Abstract
In order to explore bacteria resources that are applicable for purification of viaduct rainwater, a carbon-fixing bacteria strain numbered 1C-1 was isolated from the sediment of a viaduct rainwater tank. The strain was identified through morphological characteristics and 16S rDNA sequences. The effects of three main factors (the simulated viaduct rainwater concentration, the carbon source dosage, and the inoculation amount) on the nitrogen and phosphorus removal rate of the strain were tested using simulated viaduct rainwater. Based on this, the nitrogen and phosphorus removal efficiencies for the actual viaduct rainwater were verified. The results showed that the strain belonged to Streptomyces sp. Under different simulated viaduct rainwater concentrations, the strain exhibited relatively high efficiency for nitrogen and phosphorus removal at the original concentration of simulated viaduct rainwater; other conditions remaining unchanged, the purification efficiency was relatively high when the glucose dosage was 800 mg, and the removal rates of ammonia nitrogen (NH4+-N), total nitrogen (TN), and total phosphorus (TP) were 71.48%, 47.86%, and 10.43%, respectively; other conditions remaining unchanged, the purification efficiency was relatively high when the inoculation amount was 1%, and the removal rates of NH4+-N, TN, and TP reached 58.62%, 58.35%, and 27.32%, respectively. Under the above optimal process conditions of an original concentration of viaduct rainwater, a carbon source dosage of 800 mg, and an inoculation amount of 1%, the strain removed 92.62%, 6.98%, and 6.16% of NH4+-N, TN, and TP, respectively from the actual viaduct rainwater; more interestingly, the removal rates of NH4+-N and TN were 43.26% and 78.02%, respectively, even without carbon source addition. It seems that there is no need for carbon source addition to remove nitrogen from the actual viaduct rainwater for the strain. To sum up, the carbon-fixing bacteria 1C-1 presents an obvious nitrogen and phosphorus removal effect (especially for nitrogen) for viaduct rainwater treatment and has application potential.
1. Introduction
In recent years, viaduct transportation has developed rapidly worldwide. In order to increase traffic capacity, divert traffic, and ease congestion, numerous viaducts have been built all over the world. Because of a relatively independent system, the pollutants on the viaduct surface are difficult to evacuate, with serious accumulation. Once it rains, large amounts of suspended solids, pathogens, heavy metals, microplastics, automobile exhaust, petroleum hydrocarbons, and other organic and inorganic pollutants mix in viaduct rainwater [1,2,3,4]. The pollution level of the actual viaduct rainwater is high with 15–320 mg/L of chemical oxygen demand (COD), 0.15–2.00 mg/L of NH4+-N, 0.1–7.5 mg/L of total nitrogen (TN), and 0.05–20.0 mg/L of total phosphorus (TP), generally exceeding the level of sewage [5,6,7]. According to statistics, the total amount of pollutants from road and bridge surfaces accounts for over 15% of the total non-point source pollution load of rivers [8]. The latest research report indicates that urban non-point source pollution still contributes up to 50% of the water pollution load in China’s central cities [9]. If viaduct rainwater is not treated, pollutants on the viaduct surface will be discharged into the surrounding water bodies, seriously impacting the water environment [7,10].
In order to reduce the water environment problems caused by viaduct rainwater, viaduct construction usually contains on-site rainwater treatment devices such as rainwater treatment tanks, which can achieve the purification effect through natural sedimentation, material filtration, and plant purification [7,11]. However, affected by unstable water quality and quantity and the high instantaneous flow rate of viaduct rainwater, these devices often encounter a problem of long-term operation stability, resulting in poor purification efficiency [5]. In order to achieve efficient purification, multi-stage treatment structures are often adopted, but they encounter issues of occupying a large area, high construction cost, difficult operation, and high maintenance cost, which greatly limit the application of these devices in viaduct rainwater treatment [11,12,13]. Bioaugmentation is a highly efficient biotechnology using the addition of bacterial cultures required to speed up the rate of contaminant degradation. It is commonly used in wastewater treatment for its simple and easy operation, mild reaction condition, lack of new construction or major process condition changes, and no secondary pollution. Hence, bioaugmentation is suitable for viaduct rainwater treatment. However, there are few studies on the usage of bioaugmentation for viaduct rainwater treatment. Zhang et al. investigated the purification of stormwater in a storage pond by adding nine bacteria (Bacillus subtilis, Rhodopseudomonas palustris, Nocardia corallina, Bacillus pumilus, Micrococcus luteus, Proteus vulgaris, Pseudomonas mendocina, Pseudomonas fluorescens, and Pseudomonas chlororaphis) and two fungi (Candida tropicalis, Candida boidinii), and the removal rates of COD, NH4+-N, TN, and TP increased from 53.4%, 37.9%, 28.0%, and 65.2% to 71.2%, 55.3%, 51.1%, and 82.6%, respectively [14]. Masy et al. implemented in permeable pavement systems Rhodococcus erythropolis for resilience and improvement of the degradation capacity of hydrocarbons in road runoff, and thin and drought-resistant biofilms were formed on gravel, in which Rhodococcus erythropolis was maintained over the long-term at substantial levels (104 to 106 cells·g−1 of gravel) under real environmental conditions and even in the presence of numerous other microorganisms, in comparison with the maximum concentration obtained in lab-scale experiments with pure cultures (max. 107 cells·g−1 of gravel), preventing a release of organic pollutants into the groundwater [15]. Zhou et al. added sediment from Lake Chaohu aiming at introducing nitrifiers and denitrifiers into the biofilter for stormwater treatment, and the TN removal efficiency was increased by 25.2%, attributed to higher amoA and nirS gene abundances, indicating the successful induction of nitrifiers and denitrifiers as well as their effective functions [16]. It can be seen that the purification effect of rainwater can be significantly improved by bioaugmentation. As far as we know, there have been no similar research reports afterwards, and there is a lack of bacteria resources that are applicable for the purification of viaduct rainwater. Therefore, finding functional strains and integrating them into existing treatment devices to improve their purification efficiency is urgently needed in the field of rainwater treatment.
Carbon-fixing bacteria are microorganisms that enhance the carbon absorption capacity of ecosystems and capture CO2 in the atmosphere. Those microorganisms have been extensively studied. They are mainly autotrophic bacteria, widely distributed in different ecological environments such as ocean [17,18], volcanic crater [19], glacier [20], desert [21], and soil [22,23,24,25]. These autotrophic bacteria can be divided into two types: photoautotrophic and chemoautotrophic. In the field of wastewater treatment, the former has been extensively studied, and the typical representative is algae [26,27]. In fact, the latter also plays an important role, and the typical representatives are ammonia oxidizing bacteria [28], nitrifying bacteria [29], hydrogen bacteria [30], sulphur bacteria [31], and iron bacteria [32]. An insufficient organic carbon source is often encountered in wastewater treatment [33,34], while the latter can use CO2 in wastewater as the carbon source coupled with the energy that released during the oxidation of inorganic compounds such as NH4+-N, nitrite nitrogen, H2, H2S, and Fe2+. It can not only overcome the problem of an insufficient organic carbon source but also promote the removal of inorganic pollutants. Among the functional bacteria with carbon-fixing advantage, Streptomyces is a dominant genus, and the typical strain includes Streptomyces thermoautotrophicus and Streptomyces kunmingensis [35,36]. A large amount of research data indicate that viaduct rainwater is also a typical wastewater with carbon source deficiency [37,38,39]. Hence, the carbon-fixing bacteria may play a certain role in viaduct rainwater purification. However, to our knowledge, there have been no reports of usage of carbon-fixing bacteria for viaduct rainwater treatment.
In our previous study, we obtained a strain numbered 1C-1 with carbon-fixing ability from the sediment of the viaduct rainwater tank of a bridge located in Guangzhou. The preliminary experiment found that it also had certain water purification functions. This study aims to identify the species of the strain 1C-1, optimize the purification process using simulated viaduct rainwater, and test its nitrogen and phosphorus removal efficiencies for actual viaduct rainwater. We hope a functional bacteria resource can be explored for rainwater treatment.
2. Materials and Methods
2.1. Materials
2.1.1. Sediment Sample
The sediment was shoveled from the bottom of a viaduct rainwater tank (2 m × 1 m × 1 m, E113°29′08″, N23°05′07″, Figure 1) of the Huangpu bridge located in Guangzhou, was placed in clean sealed plastic bags, and was brought back to the laboratory for temporary storage in a 4 °C refrigerator.

Figure 1.
Sampling point. (a) GPS of the sampling point on map, (b) Huangpu bridge, and (c) viaduct rainwater tank.
2.1.2. Carbon-Free Inorganic Medium
Both the bacterial separation medium and the bacterial screening medium referred to the formula of a carbon-free inorganic medium proposed by Guo et al. [25]. The main components of the medium were MnSO4 0.02 g, Na2HPO4 1 g, KH2PO4 1.8 g, MgSO4 0.4 g, CaCl2 0.05 g, NaCl 1 g, NH4Cl 0.5 g, FeCl3 0.02 g, Na2S2O3 10 g, agar 2 g, and distilled water 1000 mL. The medium was sterilized at 121 °C for 30 min.
2.1.3. Simulated Viaduct Rainwater
Simulated viaduct rainwater followed the formula of Peng et al. [40]. The main components were KH2PO4 8.77 mg, KNO3 66.86 mg, NH4Cl 61.14 mg, glucose 373.83 mg, and distilled water 1000 mL. The water quality indicators of this simulated rain were 16 mg·L−1 of NH4+-N, 24 mg·L−1 of TN, 2 mg·L−1 of TP, and 400 mg·L−1 of COD.
2.1.4. Actual Viaduct Rainwater
Actual viaduct rainwater was collected from an inlet of the same bridge tank at 4:30 a.m., 23 November 2022. The water quality indicators for this actual viaduct rainwater were 1.47 mg·L−1 of NH4+-N, 19.02 mg·L−1 of TN, 1.03 mg·L−1 of TP, and 142.49 mg·L−1 of COD. The ratio of COD:TN was 7.5:1.
2.2. Methods
2.2.1. Isolation and Identification of the Carbon-Fixing Bacteria
- (1)
- Isolation, purification, screening of carbon-fixing bacterial strains
Ultra-pure water 90 mL was added into a 250 mL triangular flask, then sterilized at 121 °C for 30 min. Next, 10 g of sediment was added into the flask, then placed in a shaker at 30 °C, using 150 r·min−1 for 20 min to generate a bacterial suspension. A ten-fold dilution method was used to generate a gradient dilution of the bacterial suspension. Under sterile conditions, 1 mL of the diluted bacterial suspension was injected into a sterile culture dish containing the carbon-free inorganic medium. The bacterial suspension was evenly spread. The dish was inverted and cultured at 28 °C for 7–14 days. The colonies with different morphologies were picked out, and a total of 11 strains were obtained and numbered. After repeated purification through streaking, they were preserved on slant with carbon-free inorganic medium in a 4 °C refrigerator.
The 11 strains were inoculated into the liquid carbon-free inorganic medium (without agar), and their growth was determined by biomass yield. The five strains with higher growth rates were selected and individually inoculated into the simulated viaduct rainwater to test the purification effect. The strain 1C-1 showed the best purification effect and was screened for the subsequent experiment.
- (2)
- Identification of the carbon-fixing bacterial strain
The identification of the strain 1C-1 was carried out based on the morphological characteristics and 16S rDNA sequences. After observations of the bacterial morphology on the bacterial separation medium and under the optical microscope, the strain 1C-1 was further observed using a tungsten filament scanning electron microscope (SEM, Q25, FEI, USA) to study its morphological structures. In accordance with the extraction steps of the Magen bacterial DNA extraction reagent kit (MGBio, Shanghai, China), the bacterial DNA of the strain 1C-1 was amplified as a template using universal primers 27F and 1492R. The PCR reaction program was as follows: pre-denaturation at 95 °C for 3 min, denaturation at 95 °C for 10 s, annealing at 56 °C for 40 s, extension at 72 °C for 1 min, with a total of 28 cycles, and finally, extension at 72 °C for 5 min. The PCR reaction system used a 25 μL system: 2 μL DNA template, 1 μL upstream primer, 1 μL downstream primer, 12.5 μL 2xTaq PCR Master mix, and 8.5 μL distilled de-ionized water. The primers were purchased from Shanghai Bioengineering Co. Ltd. (Shanghai, China). The amplified fragments were detected by 1% agarose gel electrophoresis at 100 V for 25 min and were photographed under a gel imaging system. The amplified products were sent to Shanghai Bioengineering Co. Ltd. (Shanghai, China) for sequencing, and the results were analyzed with the GenBank database of the National Center for Biotechnology Information (NCBI). A phylogenetic tree was constructed based on the 16S rDNA gene.
2.2.2. Optimization of Purification Process of the Carbon-Fixing Bacteria Using the Simulated Viaduct Rainwater
Due to issues such as fluctuating water quality, difficult sampling, and hard preservation of the actual viaduct rainwater, simulated viaduct rainwater was used for the optimization experiment to obtain the best purification process for the strain 1C-1 and provide a reference for the subsequent actual viaduct rainwater treatment. The experiment started under the initial conditions: simulated viaduct rainwater at original concentration, a carbon source dosage of 400 mg, and an inoculation amount of 10%. Single factor experiments (simulated viaduct rainwater concentration, carbon source dosage, and inoculation amount) were conducted to explore the influence of each factor on the nitrogen and phosphorus removal rate of the strain 1C-1.
- (1)
- Effect of different simulated rainwater concentrations on the purification efficiency of 1C-1
Simulated viaduct rainwater 100 mL with different concentrations (1/2 diluent, original concentration, 2 times concentrate, 5 times concentrate) was injected into a 250 mL triangular flask and was sterilized at 121 °C for 30 min. After an inoculation amount of 10%, the flask was placed on a shaker at 30 °C, 150 r·min−1 for 3 days. Three parallel experiments were performed for each treatment. After treatment, NH4+-N, TN, and TP in the simulated viaduct rainwater were measured to determine the effect of simulated viaduct rainwater concentration on purification efficiency and to find the optimal simulated viaduct rainwater concentration.
- (2)
- Effect of different carbon source dosages on the purification efficiency of 1C-1
Different carbon source dosages (glucose dosage of 100 mg, 200 mg, 400 mg, and 800 mg) were tested following the same procedures in Section 2.2.2 (1) to determine the effect of carbon source dosage on the purification efficacy and to find the optimal carbon source dosage.
- (3)
- Effect of different inoculation amounts on the purification effect of 1C-1
Different inoculation amounts (1%, 5%, 10%, and 20%) were tested following the same procedures in Section 2.2.2 (1) to determine the effect of inoculation amount on the purification efficacy and to find the optimal inoculation amount.
The removal rate was calculated according to the following equation:
Removal rate (%) = (Pollutant concentration before treatment − Pollutant concentration after treatment)/Pollutants concentration before treatment × 100
2.2.3. The Efficiency of the Carbon-Fixing Bacteria for Actual Viaduct Rainwater Treatment
Based on the above optimal conditions in Section 2.2.2, actual viaduct rainwater was used to verify the nitrogen and phosphorus removal efficiencies of 1C-1.
3. Results and Discussion
3.1. Isolation and Identification of the Carbon-Fixing Bacteria
3.1.1. Morphology Observation of 1C-1
The colony was small, dense, dry, and opaque, with a smooth surface and uneven edges, was difficult to be picked up when young, and became a hairy structure with a powdery surface when old (Figure 2). It is suspected to be a species of Actinomycete.
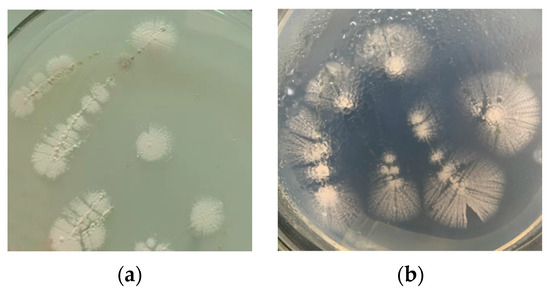
Figure 2.
Colony morphology of the strain 1C-1. (a) young colony, and (b) old colony.
Under the optical microscope, the hyphae were thin and branched. The hyphal body was well developed and was often radiating with various shapes (Figure 3). This also indicates a species of Actinomycete.
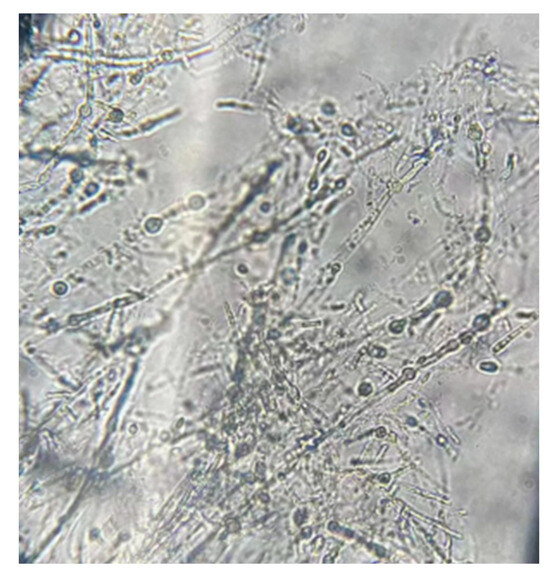
Figure 3.
Micromorphology of the strain 1C-1 (×200).
Under the SEM, the hyphae were thin. The surface of the hyphae was rough and full of irregular lines. Circular rings were present at irregular intervals on the hyphae, and both ends of the hyphae were smooth (Figure 4). This indicates a species of Streptomyces [41,42].
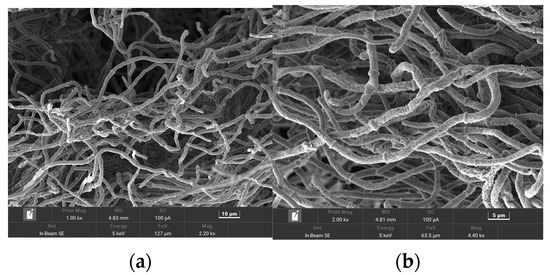
Figure 4.
Scanning electron microscopy of the strain 1C-1. (a) thin hyphae, and (b) circular rings at irregular intervals on the hyphae.
3.1.2. 16S rDNA Analysis of 1C-1
After a PCR amplification of the strain 1C-1 (Figure 5), the length of the sequenced fragment was 1431 bp. The sequence was aligned and analyzed in NCBI and BLAST databases, the 16S rDNA gene sequences of closely related species within the same genus were selected, and a phylogenetic tree was constructed using MEGA11 software (Figure 6). The phylogenetic tree shows that the strain 1C-1 is clustered together with the genus Streptomyces, with a close relationship and a similarity of more than 99%.
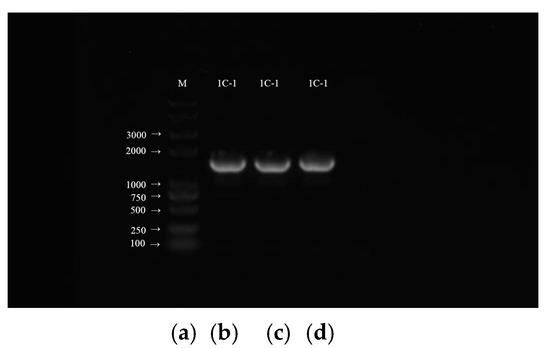
Figure 5.
PCR amplification of the strain 1C-1. (a) the marker, and (b–d) three duplications of the strain 1C-1.
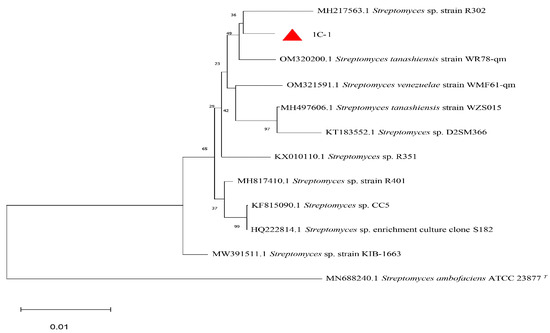
Figure 6.
Phylogenetic tree of the strain 1C-1.
In summary, based on the morphology observation and 16S rDNA analysis, the strain 1C-1 belongs to Streptomyces sp. The strain 1C-1 has been preserved in Guangdong Institute of Microbiology.
3.2. Optimization of the Purification Process of the Carbon-Fixing Bacteria Using Simulated Viaduct Rainwater
3.2.1. The Effect of Different Simulated Rainwater Concentrations on the Purification Efficiency of 1C-1
The substrate concentration directly affects bacterial growth as well as pollutant removal efficiency [43]. Therefore, taking simulated viaduct rainwater concentration as a variable with other conditions unchanged, the effect of different simulated rainwater water concentrations on the removal of NH4+-N, TN, and TP was examined, as shown in Figure 7. As the simulated viaduct rainwater concentration increased, the removal rates of NH4+-N and TN showed an overall downward trend, while TP initially increased and then decreased. When the simulated viaduct rainwater concentration was 2 times concentrate, the removal rates of NH4+-N and TP were relatively high, achieving 37.95% and 15.13%, respectively. For TN, the removal rate was relatively high at 1/2 diluent, achieving 58.28%. To balance the nitrogen and phosphorus removal efficiencies and to ensure practicability, the original concentration was chosen as the optimal simulated viaduct rainwater concentration, and the removal rates of NH4+-N and TN were 29.00% of NH4+-N, 50.73% of TN, and 9.55% of TP, respectively.
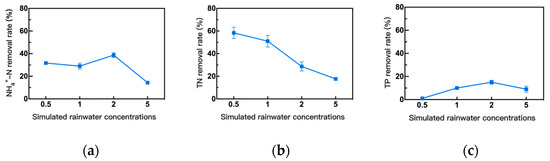
Figure 7.
Effects of different concentrations of simulated viaduct rainwater on the removal efficiency of the strain carbon-fixing bacteria. (a) the NH4+-N removal rate, (b) the TN removal rate, and (c) the TP removal rate.
3.2.2. The Effect of Different Carbon Source Dosages on the Purification Efficiency of 1C-1
Carbon source concentration directly affects pollutant removal [44,45]. Therefore, taking carbon source (glucose) dosage as a variable with other conditions unchanged, the effect of different carbon source dosages on the removal of NH4+-N, TN, and TP was examined, as shown in Figure 8. As the glucose dosage increased, the removal rates of NH4+-N and TN showed an upward trend. When the glucose dosage was 800 mg, the removal rates of NH4+-N and TN reached 71.48% and 47.86%, respectively. In contrast, as the glucose dosage increased, the removal rate of TP firstly decreased and then slowly recovered to 10.43%. This phenomenon is interesting, but the reason is not clear. It may be attributed to a mixed nutrient growth based on carbon-fixing and carbon source addition [46]. Here, the optimal carbon source dosage for glucose is 800 mg.
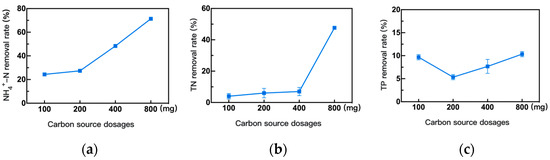
Figure 8.
Effects of different carbon source dosages on the removal efficiency of the strain 1C-1. (a) the NH4+-N removal rate, (b) the TN removal rate, and (c) the TP removal rate.
3.2.3. The Effect of Different Inoculation Amounts on the Purification Effect of 1C-1
The inoculation amount often directly determines the biomass of functional bacteria, thereby affecting pollutant removal. However, an excessive inoculation amount not only increases the inoculation cost but also hinders bacterial growth and may even result in poor pollutant removal [47]. Therefore, taking the inoculation amount as a variable with other conditions unchanged, the effect of different inoculation amounts on the removal of NH4+-N, TN, and TP was examined, as shown in Figure 9. As the inoculation amount increased, the removal rates of NH4+-N, TN, and TP showed an overall declining trend. When the inoculation amount was 1%, the removal rates of NH4+-N, TN, and TP were 58.62%, 58.35%, and 27.32%, respectively. It is thought that when the inoculation amount is too large, the nutrients in the rainwater cannot meet the normal growth of 1C-1, and the pollutant removal cannot be achieved, even with a release of NH4+-N at the inoculation amount of 20% (Figure 9a). Therefore, the optimum inoculation amount is 1%.
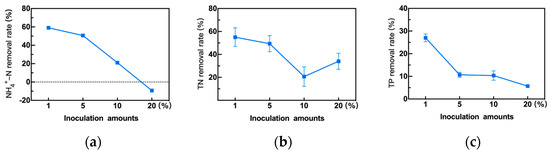
Figure 9.
Effect of different inoculation amounts on the removal efficiency of the strain 1C-1. (a) the NH4+-N removal rate, (b) the TN removal rate, and (c) the TP removal rate.
3.3. The Efficiency of the Carbon-Fixing Bacteria for Actual Viaduct Rainwater Treatment
Referring to the above results in Section 3.2, we selected the optimal process conditions of an original concentration of the viaduct rainwater, a carbon source dosage of 800 mg, and an inoculation amount of 1% for the actual viaduct rainwater treatment (Figure 10a). The removal rates of NH4+-N, TN, and TP were 92.62%, 6.98%, and 6.16%, respectively, and the mycelial pellet appeared after 3 days (Figure 10b). More interestingly, the removal rates of NH4+-N, TN, and TP achieved 43.26%, 78.02%, and 0%, respectively, even without carbon source addition (Figure 10c). It seems that there is no need for carbon source addition for the nitrogen removal from the actual viaduct rainwater for this carbon-fixing bacteria 1C-1. So far, the usage of pure bacteria for rainwater treatment has not been reported, and there is a lack of empirical data for reference. In contrast, the empirical data of the bioretention system for rainwater treatment are available. For example, in a typical zeolite-based bioretention system, the average removal efficiencies for NH4+-N, TN, and TP were 98.7%, 47.1%, and 47.5%, respectively [48]. It seems that the strain 1C-1 can attain the anticipated result. Moreover, it was reviewed that the genus Streptomyces is a powerhouse of various enzymes of commercial interest, such as the small laccases that can work under a wide range of pH, substrate specificity, and unusual resistance to inhibitors, which allows Streptomyces to perform wastewater treatment under extreme conditions [49]. It is believed that the strain 1C-1 can play a role in actual test wastewater in locations with different geographical and climatic conditions.
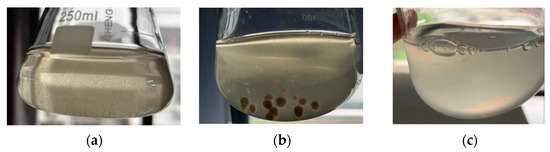
Figure 10.
Purification effect of the actual viaduct rainwater with the strain 1C-1. (a) the initial actual viaduct rainwater, (b) the actual viaduct rainwater after treatment under the optimal process conditions, with an appearance of mycelial pellet, and (c) the actual viaduct rainwater after treatment without carbon source addition.
4. Conclusions
- (1)
- Through isolation, purification, screening, and identification, a carbon-fixing bacteria strain numbered 1C-1 with water purification effects was obtained from the sediment of the viaduct rainwater tank of the bridge in Guangzhou. Based on its morphological characteristics and 16S rDNA gene molecular biology identification, the strain 1C-1 belongs to Streptomyces sp.
- (2)
- Under different simulated viaduct rainwater concentrations, the strain 1C-1 had a relatively high TN removal rate at 1/2 diluent of simulated viaduct rainwater, and a relatively high TP removal rate at 2 times concentrate. To balance the nitrogen and phosphorus removal efficiencies and to ensure practicability, the original concentration was chosen as the optimal simulated viaduct rainwater concentration with removal rates of 29.00%, 50.73%, and 9.55% for NH4+-N, TN, and TP, respectively. Under different carbon source dosages, a glucose dosage of 800 mg showed a relatively high purification efficiency with removal rates of 71.48%, 47.86%, and 10.43% for NH4+-N, TN, and TP, respectively. Under different inoculation amounts, the inoculation amount of 1% showed a relatively high efficiency with removal rates of 58.62%, 58.35%, and 27.32% for NH4+-N, TN, and TP, respectively.
- (3)
- Under the above optimal process conditions of an original concentration of the viaduct rainwater, a carbon source dosage of 800 mg, and an inoculation amount of 1%, the strain 1C-1 removed 92.62%, 6.98%, and 6.16% of NH4+-N, TN, and TP respectively from the actual viaduct rainwater. More interestingly, the removal rates of NH4+-N and TN achieved 43.26% and 78.02%, respectively, for the actual viaduct rainwater, even without carbon source addition.
To sum up, the carbon-fixing bacteria 1C-1 obtained in this study shows good nitrogen and phosphorus removal efficiencies for viaduct rainwater, especially in terms of nitrogen removal, and has application potential in viaduct rainwater treatment.
Author Contributions
Software, investigation and sampling, Q.D., H.L., X.Z. and C.X.; data curation, Q.D. and Y.L.; writing—original draft preparation, Q.D.; writing—review and editing, Y.L.; supervision Y.L.; project administration, S.B., Q.Z., W.L. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by China Transportation Fourth Navigation Engineering Institute Co., Ltd. (2021-ZJKJ-QNCX01), Key Fields of Ordinary Colleges and Universities in Guangdong Province (2022ZDZX4018 and 2022ZDZX4020), and Key Research and Development Plans in Guangzhou City (2023B03J1362).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to confidentiality issues.
Acknowledgments
The authors give many thanks to Liwen Hu for his kind help during the paper revision, and are grateful to the Guangdong Institute of Microbiology for preserving the strains.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tenebe, I.T.; Emenike, P.C.; Nnaji, C.C.; Babatunde, E.O.; Ogarekpe, N.M.; Dede-Bamfo, N.; Omole, D.O. Bacterial characterization and quantification of rainwater harvested in a rural community in Nigeria. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100370. [Google Scholar] [CrossRef]
- Huber, M.; Welker, A.; Helmreich, B. Critical review of heavy metal pollution of traffic area runoff: Occurrence, influencing factors, and partitioning. Sci. Total Environ. 2015, 541, 895–919. [Google Scholar] [CrossRef]
- Sun, X.; Jia, Q.; Ye, J.; Zhu, Y.; Song, Z.; Guo, Y.; Chen, H. Real-time variabilities in microplastic abundance and characteristics of urban surface runoff and sewer overflow in wet weather as impacted by land use and storm factors. Sci. Total Environ. 2023, 859, 160148. [Google Scholar] [CrossRef]
- Azimi, A.; Bakhtiari, A.R.; Tauler, R. Chemometrics analysis of petroleum hydrocarbons sources in the street dust, runoff and sediment of urban rivers in Anzali port—South of Caspian Sea. Environ. Pollut. 2018, 243, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sang, M.; Che, W.; Sun, H. Nutrient removal from urban stormwater runoff by an up-flow and mixed-flow bioretention system. Environ. Sci. Pollut. Res. Int. 2019, 26, 17731–17739. [Google Scholar] [CrossRef] [PubMed]
- Marszałek, A.; Dudziak, M. Application of the ultrafiltration and photooxidation process for the treatment of rainwater. Water Air Soil Pollut. 2021, 232, 504. [Google Scholar] [CrossRef]
- Zubala, T.; Patro, M. Time and spatial variability in concentrations of selected pollutants in the new bypass rainwater harvesting system. Water Air Soil Pollut. 2021, 232, 211. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Jiang, W.; Chen, L. Design of rainwater collection and treatment system for urban express way. China Water Wastewater 2007, 23, 35–39. [Google Scholar]
- Gao, Z.; Zhang, Q.; Li, J.; Wang, Y.; Dzakpasu, M.; Wang, X. First flush stormwater pollution in urban catchments: A review of its characterization and quantification towards optimization of control measures. J. Environ. Manag. 2023, 340, 117976. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, R.Y. Initial stormwater pollution control technology in the Shuangqiao River basin of Chaohu City. China Munic. Eng. 2020, 2, 56–59. [Google Scholar] [CrossRef]
- Zubala, T. The working conditions and optimisation of a large rainwater harvesting and treatment system in an area at a risk of erosion. Water Resour. Manag. 2022, 36, 135–152. [Google Scholar] [CrossRef]
- Tran, S.H.; Dang, H.T.; Dao, D.A.; Nguyen, V.A.; Nguyen, L.T.; Han, M. On-site rainwater harvesting and treatment for drinking water supply: Assessment of cost and technical issues. Environ. Sci. Pollut. Res. 2021, 28, 11928–11941. [Google Scholar] [CrossRef]
- Morales-Figueroa, C.; Castillo-Suárez, L.A.; Linares-Hernández, I.; Martínez-Miranda, V.; Teutli-Sequeira, E.A. Treatment processes and analysis of rainwater quality for human use and consumption regulations, treatment systems and quality of rainwater. Int. J. Environ. Sci. Technol. 2023, 20, 9369–9392. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Zheng, G.; Ding, G. Purification of initial rainwater stored in a storage pond by bacterial injection. J. Shanghai Univ. 2010, 16, 572–576. [Google Scholar]
- Masy, T.; Bertrand, C.; Xavier, P.M.; Vreuls, C.; Wilmot, A.; Cludts, M.; Renard, P.; Mawet, P.; Smets, S.; Dethy, B.; et al. Stable biofilms of Rhodococcus erythropolis T902.1 in draining pavement structures for runoff water decontamination. Int. Biodeterior. Biodegrad. 2016, 112, 108–118. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, P.; Cao, X.; Zhou, Y.; Song, C. Efficiency promotion and its mechanisms of simultaneous nitrogen and phosphorus removal in stormwater biofilters. Bioresour. Technol. 2016, 218, 842–849. [Google Scholar] [CrossRef]
- Hu, J.; Wang, L.; Zhang, S.; Wang, Y.; Jin, F.; Fu, X.; Li, H. Universally improving effect of mixed electron donors on the CO2 fixing efficiency of non-photosynthetic microbial communities from marine environments. J. Environ. Sci. 2014, 26, 1709–1716. [Google Scholar] [CrossRef]
- Chi, X.; Zhao, Z.; Han, Q.; Yan, H.; Ji, B.; Chai, Y.; Li, S.; Liu, K. Insights into autotrophic carbon fixation strategies through metagonomics in the sediments of seagrass beds. Mar. Environ. Res. 2023, 188, 106002. [Google Scholar] [CrossRef]
- Isobe, K.; Suetsugu, R.; Kaneko, M.; Ise, Y.; Oda, T.; Hobara, S. Chemolithotrophic microbiome of buried soil layers following volcanic eruptions: A potential huge carbon sink. Soil Biol. Biochem. 2023, 183, 109055. [Google Scholar] [CrossRef]
- Mccrimmon, D.O.; Bizimis, M.; Holland, A.; Ziolkowski, L.A. Supraglacial microbes use young carbon and not aged cryoconite carbon. Org. Geochem. 2017, 118, 63–72. [Google Scholar] [CrossRef]
- Zhao, K.; Kong, W.; Wang, F.; Long, X.; Guo, C.; Yue, L.; Yao, H.; Dong, X. Desert and steppe soils exhibit lower autotrophic microbial abundance but higher atmospheric CO2 fixation capacity than meadow soils. Soil Biol. Biochem. 2018, 127, 230–238. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, B.; Fang, X.; Fa, K.; Liu, Z. Dryland farm soil may fix atmospheric carbon through autotrophic microbial pathways. Catena 2022, 214, 106299. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Xiao, Y.; Cheng, A.; Shen, T.; Zhu, M.; Yu, L. Abundance and diversity of carbon-fixing bacterial communities in karst wetland soil ecosystems. Catena 2021, 204, 105418. [Google Scholar] [CrossRef]
- Li, Z.; Tong, D.; Nie, X.; Xiao, H.; Jiao, P.; Jiang, J.; Li, Q.; Liao, W. New insight into soil carbon fixation rate: The intensive co-occurrence network of autotrophic bacteria increases the carbon fixation rate in depositional sites. Agric. Ecosyst. Environ. 2021, 320, 107579. [Google Scholar] [CrossRef]
- Guo, J.; Fan, F.; Wang, L.; Wu, A.; Zheng, J. Isolation, identification of carbon-fixing bacteria and determination of their carbon-fixing abilities. Biotechnol. Bull. 2019, 35, 90–97. [Google Scholar]
- Kong, W.; Shen, B.; Lyu, H.; Kong, J.; Ma, J.; Wang, Z.; Feng, S. Review on carbon dioxide fixation coupled with nutrients removal from wastewater by microalgae. J. Clean. Prod. 2021, 292, 125975. [Google Scholar] [CrossRef]
- Li, M.; Ge, S.; Zhang, J.; Wu, S.; Wu, H.; Zhuang, L. Mechanism and performance of algal pond assisted constructed wetlands for wastewater polishing and nutrient recovery. Sci. Total Environ. 2022, 840, 156667. [Google Scholar] [CrossRef] [PubMed]
- Kin, I.; Ahn, K.; Lee, Y.; Jeong, Y.; Park, J.; Shin, D.; Jung, J. An experimental study on the biological fixation and effective use of carbon using biogas and bacterial community dominated by methanotrophs, methanol-oxidizing bacteria, and ammonia-oxidizing bacteria. Catalysts 2021, 11, 1342. [Google Scholar]
- Xia, W.; Bowatte, S.; Jia, Z.; Newton, P. Offsetting N2O emissions through nitrifying CO2 fixation in grassland soil. Soil Biol. Biochem. 2022, 165, 108528. [Google Scholar] [CrossRef]
- Yu, J.; Dow, A.; Pingali, S. The energy efficiency of carbon dioxide fixation by a hydrogen-oxidizing bacterium. Int. J. Hydrog. Energy 2013, 38, 8683–8690. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Tsang, Y.F.; Fu, X.; Hu, J.; Li, H.; Le, Y. Response of cbb gene transcription levels of four typical sulfur-oxidizing bacteria to the CO2 concentration and its effect on their carbon fixation efficiency during sulfur oxidation. Enzym. Microb. Technol. 2016, 92, 31–40. [Google Scholar] [CrossRef]
- Mishra, S.; Chauhan, P.; Gupta, S.; Raghuvanshi, S.; Singh, R.P.; Jha, P.N. CO2 sequestration potential of halo-tolerant bacterium Pseudomonas aeruginosa SSL-4 and its application for recovery of fatty alcohols. Process Saf. Environ. Prot. 2017, 111, 582–591. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Wang, G.; Lan, W.; Lin, J.; Bi, Q.; Shen, H.; Liang, S. Screening pretreatment methods for sludge disintegration to selectively reclaim carbon source from surplus activated sludge. Chem. Eng. J. 2014, 255, 365–371. [Google Scholar] [CrossRef]
- Hu, X.; Chen, H.; Zhang, S.; Song, W.; Li, J.; Wang, K. Study on performance of carbon source released from fruit shells and the effect on biological denitrification in the advanced treatment. Chemosphere 2022, 307, 136173. [Google Scholar] [CrossRef] [PubMed]
- Meyer, O.; Frunzke, K.; Gadkari, D.; Jacobitz, S.; Hugendieck, I.; Kraut, M. Utilization of carbon monoxide by aerobes: Recent advances. FEMS Microbiol. Lett. 1990, 87, 253–260. [Google Scholar] [CrossRef]
- Sangeetha, M.; Sivarajan, A.; Radhakrishnan, M.; Siddharthan, N.; Balagurunathan, R. Biosequestration of carbon dioxide using carbonic anhydrase from novel Streptomyces kunmingensis. Arch. Microbiol. 2022, 204, 270. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, D.; Wang, Z. The potential of using biological nitrogen removal technique for stormwater treatment. Ecol. Eng. 2017, 106, 482–495. [Google Scholar] [CrossRef]
- Wang, S.; Lin, X.; Yu, H.; Wang, Z.; Xia, H.; An, J.; Fan, G. Nitrogen removal from urban stormwater runoff by stepped bioretention systems. Ecol. Eng. 2017, 106, 340–348. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Tan, C.; Zhang, X.; Zhang, Z.; Bai, X.; Wu, L.; Yang, H. Efficient nitrogen removal from stormwater runoff by bioretention system: The construction of plant carbon source-based heterotrophic and sulfur autotrophic denitrification process. Bioresour. Technol. 2022, 349, 126803. [Google Scholar] [CrossRef]
- Peng, B.; Peng, S.; Kuang, W.; Zheng, X.; Zhang, L. Turbidity on removal of phosphorus in simulation initial rainwater by modified diatomite. Environ. Sci. Technol. 2015, 38, 36–40. [Google Scholar]
- Lu, Y.; Yan, X. Study on the classification of thermophilic Actinomycetes--I. determination of thermophilic Strepomycetes (2). J. Microbiol. 1981, 21, 414–420. [Google Scholar]
- Qian, Y.; Pan, Y.; Li, E.; Jia, H.; Wei, Y.; Liu, G. Classification of Streptomyces sp. IMS002 and identification of its antifungal metabolite. J. Microbiol. 2020, 60, 60–68. [Google Scholar]
- Singh, R.; Kumar, A.; Kirrolia, A.; Kumar, R.; Yadav, N.; Bishnoi, N.R.; Lohchab, R.K. Removal of sulphate, COD and Cr(VI) in simulated and real wastewater by sulphate reducing bacteria enrichment in small bioreactor and FTIR study. Bioresour. Technol. 2011, 102, 677–682. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Y.; Liu, Z.; Liu, J.; Cai, J. Optimum removal conditions of aniline compounds in simulated wastewater by laccase from white-rot fungi. J. Environ. Health Sci. Eng. 2019, 17, 135–140. [Google Scholar] [CrossRef]
- Wang, H.; Chen, N.; Feng, C.; Deng, Y. Insights into heterotrophic denitrification diversity in wastewater treatment systems: Progress and future prospects based on different carbon sources. Sci. Total Environ. 2021, 780, 146521. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Tao, Y.; Liu, J.; Lyu, T.; Yang, X.; Ma, S.; Dong, J.; Dou, H.; Zhang, H. Effects of emergent plants on soil carbon-fixation and denitrification processes in freshwater and brackish wetlands in a watershed in northern China. Geoderma 2023, 430, 116311. [Google Scholar] [CrossRef]
- Ibrahim, A.J. Effective biodeterioration of a common endocrine disruptor 17β-estradiol using mixed microbial cultures isolated from waste water. Environ. Res. 2021, 206, 112559. [Google Scholar]
- Chen, Y.; Shao, Z.; Kong, Z.; Gu, L.; Fang, J.; Chai, H. Study of pyrite based autotrophic denitrification system for low-carbon source stormwater treatment. J. Water Process Eng. 2020, 37, 101414. [Google Scholar] [CrossRef]
- Kaur, R.; Salwan, R.; Sharma, V. Structural properties, genomic distribution of laccases from Streptomyces and their potential applications. Process Biochem. 2022, 118, 133–144. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).