Lawton, J.H.; Eversham, B.C.; Gibbons, D.W. Rare species, the coincidence of diversity hotspots and conservation strategies. Nature 1993, 365, 335–337. [Google Scholar] [CrossRef]
- Duchardt, C.J.; Monroe, A.P.; Heinrichs, J.A.; O’Donnell, M.S.; Edmunds, D.R.; Aldridge, C.L. Prioritizing restoration areas to conserve multiple sagebrush-associated wildlife species. Biol. Conserv. 2021, 260, 109212. [Google Scholar] [CrossRef]
- Remington, T.E.; Welty, J.L.; Aldridge, C.L.; Jakes, A.F.; Pilliod, D.S.; Rachlow, J.L.; Smith, I.T. Chapter Q. Sage-grouse Management as an Umbrella for Conservation of Sagebrush. In Sagebrush Conservation Strategy—Challenges to Sagebrush Conservation; Remington, T.E., Deibert, P.A., Hanser, S.E., Davis, D.M., Robb, L.A., Welty, J.L., Eds.; U.S. Geological Survey Open-File Report; 2020-1125; U.S. Geological Survey, 2021; pp. 163–178. [Google Scholar] [CrossRef]
- Aldridge, C.L.; Nielsen, S.E.; Beyer, H.L.; Boyce, M.S.; Connelly, J.W.; Knick, S.T.; Schroeder, M.A. Range-wide patterns of greater sage-grouse persistence. Divers. Distrib. 2008, 14, 983–994. [Google Scholar] [CrossRef]
- Connelly, J.W.; Rinkes, E.T.; Braun, C.E. Characteristics of greater sage-grouse habitat—A landscape species at micro- and macroscales. In Greater Sage-Grouse: Ecology and Conservation of a Landscape Species and Its Habitats; Studies in Avian Biology; Knick, S.T., Connelly, J.W., Eds.; University of California Press: Berkeley, CA, USA, 2011; Volume 38, pp. 69–83. [Google Scholar]
- Knick, S.T.; Hanser, S.E. Connecting pattern and process in greater sage-grouse populations and sagebrush landscapes. In Greater Sage-Grouse: Ecology and Conservation of a Landscape Species and Its Habitats; Studies in Avian Biology; Knick, S.T., Connelly, J.W., Eds.; University of California Press: Berkeley, CA, USA, 2011; Volume 38, pp. 383–405. [Google Scholar]
- Wisdom, M.J.; Meinke, C.W.; Knick, S.T.; Schroeder, M.A. Factors associated with extirpation of sage-grouse. In Greater Sage-Grouse: Ecology and Conservation of a Landscape Species and Its Habitats; Studies in Avian Biology; Knick, S.T., Connelly, J.W., Eds.; University of California Press: Berkeley, CA, USA, 2011; Volume 38, pp. 451–472. [Google Scholar]
- Schroeder, M.A.; Aldridge, C.L.; Apa, A.D.; Bohne, J.R.; Braun, C.E.; Bunnell, S.D.; Connelly, J.W.; Deibert, P.A.; Gardner, S.C.; Hilliard, M.A.; et al. Distribution of sage-grouse in North America. Condor 2004, 106, 363–376. [Google Scholar] [CrossRef]
- Coates, P.S.; Prochazka, B.G.; O’Donnell, M.S.; Aldridge, C.L.; Edmunds, D.R.; Monroe, A.P.; Ricca, M.A.; Wann, G.T.; Hanser, S.E.; Wiechman, L.A.; et al. Range-Wide Greater Sage-Grouse Hierarchical Monitoring Framework—Implications for Defining Population Boundaries, Trend Estimation, and a Targeted Annual Warning System; U.S. Geological Survey Open-File Report 2020–1154; U.S. Geological Survey: Reston, VA, USA, 2021; p. 239. [Google Scholar] [CrossRef]
- Remington, T.E.; Deibert, P.A.; Hanser, S.E.; Davis, D.M.; Robb, L.A.; Welty, J.L. Sagebrush Conservation Strategy—Challenges to Sagebrush Conservation; U.S. Geological Survey Open-File Report 2020-1125; U.S. Geological Survey: Reston, VA, USA, 2021; p. 327. [Google Scholar] [CrossRef]
- U.S. Fish and Wildlife Service. Greater Sage-Grouse (Centrocercus urophasianus) Conservation Objectives; Final Report; U.S. Department of the Interior, Fish and Wildlife Service: Denver, CO, USA, 2013; p. 91. [Google Scholar]
- Wisdom, M.J.; Rowland, M.M.; Suring, L.H. Habitat Threats in the Sagebrush Ecosystem—Methods of Regional Assessment and Applications in the Great Basin; Alliance Communications Group: Lawrence, KA, USA, 2005; p. 301. [Google Scholar]
- Rich, T.; Altman, B. Under the sage-grouse umbrella: Bird Conservation. Mag. Am. Bird Conserv. 2001, 14, 10. [Google Scholar]
- Braun, C.E. Multi-species benefits of the proposed North American Sage-Grouse Management plan. In Bird Conservation Implementation and Integration in the Americas, Proceedings of the Third International Partners in Flight Symposium; Asilomar, CA, USA, 20–24 March 2005, Ralph, C.J., Rich, T.D., Eds.; General Technical Report PSW-GTR-191; USDA Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2005; pp. 1162–1164. [Google Scholar]
- Rich, T.D.; Wisdom, M.J.; Saab, V.A. Conservation of priority birds in sagebrush ecosystems. In Proceedings of the Third International Partners in Flight Symposium; General Technical Report PSW-GTR-191; USDA Forest Service, Pacific Southwest Research Station: Albany, CA, USA, 2005; pp. 598–606. [Google Scholar]
- Rowland, M.M.; Wisdom, M.J.; Suring, L.H.; Meinke, C.W. Greater sage-grouse as an umbrella species for sagebrush-associated vertebrates. Biol. Conserv. 2006, 129, 323–335. [Google Scholar] [CrossRef]
- Hanser, S.E.; Knick, S.T. Greater sage-grouse as an umbrella species for shrubland passerine birds: A multiscale assessment. In Greater Sage-Grouse: Ecology and Conservation of a Landscape Species and Its Habitats; Studies in Avian Biology; Knick, S.T., Connelly, J.W., Eds.; University of California Press: Berkeley, CA, USA, 2011; Volume 38, pp. 451–472. [Google Scholar]
- Simberloff, D. Flagships, umbrellas, and keystones: Is single-species management passé in the landscape era? Biol. Conserv. 1998, 83, 247–257. [Google Scholar] [CrossRef]
- Caro, T. Conservation by Proxy: Indicator, Umbrella, Keystone, Flagship, and Other Surrogate Species; Island Press: Washington, DC, USA, 2010. [Google Scholar]
- Caro, T.M.; O’Doherty, G. On the use of surrogate species in conservation biology. Conserv. Biol. 1999, 13, 805–814. [Google Scholar] [CrossRef]
- Fleishman, E.; Murphy, D.D.; Brussard, P.F. A new method for selection of umbrella species for conservation planning. Ecol. Appl. 2000, 10, 569–579. [Google Scholar] [CrossRef]
- Rubinoff, D. Evaluating the California Gnatcatcher as an umbrella species for conservation of southern California coastal sage scrub. Conserv. Biol. 2001, 15, 1374–1383. [Google Scholar] [CrossRef]
- Hanser, S.E.; Leu, M.; Knick, S.T.; Aldridge, C.L. Sagebrush Ecosystem Conservation and Management: Ecoregional Assessment Tools and Models for the Wyoming Basins; Allen Press: Lawrence, KS, USA, 2011. [Google Scholar]
- Leu, M.; Hanser, S.E.; Aldridge, C.L.; Nielsen, S.E.; Cade, B.S.; Knick, S.T. Chapter 4: A sampling and analytical approach to develop spatial distribution models for sagebrush-associated species. In Sagebrush Ecosystem Conservation and Management: Ecoregional Assessment Tools and Models for the Wyoming Basins; Hanser, S.E., Leu, M., Knick, S.T., Aldridge, C.L., Eds.; Allen Press: Lawrence, KS, USA, 2011; pp. 88–111. [Google Scholar]
- WBEA Model Outputs. 2011. Available online: https://www.sciencebase.gov/catalog/item/64a5e82ed34ef77fcb0624f9?community=Forest+and+Rangeland+Ecosystem+Science+Center+%28FRESC%29 (accessed on 15 September 2023).
- Smith, I.T.; Rachlow, J.L.; Svancara, L.K.; McMahon, L.A.; Knetter, S.J. Habitat specialists as conservation umbrellas: Do areas managed for greater sage-grouse also protect pygmy rabbits? Ecosphere 2019, 10, e02827. [Google Scholar] [CrossRef]
- Pilliod, D.S.; Jeffries, M.I.; Arkle, R.S.; Olson, D.H. Reptiles under the conservation umbrella of the greater sage-grouse. J. Wildl. Manag. 2020, 84, 478–491. [Google Scholar] [CrossRef]
- Fleishman, E.; Noss, R.F.; Noon, B.R. Utility and limitations of species richness metrics for conservation planning. Ecol. Indic. 2006, 6, 543–553. [Google Scholar] [CrossRef]
- LANDFIRE, LANDFIRE 1.0.0 Existing Vegetation Type Layer. U.S. Department of Interior, Geological Survey. 2007. Available online: http://landfire.cr.usgs.gov/viewer/ (accessed on 20 September 2011).
- Rowland, M.M.; Leu, M. Study area description. In Sagebrush Ecosystem Conservation and Management: Ecoregional Assessment Tools and Models for the Wyoming Basins; Hanser, S.E., Leu, M., Knick, S.T., Aldridge, C.L., Eds.; Allen Press: Lawrence, KS, USA, 2011; pp. 10–45. [Google Scholar]
- Knick, S.T.; Hanser, S.E.; Leu, M.; Aldridge, C.L.; Wisdom, M.J. Introduction: An Ecoregional Assessment of the Wyoming Basins. In Sagebrush Ecosystem Conservation and Management: Ecoregional Assessment Tools and Models for the Wyoming Basins; Hanser, S.E., Leu, M., Knick, S.T., Aldridge, C.L., Eds.; Allen Press: Lawrence, KS, USA, 2011; pp. 1–9. [Google Scholar]
- Hanser, S.E.; Aldridge, C.L.; Leu, M.; Rowland, M.M.; Nielsen, S.E.; Knick, S.T. Greater Sage-Grouse: General Use and Roost Site Occurrence with Pellet Counts as a Measure of Relative Abundance. In Sagebrush Ecosystem Conservation and Management: Ecoregional Assessment Tools and Models for the Wyoming Basins; Hanser, S.E., Leu, M., Knick, S.T., Aldridge, C.L., Eds.; Allen Press: Lawrence, KS, USA, 2011; pp. 112–140. [Google Scholar]
- Aldridge, C.L.; Hanser, S.E.; Nielsen, S.E.; Leu, M.; Cade, B.S.; Saher, D.J.; Knick, S.T. Detectability Adjusted Count Models of Songbird Abundance. In Sagebrush Ecosystem Conservation and Management: Ecoregional Assessment Tools and Models for the Wyoming Basins; Hanser, S.E., Leu, M., Knick, S.T., Aldridge, C.L., Eds.; Allen Press: Lawrence, KS, USA, 2011; pp. 141–220. [Google Scholar]
- Wyoming Game and Fish Department [WGFD]. State Wildlife Action Plan; Wyoming Game and Fish Department: Cheyenne, WY, USA, 2017. [Google Scholar]
- Leu, M.; Hanser, S.E.; Aldridge, C.L.; Nielsen, S.E.; Suring, L.H.; Knick, S.T. Chapter 8: Occurrence of large and medium-sized mammals: Occurrence but not count models predict pronghorn distribution. In Sagebrush Ecosystem Conservation and Management: Ecoregional Assessment Tools and Models for the Wyoming Basins; Hanser, S.E., Leu, M., Knick, S.T., Aldridge, C.L., Eds.; Allen Press: Lawrence, KS, USA, 2011; pp. 315–336. [Google Scholar]
- Ngugi, K.R.; Powell, J.; Hinds, F.C.; Olson, R.A. Range animal diet composition in southcentral Wyoming. J. Range Manag. 1992, 45, 542–545. [Google Scholar] [CrossRef]
- Jakes, A.F. Chapter, F. Pronghorn. In Sagebrush Conservation Strategy—Challenges to Sagebrush Conservation; Remington, T.E., Deibert, P.A., Hanser, S.E., Davis, D.M., Robb, L.A., Welty, J.L., Eds.; U.S. Geological Survey Open-File Report 2020-1125; U.S. Geological Survey: Reston, VA, USA, 2021; pp. 37–42. [Google Scholar] [CrossRef]
- Outdoor Industry Association. The Outdoor Recreation Economy: Wyoming; Outdoor Industry Association: Boulder, CO, USA, 2017; Available online: https://outdoorindustry.org/wp-content/uploads/2017/07/OIA_RecEcoState_WY.pdf (accessed on 19 November 2020).
- Wyoming Game and Fish Department [WGFD]. Annual Reports of Big and Trophy Game Harvest: Antelope; Wyoming Game and Fish Department: Cheyenne, WY, USA, 2019. Available online: https://wgfd.wyo.gov/WGFD/media/content/PDF/Hunting/Harvest%20Reports/HR2019_Antelope.pdf (accessed on 19 November 2020).
- Knick, S.T.; Dobkin, D.S.; Rotenberry, J.T.; Schroeder, M.A.; Vander Haegen, W.M.; van Riper, C., III. Teetering on the edge or too late? Conservation and research issues for avifauna of sagebrush habitats. Condor 2003, 105, 611–634. [Google Scholar] [CrossRef]
- Green, A.W.; Aldridge, C.L.; O’Donnell, M.S. Investigating impacts of oil and gas development on Greater sage-grouse. J. Wildl. Manag. 2017, 81, 46–57. [Google Scholar] [CrossRef]
- Naugle, D.E.; Doherty, K.E.; Walker, B.L.; Copeland, H.E.; Holloran, M.J.; Tack, J.D. Sage-grouse and cumulative impacts of energy development. In Energy Development and Wildlife Conservation in North America; Naugle, D.E., Ed.; Island Press: Washington, DC, USA, 2011. [Google Scholar]
- Smith, J.T.; Evans, J.S.; Martin, B.H.; Baruch-Mordo, S.; Kiesecker, J.M.; Naugle, D.E. Reducing cultivation risk for at-risk species: Predicting outcomes of conservation easements for sage-grouse. Biol. Conserv. 2016, 201, 10–19. [Google Scholar] [CrossRef]
- Ingelfinger, F.; Anderson, S. Passerine response to roads associated with natural gas extraction in a sagebrush steppe habitat. West. N. Am. Nat. 2004, 64, 385–395. [Google Scholar]
- Gilbert, M.M.; Chalfoun, A.D. Energy development affects populations of sagebrush songbirds in Wyoming. J. Wildl. Manag. 2011, 75, 816–824. [Google Scholar] [CrossRef]
- Mutter, M.; Pavlacky, D.C.; Van Lanen, N.J.; Grenyer, R. Evaluating the impact of gas extraction infrastructure on the occupancy of sagebrush-obligate songbirds. Ecol. Appl. 2015, 25, 1175–1186. [Google Scholar] [CrossRef]
- Carlisle, J.D.; Chalfoun, A.D.; Smith, K.T.; Beck, J.L. Nontarget effects on songbirds from habitat manipulation for greater sage-grouse: Implications for the umbrella species concept. Condor Ornithol. Appl. 2018, 120, 439–455. [Google Scholar] [CrossRef]
- Hanser, S.E.; Leu, M.; Aldridge, C.L.; Nielsen, S.E.; Rowland, M.M.; Knick, S.T. Occurrence and Abundance of Ants, Reptiles, and Mammals. In Sagebrush Ecosystem Conservation and Management: Ecoregional Assessment Tools and Models for the Wyoming Basins; Hanser, S.E., Leu, M., Knick, S.T., Aldridge, C.L., Eds.; Allen Press: Lawrence, KS, USA, 2011; pp. 221–314. [Google Scholar]
- Dobbs, R.C.; Martin, P.R.; Martin, T.E. Green-tailed Towhee (Pipilo chlorurus), Version 1.0. In Birds of the World; Poole, A.F., Ed.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Holmes, A.L.; Maestas, J.D.; Naugle, D.E. Bird responses to removal of western juniper in sagebrush-steppe. Rangel. Ecol. Manag. 2017, 70, 87–94. [Google Scholar] [CrossRef]
- Ridgely, R.S.; Allnutt, T.F.; Brooks, T.; McNicol, D.K.; Mehlman, D.W.; Young, B.E.; Zook, J.R. Digital Distribution of the Birds of the Western Hemisphere, Version 1.0; NatureServe: Arlington, VA, USA, 2003; Available online: http://www.natureserve.org/getData/birdMaps.jsp (accessed on 20 September 2001).
- Stebbins, R.C. A Field Guide to Western Reptiles and Amphibians, 3rd ed.; Houghton Mifflin Company: Boston, MA, USA, 2003. [Google Scholar]
- Rowland, M.M.; Suring, L.H.; Leu, M.; Knick, S.T. Sagebrush-associated species of conservation concern. In Sagebrush Ecosystem Conservation and Management: Ecoregional Assessment Tools and Models for the Wyoming Basins; Hanser, S.E., Leu, M., Knick, S.T., Aldridge, C.L., Eds.; Allen Press: Lawrence, KS, USA, 2011; pp. 46–68. [Google Scholar]
- Gaston, K.J. How large is a species’ geographic range? Oikos 1991, 61, 434–438. [Google Scholar] [CrossRef]
- Fertig, W.; Reiners, W.A. Predicting presence/absence of plant species for range mapping: A case study from Wyoming. In Predicting Species Occurrences: Issues of Accuracy and Scale; Scott, J.M., Heglund, P.J., Morrison, M.L., Haufler, J.B., Raphael, M.G., Wall, W.A., Sanson, F.B., Eds.; Island Press: Washington, DC, USA, 2002; pp. 483–489. [Google Scholar]
- Dobkin, D.S.; Sauder, J.D. Shrub-Steppe Landscapes in Jeopardy. In Distributions, Abundances, and the Uncertain Future of Birds and Small Mammals in the Intermountain West; High Desert Ecological Research Institute: Bend, OR, USA, 2004. [Google Scholar]
- Doherty, K.E.; Naugle, D.E.; Copeland, H.E.; Pocewicz, A.; Kiesecker, J.M. Energy development and conservation tradeoffs: Systematic planning for Greater Sage-Grouse in their eastern range. In Greater Sage-Grouse: Ecology and Conservation of a Landscape Species and Its Habitats; Studies in Avian Biology; Knick, S.T., Connelly, J.W., Eds.; University of California Press: Berkeley, CA, USA, 2011; Volume 38, pp. 505–516. [Google Scholar]
- Fedy, B.C.; Doherty, K.E.; Aldridge, C.L.; O’Donnell, M.S.; Beck, J.L.; Bedrosian, B.; Gummer, D.L.; Holloran, M.J.; Johnson, G.D.; Kaczor, N.W.; et al. Habitat prioritization across large landscapes, multiple seasons, and novel areas: An example using greater sage-grouse in Wyoming. Wildl. Monogr. 2014, 190, 2795–2813. [Google Scholar] [CrossRef]
- Connelly, J.W.; Knick, S.T.; Schroeder, M.A.; Stiver, S.J. Conservation Assessment of Greater Sage-Grouse and Sagebrush Habitats; Western Association of Fish and Wildlife Agencies: Cheyenne, WY, USA, 2004. [Google Scholar]
- McGarigal, K.; Wan, H.Y.; Zeller, K.A.; Timm, B.C.; Cushman, S.A. Multi-scale habitat selection modeling: A review and outlook. Landsc. Ecol. 2016, 31, 1161–1175. [Google Scholar] [CrossRef]
- Van Lanen, N.J.; Shyvers, J.E.; Duchardt, C.J.; Aldridge, C.L. A multi-ecosystem prioritization framework to balance competing habitat conservation needs of multiple species in decline. Landsc. Ecol. 2023, 38, 1–19. [Google Scholar] [CrossRef]
- Sauer, J.R.; Hines, J.E.; Fallon, J.E.; Pardieck, K.L.; Ziolkowski, D.J., Jr.; Link, W.A. The North American Breeding Bird Survey, Results and Analysis 1966–2009; Version 3.23.2011; USGS Patuxent Wildlife Research Center: Laurel, MD, USA, 2011. [Google Scholar]
- Van Horne, B. Density as a misleading indicator of habitat quality. J. Wildl. Manag. 1983, 47, 893–901. [Google Scholar] [CrossRef]
- Dinkins, J.B.; Beck, J.L. Comparison of conservation policy benefits for and umbrella and related sagebrush-obligate species. Hum. Wildl. Interact. 2019, 13, 447–458. [Google Scholar] [CrossRef]
- Carlisle, J.D.; Stewart, D.R.; Chalfoun, A.D. An invertebrate ecosystem engineer under the umbrella of sage-grouse conservation. West. N. Am. Nat. 2017, 77, 450–463. [Google Scholar] [CrossRef]
- Duchardt, C.J.; Monroe, A.P.; Edmunds, D.R.; Holloran, M.J.; Holloran, A.G.; Aldridge, C.L. Using neutral landscape models to evaluate the umbrella species concept in an ecotone. Landsc. Ecol. 2023, 38, 1447–1462. [Google Scholar] [CrossRef]
- Van Lanen, N.J.; Monroe, A.P.; Aldridge, C.L. A hidden cost of single species management: Habitat-relationships reveal potential negative effects of conifer removal on a non-target species. Biol. Conserv. 2023, 280, 109959. [Google Scholar] [CrossRef]
- Aldridge, C.L.; Saher, D.J.; Childers, T.; Stahlnecker, K.E.; Bowen, Z.H. Crucial nesting habitat for Gunnison sage-grouse: A spatially explicit hierarchical approach. J. Wildl. Manag. 2012, 76, 391–406. [Google Scholar] [CrossRef]
- Kirol, C.P.; Beck, J.L.; Huzurbazar, S.V.; Holloran, M.J.; Miller, S.N. Identifying greater sage-grouse source and sink habitats for conservation planning in an energy development landscape. Ecol. Appl. 2015, 25, 968–990. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, C.L.; Boyce, M.S. Linking occurrence and fitness to persistence: Habitat-based approach for endangered Greater Sage-Grouse. Ecol. Appl. 2007, 117, 508–526. [Google Scholar] [CrossRef] [PubMed]
- Lennon, J.J.; Koleff, P.; Greenwood, J.J.D.; Gaston, K.J. Contribution of rarity and commonness to patterns of species richness. Ecol. Lett. 2004, 7, 81–87. [Google Scholar] [CrossRef]
- Pilliod, D.S.; Jeffries, M.I. 2021. Chapter I. Sagebrush Amphibians and Reptiles. In Sagebrush Conservation Strategy—Challenges to Sagebrush Conservation; Remington, T.E., Deibert, P.A., Hanser, S.E., Davis, D.M., Robb, L.A., Welty, J.L., Eds.; U.S. Geological Survey Open-File Report 2020-1125; U.S. Geological Survey: Reston, VA, USA, 2021; pp. 67–75. [Google Scholar] [CrossRef]
- Andelman, S.J.; Fagan, W.F. Umbrellas and flagships: Efficient conservation surrogates or expensive mistakes? Proc. Natl. Acad. Sci. USA 2000, 97, 5954–5959. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.; Brammer-Robbins, E.; Aschehoug, E.; Haddad, N. Do substitute species help or hinder endangered species management? Biol. Conserv. 2019, 232, 127–130. [Google Scholar] [CrossRef]
- Zipkin, E.F.; DeWan, A.; Royle, J.A. Impacts of forest fragmentation on species richness: A hierarchical approach to community modelling. J. Appl. Ecol. 2009, 46, 815–822. [Google Scholar] [CrossRef]
- Monroe, A.P.; Edmunds, D.R.; Aldridge, C.L.; Holloran, M.J.; Assal, T.J.; Holloran, A.G. Prioritizing landscapes for grassland bird conservation with hierarchical community models. Landsc. Ecol. 2021, 36, 1023–1038. [Google Scholar] [CrossRef]
- Pacifici, K.; Zipkin, E.F.; Collazo, J.A.; Irizarry, J.I.; DeWan, A. Guidelines for a priori grouping of species in hierarchical community models. Ecol. Evol. 2014, 4, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, J.D.; Chalfoun, A.D. The abundance of Greater sage-grouse as a proxy for the abundance of sagebrush-associated songbirds in Wyoming, USA. Avian Conserv. Ecol. 2020, 15, 16. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Manning, A.D.; Smith, P.L.; Possingham, H.P.; Fischer, J.; Oliver, I.; McCarthy, M.A. The focal-species approach and landscape restoration: A critique. Conserv. Biol. 2002, 16, 338–345. [Google Scholar] [CrossRef]
- Gregg, M.A.; Crawford, J.A. Survival of Greater sage-grouse chicks and broods in the Northern Great Basin. J. Wildl. Manag. 2009, 73, 904–913. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Luna, T.; Pinto, J.R.; Landis, T.D. Forbs: Foundation for restoration of monarch butterflies, other pollinators, and greater sage-grouse in the Western United States. Nat. Areas J. 2016, 36, 499–511. [Google Scholar] [CrossRef]
- Copeland, H.E.; Sawyer, H.; Monteith, K.L.; Naugle, D.E.; Pocewicz, A.; Graf, N.; Kauffman, M.J. Conserving migratory mule deer through the umbrella of sage-grouse. Ecosphere 2014, 5, 117. [Google Scholar] [CrossRef]
- Johnson, M.D. Measuring habitat quality: A review. Condor 2007, 109, 489–504. [Google Scholar] [CrossRef]
- Timmer, J.M.; Aldridge, C.L.; Fernández-Giménez, M.E. Managing for multiple species: Greater sage-grouse and sagebrush songbirds. J. Wildl. Manag. 2019, 83, 1043–1056. [Google Scholar] [CrossRef]
- Lukacs, P.M.; Seglund, A.; Boyle, S. Effects of Gunnison Sage-Grouse habitat treatment efforts on associated avifauna and vegetation structure. Avian Conserv. Ecol. 2015, 10, 7. [Google Scholar] [CrossRef]
- Barlow, N.L.; Kirol, C.P.; Doherty, K.E.; Fedy, B.C. Evaluation of the umbrella species concept at fine spatial scales. J. Wildl. Manag. 2020, 84, 237–248. [Google Scholar] [CrossRef]
- Johnson, D.H. The comparison of usage and availability measurements for evaluating resource preference. Ecology 1980, 61, 65–71. [Google Scholar] [CrossRef]
- Manier, D.J.; Wood, D.J.A.; Bowen, Z.H.; Donovan, F.M.; Holloran, M.J.; Juliusson, L.M.; Oyler-McCance, S.J.; Quamen, F.R.; Saher, D.J.; Titolo, A.J. Summary of Science Activities, Programs, and Policies That Influence the Rangewide Conservation of Greater Sage-Grouse (Centrocercus urophasianus); U.S. Geological Survey Open-File Report 2013-1098; U.S. Geological Survey: Reston, VA, USA, 2013; p. 170. Available online: http://pubs.usgs.gov/of/2013/1098/ (accessed on 15 September 2023).
- Garman, S.L. A simulation framework for assessing physical and wildlife impacts of oil and gas development scenarios in southwestern Wyoming. Environ. Model. Assess. 2018, 23, 39–56. [Google Scholar] [CrossRef]
- Brooks, M.L.; D’Antonio, C.M.; Richardson, D.M.; Grace, J.B.; Keeley, J.E.; DiTomaso, J.M.; Hobbs, R.J.; Pellant, M.; Pyke, D. Effects of invasive alien plants on fire regimes. Bioscience 2004, 54, 677–688. [Google Scholar] [CrossRef]
- Brooks, M.L.; Matchett, J.R.; Shinneman, D.J.; Coates, P.S. Fire Patterns in the Range of the Greater Sage-Grouse, 1984–2013—Implications for Conservation and Management; U.S. Geological Survey Open-File Report 2015-1167; U.S. Geological Survey: Reston, VA, USA, 2015; p. 66. [Google Scholar] [CrossRef]
- Balch, J.K.; Bradley, B.A.; D’Antonio, C.M.; Gómez-Dans, J. Introduced annual grass increases regional fire activity across the arid western USA (1980–2009). Glob. Chang. Biol. 2013, 19, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Homer, C.G.; Xian, G.; Aldridge, C.L.; Meyer, D.K.; Loveland, T.R.; O’Donnell, M.S. Forecasting sagebrush ecosystem components and greater sage-grouse habitat for 2050: Learning from past climate patterns and Landsat imagery to predict the future. Ecol. Indic. 2015, 55, 131–145. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Naugle, D.E.; Hagen, C.A.; Maestas, J.D. Public lands and private waters: Scarce mesic resources structure land tenure and sage-grouse distributions. Ecosphere 2016, 7, e01208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).