Abstract
Water contamination by antibiotics has become a critical environmental and public health issue. Among emerging technologies for their removal, heterogeneous photocatalysis has shown remarkable potential. This review provides a systematic analysis of 40 recent studies (2019–2025) that employed green synthesis routes—including sol–gel, hydrothermal, combustion, pyrolysis and co-precipitation methods—for the photocatalytic degradation of antibiotics. The comparison of these techniques revealed that biogenic metal oxides and ferrites synthesized with plant extracts achieved outstanding photocatalytic performance, with degradation efficiencies often exceeding 90–100% for antibiotics such as ciprofloxacin and tetracycline. These results are attributed to the phytochemical composition of the extracts, which are rich in flavonoids, phenols, saponins, tannins, and alkaloids, which act as natural reducing, capping, and stabilizing agents, promoting uniform nucleation, smaller particle sizes, and enhanced crystallinity. The review also highlights the synergistic relationship between biomolecule-mediated reduction and controlled synthesis conditions, which enables the design of sustainable, reusable, and high-efficiency photocatalysts for wastewater treatment and environmental remediation.
1. Introduction
The growing accumulation of antibiotics in aquatic environments, even at trace levels, has emerged as a major environmental and public health concern due to their persistence, bioactivity, and contribution to the spread of antibiotic resistance genes [1,2]. These compounds, continuously released from domestic, hospital, and agricultural sources, resist conventional wastewater treatment systems, which are often unable to achieve full mineralization due to the structural complexity and low biodegradability of these molecules [3,4]. As a result, alternative treatment methods with higher efficiency and sustainability have become a global research priority.
Among these methods, heterogeneous photocatalysis stands out as an advanced oxidation process capable of degrading antibiotics into harmless end-products through the generation of reactive oxygen species such as hydroxyl radicals (•OH) and superoxide anions (O2•–) under light irradiation [5,6,7]. Conventional photocatalysts, including TiO2 and ZnO, exhibit remarkable activity and stability; however, traditional synthesis routes often require toxic solvents, high temperatures, and energy-intensive steps, limiting their environmental viability and scalability [4,8].
To overcome these limitations, the integration of green synthesis principles into photocatalyst production has attracted increasing attention. This approach employs plant-derived extracts as natural reducing, chelating, and stabilizing agents that promote nanoparticle formation under mild and environmentally benign conditions [9,10,11]. The efficiency of these bioextracts is closely linked to their phytochemical composition, which typically includes flavonoids, phenols, tannins, saponins, terpenoids, and alkaloids. These molecules contain functional groups such as hydroxyl (–OH), carbonyl (C=O), and ether (C–O), capable of reducing metal ions, facilitating homogeneous nucleation, and stabilizing nanostructures by preventing agglomeration. The presence and relative abundance of these phytochemicals directly influence the crystallinity, morphology, and optical behavior of the synthesized nanomaterials, ultimately determining their photocatalytic performance [6,7,12].
Recent studies have demonstrated that the synergistic interaction between biochemical reducing capacity and controlled synthesis conditions—such as hydrothermal, sol–gel, combustion, and co-precipitation routes—plays a decisive role in achieving high photocatalytic efficiencies, often surpassing 90–100% antibiotic degradation under UV or solar irradiation. Building upon these findings, the present review expands the analysis to 40 representative works published between 2019 and 2025, providing a comparative discussion on green synthesis methods, plant-based precursors, and structure–property–activity relationships in biogenic photocatalysts [12,13,14,15].
Instead of the traditional tabular overview, Figure 1 now presents a keyword cloud summarizing the main research trends and conceptual interconnections identified across the analyzed studies, offering a comprehensive visual synthesis of this rapidly evolving field.
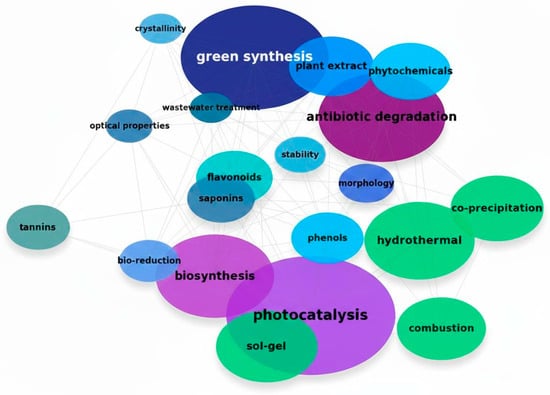
Figure 1.
Visual representation of the most frequently occurring keywords.
The thematic cloud highlights the centrality of photocatalysis, green synthesis, and antibiotic degradation, reflecting the strong current demand for sustainable technologies for wastewater treatment. Terms such as plant extract, phytochemicals, biosynthesis, flavonoids, and tannins emphasize the growing interest in the use of phytochemicals as reducing and stabilizing agents in the production of nanoparticles. Hydrothermal, sol–gel, combustion, and co-precipitation reinforce the diversity of synthesis routes studied to optimize structural, adsorptive, and photocatalytic properties, focusing on materials for environmental remediation. The presence of morphology, crystallinity, stability, and optical properties indicates a focus on the relationship between structure and performance of these materials.
2. Methodology
The research and selection criteria were defined in alignment with the central scope of this review, and the literature search focused on studies explicitly addressing green synthesis routes applied to the photocatalytic degradation of antibiotics. Searches were conducted across major scientific publishers—MDPI, Elsevier, Springer, RSC, and IOP—covering the period 2019 to 2025, using the retrieval strategy combined the descriptors green synthesis, photocatalysis, antibiotic degradation, plant extract, hydrothermal, sol–gel, combustion, and co-precipitation.
Only peer-reviewed articles reporting photocatalysts synthesized through eco-friendly or biogenic approaches were included. In total, 31 studies met the inclusion criteria and were examined according to the following: (i) photocatalyst type, (ii) synthesis route, (iii) plant extract or biogenic agent, (iv) target antibiotic, and (v) photocatalytic efficiency. In addition, the discussion throughout this review highlights the influence of key synthesis parameters—such as phytochemical composition, precursor concentration, reaction temperature, and calcination conditions—on the resulting photocatalytic performance, emphasizing how plant-derived compounds can promote synergistic effects with semiconductor materials and enhance pollutant degradation efficiency.
Table 1, presented below, summarizes the selected studies and consolidates their key characteristics, forming the basis for the comparative analyses discussed throughout this review.

Table 1.
Summary of the Reviewed Studies on Green Synthesis Routes Applied to Antibiotic Photodegradation and Reported Efficiencies.
3. Discussion
Among the green synthesis routes, the sol–gel method stood out for enabling precise control over particle morphology, crystallinity, and phase dispersion—key parameters in optimizing photocatalytic performance. This method relies on hydrolysis and polycondensation of metal alkoxides or salts in the presence of aqueous or alcoholic solvents, where plant extracts serve as reducing, stabilizing, or complexing agents [17,28,45,46].
A CuFe2O4/CuO-rGO heterostructure was synthesized via sol–gel using basil seeds as natural reactors, resulting in efficient degradation of oxytetracycline due to the formation of a heterojunction that enhanced charge separation and visible light activation [17]. Similarly, CdS nanoparticles were obtained through a sol–gel route assisted by Camellia sinensis (tea leaf) extract, leading to significant photodegradation of ciprofloxacin; in this case, both doping and green synthesis contributed to bandgap narrowing [45].
Another relevant case involved the synthesis of Nb-doped ZnO using Vernonia amygdalina leaf extract via co-precipitation. This method promoted better structural integration and improved photocatalytic degradation of tetracycline, highlighting the role of dopants in suppressing electron-hole recombination and extending light absorption [47].
The TiO2/GO/chitosan composite, synthesized using Olea europaea extract, showed effective removal of cefixime trihydrate under UV-A light, mainly due to the synergistic interaction among TiO2, GO, and the biopolymer chitosan, which increased the surface area, improved pollutant adsorption, and facilitated hydroxyl radical generation [16].
The incorporation of natural polymers also proved beneficial. For instance, ZnCoFe2O4@Ch nanohybrids were obtained using chitosan through a microwave-assisted co-precipitation route, demonstrating excellent tetracycline adsorption and recyclability while combining photocatalytic activity with magnetic separability.
Bio-graphenes synthesized via pyrolysis of biogenic precursors such as xanthan gum, chitosan, Boswellia resin, and tragacanth gum exhibited high performance in tetracycline removal. The presence of functional groups from the original biomaterials enabled N, S, and P doping, thereby enhancing charge mobility and visible-light responsiveness [48].
The synthesis of Ni-, Co-, and Mn-doped ZnO nanoparticles using Bauhinia racemosa extract via hydrothermal method resulted in superior degradation of tetracycline, ampicillin, and amoxicillin, reinforcing the role of metal doping in improving light absorption and charge carrier mobility in ZnO matrices [49].
Further evidence of heterostructure advantages was provided by the development of Ag-decorated ZnO–CeO2 nanostructures using Moringa oleifera extract. The synergistic effects between Ag and CeO2 enhanced visible-light activation and ciprofloxacin degradation, illustrating the benefit of multi-component systems in extending the light absorption spectrum [50].
Mo-doped SnO2 quantum dots were also successfully synthesized via hydrothermal method using non-toxic reagents, showing efficient tetracycline hydrochloride degradation under visible light, and the introduction of molybdenum altered the band structure, improving light absorption and promoting the generation of reactive species [50].
In another case, Se-doped Ag/AgO-ZnO nanocomposites were prepared by sol–gel using Pinus nigra pollen extract, and the incorporation of selenium significantly improved the photodegradation of tetracycline, likely due to bandgap narrowing and synergistic effects among the constituent metal oxides [28].
Overall, sol–gel and co-precipitation routes have consistently stood out among green synthesis methods due to their versatility, eco-compatibility, and reliable reproducibility. Beyond merely replacing hazardous reagents, these approaches embody a broader paradigm shift toward sustainable materials engineering. By employing plant extracts, natural chelators, or mild reaction conditions, green synthesis pathways not only reduce chemical waste and energy consumption but also contribute to lowering carbon emissions along the photocatalyst fabrication chain. Such eco-designed photocatalysts have shown remarkable synergistic effects when applied to bioremediation strategies, particularly in heterogeneous photocatalysis aimed at the degradation of contaminants of emerging concern [19,21,33,51].
In this context, antibiotics represent a critical class of pollutants due to their persistence, incomplete degradation in wastewater systems, and their role in accelerating the emergence of multidrug-resistant microorganisms, a global public health threat. The data compiled in this review underscores this issue: the antibiotics most frequently targeted in the analyzed studies reflect the rising environmental prevalence of compounds such as ciprofloxacin, tetracycline, and amoxicillin, which are commonly detected in rivers, effluents, groundwater, and even drinking water [23,31,32,36,37,39,40,42].
3.1. Photocatalysts Synthesized by Sol–Gel Method
The sol–gel technique has been widely adopted as a green synthesis route due to its versatility in producing homogeneous, highly porous, and nanostructured photocatalysts under mild conditions. This method allows for the integration of plant-derived compounds as natural chelating and stabilizing agents, enabling eco-friendly control over particle morphology and surface properties.
CdS nanoparticles were synthesized through a sol–gel approach assisted by Camellia sinensis (tea leaf) extract, which contributed to metal precursor reduction and particle dispersion stabilization, and the resulting material exhibited enhanced visible-light responsiveness and successfully degraded ciprofloxacin [45].
In another application of this method, basil seed extract was used in the sol–gel synthesis of CuFe2O4/CuO-rGO heterostructures. The mucilage from the seeds acted as a reducing agent and structuring biopolymer, enabling the formation of stable two-dimensional nanocomposites with significant oxytetracycline degradation under visible-light irradiation [17].
A sol–gel route assisted by Citrus limon (lemon) extract was also employed to synthesize ZnO and TiO2 nanoparticles for the simultaneous removal of estrogenic hormones. The phytochemical constituents of the extract played a key role in forming fine-grained semiconductors with improved photoactivity [46].
Se@Ag/AgO-ZnO nanocomposites were produced by sol–gel processing using Pinus nigra extract, which facilitated the incorporation of selenium and silver while stabilizing particle surfaces and interfaces. The final material demonstrated effective tetracycline degradation along with antibacterial properties [28].
These findings demonstrate that the sol–gel method, when combined with plant-based extracts, enables the development of multifunctional materials with improved photocatalytic activity and environmental compatibility. The incorporation of natural chelators and templating agents enhances morphology control, band gap modulation, and charge separation efficiency.
3.2. Photocatalysts Synthesized by Co-Precipitation
The co-precipitation method has proven to be a promising green route for producing photocatalysts with well-defined structures and controlled chemical compositions. Its main advantages include low cost, operational simplicity, and the ability to incorporate dopants and natural extracts, which act as reducing and stabilizing agents during the formation of catalytic phases. In this process, these extracts, rich in biomolecules (such as polyphenols), replace toxic chemicals, acting as reducing agents to convert precursor metal ions into oxides. More importantly, they function as stabilizing agents, enveloping the newly formed nanoparticles. This prevents agglomeration and allows precise control of the size and morphology of the crystals, which proves essential for optimizing the surface area and, consequently, the photocatalytic efficiency of the final material.
TiO2/GO/chitosan nanocomposites were synthesized using Olea europaea leaf extract, achieving effective degradation of cefixime trihydrate under UV-A irradiation. The natural extract improved phase dispersion and crystallinity, enhancing photocatalytic performance through increased charge separation and reactive oxygen species (ROS) generation [16].
Ag-decorated ZnO–CeO2 nanostructures were obtained via a green co-precipitation route using Moringa oleifera extract. Phytochemicals in the extract acted as natural reducing and stabilizing agents, enabling the formation of uniform, well-dispersed nanostructures. The resulting photocatalyst exhibited 91.5% ciprofloxacin degradation under visible light, attributed to the synergistic effects of Ag doping and CeO2 incorporation, which enhanced charge separation and expanded the light absorption range [18].
ZnCoFe2O4@chitosan nanohybrids were developed through microwave-assisted co-precipitation, in which chitosan functioned as a green matrix and stabilizer. The material exhibited strong adsorption of tetracycline via surface interactions and showed recyclability and magnetic properties that facilitated its recovery for reuse [10,52].
The synthesized material proved to be significantly more efficient than pure zinc oxide (ZnO), a traditionally used semiconductor. The maximum decontamination efficiency of rifampicin achieved by the optimized composite was 94.72% in 90 minutes of reaction, using a dosage of 0.20 g of catalyst. In contrast, pure ZnO reached 74.2% under the same experimental conditions. The advantage of the ZnO/MnFe2O4 composite is its magnetic nature, conferred by the incorporation of manganese ferrite. This characteristic allows for the accelerated separation and recovery of the catalyst from the treated solution after the photocatalytic process, through the application of a magnetic field, which is essential for the viability of large-scale application [10]. A Fe3O4-ZnO-Chitosan/Alginate nanocomposite was prepared using Camellia sinensis extract, resulting in successful degradation of ciprofloxacin and sulfamethoxazole in aqueous media, where the biopolymer components contributed to morphological stability and electron transfer across heterojunction interfaces [20]. A chitosan/PVP/Fe3O4 composite was synthesized via co-precipitation for the removal of amoxicillin trihydrate. The method enabled uniform dispersion of magnetic particles within the biopolymeric matrix, promoting a balance between adsorption efficiency and magnetic recoverability [11]. ZnO/carbon nanofiber composites were prepared using Thymus daenensis and Stachys pilifera Benth extracts. These materials demonstrated effective photocatalytic degradation of tetracycline, attributed to the high surface area, porosity of the carbon phase, and active functional groups provided by the extracts [37]. ZnO/nanocellulose composites were synthesized using Tinospora cordifolia and sugarcane bagasse extracts via co-precipitation. It was observed that the resulting hybrid system effectively degraded enrofloxacin, benefitting from the light-absorbing capacity and biocompatibility of nanocellulose [53]. Co-precipitation was also used to synthesize ZnS nanoparticles with Sutherlandia frutescens extract, where these nanoparticles showed notable photocatalytic removal of sulfisoxazole and sulfamethoxazole, supported by the presence of bioactive compounds in the extract [54]. Nb-doped ZnO was synthesized using Vernonia amygdalina extract through co-precipitation, and the doped system presented enhanced degradation of tetracycline under visible light, attributed to improved charge carrier mobility and reduced electron-hole recombination [55]. A common observation across the reviewed studies is that parameters such as the reaction medium pH, the molar ratio of precursors to extract, and the aging time significantly affect particle size, crystallinity, and surface functionality. Extracts contribute functional groups—such as hydroxyl, amine, and carbonyl—which improve antibiotic adsorption and facilitate interfacial electron transfer, ultimately increasing overall photocatalytic efficiency. Although less frequently reported than sol–gel or hydrothermal methods, green co-precipitation offers distinct advantages in terms of simplicity, reproducibility, and scalability. The incorporation of magnetic or doped phases further supports its application in environmental remediation, particularly for the treatment of complex hospital wastewater containing diverse pharmaceutical residues.
3.3. Photocatalysts Synthesized by Hydrothermal Route
The hydrothermal synthesis method has been widely recognized as an effective green route for obtaining photocatalysts with controlled morphology, crystallinity, and enhanced stability—all of which are essential for environmental remediation applications. This technique, typically conducted under moderate temperature and pressure, enables the growth of nanostructures with the aid of plant extracts acting as reducing and stabilizing agents.
Metal-doped ZnO nanoparticles (Ni-ZnO, Mn-ZnO, and Co-ZnO) were synthesized via hydrothermal treatment using Bauhinia racemosa leaf extract. The resulting photocatalysts achieved removal efficiencies above 90% for tetracycline, ampicillin, and amoxicillin. The doping elements improved visible light absorption, while the plant extract served as a reducing and capping agent during nanoparticle formation [49].
Mo-doped SnO2 quantum dots were produced using a hydrothermal route and non-toxic reagents, yielding high photocatalytic activity under visible light and achieving 91.4% degradation of tetracycline hydrochloride, and the incorporation of molybdenum contributed to bandgap narrowing and enhanced charge carrier separation [5,50].
Aloe vera extract was used to synthesize three-dimensional tripyramid TiO2 structures via hydrothermal synthesis, where the resulting photocatalyst exhibited 93% ciprofloxacin degradation under visible light, and the unique morphology increased surface area and promoted light harvesting, while the extract acted as a bio-template and stabilizer during crystal formation [5].
Altogether, these studies confirm that the hydrothermal method, when combined with plant-based agents, offers a promising and eco-friendly route for developing photocatalysts with high performance. Its compatibility with doping strategies and its ability to yield recyclable nanomaterials supports its application in sustainable water treatment systems.
As illustrated in Figure 2 [22], which clearly depicts the process schematically, this synthesis follows a green hydrothermal route mediated by plant extract, in which dried and powdered lemon peel is employed as a natural source of reducing and stabilizing compounds. The aqueous extract is obtained through heating and filtration, then combined with a 0.1 M zinc nitrate solution under stirring at 50 °C for 30 min. The resulting mixture undergoes hydrothermal treatment at 180 °C for 18 h in a Teflon-lined autoclave, enabling controlled nucleation and growth of ZnO nanoparticles. After sequential washing, drying at 120 °C, and calcination at 500 °C, highly crystalline and stable ZnO nanoparticles (LP-ZnO NPs) are produced. This process exemplifies a simple, sustainable, and efficient approach, in which the flavonoids, phenols, and alkaloids present in the lemon peel extract act as natural reducing and capping agents, yielding biogenic ZnO nanoparticles of high purity and low cost.
3.4. Photocatalysts Synthesized by Combustion Method
The solution combustion synthesis (SCS) technique has been explored as a rapid and eco-friendly route to fabricate photocatalysts with high crystallinity and surface reactivity. This method typically involves exothermic redox reactions between oxidizers (e.g., metal nitrates) and green fuels—such as plant extracts—leading to in situ formation of nanostructured oxides without the need for high-temperature calcination or hazardous reagents [19,56].
ZnO nanoparticles with a spongy morphology were synthesized via SCS using pomegranate seed extract as a natural fuel. The combustion process promoted the generation of hydroxyl radicals under solar irradiation, enabling efficient degradation of flumequine in antibiotic-enriched wastewater, and the porous structure, formed due to rapid gas evolution during combustion, contributed to a high surface area and improved photocatalytic performance under visible light [19,56].
These findings reinforce the viability of SCS as a green and scalable route for producing ZnO-based photocatalysts using biomass-derived agents, supporting the development of sustainable technologies for water remediation.
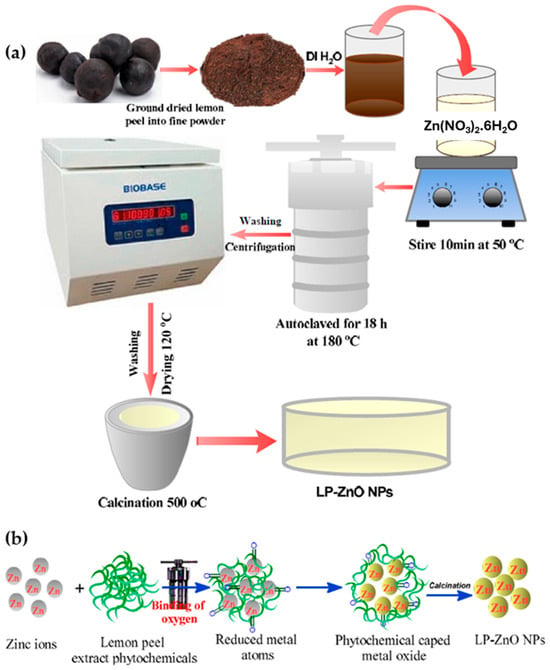
Figure 2.
(a) Schematic representation of the preparation of LP-ZnO nanoparticles (NPs); (b) Possible mechanism of LP-ZnO NP formation using lemon peel aqueous extract. Extracted and adapted from [22].
3.5. Photocatalysts Synthesized by Pyrolysis
Pyrolysis has emerged as a versatile and eco-friendly technique for the green synthesis of photocatalysts, particularly when combined with natural polymers, plant-based materials, or biowaste. This thermal process involves the decomposition of organic matter under limited oxygen conditions, typically at moderate to high temperatures, resulting in the formation of carbonaceous nanostructures, metal oxides, or doped composites with tailored physicochemical properties. Its simplicity, scalability, and compatibility with biomass feedstocks make pyrolysis an attractive route for fabricating photocatalysts intended for antibiotic degradation in wastewater treatment.
Novel N,P,S co-doped graphenic SiC layers (g-SiC) were developed using gelatin as both a green carbon source and a doping precursor. The resulting photocatalyst exhibited high visible-light-driven activity for the simultaneous degradation of tetracycline, amoxicillin, and ciprofloxacin. The co-doping with nitrogen, phosphorus, and sulfur introduced lattice distortions and enhanced charge carrier mobility, while the porous structure favored light scattering and radical generation. These synergistic effects contributed to extended light absorption and improved charge separation, increasing quantum efficiency and reaction kinetics [14].
In another study, pyrolysis of Brucea javanica seeds was applied to fabricate Ag/Ni/Fe3O4-activated carbon beads (Ag/Ni/MACB). The photocatalyst showed efficient degradation of enrofloxacin and concurrent antibacterial performance. The Fe3O4 component imparted magnetic properties that enabled easy recovery, while Ag and Ni nanoparticles created redox-active sites for Fenton-like reactions, boosting hydroxyl radical generation, and the carbon matrix derived from biomass provided a high-surface-area support, enhancing adsorption and preserving the catalyst’s structural integrity [47].
Further advancement was achieved through the synthesis of doped bio-graphenes using pyrolysis of natural gums, including xanthan gum, chitosan, Boswellia resin, and tragacanth gum. These biopolymers served as both carbon sources and heteroatom donors, resulting in N- and O-doped graphene-like structures with superior catalytic properties, and the metal-free photocatalyst exhibited high tetracycline affinity, improved conductivity, and active defect sites that facilitated charge transfer and radical production under visible light [48].
Altogether, pyrolysis-based routes offer a flexible, cost-effective, and sustainable strategy for producing high-performance photocatalysts. These methods enable the incorporation of heteroatoms and complex morphologies without reliance on hazardous solvents or costly reagents. Photocatalysts derived from biomass pyrolysis demonstrate promising capabilities in antibiotic degradation, with stability, reusability, and enhanced photocatalytic performance. As the field progresses, combining pyrolysis with advanced precursor design—such as molecular tailoring of biopolymers—may further enhance the efficiency and selectivity of green photocatalysts for environmental applications.
3.6. Similarities in the Use of Plant Extracts
The analysis of the 40 selected studies revealed a recurring pattern in the preparation of plant-based extracts used for green photocatalyst synthesis. Most protocols involve heating plant material—typically leaves or fruit peels—in distilled water at temperatures ranging from 60 °C to 90 °C for 30 to 60 min, followed by simple filtration. This aqueous extraction method avoids the use of organic solvents and preserves key phytochemicals such as polyphenols, flavonoids, and tannins, which function as reducing, chelating, and stabilizing agents during nanoparticle formation [5,15,36,39,40,42,43,44].
Short extraction durations, generally under one hour, combined with moderate thermal treatment, appear to ensure reproducibility while minimizing the degradation of sensitive compounds [49,53]. Several studies using extracts from Camellia sinensis, Citrus sinensis, and Moringa oleifera report consistent outcomes under these conditions. In particular, C. sinensis (green tea) extract proved highly effective due to its rich catechin content, which enhances metal ion reduction and nanostructure stabilization [12,25,35,41,57,58].
Frequently used plant sources include Moringa oleifera, Azadirachta indica, Citrus sinensis, and Passiflora edulis, in which these plants are favored not only for their high antioxidant contents but also for their availability and ease of processing. For example, Moringa oleifera leaf extract was employed in the green synthesis of ZnO@CuO nanocomposites and enabled the degradation of ofloxacin and other antibiotics above 90%. This performance was attributed to the presence of bio-reducing polyphenols that promoted rapid nucleation and prevented nanoparticle agglomeration [16,29,31,32,37,38,58,59,60,61,62].
Citrus sinensis peels, rich in gallic acid and flavonoids, were also widely applied in the synthesis of Ag–TiO2 and Ni-doped ZnO photocatalysts, where these extracts functioned as morphology-controlling agents, facilitating the formation of porous nanostructures and enhancing visible-light absorption. Similarly, Azadirachta indica (neem) and Passiflora edulis (passion fruit) extracts were shown to improve crystallinity and photocatalytic efficiency in Fe2O3/TiO2 and CuO–ZnO composites, respectively [3,16,23,24,26,30,34,63,64].
A consistent preference for leaf and peel extracts over roots or seeds was noted, likely due to their higher antioxidant content enhanced by light exposure. These tissues also pose a lower risk of contamination by soil-borne pathogens or heavy metals, making them safer and more reproducible sources of phytochemicals [14,20,27,33,51].
In several studies, both the concentration of plant extract and its resting time before precursor mixing significantly influenced the size, shape (e.g., nanorods, nanosheets), and crystallinity of the resulting photocatalysts, and these morphological factors directly impacted photocatalytic performance. For instance, higher extract concentrations generally produced smaller particles with greater surface area and more active sites, while excessive concentration could lead to over-capping and reduced activity [5,54,65,66].
In summary, plant extracts fulfill multifunctional roles in green photocatalyst synthesis—as reducing, chelating, templating, and stabilizing agents. Their standardized and reproducible use contributes to morphology control, improved photocatalytic efficiency, and overall environmental sustainability in nanomaterials development [56,67,68].
3.7. Phytochemistry in Green Synthesis
The phytochemical composition of plant extracts used in green synthesis exerts a decisive influence on the structural and functional characteristics of the resulting nanomaterials. Bioactive molecules such as flavonoids, tannins, phenols, saponins, alkaloids, and steroids provide hydroxyl, carbonyl, and ether functional groups (O–H, C=O, C–O) capable of reducing metallic ions, promoting uniform nucleation, and stabilizing particle growth. These metabolites act in a synergistic manner as natural reducing, complexing, and capping agents, limiting agglomeration and improving colloidal stability, while their chemical diversity directly shapes crystallinity, morphology, and electronic conductivity—properties that are strongly associated with enhanced photocatalytic behavior [12,15].
Green-mediated hydrothermal syntheses have demonstrated particularly significant outcomes, producing nanoparticles with high crystallinity, reduced particle size, and stable catalytic reuse performance. Examples include ZnO synthesized via citrus peel extract with efficiencies above 90% in ciprofloxacin degradation, Ag–CuFe2O4 produced using M. burkeana extract exhibiting strong charge separation and high activity under UV light, and ZnO synthesized with Senna siamea extract achieving complete degradation of tetracycline under UV and natural sunlight with excellent structural stability over repeated photocatalytic cycles [22,36,37].
Similar effects were observed in sol–gel, co-precipitation, and green combustion approaches, where the phytochemical environment favored the formation of catalysts with refined nanostructural attributes and improved surface properties. Nanostructured CeO2 obtained from Manilkara zapota extract achieved rapid antibiotic degradation due to phenolic and amino functional groups assisting surface activation; ZnO synthesized via co-precipitation exhibited controlled band gap and minimized electron–hole recombination, improving sulfisoxazole degradation; and Fe3O4/biochar composites generated through combustion–pyrolysis displayed enhanced electron transport and active oxygen vacancy interfaces promoting high photocatalytic activity [34,38,39].
Structurally, green-synthesized photocatalysts consistently presented high crystallinity, homogeneous morphology, and abundant reactive surface sites, supporting efficient separation of charge carriers and rapid radical formation. The interplay between phytochemical reducing environments and controlled low-temperature processing resulted in nanocatalysts that are stable, reusable, and highly active, with degradation efficiencies commonly ranging from 46% to values exceeding 90%. These trends align with structure–property correlations and radical-driven degradation mechanisms reported in the literature, reinforcing that phytochemistry-based synthesis provides a sustainable, scalable, and high-performance route for designing photocatalysts for antibiotic degradation in aqueous systems [12,15].
3.8. Changes in Crystallinity as a Function of the Synthesis Route
The crystallinity of photocatalysts is strongly influenced by the synthesis route, which directly impacts their performance. The hydrothermal route and pyrolysis generally result in materials with high crystallinity. Treatment at high temperature and pressure (hydrothermal) or simply at high temperature (pyrolysis) favors the growth of well-defined crystals. On the other hand, the sol–gel method and co-precipitation typically lead to products with lower crystallinity or an amorphous/nanocrystalline structure, as they are low-temperature processes that may not provide sufficient energy for extensive crystalline ordering. The combustion method (such as solution combustion) can generate crystallinity ranging from medium to high, depending on the post-synthesis calcination temperature, but often results in nanoparticles with purer crystalline phases due to its exothermic and rapid nature. In general, high-temperature routes (pyrolysis, hydrothermal) promote greater crystallinity than low-temperature routes (sol–gel, co-precipitation) [10,16,17].
For sol–gel method, the Se-Doped Nanocomposites Se@Ag, Se@ZnO and Se@Ag/AgO-ZnO were synthesized via a green route using plant extract, a method which, while not strictly sol–gel, requires a final calcination step at 400 °C to achieve crystallinity. X-ray diffraction reveals that after this thermal treatment, the material exhibits high crystallinity, evidenced by intense and sharp diffraction peaks. These peaks allow for the precise identification of the crystalline phases of the metal oxide and silver nanoparticles. Although the initial green synthesis (or sol–gel-like route) is a low-temperature technique, the controlled final calcination ensures the ordered growth of crystallites, resulting in a nanostructure with the necessary phase purity and structural order for high photocatalytic activity [19].
Considering co-precipitation, in Ref. [21], it was noted that the crystallinity of TiO2 was confirmed by XRD, which showed intense and narrow peaks characteristic of the anatase phase, indicating high crystallinity. This phase is the most desirable for photocatalysis due to its reactivity. The presence of GO and chitosan was confirmed by the attenuation of the GO peak, but the presence of TiO2 remained high, which was largely responsible for the photocatalytic effect. For hydrothermal method, Ref. [5] synthesized three-dimensional (3D) tripyramidal TiO2 architectures is carried out by a green route that combines the use of Aloe extract. XRD confirms that TiO2 is formed predominantly in the Anatase phase also, which, combined with the 3D architecture induced by the Aloe extract, results in a structure with excellent crystalline order.
The synthesis of nanostructured and spongy ZnO is carried out via a green method, also using Aloe extract, but followed by a calcination method. Characteristic peaks were identified by XRD referring to the hexagonal Wurtzite structure of ZnO, indicating high phase purity and excellent crystalline ordering, since the material, even with spongy morphology, maintains the integrity of the network, minimizing defects that could be recombination sites, and this characteristic supports the high production of hydroxyl radicals under sunlight for water remediation [30].
Finally, in terms of the pyrolytic method, commonly used for photocatalyst composites, even with an amorphous base structure such as coals [20], they identified that the synthesis of the Ag/Ni/Fe3O4 composite supported on activated carbon (AC) using plant extract as a reducing and stabilizing agent indicated, by XRD, the presence of multiple phases with peaks consistently indexed to the magnetite phase, with an inverted cubic spinel structure, and also to the Ag/Ni metallic phases. The high crystallinity of the Fe3O4 nanoparticles was essential for their magnetic and catalytic properties, while the Ag phase increased photoactivity.
4. Challenges and Future Directions in Green-Synthesized Photocatalysts for Antibiotic Degradation
Despite meaningful advances in green synthesis for photocatalytic antibiotic removal, several persistent limitations hinder the large-scale and safe application of these technologies.
A major concern lies in the limited data regarding the toxicity of intermediate degradation products. While many studies reported high removal efficiencies for antibiotics such as ciprofloxacin, tetracycline, and amoxicillin, only a minority examined the chemical identity or environmental fate of transformation byproducts. For example, the use of N,P,S co-doped graphenic SiC demonstrated promising antibacterial performance, yet the ecotoxicological risks and transformation pathways of resulting metabolites were not fully assessed [48]. Similarly, although Ag/Ni/Fe3O4-activated carbon beads showed effective degradation of enrofloxacin, no study from the dataset systematically identified potential nitro-aromatic or halogenated byproducts formed during treatment [47]. These observations highlight the need for advanced analytical techniques, such as LC-MS/MS, coupled with standardized toxicity assays.
Stability and recyclability of green-synthesized photocatalysts also remain insufficiently explored. Although materials such as Nb-doped ZnO and ZnO-based heterostructures achieved high degradation performance, most studies lacked long-term evaluation across multiple reuse cycles [49,55]. Parameters such as leaching of active species, photocorrosion, and surface fouling were rarely monitored, making it difficult to assess operational durability under realistic conditions.
Furthermore, the transition from laboratory-scale testing to real wastewater treatment scenarios remains underdeveloped. Nearly all selected studies relied on synthetic or isolated antibiotic solutions, without considering the influence of competing contaminants, organic load, or ionic strength. For instance, although bio-graphenes derived from plant resins demonstrated efficient tetracycline degradation, their behavior in complex effluents such as hospital or municipal wastewater was not investigated [48]. This gap limits the extrapolation of laboratory results to field applications.
Reactor configuration and process integration were also overlooked. None of the reviewed studies evaluated photocatalysts in continuous-flow systems, solar-assisted setups, or hybrid reactors under open-environment conditions. Fixed-bed and pilot-scale designs, which are critical for industrial scaling, were entirely absent. As an example, the CdS photocatalyst synthesized via sol–gel exhibited excellent ciprofloxacin removal, but was only tested under batch-mode irradiation [46], and advancing from material development to reactor engineering remains a key step for deployment.
Economic and environmental assessments were likewise limited. Although the use of low-cost and biodegradable precursors—such as basil seeds or Brucea javanica—was often emphasized, few studies conducted life cycle assessments or cost–benefit analyses to validate scalability [17,47].
Finally, there is a notable concentration of studies targeting only a few antibiotic classes—particularly tetracycline and ciprofloxacin, in which broader pharmaceutical groups, including macrolides, sulfonamides, and antivirals—remain underexplored. Among the reviewed works, only a limited subset addressed multiple antibiotics simultaneously, such as ampicillin and sulfamethoxazole, indicating a substantial opportunity for the development of multicomponent or adaptive photocatalysts capable of broader pollutant coverage. A further challenge lies in advancing synthesis strategies that maximize performance while minimizing environmental impact. Future research must prioritize routes that reduce or eliminate hazardous solvents, rely on low-energy processing, optimize phytochemical utilization, and enhance material efficiency without increasing toxicity. The pursuit of greener synthesis pathways—leaning on renewable feedstocks, reduced waste generation, and improved biodegradability—remains essential for ensuring that emerging photocatalysts are not only effective in pollutant removal but also sustainable, safe, and scalable for real-world water treatment applications [19,49].
5. Conclusions
Green synthesis applied to the photocatalytic degradation of antibiotics represents a significant step forward in the pursuit of sustainable technologies for water purification. By aligning the principles of green chemistry with materials engineering, synthetic routes such as sol–gel, hydrothermal, combustion, and co-precipitation have demonstrated outstanding potential in the removal of persistent antibiotics, particularly when mediated by plant extracts rich in bioactive compounds. These phytochemical constituents—such as flavonoids, phenols, saponins, and alkaloids—act synergistically as natural reducing, complexing, and capping agents, contributing to uniform nucleation, morphology control, and enhanced stability of the resulting nanostructures.
The studies reviewed confirm the technical viability of these green approaches, with several works reporting degradation efficiencies exceeding 90% for antibiotics like ciprofloxacin, tetracycline, and sulfamethoxazole. The incorporation of plant-derived metabolites during synthesis has proven decisive in improving the optical response, charge carrier separation, and durability of photocatalysts under irradiation. Moreover, the diversity of biological precursors and synthesis conditions has enabled tunable material properties, allowing for the design of multifunctional and reusable catalysts.
Nonetheless, the transition from laboratory-scale success to practical application remains a central challenge. Key aspects such as the toxicity of intermediate degradation products, the long-term reusability of catalysts, and their behavior in complex wastewater matrices require further elucidation. In addition, the integration of green photocatalysts into continuous-flow or hybrid treatment systems, combined with biological and adsorption processes, offers promising avenues that remain underexplored.
Future research should also prioritize comprehensive economic and environmental evaluations, including life-cycle assessment and energy balance studies, to validate the real sustainability of these technologies compared to conventional alternatives. The valorization of plant residues, scalable synthesis routes, and intelligent process monitoring will play pivotal roles in transforming green photocatalysis into an accessible and efficient solution for emerging contaminants.
In conclusion, the adoption of phytochemical-assisted green synthesis not only minimizes the ecological footprint of nanomaterial fabrication but also enhances photocatalytic performance through natural molecular design. As interdisciplinary efforts continue to evolve and standardized evaluation criteria emerge, these technologies hold substantial promise for industrial-scale implementation and for contributing to global objectives on water quality, environmental protection, and sustainable development.
Author Contributions
Conceptualization, F.S.D. and R.O.; methodology, F.S.D.; software, A.M. and L.O.; validation, F.S.D., A.M. and J.D.; formal analysis, F.S.D.; investigation, F.S.D.; resources, R.O.; data curation, F.S.D.; writing—original draft preparation, F.S.D.; writing—review and editing, J.D.; visualization, L.O.; supervision, J.D.; project administration, J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were generated or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank the National Council for Scientific and Technological Development (CNPq/Brazil) and the Coordination for the Improvement of Higher Education Personnel (CAPES/Brazil).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xing, Z.; Wang, Z.; Chen, W.; Zhang, M.; Fu, X.; Gao, Y. Degradation of Levofloxacin in Wastewater by Photoelectric and Ultrasonic Synergy with TiO2/g-C3N4@AC Combined Electrode. J. Environ. Manag. 2023, 330, 117168. [Google Scholar] [CrossRef]
- Singh, P.P.; Pandey, G.; Murti, Y.; Gairola, J.; Mahajan, S.; Kandhari, H.; Tivari, S.; Srivastava, V. Light-Driven Photocatalysis as an Effective Tool for Degradation of Antibiotics. RSC Adv. 2024, 14, 20492–20515. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yang, Y. Hot Water Extraction of Antioxidants from Tea Leaves—Optimization of Brewing Conditions for Preparing Antioxidant-Rich Tea Drinks. Molecules 2023, 28, 3030. [Google Scholar] [CrossRef]
- Hayat, A.; Duarte, J.L.S.; Cruz-Gómez, F.; Domínguez, C.M.; Santos, A.; Cotillas, S. Electrochemical Degradation of Levofloxacin in Synthetic Hospital Effluents: Insights into Operating Parameters, by-Products Formation and Toxicity. Electrochim. Acta 2025, 530, 146390. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Zhu, M. Green Synthesis of 3D Tripyramid TiO2 Architectures with Assistance of Aloe Extracts for Highly Efficient Photocatalytic Degradation of Antibiotic Ciprofloxacin. Appl. Catal. B 2020, 260, 118149. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.; Jiang, L.; Yu, H.; Zhao, Y.; Chen, H.; Yuan, X.; Liang, J.; Li, H.; Wu, Z. Defective Polymeric Carbon Nitride: Fabrications, Photocatalytic Applications and Perspectives. Chem. Eng. J. 2022, 427, 130991. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, J.; Zhou, S.; Yu, H.; Liang, J.; Chu, W.; Li, H.; Wang, H.; Wu, Z.; Yuan, X. Strategies to Extend Near-Infrared Light Harvest of Polymer Carbon Nitride Photocatalysts. Coord. Chem. Rev. 2021, 439, 213947. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, S.; Gan, Y.; Wu, J.; Dai, J.; Chao, H.J.; Yan, D. Dichlorodiphenyltrichloroethane Inhibits Soil Ammonia Oxidation by Altering Ammonia-Oxidizing Archaeal and Bacterial Communities. Environ. Pollut. 2023, 333, 122063. [Google Scholar] [CrossRef]
- Melo, A.L.M.S.; Duarte, F.S.; Ferro, A.B.; Motta, R.J.B.; Zanta, C.L.P.S.; Oliveira, L.M.T.M.; Duarte, J.L.S.; Oliveira, R.M.P.B. Synthesis and Characterization of a Magnetic TiO2 for Propylparaben Degradation. Water Air Soil Pollut. 2025, 236, 17. [Google Scholar] [CrossRef]
- Duarte, F.D.S.; Melo, A.L.M.D.S.; Ferro, A.D.B.; Zanta, C.L.D.P.E.S.; Duarte, J.L.D.S.; Oliveira, R.M.P.B. Magnetic Zinc Oxide/Manganese Ferrite Composite for Photodegradation of the Antibiotic Rifampicin. Materials 2022, 15, 8185. [Google Scholar] [CrossRef]
- Mangla, D.; Sharma, A.; Ikram, S. Synthesis of Ecological Chitosan/PVP Magnetic Composite: Remediation of Amoxicillin Trihydrate from Its Aqueous Solution, Isotherm Modelling, Thermodynamic, and Kinetic Studies. React. Funct. Polym. 2022, 175, 105261. [Google Scholar] [CrossRef]
- Gendo, K.M.; Feyisa Bogale, R.; Kenasa, G. Green Synthesis, Characterization, and Evaluation of Photocatalytic and Antibacterial Activities of Co3O4-ZnO Nanocomposites Using Calpurnia Aurea Leaf Extract. ACS Omega 2024, 9, 28354–28371. [Google Scholar] [CrossRef] [PubMed]
- Jahan, N.; Rasheed, K.; Rahman, K.-U.; Hazafa, A.; Saleem, A.; Alamri, S.; Iqbal, M.O.; Rahman, M.A. Green Inspired Synthesis of Zinc Oxide Nanoparticles Using Silybum marianum (Milk Thistle) Extract and Evaluation of Their Potential Pesticidal and Phytopathogens Activities. PeerJ 2023, 11, e15743. [Google Scholar] [CrossRef] [PubMed]
- Afsharpour, M.; Behtooei, H.R.; Shakiba, M.; Martí, V.; Parizi, S.S. Novel N,P,S Co-Doped Graphenic SiC Layers (g-SiC) in Visible-Light Photodegradation of Antibiotics and Inactivating the Bacteria. Process Saf. Environ. Prot. 2022, 166, 704–717. [Google Scholar] [CrossRef]
- Chanthapong, P.; Maensiri, D.; Rangsrisak, P.; Jaiyan, T.; Rahaeng, K.; Oraintara, A.; Ratchaphonsaenwong, K.; Sanitchon, J.; Theerakulpisut, P.; Mahakham, W. Plant-Based ZnO Nanoparticles for Green Nanobiocontrol of a Highly Virulent Bacterial Leaf Blight Pathogen: Mechanistic Insights and Biocompatibility Evaluation. Nanomaterials 2025, 15, 1011. [Google Scholar] [CrossRef]
- Erim, B.; Ciğeroğlu, Z.; Bayramoğlu, M. Green Synthesis of TiO2/GO/Chitosan by Using Leaf Extract of Olea Europaea as a Highly Efficient Photocatalyst for the Degradation of Cefixime Trihydrate under UV-A Radiation Exposure: An Optimization Study with D-Optimal Design. J. Mol. Struct. 2021, 1234, 130194. [Google Scholar] [CrossRef]
- Paghaleh, E.S.; Dashtian, K.; Seyf, J.Y.; Seidi, F.; Kolvari, E. Green Synthesis of Stable CuFe2O4/CuO-RGO Heterostructure Photocatalyst Using Basil Seeds as Chemo-Reactors for Improved Oxytetracycline Degradation. J. Environ. Chem. Eng. 2023, 11, 110676. [Google Scholar] [CrossRef]
- Iqbal, Y.; Ahmed, S.; Aziz, M.H.; Alam, M.; Asif, M.; Huang, Q. Greener Approach for the Synthesis of Ag Decorated ZnO–CeO2 Nanostructure Using Moringa Oleifera LE and Its Investigation as Photocatalyst for Degradation of Ciprofloxacin and Methylene Orange. Mater. Chem. Phys. 2024, 318, 129299. [Google Scholar] [CrossRef]
- Essawy, A.A.; Alsohaimi, I.H.; Alhumaimess, M.S.; Hassan, H.M.A.; Kamel, M.M. Green Synthesis of Spongy Nano-ZnO Productive of Hydroxyl Radicals for Unconventional Solar-Driven Photocatalytic Remediation of Antibiotic Enriched Wastewater. J. Environ. Manag. 2020, 271, 110961. [Google Scholar] [CrossRef]
- Roy, N.; Kannabiran, K.; Mukherjee, A. Studies on Photocatalytic Removal of Antibiotics, Ciprofloxacin and Sulfamethoxazole, by Fe3O4-ZnO-Chitosan/Alginate Nanocomposite in Aqueous Systems. Adv. Powder Technol. 2022, 33, 103691. [Google Scholar] [CrossRef]
- González-Ballesteros, N.; Martins, P.M.; Tavares, C.J.; Lanceros-Méndez, S. Quercetin-Mediated Green Synthesis of Au/TiO2 Nanocomposites for the Photocatalytic Degradation of Antibiotic Ciprofloxacin. J. Ind. Eng. Chem. 2025, 143, 526–537. [Google Scholar] [CrossRef]
- Batterjee, M.G.; Nabi, A.; Kamli, M.R.; Alzahrani, K.A.; Danish, E.Y.; Malik, M.A. Green Hydrothermal Synthesis of Zinc Oxide Nanoparticles for UV-Light-Induced Photocatalytic Degradation of Ciprofloxacin Antibiotic in an Aqueous Environment. Catalysts 2022, 12, 1347. [Google Scholar] [CrossRef]
- Meneceur, S.; Bouafia, A.; Laouini, S.E.; Mohammed, H.A.; Daoudi, H.; Hasan, G.G.; Salmi, C. High-Efficiency Photocatalytic Degradation of Antibiotics and Molecular Docking Study to Treat the Omicron Variant of COVID-19 Infection Using Biosynthesized ZnO@Fe3O4 Nanocomposites. Phys. Scr. 2023, 98, 115926. [Google Scholar] [CrossRef]
- Farheen; Parveen, A. Enhanced Visible Light Energy Harvesting and Efficient Photocatalytic Antibiotic Drug Degradation over Egg Albumen Mediated Sr Doped Fe2O3 Nanoparticles. Mater. Sci. Semicond. Process. 2022, 148, 106804. [Google Scholar] [CrossRef]
- Yadav, S.; Shah, A.; Malhotra, P. Orange Peel-Derived Cu2O/RGO Nanocomposite: Mesoporous Binary System for Degradation of Doxycycline in Water. Environ. Dev. Sustain. 2024, 26, 4505–4532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Ruan, Z.; Yuan, Y.; Lin, K. Extensive Solar Light Utilizing by Ternary C-Dots/Cu2O/SrTiO3: Highly Enhanced Photocatalytic Degradation of Antibiotics and Inactivation of E. Coli. Chemosphere 2022, 290, 133340. [Google Scholar] [CrossRef] [PubMed]
- Alfred, M.O.; Olorunnisola, C.G.; Oyetunde, T.T.; Dare, P.; Vilela, R.R.C.; de Camargo, A.; Oladoja, N.A.; Omorogie, M.O.; Olukanni, O.D.; Motheo, A.D.J.; et al. Sunlight-Driven Photocatalytic Mineralization of Antibiotic Chemical and Selected Enteric Bacteria in Water via Zinc Tungstate-Imprinted Kaolinite. Green Chem. Lett. Rev. 2022, 15, 705–723. [Google Scholar] [CrossRef]
- Ulukuş, D.; Mirza, S.; Hussaini, A.A.; Öztürk, T.; Toprak, A.; Uysal, A.; Yıldırım, M. Green Synthesis of Selenium-Doped Nanocomposites for Photocatalytic Degradation of Dyes, Antibiotics, and Antibacterial Activities. J. Inorg. Organomet. Polym. Mater. 2025, 35, 7700–7716. [Google Scholar] [CrossRef]
- Chin, J.Y.; Ahmad, A.L.; Low, S.C. Green Synthesis of TiO2 Phases for Efficient Photocatalytic Degradation of Oxytetracycline in Real Aquaculture Wastewater. J. Water Process Eng. 2025, 69, 106644. [Google Scholar] [CrossRef]
- Ganeshbabu, M.; Priya, J.S.; Manoj, G.M.; Puneeth, N.P.N.; Shobana, C.; Shankar, H.; Selvan, R.K. Photocatalytic Degradation of Fluoroquinolone Antibiotics Using Chitosan Biopolymer Functionalized Copper Oxide Nanoparticles Prepared by Facile Sonochemical Method. Int. J. Biol. Macromol. 2023, 253, 127027. [Google Scholar] [CrossRef]
- Davarnejad, R.; Hassanvand, Z.R.; Mansoori, S.; Kennedy, J.F. Metronidazole Elimination from Wastewater through Photo-Fenton Process Using Green-Synthesized Alginate-Based Hydrogel Coated Bimetallic Iron-copper Nanocomposite Beads as a Reusable Heterogeneous Catalyst. Bioresour. Technol. Rep. 2022, 18, 101068. [Google Scholar] [CrossRef]
- Arabkhani, P.; Saeedi, N.; Sadeghi, H.; Nouripour-Sisakht, S.; Gharaghani, M.; Asfaram, A. Plant Extracts-Mediated Green Synthesis of Zinc Oxide/Carbon Nanofiber Nanocomposites with Highly Efficient Photocatalytic and Antimicrobial Properties for Wastewater Treatment. J. Water Process Eng. 2023, 54, 104020. [Google Scholar] [CrossRef]
- Nimshi, R.E.; Vijaya, J.J.; Kennedy, L.J.; Selvamani, P.S.; Bououdina, M.; Sophia, P.J. Effective Microwave Assisted Synthesis of CoFe2O4@TiO2@rGO Ternary Nanocomposites for the Synergic Sonophotocatalytic Degradation of Tetracycline and c Antibiotics. Ceram. Int. 2023, 49, 13762–13773. [Google Scholar] [CrossRef]
- Zulfiqar, N.; Nadeem, R.; Musaimi, O.A. Photocatalytic Degradation of Antibiotics via Exploitation of a Magnetic Nanocomposite: A Green Nanotechnology Approach toward Drug-Contaminated Wastewater Reclamation. ACS Omega 2023, 9, 7986–8004. [Google Scholar] [CrossRef]
- Naeimi, A.; Honarmand, M.; Ali Chaji, M.; Khosravi, S. Green Synthesis of Bentonite/Cellulose@lead Oxide Bio-Nanocomposite with Assistance of Pistacia Atlantica Extract for Efficient Photocatalytic Degradation of Ciprofloxacin. Adv. Powder Technol. 2022, 33, 103441. [Google Scholar] [CrossRef]
- Makofane, A.; Motaung, D.E.; Hintsho-Mbita, N.C. Green Synthesis of Silver Deposited on Copper Ferrite Nanoparticles for the Photodegradation of Dye and Antibiotics. Appl. Surf. Sci. Adv. 2024, 21, 100601. [Google Scholar] [CrossRef]
- Thangsan, P.; Wannakan, K.; Nanan, S. Biosynthesis of ZnO Using Senna Siamea Leaf Extract for Photodegradation of Tetracycline Antibiotic and Azo Dye in Wastewater. OpenNano 2024, 16, 100202. [Google Scholar] [CrossRef]
- Bopape, D.A.; Motaung, D.E.; Hintsho-Mbita, N.C. Green Synthesis of ZnO: Effect of Plant Concentration on the Morphology, Optical Properties and Photodegradation of Dyes and Antibiotics in Wastewater. Optik 2022, 251, 168459. [Google Scholar] [CrossRef]
- Ayodhya, D.; Ambala, A.; Balraj, G.; Pradeep Kumar, M.; Shyam, P. Green Synthesis of CeO2 NPs Using Manilkara Zapota Fruit Peel Extract for Photocatalytic Treatment of Pollutants, Antimicrobial, and Antidiabetic Activities. Results Chem. 2022, 4, 100441. [Google Scholar] [CrossRef]
- Martins Bernardes Ramos, R.; Paludo, L.C.; Monteiro, P.I.; Maurat da Rocha, L.V.; Veiga de Moraes, C.; Santos, O.O.; Alves, E.R.; Porto Dantas, T.L. Amoxicillin Degradation by Iron Photonanocatalyst Synthetized by Green Route Using Pumpkin (Tetsukabuto) Peel Extract. Talanta 2023, 260, 124658. [Google Scholar] [CrossRef]
- Ahmad, W.; Kaur, N. Microwave-Assisted Single Step Green Synthesis of NiO Nanoparticles Using Coleus scutellariodes Leaf Extract for the Photocatalytic Degradation of Rufloxacin. MRS Adv. 2023, 8, 835–842. [Google Scholar] [CrossRef]
- Pakzad, K.; Alinezhad, H.; Nasrollahzadeh, M. Euphorbia Polygonifolia Extract Assisted Biosynthesis of Fe3O4@CuO Nanoparticles: Applications in the Removal of Metronidazole, Ciprofloxacin and Cephalexin Antibiotics from Aqueous Solutions under UV Irradiation. Appl. Organomet. Chem. 2020, 34, e5910. [Google Scholar] [CrossRef]
- Laddha, H.; Yadav, P.; Sharma, M.; Agarwal, M.; Gupta, R. Waste to Value Transformation: Converting Carica Papaya Seeds into Green Fluorescent Carbon Dots for Simultaneous Selective Detection and Degradation of Tetracycline Hydrochloride in Water. Environ. Res. 2023, 227, 115820. [Google Scholar] [CrossRef]
- Meky, A.I.; Hassaan, M.A.; Fetouh, H.A.; Ismail, A.M.; El Nemr, A. Cube-Shaped Cobalt-Doped Zinc Oxide Nanoparticles with Increased Visible-Light-Driven Photocatalytic Activity Achieved by Green Co-Precipitation Synthesis. Sci. Rep. 2023, 13, 19329. [Google Scholar] [CrossRef] [PubMed]
- Faizah, A.H.; Gunawan; Khabibi; Wijaya, R.A. Effect of Calcination Temperature Variation on Green Synthesis of Cadmium Sulfide for Ciprofloxacin Photodegradation. Int. J. Res.-Granthaalayah 2024, 12, 17–30. [Google Scholar] [CrossRef]
- Yasir, M.; Šopík, T.; Ali, H.; Kimmer, D.; Sedlařík, V. Green Synthesis of Titanium and Zinc Oxide Nanoparticles for Simultaneous Photocatalytic Removal of Estrogens in Wastewater. In Proceedings of the NANOCON Conference Proceedings—International Conference on Nanomaterials, Brno, Czech Republic, 15–17 October 2025; TANGER Ltd.: Greensboro, NC, USA; pp. 189–196. [Google Scholar]
- Hoang, V.H.; Phan, T.N.B.; Nguyen, V.T.; Le, T.T.; Do, M.H.; Luu, V.T.; Tran, V.A.; Doan, V.D.; Le, V.T. One-Pot Green Synthesis of Ag/Ni/Fe3O4-Activated Carbon Beads for Recyclable Photo-Fenton Antibiotic Removal and Antibacterial Action: Mechanistic Study and Optimization. RSC Adv. 2025, 15, 13478–13496. [Google Scholar] [CrossRef]
- Afsharpour, M.; Radmanesh, L.; Yang, C. In Situ Synthesis of Doped Bio-Graphenes as Effective Metal-Free Catalysts in Removal of Antibiotics: Effect of Natural Precursor on Doping, Morphology, and Catalytic Activity. Molecules 2023, 28, 7212. [Google Scholar] [CrossRef]
- Mariappan, A.; Harikrishnan, L.; Eswaran, J.; Arumugham, N.; Balasubramaniam, Y.; Daniel, S.; Kanthapazham, R. Green Synthesis of Metal-Doped ZnO Nanoparticles Using Bauhinia Racemosa Lam. Extract and Evaluation of Their Photocatalysis and Biomedical Applications. ACS Appl. Bio Mater. 2024, 7, 2519–2532. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Liu, J.; Zhai, Z.; Yang, Z.; Xia, J.; Deng, S.; Qu, X.; Zhang, H.; Wu, D.; et al. Mo-Modified Band Structure and Enhanced Photocatalytic Properties of Tin Oxide Quantum Dots for Visible-Light Driven Degradation of Antibiotic Contaminants. J. Environ. Chem. Eng. 2022, 10, 107091. [Google Scholar] [CrossRef]
- Özkal, C.B. Synthesis of CuFe2O4-Ti and CuFe2O4-Ti-GO Nanocomposite Photocatalysts Using Green-Synthesized CuFe2O4: Determination of Photocatalytic Activity, Bacteria Inactivation and Antibiotic Degradation Potentials under Visible Light. J. Chem. Technol. Biotechnol. 2022, 97, 1842–1859. [Google Scholar] [CrossRef]
- Nasiri, A.; Golestani, N.; Rajabi, S.; Hashemi, M. Facile and Green Synthesis of Recyclable, Environmentally Friendly, Chemically Stable, and Cost-Effective Magnetic Nanohybrid Adsorbent for Tetracycline Adsorption. Heliyon 2024, 10, e24179. [Google Scholar] [CrossRef]
- Nahi, J.; Radhakrishnan, A.; Beena, B. Green Synthesis of Zinc Oxide Incorporated Nanocellulose with Visible Light Photocatalytic Activity and Application for the Removal of Antibiotic Enrofloxacin from Aqueousmedia. Mater. Today Proc. 2020, 41, 583–589. [Google Scholar] [CrossRef]
- Munyai, S.; Mahlaule-Glory, L.M.; Hintsho-Mbita, N.C. Green Synthesis of Zinc Sulphide (ZnS) Nanostructures Using S. Frutescences Plant Extract for Photocatalytic Degradation of Dyes and Antibiotics. Mater. Res. Express 2022, 9, 015001. [Google Scholar] [CrossRef]
- Nguyen, T.H.A.; Le, V.T.; Doan, V.-D.; Tran, A.V.; Nguyen, V.C.; Nguyen, A.-T.; Vasseghian, Y. Green Synthesis of Nb-Doped ZnO Nanocomposite for Photocatalytic Degradation of Tetracycline Antibiotic under Visible Light. Mater. Lett. 2022, 308, 131129. [Google Scholar] [CrossRef]
- Gyulasaryan, H.; Kuzanyan, A.; Manukyan, A.; Mukasyan, A.S. Combustion Synthesis of Magnetic Nanomaterials for Biomedical Applications. Nanomaterials 2023, 13, 1902. [Google Scholar] [CrossRef]
- Abdelbaky, A.S.; Mohamed, A.M.H.A.; Sharaky, M.; Mohamed, N.A.; Diab, Y.M. Green Approach for the Synthesis of ZnO Nanoparticles Using Cymbopogon Citratus Aqueous Leaf Extract: Characterization and Evaluation of Their Biological Activities. Chem. Biol. Technol. Agric. 2023, 10, 63. [Google Scholar] [CrossRef]
- Hokonya, N.; Mahamadi, C.; Mukaratirwa-Muchanyereyi, N.; Gutu, T.; Zvinowanda, C. Green Synthesis of P−ZrO2CeO2ZnO Nanoparticles Using Leaf Extracts of Flacourtia Indica and Their Application for the Photocatalytic Degradation of a Model Toxic Dye, Congo Red. Heliyon 2022, 8, e10277. [Google Scholar] [CrossRef] [PubMed]
- Baykut, F.; Benlioğlu, G.; Baykut, G. Photocatalytic Production of Ascorbic Acid. A Secondary Photosynthesis in Plants. In Homogeneous and Heterogeneous Photocatalysis; Springer: Dordrecht, The Netherlands, 1986; pp. 161–173. [Google Scholar]
- Fatimah, I.; Anggraini, F.; Nurlaela, N.; Akbar, S.A.F.; Sagadevan, S.; bin Johan, M.R.; Doong, R.; Oh, W.-C. Plant-Mediated Synthesis of W18O49 and W18O49/g-C3N4 Photocatalysts for Tetracycline Removal. J. Environ. Chem. Eng. 2025, 13, 117160. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, S.; Tan, M.; Shen, J.; Zhao, H.; Wu, D. Occurrence, Removal, and Risk Assessment of Emerging Contaminants in Aquatic Products Processing Sewage Treatment Plants. Environ. Sci. Pollut. Res. 2023, 30, 117772–117784. [Google Scholar] [CrossRef]
- Villagrán, Z.; Anaya-Esparza, L.M.; Velázquez-Carriles, C.A.; Silva-Jara, J.M.; Ruvalcaba-Gómez, J.M.; Aurora-Vigo, E.F.; Rodríguez-Lafitte, E.; Rodríguez-Barajas, N.; Balderas-León, I.; Martínez-Esquivias, F. Plant-Based Extracts as Reducing, Capping, and Stabilizing Agents for the Green Synthesis of Inorganic Nanoparticles. Resources 2024, 13, 70. [Google Scholar] [CrossRef]
- Hashem, A.M.; Abuzeid, H.; Kaus, M.; Indris, S.; Ehrenberg, H.; Mauger, A.; Julien, C.M. Green Synthesis of Nanosized Manganese Dioxide as Positive Electrode for Lithium-Ion Batteries Using Lemon Juice and Citrus Peel. Electrochim. Acta 2018, 262, 74–81. [Google Scholar] [CrossRef]
- Jawad, A.H.; Sabar, S.; Ishak, M.A.M.; Wilson, L.D.; Ahmad Norrahma, S.S.; Talari, M.K.; Farhan, A.M. Microwave-Assisted Preparation of Mesoporous-Activated Carbon from Coconut (Cocos Nucifera) Leaf by H3PO4 Activation for Methylene Blue Adsorption. Chem. Eng. Commun. 2017, 204, 1143–1156. [Google Scholar] [CrossRef]
- Azad, A.; Zafar, H.; Raza, F.; Sulaiman, M. Factors Influencing the Green Synthesis of Metallic Nanoparticles Using Plant Extracts: A Comprehensive Review. Pharm. Front. 2023, 05, e117–e131. [Google Scholar] [CrossRef]
- Narh, D.; Sampson, B.; Ocrah Junior, S.; Pokuaa Manu, G.; Agyei-Tuffour, B.; Nyankson, E.; Kwame Efavi, J. Green Synthesis of Citrus Sinensis Peel Extract-Mediated Ag-TiO2 and Its Application as a Photocatalyst for Organic Molecules and Antimicrobial Agent. J. Nanotechnol. 2024, 2024, 9169241. [Google Scholar] [CrossRef]
- da Silva Duarte, J.L.; Solano, A.M.S.; Arguelho, M.L.; Tonholo, J.; Martínez-Huitle, C.A.; e Silva, C.L.D.P. Evaluation of Treatment of Effluents Contaminated with Rifampicin by Fenton, Electrochemical and Associated Processes. J. Water Process Eng. 2018, 22, 250–257. [Google Scholar] [CrossRef]
- Tavares, M.G.; Duarte, J.L.D.S.; Oliveira, L.M.; Fonseca, E.J.; Tonholo, J.; Ribeiro, A.S.; Zanta, C.L. Reusable Iron Magnetic Catalyst for Organic Pollutant Removal by Adsorption, Fenton and Photo Fenton Process. J. Photochem. Photobiol. A Chem. 2022, 432, 114089. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.