Establishing Total Phosphorus Boundaries to Support Good Ecological Status of Greek Lakes and Reservoirs in Accordance with the Water Framework Directive
Abstract
1. Introduction
2. Materials and Methods
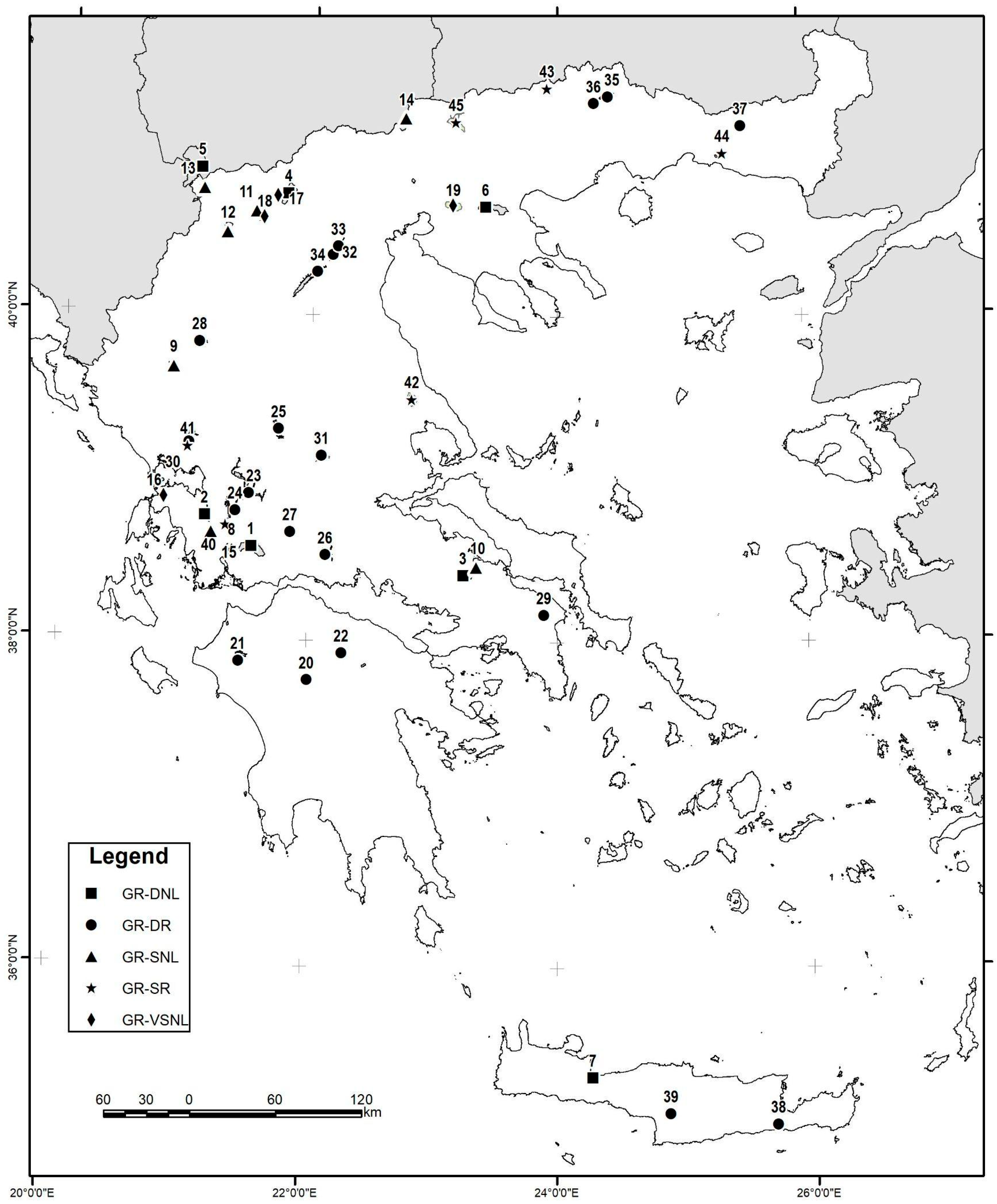
2.1. Datasets
2.2. Data Check
2.3. Statistical Approaches
2.4. Confusion Matrix
2.5. Selection of the Most Appropriate Approach
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poikane, S.; Kelly, M.G.; Várbíró, G.; Borics, G.; Erős, T.; Hellsten, S.; Kolada, A.; Lukács, B.A.; Solheim, A.L.; López, J.P.; et al. Estimating nutrient thresholds for eutrophication management: Novel insights from understudied lake types. Sci. Total Environ. 2022, 827, 154242. [Google Scholar] [CrossRef]
- Poikane, S.; Portielje, R.; Van Den Berg, M.; Phillips, G.; Brucet, S.; Carvalho, L.; Mischke, U.; Ott, I.; Soszka, H.; Van Wichelen, J. Defining ecologically relevant water quality targets for lakes in Europe. J. Appl. Ecol. 2014, 51, 592–602. [Google Scholar] [CrossRef]
- Jilbert, T.; Couture, R.M.; Huser, B.J.; Salonen, K. Preface: Restoration of eutrophic lakes: Current practices and future challenges. Hydrobiologia 2020, 847, 4343–4357. [Google Scholar] [CrossRef]
- Phillips, G.; Teixeira, H.; Kelly, M.G.; Salas Herrero, F.; Várbíró, G.; Lyche Solheim, A.; Kolada, A.; Free, G.; Poikane, S. Setting nutrient boundaries to protect aquatic communities: The importance of comparing observed and predicted classifications using measures derived from a confusion matrix. Sci. Total Environ. 2024, 912, 168872. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Peder Jensen, J.; Søndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic structure, species richness and biodiversity in Danish lakes: Changes along a phosphorus gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. WIREs Water 2019, 6, e1373. [Google Scholar] [CrossRef]
- Poikane, S.; Phillips, G.; Birk, S.; Free, G.; Kelly, M.G.; Willby, N.J. Deriving nutrient criteria to support ‘good’ ecological status in European lakes: An empirically based approach to linking ecology and management. Sci. Total Environ. 2019, 650, 2074–2084. [Google Scholar] [CrossRef]
- European Commission. Directive 2000/60/ECof the European Parliament of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off. J. Eur. Communities 2000, L 327, 1–73. [Google Scholar]
- Poikane, S.; Birk, S.; Böhmer, J.; Carvalho, L.; de Hoyos, C.; Gassner, H.; Hellsten, S.; Kelly, M.; Lyche Solheim, A.; Olin, M.; et al. A hitchhiker’s guide to European lake ecological assessment and intercalibration. Ecol. Indic. 2015, 52, 533–544. [Google Scholar] [CrossRef]
- Poikane, S.; Salas Herrero, F.; Kelly, M.G.; Borja, A.; Birk, S.; Van De Bund, W. European aquatic ecological assessment methods: A critical review of their sensitivity to key pressures. Sci. Total Environ. 2020, 740, 140075. [Google Scholar] [CrossRef]
- Nikolaidis, N.P.; Poikane, S.; Bouraoui, F.; Herrero, F.S.; Free, G.; Varkitzi, I.; van de Bund, W.; Kelly, M.G. Comparison of eutrophication assessment for the Nitrates and Water Framework Directives: Impacts and opportunities for streamlined approaches. Ecol. Indic. 2025, 177, 113375. [Google Scholar] [CrossRef]
- Ntislidou, C.; Lazaridou, M.; Tsiaoussi, V.; Bobori, D.C. A new multimetric macroinvertebrate index for the ecological assessment of Mediterranean lakes. Ecol. Indic. 2018, 93, 1020–1033. [Google Scholar] [CrossRef]
- Zervas, D.; Tsiaoussi, V.; Tsiripidis, I. HeLM: A macrophyte-based method for monitoring and assessment of Greek lakes. Environ. Monit. Assess. 2018, 190, 326. [Google Scholar] [CrossRef] [PubMed]
- Latinopoulos, D.; Spiliotis, M.; Ntislidou, C.; Kagalou, I.; Bobori, D.; Tsiaoussi, V.; Lazaridou, M. “One Out–All Out” Principle in the Water Framework Directive 2000—A New Approach with Fuzzy Method on an Example of Greek Lakes. Water 2021, 13, 1776. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Solimini, A.; Premazzi, G.; Carvalho, L.; Lyche, A.; Rekolainen, S. Phosphorus reference concentrations in European lakes. Hydrobiologia 2007, 584, 3–12. [Google Scholar] [CrossRef]
- Nikolopoulou, I.; Mavromati, E.; Moschandreou, K.; Navrozidou, V.; Kemitzoglou, D.; Tsiaoussi, V. Lake phytoplankton status and trends: A case study from Greek lakes, Eastern Mediterranean. Environ. Monit. Assess. 2025, 197, 733. [Google Scholar] [CrossRef]
- Lyche-Solheim, A.; Feld, C.K.; Birk, S.; Phillips, G.; Carvalho, L.; Morabito, G.; Mischke, U.; Willby, N.; Søndergaard, M.; Hellsten, S.; et al. Ecological status assessment of European lakes: A comparison of metrics for phytoplankton, macrophytes, benthic invertebrates and fish. Hydrobiologia 2013, 704, 57–74. [Google Scholar] [CrossRef]
- Phillips, G.; Pitt, J.A. A Comparison of European Freshwater Nutrient Boundaries Used for the Water Framework Directive: A Report to WG ECOSTAT; Ensis Ltd.: London, UK, 2016. [Google Scholar]
- Dolman, A.M.; Mischke, U.; Wiedner, C. Lake-type-specific seasonal patterns of nutrient limitation in German lakes, with target nitrogen and phosphorus concentrations for good ecological status. Freshw. Biol. 2016, 61, 444–456. [Google Scholar] [CrossRef]
- Free, G.; Tierney, D.; Little, R.; Kelly, F.L.; Kennedy, B.; Plant, C.; Trodd, W. Lake ecological assessment metrics in Ireland: Relationships with phosphorus and typology parameters and the implications for setting nutrient standards. Biol. Environ. Proc. R. Ir. Acad. 2016, 116B, 191–204. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jeppesen, E.; Jensen, J.P.; Amsinck, S.P. Water Framework Directive: Ecological Classification of Danish Lakes. J. Appl. Ecol. 2005, 42, 616–629. [Google Scholar] [CrossRef]
- Kagalou, I.; Ntislidou, C.; Latinopoulos, D.; Kemitzoglou, D.; Tsiaoussi, V.; Bobori, D.C. Setting the Phosphorus Boundaries for Greek Natural Shallow and Deep Lakes for Water Framework Directive Compliance. Water 2021, 13, 739. [Google Scholar] [CrossRef]
- Phillips, G.; Teixeira, H.; Kelly, M.; Lyche-Solheim, A.; Free, G.; Salas Herrero, F.; Poikane, S. Establishing Supporting Element Standards: A Revised Approach and Applications; European Commission: Joint Research Centre, Publications Office of the European Union: Brussels, Belgium, 2024. [Google Scholar]
- Perivolioti, T.M.; Apostolakis, A.; Papadimos, D.; Tsiaoussi, V. Bathymetry and morphometric analysis of Greek natural lakes through a hybrid GIS-acoustic methodology. Inland Waters 2025, 15, 2447173. [Google Scholar] [CrossRef]
- Tsiaoussi, V.; Mavromati, E.; Kemitzoglou, D. Report on the Development of the National Method for the Assessment of the Ecological Status of Natural Lakes in Greece, Using the Biological Quality Element “Phytoplankton”, 1st ed.; Greek Biotope/Wetland Centre (EKBY) and Special Secretariat for Waters, Ministry of Environment: Athens, Greece, 2017; p. 16.
- Pahissa, J.; Catalan, J.; Morabito, G.; Dörflinger, G.; Ferreira, J.; Laplace-Treyture, C.; Gîrbea, R.; Marchetto, A.; Polykarpou, P.; de Hoyos, C. Benefits and limitations of an in-tercalibration of phytoplankton assessment methods based on the Mediterranean GIG reservoir experience. Sci. Total Environ. 2015, 538, 169–179. [Google Scholar] [CrossRef]
- de Hoyos, C.; Catalan, J.; Dörflinger, G.; Ferreira, J.; Kemitzoglou, D.; Laplace-Treyture, C.; López, J.P. Water Framework Directive intercalibration technical report. In Mediterranean Lake Phytoplankton Ecological Assessment Methods; LU Publications Office: Luxembourg, 2014. [Google Scholar]
- APHA Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington DC, USA, 2017.
- European Commission. Commission Decision (EU) 2018/229 of 12 February 2018 establishing pursuant to Directive 2000/60/ECof the European Parliament of the Council the values of the Member State monitoring system classifications as a result of the intercalibration exercise repealing Commission Decision 2013/480/EU. Off. J. Eur. Union. 2018, 47, 1–91. [Google Scholar]
- Kelly, M.; Juggins, S. Revision of Phytoplankton Metrics for Greek Lakes; Technical Report; The Goulandris Natural History Museum, Greek Biotope—Wetland Centre: Thessaloniki, Greece, 2023; p. 49. [Google Scholar]
- Kelly, M.G.; Phillips, G.; Teixeira, H.; Várbíró, G.; Salas Herrero, F.; Willby, N.J.; Poikane, S. Establishing ecologically-relevant nutrient thresholds: A tool-kit with guidance on its use. Sci. Total Environ. 2022, 807, 150977. [Google Scholar] [CrossRef]
- Phillips, G.; Teixeira, H.; Poikane, S.; Salas Herrero, F.; Kelly, M.G. Establishing nutrient thresholds in the face of uncertainty and multiple stressors: A comparison of approaches using simulated datasets. Sci. Total Environ. 2019, 684, 425–433. [Google Scholar] [CrossRef]
- Zhou, J.; Leavitt, P.R.; Zhang, Y.; Qin, B. Anthropogenic eutrophication of shallow lakes: Is it occasional? Water Res. 2022, 221, 118728. [Google Scholar] [CrossRef]
- Verburg, P.; Schallenberg, M.; Elliott, S.; McBride, C.G. Nutrient Budgets in Lakes. In Lake Restoration Handbook; Hamilton, D.P., Collier, K.J., Quinn, J.M., Howard-Williams, C., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 129–163. [Google Scholar]
- Zhao, L.; Zhu, R.; Zhou, Q.; Jeppesen, E.; Yang, K. Trophic status and lake depth play important roles in determining the nutrient-chlorophyll a relationship: Evidence from thousands of lakes globally. Water Res. 2023, 242, 120182. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewska-Madura, K.; Dondajewska-Pielka, R.; Gołdyn, R. The Assessment of External and Internal Nutrient Loading as a Basis for Lake Management. Water 2022, 14, 2844. [Google Scholar] [CrossRef]
- Kirol, A.P.; Morales-Williams, A.M.; Braun, D.C.; Marti, C.L.; Pierson, O.E.; Wagner, K.J.; Schroth, A.W. Linking Sediment and Water Column Phosphorus Dynamics to Oxygen, Temperature, and Aeration in Shallow Eutrophic Lakes. Water Resour. Res. 2024, 60, e2023WR034813. [Google Scholar] [CrossRef]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Free, G.; Poikane, S.; Solheim, A.L.; Bussettini, M.; Bradley, C.; Smith, J.; Caroni, R.; Bresciani, M.; Pinardi, M.; Giardino, C.; et al. Climate change and ecological assessment in Europe under the WFD—Hitting moving targets with shifting baselines? J. Environ. Manag. 2024, 370, 122884. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Pierson, D.; Jennings, E. Effect of Extreme Climate Events on Lake Ecosystems. Water 2021, 13, 282. [Google Scholar] [CrossRef]
- Frost, P.C.; Pearce, N.J.T.; Berger, S.A.; Gessner, M.O.; Makower, A.K.; Marzetz, V.; Nejstgaard, J.C.; Pralle, A.; Schälicke, S.; Wacker, A.; et al. Interactive effects of nitrogen and phosphorus on growth and stoichiometry of lake phytoplankton. Limnol. Oceanogr. 2023, 68, 1172–1184. [Google Scholar] [CrossRef]
- Liang, Z.; Soranno, P.A.; Wagner, T. The role of phosphorus and nitrogen on chlorophyll a: Evidence from hundreds of lakes. Water Res. 2020, 185, 116236. [Google Scholar] [CrossRef]
- Latinopoulos, D.; Ntislidou, C.; Kagalou, I. Multipurpose Plans for the Sustainability of the Greek Lakes: Emphasis on Multiple Stressors. Environ. Process. 2016, 3, 589–602. [Google Scholar] [CrossRef]
- Jeppesen, E.; Kronvang, B.; Olesen, J.E.; Audet, J.; Søndergaard, M.; Hoffmann, C.C.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Larsen, S.E.; et al. Climate change effects on nitrogen loading from cultivated catchments in Europe: Implications for nitrogen retention, ecological state of lakes and adaptation. Hydrobiologia 2011, 663, 1–21. [Google Scholar] [CrossRef]
- Stefanidis, K.; Varlas, G.; Papadopoulos, A.; Dimitriou, E. Four Decades of Surface Temperature, Precipitation, and Wind Speed Trends over Lakes of Greece. Sustainability 2021, 13, 9908. [Google Scholar] [CrossRef]
- Doulgeris Ch Papadimos, D.; Kapsomenakis, J. Impacts of climate change on the hydrology of two Natura 2000 sites in Northern Greece. Reg. Environ. Change 2016, 16, 1941–1950. [Google Scholar] [CrossRef]
- Zervas, D.; Tsiaoussi, V.; Kallimanis, A.; Dimopoulos, P.; Bergmeier, E.; Tsiripidis, I. Multiple-facet diversity patterns of aquatic vegetation in lakes along a trophic gradient. Water 2021, 13, 2281. [Google Scholar] [CrossRef]
- Soria, J.; Apostolova, N. Decrease in the water level of lake Prespa (North Macedonia) studied by remote sensing methodology: Relation with hydrology and agriculture. Hydrology 2022, 9, 99. [Google Scholar] [CrossRef]
- WISE-Water Information System for Europe. 2024. Available online: https://water.europa.eu/freshwater/resources/metadata/wfd-dashboards/surface-water-bodies-ecological-status-or-potential-bycategory-chart (accessed on 19 March 2025).
- Mavromati, E.; Kagalou, I.; Kemitzoglou, D.; Apostolakis, A.; Seferlis, M.; Tsiaoussi, V. Relationships among land use patterns, hydromorphological features and physicochemical parameters of surface waters: WFD lake monitoring in Greece. Environ. Process. 2018, 5 (Suppl. S1), 139–151. [Google Scholar] [CrossRef]
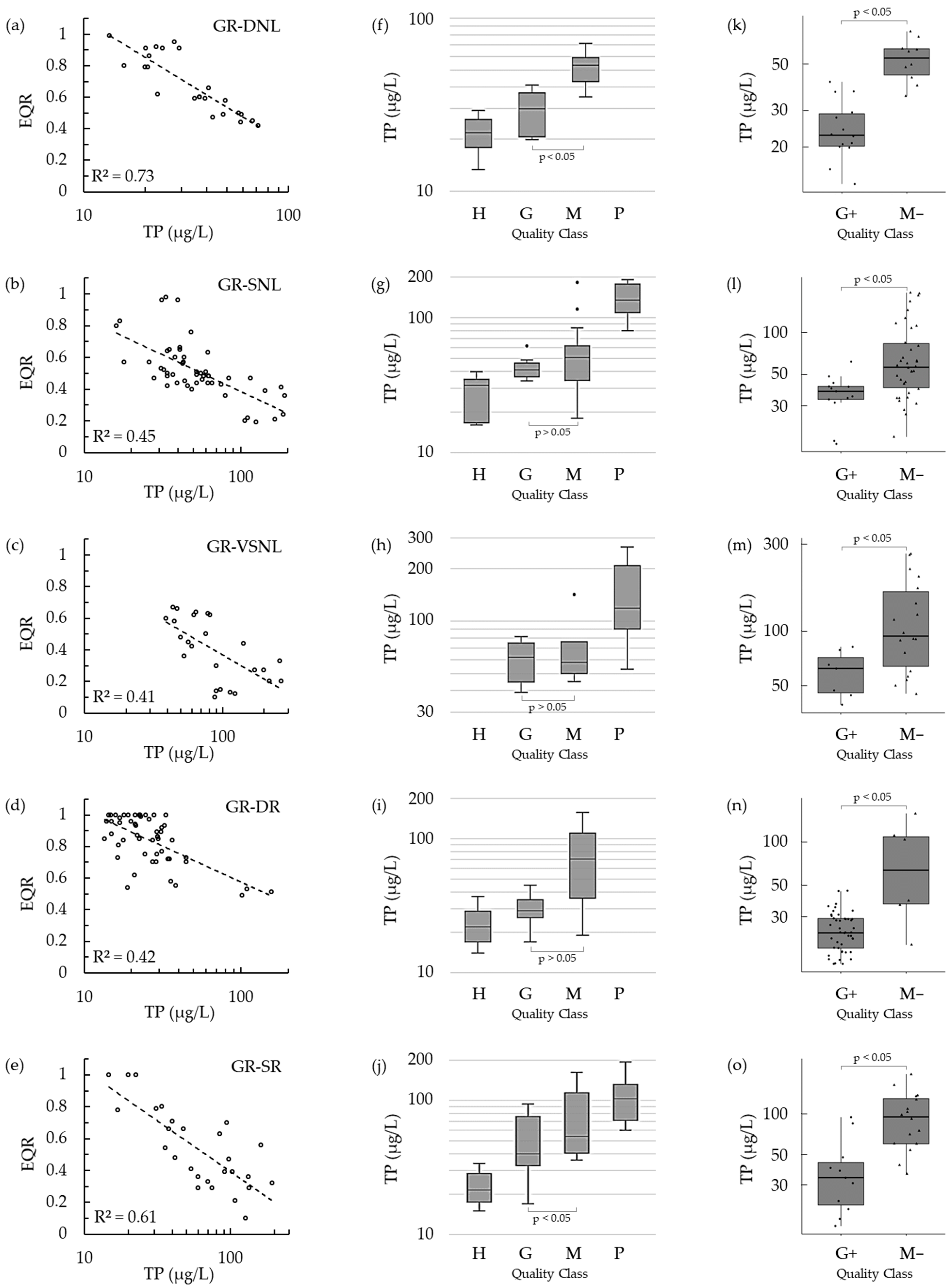

| Lake Type | Method | N | GM | GML | GMU | AUC | R2 (Pseudo-r2) | Prob | Prev | CCR | Misclass | Omis | Comm | kp |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GR-DNL | BLM | 24 | 39 | 30 | 50 | 0.97 | (0.84) | 0.43 | 0.58 | 0.92 | 0.08 | 0.07 | 0.10 | 0.83 |
| GR-SNL | BLM | 51 | 42 | 28 | 73 | 0.77 | (0.27) | 0.28 | 0.26 | 0.75 | 0.25 | 0.23 | 0.26 | 0.43 |
| GR-VSNL | BLM | 25 | 76 | 51 | 266 | 0.82 | (0.36) | 0.26 | 0.28 | 0.68 | 0.32 | 0.29 | 0.28 | 0.39 |
| GR-DR | BLM | 52 | 32 | 18 | 49 | 0.86 | (0.51) | 0.89 | 0.89 | 0.85 | 0.15 | 0.15 | 0.17 | 0.48 |
| GR-SR | RMA | 27 | 50 | 31 | 65 | NA | 0.61 | NA | 0.41 | 0.85 | 0.15 | 0.18 | 0.13 | 0.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zioga, M.; Kemitzoglou, D.; Zerva, I.; Katsavouni, S.; Kagalou, I.; Tsiaoussi, V. Establishing Total Phosphorus Boundaries to Support Good Ecological Status of Greek Lakes and Reservoirs in Accordance with the Water Framework Directive. Water 2025, 17, 3349. https://doi.org/10.3390/w17233349
Zioga M, Kemitzoglou D, Zerva I, Katsavouni S, Kagalou I, Tsiaoussi V. Establishing Total Phosphorus Boundaries to Support Good Ecological Status of Greek Lakes and Reservoirs in Accordance with the Water Framework Directive. Water. 2025; 17(23):3349. https://doi.org/10.3390/w17233349
Chicago/Turabian StyleZioga, Marianthi, Dimitra Kemitzoglou, Ioanna Zerva, Sotiria Katsavouni, Ifigenia Kagalou, and Vasiliki Tsiaoussi. 2025. "Establishing Total Phosphorus Boundaries to Support Good Ecological Status of Greek Lakes and Reservoirs in Accordance with the Water Framework Directive" Water 17, no. 23: 3349. https://doi.org/10.3390/w17233349
APA StyleZioga, M., Kemitzoglou, D., Zerva, I., Katsavouni, S., Kagalou, I., & Tsiaoussi, V. (2025). Establishing Total Phosphorus Boundaries to Support Good Ecological Status of Greek Lakes and Reservoirs in Accordance with the Water Framework Directive. Water, 17(23), 3349. https://doi.org/10.3390/w17233349







