Analysis of Resource Potential of Emergent Aquatic Vegetation in the Curonian Lagoon of the Baltic Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sampling Procedure
2.2. Determination of the Physicochemical Composition of Aquatic Plants
3. Results and Discussion
3.1. Results of Emergent Aquatic Vegetation Species Diversity and Biomass Assessment
3.2. Assessment of the Physicochemical Properties of Emergent Aquatic Vegetation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christian, F.; Arturas, R.; Saulius, G.; Georg, U.; Lina, B. Hydraulic regime-based zonation scheme of the Curonian Lagoon. Hydrobiologia 2008, 611, 133–146. [Google Scholar] [CrossRef]
- Shibaev, S.V.; Khlopnikov, M.M.; Sokolov, A.V. Fishery Cadastre of Trans-Boundary Reservoirs of Russia (the Kaliningrad Region) and Lithuania; IP Mishutkina: Kaliningrad, Russia, 2008; pp. 84–165. (In Russian) [Google Scholar]
- Aleksandrov, S.V. Long-term variability of the trophic status of the Curonian and Vistula Lagoons of the Baltic Sea. Inland Water Biol. 2009, 2, 319–326. [Google Scholar] [CrossRef]
- Aleksandrov, S.V. Biological production and eutrophication of Baltic Sea estuarine ecosystems: The Curonian and Vistula Lagoons. Mar. Pollut. Bull. 2010, 61, 205. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, S.; Krek, A.; Bubnova, E.; Danchenkov, A. Eutrophication and effects of algal bloom in the south-western part of the Curonian Lagoon alongside the Curonian spit. Baltica 2018, 31, 1–12. [Google Scholar] [CrossRef]
- Bartoli, M.; Zilius, M.; Bresciani, M.; Vaiciute, D.; Vybernaite-Lubiene, I.; Petkuviene, J.; Giordani, G.; Daunys, D.; Ruginis, T.; Benelli, S.; et al. Drivers of Cyanobacterial Blooms in a Hypertrophic Lagoon. Front. Mar. Sci. 2018, 5, 434. [Google Scholar] [CrossRef]
- Komulainen, M.; Simi, P.; Hagelberg, E.; Ikonen, I.; Lyytinen, S. Reed Energy. Possibilities of Using the Common Reed for Energy Generation in Southern Finland. Repo. Turku Uni. Appl. Sci. 2008, 67, 80–81. [Google Scholar]
- Iital, A.; Klõga, M.; Kask, Ü.; Voronova, V.; Cahill, B. Reed harvesting. In Compendium: An Assessment of Innovative and Sustainable Uses of Baltic Marine Resources; Maritime Institute in Gdansk: Gdansk, Poland, 2012; pp. 103–124. [Google Scholar]
- Morkūnė, R.; Petkuvienė, J.; Bružas, M.; Morkūnas, J.; Bartoli, M. Monthly Abundance Patterns and the Potential Role of Waterbirds as Phosphorus Sources to a Hypertrophic Baltic Lagoon. Water 2020, 12, 1392. [Google Scholar] [CrossRef]
- Kaziukonytė, K.; Lesutienė, J.; Gasiūnaitė, Z.R.; Morkūnė, R.; Elyaagoubi, S.; Razinkovas-Baziukas, A. Expert-based assessment and mapping of ecosystem services potential in the Nemunas Delta and Curonian Lagoon region, Lithuania. Water 2021, 13, 2728. [Google Scholar] [CrossRef]
- Karstens, S.; Buczko, U.; Glatzel, S. Phosphorus storage and mobilization in coastal Phragmites wetlands: Influence of local-scale hydrodynamics. Estuar. Coast Shelf Sci. 2015, 164, 124–133. [Google Scholar] [CrossRef]
- Allirand, J.-M.; Gosse, G. An aboveground biomass production model for a common reed (Phragmites communis Trin.) stand. Biomass Bioenergy 1995, 9, 441–448. [Google Scholar] [CrossRef]
- Köbbing, J.; Thevs, N.; Zerbe, S. The utilization of reed (Phragmites australis): A review. Mires. Peat. 2013, 13, 1–14. [Google Scholar]
- Katanskaya, V.M. Higher Aquatic Vegetation of the Continental Reservoirs of the USSR. Methods of Study; Nauka: Leningrad, Russia, 1981; pp. 34–187. (In Russian) [Google Scholar]
- Tsvelev, N.N. Determinant of Vascular Plants of North-Western Russia; SPHFA Publishing House: St. Petersburg, Russia, 2000; pp. 20–750. [Google Scholar]
- Smart, M.M.; Reid, F.A.; Jones, J.R. A Comparison of a Persulfate Digestion and the Kjeldahl Procedure for Determination of Total Nitrogen in Freshwater Samples. Water Res. 1981, 15, 919–921. [Google Scholar] [CrossRef]
- Holman, B.; Bailes, K.; Meyer, R.; Hopkins, D. Effect of modified Soxhlet and Folch extraction method selection on the total lipid determination of aged beef. J. Food Sci. Technol. 2019, 56, 3957–3961. [Google Scholar] [CrossRef] [PubMed]
- Durynina, E.P.; Egorov, V.S. Agrochemical Analysis of Soils, Plants, Fertilizers; MSU Publishing House: Moscow, Russia, 1998; pp. 87–90. (In Russian) [Google Scholar]
- Stashko, A.V.; Aleksandrov, S.V. Specific features of spatial distribution of hydrochemical parameters in the Curonian Lagoon of the Baltic Sea in 2018–2022. Aquat. Biores. Environ. 2023, 6, 48–61. [Google Scholar]
- Hansson, P.-A.; Fredriksson, H. Use of summer harvested common reed (Phragmites Australis) as nutrient source for organic crop production in Sweden. Agric. Ecosyst. Environ. 2004, 102, 365–375. [Google Scholar] [CrossRef]
- Karstens, S.; Inácio, M.; Schernewski, G. Expert-based evaluation of ecosystem service provision in coastal reed wetlands under different management regimes. Front. Environ. Sci. 2019, 7, 63. [Google Scholar] [CrossRef]
- Jakimavičius, D.; Kriaučiūnienė, J.; Šarauskienė, D. Impact of climate change on the Curonian Lagoon water balance components, salinity and water temperature in the 21st century. Oceanologia 2018, 60, 378–389. [Google Scholar] [CrossRef]
- Huang, M.; Sheng, Q.; Wu, J.; Pan, X. Effects of winter harvesting and salinity on the structure of Regrowing Reed stands. Am. J. Plant Sci. 2014, 5, 3250–3257. [Google Scholar] [CrossRef]
- Lissner, J.; Schierup, H.-H. Effects of salinity on the growth of phragmites australis. Aquat. Bot. 1997, 55, 247–260. [Google Scholar] [CrossRef]
- Andersen, J.; Laamanen, M.; Aigars, J.; Axe, P.; Blomqvist, M.; Carstensen, J.; Claussen, U.; Josefson, A.B.; Fleming-Lehtinen, V.; Järvinen, M.; et al. HELCOM Eutrophication in the Baltic Sea—An integrated thematic assessment of the effects of nutrient enrichment and eutrophication in the Baltic Sea region. Balt. Sea Environ. Proc. 2009, 115, 1–148. [Google Scholar] [CrossRef]
- Portnov, D.; Subbotin, D.; Kazakov, A.; Zavorin, A. The Peat and Wood Gasification at Different Conditions of the Pyrolysis Process. MATEC Web Conf. 2015, 37, 01043. [Google Scholar] [CrossRef]
- Cai, H.; Yang, K.; Zhang, Q.; Zhao, K.; Gu, S. Pyrolysis Characteristics of Typical Biomass Thermoplastic Composites. Results Phys. 2017, 7, 3230–3235. [Google Scholar] [CrossRef]
- Beyzi, S.B.; Ülger, İ.; Konca, Y. Chemical, Fermentative, Nutritive and Anti-nutritive Composition of Common Reed (Phragmites australis) Plant and Silage. Waste Biomass Valor. 2023, 14, 927–936. [Google Scholar] [CrossRef]
- Pimchan, P.; Usoungnern, S.; Bidon, S.; Duangde, S.; Utaiku, A. Study on chemical composition of Typha angustifalia L and extracted cellulose from Typha angustifalia L for Food Applications. J. Appl. Sci. 2020, 19, 116–128. [Google Scholar] [CrossRef]
- Little, E.C.S. Handbook of Utilization of Aquatic Plants: A Compilation of the World’s Publications; Food and Agriculture Organization of the UNO: Rome, Italy, 1968; pp. 56–58. [Google Scholar]
- Cao, W.; Li, J.; Martí-Rosselló, T.; Zhang, X. Experimental study on the ignition characteristics of cellulose, hemicellulose, lignin and their mixtures. J. Energy Inst. 2019, 92, 1303–1312. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, H.; Ozao, R.; Cao, Y.; Chen, B.I.-T.; Wang, C.-W.; Pan, W.-P. Characterization of Activated Carbon Prepared from Chicken Waste and Coal. Energy Fuels 2007, 21, 3735–3739. [Google Scholar] [CrossRef]
- Stanislav, V.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Petráš, R.; Mecko, J.; Kukla, J.; Kuklová, M.; Krupová, D.; Pástor, M.; Raček, M.; Pivková, I. Energy stored in above-ground biomass fractions and model trees of the main coniferous woody plants. Sustainability 2021, 13, 12686. [Google Scholar] [CrossRef]
- Pocienė, L.; Kadžiulienė, Ž. Biomass yield and fibre components in reed canary grass and tall fescue grown as feedstock for combustion. Zemdirbyste-Agric. 2016, 103, 297–304. [Google Scholar] [CrossRef]
- Valkama, E.; Lyytinen, S.; Koricheva, J. The Impact of Reed Management on Wildlife, a Meta-Analytical Review of European Studies. Biol. Conserv. 2008, 141, 364–374. [Google Scholar] [CrossRef]
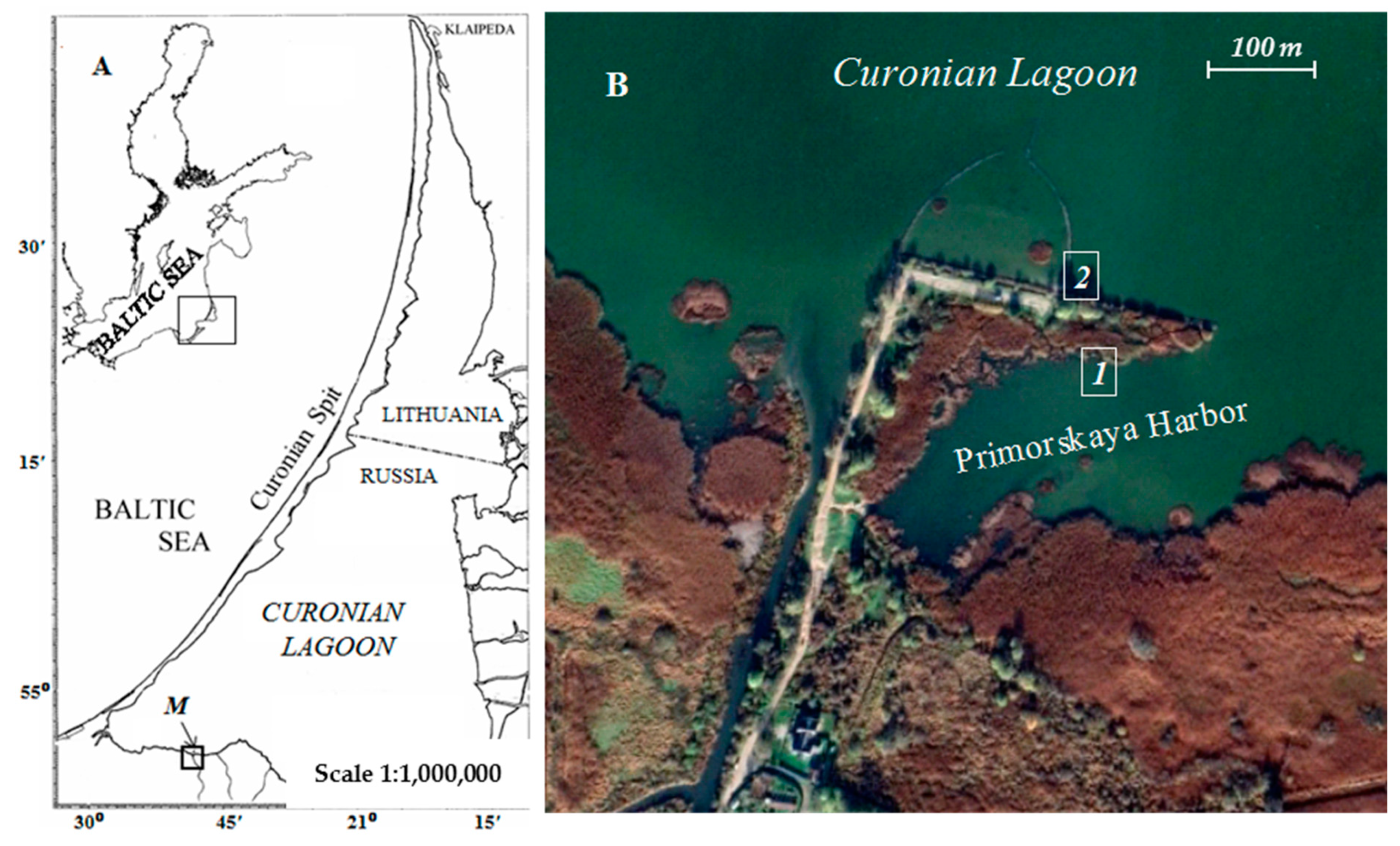

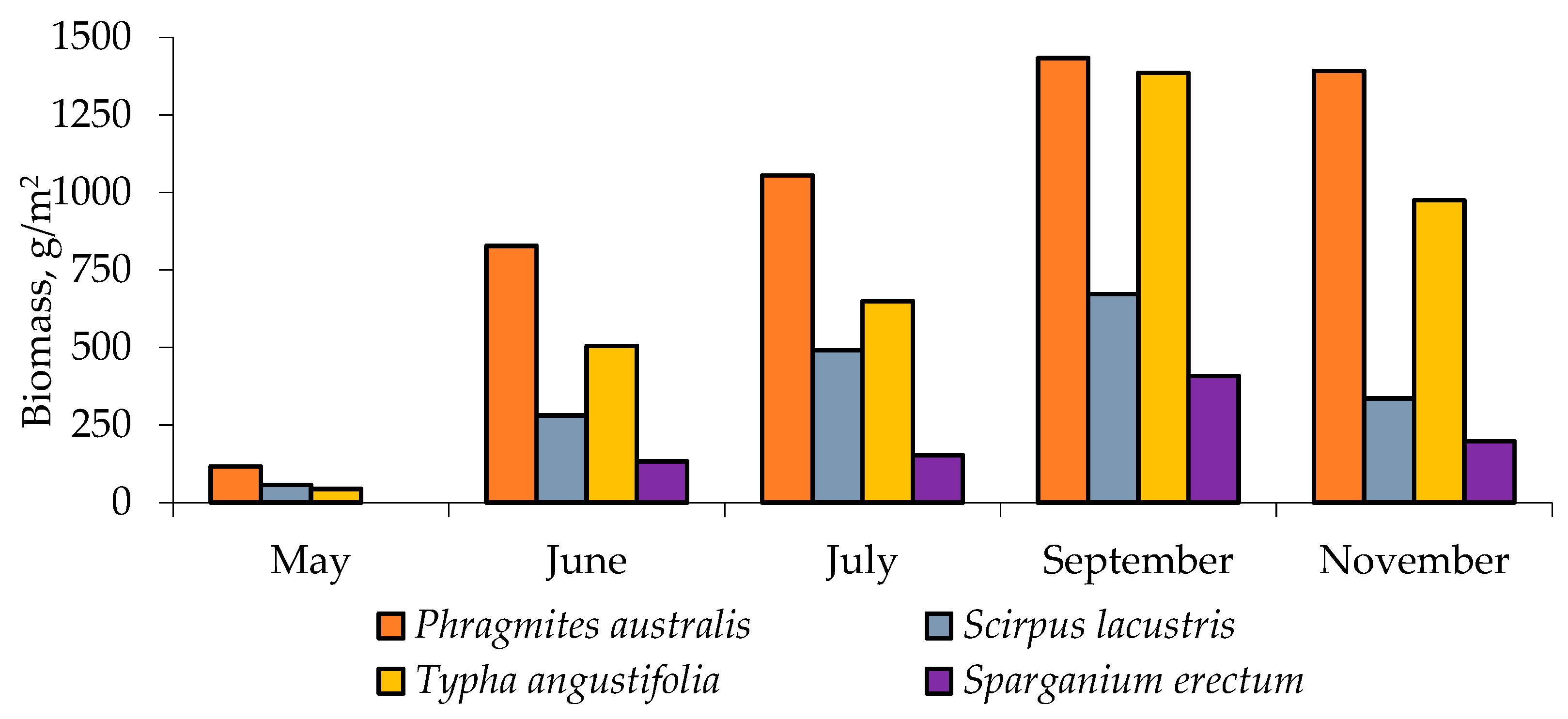



| Species | Air-Dry Biomass, g/m2 | |
|---|---|---|
| September | Average (May–November) | |
| Phragmites australis (Cav.) Trin.ex Stend. | 1433 | 965 |
| Scirpus lacustris L. | 672 | 367 |
| Typha angustifolia L. | 1386 | 712 |
| Sparganium erectum L. | 408 | 223 |
| Butomus umbellatus L. | 85 | 102 |
| Nuphar luteum (L.) Smith | - | 75 |
| Potamogeton perfoliatus L. | - | 35 |
| Ceratophyllum demersum L. | - | 15 |
| Sample | Source | Content, % | ||||
|---|---|---|---|---|---|---|
| C | H | N | S | O (calc.) | ||
| Phragmites australis | Experimental results | 49.56 ± 0.98 | 7.09 ± 0.08 | 1.25 ± 0.57 | 0.08 ± 0.01 | 42.01 |
| Scirpus lacustris | 45.65 ± 0.93 | 6.67 ± 0.12 | 0.55 ± 0.03 | 0.41 ± 0.17 | 46.73 | |
| Typha angustifolia | 45.66 ±3.13 | 6.55 ± 0.28 | 0.95 ± 0.10 | 0.37 ± 0.06 | 46.47 | |
| Sparganium erectum | 44.79 ± 0.53 | 6.48 ± 0.11 | 1.78 ± 0.25 | 0.32 ± 0.20 | 46.63 | |
| Butomus umbellatus | 36.82 ± 0.87 | 5.28 ± 0.09 | 1.42 ± 0.11 | 0.27 ± 0.05 | 56.21 | |
| Coniferous wood | [26] | 48.56 | 11.84 | 0.7 | 0.06 | 38.84 |
| Corn stalk | [27] | 49.30 | 6.00 | 0.70 | 0.11 | 43.89 |
| Sample | Protein Content | Lipid Content | Carbohydrates | Ash Content, d.m.% |
|---|---|---|---|---|
| Ash-Free Dry Mass, % | ||||
| Phragmites australis | 5.85 ± 0.29 | 3.03 ± 0.28 | 81.30 | 8.83 ± 0.04 |
| Scirpus lacustris | 4.84 ± 0.01 | 3.81 ± 0.95 | 82.69 | 8.66 ± 0.07 |
| Typha angustifolia | 4.65 ± 0.16 | 3.1 ± 0.29 | 84.51 | 7.75 ± 0.48 |
| Sparganium erectum | 6.25 ± 0.09 | 2.81 ± 0.21 | 81.92 | 9.02 ± 0.28 |
| Butomus umbellatus | 5.20 ± 0.08 | 3.47 ± 0.26 | 81.48 | 9.85 ± 0.07 |
| Sample | Number of Main Stages | t1 | t2 | tmax | ∆m, % | Rest, % | NCV, KJ/g |
|---|---|---|---|---|---|---|---|
| Phragmites australis | 1 | 220 | 380 | 351 | 60.8 | 7.6 | 12.62 |
| 2 | 380 | 700 | 464 | 28.9 | |||
| Scirpus lacustris | 1 | 250 | 380 | 345 | 66.2 | 6.6 | 12.55 |
| 2 | 380 | 450 | 421 | 22.5 | |||
| 3 | 450 | 560 | 463 | ||||
| Typha angustifolia | 1 | 210 | 380 | 344 | 57.7 | 8.8 | 12.55 |
| 2 | 380 | 590 | 453 | 28.5 | |||
| Sparganium erectum | 1 | 240 | 410 | 338 | 56.7 | 10.4 | 11.57 |
| 2 | 410 | 540 | 487 | 24.6 | |||
| Butomus umbellatus | 1 | 130 | 370 | 323 | 42.8 | 19.1 | 10.31 |
| 2 | 370 | 510 | 468 | 26.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikova, Y.; Gorbunova, J.; Aleksandrov, S.; Krasnovskih, M.; Gurchenko, V.; Babich, O. Analysis of Resource Potential of Emergent Aquatic Vegetation in the Curonian Lagoon of the Baltic Sea. Water 2023, 15, 2136. https://doi.org/10.3390/w15112136
Kulikova Y, Gorbunova J, Aleksandrov S, Krasnovskih M, Gurchenko V, Babich O. Analysis of Resource Potential of Emergent Aquatic Vegetation in the Curonian Lagoon of the Baltic Sea. Water. 2023; 15(11):2136. https://doi.org/10.3390/w15112136
Chicago/Turabian StyleKulikova, Yuliya, Julia Gorbunova, Sergey Aleksandrov, Marina Krasnovskih, Valentin Gurchenko, and Olga Babich. 2023. "Analysis of Resource Potential of Emergent Aquatic Vegetation in the Curonian Lagoon of the Baltic Sea" Water 15, no. 11: 2136. https://doi.org/10.3390/w15112136
APA StyleKulikova, Y., Gorbunova, J., Aleksandrov, S., Krasnovskih, M., Gurchenko, V., & Babich, O. (2023). Analysis of Resource Potential of Emergent Aquatic Vegetation in the Curonian Lagoon of the Baltic Sea. Water, 15(11), 2136. https://doi.org/10.3390/w15112136








