Xylem Hydraulic Characteristics and Soil Water Content Drive Drought Sensitivity Differences in Afforestation Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location and Materials
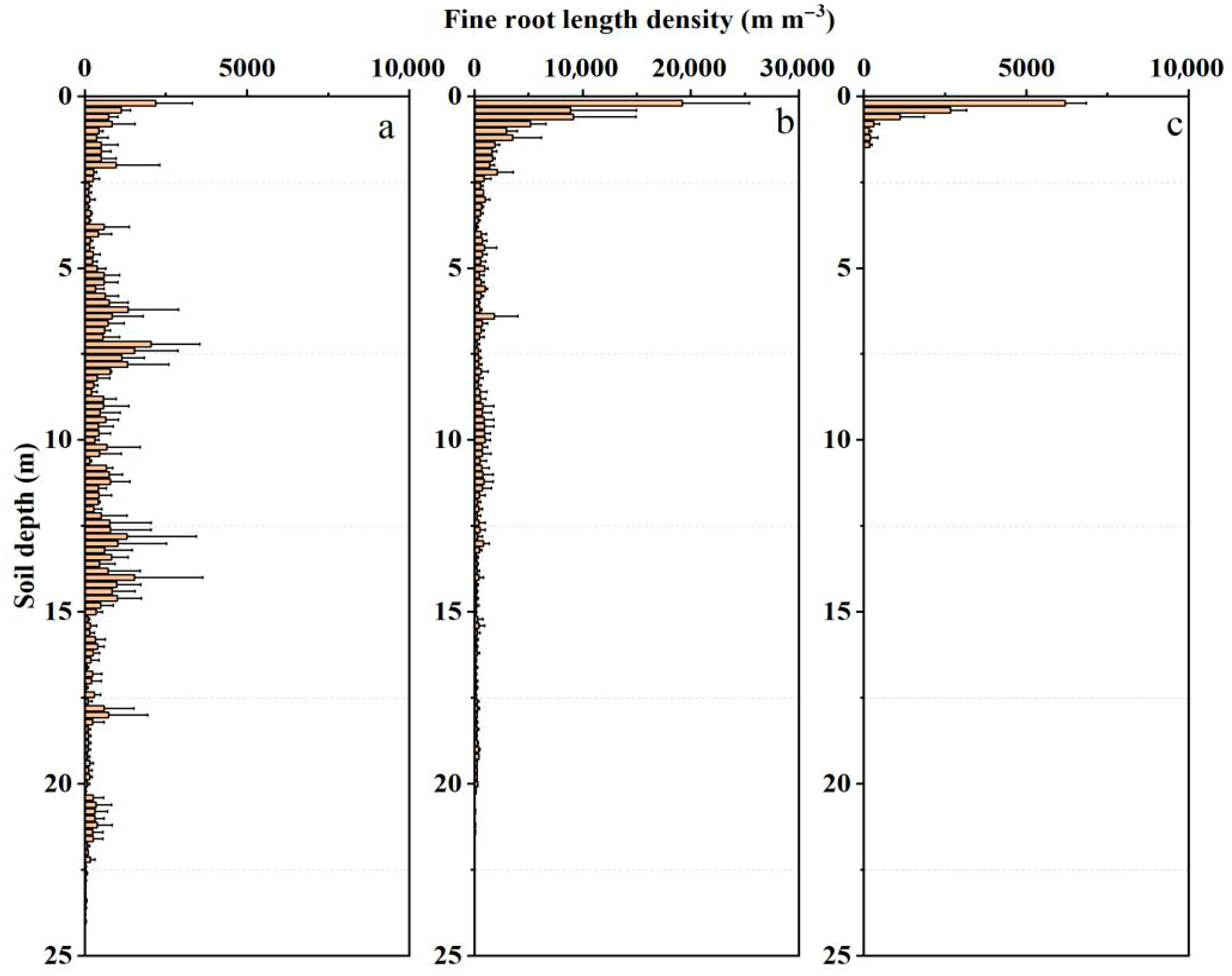
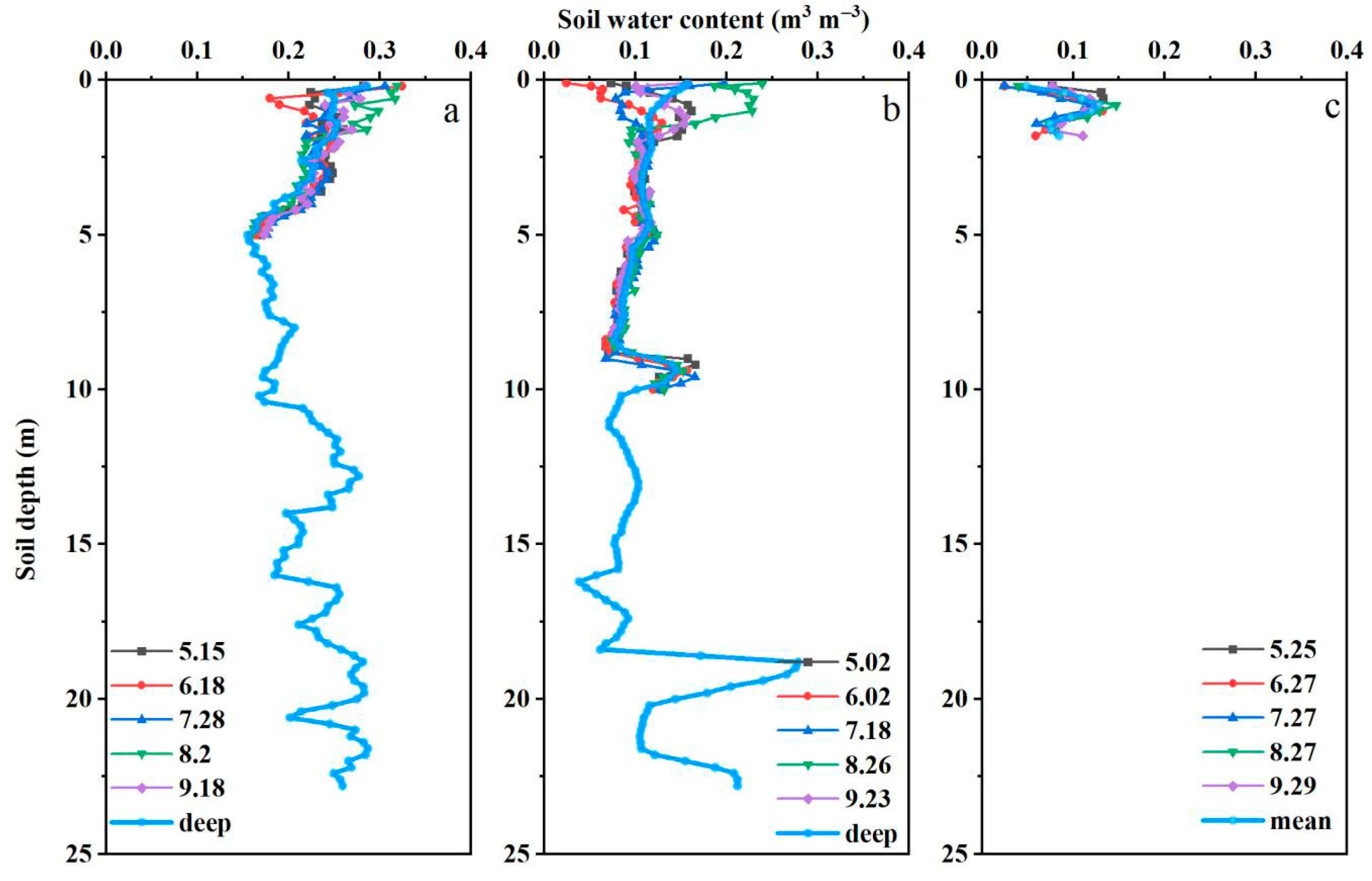
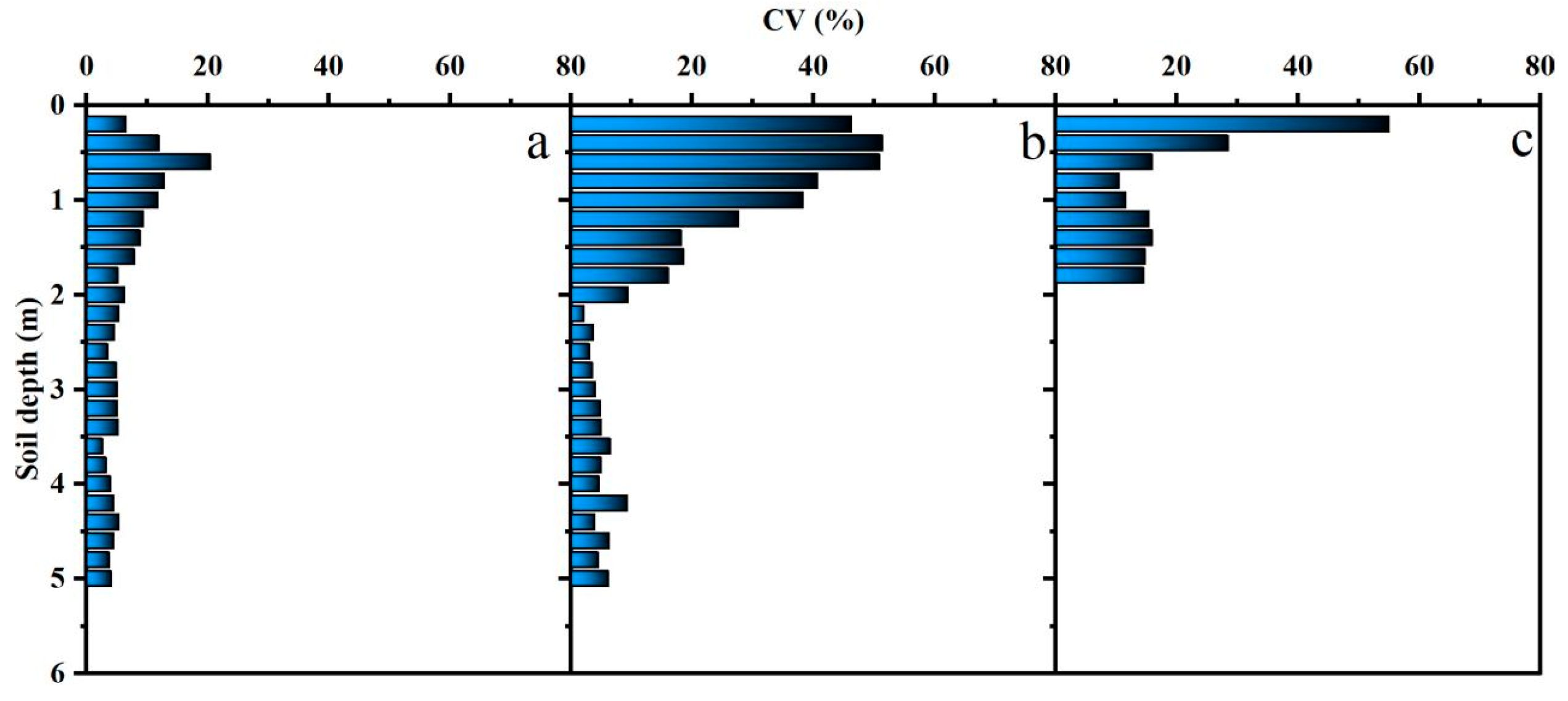
2.2. Soil Water Content, Texture, and Fine Roots
2.3. Leaf Area Index and Leaf Water Potential
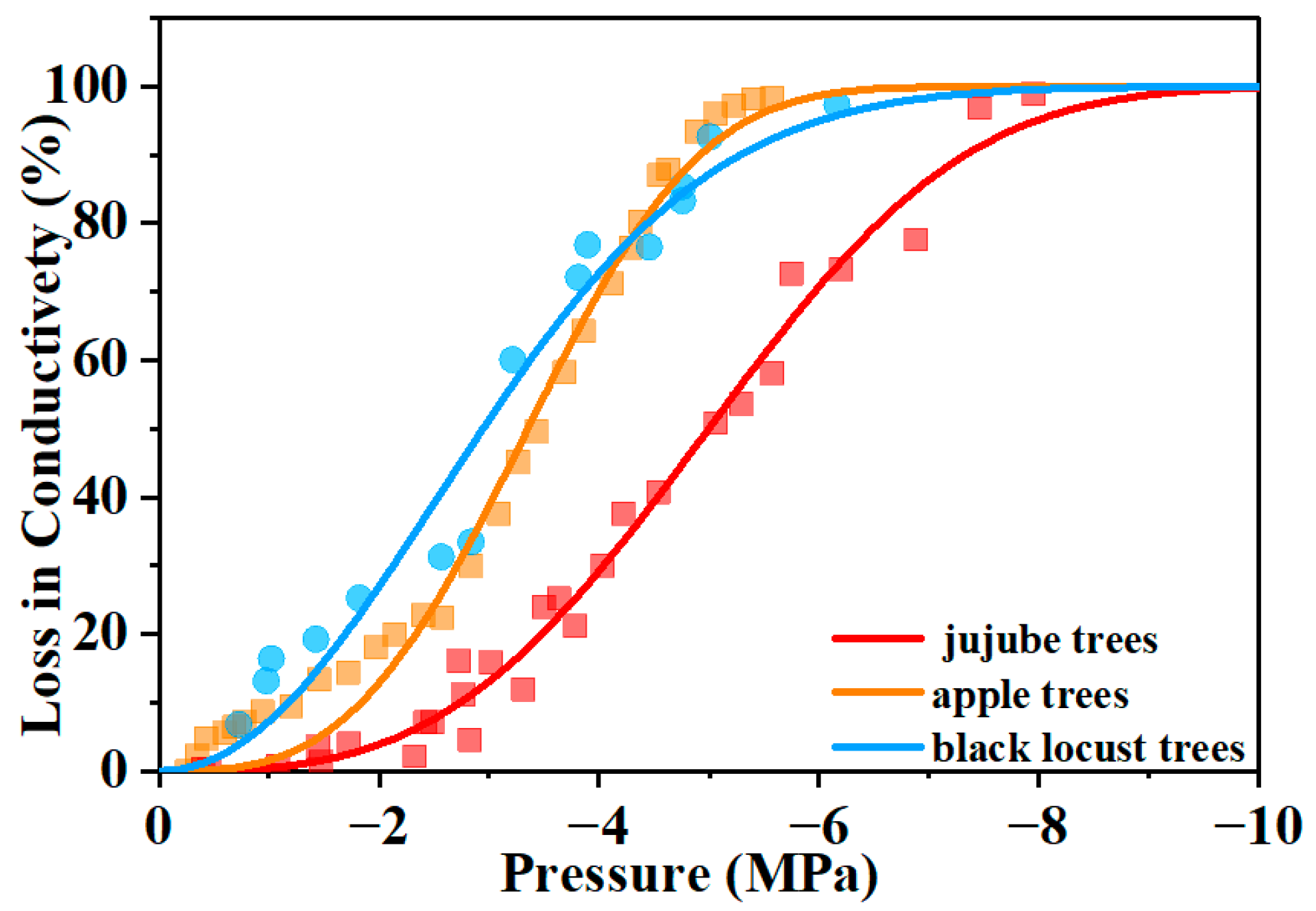
2.4. Xylem Conductivity and Embolism Vulnerability Curves
2.5. Sap Flow and Meteorological Data
2.6. Data Analysis
3. Results
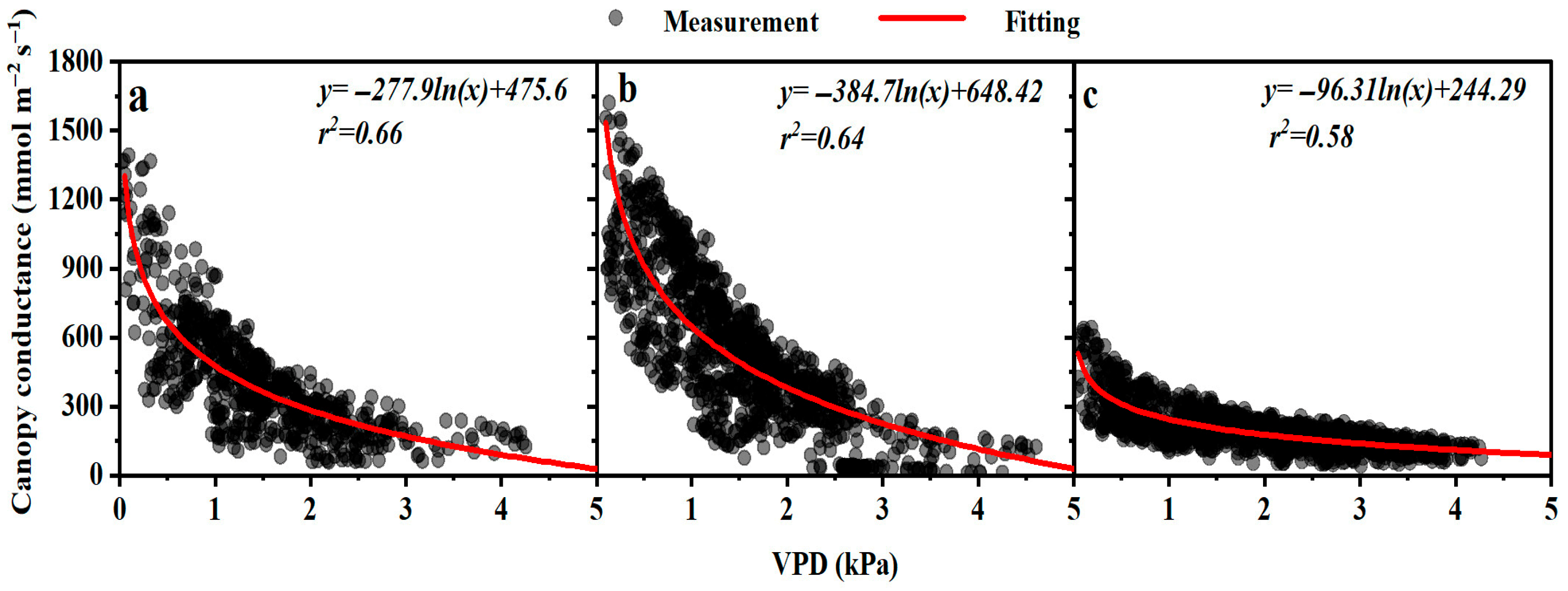
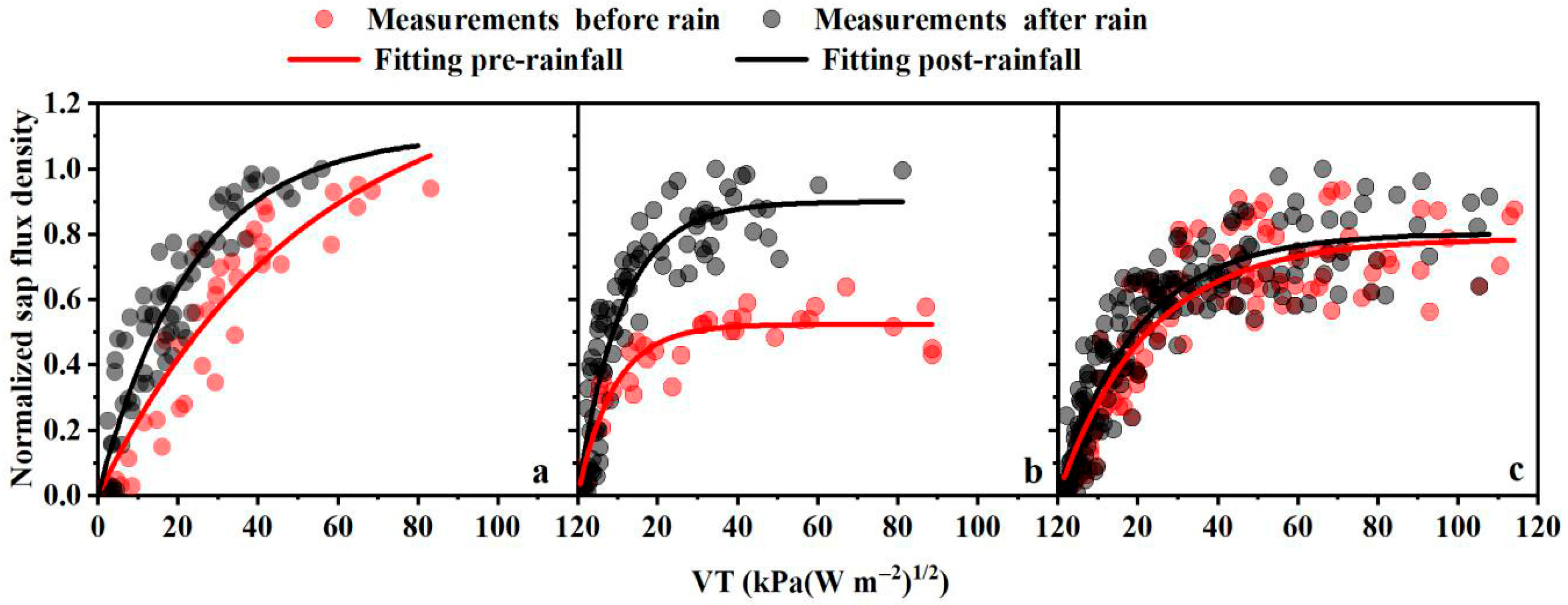
3.1. Sensitivity of Canopy Conductance to VPD and Response of Sap Flow to Precipitation
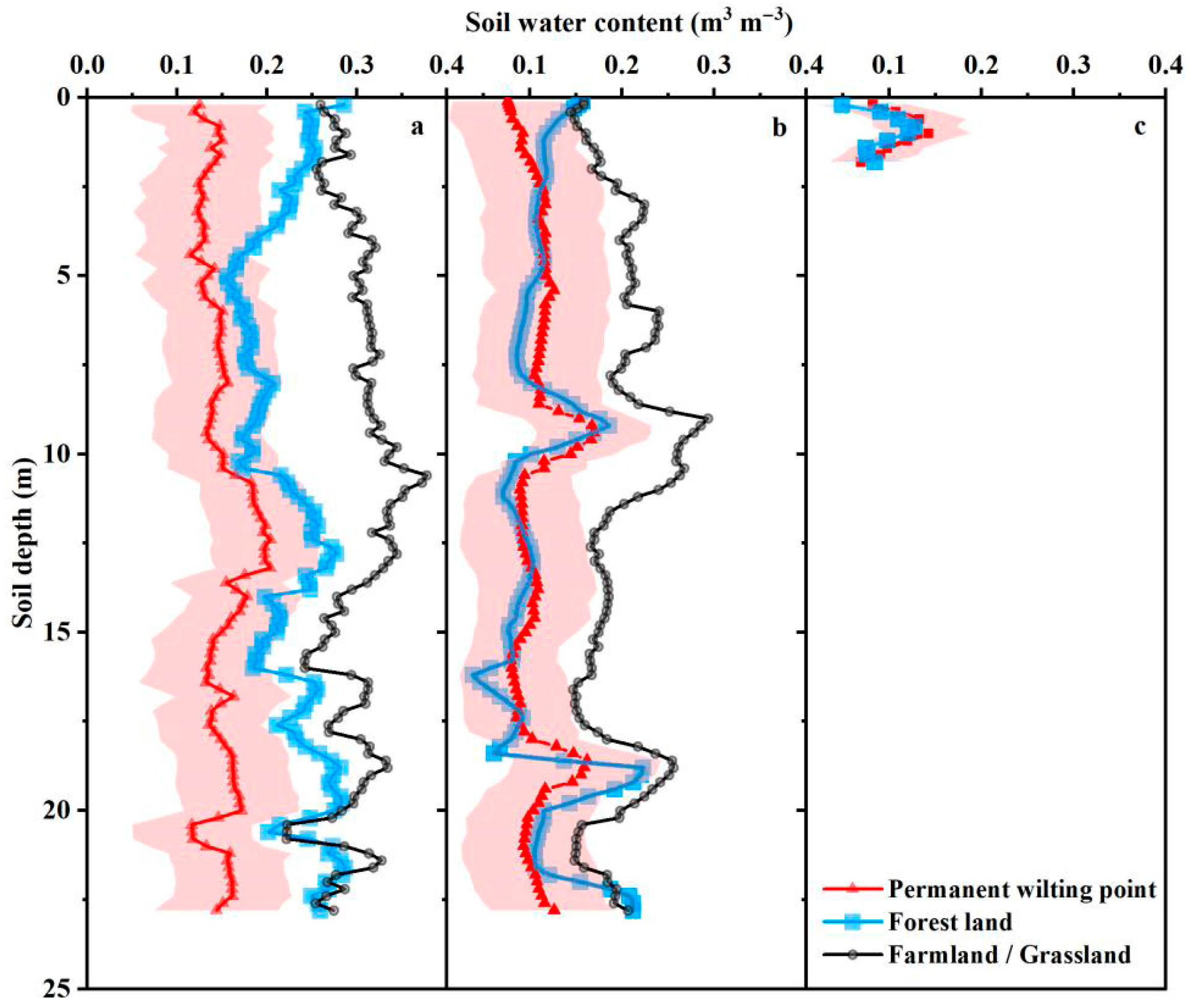
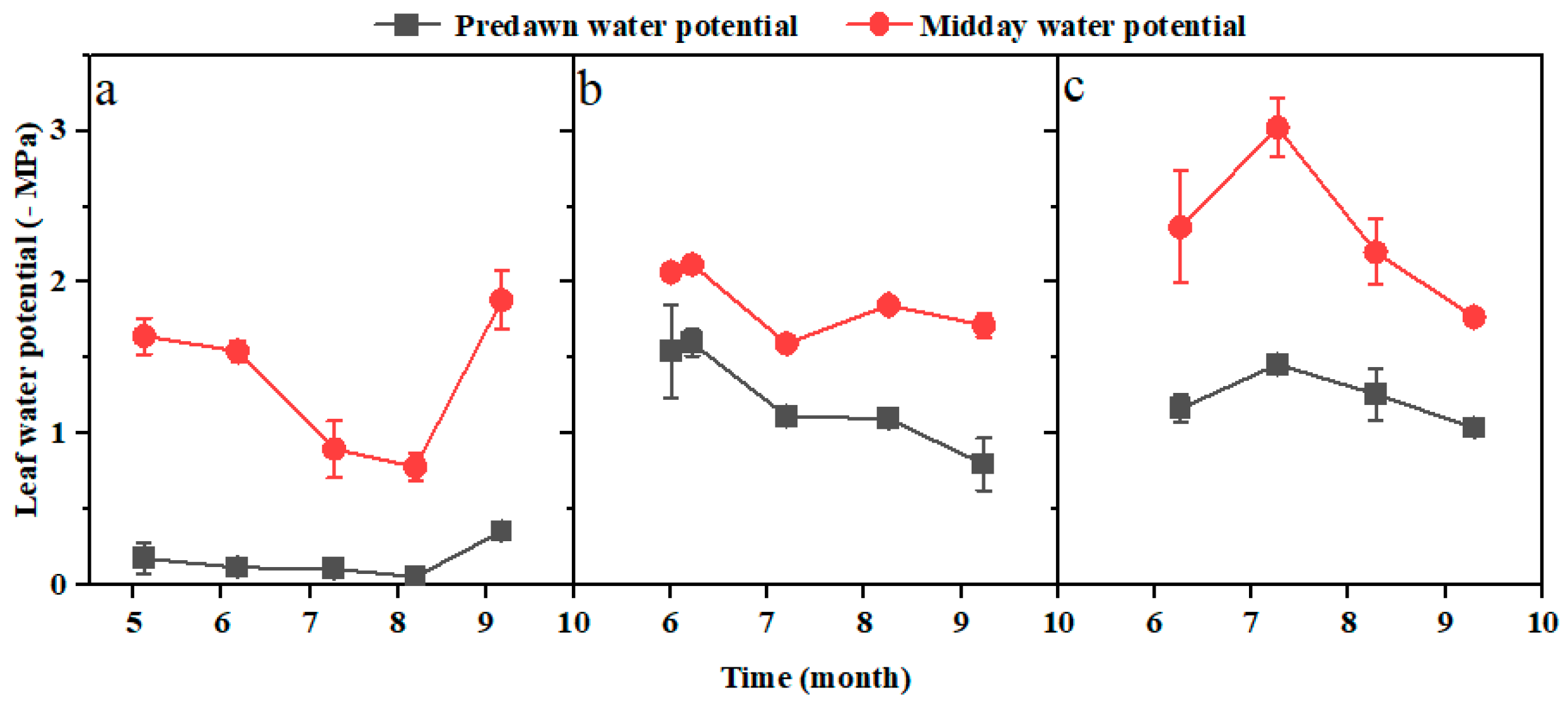
3.2. Soil Water Content and Physiological Characteristics in the Rhizosphere
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VPD | Vapor pressure deficit |
| Gc | Canopy conductance |
| Gcref | Reference canopy conductivity |
| VT | Composite function of solar radiation and saturated vapor pressure deficit |
| NFD | Standardized sap flow |
| PLC | Percentage loss of conductivity |
Appendix A
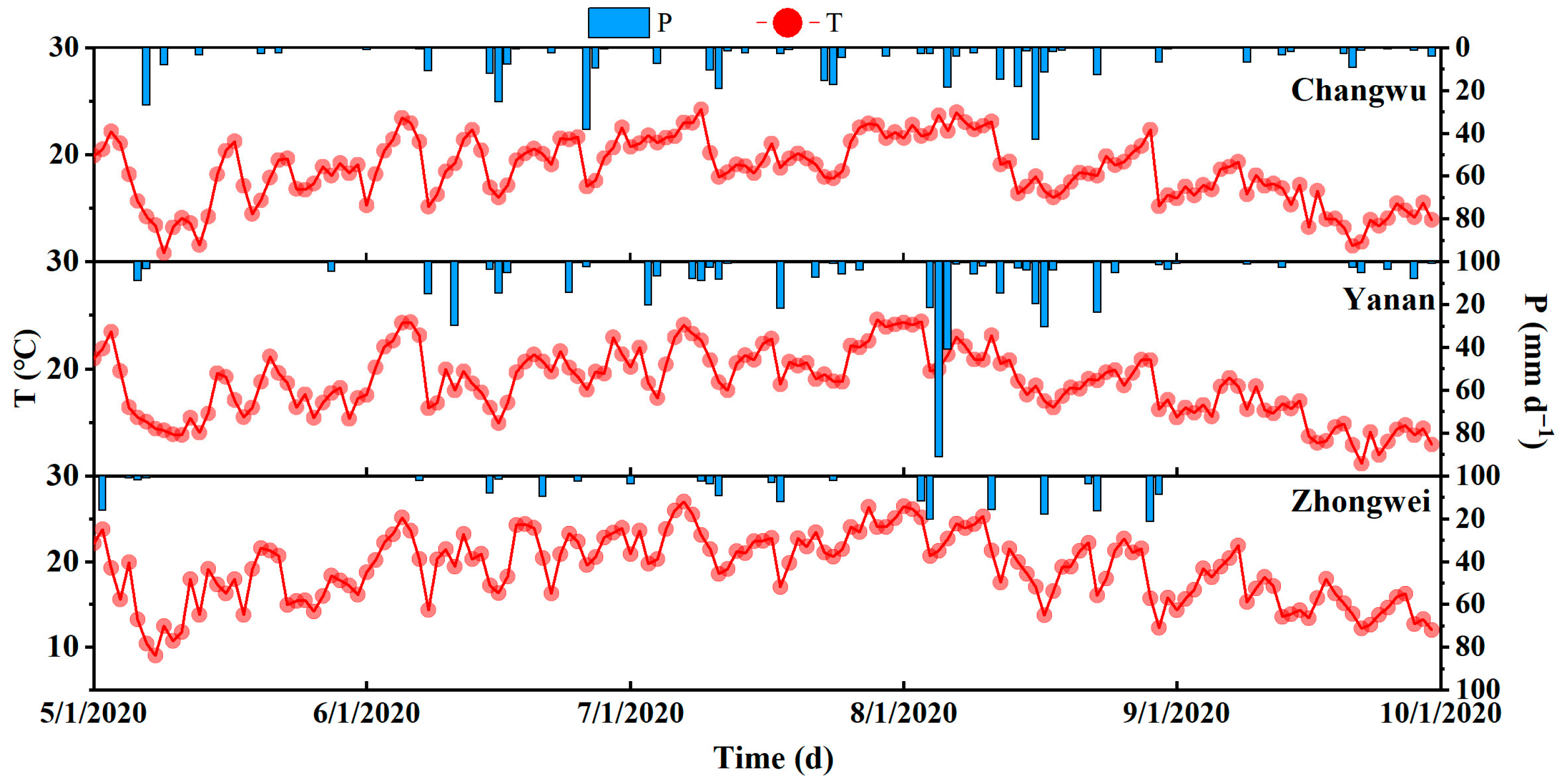
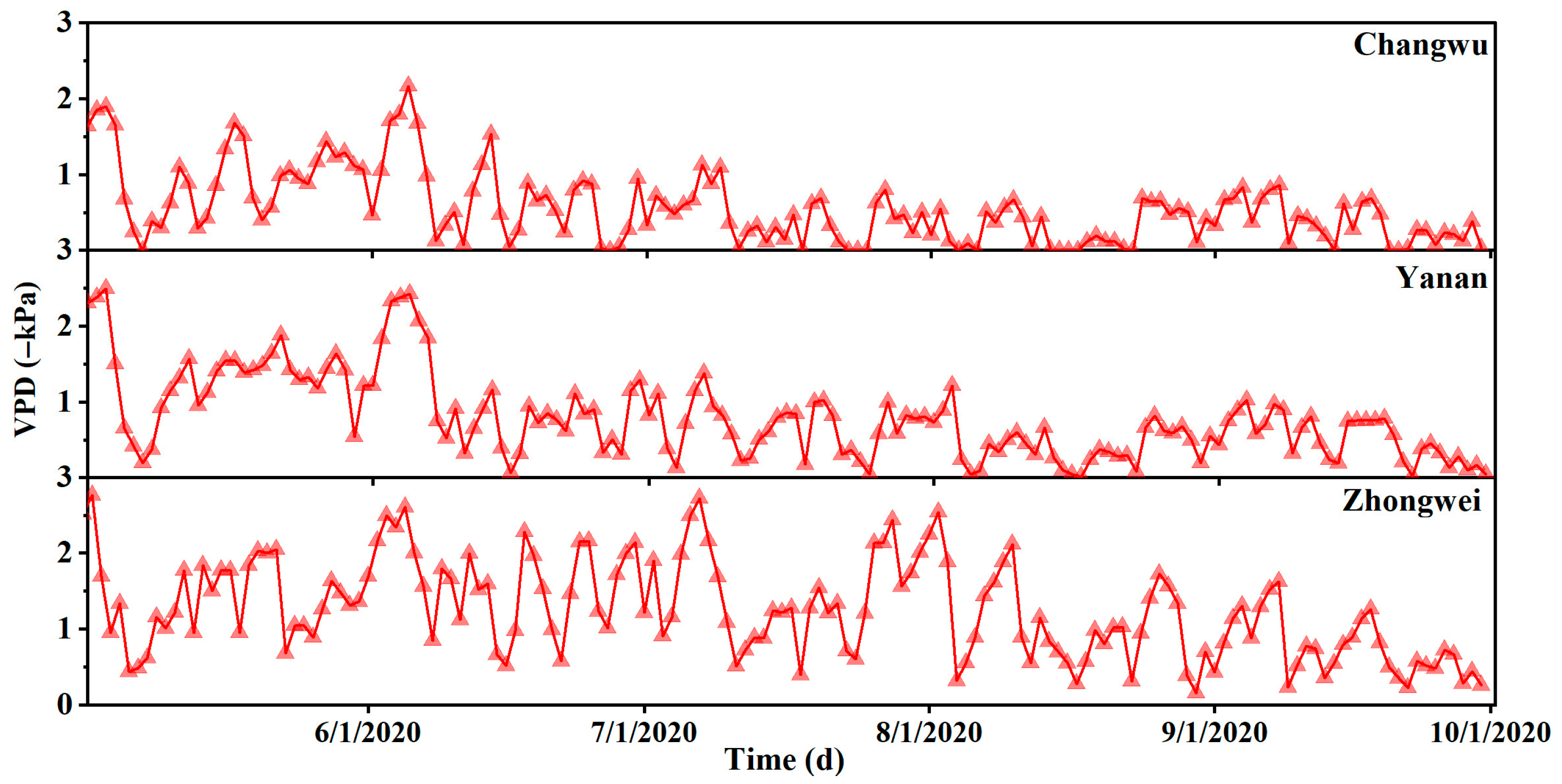
Appendix B

References
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Huang, L.; He, B.; Chen, A.F.; Wang, H.Y.; Liu, J.J.; Lu, A.F.; Chen, Z.Y. Drought dominates the interannual variability in global terrestrial net primary production by controlling semi-arid ecosystems. Sci. Rep. 2016, 6, 24639. [Google Scholar] [CrossRef]
- Xu, L.; Chen, N.C.; Zhang, X. Global drought trends under 1.5 and 2 degrees C warming. Int. J. Clim. 2019, 39, 2375–2385. [Google Scholar] [CrossRef]
- Pan, Y.D.; Birdsey, R.A.; Fang, J.Y.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Feng, X.M.; Fu, B.J.; Piao, S.; Wang, S.H.; Ciais, P.; Zeng, Z.Z.; Lu, Y.H.; Zeng, Y.; Li, Y.; Jiang, X.H.; et al. Revegetation in China’s Loess Plateau is approaching sustainable water resource limits. Nat. Clim. Change 2016, 6, 1019–1022. [Google Scholar] [CrossRef]
- Lu, F.; Hu, H.; Sun, W.; Zhu, J.; Liu, G.; Zhou, W.; Zhang, Q.; Shi, P.; Liu, X.; Wu, X.; et al. Effects of national ecological restoration projects on carbon sequestration in China from 2001 to 2010. Proc. Natl. Acad. Sci. USA 2018, 115, 4039–4044. [Google Scholar] [CrossRef]
- Liu, M.X.; Xu, X.L.; Sun, A.Y. New drought index indicates that land surface changes might have enhanced drying tendencies over the Loess Plateau. Ecol. Indic. 2018, 89, 716–724. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Jia, X.X.; Shao, M.G.; Zhang, C.C.; Li, X.D.; Ma, C.K. Sap flow of black locust in response to short-term drought in southern Loess Plateau of China. Sci. Rep. 2018, 8, 6222. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.X.; Shao, M.A.; Zhu, Y.J.; Luo, Y. Soil moisture decline due to afforestation across the Loess Plateau, China. J. Hydrol. 2017, 546, 113–122. [Google Scholar] [CrossRef]
- Zhao, C.; Jia, X.; Gongadze, K.; Shao, M.; Wu, L.; Zhu, Y. Permanent dry soil layer a critical control on soil desiccation on China’s Loess Plateau. Sci. Rep. 2019, 9, 3296. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.P.; Ibanez, I.; D’Orangeville, L.; Hanson, P.J.; Ryan, M.G.; McDowell, N.G. A belowground perspective on the drought sensitivity of forests: Towards improved understanding and simulation. For. Ecol. Manag. 2016, 380, 309–320. [Google Scholar] [CrossRef]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Wolf, A.; Anderegg, W.R.L.; Pacala, S.W. Optimal stomatal behavior with competition for water and risk of hydraulic impairment. Proc. Natl. Acad. Sci. USA 2016, 113, E7222–E7230. [Google Scholar] [CrossRef]
- Fiorin, L.; Brodribb, T.J.; Anfodillo, T. Transport efficiency through uniformity: Organization of veins and stomata in angiosperm leaves. New Phytol. 2016, 209, 216–227. [Google Scholar] [CrossRef]
- Mitchell, P.J.; O’Grady, A.P.; Tissue, D.T.; White, D.A.; Ottenschlaeger, M.L.; Pinkard, E.A. Drought response strategies define the relative contributions of hydraulic dysfunction and carbohydrate depletion during tree mortality. New Phytol. 2013, 197, 862–872. [Google Scholar] [CrossRef]
- Sperry, J.S. Hydraulic constraints on plant gas exchange. Agric. For. Meteorol. 2000, 104, 13–23. [Google Scholar] [CrossRef]
- Oren, R.; Sperry, J.S.; Katul, G.G.; Pataki, D.E.; Ewers, B.E.; Phillips, N.; Schafer, K.V.R. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 1999, 22, 1515–1526. [Google Scholar] [CrossRef]
- Naithani, K.J.; Ewers, B.E.; Pendall, E. Sap flux-scaled transpiration and stomatal conductance response to soil and atmospheric drought in a semi-arid sagebrush ecosystem. J. Hydrol. 2012, 464, 176–185. [Google Scholar] [CrossRef]
- McCulloh, K.A.; Woodruff, D.R. Linking stomatal sensitivity and whole-tree hydraulic architecture. Tree Physiol. 2012, 32, 369–372. [Google Scholar] [CrossRef]
- Bush, S.E.; Pataki, D.E.; Hultine, K.R.; West, A.G.; Sperry, J.S.; Ehleringer, J.R. Wood anatomy constrains stomatal responses to atmospheric vapor pressure deficit in irrigated, urban trees. Oecologia 2008, 156, 13–20. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Litvak, E.; McCarthy, H.R.; Pataki, D.E. Transpiration sensitivity of urban trees in a semi-arid climate is constrained by xylem vulnerability to cavitation. Tree Physiol. 2012, 32, 373–388. [Google Scholar] [CrossRef]
- Addington, R.N.; Mitchell, R.J.; Oren, R.; Donovan, L.A. Stomatal sensitivity to vapor pressure deficit and its relationship to hydraulic conductance in Pinus palustris. Tree Physiol. 2004, 24, 561–569. [Google Scholar] [CrossRef]
- Siddiq, Z.; Chen, Y.-J.; Zhang, Y.-J.; Zhang, J.-L.; Cao, K.-F. More sensitive response of crown conductance to VPD and larger water consumption in tropical evergreen than in deciduous broadleaf timber trees. Agric. For. Meteorol. 2017, 247, 399–407. [Google Scholar] [CrossRef]
- Oren, R.; Sperry, J.S.; Ewers, B.E.; Pataki, D.E.; Phillips, N.; Megonigal, J.P. Sensitivity of mean canopy stomatal conductance to vapor pressure deficit in a flooded Taxodium distichum L. forest: Hydraulic and non-hydraulic effects. Oecologia 2001, 126, 21–29. [Google Scholar] [CrossRef]
- Klein, T.; Shpringer, I.; Fikler, B.; Elbaz, G.; Cohen, S.; Yakir, D. Relationships between stomatal regulation, water-use, and water-use efficiency of two coexisting key Mediterranean tree species. For. Ecol. Manag. 2013, 302, 34–42. [Google Scholar] [CrossRef]
- Song, L.N.; Zhu, J.J.; Zheng, X.; Wang, K.; Zhang, J.X.; Hao, G.Y.; Wang, G.C.; Liu, J.H. Comparison of canopy transpiration between Pinus sylvestris var. mongolica and Pinus tabuliformis plantations in a semiarid sandy region of Northeast China. Agric. For. Meteorol. 2022, 314, 108784. [Google Scholar] [CrossRef]
- Igarashi, Y.; Kumagai, T.; Yoshifuji, N.; Sato, T.; Tanaka, N.; Tanaka, K.; Suzuki, M.; Tantasirin, C. Environmental control of canopy stomatal conductance in a tropical deciduous forest in northern Thailand. Agric. For. Meteorol. 2015, 202, 1–10. [Google Scholar] [CrossRef]
- Du, S.; Wang, Y.-L.; Kume, T.; Zhang, J.-G.; Otsuki, K.; Yamanaka, N.; Liu, G.-B. Sapflow characteristics and climatic responses in three forest species in the semiarid Loess Plateau region of China. Agric. For. Meteorol. 2011, 151, 1–10. [Google Scholar] [CrossRef]
- Lyu, J.L.; He, Q.Y.; Chen, Q.W.; Cheng, R.R.; Li, G.Q.; Otsuki, K.; Yamanaka, N.; Du, S. Distinct transpiration characteristics of black locust plantations acclimated to semiarid and subhumid sites in the Loess Plateau, China. Agric. Water Manag. 2022, 262, 107402. [Google Scholar] [CrossRef]
- Weithmann, G.; Schuldt, B.; Link, R.M.; Heil, D.; Hoeber, S.; John, H.; Muller-Haubold, H.; Schuller, L.M.; Schumann, K.; Leuschner, C. Leaf trait modification in European beech trees in response to climatic and edaphic drought. Plant Biol. 2021, 24, 1272–1286. [Google Scholar] [CrossRef] [PubMed]
- Schuldt, B.; Knutzen, F.; Delzon, S.; Jansen, S.; Muller-Haubold, H.; Burlett, R.; Clough, Y.; Leuschner, C. How adaptable is the hydraulic system of European beech in the face of climate change-related precipitation reduction? New Phytol. 2016, 210, 443–458. [Google Scholar] [CrossRef]
- Kume, T.; Takizawa, H.; Yoshifuji, N.; Tanaka, K.; Tantasirin, C.; Tanaka, N.; Suzuki, M. Impact of soil drought on sap flow and water status of evergreen trees in a tropical monsoon forest in northern Thailand. For. Ecol. Manag. 2007, 238, 220–230. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Shangguan, Z. Rapid response of the carbon balance strategy in Robinia pseudoacacia and Amorpha fruticosa to recurrent drought. Environ. Exp. Bot. 2017, 138, 46–56. [Google Scholar] [CrossRef]
- Weber, P.; Bugmann, H.; Pluess, A.R.; Walthert, L.; Rigling, A. Drought response and changing mean sensitivity of European beech close to the dry distribution limit. Trees 2012, 27, 171–181. [Google Scholar] [CrossRef]
- He, Q.-Y.; Yan, M.-J.; Miyazawa, Y.; Chen, Q.-W.; Cheng, R.-R.; Otsuki, K.; Yamanaka, N.; Du, S. Sap flow changes and climatic responses over multiple-year treatment of rainfall exclusion in a sub-humid black locust plantation. For. Ecol. Manag. 2020, 457, 117730. [Google Scholar] [CrossRef]
- Yan, M.J.; Zhang, J.G.; He, Q.Y.; Shi, W.Y.; Otsuki, K.; Yamanaka, N.; Du, S. Sapflow-Based Stand Transpiration in a Semiarid Natural Oak Forest on China’s Loess Plateau. Forests 2016, 7, 227. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Chen, Y. Sap flow characteristics and physiological adjustments of two dominant tree species in pure and mixed plantations in the semi-arid Loess Plateau of China. J. Arid Land 2018, 10, 833–849. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, L.; Zhang, S.; Cai, J.; Tyree, M.T. Water relations of Robinia pseudoacacia L.: Do vessels cavitate and refill diurnally or are R-shaped curves invalid in Robinia? Plant Cell Environ. 2014, 37, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Feng, F.; Wang, Y.; Tyree, M.T. Do nano-particles cause recalcitrant vulnerability curves in Robinia? Testing with a four-cuvette Cochard rotor and with water extraction curves. Tree Physiol. 2019, 39, 156–165. [Google Scholar] [CrossRef]
- Oogathoo, S.; Houle, D.; Duchesne, L.; Kneeshaw, D. Vapour pressure deficit and solar radiation are the major drivers of transpiration of balsam fir and black spruce tree species in humid boreal regions, even during a short-term drought. Agric. For. Meteorol. 2020, 291, 108063. [Google Scholar] [CrossRef]
- Zhang, H.P.; Simmonds, L.P.; Morison, J.I.L.; Payne, D. Estimation of transpiration by single trees: Comparison of sap flow measurements with a combination equation. Agric. For. Meteorol. 1997, 87, 155–169. [Google Scholar] [CrossRef]
- Bovard, B.D.; Curtis, P.S.; Vogel, C.S.; Su, H.B.; Schmid, H.P. Environmental controls on sap flow in a northern hardwood forest. Tree Physiol. 2005, 25, 31–38. [Google Scholar] [CrossRef]
- Fonti, P.; Heller, O.; Cherubini, P.; Rigling, A.; Arend, M. Wood anatomical responses of oak saplings exposed to air warming and soil drought. Plant Biol. 2013, 15, 210–219. [Google Scholar] [CrossRef]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Moura, J.; Bonine, C.A.V.; Viana, J.D.F.; Dornelas, M.C.; Mazzafera, P. Abiotic and Biotic Stresses and Changes in the Lignin Content and Composition in Plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Zhang, L.; Merlin, I.; Pascal, S.; Bert, P.F.; Domergue, F.; Gambetta, G.A. Drought activates MYB41 orthologs and induces suberization of grapevine fine roots. Plant Direct 2020, 4, e00278. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E. Water uptake by roots: Effects of water deficit. J. Exp. Bot. 2000, 51, 1531–1542. [Google Scholar] [CrossRef]
- Cuneo, I.F.; Knipfer, T.; Brodersen, C.R.; McElrone, A.J. Mechanical Failure of Fine Root Cortical Cells Initiates Plant Hydraulic Decline during Drought. Plant Physiol. 2016, 172, 1669–1678. [Google Scholar] [CrossRef]
- Mackay, D.S.; Savoy, P.R.; Grossiord, C.; Tai, X.; Pleban, J.R.; Wang, D.R.; McDowell, N.G.; Adams, H.D.; Sperry, J.S. Conifers depend on established roots during drought: Results from a coupled model of carbon allocation and hydraulics. New Phytol. 2020, 225, 679–692. [Google Scholar] [CrossRef]
- Carminati, A.; Javaux, M. Soil Rather Than Xylem Vulnerability Controls Stomatal Response to Drought. Trends Plant Sci. 2020, 25, 868–880. [Google Scholar] [CrossRef]
- Cai, G.; Ahmed, M.A.; Abdalla, M.; Carminati, A. Root hydraulic phenotypes impacting water uptake in drying soils. Plant Cell Environ. 2022, 45, 650–663. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; Lopez, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Klein, T.; Bartlett, M.; Sack, L.; Pellegrini, A.F.A.; Choat, B.; Jansen, S. Meta-analysis reveals that hydraulic traits explain cross-species patterns of drought-induced tree mortality across the globe. Proc. Natl. Acad. Sci. USA 2016, 113, 5024–5029. [Google Scholar] [CrossRef]
- McDowell, N.G.; Sevanto, S. The mechanisms of carbon starvation: How, when, or does it even occur at all? New Phytol. 2010, 186, 264–266. [Google Scholar] [CrossRef]
- Fan, Z.-X.; Zhang, S.-B.; Hao, G.-Y.; Ferry Slik, J.W.; Cao, K.-F. Hydraulic conductivity traits predict growth rates and adult stature of 40 Asian tropical tree species better than wood density. J. Ecol. 2012, 100, 732–741. [Google Scholar] [CrossRef]
- Zhang, J.L.; Cao, K.F. Stem hydraulics mediates leaf water status, carbon gain, nutrient use efficiencies and plant growth rates across dipterocarp species. Funct. Ecol. 2009, 23, 658–667. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Change 2012, 3, 52–58. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, L.; Wu, P.; Ji, P.; Sheffield, J.; Zhang, M. Anthropogenic shift towards higher risk of flash drought over China. Nat. Commun. 2019, 10, 4661. [Google Scholar] [CrossRef]
- Tomasella, M.; Beikircher, B.; Haberle, K.H.; Hesse, B.; Kallenbach, C.; Matyssek, R.; Mayr, S. Acclimation of branch and leaf hydraulics in adult Fagus sylvatica and Picea abies in a forest through-fall exclusion experiment. Tree Physiol. 2018, 38, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Tng, D.Y.P.; Apgaua, D.M.G.; Ishida, Y.F.; Mencuccini, M.; Lloyd, J.; Laurance, W.F.; Laurance, S.G.W. Rainforest trees respond to drought by modifying their hydraulic architecture. Ecol. Evol. 2018, 8, 12479–12491. [Google Scholar] [CrossRef] [PubMed]

| Species | Locations | Slope | D (ha−1) | Age (Years) | DBH (cm) | H (m) | MLAI (m−2 m−2) |
|---|---|---|---|---|---|---|---|
| apple tree | Changwu (CW) | 0° | 952 | 22 | 20.0 ± 0.3 | 3.0 ± 0.2 | 2.38 |
| black locust tree | Yan’an (YA) | 35° (southwest) | 2500 | 17 | 10.2 ± 0.5 | 9.6 ± 0.2 | 3.83 |
| jujube tree | Zhongwei (ZW) | 0° | 417 | 15 | 10.6 ± 0.5 | 4.3 ± 0.2 | 0.98 |
| Species | Pre-Rainfall | Post-Rainfall | Difference Between Coefficients |
|---|---|---|---|
| apple trees | a = 1.298 | a = 1.107 | p < 0.05 |
| b = 0.0194 | b = 0.0426 | p < 0.01 | |
| r2 = 0.8910 | r2 = 0.8436 | ||
| p < 0.01 | p < 0.01 | ||
| black locust trees | a = 0.523 | a = 0.899 | p < 0.01 |
| b = 0.109 | b = 0.0914 | p < 0.01 | |
| r2 = 0.8016 | r2 = 0.8551 | ||
| p < 0.01 | p < 0.01 | ||
| jujube trees | a = 0.786 | a = 0.802 | p < 0.01 |
| b = 0.045 | b = 0.051 | Not significant | |
| r2 = 0.8050 | r2 = 0.8764 | ||
| p < 0.01 | p < 0.01 |
| Species | Apple Trees | Black Locust Trees | Jujube Trees |
|---|---|---|---|
| Xylem specific conductivity (Ks) (kg m−1 Mpa−1 s−1) | 1.47 ± 0.34 | 7.75 ± 1.77 | 0.03 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.; Xing, Z.; Lei, M.; Li, G.; Liu, X.; Fang, J.; Lei, D.; Zou, X. Xylem Hydraulic Characteristics and Soil Water Content Drive Drought Sensitivity Differences in Afforestation Species. Water 2025, 17, 2445. https://doi.org/10.3390/w17162445
He R, Xing Z, Lei M, Li G, Liu X, Fang J, Lei D, Zou X. Xylem Hydraulic Characteristics and Soil Water Content Drive Drought Sensitivity Differences in Afforestation Species. Water. 2025; 17(16):2445. https://doi.org/10.3390/w17162445
Chicago/Turabian StyleHe, Ruimin, Zhenguo Xing, Mingzhe Lei, Guanjie Li, Xiaoqing Liu, Jie Fang, Da Lei, and Xin Zou. 2025. "Xylem Hydraulic Characteristics and Soil Water Content Drive Drought Sensitivity Differences in Afforestation Species" Water 17, no. 16: 2445. https://doi.org/10.3390/w17162445
APA StyleHe, R., Xing, Z., Lei, M., Li, G., Liu, X., Fang, J., Lei, D., & Zou, X. (2025). Xylem Hydraulic Characteristics and Soil Water Content Drive Drought Sensitivity Differences in Afforestation Species. Water, 17(16), 2445. https://doi.org/10.3390/w17162445






