Abstract
Adsorption using biochar is a high-efficient method for removing dyes from wastewater, and it has become a hot research topic in recent years. Biochar produced from organic wastes through pyrolysis is a promising way to combine bioenergy recovery and dye removal. In this study, durian shell (DS) was used as a feedstock for biochar and bio-oil production under different pyrolysis temperatures (400, 500, and 600 °C) for bioenergy recovery. Then, the biochar was applied as the absorbent for methylene blue (MB) removal from wastewater under batch and continuous experiments. It was found that the bio-oil production was slightly affected by temperature, while the productivity of biochar decreased from 42.05% to 30.65% with the increase in pyrolysis temperature from 400 to 600 °C. Compared with the biochar produced at 500 °C (DS-500) and 600 °C (DS-600), the biochar obtained at 400 °C (DS-400) exhibited higher MB removal efficiency and adsorption capacity under various pH conditions due to the superior microstructure. A high pH condition was beneficial for the adsorption process with DS-400. Additionally, the MB removal efficiencies increased with the increase in biochar dosage by providing more activated sites. A high MB content can promote the adsorption process, but a too high MB content negatively affects the removal efficiency due to the sorption saturation. Adsorption processes are more likely to match a pseudo-second-order model by chemical reactions. In the long-term continuous experiment, MB can be effectively removed to match the discharge standard by DS-400. This study provided a sustainable pathway for organic waste disposal and dye wastewater treatment.
1. Introduction
Dyes have been widely used in many fields such as printing, textile, leather, and food industries [1], generating large amounts of wastewater with high contents of dye, causing adverse effects on human health and aquatic life [2,3]. Methylene blue (MB), one of the commonly used dyes, cannot totally adhere to fibers, which finally results in large amounts of MB being discharged into the water streams [4,5]. The release of toxic MB into natural water sources will cause serious harm to the aquatic life and human public health due to its adverse impacts on the respiratory system and metabolic processes [6]. Furthermore, MB can prevent the penetration of sunlight and consume dissolved oxygen, which disturbs the aquatic ecosystem [7]. Moreover, the persistence of MB in the environment and its potential to bioaccumulate will cause long-term risks [8]. Commonly, the concentration of MB in water streams largely depends on industrial activities. For instance, the MB content in the effluents of textile and printing industries may range from 10 mg/L to 1000 mg/L [1]. However, according to the environmental protection agency, the allowable MB concentration should be lower than 0.2 mg/L [9]. Therefore, the high-efficiency treatment of MB-containing wastewater is crucial in order to mitigate the potential adverse impacts on both the environment and human health.
MB removal through traditional biological, chemical, and physical treatment processes such as ion exchange [10], photocatalysis [11], electrocoagulation [12], membrane filtration [13], and activated sludge has been largely explored, but they are not applicable due to their high operation costs and low removal efficiencies [14]. Adsorption is a commonly used method for MB removal [15,16,17]. It is a simple, green, and efficient process [1,18]. However, the high cost of traditional adsorbents, such as activated carbon, graphene oxide, and zeolite, and the hazards of chemicals used in their preparation suppress the practical application of this technology [1]. It has been reported that biochar characterizes high surface areas and complex pore structures, is an ideal alternative to other absorbents, and can be effectively utilized for dye removal; it has been widely studied in recent years [16,19,20,21].
Biochar can be produced from various organic wastes such as agricultural wastes, municipal solid wastes, and also industrial wastes via pyrolysis. In this process, the sustainable disposal of organic wastes can be achieved, and the produced carbon-based materials (biochar) can also be obtained as adsorbents for the removal of dyes [22,23]. In addition, mixed gas and bio-oil produced during pyrolysis can be collected and utilized as bioenergy [24]. Messaoudi et al. (2023) prepared biochar from the citrus sinensis leaf for the adsorption of acid blue 25 and obtained a removal efficiency of 98.64% after 60 min using 0.4 g/L of the adsorbent [25]. In another study, El Khomri et al. (2022) employed argan nutshell wood agricultural solid waste for the adsorption of dye and obtained an adsorption capacity of 12.24 and 12.06 mg/g in the case of methylene blue and crystal violet, respectively [26]. Yang et al. (2023) synthesized biochar from food waste digestate for the adsorption of methylene, and reported that the adsorption capacity reached 1123.5 mg/g [27]. Thus, the utilization of carbon-based materials (e.g., biochar) synthesized from organic wastes as adsorbents can facilitate the application of adsorption process and also waste disposal. However, in these studies, the properties of bioenergy recovery during pyrolysis were not mentioned, which needs further investigation.
Durian, as a favorable fruit in China, is largely imported and consumed every year. It discharges large amounts of shells as waste. Durian shell (DS), particularly the thorny layer, is classified as a lignocellulosic material that exhibits a complex uneven fiber bundle structure, primarily composed of cellulose (45–65%), hemicellulose (20–30%), and lignin (10–15%) [28]. Without proper disposal, these husk wastes would lead to serious environmental pollution. The traditional treatment methods such as landfill disposal or incineration may result in the release of greenhouse gases like CO2 and CH4, leading to air pollution. Anaerobic digestion is a promising method for energy recovery from DS, but the lignocellulosic recalcitrance seriously reduces the bioconversion efficiency and requires a complicated pretreatment [29]. In this context, thermochemical treatments such as torrefaction, pyrolysis, and hydrothermal methods have been hotly explored in recent years due to their simplicity and ease of large-scale treatment [30]. Additionally, as DS biomass possesses an inherent porous structure, biochar produced from DS will characterize a high surface area and a large pore volume, which is beneficial for adsorption process [31]. It has been verified that biochar from DS characteristics can be effectively used as adsorbents for removing heavy metals, dyes, and also other pollutants [30]. Therefore, pyrolysis of DS can not only reduce environmental pollution and waste stream volume but can also alleviate our reliance on fossil fuels and improve the decentralized energy infrastructure. Bio-oils, mainly composed of water and oxygen-containing organic matter, are the main by-products of biomass pyrolysis and can be further processed to obtain biofuels and high-value-added chemicals. The pyrolysis of DS biomass presents significant opportunities for the production of bio-oils, the acquisition of major chemical raw materials, and the reduction of environmental pollution [32].
Although some studies have explored the properties of biochar production from DS through pyrolysis [33], the bio-oil yield and biochar structures were significantly affected by the pyrolysis temperatures. In addition, the removal of dyes using DS biochar prepared at different temperatures was seldom reported. In this study, DS was used as feedstocks for producing biochar and bioenergy under different temperatures, and the energy recovery performance and biochar structures were analyzed. Then, biochar was used as absorbent for removing dye from wastewater. The influencing factors (pH, biochar dosage, and MB content) on adsorption process were explored. Finally, the dye removal properties during long-term operations were also explored. The results of this study will provide useful information for combining solid waste disposal and wastewater treatment, and contribute to achieve the goal of the United Nations Sustainable Development Group (UNSDG) regarding energy saving and environment protection.
2. Materials and Methods
2.1. Pretreatment of DS Biomass
The DS biomass was collected from local supermarkets in Chengdu city, China. After being transported to the laboratory, raw DS biomasses were washed and dried in an oven (DHG-9203A, Shanghai Jinghong Laboratory Instrument Co., Ltd., Shanghai, China) at 80 °C until constant weight. Then, they were ground, sieved (0.3 mm), and stored in the desiccator. To avoid the negative effects of moisture on biochar and bio-oil production, the feedstocks were further dried at 80 °C for 8 h to ensure complete dryness before pyrolysis.
2.2. Bioengery Recovery from DS
Biochar and bio-oil were produced through pyrolysis. The pyrolysis system used in this study contained a heating furnace and biodiesel collectors, as reported in a previous study [34]. The pyrolysis temperature was determined according to the TG results of DS, while other conditions were determined by pre-experiments, as described previously [34]. Briefly, 5 g of the feedstocks was weighted into a ceramic crucible, and nitrogen gas with a flow rate of 80 mL/min was used to flush the reactor tube to create an oxygen-free environment. After being flushed (5 min) with nitrogen gas, the furnace was heated up to the setting temperature (400 °C, 500 °C, or 600 °C), using an increase rate of 15 °C/min and retained for 20 min. After that, the tube furnace was naturally cooled down to room temperature, and the weight of biochar was measured as the change of crucible weight before and after pyrolysis. The bio-oil yield was calculated by measuring the changes in the weight of condenser bottles, connections, and the reactor tube; then, the gas yield was calculated by difference. The bio-oil was collected and further used as green fuel. After the experiments, biochar was collected and washed with pure water, and then dried at 105 °C until constant value. The biochar produced with DS at 400 °C, 500 °C, and 600 °C was marked as DS-400, DS-500, and DS-600, and used in the next experiments.
2.3. Adsorption of MB Using Biochar
2.3.1. Effect of pH on MB Adsorption
To investigate the effect of pH on MB removal through adsorption, a series of tests were conducted at a pH range from 2 to 10. The experiments were carried out in tubes (50 mL) with 40 mL of synthetic dye wastewater and an initial MB content of 100 mg/L. Firstly, 0.1 g of DS-400, DS-500, and DS-600 was added in three groups of tubes, respectively. Then, the dye wastewater was added into the tubes and the pH was adjusted to setting value using 5 M hydrochloric acid (HCl, analytical reagent) and sodium hydroxide (NaOH, analytical reagent). Thereafter, all tubes were placed in the shaker (100 rpm) and operated at a temperature of 25 °C for 24 h. A group with biochar addition but without pH adjustment was also conducted as the control.
2.3.2. Effect of Biochar Dosage on MB Adsorption
Biochar dosage significantly affects the MB removal processes. To explore the adsorption of MB by biochar amount, experiments of different biochar dosage on MB removal were conducted. Different amounts of biochar were added to the tubes to achieve biochar dosages of 0.25 g/L, 0.5 g/L, 1 g/L, 1.5 g/L, 2 g/L, and 2.5 g/L, respectively, for DS-400. For DS-500 and DS-600, the final biochar content was fixed at 1.25 g/L, 2.5 g/L, 3.75 g/L, and 5 g/L. Thereafter, the MB wastewater was added into each tube and the pH was adjusted to 10 using 5 M HCl and NaOH. Then, all tubes were put in the shaker (100 rpm, 25 °C), and the MB content was detected after 24 h.
2.3.3. Effect of Initial MB Content on Adsorption
Based on the results of pH and biochar dosage experiments, DS-400 exhibited better MB removal performance, so it was used to explore the impacts of initial MB content on adsorption processes. Firstly, 0.05 g biochar (DS-400) was added into each tube, then MB was added into the tubes to realize an initial MB content between 80 and 400 mg/L. After the pH was adjusted to 10, all tubes were set in a shaker (100 rpm, 25 °C). Samples were obtained periodically from each tube to measure the MB content. Adsorption capacity and kinetics were determined based on the variations of the MB content.
2.3.4. Long-Term MB Removal Using Biochar
To further explore the MB removal properties with biochar in the continuous system for practical use, DS-400 biochar (2 g) was added to the adsorption reactor and the MB wastewater (100 mg/L) was continuously pumped (2.5 mL/min) to the reactor to realize a hydrolytic retention time of 4 h. A membrane filter with a pore size of 1 μM was installed under the reactor to trap biochar, and a peristaltic pump was used to suck the water from the filter. The MB content in the effluent was detected and recorded until the MB content reached constant. The experiment was conducted for approximately 18 h.
2.4. Analysis Method
2.4.1. Thermogravimetry (TG) Analyses of Organic Wastes
Thermal analysis in TG-DTA mode was carried out using a NETZSCH STA 449 F3 Jupiter (NETZSCH GmbH, Selb, Germany) device. A one-time experiment was performed on 10 mg of an air-dried sample material heated in Al2O3 crucible pans from ambient temperature to 800 °C. The pyrolysis was investigated under non-isothermal conditions at heating rates of 10 °C/min. The tests were performed in a dry nitrogen atmosphere with the gas flow of 30 mL/min (protective) and 50 mL/min (purge), respectively.
2.4.2. Scanning Electron Microscope (SEM)
Scanning electron microscope (SEM) imaging (GeminiSEM 300, ZEISS, Oberkochen, German) was used to analyze the microstructure and surface morphology of the biochar. This investigation was carried out using field emission scanning electron microscopy (FE-SEM), which can provide magnifications of up to 106 times. The biochar was firstly dissolved in ethanol (analytical reagent), followed by a 10 min ultrasonication process to ensure uniform dispersion of biochar particles within the solution. A few drops of the prepared solution were applied on copper tape with a dropper and left to evaporate at room temperature for 24 h. Following that, a SEM examination was performed.
2.4.3. Analysis Methods of Dye
The water samples were obtained from each reactor and centrifuged using a centrifuge (TG19, Shanghai Lu Xiangyi Centrifuge Instrument Co., Ltd., Shanghai, China) at 4000 rpm for 5 min. Then, the supernatant was filtrated using the membrane filters (0.45 μm) to remove the biochar particles. The MB content in the solution was determined by a spectrophotometer (UV-1800PC, Shanghai Mapada instruments, Shanghai, China) at 664 nm. All experiments were triplicated.
3. Results and Discussions
3.1. Properties of Bioenergy Recovery from DS
TG is a useful tool for analyzing the relationships between organic degradation and temperatures. As shown in Figure 1a, a sharp decrease in TG was observed between 165 and 350 °C, which was mainly attributed to the thermal conversion of the organics in the DS biomass. Thereafter, TG gradually decreased to 34.98% until 450 °C, and remained almost constant when the pyrolysis temperature exceeded 600 °C, indicating that the organics in the feedstocks were almost converted. These results verified that the pyrolysis temperature of DS can be controlled at less than 600 °C. The DTG curve remained stable at a temperature lower than 170 °C, but sharply decreased at 303 °C, and then increased until 600 °C. It was reported that the weight loss between 198 °C and 487 °C was due to the conversion of cellulose, lignin, and hemicellulose, while the mass loss at higher temperatures was probably caused by the decomposition of the residues formed during the disintegration of lignocellulose [9]. The distinct temperature ranges of the TG reduction was mainly related to the different components in the biomass [35]. In addition, the final TG value was very low (33.3%), indicating that large amounts of organics in DS can be converted through pyrolysis, and provide a high potential for bioenergy (bio-oil and mixed gas) recovery.
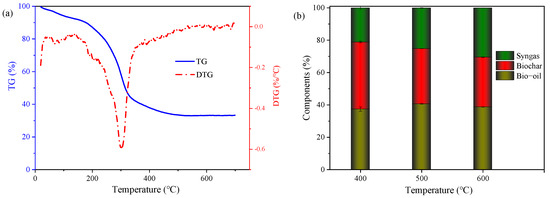
Figure 1.
Variations of TG of durian shell (a) and components of pyrolysis products under different temperatures (b).
The pyrolysis products under different temperatures are shown in Figure 1b. It was found that the biochar content decreased with the increase in the pyrolysis temperature, while the content of the syngas exhibited increasing trends, indicating that a higher temperature would transform organics into gas forms, which is consistent with previous studies [36]. The increase in gas yield with increasing temperature was mainly attributed to bond splitting, isomerization, and small molecule polymerization of macromolecules [36]. When the pyrolysis temperature was 400 °C, the biochar was around 41.5%, while it decreased to 34.18% and 30.83% when the temperature increased to 500 °C and 600 °C, indicating that a higher temperature can transform the organics in DS into biodiesel and syngas, which is consistent with the previous studies [28,36]. It was reported that the structures of organics in biomass can be damaged by increased temperatures, which may also affect the physical/chemical properties of biochar. Meanwhile, the content of syngas increased with the increase in temperature. It was only 21.08% at 400 °C, while it sharply increased to 25.18% and 30.43% when the temperature increased to 500 °C and 600 °C, respectively. The increase in syngas content further explained the reduction in biochar content with temperatures. The bio-oil content at 500 °C was the highest (40.65%), but it was 37.43% and 38.75% at 400 °C and 600 °C, which indicates that 500 °C is better for bio-oil production. As it has been reported that bio-oil as a valuable green energy can be widely utilized in industrial fields after refining, the production of biochar and biodiesel using DS is a promising pathway for organic waste disposal and also high-value product recovery. At a lower temperature, the syngas production was lower, indicating that more energy (bio-oil) and valuable products (biochar) can be obtained. In addition, less electric power for heating was consumed during pyrolysis processes; thus, it is more effective to conduct at a lower temperature. In addition, the quality of bio-oil will be influenced by its components, and the components of bio-oil obtained through pyrolysis will be further studied in the future.
3.2. Properties of Biochar
The DS biomass can produce a high content of biodiesel at a relatively lower temperature, indicating that more energy can be recovered through pyrolysis. Therefore, DS was regarded as a suitable feedstock for bioenergy production, and the DS biochar generated under different temperatures was selected and utilized for the removal of dye from wastewater. It was found that the DS-400 biochar particles had a honeycomb-like structure on the surface and were highly porous (Figure 2), which facilitated the absorption of MB from the water. The honeycomb structure was attributed to the decomposition of volatile components from the DS biomass. However, increasing the pyrolysis temperature to 500 °C resulted in irregular pores, and the pores were obviously crashed, which might have been due to the fact that the high temperature damaged the structures of the organics and led to the pore collapse. Furthermore, pores could not be found on the surface of DS-600 and the pore structures were totally damaged. The differences in morphological images under different temperatures resulted from the thermal decomposition of the organics, which is consistent with the previous studies [3,27]. The different surface microstructures of biochar under different temperatures will affect the MB removal processes, which will be discussed in the next section.

Figure 2.
The SEM images of the biochar produced under different temperatures.
3.3. Effect of Key Factors on MB Removal Using Biochar
Operating parameters are decisive for the adsorption of MB. To investigate the properties of biochar as an adsorbent for MB removal, some important operation parameters such as pH, biochar dosage, and initial MB concentration were firstly explored, and the adsorption capacity under different conditions was evaluated.
3.3.1. Effect of pH on MB Removal
pH is an important parameter that determines the charge state of adsorbents, the dissociation of dye molecules, and directly affects the adsorption processes of dyes on biochar [37]. As shown in Figure 3, DS-400 biochar could completely remove the MB under various pH conditions during 24 h, showing a removal efficiency of 100%. Interestingly, in the reactor with uncontrolled pH (UN), the pH value after adding biochar was around 10, which also resulted in a high MB removal efficiency. However, at pH 2, the MB removal efficiency was slightly lower (96.5%), which might have been due to the fact that the extremely low pH condition changed the properties of the biochar surface and resulted in lower MB removal. It has been verified that, at a low pH condition, the surface of biochar is protonated, which results in increased repulsion force. The decrease in removal efficiency and adsorption capacity at low pH values was mainly due to the repulsion between MB molecules and the positively charged surface of biochar [38]. Additionally, at low pH conditions, the competition between H+ ions and MB on the negatively charged biochar surface was high, which inhibited the efficient adsorption of MB on the biochar’s surface. The same phenomenon was also reported by other researchers [38]. Overall, the surface ions on biochar will be changed by pH adjustment and finally affect the adsorption properties. For other pH conditions, the final MB content was lower than the detection level, indicating that MB can be completely removed by DS-400 during the experiment period.
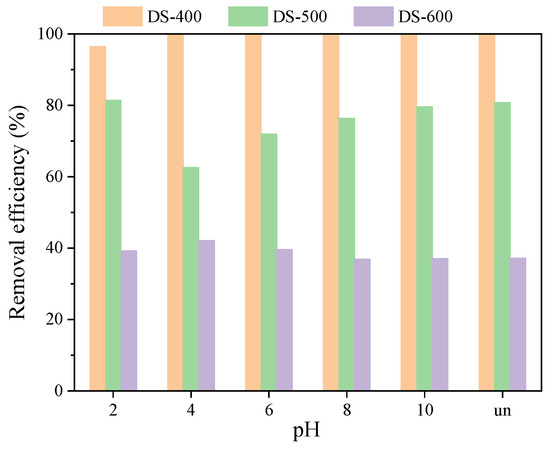
Figure 3.
Effect of pH on MB removal with different biochar (biochar dosage = 0.1 g, MB content = 100 mg/L).
Compared with the DS-400 biochar, MB removal efficiencies with DS-500 and DS-600 were relatively lower under various pH conditions. It was found that the MB removal efficiency by DS-500 slightly increased with the increase in pH from 4 to 10, indicating that an alkaline pH was favorable for MB removal using this biochar, which was mainly due to the fact that a higher pH can affect the zeta potential of biochar and influence the chemical adsorption processes. As the pH increased, the biochar surface was progressively deprotonated, which enhanced the attractive forces between the biochar and the MB dye. The increase in negative charges of the adsorbent surface improved the attraction forces for the MB ions. As has been reported, the increase in pH could increase the deprotonation of functional groups such as -OH and -COOH, which improved the adsorption of MB [37].
However, the MB removal efficiency using DS-600 was much lower (around 40%) than that of DS-400 and DS-500, further indicating that the DS-600 had a lower MB adsorption capacity. Unlike other reactors, the MB removal efficiencies in the reactors with DS-600 gradually decreased with the increase in pH, and the highest value (42.1%) was observed at pH 4, which indicated that the adsorption properties of DS-600 was very different to that of DS-500 and DS-400. The differences in MB removal efficiencies can be explained by the different surface properties. It has been reported that temperatures can change the properties of the biochar surface and also the pore structures [39], which finally affect the MB removal processes. In addition, the destruction of pores on the surface of DS-600 was another cause for lower MB removal efficiency.
3.3.2. Effect of Biochar Dosage on MB Removal
Biochar dosage is another influencing factor for MB removal. It was reported that a low biochar dosage would lead to low MB removal due to the lack of active sites, while a too high biochar amount would also restrict the MB removal processes as the biochar particles might agglomerate, which results in the reduction in adsorption sites and negatively affects the diffusion process [40]. As shown in Figure 4a, the MB removal efficiency was only 51.77% at a DS-400 dosage of 0.25 g/L, but it increased to 99.32% when the biochar dosage increased to 0.5 g/L, indicating that increasing biochar dosage could obviously enhance the MB removal. When the DS-400 dosage further increased to higher than 1.0 g/L, the MB content in the liquid was lower than the detection level, showing that DS-400 can effectively remove the MB from wastewater even at a low biochar dosage (<1 g/L). The reason for enhancing the sorption efficiency by increasing the biochar dosage could be owing to more active sites provided by the biochar addition, but, at high concentrations, the low MB content became the restriction and limited the diffusion process, which finally reduced the adsorption capacity. This result was similar to the previous study regarding methyl violet being removed by biochar produced from lotus leaves [20].
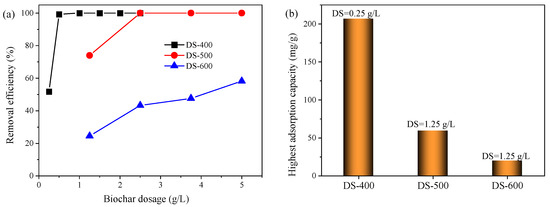
Figure 4.
Effect of biochar dosage on the MB removal efficiency (a) and highest adsorption capacity (b).
However, the DS-500 and DS-600 exhibited relatively lower removal efficiencies. At a biochar dosage of 1.25 g/L, the MB removal efficiency using DS-500 was 74.03%, but it was only 24.50% with DS-600, showing that the pyrolysis temperature would significantly affect the biochar adsorption properties, which might be relative to the distinct pore structures and surface charge [39,41]. Further increasing the biochar dosage to above 2.5 g/L, the MB removal efficiencies using DS-500 increased to around 100%, further showing that a high biochar dosage could promote MB removal efficiency. However, compared with the other two types of biochar, DS-600 exhibited much lower MB removal efficiencies even at a higher biochar dosage. The MB removal efficiency was just 58.25%, even at a biochar dosage of 5 g/L, indicating that the DS-600 was not suitable for MB removal.
As shown in Figure 4b, the highest adsorption capacity of DS-400 (207.01 mg/g) was observed at the biochar dosage of 0.25 g/L, which was much higher than that of DS-500 (59.23 mg/g) and DS-600 (19.60 mg/g). It was reported that a higher temperature would affect the surface areas and also the pore structures [42], which would further affect the MB removal efficiency. This conclusion can be further verified by the biochar surface images (Figure 2). However, some studies showed that the porosity of biochar increased with the increase in pyrolysis temperatures, and finally resulted in a higher adsorption capacity. This contradiction was probably attributed to the different biomasses for biochar production and also the pyrolysis conditions.
3.3.3. Effect of MB Content on Removal Efficiency
It was reported that the initial MB concentration would affect the adsorption capacity of biochar and also adsorption kinetics. In this study, the MB concentration from 80 to 400 mg/L was investigated using DS-400. Obviously, the MB content decreased with the operation time and exhibited different tendencies (Figure 5a). In the reactors with a content of 80 and 100 mg/L, the MB could be completely removed in 210 min and 330 min, respectively. While, in other reactors with higher MB concentrations, MB could not be completely removed, and the residual value increased with the initial MB concentration. Meanwhile, the MB content sharply decreased during the first 60 min in all reactors, and then reduced at a lower rate, probably due to the fact that the active sites on biochar were occupied by the MB molecules and the adsorption would occur in deep pores by diffusion processes [43]. In addition, due to the rapid accumulation of MB on the biochar surface, the charge of biochar surface was changed, which may also have slown down the adsorption processes.
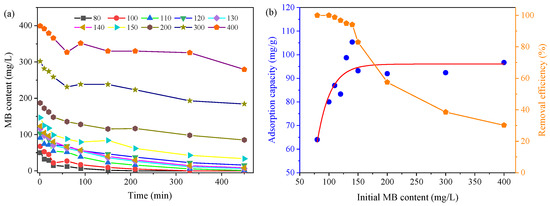
Figure 5.
Effect of initial MB content on adsorption processes. (a) Variations of MB content; (b) adsorption capacity.
The decreased removal efficiency with increasing MB concentration (Figure 5b) was mainly due to the unavailability of active sites to adsorb a high number of MB molecules [44]. Abdelwahab et al. (2008) observed the same phenomena during the adsorption of reactive orange using loofa-activated carbon [45]. In addition, the increase in initial MB concentrations could increase the adsorption uptake, as the raising of the initial concentration could overcome the mass transfer resistance and act as a driving force to accelerate the adsorption of MB on the biochar surface [46]. Ebrahimian Pirbazari et al. (2014) found the adsorption capacity increased from 15.2 mg/g to 62.2 mg/g with the increase in the initial concentration of MB from 50 mg/L to 200 mg/L [47]. In this study, the adsorption capacity was 64 mg/g when the MB content was 80 mg/L, and it increased with the increase in MB content to 140 mg/L (105.37 mg/g), showing that a high initial MB content could promote the adsorption processes. However, further increasing the MB content would not further increase the MB adsorption capacity, which verified that a too high MB content may restrict the MB removal, which was mainly due to the limitation of an activated site on the biochar surface. Based on the simulation, the adsorption capacity of MB with DS-400 was around 96.04 mg/g, which is comparable with previous studies [21].
3.4. Adsorption Kinetics
To perceive the adsorption data and evaluate the efficiency, rate, and rate-controlling step, adsorption kinetic models were implemented. The pseudo-first-order and second-order kinetic models were introduced to analyze the sorption processes, respectively. The pseudo-second-order model accurately described the adsorption of MB on the biochar surface, as indicated by R2 presented in Table 1. This finding demonstrated that the adsorption process may be predominantly chemical. A chemical reaction between the biochar and MB by sharing electrons could change the valence forces, which would be a rate-limiting step in the sorption method [21]. Thus, both the reaction circumstances and the physical and chemical characteristics of the adsorbent may influence the adsorption process.

Table 1.
Kinetic model and their parameters for the MB dye sorption on DS-400.
The adsorption of the dye on the MBC surface could be due to the attractive forces and surface complexation between the NH+ group in the MB molecules and the negative-charge deprotonated COO-group on the biochar’s surface. Further, the adsorption could occur because of the hydrogen bonding between the OH, COOH, and CH groups in the biochar and the NH group in the dye [1]. Additionally, the π-π stacking could be attained between the aromatic group (C=C) in the MB and the aromatic group (C=C) in the biochar. Moreover, the adsorption might be because of the π-π stacking between the benzene ring in MB and oxygen in the OH group in the fabricated biochar [48].
3.5. Comparison of Dye Removal with Different Adsorbents Reported in the Literature
Recently, many researchers have developed novel biochar materials using different raw biomass materials for removing dyes from wastewater. By comparing the maximum adsorption capacity of DS-400 and other reported results (Table 2), it can be found that DS-400 exhibited a satisfactory adsorption capacity and is more suitable for treating high-concentration dye wastewater. Moreover, DS-400 has a relatively simple preparation procedure without the need for additional acid or metallic salt pretreatment.

Table 2.
Dye removal using biochar produced from different biomasses.
3.6. Long-Term MB Removal Using DS Biochar
To further investigate the MB removal using biochar for practical application, a long-term lab-scale continuous reactor was developed and conducted. The experiment devices are shown in Figure 6a. It should be noted that, without biochar adsorption, the membrane exhibited no removal for MB. However, as shown in Figure 6b, a three-stage MB removal profile was observed during the whole experiment period. At the complete removal stage (0–400 min), the MB content in the effluent was lower than the detection level, indicating that the DS-400 biochar can completely remove the MB in this phase. At the second stage, the MB content in the effluent linearly increased and reached 92.97 mg/L at 900 min, which indicated that the biochar can partially remove the MB from wastewater, but the MB content exceeded the discharge standard. At the saturation stage, the MB content in the effluent remained almost constant, showing that biochar achieved adsorption saturation and could not further remove MB from the wastewater. The color of the effluent from each stage (Figure 6c) also verified the removal efficiency during the experiment. Based on the discharge level (0.1 mg/L), the reactor could be safely conducted for 400 min with 2 g of DS-400 biochar. Based on the MB removal properties, it can be calculated that the adsorption capacity was close to that of batch tests, indicating that the biochar can be effectively used as an absorbent for MB removal during long-term continuous experiments.
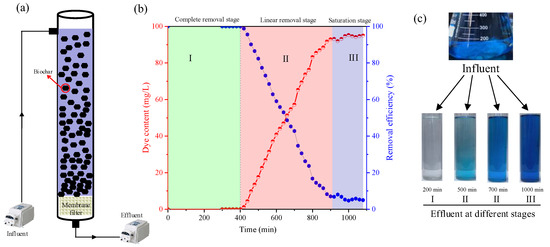
Figure 6.
MB removal performance during the long-term continuous operation. (a) Reactor diagram, (b) dye removal properties, and (c) photos of the effluent at different stages.
4. Conclusions
DS was used as the feedstock for bioenergy recovery and high adsorption material production. It was found that the bio-oil production was not significantly influenced by the pyrolysis temperature, while the biochar yield decreased from 42.05% to 30.65% with the increase in temperature from 400 to 600 °C. The DS-400 biochar had superior pore structures and larger porosity, and exhibited higher MB removal efficiency than that of DS-500 and DS-600. The MB adsorption efficacy increased with pH and biochar dosage, but remained constant when initial MB content was higher than 140 mg/L, showing an adsorption capacity of 100.4 mg/g. In addition, the adsorption process matched the pseudo second order. During the long-term continuous operation, the MB in wastewater can be effectively removed by biochar and matched the discharge standard during a long-term operation. This work provided a promising pathway for organic solid waste disposal and dye wastewater treatment through a sustainable way. In the future, the MB removal efficiency in a large-scale application will be studied, and the economical assessment of this processes will be investigated. Additionally, using DS biochar to remove heavy metals and other organics will be promising research fields.
Author Contributions
Conceptualization, Y.P. and J.T.; investigation, N.J., Y.X., J.C. and R.L.; writing—original draft preparation, Y.P.; writing—review and editing, J.T., Q.W. and A.A.; funding acquisition, J.T. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by State Key Laboratory of Pollution Control and Resource Reuse Foundation (PCRRF21017), Science and Technology Program of Sichuan (2020YJ0196) and Solid-state Fermentation Resource Utilization Key Laboratory of Sichuan Province (2018GTJ008).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abdel Azim, E.; Samy, M.; Hanafy, M.; Mahanna, H. Novel mint-stalks derived biochar for the adsorption of methylene blue dye: Effect of operating parameters, adsorption mechanism, kinetics, isotherms, and thermodynamics. J. Environ. Manag. 2024, 357, 120738. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-N.; Yu, K.; He, J.-H.; Chen, Y.; Guo, J.-Z.; Li, B. Multiple roles of ferric chloride in preparing efficient magnetic hydrochar for sorption of methylene blue from water solutions. Bioresour. Technol. 2023, 373, 128715. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-B.; Do, Q.-H.; Chen, C.-W.; Chen, W.-H.; Bui, X.-T.; Dong, C.-D. Pyrolysis temperature effect on biochar-derived cow manure: Physicochemical properties and adsorption behavior toward organic dyes. J. Taiwan Inst. Chem. Eng. 2024, 164, 105675. [Google Scholar] [CrossRef]
- Li, X.; Natsuki, J.; Natsuki, T. A recyclable silver nanoparticles/graphene oxide nanoscroll composite photocatalyst. Environ. Technol. Innov. 2021, 21, 101210. [Google Scholar] [CrossRef]
- Chandrasekar, R.; Prakash, P.; Ghosh, D.; Narayanasamy, S. Heteroatom doped biochar-aluminosilicate composite as a green alternative for the removal of hazardous dyes: Functional characterization and modeling studies. Environ. Res. 2024, 260, 119579. [Google Scholar] [CrossRef] [PubMed]
- Dimbo, D.; Abewaa, M.; Adino, E.; Mengistu, A.; Takele, T.; Oro, A.; Rangaraju, M. Methylene blue adsorption from aqueous solution using activated carbon of spathodea campanulata. Results Eng. 2024, 21, 101910. [Google Scholar] [CrossRef]
- Yang, P.; Lu, Y.; Zhang, H.; Li, R.; Hu, X.; Shahab, A.; Elnaggar, A.Y.; Alrefaei, A.F.; AlmutairiI, M.H.; Ali, E. Effective removal of methylene blue and crystal violet by low-cost biomass derived from eucalyptus: Characterization, experiments, and mechanism investigation. Environ. Technol. Innov. 2024, 33, 103459. [Google Scholar] [CrossRef]
- Khandelwal, D.; Rana, I.; Mishra, V.; Ranjan, K.R.; Singh, P. Unveiling the impact of dyes on aquatic ecosystems through zebrafish—A comprehensive review. Environ. Res. 2024, 261, 119684. [Google Scholar] [CrossRef]
- Thabede, P.M.; Shooto, N.D.; Naidoo, E.B. Removal of methylene blue dye and lead ions from aqueous solution using activated carbon from black cumin seeds. S. Afr. J. Chem. Eng. 2020, 33, 39–50. [Google Scholar] [CrossRef]
- Alatabe, M.J.A.; Ghorbanpour, M. A performance comparison of photo-fenton decolorization of methylene blue by using bentonite/iron composites prepared by liquid phase and solid phase ion exchange method. Desalination Water Treat. 2024, 317, 100027. [Google Scholar] [CrossRef]
- Shaheen, I.; Ata, S.; Aslam, H.; Farooq, H.; Ali, A.; Elqahtani, Z.M.; Alwadai, N.; Iqbal, M.; Arif, H.; Nazir, A. Photocatalytic removal of methylene blue and Victoria blue R dyes using Tb and La-doped BaZnO2. Desalination Water Treat. 2024, 318, 100389. [Google Scholar] [CrossRef]
- Teresa Jose, J.; Priya, K.L.; Chellappan, S.; Sreelekshmi, S.; Remesh, A.; Venkidesh, V.; Krishna, A.J.; Pugazhendhi, A.; Selvam, S.; Baiju, V.; et al. A hybrid electrocoagulation-biocomposite adsorption system for the decolourization of dye wastewater. Environ. Res. 2024, 252, 118759. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-y.; Li, L.; Feng, M.; Huang, T.; Zhang, N.; Wang, Y. UV-activated superwetting ability of electrospun polysulfone/titanium dioxide membranes toward highly efficient methylene blue removal and oil/water separation. J. Membr. Sci. 2024, 695, 122450. [Google Scholar] [CrossRef]
- Hashemi, E.; Norouzi, M.-M.; Sadeghi-Kiakhani, M. Magnetic biochar as a revolutionizing approach for diverse dye pollutants elimination: A comprehensive review. Environ. Res. 2024, 261, 119548. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Z.; Li, X.; Zeng, L.; Xu, W.; Ma, Y.; Cai, J. Enhanced removal of methylene blue from water by mesopore-dominant biochar from kelp: Kinetic, equilibrium and thermodynamic studies. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133652. [Google Scholar] [CrossRef]
- Xu, R.; Wei, J.; Cheng, D.; Wang, W.; Hong, L.; Chen, Y.; Guo, Y. Abundant porous biochar derived from luffa vine for removal of methylene blue: Selective adsorption and mechanistic studies. Ind. Crops Prod. 2024, 219, 119114. [Google Scholar] [CrossRef]
- Chaoui, A.; Farsad, S.; Ben Hamou, A.; Amjlef, A.; Nouj, N.; Ezzahery, M.; El Alem, N. Reshaping environmental sustainability: Poultry by-products digestate valorization for enhanced biochar performance in methylene blue removal. J. Environ. Manag. 2024, 351, 119870. [Google Scholar] [CrossRef]
- Mensah, K.; Mahmoud, H.; Fujii, M.; Samy, M.; Shokry, H. Dye removal using novel adsorbents synthesized from plastic waste and eggshell: Mechanism, isotherms, kinetics, thermodynamics, regeneration, and water matrices. Biomass Convers. Biorefinery 2024, 14, 12945–12960. [Google Scholar] [CrossRef]
- Jiang, P.; Zhou, L.; Han, Y.; Fu, W.; Su, S.; Zeng, M. Utilizing waste corn straw to photodegrade methyl orange and methylene blue: Photothermal effect of biochar enhances photodegradation efficiency. J. Environ. Chem. Eng. 2024, 12, 112914. [Google Scholar] [CrossRef]
- Chen, J.; Yan, Z.; Zhang, S.; Yue, L.; Xu, Z. Fabrication of agro–waste lotus leaf–derived adsorbent for effective removal of organic pollutants from water. Chem. Eng. Sci. 2024, 283, 119426. [Google Scholar] [CrossRef]
- Vyavahare, G.; Patil, R.; Gurav, R.; Shorobi, F.M.; Kadam, S.; Jadhav, J.; Park, J.H. Investigating the efficacy of biochar produced from agro-waste for basic fuchsin dye removal: Kinetics, isotherm, and thermodynamic studies. J. Indian Chem. Soc. 2024, 101, 101278. [Google Scholar] [CrossRef]
- Liu, J.; Huang, S.; Chen, K.; Wang, T.; Mei, M.; Li, J. Preparation of biochar from food waste digestate: Pyrolysis behavior and product properties. Bioresour. Technol. 2020, 302, 122841. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gamliel, D.P.; Markunas, B.; Valla, J.A. A Promising Solution for Food Waste: Preparing Activated Carbons for Phenol Removal from Water Streams. ACS Omega 2021, 6, 8870–8883. [Google Scholar] [CrossRef]
- Chatterjee, R.; Sajjadi, B.; Chen, W.-Y.; Mattern, D.L.; Hammer, N.; Raman, V.; Dorris, A. Effect of Pyrolysis Temperature on PhysicoChemical Properties and Acoustic-Based Amination of Biochar for Efficient CO2 Adsorption. Front. Energy Res. 2020, 8, 85. [Google Scholar] [CrossRef]
- Messaoudi, N.E.; Mouden, A.E.; Khomri, M.E.; Bouich, A.; Fernine, Y.; Ciğeroğlu, Z.; Américo-Pinheiro, J.H.P.; Labjar, N.; Jada, A.; Sillanpää, M.; et al. Experimental study and theoretical statistical modeling of acid blue 25 remediation using activated carbon from Citrus sinensis leaf. Fluid Phase Equilibria 2023, 563, 113585. [Google Scholar] [CrossRef]
- El Khomri, M.; El Messaoudi, N.; Dbik, A.; Bentahar, S.; Lacherai, A.; Chegini, Z.G.; Bouich, A. Removal of Congo red from aqueous solution in single and binary mixture systems using Argan nutshell wood. Pigment Resin Technol. 2022, 51, 477–488. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Li, Y.; Guo, C.; Han, Y.; Zhang, Y.; Song, B. Valorization of food waste digestate to ash and biochar composites for high performance adsorption of methylene blue. J. Clean. Prod. 2023, 397, 136612. [Google Scholar] [CrossRef]
- Manmeen, A.; Kongjan, P.; Palamanit, A.; Jariyaboon, R. Biochar and pyrolysis liquid production from durian peel by using slow pyrolysis process: Regression analysis, characterization, and economic assessment. Ind. Crops Prod. 2023, 203, 117162. [Google Scholar] [CrossRef]
- Wang, L.; Wei, B.; Cai, F.; Chen, C.; Liu, G. Recycling durian shell and jackfruit peel via anaerobic digestion. Bioresour. Technol. 2022, 343, 126032. [Google Scholar] [CrossRef]
- Nguyen, N.T.H.; Nguyen, T.T.T.; Nguyen, D.T.C.; Tran, T.V. A comprehensive review on the production of durian fruit waste-derived bioadsorbents for water treatment. Chemosphere 2024, 363, 142801. [Google Scholar] [CrossRef]
- Ly, T.B.; Trinh, A.M.H.; Tran, H.P.T.; Dang, K.N.; Nguyen, T.D.T.; Tran, V.T.; Le, P.K. Evaluation of an operating durian shell charcoal briquette manufacturing line and development of a biorefinery process for higher value products. Energy 2024, 307, 132727. [Google Scholar] [CrossRef]
- Liu, H.; Liu, J.; Huang, H.; Evrendilek, F.; Wen, S.; Li, W. Optimizing bioenergy and by-product outputs from durian shell pyrolysis. Renew. Energy 2021, 164, 407–418. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Wei, X.; Chen, Z.; Yuan, H.; Evrendilek, F.; Huang, S.; Chen, T.; Xie, W.; Zhong, S.; et al. Co-thermal conversion, atmosphere, and blend type controls over heavy metals in biochars and bottom slags of textile dyeing sludge and durian shell. Fuel 2023, 352, 129017. [Google Scholar] [CrossRef]
- Xiong, M.; Huang, J.; He, X.; Zhou, Z.; Qu, X.; Faisal, S.; Abomohra, A. Evaluation of bio-oil/biodiesel production from co-pyrolysis of corn straw and natural hair: A new insight towards energy recovery and waste biorefinery. Fuel 2023, 331, 125710. [Google Scholar] [CrossRef]
- Li, X.; Cen, K.; Wang, L.; Jia, D.; Zhu, X.; Chen, D. Co-pyrolysis of cellulose and lignin: Effects of pyrolysis temperature, residence time, and lignin percentage on the properties of biochar using response surface methodology. Ind. Crops Prod. 2024, 219, 119071. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, Z.; Zhao, L.; Yu, F.; Li, Z.; Yi, W.; Fu, P.; Jia, J.; Zhao, Y. Effects of various pyrolysis temperatures on the physicochemical characteristics of crop straw-derived biochars and their application in tar reforming. Catal. Today 2024, 433, 114663. [Google Scholar] [CrossRef]
- Zhou, P.; Li, X.; Zhou, J.; Peng, Z.; Shen, L.; Li, W. Insights of the adsorption mechanism of methylene blue on biochar from phytoextraction residues of Citrus aurantium L.: Adsorption model and DFT calculations. J. Environ. Chem. Eng. 2023, 11, 110496. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, B.; Liu, Q.; Wu, C.; Li, Z. Preparation of porous biochar from heavy bio-oil for adsorption of methylene blue in wastewater. Fuel Process. Technol. 2022, 238, 107485. [Google Scholar] [CrossRef]
- Wu, Z.-F.; Wang, Z.-K.; Li, J.-B.; Qiu, Y.-H.; Chen, Z.-L.; Owens, G.; Yang, Z.-M. Effects of biochars derived from different feedstocks and pyrolysis temperatures on the anaerobic digestion of kitchen waste. Renew. Energy 2024, 230, 120833. [Google Scholar] [CrossRef]
- Costa Louzada, T.C.; Weschenfelder, S.E.; dos Passos, B.T.; Mazur, L.P.; Marinho, B.A.; da Cunha, M.d.F.R.; da Silva, A.; Ulson de Souza, A.A.; Guelli Ulson de Souza, S.M.A. New insights in the treatment of real oilfield produced water: Feasibility of adsorption process with coconut husk activated charcoal. J. Water Process Eng. 2023, 54, 104026. [Google Scholar] [CrossRef]
- Tao, J.; Wu, W.; Lin, D.; Yang, K. Role of biochar pyrolysis temperature on intracellular and extracellular biodegradation of biochar-adsorbed organic compounds. Environ. Pollut. 2024, 346, 123583. [Google Scholar] [CrossRef] [PubMed]
- Gotore, O.; Masere, T.P.; Muronda, M.T. The immobilization and adsorption mechanisms of agro-waste based biochar: A review on the effectiveness of pyrolytic temperatures on heavy metal removal. Environ. Chem. Ecotoxicol. 2024, 6, 92–103. [Google Scholar] [CrossRef]
- Tamjidi, S.; Moghadas, B.K.; Esmaeili, H.; Shakerian Khoo, F.; Gholami, G.; Ghasemi, M. Improving the surface properties of adsorbents by surfactants and their role in the removal of toxic metals from wastewater: A review study. Process Saf. Environ. Prot. 2021, 148, 775–795. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Bi, F.; Zheng, Z.; Sheng, L.; Xu, J.; Wang, Z.; Yang, Y. Effective toluene adsorption over defective UiO-66-NH2: An experimental and computational exploration. J. Mol. Liq. 2020, 316, 113812. [Google Scholar] [CrossRef]
- Abdelwahab, O. Evaluation of the use of loofa activated carbons as potential adsorbents for aqueous solutions containing dye. Desalination 2008, 222, 357–367. [Google Scholar] [CrossRef]
- Mahanna, H.; Azab, M. Adsorption of Reactive Red 195 dye from industrial wastewater by dried soybean leaves modified with acetic acid. Desalination Water Treat. 2020, 178, 312–321. [Google Scholar] [CrossRef]
- Ebrahimian Pirbazari, A.; Saberikhah, E.; Habibzadeh Kozani, S.S. Fe3O4–wheat straw: Preparation, characterization and its application for methylene blue adsorption. Water Resour. Ind. 2014, 7–8, 23–37. [Google Scholar] [CrossRef]
- Mensah, K.; Mahmoud, H.; Fujii, M.; Shokry, H. Novel nano-ferromagnetic activated graphene adsorbent extracted from waste for dye decolonization. J. Water Process Eng. 2022, 45, 102512. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Liu, G.; Li, C. Biochar obtained from alkaline earth metal-treated mushroom residue: Thermal behavior and methyl orange adsorption capability. J. Environ. Manag. 2024, 351, 119669. [Google Scholar] [CrossRef]
- Zhao, F.; Shan, R.; Li, S.; Yuan, H.; Chen, Y. Characterization and Co-Adsorption Mechanism of Magnetic Clay-Biochar Composite for De-Risking Cd(II) and Methyl Orange Contaminated Water. Int. J. Mol. Sci. 2023, 24, 5755. [Google Scholar] [CrossRef]
- Xu, J.; Fu, M.; Ma, Q.; Zhang, X.; You, C.; Shi, Z.; Lin, Q.; Wang, X.; Feng, W. Modification of biochar by phosphoric acid via wet pyrolysis and using it for adsorption of methylene blue. RSC Adv. 2023, 13, 15327–15333. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).