Effects of Coal Fly Ash Addition on the Carbon Mineralization of Agricultural Soil Under Different Moisture Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection and Preparation
2.2. Incubation Experiments
2.3. Soil C Mineralization Monitoring
2.4. WSOC and MBC Measurement
2.5. Enzyme Activity and Microbial Diversity Determination
2.6. Data Processing
3. Results
3.1. Soil C Mineralization Characteristics
3.2. Variation in pH, EC, WSOC, and MBC
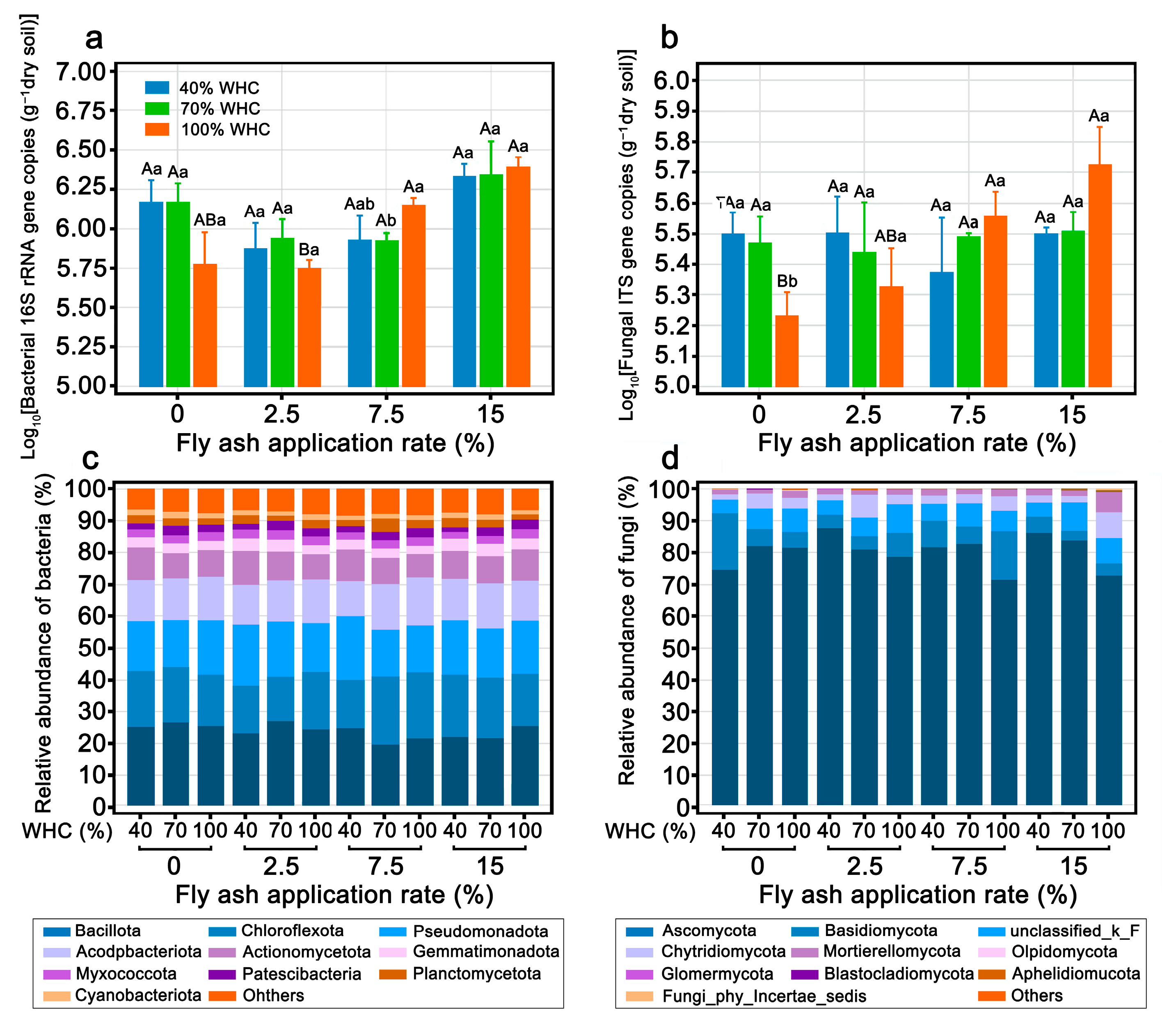
3.3. Enzyme Activit and Microbial Quantity and Diversity
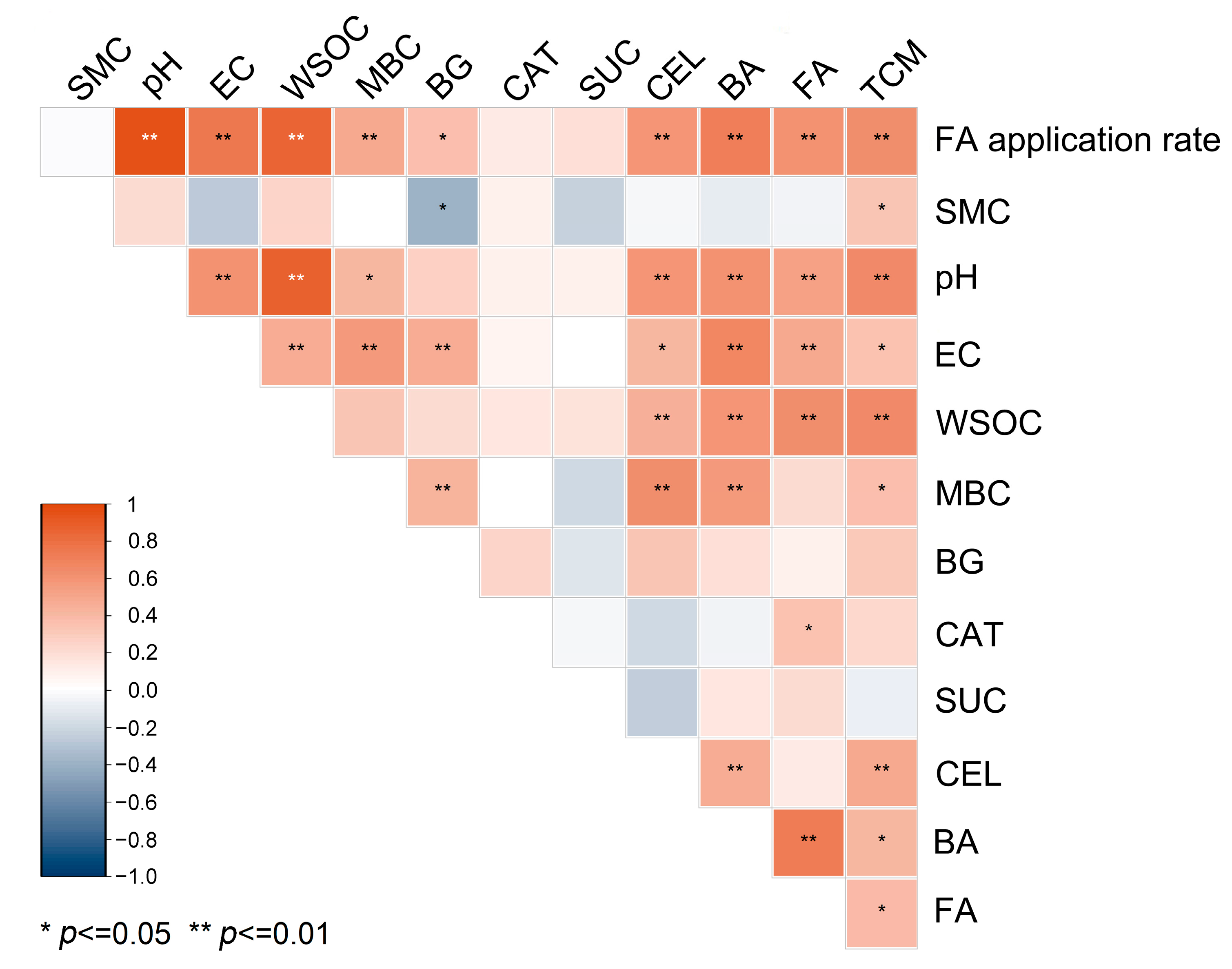
3.4. Relationship Between CO2 Release and Soil Properties
4. Discussion
4.1. Soil C Mineralization Characteristics and the Influence of Fly Ash Addition
4.2. Influence of Moisture Conditions on C Mineralization
4.3. Influence on Enzyme Activities
4.4. Relationship Between Microbial Quantity and C Mineralization
4.5. Relationship Between Bacterial Community Characteristics and C Mineralization
4.6. Benefits and Potential Risk Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Millati, R.; Cahyono, R.B.; Ariyanto, T.; Azzahrani, I.N.; Putri, R.U.; Taherzadeh, M.J. Chapter 1—Agricultural, Industrial, Municipal, and Forest Wastes: An Overview. In Sustainable Resource Recovery and Zero Waste Approaches; Taherzadeh, M.J., Bolton, K., Wong, J., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–22. ISBN 978-0-444-64200-4. [Google Scholar]
- Lehmann, J.; Rilig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Saadaoui, E.; Ghazel, N.; Ben Romdhane, C.; Massoudi, N. Phosphogypsum: Potential uses and problems—A review. Int. J. Environ. Stud. 2017, 74, 558–567. [Google Scholar] [CrossRef]
- Kumar, A.; Chopra, A.K.; Kumar, V. A Review on sewage sludge (biosolids) a resource for sustainable agriculture. Arch. Agric. Environ. Sci. 2017, 2, 340–347. [Google Scholar] [CrossRef]
- Basu, M.; Pande, M.; Bhadoria, P.; Mahapatra, S. Potential fly-ash utilization in agriculture: A global review. Prog. Nat. Sci. 2009, 19, 1173–1186. [Google Scholar] [CrossRef]
- Cristelo, N.; Glendinning, S.; Fernandes, L.; Pinto, A.T. Effects of alkaline-activated fly ash and Portland cement on soft soil stabilisation. Acta Geotech. 2013, 8, 395–405. [Google Scholar] [CrossRef]
- Liu, H.B.; Liu, Z.L. Recycling utilization patterns of coal mining waste in China. Resour. Conserv. Recycl. 2010, 54, 1331–1340. [Google Scholar] [CrossRef]
- Zacco, A.; Borgese, L.; Gianoncelli, A.; Struis, R.P.; Depero, L.E.; Bontempi, E. Review of fly ash inertisation treatments and recycling. Environ. Chem. Lett. 2014, 12, 153–175. [Google Scholar] [CrossRef]
- Usman, M.; Anastopoulos, I.; Hamid, Y.; Wakeel, A. Recent trends in the use of fly ash for the adsorption of pollutants in contaminated wastewater and soils: Effects on soil quality and plant growth. Environ. Sci. Pollut. Res. 2023, 30, 124427–124446. [Google Scholar] [CrossRef]
- He, H.H.; Dong, Z.G.; Peng, Q.; Wang, X.; Fan, C.B.; Zhang, X.C. Impacts of coal fly ash on plant growth and accumulation of essential nutrients and trace elements by alfalfa (Medicago sativa) grown in a loessial soil. J. Environ. Manag. 2017, 197, 428–439. [Google Scholar] [CrossRef]
- Le, T.V.; Ngo, C.N.T.; Futamata, H. Effect of fly ash amendment on sandy soil properties and peanut yields. Sci. Asia. 2021, 47, 357–365. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Yin, J.; Qi, L.; Feng, Y. Combined amendments of nano-hydroxyapatite immobilized cadmium in contaminated soil-potato (Solanum tuberosum L.) system. Bull. Environ. Contam. Toxicol. 2018, 100, 581–587. [Google Scholar] [CrossRef]
- Du, Y.J.; Wei, M.L.; Reddy, K.R.; Liu, Z.P.; Jin, F. Effect of acid rain pH on leaching behavior of cement stabilized lead-contaminated soil. J. Hazard. Mater. 2014, 271, 131–140. [Google Scholar] [CrossRef]
- Komonweeraket, K.; Cetin, B.; Aydilek, A.H.; Benson, C.H.; Edil, T.B. Effects of pH on the leaching mechanisms of elements from fly ash mixed soils. Fuel 2015, 140, 788–802. [Google Scholar] [CrossRef]
- Nayak, A.K.; Raja, R.; Rao, K.S.; Shukla, A.K.; Mohanty, S.; Shahid, M.; Tripathi, R.; Panda, B.B.; Bhattacharyya, P.; Kumar, A.; et al. Effect of fly ash application on soil microbial response and heavy metal accumulation in soil and rice plant. Ecotoxicol. Environ. Saf. 2015, 114, 257–262. [Google Scholar] [CrossRef]
- Saidy, A.R.; Hayati, A.; Septiana, M. Different effects of ash application on the carbon mineralization and microbial biomass carbon of reclaimed mining soils. J. Soil Sci. Plant Nutr. 2020, 20, 1001–1012. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, N. Impact of fly ash incorporation in soil systems. Agric. Ecosyst. Environ. 2010, 136, 16–27. [Google Scholar] [CrossRef]
- Usmani, Z.; Kumar, V.; Gupta, P.; Gupta, G.; Rani, R.; Chandra, A. Enhanced soil fertility, plant growth promotion and microbial enzymatic activities of vermicomposted fly ash. Sci. Rep. 2019, 9, 10455. [Google Scholar] [CrossRef]
- Jambhulkar, H.P.; Shaikh, S.M.S.; Kumar, M.S. Fly ash toxicity, emerging issues and possible implications for its exploitation in agriculture; Indian scenario: A review. Chemosphere 2018, 3, 333–344. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon and organic matter. In Methods of Soil Analysis, Part 3, Chemical Methods; Soil Science Society of America Book Series; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America: Madison, WI, USA, 2001; pp. 961–1010. [Google Scholar]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Klute, A.; Page, A.L. Methods of Soil Analysis. In Part 1. Physical and Mineralogical Methods; Part 2. Chemical and Microbiological Properties; American Society of Agronomy, Inc.: Madison, WI, USA, 1986. [Google Scholar]
- Angers, D.A.; Recous, S. Decomposition of wheat straw and rye residues as affected by particle size. Plant Soil 1997, 189, 197–203. [Google Scholar] [CrossRef]
- Stanford, G.; Smith, S. Nitrogen mineralization potentials of soils. Soil Sci. Soc. Am. J. 1972, 3, 465–472. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Guan, S. Soil Enzymes and Its Research Methods; Agriculture Press: Beijing, China, 1986. (In Chinese) [Google Scholar]
- Kumar, M.; Yadav, V.; Tuteja, N.; Johri, A.K. Antioxidant enzyme activities in maize plants colonized with Piriformospora indica. Microbiology 2009, 155, 780–790. [Google Scholar] [CrossRef]
- Krämer, S.; Green, D.M. Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland. Soil Biol. Biochem. 2000, 32, 179–188. [Google Scholar] [CrossRef]
- Ma, C.; Chen, X.Y.; Zheng, G.D.; Liu, N.; Zhao, J.H.; Zhang, H.Z. Exploring the influence mechanisms of polystyrene-microplastics on sewage sludge composting. Bioresour. Technol. 2022, 362, 127798. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, X.; Wang, D.; Wang, M.; Liao, C.; Yang, X.; Liu, F. Decoupled linkage between soil carbon and nitrogen mineralization among soil depths in a subtropical mixed forest. Soil Biol. Biochem. 2017, 109, 135–144. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, X.; Zhu, L.; Ahmed, N.; Gao, Y. Laboratory investigation on the effects of root-secreted organic acids on carbon and nitrogen mineralization in alpine soils caused by invasive shrubs. J. Environ. Chem. Eng. 2025, 13, 117271. [Google Scholar] [CrossRef]
- Pati, S.S.; Sahu, S.K. CO2 evolution and enzyme activities (dehydrogenase, protease and amylase) of fly ash amended soil in the presence and absence of earthworms (Drawida willsi Michaelsen) under laboratory conditions. Geoderma 2004, 118, 289–301. [Google Scholar] [CrossRef]
- Singh, R.P.; Sharma, B.; Sarkar, A.; Sengupta, C.; Singh, P.; Ibrahim, M.H. Biological responses of agricultural soils to fly-ash amendment. Rev. Environ. Contam. Toxicol. 2014, 232, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.H.; Wong, J. Effects of fly ash on soil microbial activity. Enviro. Pollut. Ser. A Ecol. Biol. 1986, 40, 127–144. [Google Scholar] [CrossRef]
- Lim, S.S.; Choi, W.J. Changes in microbial biomass, CH4 and CO2 emissions, and soil carbon content by fly ash co-applied with organic inputs with contrasting substrate quality under changing water regimes. Soil Biol. Biochem. 2014, 68, 494–502. [Google Scholar] [CrossRef]
- Curtin, D.; Beare, M.H.; Hernandez-Ramirez, G. Temperature and moisture effects on microbial biomass and soil organic matter mineralization. Soil Sci. Soc. Am. J. 2012, 76, 2055–2067. [Google Scholar] [CrossRef]
- Yeledhalli, N.; Prakash, S.; Gurumurthy, S.; Ravi, M. Coal fly ash as modifier of physico-chemical and biological properties of soil. Karn. J. Agric. Sci. 2007, 20, 531–534. [Google Scholar]
- Kudeyarov, V.N. Soil respiration and carbon sequestration: A review. Euras. Soil Sci. 2023, 56, 1191–1200. [Google Scholar] [CrossRef]
- Fang, H.; Dong, B.; Yan, H.; Tang, F.; Wang, B.; Yu, Y. Effect of vegetation of transgenic Bt rice lines and their straw amendment on soil enzymes, respiration, functional diversity and community structure of soil microorganisms under field conditions. J. Environ. Sci. 2012, 24, 1259–1270. [Google Scholar] [CrossRef]
- Schutter, M.; Fuhrmann, J. Soil microbial community responses to fly ash amendment as revealed by analyses of whole soils and bacterial isolates. Soil Biol. Biochem. 2001, 33, 1947–1958. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Fan, Z.; Shi, J.; Huo, C.; Zhang, Z.; Wang, X. Microbial associations with soil organic carbon pool composition and stabilization in eroding landscapes. CATENA 2025, 258, 109302. [Google Scholar] [CrossRef]
- Tang, J.; Liu, E.; Li, Y.; Tang, Y.; Tian, Y.; Du, S.; Li, H.; Wan, L.; Zhang, Q. Afforestation promotes soil organic carbon and soil microbial residual carbon accrual in a seasonally flooded marshland. Forests 2024, 15, 1542. [Google Scholar] [CrossRef]
- GB 1561-2018; Soil Environment Quality Risk Control Standard for Soil Contamination of Agriculture Land. Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2018. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/trhj/201807/t20180703_446029.shtml (accessed on 26 September 2025).




| pH(H2O) | SBD g cm−3 | CEC (cmol(+) kg−1) | TOC g kg−1 | WSOC mg kg−1 | MBC mg kg−1 | Mechanical Composition (%) | ||
|---|---|---|---|---|---|---|---|---|
| 2–0.02 mm | 0.02–0.002 mm | <0.002 mm | ||||||
| 5.09 | 1.45 | 7.97 | 13.12 | 37.17 | 236.79 | 62.85 | 14.27 | 22.88 |
| pH(H2O) | WSOC mg kg−1 | TN g kg−1 | TP g kg−1 | TK g kg−1 | Fe g kg−1 | Mn g kg−1 | Ca g kg−1 | Mg g kg−1 | S g kg−1 |
|---|---|---|---|---|---|---|---|---|---|
| 8.02 | 17.9 | 0.03 | 0.34 | 6.2 | 3.63 | 0.19 | 158.0 | 9.34 | 47.18 |
| B mg kg−1 | Mo mg kg−1 | Na g kg−1 | Cu mg kg−1 | Pb mg kg−1 | Zn mg kg−1 | Cd mg kg−1 | Cr mg kg−1 | As mg kg−1 | Hg mg kg−1 |
| 77.7 | 1.62 | 3.63 | 62.0 | 11.0 | 73.0 | 0.25 | 60.0 | 6.01 | 0.16 |
| Fly Ash Application Rate | Moisture Condition | C0 | k | R2 | p |
|---|---|---|---|---|---|
| 0% | 40% WHC | 13.85 | 0.054 | 0.9966 | <0.01 |
| 70% WHC | 18.70 | 0.041 | 0.9960 | <0.01 | |
| 100%WHC | 15.40 | 0.053 | 0.9972 | <0.01 | |
| 2.5% | 40% WHC | 15.42 | 0.058 | 0.9964 | <0.01 |
| 70% WHC | 17.13 | 0.050 | 0.9961 | <0.01 | |
| 100% WHC | 15.31 | 0.072 | 0.9934 | <0.01 | |
| 7.5% | 40% WHC | 13.59 | 0.072 | 0.9933 | <0.01 |
| 70% WHC | 18.99 | 0.058 | 0.9953 | <0.01 | |
| 100%WHC | 17.74 | 0.064 | 0.9887 | <0.01 | |
| 15% | 40% WHC | 16.18 | 0.081 | 0.9877 | <0.01 |
| 70% WHC | 18.56 | 0.079 | 0.9822 | <0.01 | |
| 100%WHC | 17.63 | 0.091 | 0.9633 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, M.; Jiang, H.; Zou, X.; Ma, D.; Cheng, J.; Jiang, X.; Yuan, Z.; Huang, B. Effects of Coal Fly Ash Addition on the Carbon Mineralization of Agricultural Soil Under Different Moisture Conditions. Water 2025, 17, 2912. https://doi.org/10.3390/w17192912
Rao M, Jiang H, Zou X, Ma D, Cheng J, Jiang X, Yuan Z, Huang B. Effects of Coal Fly Ash Addition on the Carbon Mineralization of Agricultural Soil Under Different Moisture Conditions. Water. 2025; 17(19):2912. https://doi.org/10.3390/w17192912
Chicago/Turabian StyleRao, Mumin, Heng Jiang, Xiangbo Zou, Dequn Ma, Jiong Cheng, Xinyu Jiang, Zaijian Yuan, and Bin Huang. 2025. "Effects of Coal Fly Ash Addition on the Carbon Mineralization of Agricultural Soil Under Different Moisture Conditions" Water 17, no. 19: 2912. https://doi.org/10.3390/w17192912
APA StyleRao, M., Jiang, H., Zou, X., Ma, D., Cheng, J., Jiang, X., Yuan, Z., & Huang, B. (2025). Effects of Coal Fly Ash Addition on the Carbon Mineralization of Agricultural Soil Under Different Moisture Conditions. Water, 17(19), 2912. https://doi.org/10.3390/w17192912







