Abstract
The potential interactions of rhizobium bacteria in enhancing nodulation, nitrogen (N) fixation for boosting N availability, and the yield of black gram under a temperate environment continue to remain unexplored. Therefore, this study aimed to evaluate the agronomic performance of black gram cultivars, their yield comparisons, and shoot–grain–soil N dynamics in a prevalently rainfed farming system. Two black gram cultivars, NARC Mash-I and NARC Mash-II, were subjected to rhizobia inoculation combined with different N doses (0, 25, 50, 75, 100 kg ha−1). The response variables included root nodulation, agronomic yield attributes, grain yield, shoot–grain and soil N dynamics, and biological productivity. Black gram cultivar NARC Mash-II showed the maximum nodule formation (41 per plant), while each nodule obtained 0.69 g weight in response to RI combined with 25 kg N ha−1. Additionally, this combination showed the highest pods per plant and thousand grain weight, which maximized the grain yield (1777 kg ha−1) and biological productivity (3007 kg ha−1). In contrast, NARC Mash-I under 50 kg N recorded the highest shoot N content, while the same cultivar under 100 kg N exhibited the maximum soil N content. The correlation analyses indicated a significantly robust association among the nodule numbers, grain weight, and N contents in different plant organs. These results give mechanistic insights into plant–microbe interactions based on the eco-friendly, sustainable, and smart agricultural practice of black gram production in a temperate environment.
1. Introduction
Declining soil fertility due to agricultural practices, unpredictable climate changes, and shortages of water and minerals is challenging for an eco-friendly modern agricultural system [1]. A gradual decrease in soil fertility has reduced the production of food crops, leading to food and nutritional insecurity for an increasing number of the population, especially in the Indo-Pak subcontinent [2]. Among the strategically crucial plant nutrients required to achieve potential crop yields, N ranks at the top as it is required by crop plants in larger quantities to attain potential vegetative growth, promote chlorophyll development, and trigger the biosynthesis of amino acids, proteins, and nucleotides [3]. However, resource-poor growers with small land holdings can ill-afford the skyrocketing cost of inorganic fertilizers to compensate for N deficiency in agricultural soils. Furthermore, a variety of processes, especially sub-optimized N doses, cause losses of over 50% of soil-applied N fertilizers, making it a prime source of environmental pollution [4]. Interestingly, numerous studies have reported contrasting findings on N fertilization regimes for black gram under tropical and subtropical environments, including Reddy et al. [5], Rathore et al. [6], and Prasad et al. [7] who suggested 20 kg N ha−1, while Marimuthu and Surendran [8] recommended 25 kg N ha−1. However, Chandrasekar and Bangarusamy [9] and Ahmed et al. [10] suggested even higher quantities of N (30–55 kg ha−1). Research findings are scant for temperate conditions; thus, increasing prices of N fertilizers and emerging agro-ecological pollution have renewed research interest in scouting for optimal N doses and finding biologically viable strategies to boost the crop yield and soil fertility status.
Several strategies have been applied for boosting mineral nutrition, abiotic stress tolerance, and plant impartments in soils [11,12,13,14,15]. In place of mineral N fertilizers, legume–rhizobium symbiosis might serve as a potent source of N and may reduce plants’ N requirements and maintain crop yields as per varietal potential [16,17]. In addition, N fixed through the biological N fixation (BNF) process has the advantage of being a renewable source and there is scant probability of loss through leaching and volatilization [18]. The other associated advantage offered by rhizobia symbiosis is its potential for building up soil fertility and enhancing crop productivity in a sustainable and eco-friendly manner [4]. Previous studies have reported the positive effects of rhizobia inoculation for boosting the crop productivity of Rhizobium trifolii in clover, Bradyrhizobium japonicum in common beans, soybean, etc., Azorhizobium caulinodans in sesbania, sinorhizobium/Ensifer meliloti comb in alfalfa, and Mesorhizobium mediterraneum in chickpeas [4,19,20,21].
For sustainable legume–rhizobium symbiosis, seed inoculation must bolster root nodulation, which significantly improves N uptake and crop production [22,23]. Rhizobia participate in the BNF process in the root nodules of legumes [16], leading to the conversion of molecular N into ammonia [24]. Interestingly, non-inoculated plants are exposed to multiple rhizobia, which significantly vary in their capacity to promote nodulation and N fixation. Despite the wide abundance and effectiveness of natural rhizobia populations, maximized N fixation by legume crops has rarely been achieved. Sánchez–Navarro et al. [18] inferred that a wide array of microbiomes exist in the rhizosphere; however, rhizobium inoculation could be useful even for soils where legumes have been cultivated for many years, owing to the absence of crop-specific rhizobia [25]. It was also inferred that inoculation increased the rhizobia population in the rhizosphere, which led to enhanced N fixation by legumes in soil where there was a scarcity of crop-specific rhizobia. Additionally, it has been suggested that rhizobia must survive in soil and utilize ecological niches created by plant roots for fixing atmospheric N [26]. However, poorly fixed naturally occurring rhizobia strains may become dominant and gain an advantage over inoculated strains [27]. Therefore, Safronova et al. [28] suggested that the choice of rhizobia strains for seed inoculation must be made keeping in mind their host specificity and the microbial genera present in the rhizosphere. In order to maximize BNF, the inoculant strain must be efficient and match the desired legume variety in a growing agro-ecological zone. The adaptation of host-specific rhizobia strains to the local environment and soil conditions increases the chance of effective nodulation [26]. However, research and knowledge gaps exist pertaining to the impact of inoculation on N dynamics in the shoots and grains of legumes under temperate conditions, which necessitate conducting fresh studies.
Black gram (Vigna mungo (L.) Hepper) is a mash bean that is widely cultivated in the Indo-Pak sub-continent and Bangladesh [29] and ranks as the third major pulse crop of Pakistan [30]. Black gram is an excellent source of protein for human and animal nutrition. It comprises over 24% protein, 60% carbohydrates, and 1.5% fat, and finds use as whole seed and dehusked splits (cotyledons), as well as in a variety of fermented products, like dosa. Interestingly, it is subjected to fermentation with rice blends for preparing fermented steamed products and different types of roasted pancakes [31]. In black gram, the assimilated translocation to the constantly growing vegetative sinks even after the initiation of the reproductive phase considerably reduces the grain yield, owing to N supply limitations [26]. This might be rectified by optimizing N doses and performing seed inoculation with appropriate rhizobia strains. Another serious constraint in black gram production is flower and fruit drop caused by N deficiency, which requisites conducting fresh studies on optimizing N fertilization regimes.
Thus, under a changing climate, it is of strategic pertinence to bridge the research and knowledge gaps pertaining to N dose optimization to enhance biological N fixation through the inoculation of rhizobia strains under a temperate environment. Additionally, the field testing of black gram cultivars for assessing the grain yield potential offers a bright perspective to enhance productivity and profitability. Thus, this field research tested the hypothesis that the optimization of N fertilization regimes and seed inoculation of rhizobia might increase the black gram grain yield, N dynamics, and soil fertility. Thus, the objectives of this study were to explore the genetic potential of black gram cultivars under a temperate environment through the optimization of N doses and rhizobia inoculation to enhance the nodulation properties, agronomic yield traits, and productivity, along with plant and soil N dynamics.
2. Materials and Methods
2.1. Experimental Site’s Meteorological Features
The field trial was conducted during the crop growth seasons of 2019 and 2020 in the farm area of the Faculty of Agriculture, University of Poonch Rawalakot, Azad Jammu and Kashmir, Pakistan. The geographical coordinates of the study locality are depicted in Figure 1. The study site has a sub-mountainous topography of valleys, temperate climatic characteristics, and an altitude of 1633 m. The average temperature and the mean annual precipitation of the region are 15 °C and 360 mm, respectively [32]. The texture of the experimental block was silty clay loam. Pre-sowing soil sampling was performed in order to estimate the physico-chemical properties of experimental sites which exhibited a pH and electrical conductivity of 7.4 and 0.37, respectively. Additionally, the available phosphorous and N content were 2.56 mg kg−1 and 0.41%, respectively, while organic matter was 1.21%. Moreover, soil organic carbon, bulk density, and soil porosity were 6.38 g ka−1, 1.34%, and 43%, respectively.
Figure 1.
The trial’s location (Rawalakot, Azad Kashmir, Pakistan) map prepared with the help of QGIS software (version 3.24.3, Bern, Switzerland), whereby the black point highlights the trial’s location while the half-arrow is pointed toward the north direction.
2.2. Experimental Design, Rhizobia Inoculum, and Nitrogen Supplementation
The experiment was comprised of a control treatment (without inoculation and fertilization), two cultivars of black gram (V1 = NARC Mash-I and V2 = NARC Mash-II), and four N fertilization regimes (25, 50, 75, and 100 kg N ha−1). The details of the employed treatments are presented in Table 1. The trial was conducted using a randomized complete block design (RCBD) with split plot arrangement and there were three replications. The trial was comprised of 36 experimental plots in total, while the net size of each plot was 9 m2 (after excluding the walking paths and plot boundaries). Black gram varieties (NARC Mash-I and NARC Mash-II) were used as planting materials and their seeds were acquired from the National Agriculture Research Center (NARC), Islamabad, Pakistan. Both cultivars were bold-seeded, high-yielding, and resistant to yellow mosaic virus (YMV) and urdbean leaf crinkle virus (ULCV). These cultivars are being grown on a wide scale in the irrigated and rainfed areas of the Punjab and Sindh provinces of Pakistan [33]; however, no research-based findings are available regarding their performance under the temperate environment of the Azad Jammu and Kashmir region of Pakistan. A low soil temperature delays germination, and thus seed hydro-priming was performed by dipping the seeds into water for 12 h followed by shade drying for 5 h, as recommended by Golezani et al. [34]. The sowing was conducted on 27 and 29 April of 2019 and 2020, respectively, and harvesting was manually performed on 23 and 24 August using sickles.

Table 1.
Details of treatments regarding Rhizobia inoculation and N fertilization regimes for black gram cultivars tested during the course of this study.
The crop was sown using 30 cm R × R and 10 cm P × P spacing (30 rows per plot). The sowing was conducted using a hand drill. The phosphorous (P = 60 kg ha−1) fertilizer was applied as a basal dose in a slightly higher quantity than suggested by Qayyum et al. [30] (50 kg ha−1 in order to compensate for the significantly lower available P of 2.56 mg kg−1 of soil in all experimental plots). All agronomic management practices (e.g., land preparation, priming duration, seed rate, plot size sowing time and technique, plant–plant and row–row spacings, weeding frequency, etc.) except those employed as treatments (N dose, cultivars, and rhizobium inoculation) were kept uniform to inhibit the influence of any external factor which could have altered the treatment effects on the response variables under investigation. The seeds of black gram cultivars were inoculated with 10% brown sugar (to attain the dual purposes of thickening the solution for enhancing adherence to the grain surface and to provide an instant source of energy for microbes) solution containing the Bradyrhizobium strain of TAL-169 (2 weeks old rhizobia culture acquired from NARC, Islamabad, Pakistan). It was prepared by following the protocol of Saleem et al. [2] in such a way that the inoculum was completely stuck on the grains of the black gram [14,15]. The solution was prepared by adding sugar to water, while black gram grains were placed in a large open-top tub. The grains were mixed with the inoculant using an inoculant/grain weight ratio of 1:100, as suggested by Temprano et al. [35]. The continuous stirring of grains in the solution was performed by mixing a cup of the sugar solution at continuous intervals. The stirring was continued until the seeds were damp but not saturated by ensuring that there was no standing liquid. Once the grains were thoroughly mixed with the thick solution, the inoculant powder was gradually added. This procedure was performed using small batches of grains because a mechanical mixer was not available for this purpose. After inoculation, grains were sown within eight hours to ensure the viability of the rhizobia.
2.3. Collection of Root Nodules and Plant Samples
In the grain filling stage, ten plants were randomly selected from the middle rows of each experimental plot and uprooted with the help of a spade. For the purpose of loosening the sticky soil from roots, plants were placed into the plastic buckets filled with water. Thereafter, soil adhering to the roots was manually removed. Subsequently, plants’ roots were separated and nodules were picked from roots for data recording. In the maturity stage, plant samples from an area of one square meter were randomly harvested from all experimental plots for the estimation of different agronomical traits, including the number of pods per plant, thousand grain weight, biological and grain yields, etc.
2.4. Estimation of Physio-Chemical Parameters of the Soils
After crop harvesting, soil sampling was conducted from 0 to 15 cm and 15 to 30 cm depths, while the soil sampling points included the four corners and middle of the experimental block. Thereafter, the soil samples were thoroughly mixed and preserved in zip-lockable bags for N estimation, following the Kjeldahl method [36]. The soil organic carbon (SOC) and organic matter (SOM) were analyzed by following the standard methods [37].
2.5. Determination of Shoot and Grain N
For the estimation of the N content of the shoot and grains, 1 g of crushed matter (using mortar and pestle) was mixed with 50 mL of H2SO4 and 10 g of Kjeldahl Catalyst (Cu-Se) that was subsequently placed in the digestion flasks for two hrs. Thereafter, 100 mL of water was added in the flasks of each sample, which was shaken using a shaker for 10 min. Finally, all samples were put in the analyzer (Kjeltec Auto 1030 Analyzer) for N estimation [38].
2.6. Statistical Analysis
The collected data were subjected to Bartlett’s test which exhibited a non-significant effect of the year, and thus data were transformed into mean values. Subsequently, the transformed data were analyzed using SPSS statistics computer software (version 17.0) by employing the analysis of variance (ANOVA) technique to measure the significant difference at p ≤ 0.05. Additionally, two-way factor interaction (treatments × cultivars) was conducted and comparisons of means were performed using Tukey’s honest significant difference (HSD) test at a 5 percent probability level [39]. Moreover, to determine the relationship among response variables, the Pearson correlation coefficient was found using the same statistical package. Furthermore, for the exploration of the multivariate variability caused by employed treatments for the grain, shoot, and soil N accumulation in the plant–soil system, principal component analysis (PCA) was employed using PAST-Paleontological Statistics [40].
3. Results
3.1. Rhizobia Inoculation Combined with N Supplementation Enhanced Root Nodulation
The nodulation potential of the black gram cultivars was evaluated in terms of number and dry weight of nodules under rhizobia inoculation (RI) and varying N fertilization regimes, while the results exhibited noticeable diversity in these traits (Table 2). Overall, the mean values of the nodulation characteristics under N doses with RI were considerably higher than for the unamended control treatment. It was revealed that the highest number of nodules per plant (41 ± 2.5) was recorded for the N25RI treatment (NARC Mash-I cultivar), which remained statistically at par with N0RI for NARC Mash-II; however, it was 32% higher than for the unamended control treatment. It was followed by N25RI, which performed at par with N50RI for NARC Mash-II. Likewise, the maximum nodule dry weight was recorded for NARC Mash-II in response to the treatment of N25RI (0.69 ± 0.02), which was 37% higher than for the control treatment. The minimum nodule number (27 ± 2.0) was recorded in the control treatment with the lowest nodule dry weight (0.41 ± 0.03) for NARC Mash-I, which was statistically at par with the N100RI treatment. Moreover, N100RI could not match the nodulation recorded by N75RI, which in turn remained inferior to N50RI. Furthermore, N50RI exhibited significantly lower nodule numbers and dry weight than N25RI. To sum up, a significant genotypic difference was recorded, because NARC Mash-I depicted the maximum number of nodules, while NARC Mash-II remained superior for nodule dry weight when inoculated with rhizobia under limited N supply.

Table 2.
The variations in the root nodules of black gram cultivars under rhizobia inoculation and N fertilization regimes in a temperate climate.
3.2. Yield Attributes under Rhizobia Inoculation and N Fertilization Regimes
The effect of cultivars and employed treatments (rhizobia inoculation and N fertilization regimes) was significant, as depicted in Table 3. The maximum pod numbers per plant (46 ± 3.1) and thousand grain weight (62 ± 5.5) were exhibited by N25RI for NARC Mash-II and were 33% and 19%, respectively, higher than for the unamended control treatment. In addition, the N0RI and N50RI treatments recorded statistically similar results in terms of pod numbers and thousand grain weight. In contrast, the minimum number of pods per plant and thousand grain weight were recorded for NARC Mash-I in the control treatment, while the N50RI and N75RI treatments remained statistically at par with each other (60 ± 4.5 and 59 ± 2.1, respectively) for thousand grain weight in NARC Mash-II. Likewise, NARC Mash-II in response to the N25RI treatment showed the highest pod numbers and thousand grain weight; however, it remained at par with NARC Mash-I under the same treatment. In terms of the pod numbers per plant, the N25RI and N50RI treatments exhibited unmatched results for NARC Mash-II, while both cultivars under the control treatment recorded the minimum pod numbers and thousand grain weight.

Table 3.
The grain yield attributes of black gram cultivars under rhizobia inoculation and N fertilization regimes in a temperate climate.
3.3. Grain and Biological Yields under Rhizobia Inoculation and N Fertilization
Black gram cultivars depicted significant diversity in terms of grains and biological yields under the influence of RI and N fertilization regimes (Table 4). All of the fertilization regimes performed superiorly over the unfertilized control treatment, especially for the NARC Mash-II cultivar. The results revealed that the maximum grain yield (1777 ± 118) and biological yield (3007 ± 105) were recorded for NARC Mash-II in response to N25RI, and were 58% and 37% higher compared to the unfertilized control treatment. Interestingly, NARC Mash-II remained statistically at par with N25RI, N0RI, and N75RI in terms of grain yield. In contrast, NARC Mash-II under N25RI exhibited a statistically similar biological yield (3007 ± 105) as that of N50RI (2893 ± 110). Among the fertilizer regimes, N100RI could not perform at par to the rest of the treatments; however, it remained statistically equal to the control treatment in terms of both grain yield and biological productivity. Following the trend, the Mash-II cultivar surpassed the Mash-I cultivar under N25RI by recording significantly higher grains and biological yields.

Table 4.
Grain yield and biomass productivity of black gram cultivars under rhizobia inoculation and N fertilization regimes in a temperate climate.
3.4. Nitrogen Accumulation Grains and Shoots
The findings regarding grain and shoot N content displayed significant variation among black gram cultivars and fertilization regimes (Figure 2). NARC Mash-II outperformed NARC Mash-I, especially under the fertilization regime of N25RI, by recording a 47% higher grain N content than the unfertilized control treatment, while this treatment combination performed statistically at par to N0RI (Figure 2A). In contrast, NARC Mash-I under the fertilization regime of N25RI gave maximum grain N content that was 33% higher compared to the control treatment and thus, in this way, NARC Mash-II accumulated 14% higher grain N content than NARC Mash-I under N25RI. Overall, all of the fertilization regimes recorded comparatively higher N content of grain compared to the unfertilized control treatment. As far as shoot N content of black gram was concerned, NARC Mash-I in response to N25RI exhibited the maximum shoot N value, which was 112% higher than the control treatment (Figure 2B). Moreover, this treatment combination remained statistically non-significant compared to the same cultivar under the N75RI fertilization regime. Moreover, the minimum N content of the shoot was recorded for NARC Mash-I under the unfertilized control treatment that performed statistically similarly to NARC Mash-II under no fertilization, as well as the N100RI fertilization regime.
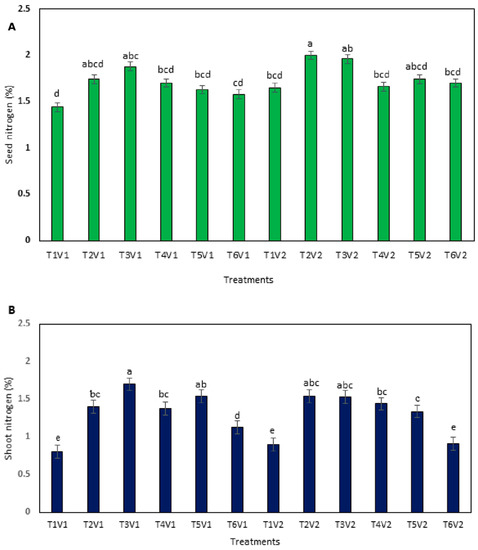
Figure 2.
Content of grain nitrogen (A) and shoot nitrogen (B) of black gram cultivars supplemented with rhizobia inoculation and N fertilization in a temperate climate. Different letters on the bar column indicate significant differences at p ≤ 0.05. All of the results are presented as mean ± SE of at least three independent replications. Here, T1 = control, no inoculation and fertilization, T2 = inoculation but no fertilization, T3 = 25 kg N ha−1, T4 = 50 kg N ha−1, T5 = 75 kg N ha−1, T6 = 100 kg N ha−1, V1 = NARC Mash-I, V2 = NARC Mash-II, RI = rhizobia inoculation.
3.5. Analysis of Soil N Dynamics
The results revealed that all of the N fertilization regimes outperformed the unfertilized control treatment in terms of soil N content (Figure 3). The maximum soil N buildup (40% higher N compared to the unfertilized control treatment) was noted for experimental plots where NARC Mash-I was sown under the fertilization regime of N100RI. It performed statistically at par with NARC Mash-II under the same fertilization regime that recorded 38% higher soil N compared to the unfertilized control treatment. Interestingly, NARC Mash-II sown in unfertilized plots remained statistically at par with the inoculated but unfertilized treatment, while NARC Mash-I and NARC Mash-II cultivars under the fertilization regimes of N25RI, N50RI, and N75RI remained at par in terms of soil N content.
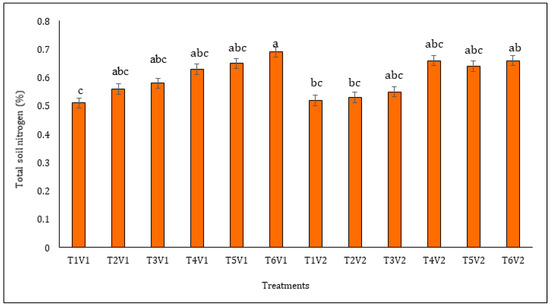
Figure 3.
Accumulation of total nitrogen in soil under black gram cultivars supplemented with rhizobia inoculation and N fertilization in a temperate climate. Different letters of the bar column indicate significant differences at p ≤ 0.05. All of the results are presented as mean ± SE of at least three independent replications. Here, T1 = control, no inoculation and fertilization, T2 = inoculation but no fertilization, T3 = 25 kg N ha−1, T4 = 50 kg N ha−1, T5 = 75 kg N ha−1, T6 = 100 kg N ha−1, V1 = NARC Mash-I, V2 = NARC Mash-II, RI = rhizobia inoculation.
3.6. Principal Component Analysis and Correlation of Different Yield Attributes
The correlation analysis indicated a highly significant association among yield attributes (number of pods per plant, thousand grain weight, etc.) with the grain yield as well as the biological yield of black gram (Table 5). Moreover, in accordance with principal component analysis (PCA), the observation point made by a combination of PC1/PC2 and PC1/PC3 depicts the general variance described by the five major components. The maximum loading was noted for the PC1 component, while the minimum loading was observed in the PC3 component for the N content of the grain, shoot, and soil. Furthermore, PC4 and PC5 were not plotted as they do not furnish any further information. Obviously, the PCA showed that the control treatments of both genotypes were less affected by the grain, shoot, and soil N accumulation in the plant–soil system in comparison with RI alone or in association with the N fertilization treatments (Figure 4A,B). Actually, the points of the control treatments found were closer compared with the other amended treatments regarding both genotypes, and also nearer to the central point of the PCA components.

Table 5.
Correlation analyses of different agricultural attributes of black gram (mean values of both cultivars and years) tested under rhizobia inoculation and N fertilization regimes in a temperate climate.
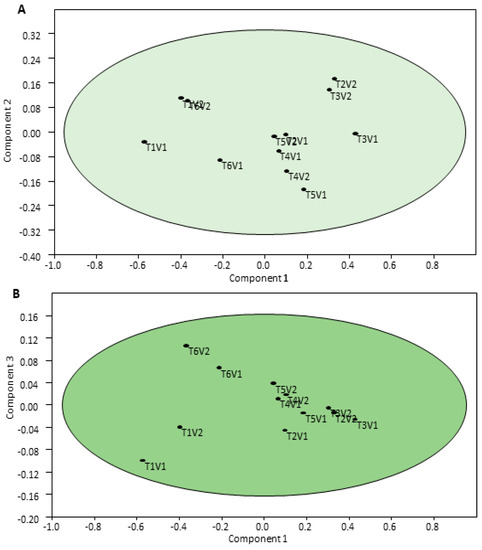
Figure 4.
The principal component analysis (PCA) of black gram cultivars subjected to rhizobia inoculation (A) and varying N fertilization regimes (B) in a temperate climate. Here, T1 = control, no inoculation and fertilization, T2 = inoculation but no fertilization, T3 = 25 kg N ha−1, T4 = 50 kg N ha−1, T5 = 75 kg N ha−1, T6 = 100 kg N ha−1, V1 = NARC Mash-I, V2 = NARC Mash-II, RI = rhizobia inoculation.
4. Discussion
Increasing crop yields through microbial inoculation and N fertilizer supplementation is a biologically viable way to move toward modern agricultural production systems. The RI of leguminous crops, especially on virgin soils, constitutes a sustainable approach toward boosting crop productivity [17,20]. In this study, the RI and N fertilization regimes significantly boosted the nodulation of black gram cultivars. The RI tends to promote the grain yield of leguminous crops by boosting root nodulation, which results in the higher fixation of atmospheric N through the BNF process [41]. Similar to our findings (sole RI and N fertilization in association with RI significantly promoted black gram nodulation), it was reported that RI proved to be an eco-friendly and cost-effective technique for boosting root nodulation in mung bean, peas, and red clover [42,43]. However, a previous study suggested that RI reduces the requirement of N fertilizer due to increased N fixation in many crops [44]. Additionally, it helped to activate the response of ineffective rhizobium strains present in the soils which later on became involved in the N fixation process [45].
Interestingly, RI along with low N supply improved N fixation by producing a greater number of functional nodules [46]. In this present study, the better response of RI even without N supplementation opened a new avenue for sustainable, eco-friendly, and smart agriculture by utilizing rhizobia for promoting N fixation. In contrast, the suppression of nodulation in response to a high N dose indicates the inhibitory effects of high N content on nitrogenase activity in the root nodules. A negative correlation was found between the supplementation of N and the number of formed root nodules [47]. Robust vegetative growth was recorded as seen through higher N doses in legumes and grain crops [48]; however, nodule formation was governed by soil rhizobia population and soil N content [49]. In this study, the nodulation was influenced by RI combined with N supplementation in black gram. A number of studies reported that RI considerably improved plant growth, nutritional accumulation, and plant fitness to changing environments [9,13,50,51]. Moreover, varying genotypic potential existed among soybean cultivars, along with RI which significantly increased the nodule dry weight [52,53]. However, the nodule number could vary due to genetic potential, and agronomic management practices might impart significant influence on the nodule development of leguminous crops [54]. Likewise, it has also been inferred that genotypic divergence in black gram cultivars existed, which resulted in varying levels of nodulation in terms of the number of functional nodules along with fresh and dry weight of nodules per plant [54,55,56,57].
Legumes are the most effective crops which contribute N to soils through N fixation and ultimately improve soil fertility. Our study also reports the benefit of exogenous N supplementation and RI, which significantly enhanced the pod number per plant, thousand grain weight, grain yield, and biological biomass production in black gram cultivars. Thus, effective plant–microbe interactions, the enhancement of root nodulation, and increase in N fixation tend to improve the growth and yield attributes of black gram [58]. In contrast, the mismatching of RI with higher N doses might reduce root development and water and nutrient uptake in soils. Furthermore, RI under limited N supply tends to trigger various vital processes such as N fixation, nitrate reductase activity, the biosynthesis of crucial plant hormones such as auxins, gibberellins, and cytokinins, and the boosting of phosphate solubilization, which enhanced the legume yield [59]. In this study, shoot N status showed that the least N supply with RI significantly improved the black gram grain yield and shoot N content compared to the control treatment. It might be concluded that RI in association with reduced N doses remained effective in boosting the BNF fixation process, and ultimately, a greater concentration of N accumulated in the grains and shoots. A previous study has suggested that RI with N supplementation enhanced nitrogenase activity, which improved the total N content in plants [40]. In addition, it was also inferred that a limited supply of inorganic N fulfilled the immediate N needs of crop plants, while a slow and steady provision of N produced through the BNF process resulted in greater N content of grains. Moreover, meager N supply triggered rhizobia activity and ultimately better N accumulation was recorded in the leaves, shoots, and grains of the crops [1]. However, RI with a limited N supply increased the N content of the plant–soil system, owing to improved N absorption and higher N use efficiency [60].
Soil N content is a vital factor that determines rhizobia’s ability to fix N through the BNF process. The higher doses of inorganic N with RI improved the soil N status in comparison to lower N doses, indicating that higher N doses were over and above the crop plants’ requirements and remained unutilized, which led to N buildup in the soil. These results might be linked to the black gram tendency to uptake lower amounts of supplemented N, which ultimately resulted in the accumulation of higher N in the rhizosphere [53]. However, RI combined with a moderate N supply improved the total soil N contents compared to limited N doses [61,62]. Interestingly, a lower N dose with RI has been reported to increase shoot and soil N contents [60]. The Pearson correlation analysis amongst black gram nodulation, yield attributes such as number of pods per plant and their dry weight, and grain and biological yields, along with plant and soil N, indicated a stronger linear relationship, which highlights the role of these yield components in boosting the grain yield of black gram. These traits have been reported to be of great pertinence for the selection and establishment of breeding criteria to increase the grain yield of legumes. These findings are in accordance with a previous study, where it was reported that the determination of pod numbers per plant and thousand grain weight were the most crucial strategies for projecting the grain yield, because these were linearly associated with the grain yield of legumes [63,64]. Contrastingly, a greater number of pods per plant contained a lower number of grains per pod, and thus this was negatively associated with the thousand grain weight of black gram. However, our findings were supported by previous studies where the yield attributes including the pod numbers and grain weight were linearly associated with the grain yield and N content of the shoots and grains in black gram [65,66,67,68]. Moreover, shoot N increased in the presence of greater soil N content. However, the legumes’ grain yield increment was directly associated with the robustness of the nodulation and amount of N fixed through the BNF process [41].
PC analysis can identify the traits that account for variation in crop yields [64,65,66,67,68,69], and thus, it may be employed for confirming the most influential response variables [70,71,72]. According to the PC1/PC2 combination analysis in this study, the unamended control treatment remained the least affected compared with the amended treatments for both cultivars of black gram. Furthermore, higher N doses applied with RI recorded significantly less N content in the shoots and grains of black gram. However, yield traits had a negative correlation with soil total N contents. Similarly to our findings, El-Sorady et al. [40] inferred that two PCs accounted for over 92% of the total variation among employed treatments. Likewise, our findings also remained in concurrence with the results of a previous study whereby PC analysis highlighted the major variability among the treatments [73,74]. These research findings can be of great assistance to black gram growers for boosting productivity and soil fertility in a sustainable and eco-friendly way.
5. Conclusions
The research findings provide a mechanistic insight into plant–microbe interactions and N-fertilization-based improvement in the yield attributes and grain yield of black gram. This study found that considerable yield potential variation existed among black gram cultivars which was significantly influenced by RI and different doses of N fertilization under a temperate environment. The NARC Mash-II cultivar remained superior under the N25RI fertilization regime by recording significantly higher root nodulation, pod numbers per plant, and thousand grain weight than the unfertilized control treatment did. The same treatment combination outmatched the control treatment in terms of grain yield and biological yield, along with the N content of the grain, while NARC Mash-I resulted in higher N accumulation in the shoot and soil. This study further suggests that a high level of N supply (>25 kg N ha−1) reduced the nodule formation and pod setting, while 25 kg N ha−1 combined with RI improved all of the response variables except for shoot and soil N content. This combination of cultivar (NARC Mash-II) and 25 kg N ha−1 combined with RI might be recommended to growers for boosting the nodulation, N dynamics, agronomic yield attributes, and productivity of black gram. However, future research needs to focus on determining the influence of higher N doses on nodulation and soil–plant N dynamics to assess the possible negative effects of nitrate on nodule formation and root N levels.
Author Contributions
Conceptualization, M.S., N.R., M.M.T. and M.A.I.; methodology, M.S., A.M. and M.A.I.; validation, M.A.I. and R.A.; formal analysis, R.A., M.D.A. and M.A.I.; writing—original draft preparation, M.A.I., M.M.T., N.R. and R.A.; writing—review and editing, M.A.I., M.D.A. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project (number PNURSP2023R355), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Acknowledgments
All of the authors convey the highest appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project (number PNURSP2023R355), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chattha, M.U.; Arif, W.; Khan, I.; Soufan, W.; Bilal Chattha, M.; Hassan, M.U.; Ullah, N.; Sabagh, A.E.; Qari, S.H. Mitigation of cadmium induced oxidative stress by using organic amendments to improve the growth and yield of mash beans [Vigna mungo (L.)]. Agronomy 2021, 11, 2152. [Google Scholar] [CrossRef]
- Saleem, R.; Ahmad, Z.I.; Ashraf, M.; Anees, M.A.; Javed, H.I. Impact of different fertility sources intercropping on productivity of black gram. Int. J. Biol. Biotechnol. 2016, 13, 89–99. [Google Scholar]
- Simon, Z.; Mtei, K.; Gessesse, A.; Ndakidemi, P.A. Isolation characterization of nitrogen fixing rhizobia from cultivated uncultivated soils of Northern Tanzania. Am. J. Plant Sci. 2014, 5, 4050–4067. [Google Scholar] [CrossRef]
- Roychowdhury, R.; Banerjee, U.; Sofkova, S.; Tah, J. Organic farming for crop improvement sustainable agriculture in the Era of climate change Online. J. Biol. Sci. 2013, 13, 50–65. [Google Scholar] [CrossRef]
- Reddy, A.; Kavitha Priya, M.S.; Reddy, D.M.; Reddy, B.R. Principal Component Analysis for Yield in blackgram (Vigna mungo L. Hepper) under organic and inorganic fertilizer managements. Int. J. Plant Soil Sci. 2021, 33, 26–34. [Google Scholar] [CrossRef]
- Rathore, R.S.; Singh, R.P.; Nawange, D.D. Effect of land configuration, seed rates and fertilizer doses on growth and yield of black gram [Vigna Mungo (L.) Hepper]. Legume Res. 2010, 33, 274–278. [Google Scholar]
- Prasad, J.D.; Sharma, S.K.; Amarawat, T. Effect of organic and inorganic sources of nutrients on yield and economics of blackgram (Vigna mungo L.) grown during kharif. Agric. Sci. Digest. 2015, 35, 224–228. [Google Scholar] [CrossRef]
- Marimuthu, S.; Surendran, U. Effect of nutrients and plant growth regulators on growth and yield of black gram in sandy loam soils of Cauvery new delta zone, India. Cogent Food Agri. 2015, 1, 1010415. [Google Scholar] [CrossRef]
- Chandrasekar, C.N.; Bangarusamy, U. Maximizing the yield of mungbean by foliar application of growth regulating chemicals and nutrients. Madras Agric. J. 2003, 90, 142–145. [Google Scholar]
- Ahmed, Z.I.; Ansar, M.; Saleem, A.; Arif, Z.U.; Javed, H.I.; Saleem, R. Improvement of mash bean production under rainfed conditions by Rhizobium inoculation lower rates of starter nitrogen Pak. J. Agric. Res. 2012, 25, 154–356. [Google Scholar]
- Rahman, M.A.; Lee, S.-H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.-W. Importance of mineral nutrition for mitigating aluminum toxicity in plants on acidic soils: Current status opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef]
- Rahman, M.A.; Parvin, M.; Das, U.; Ela, E.J.; Lee, S.-H.; Lee, K.-W.; Kabir, A.H. Arbuscular mycorrhizal symbiosis mitigates iron (Fe)-deficiency retardation in alfalfa (Medicago sativa L.) through the enhancement of Fe accumulation sulfur-assisted antioxidant defense. Int. J. Mol. Sci. 2020, 21, 2219. [Google Scholar] [CrossRef]
- Kabir, A.H.; Rahman, M.A.; Rahman, M.M.; Brailey-Jones, P.; Lee, K.W.; Bennetzen, J.L. Mechanistic assessment of tolerance to iron deficiency mediated by Trichoderma harzianum in soybean roots. J. Appli. Microbiol. 2022, 133, 2760–2778. [Google Scholar] [CrossRef]
- Khan, I.; Muhammad, A.; Chattha, M.U.; Skalicky, M.; Bilal Chattha, M.; Ayub, M.A.; Anwar, M.R.; Soufan, W.; Hassan, M.U.; Rahman, M.A.; et al. Mitigation of salinity-induced oxidative damage, growth, and yield reduction in fine rice by sugarcane press mud application. Front. Plant. Sci. 2022, 13, 840900. [Google Scholar] [CrossRef]
- Khan, Q.A.; Sardar, A.C.; Muhammad, F.; Abdul, W.; Fasih, U.H. Monitoring the role of molybdenum seed priming on productivity of mung bean (Vigna radiata L.). J. Res. Ecol. 2019, 7, 2417–2427. [Google Scholar]
- Allito, B.B.; Ewusi-Mensah, N.; Logah, V. Legume-Rhizobium strain specificity enhances nutrition and nitrogen fixation in faba bean (Vicia faba L.). Agronomy 2020, 10, 826. [Google Scholar] [CrossRef]
- Rahman, M.A.; Alam, I.; Kim, Y.-G.; Ahn, N.-Y.; Heo, S.-H.; Lee, D.-G.; Liu, G.; Lee, B.-H. Screening for salt-responsive proteins in two contrasting alfalfa cultivars using a comparative proteome approach. Plant Physiol. Biochem. 2015, 89, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Navarro, V.; Zornoza, R.; Faz, Á.; Egea-Gilabert, C.; Ros, M.; Pascual, J.A.; Fernández, J.A. Inoculation with different nitrogen-fixing bacteria and Arbuscular mycorrhiza affects grain protein content and nodule bacterial communities of a fava bean crop. Agronomy 2020, 10, 768. [Google Scholar] [CrossRef]
- Htwe, A.Z.; Moa, S.M.; Seo, K.M.; Moe, K.; Yamakawa, T. Effects of bio-fertilizer produced from Bradyrhizobium and Streptomyces griseoflavus on plant growth, nodulation, nitrogen fixation, nutrient uptake and seed yield of mung Bean, cowpea, and soybean. Agronomy 2019, 9, 77. [Google Scholar] [CrossRef]
- de Carvalho, R.H.; da Conceição Jesus, E.; Favero, V.O. The Co-inoculation of Rhizobium and Bradyrhizobium increases the early nodulation and development of common beans. J. Soil. Sci. Plant Nutr. 2020, 20, 860–864. [Google Scholar] [CrossRef]
- Kebede, E. Competency of Rhizobial inoculation in sustainable agricultural production and biocontrol of plant diseases. Front. Sustain. Food Syst. 2021, 5, 728014. [Google Scholar] [CrossRef]
- Abbas, R.N.; Arshad, M.A.; Iqbal, A.; Iqbal, M.A.; Imran, M.; Raza, A.; Chen, J.-T.; Alyemeni, M.N.; Hefft, D.I. Weeds spectrum, productivity and land-use efficiency in maize-gram intercropping systems under semi-arid environment. Agronomy 2021, 11, 1615. [Google Scholar] [CrossRef]
- Amine-Khodja, I.R.; Boscari, A.; Riah, N.; Kechid, M.; Maougal, R.T.; Belbekri, N.; Djekoun, A. Impact of two strains of rhizobium leguminosarum on the adaptation to terminal water deficit of two cultivars Vicia faba. Plants 2022, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kumar, D.; Sharma, P. Effect of organics, bio-fertilizers and crop residue application on soil microbial activity in rice-wheat and rice-wheat mung bean cropping systems in the Indo Gangetic Plains. Cogent Geosci. 2015, 1, 1085296. [Google Scholar] [CrossRef]
- Toledo, C.B. Effect of Rhizobium inoculation on tomato (Solanum lycopersicum L.) Yield in protected crops. Biol. Life Sci. Forum 2021, 3, 52. [Google Scholar] [CrossRef]
- Jalal, A.; Galindo, F.S.; Boleta, E.H.M.; Oliveira, C.E.d.S.; Reis, A.R.d.; Nogueira, T.A.R.; Moretti Neto, M.J.; Mortinho, E.S.; Fernandes, G.C.; Teixeira Filho, M.C.M. Common bean yield and zinc use efficiency in association with diazotrophic bacteria co-inoculations. Agronomy 2021, 11, 959. [Google Scholar] [CrossRef]
- Genetu, G.; Yli-Halla, M.; Asrat, M.; Alemayehu, M. Rhizobium inoculation and chemical fertilisation improve faba bean yield and yield components in northwestern Ethiopia. Agriculture 2021, 11, 678. [Google Scholar] [CrossRef]
- Safronova, V.; Sazanova, A.; Kuznetsova, I.; Belimov, A.; Guro, P.; Karlov, D.; Yuzikhin, O.; Chirak, E.; Verkhozina, A.; Afonin, A.; et al. Increasing the legume–rhizobia symbiotic efficiency due to the synergy between commercial strains and strains isolated from relict symbiotic systems. Agronomy 2021, 11, 1398. [Google Scholar] [CrossRef]
- Malhi, G.S.; Rana, M.C.; Kumar, S.; Rehmani, M.I.A.; Hashem, A.; Abd_Allah, E.F. Efficacy, energy budgeting, and carbon footprints of weed management in black gram (Vigna mungo L.). Sustainability 2021, 13, 13239. [Google Scholar] [CrossRef]
- Qayyum, A.; Iqbal, L.J.; Barbanti, A.; Sher, G.; Shabbir, G.; Rabbani, M.K.; Rafiq, M.N.; Tareen, M.J.; Amin, B.A. Mash bean [Vigna mungo (L.) Hepper] Germplasm evaluation at different ecological conditions of Pakistan. Appl. Ecol. Environ. Res. 2019, 17, 6643–6654. [Google Scholar] [CrossRef]
- Banerjee, P.; Venugopalan, V.K.; Nath, R.; Althobaiti, Y.S.; Gaber, A.; Al-Yasi, H.; Hossain, A. Physiology growth productivity of spring–summer black gram (Vigna mungo, L. Hepper) as influenced by heat and moisture stresses in different dates of sowing and nutrient management conditions. Agronomy 2021, 11, 2329. [Google Scholar] [CrossRef]
- Khaliq, A.; Muhammad Aamir Iqbal Zafar, M.; Gulzar, A. Appraising economic dimension of maize production under coherent fertilization in Azad Kashmir, Pakistan. Custos Agronegocio 2019, 15, 243–253. [Google Scholar]
- Zia-Ul-Haq, M.; Ahmad, S.; Bukhari, S.A.; Amarowicz, R.; Ercisli, S.; Jaafar, H.Z.E. Compositional studies and biological activities of some mash bean (Vigna mungo (L.) Hepper) cultivars commonly consumed in Pakistan. Biol. Res. 2014, 47, 23. [Google Scholar] [CrossRef] [PubMed]
- Golezani, K.G.; Saeid, H.B.; Ali, B.H.; Salar, F.A. Seed hydro-priming a simple way for improving mungbean performance under water stress. Int. J. Biosci. 2014, 4, 12–18. [Google Scholar] [CrossRef]
- Temprano, F.J.; Albareda, M.; Camacho, M.; Daza, A.; Santamaría, C.; Rodríguez-Navarro, D.N. Survival of several Rhizobium/Bradyrhizobium strains on different inoculant formulations and inoculated seeds. Int. Microbiol. 2002, 5, 81–86. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; Agronomy 9; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–594. [Google Scholar]
- Apesteguia, M.; Plante, A.F.; Virto, I. Methods assessment for organic and inorganic carbon quantification in calcareous soils of the Mediterranean region. Geoderma Reg. 2018, 12, 39–48. [Google Scholar] [CrossRef]
- Ghaemi, A.; Rahimi, A.; Banihashemi, Z. Effects of water stress and Fusarium oxysporum f. sp. Lycoperseci on growth (leaf area, plant height, shoot dry matter) and shoot nitrogen content of tomatoes under greenhouse conditions. Iran Agric. Res. 2009, 28, 51–62. [Google Scholar]
- Iqbal, M.A.; Raza, R.Z.; Zafar, M.; Ali, O.M.; Ahmed, R.; Rahim, J.; Ijaz, R.; Ahmad, Z.; Bethune, B.J. Integrated fertilizers synergistically bolster temperate soybean growth, yield, and oil content. Sustainability 2022, 14, 2433. [Google Scholar] [CrossRef]
- El-Sorady, G.A.; El-Banna, A.A.A.; Abdelghany, A.M.; Salama, E.A.A.; Ali, H.M.; Siddiqui, M.H.; Hayatu, N.G.; Paszt, L.S.; Lamlom, S.F. Response of bread wheat cultivars inoculated with azotobacter species under different nitrogen application rates. Sustainability 2022, 14, 8394. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Chapagain, T.; Ghimire, B.; Pudasaini, R.; Tamang, B.B.; Gurung, K.; Choi, K.; Rai, L.; Magar, S.; Bishnu, B.K.; et al. Evaluating the effectiveness of rhizobium inoculants and micronutrients as technologies for Nepalese common bean smallholder farmers in the real-world context of highly variable hillside environments and indigenous farming practices. Agriculture 2019, 9, 9010020. [Google Scholar] [CrossRef]
- Dacko, M.; Zajac, T.; Synowiec, A.; Oleksy, A.; Klimek-Kopyra, A.; Kulig, B. New approach to determine biological and environmental factors influencing mass of a single pea (Pisum sativum L.) seed in Silesiaregion in Poland using a CART model. Eur. J. Agron. 2016, 74, 29–37. [Google Scholar] [CrossRef]
- Furtak, K.; Gawryjołek, K.; Gałązka, A.; Grządziel, J. The response of red clover (Trifolium pratense L.) to separate mixed inoculations with Rhizobium leguminosarum Azospirillum brasilense in presence of polycyclic aromatic hydrocarbons. Int. J. Environ. Res. Public Health 2020, 17, 5751. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, M.S.; Raizada, M.N. A review of nutrient management studies involving finger millet in the semi-arid tropics of Asia and Africa. Agronomy 2015, 5, 262–290. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Raizada, M.N. Challenges in using precision agriculture to optimize symbiotic nitrogen fixation in legumes progress, limitations, and future improvements needed in diagnostic testing. Agronomy 2018, 8, 78. [Google Scholar] [CrossRef]
- Htwe, A.Z.; Moh, S.M.; Moe, K.; Yamakawa, T. Effects of co-inoculation of Bradyrhizobium japonicum SAY3-7 and Streptomyces griseoflavus P4 on plant growth, nodulation, nitrogen fixation, nutrient uptake, and yield of soybean in a field condition. Soil Sci. Plant Nutri. 2018, 64, 222–229. [Google Scholar] [CrossRef]
- Aung, T.T.; Tittaburt, P.; Boonkerd, N.; Herridge, D. Co-inoculation effects of Bradrhizobium japonicum Azospirillum sp on competitive nodulation rhizosphere bacterial community structures of soybean under rhizobia-established soil conditions. Afr. J. Biotechnol. 2013, 12, 2850–2862. [Google Scholar]
- Mbarki, S.; Talbi, O.; Skalicky, M.; Vachova, P.; Hejnak, V.; Hnilicka, F.; Al-ashkar, I.; Abdelly, C.; Rahman, M.A.; El Sabagh, A.; et al. Comparison of grain sorghum and alfalfa for providing heavy metal remediation of sandy soil with different soil amendments and salt stress. Front. Environ. Sci. 2022, 10, 1022629. [Google Scholar] [CrossRef]
- Chauhan, J.; Srivastava, J.P.; Singhal, R.K.; Soufan, W.; Dadarwal, B.K.; Mishra, U.N.; Anuragi, H.; Rahman, M.A.; Sakran, M.I.; Brestic, M.; et al. Alterations of oxidative stress indicators, antioxidant enzymes, soluble sugars, and amino acids in mustard [Brassica juncea (L.) Czern and Coss.] in response to varying sowing time, and field temperature. Front. Plant. Sci. 2022, 13, 875009. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; García-Caparrós, P.; Rahman, M.A.; Ogwugwa, V.H.; Saeed, F.; Jin, W. Melatonin-mediated temperature stress tolerance in plants. GM Crops Food 2022, 13, 196–217. [Google Scholar] [CrossRef]
- Raza, A.; Salehi, H.; Rahman, M.A.; Zahid, Z.; Haghjou, M.M.; Najafi-Kakavand, S.; Charagh, S.; Osman, H.S.; Albaqami, M.; Zhuang, Y.; et al. Plant hormones and neurotransmitter interactions mediate antioxidant defenses under induced oxidative stress in plants. Front. Plant. Sci. 2022, 13, 961872. [Google Scholar] [CrossRef]
- Yamakawa, T.; Soe, K.M. Evaluation of effective Myanmar Brady-rhizobium strains isolated from Myanmar soybean and effects of co-inoculation with Streptomyces griseoflavus P4 for nitrogen fixation. Soil Sci. Plant Nutr. 2013, 59, 361–370. [Google Scholar] [CrossRef]
- Soe, K.M.; Bhromsiri, A.; Karladee, D.; Yamakawa, T. Effects of endophytic Actinomycetes and Bradyrhizobium japonicum strains on growth, nodulation, nitrogen fixation and seed weight of soybean varieties. Soil Sci. Plant Nutr. 2012, 58, 319–325. [Google Scholar] [CrossRef]
- Nisar, M.; Ghafoor, A.; Khan, M.R.; Ahmad, H.; Qureshi, A.S.; Ali, H. Genetic diversity geographic relationship among local exotic chickpea germplasm. Pak. J. Bot. 2007, 39, 1575–1581. [Google Scholar]
- Hassan, H.M.; Hadifa, A.A.; El-leithy, S.A.; Batool, M.; Sherif, A.; Al-Ashkar, I.; Ueda, A.; Rahman, M.A.; Hossain, M.A.; Elsabagh, A. Variable level of genetic dominance controls important agronomic traits in rice populations under water deficit condition. PeerJ 2023, 11, e14833. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.-R.; Kamal, M.-M.; Hossain, M.-F.; Hossain, J.; Azam, M.-G.; Akhter, M.-M.; Hasan, M.-K.; Al-Ashkar, I.; Almutairi, K.-F.; Sabagh, A.-E.; et al. Drought tolerance in mung bean is associated with the genotypic divergence, regulation of proline, photosynthetic pigment and water relation. Phyton 2023, 92, 955–981. [Google Scholar] [CrossRef]
- Zafar, S.A.; Aslam, M.; Khan, H.J.; Sarwar, S.; Rehman, R.S.; Hassan, M.; Ahmad, R.M.; Gill, R.A.; Ali, B.; Al-Ashkar, I.; et al. Estimation of genetic divergence and character association studies in local and exotic diversity panels of soybean (Glycine max L.) genotypes. Phyton 2023, 92, 1887–1906. [Google Scholar] [CrossRef]
- Patra, R.K.; Pant, L.M.; Pradhan, K. Response of Soybean to Inoculation with Rhizobial Strains, Effect on Growth, Yield, N Uptake and Soil N Status. World J. Agric. Res. 2012, 8, 51–54. [Google Scholar]
- Consentino, B.B.; Aprile, S.; Rouphael, Y.; Ntatsi, G.; De Pasquale, C.; Iapichino, G.; Alibrandi, P.; Sabatino, L. Application of PGPB combined with variable n doses affects growth, yield-related traits, n-fertilizer efficiency and nutritional status of lettuce grown under controlled condition. Agronomy 2022, 12, 236. [Google Scholar] [CrossRef]
- Tahir, M.M.; Abbasi, M.K.; Rahim, N.; Khaliq, A.; Kazmi, M.H. Effect of Rhizobium inoculation and NP fertilization on growth, yield and nodulation of soybean (Glycine max L.) in the sub-humid hilly region of Rawalakot Azad Kashmir, Pakistan. Afr. J. Biotechnol. 2009, 8, 6191–6200. [Google Scholar] [CrossRef]
- Soe, K.M.; Yamakawa, T. Low-density co-inoculation with Bradyrhizobium japonicum, S.A.Y.3.-7.; Streptomyces griseoflavus P4 promotes plant growth nitrogen fixation in soybean cultivars. Am. J. Plant Sci. 2016, 7, 1652–1661. [Google Scholar] [CrossRef]
- Zaman, A.; Sarkar, A.S.; Sarkar, W.; Devi, P. Effect of organic inorganic sources of nutrients on productivity specific gravity processing quality of (Solanum tuberosum) Indian. J. Agric. Sci. 2011, 81, 1137–1142. [Google Scholar]
- Mudasir, S.; Sofi, P.A.; Khan, M.N.; Sofi, N.R.; Dar, Z.A. Genetic diversity variability character association in local common bean (Phaseolus vulgaris L) germplasm of Kashmir Electron. J. Plant Breed 2012, 3, 883–891. [Google Scholar]
- Yaseen, M.; Kashif, M.; Nazish, H.T.; Munir, R.; Iqbal, J.; Usman, M.; Rabbani, G. Effect of rain-fed conditions on yield of mash bean genepool by using augmented design. J. Stat. Theory Appl. 2022, 21, 186–199. [Google Scholar] [CrossRef]
- Hakim, L. Variability correlation of agronomic characters of mung bean germplasm their utilization for variety improvement program. Indones. J. Agric. Sci. 2008, 9, 24–28. [Google Scholar] [CrossRef]
- Konda, C.R.; Salimathand, P.H.; Mishra, M.N. Correlation and path coefficient analysis in black gram (Vigna mungo (L.) Hepper). Legume Res. 2008, 31, 202–205. [Google Scholar]
- Veeramani, N.; Venkatesan, M.; Thangavel, P.; Ganesan, J. Genetic variability, heritability and genetic advance analysis in segregating generation of black gram (Vigna mungo (L.) Hepper). Legume Res. 2005, 28, 49–51. [Google Scholar]
- Kumar, B.S.; Padmavathi, S.; Prakash, M.; Ganesan, J. Correlation and path analysis in black gram [Vigna mungo (L.) Hepper]. Legume Res. 2003, 26, 75–76. [Google Scholar]
- Al-Suhaibani, N.; Selim, M.; Alderfasi, A.; El-Hendawy, S. Comparative performance of integrated nutrient management between composted agricultural wastes, chemical fertilizers, and biofertilizers in improving soil quantitative and qualitative properties and crop yields under arid conditions. Agronomy 2022, 10, 1503. [Google Scholar] [CrossRef]
- Dal Cortivo, C.; Ferrari, M.; Visioli, G.; Lauro, M.; Fornasier, F.; Barion, G.; Panozzo, A.; Vamerali, T. Effects of seed-applied biofertilizers on rhizosphere biodiversity and growth of common wheat (Triticum aestivum L.) in the field. Front. Plant Sci. 2020, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Bainard, L.D.; Ma, B.; Liu, J. Bio-fertilizer and rotten straw amendments alter the rhizosphere bacterial community and increase oat productivity in a saline–alkaline environment. Sci. Rep. 2020, 10, 19896. [Google Scholar] [CrossRef]
- Rathnathilaka, T.; Premarathna, M.; Madawala, S.; Pathirana, A.; Karunaratne, K.; Seneviratne, G. Biofilm biofertilizer application rapidly increases soil quality grain yield in large scale conventional rice cultivation A case study. J. Plant. Nutr. 2022, 46, 1220–1230. [Google Scholar] [CrossRef]
- dos Santos, J.S.; dos Santos, M.L.; Conti, M.M. Comparative study of metal contents in Brazilian coffees cultivated by conventional and organic agriculture applying principal component analysis. J. Braz. Chem. Soc. 2010, 21, 1468–1476. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Y. Evaluation of the effects of irrigation and fertilization on tomato fruit yield and quality, a principal component analysis. Sci. Rep. 2017, 7, 350. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).