RETRACTED: Phytoplankton Carbon Utilization Strategies and Effects on Carbon Fixation
Abstract
1. Introduction
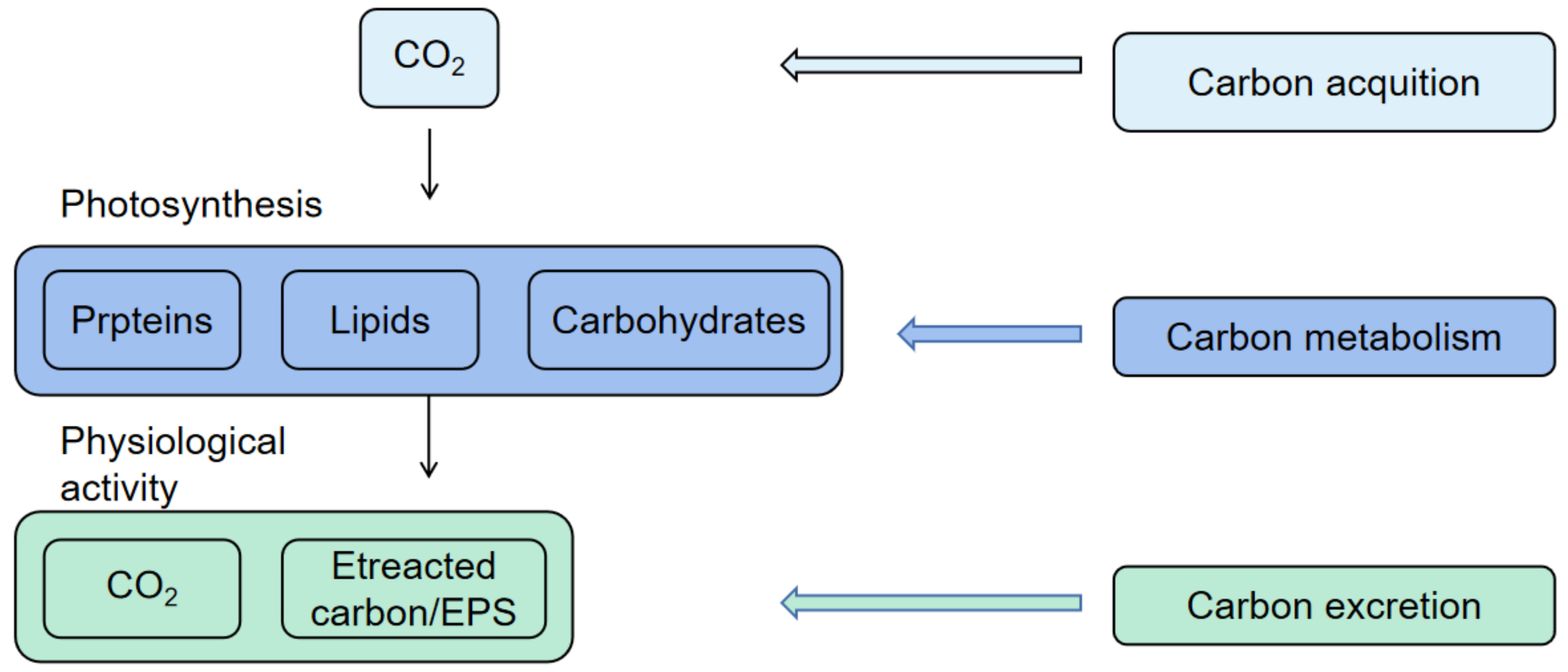
2. Carbon Acquisition
2.1. CO2 Fixation Mechanism
2.2. Phagocytosis Mechanism
2.3. Motility
3. Carbon Metabolism
3.1. Light Adaptation-Photosynthetic Pigment Regulation
3.2. Light Suppression and Light Protection
3.3. Carbon Flow Distribution-Biochemical Composition
3.3.1. Phosphorus Limitation
3.3.2. Nitrogen Limitation
3.3.3. Silicon Limitation
3.3.4. Temperature
3.3.5. Light
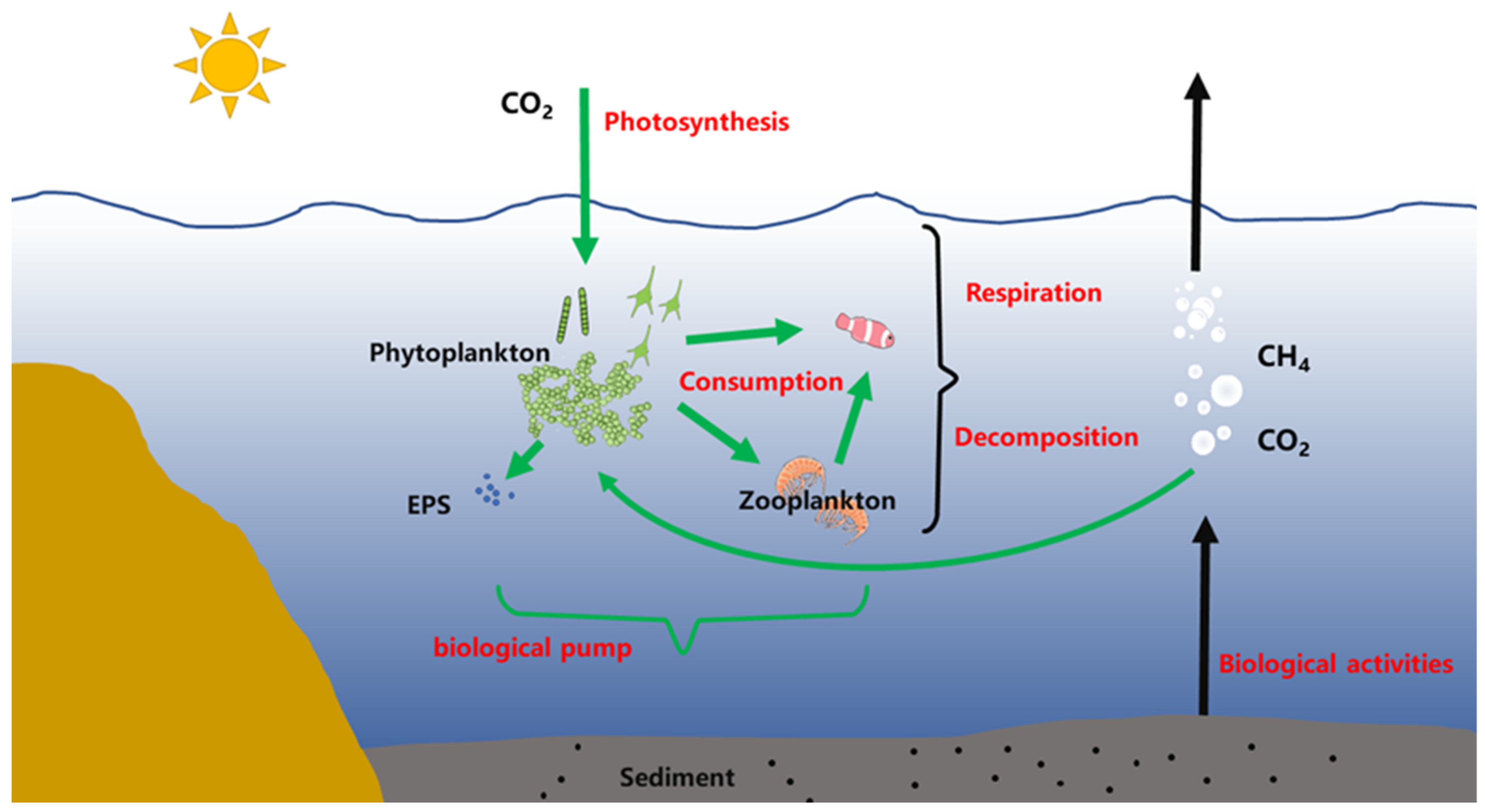
4. Carbon Excretion
5. Carbon Fixation of Phytoplankton under Different Carbon Utilization Strategies
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.A.; Cheng, H.F. The urgency of assessing the greenhouse gas budgets of hydroelectric reservoirs in China. Nat. Clim. Chang. 2013, 3, 708–712. [Google Scholar] [CrossRef]
- Gunkel, G. Hydropower—A Green Energy? Tropical Reservoirs and Greenhouse Gas Emissions. Clean-Soil Air Water 2009, 37, 726–734. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate. Blooms like it hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [PubMed]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Env. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Xiao, Q.T.; Xu, X.F.; Duan, H.T.; Qi, T.C.; Qin, B.Q.; Lee, X.; Hu, Z.H.; Wang, W.; Xiao, W.; Zhang, M. Eutrophic Lake Taihu as a significant CO2 source during 2000–2015. Water Res. 2020, 170, 115331. [Google Scholar] [CrossRef]
- Anderson, N.J.; Bennion, H.; Lotter, A.F. Lake eutrophication and its implications for organic carbon sequestration in Europe. Glob. Chang. Biol. 2014, 20, 2741–2751. [Google Scholar] [CrossRef]
- Davidson, T.A.; Audet, J.; Svenning, J.C.; Lauridsen, T.L.; Sondergaard, M.; Landkildehus, F.; Larsen, S.E.; Jeppesen, E. Eutrophication effects on greenhouse gas fluxes from shallow-lake mesocosms override those of climate warming. Glob. Chang. Biol. 2015, 21, 4449–4463. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Jansson, M.; Karlsson, J.; Jonsson, A. Carbon dioxide supersaturation promotes primary production in lakes. Ecol. Lett. 2012, 15, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Trejos, E.; Brandt, G.; Merico, A.; Smith, S.L. Biogeographical patterns of phytoplankton community size structure in the oceans. Glob. Ecol. Biogeogr. 2013, 22, 1060–1070. [Google Scholar] [CrossRef]
- Grossart, H.P. Interactions between marine bacteria and axenic diatoms (Cylindrotheca fusiformis, Nitzschia laevis, and Thalassiosira weissflogii) incubated under various conditions in the lab. Aquat. Microb. Ecol. 1999, 19, 1–11. [Google Scholar] [CrossRef]
- Boyd, P.; Laroche, J.; Gall, M.; Frew, R.; Mckay, R.M.L. Role of iron, light, and silicate in controlling algal biomass in subantarctic waters SE of New Zealand. J. Geophys. Res. Ocean. 1999, 104, 13395–13408. [Google Scholar] [CrossRef]
- Barranguet, C.; Kromkamp, J.; Peene, J. Factors controlling primary production and photosynthetic characteristics of intertidal microphytobenthos. Mar. Ecol. Prog. Ser. 1998, 173, 117–126. [Google Scholar] [CrossRef]
- Geider, R.J.; Macintyre, H.L.; Kana, T.M. A dynamic regulatory model of phytoplanktonic acclimation to light, nutrients, and temperature. Limnol. Oceanogr. 1998, 43, 679–694. [Google Scholar] [CrossRef]
- Geider, R.J.; Macintyre, H.L.; Kana, T.M. Dynamic model of phytoplankton growth and acclimation: Responses of the balanced growth rate and the chlorophyll a:carbon ratio to light, nutrient-limitation and temperature. Mar. Ecol. Prog. Ser. 1997, 148, 187–200. [Google Scholar] [CrossRef]
- Shi, D.; Xu, Y.; Morel, F.M.M. Effects of the pH/pCO(2) control method on medium chemistry and phytoplankton growth. Biogeosciences 2009, 6, 1199–1207. [Google Scholar] [CrossRef]
- Badger, M.R. Kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase from Anabaena variabilis. Arch. Biochem. Biophys. 1980, 201, 247–254. [Google Scholar] [CrossRef]
- Miller, A.G.; Espie, G.S.; Canvin, D.T. Physiological aspects of CO2 and HCO3− transport by cyanobacteria: A review. Can. J. Bot. 1990, 68, 1291–1302. [Google Scholar] [CrossRef]
- Badger, M.R.; Andrews, T.J.; Whitney, S.M.; Ludwig, M.; Yellowlees, D.C.; Leggat, W.; Price, G.D. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can. J. Bot. 1998, 76, 1052–1071. [Google Scholar] [CrossRef]
- Reinfelder, J.R.; Kraepiel, A.M.; Morel, F.M. Unicellular C4 photosynthesis in a marine diatom. Nature 2000, 407, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Morel, F.M.M.; Cox, E.H.; Kraepiel, A.M.L.; Lane, T.W.; Milligan, A.J.; Schaperdoth, I.; Reinfelder, J.R.; Tortell, P.D. Acquisition of inorganic carbon by the marine diatom Thalassiosira weissflogii. Funct. Plant Biol. 2002, 29, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Reinfelder, J.R.; Milligan, A.J.; Morel, F.M. The role of the C4 pathway in carbon accumulation and fixation in a marine diatom. Plant Physiol. 2004, 135, 2106–2111. [Google Scholar] [CrossRef]
- Badger, M.R.; Hanson, D.; Price, G.D. Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Funct. Plant Biol. 2002, 29, 161–173. [Google Scholar] [CrossRef]
- Badger, M.R.; Price, G.D. CO2 concentrating mechanisms in cyanobacteria: Molecular components, their diversity and evolution. J. Exp. Bot. 2003, 54, 609–622. [Google Scholar] [CrossRef]
- Kaplan, A.; Reinhold, L. CO2 concentrating mechanisms in photosynthetic microorganisms. Annu. Rev. Plant Phys. 1999, 50, 539–570. [Google Scholar] [CrossRef]
- Colman, B.; Huertas, I.E.; Bhatti, S.; Dason, J.S. The diversity of inorganic carbon acquisition mechanisms in eukaryotic microalgae. Funct. Plant Biol. 2002, 29, 261–270. [Google Scholar] [CrossRef]
- Pronina, N.A.; Rogova, N.B. Carbonic anhydrase activity and fatty acid composition in photosistem-deficient and high-CO(2)-required mutant of Chlamydomonas reinhardtII. Phycologia 1997, 36, 91. [Google Scholar]
- Raven, J.A. Putting the C in phycology. Eur. J. Phycol. 1997, 32, 319–333. [Google Scholar] [CrossRef]
- Raven, J.A. Inorganic carbon acquisition by marine autotrophs. Adv. Bot. Res. 1997, 27, 85–209. [Google Scholar]
- Raven, J.A. CO2-concentrating mechanisms: A direct role for thylakoid lumen acidification? Plant Cell Environ. 1997, 20, 147–154. [Google Scholar] [CrossRef]
- Van Hunnik, E.; Sultemeyer, D. A possible role for carbonic anhydrase in the lumen of chloroplast thylakoids in green algae. Funct. Plant Biol. 2002, 29, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Phagotrophy in phototrophs. Limnol. Oceanogr. 1997, 42, 198–205. [Google Scholar] [CrossRef]
- Li, J.; Chen, W.; Gao, K.; Beardall, J. Carbon concentrating mechanisms in phytoplankton: Molecular aspects and ecological implications. Aquat. Bot. 2022, 173, 103498. [Google Scholar]
- Nygaard, K.; Tobieson, A. Bacterivory in Algae: A Survival Strategy during Nutrient Limitation. Limnol. Oceanogr. 1993, 38, 273–279. [Google Scholar] [CrossRef]
- Koide, R.; Smith, D.C.; Douglas, A.E. The Biology of Symbiosis. In The Bryologist; American Bryological and Lichenological Society: Bellevue, PA, USA, 1987. [Google Scholar]
- Fenchel, T. Ecology of Heterotrophic Microflagellates. II. Bioenergetics and Growth. Mar. Ecol. Prog. Ser. 1982, 8, 225–231. [Google Scholar] [CrossRef]
- Pang, M.W.; Liu, K.L.; Liu, H.B. Evidence for mixotrophy in pico-chlorophytes from a new Picochlorum (Trebouxiophyceae) strain. J. Phycol. 2021, 58, 80–91. [Google Scholar] [CrossRef]
- Princiotta, S.D.; Smith, B.T.; Sanders, R.W. Temperature-Dependent Phagotrophy and Phototrophy in a Mixotrophic Chrysophyte. J. Phycol. 2016, 52, 432–440. [Google Scholar] [CrossRef]
- Schuergers, N.; Mullineaux, C.W.; Wilde, A. Cyanobacteria in motion. Curr. Opin. Plant Biol. 2017, 37, 109–115. [Google Scholar] [CrossRef]
- Ehlers, K.M.; Samuel, A.D.T.; Berg, H.C.; Montgomery, R. Do cyanobacteria swim using traveling surface waves? Proc. Natl. Acad. Sci. USA 1996, 93, 8340–8343. [Google Scholar] [CrossRef] [PubMed]
- Ueki, N.; Ide, T.; Mochiji, S.; Kobayashi, Y.; Tokutsu, R.; Ohnishi, N.; Yamaguchi, K.; Shigenobu, S.; Tanaka, K.; Minagawa, J.; et al. Eyespot-dependent determination of the phototactic sign in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2016, 113, 5299–5304. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Sipos, O.; Valkai, S.; Gombai, E.; Hodula, O.; Kerenyi, A.; Ormos, P.; Galajda, P. Microfluidic study of the chemotactic response of Escherichia coli to amino acids, signaling molecules and secondary metabolites. Biomicrofluidics 2015, 9, 044105. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.M.; Che, H.X.; Guo, C.; Liu, C.Z.; Low, S.C.; Chan, D.J.C.; Mohamud, R.; Lim, J. Artificial Magnetotaxis of Microbot: Magnetophoresis versus Self-Swimming. Langmuir 2018, 34, 7971–7980. [Google Scholar] [CrossRef]
- Halsey, K.H.; Milligan, A.J.; Behrenfeld, M.J. Contrasting strategies of photosynthetic energy utilization drive lifestyle strategies in ecologically important picoeukaryotes. Metabolites 2014, 4, 260–280. [Google Scholar] [CrossRef]
- Worden, A.Z.; Lee, J.H.; Mock, T.; Rouze, P.; Simmons, M.P.; Aerts, A.L.; Allen, A.E.; Cuvelier, M.L.; Derelle, E.; Everett, M.V.; et al. Green Evolution and Dynamic Adaptations Revealed by Genomes of the Marine Picoeukaryotes Micromonas. Science 2009, 324, 268–272. [Google Scholar] [CrossRef]
- Girdner, S.; Mack, J.; Buktenica, M. Impact of nutrients on photoacclimation of phytoplankton in an oligotrophic lake measured with long-term and high-frequency data: Implications for chlorophyll as an estimate of phytoplankton biomass. Hydrobiologia 2020, 847, 1817–1830. [Google Scholar] [CrossRef]
- Clementson, L.A.; Wojtasiewicz, B. Dataset on the in vivo absorption characteris-tics and pigment composition of various phytoplankton species. Data Brief 2019, 25, 104020. [Google Scholar] [CrossRef]
- Baird, M.E.; Emsley, S.M.; Mcglade, J.M. Modelling the interacting effects of nutrient uptake, light capture and temperature on phytoplankton growth. J. Plankton Res. 2001, 23, 829–840. [Google Scholar] [CrossRef]
- Heidenreich, K.M.; Richardson, T.L. Photopigment, Absorption, and Growth Responses of Marine Cryptophytes to Varying Spectral Irradiance. J. Phycol. 2020, 56, 507–520. [Google Scholar] [CrossRef]
- Geider, R. Light and Temperature Dependence of the Carbon to Chlorophyll a Ratio in Microalgae and Cyanobacteria. New Phytol. 1987, 106, 1–34. [Google Scholar] [CrossRef]
- Brown, M.A.; Mouget, J.L.; Lee, J. Temperature-dependent pigment content and growth rates of three phytoplankton species. Algal Res. 2021, 56, 102413. [Google Scholar]
- Halsey, K.H.; Jones, B.M. Phytoplankton Strategies for Photosynthetic Energy Allocation. Annu. Rev. Mar. Sci. 2015, 7, 265–297. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Lee, S.J.; Lee, W.K. Application of chlorophyll fluorescence imaging for in-situ monitoring of harmful algal blooms. Environ. Sci. Pollut. Res. 2021, 28, 29435–29446. [Google Scholar]
- Desnues, A.; Piganeau, G.; Moreau, H. Ecophysiology of picophytoplankton: What can we learn from their pigment signatures? J. Phycol. 2021, 57, 1043–1058. [Google Scholar]
- Kim, M.S.; Kim, Y.O.; Kim, S.Y. Effects of nutrient and light availability on cel-lular chlorophyll a content in three phytoplankton species. J. Ecosyst. Ecography 2021, 11, 1–7. [Google Scholar]
- Chen, H.; Zhou, W.; Chen, W.X.; Xie, W.; Jiang, L.P.; Liang, Q.L.; Huang, M.J.; Wu, Z.W.; Wang, Q. Simplified, rapid, and inexpensive estimation of water primary productivity based on chlorophyll fluorescence parameter. J. Plant Physiol. 2017, 211, 128–135. [Google Scholar] [CrossRef]
- Cermeño, P.; Lee, J.A.; Wyman, K.; Schofield, O. Variability and drivers of carbon flux attenuation in the upper ocean from the Southern Ocean to the North Atlantic. Glob. Biogeochem. Cycles 2018, 32, 708–728. [Google Scholar]
- Lee, Y.; Matrai, P.A.; Friedrichs, M.A.M.; Saba, V.S.; Antoine, D.; Ardyna, M.; Asanuma, I.; Babin, M.; Bélanger, S.; Benoît-Gagné, M.; et al. An assessment of phytoplankton primary productivity in the Arctic Ocean from satellite ocean color/in situ chlorophyll-a based models. J. Geophys. Res. Oceans 2015, 120, 6508–6541. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, H.; Liu, J. Habitat selection, adaptation and invasion: A unified framework for predicting species distributions. Ecol. Lett. 2021, 24, 34–50. [Google Scholar]
- Chowdhury, A.; Kaviraj, A. Mitigation of photodamage in microalgae under fluctuating light: An overview. Algal Res. 2022, 60, 102678. [Google Scholar]
- Wang, X.; Chen, J. Effects of light intensity on photosynthesis, growth and phys-iology of desert plants: A review. J. Arid. Land 2021, 13, 563–575. [Google Scholar]
- Paul, K.; Bandyopadhyay, S. Elevated ultraviolet-B radiation and high altitude: Interactive effects on growth, photosynthesis, and secondary metabolites in plants. Environ. Sci. Pollut. Res. 2021, 28, 29958–29968. [Google Scholar]
- Yoo, S.; Kong, C.E.; Son, Y.B.; Ishizaka, J. A critical re-assessment of the primary productivity of the Yellow Sea, East China Sea and Sea of Japan/East Sea Large Marine Ecosystems. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2019, 163, 6–15. [Google Scholar] [CrossRef]
- Laws, E.A.; Bannister, T.T. Nutrient- and light-limited growth of Thalassiosira fluviatilis in continuous culture with implications for phytoplankton growth in the ocean. Limnol. Oceanogr. 2004, 49, 2316. [Google Scholar] [CrossRef]
- Xing, X.G.; Boss, E.; Chen, S.L.; Chai, F. Seasonal and Daily-Scale Photoacclimation Modulating the Phytoplankton Chlorophyll-Carbon Coupling Relationship in the Mid-Latitude Northwest Pacific. J. Geophys. Res. Oceans 2021, 126, e2021JC017717. [Google Scholar] [CrossRef]
- Pierangelini, M.; Stojkovic, S.; Orr, P.T.; Beardall, J. Photo-acclimation to low light-Changes from growth to antenna size in the cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae 2015, 46, 11–17. [Google Scholar] [CrossRef]
- Li, L.; Aro, E.M.; Millar, A.H. Mechanisms of Photodamage and Protein Turnover in Photoinhibition. Trends Plant Sci. 2018, 23, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.E.; Larkum, A.W.D.; Raven, J.A. Photosynthesis in Algae; Kluwer Academic: Dordrecht, The Netherlands; Boston, MA, USA, 2003. [Google Scholar]
- Sonoike, K. Photoinhihition of photosystem I: Its physiological significance in the chilling sensitivity of plants. Plant Cell Physiol. 1996, 37, 239–247. [Google Scholar] [CrossRef]
- Ivanov, A.G.; Allakhverdiev, S.I.; Huner, N.P.A.; Murata, N. Genetic decrease in fatty acid unsaturation of phosphatidylglycerol increased photoinhibition of photosystem I at low temperature in tobacco leaves. Biochim. Biophys. Acta-Bioenerg. 2012, 1817, 1374–1379. [Google Scholar] [CrossRef]
- Terashima, I.; Noguchi, K.; Itoh-Nemoto, T.; Park, Y.M.; Kubo, A.; Tanaka, K. The cause of PSI photoinhibition at low temperatures in leaves of Cucumis sativus, a chilling-sensitive plant. Physiol. Plant. 1998, 103, 295–303. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kunderlikova, K.; Allakhverdiev, S.I. High temperature specifically affects the photoprotective responses of chlorophyll b-deficient wheat mutant lines. Photosynth. Res. 2016, 130, 251–266. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta-Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V.; Wilson, S. The Mechanism of Non-Photochemical Quenching in Plants: Localization and Driving Forces. Plant Cell Physiol. 2021, 62, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Magdaong, N.C.M.; Blankenship, R.E. Photoprotective, excited-state quenching mechanisms in diverse photosynthetic organisms. J. Biol. Chem. 2018, 293, 5018–5025. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestic, M.; Bussotti, F.; Calatayud, A.; Dąbrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef]
- Ruban, A.V. Nonphotochemical Chlorophyll Fluorescence Quenching: Mechanism and Effectiveness in Protecting Plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef]
- Yamori, W.; Kusumi, K.; Iba, K.; Terashima, I. Increased stomatal conductance induces rapid changes to photosynthetic rate in response to naturally fluctuating light conditions in rice. Plant Cell Environ. 2020, 43, 1230–1240. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry & Molecular Biology of Plants; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Phys. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Kono, M.; Noguchi, K.; Terashima, I. Roles of the Cyclic Electron Flow Around PSI (CEF-PSI) and O-2-Dependent Alternative Pathways in Regulation of the Photosynthetic Electron Flow in Short-Term Fluctuating Light in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 990–1004. [Google Scholar] [CrossRef]
- Chaux, F.; Peltier, G.; Johnson, X. A security network in PSI photoprotection: Regulation of photosynthetic control, NPQ and O-2 photoreduction by cyclic electron flow. Front. Plant Sci. 2015, 6, 875. [Google Scholar] [CrossRef]
- Inagaki, N. Processing of D1 Protein: A Mysterious Process Carried Out in Thylakoid Lumen. Int. J. Mol. Sci. 2022, 23, 2520. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Moon, J.C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar]
- Tamaki, S.; Mochida, K.; Suzuki, K. Diverse Biosynthetic Pathways and Protective Functions against Environmental Stress of Antioxidants in Microalgae. Plants 2021, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- Kana, R.; Kotabova, E.; Sediva, B.; Trskova, E.K. Photoprotective strategies in the motile cryptophyte alga Rhodomonas salina-role of non-photochemical quenching, ions, photoinhibition, and cell motility. Folia Microbiol. 2019, 64, 691–703. [Google Scholar] [CrossRef]
- Alderkamp, A.C.; Garcon, V.; De Baar, H.J.W.; Arrigo, K.R. Short-term photoacclimation effects on photoinhibition of phytoplankton in the Drake Passage (Southern Ocean). Deep. Sea Res. Part I Oceanogr. Res. Pap. 2011, 58, 943–955. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, X.L.; Yu, Y.; Kong, F.X. The Acclimative Changes in Photochemistry after Colony Formation of the Cyanobacteria Microcystis Aeruginosa. J. Phycol. 2011, 47, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Timm, S.; Florian, A.; Fernie, A.R.; Bauwe, H. The regulatory inter-play between photorespiration and photosynthesis. J. Exp. Bot. 2016, 67, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, L.; Li, W.; Wei, W.; Huang, B. Interactive effects of phosphorus limitation and elevated temperature on the physiology and gene expression of the harmful dinoflagellate Prorocentrum donghaiense. Sci. Total Environ. 2021, 775, 145938. [Google Scholar]
- Rocha, J.M.; Leal, M.C.; Engrola, S. Selenastrum gracile under phosphorus limitation: Changes in physiology and biochemical composition. J. Appl. Phycol. 2021, 33, 2337–2349. [Google Scholar]
- Rocha, G.S.; Parrish, C.C.; Lombardi, A.T.; Melao, M.D.G. Biochemical and physiological responses of Selenastrum gracile (Chlorophyceae) acclimated to different phosphorus concentrations. J. Appl. Phycol. 2018, 30, 2167–2177. [Google Scholar] [CrossRef]
- Kilham, S.S.; Kreeger, D.A.; Goulden, C.E.; Lynn, S.G. Effects of nutrient limitation on biochemical constituents of Ankistrodesmus falcatus. Freshwater Biol. 1997, 38, 591–596. [Google Scholar] [CrossRef]
- Meilinda, E.; Ishida, Y.; Yoshikawa, T. Response of phytoplankton fatty acid composition to nutrient limitation: A meta-analysis. Front. Mar. Sci. 2021, 8, 1839. [Google Scholar]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Morales, M.; Aflalo, C.; Bernard, O. Microalgal lipids: A review of lipids potential and quantification for 95 phytoplankton species. Biomass Bioenergy 2021, 150, 106108. [Google Scholar] [CrossRef]
- Swan, C.M.; Vogt, M.; Gruber, N.; Laufkoetter, C. A global seasonal surface ocean climatology of phytoplankton types based on CHEMTAX analysis of HPLC pigments. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2016, 109, 137–156. [Google Scholar] [CrossRef]
- Tréguer, P.; Bowler, C.; Moriceau, B.; Dutkiewicz, S.; Gehlen, M.; Aumont, O.; Bittner, L.; Dugdale, R.; Finkel, Z.; Iudicone, D.; et al. Influence of diatom diversity on the ocean biological carbon pump. Nat. Geosci. 2017, 11, 27–37. [Google Scholar] [CrossRef]
- Liu, J.; Huang, Y.; Guan, D.; Wei, X.; Liang, S. Anthropogenic perturbation enhances ocean carbon sink and net sea-air CO2 flux via increasing phytoplankton biomass. Glob. Chang. Biol. 2021, 27, 998–1009. [Google Scholar]
- Makareviciute-Fichtner, K.; Matthiessen, B.; Lotze, H.K.; Sommer, U. Phytoplankton nutritional quality is altered by shifting Si:N ratios and selective grazing. J. Plankton Res. 2021, 43, 325–337. [Google Scholar] [CrossRef]
- De Martino, A.; Meichenin, A.; Shi, J.; Pan, K.H.; Bowler, C. Genetic and phenotypic characterization of Phaeodactylum tricornutum (Bacillariophyceae) accessions. J. Phycol. 2007, 43, 992–1009. [Google Scholar] [CrossRef]
- Schoefs, B.; Hu, H.H.; Kroth, P.G. The peculiar carbon metabolism in diatoms. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160405. [Google Scholar] [CrossRef] [PubMed]
- Taucher, J.; Bach, L.T.; Prowe, A.E.F.; Boxhammer, T.; Kvale, K.; Riebesell, U. Enhanced silica export in a future ocean triggers global diatom decline. Nature 2022, 605, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Dutkiewicz, S.; Morris, J.J.; Follows, M.J.; Scott, J.; Levitan, O.; Dyhrman, S.T.; Berman-Frank, I. Impact of ocean acidification on the structure of future phytoplankton communities. Nat. Clim. Chang. 2015, 5, 1002–1006. [Google Scholar] [CrossRef]
- Bach, L.T.; Taucher, J. CO2 effects on diatoms: A synthesis of more than a decade of ocean acidification experiments with natural communities. Ocean Sci. 2019, 15, 1159–1175. [Google Scholar] [CrossRef]
- Flanjak, L.; Vrana, I.; Kusan, A.C.; Godrijan, J.; Novak, T.; Penezic, A.; Gasparovic, B. Effects of high temperature and nitrogen availability on the growth and composition of the marine diatom Chaetoceros pseudocurvisetus. J. Exp. Bot. 2022, 73, 4250–4265. [Google Scholar] [CrossRef]
- Nalley, J.O.; O’donnell, D.R.; Litchman, E. Temperature effects on growth rates and fatty acid content in freshwater algae and cyanobacteria. Algal Res. 2018, 35, 500–507. [Google Scholar] [CrossRef]
- Sayegh, F.A.; Montagnes, D.J. Temperature shifts induce intraspecific variation in microalgal production and biochemical composition. Bioresour. Technol. 2011, 102, 3007–3013. [Google Scholar] [CrossRef]
- Gatamaneni, B.L.; Orsat, V.; Lefsrud, M. Factors Affecting Growth of Various Microalgal Species. Environ. Eng. Sci. 2018, 35, 1037–1048. [Google Scholar] [CrossRef]
- Sheehan, C.E.; Baker, K.G.; Nielsen, D.A.; Petrou, K. Temperatures above thermal optimum reduce cell growth and silica production while increasing cell volume and protein content in the diatomThalassiosira pseudonana. Hydrobiologia 2020, 847, 4233–4248. [Google Scholar] [CrossRef]
- Lehmuskero, A.; Chauton, M.S.; Bostrom, T. Light and photosynthetic microalgae: A review of cellular- and molecular-scale optical processes. Prog. Oceanogr. 2018, 168, 43–56. [Google Scholar] [CrossRef]
- Harb, T.B.; Nardelli, A.; Chow, F. Physiological responses of Pterocladiella capillacea (Rhodophyta, Gelidiales) under two light intensities. Photosynthetica 2018, 56, 1093–1106. [Google Scholar] [CrossRef]
- Belkoura, M.; Benider, A.; Dauta, A. Effects of temperature, light intensity and growth phase on the biochemical composition of Chlorella sorokiniana Shihira & Krauss. Ann. Limnol.-Int. J. Lim. 1997, 33, 3–11. [Google Scholar] [CrossRef]
- Sarkar, S.; Manna, M.S.; Bhowmick, T.K.; Gayen, K. Effect of different illumination patterns on the growth and biomolecular synthesis of isolated Chlorella Thermophila in a 50 L pilot-scale photobioreactor. Process Biochem. 2021, 109, 87–97. [Google Scholar] [CrossRef]
- Xia, S.; Wan, L.L.; Li, A.F.; Sang, M.; Zhang, C.W. Effects of nutrients and light intensity on the growth and biochemical composition of a marine microalga Odontella aurita. Chin. J. Oceanol. Limn. 2013, 31, 1163–1173. [Google Scholar] [CrossRef]
- Gris, B.; Morosinotto, T.; Giacometti, G.M.; Bertucco, A.; Sforza, E. Cultivation of Scenedesmus obliquus in Photobioreactors: Effects of Light Intensities and Light-Dark Cycles on Growth, Productivity, and Biochemical Composition. Appl. Biochem. Biotech. 2014, 172, 2377–2389. [Google Scholar] [CrossRef]
- Walter, B.; Peters, J.; Van Beusekom, J.E.E. The effect of constant darkness and short light periods on the survival and physiological fitness of two phytoplankton species and their growth potential after re-illumination. Aquat. Ecol. 2017, 51, 591–603. [Google Scholar] [CrossRef]
- Kim, D.W.; Shin, W.S.; Sung, M.G.; Lee, B.; Chang, Y.K. Light intensity control as a strategy to improve lipid productivity in Chlorella sp. HS2 for biodiesel production. Biomass Bioenergy 2019, 126, 211–219. [Google Scholar] [CrossRef]
- Fernandez, E.; Fritz, J.J.; Balch, W.M. Chemical composition of the coccolithophorid Emiliania huxleyi under light-limited steady state growth. J. Exp. Mar. Biol. Ecol. 1996, 207, 149–160. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)—Part I: Structural and ecological aspects. Water Sci. Technol. 2001, 43, 1–8. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs)—Part II: Technical aspects. Water Sci. Technol. 2001, 43, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. Extracellular polymeric substances (EPS) in biofilms: Conformation, regulation and functional role. In Microbial Biofilms; Wiley: Hoboken, NJ, USA, 2021; pp. 87–127. [Google Scholar]
- Thornton, D.C.O. Dissolved organic matter (DOM) release by phytoplankton in the contemporary and future ocean. Eur. J. Phycol. 2014, 49, 20–46. [Google Scholar] [CrossRef]
- Mao, Z.; Wang, Y.; Wang, Q.; Zhao, L. Contribution of extracellular enzymes to phytoplankton-mediated carbon cycling in marine ecosystems. Mar. Environ. Res. 2022, 175, 105427. [Google Scholar]
- Alldredge, A.L.; Passow, U.; Logan, B.E. The abundance and significance of a class of large, transparent organic particles in the ocean. Deep. Sea Res. Part I Oceanogr. Res. Pap. 1993, 40, 1131–1140. [Google Scholar] [CrossRef]
- Passow, U.; Shipe, R.F.; Murray, A.; Pak, D.K.; Brzezinski, M.A.; Alldredge, A.L. The origin of transparent exopolymer particles (TEP) and their role in the sedimentation of particulate matter. Cont. Shelf Res. 2001, 21, 327–346. [Google Scholar] [CrossRef]
- Eichner, M.; Wolf, D.; Ploug, H. Carbonate chemistry in the microenvironment within cyanobacterial aggregates under present-day and future pCO(2) levels. Limnol. Oceanogr. 2022, 67, 203–218. [Google Scholar] [CrossRef]
- Sun, H.; Lu, X.; Yu, R.; Yang, J.; Liu, X.; Cao, Z.; Zhang, Z.; Li, M.; Geng, Y. Eutrophication decreased CO2 but increased CH4 emissions from lake: A case study of a shallow Lake Ulansuhai. Water Res. 2021, 201, 117363. [Google Scholar] [CrossRef]
- Lindeman, K.C.; Checkley, D.M. Seasonal and interannual variability of phyto-plankton community structure in the California Current System. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2019, 169, 104660. [Google Scholar]
- Tang, E.P.; Landry, M.R. Ecological drivers of phytoplankton aggregation and community composition in the California Current. Prog. Oceanogr. 2021, 190, 102546. [Google Scholar]
- Huang, H. Effects of Different Inorganic Nitrogen SOURCES in water Bodies on Phytoplankton Growth and Greenhouse Gas Production and Emission. Master’s Thesis, Northwest Agriculture and Forestry University, Xianyang, China, 2021. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Witvrouw, M.; Declercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. Vasc. Syst. 1997, 29, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, T.; Zhao, X.; Zan, F.; Yang, G.; Miao, Y. Cyanobacteria blooms potentially enhance volatile organic compound (VOC) emissions from a eutrophic lake: Field and experimental evidence. Environ. Res. 2021, 202, 111664. [Google Scholar] [CrossRef] [PubMed]
- Bartosiewicz, M.; Maranger, R.; Przytulska, A.; Laurion, I. Effects of phytoplankton blooms on fluxes and emissions of greenhouse gases in a eutrophic lake. Water Res. 2021, 196, 116985. [Google Scholar] [CrossRef]
- Li, Y.; Shang, J.; Zhang, C.; Zhang, W.; Niu, L.; Wang, L.; Zhang, H. The role of freshwater eutrophication in greenhouse gas emissions: A review. Sci. Total Environ. 2021, 768, 144582. [Google Scholar] [CrossRef]
- Ni, J.; Wang, H.; Ma, T.; Huang, R.; Ciais, P.; Li, Z.; Yue, Y.; Chen, J.; Li, B.; Wang, Y.; et al. Three Gorges Dam: Friend or foe of riverine greenhouse gases? Natl. Sci. Rev. 2022, 9, nwac013. [Google Scholar] [CrossRef]
- Xun, F.; Du, X.; Chen, X.F. Effects of continuous cyanobacterial salvage in autumn on greenhouse gas fluxes at the water-air interface. Lake Sci. 2020, 32, 1707–1722. [Google Scholar]
| Mechanism | CO2 Enrichment Requirement | Reference |
|---|---|---|
| C4 Pathway Inorganic C + C3 → C4 dicarboxylate in the cytosol → C3 + CO2 in plastids containing Rubisco | A high volume of Rubisco-containing high-CO2 compartments | Reinfelder (2000) [23] Morel (2002) [24] Reinfelder (2004) [25] |
| HCO3− Active Transport HCO3− catalyzed by CA to CO2, often in the carboxysome or pyrenoid (also known as the HCO3− active inflow or CA pathway) | Plasma membrane with active transport ability | Badger (1998) [22] Badger (2002) [26] Badger (2003) [27] Kaplan (1999) [28] |
| CO2 Active Transport | Relatively large CO2 compartments | Badger (1998) [22] Colman (2002) [29] Kaplan (1999) [28] |
| CO2 Passive Diffusion CO2 diffuses passively into the cyanobacterial plasma membrane and is converted to HCO by NADHdh3−, which then enters the carboxysome and is catalyzed by CA to CO2 | Badger (2002) [26] Badger (2003) [27] Kaplan (1999) [28] | |
| HCO3− Acidification HCO3− enters the acidification bin and accumulates, HCO3− is then catalyzed by CA to a high concentration of CO2 which randomly diffuses into the RUBISCO bin | Presence of acidification silos | Pronina (1997) [30] Raven (1997) [31] Raven (1997) [32] Raven (1997) [33] Van Hunnik (2002) [34] |
| Other Crassulacean acid metabolism (CAM) Only in cactus | ||
| Species | Class | Reference |
|---|---|---|
| Poterioochromonas malhamensis | Chrysophyta | Raven (1997) [31] |
| Paraphysomonas vestita | Chrysophyta | Fenchel (1982) [39] |
| Ochromonas sp. Nov. | Chrysophyta | |
| Pleuromonas jaculans | Chrysophyta | |
| Pseudobodo tremulans | Diatoms | |
| Picochlorum sp. | Chlorophyta | Pang (2021) [40] |
| Dinobryon sociale | Chrysophyta | Princiotta (2016) [41] |
| Chlorella | Chlorophyta | |
| Spirulina | Cyanophyta |
| Light Protection Method | Mechanism of Action | Citation | |
|---|---|---|---|
| Non-photochemical quenching (NPQ) | Chlorophyll fluorescence | Emission of excess photons | Ruban and Wilson (2021) [76] |
| Heat dissipation | Molecular vibration to dissipate energy | Magdaong (2018) [77] Kalaji et al. (2014) [78] Ruban (2016) [79] | |
| Photochemical reactions | Photosynthesis | Energy consumption to increase photosynthetic rate | Yamori et al. (2022) [80] |
| Light breathing | Excess energy consumption | Buchanan et al. (2015) [81] | |
| Water-water cycle | Promotion of the lutein cycle to consume excess excitation energy | Asada (1999) [82] | |
| Cyclic electron flow (CEF) | Regulated potential control of electron transfer | Kono et al. (2014) [83] Chaux et al. (2015) [84] | |
| Organism repair through rapid D1 protein turnover | Rapid replenishment of damaged D1 protein | Inagaki 2022 [85] | |
| Reactive oxygen removal | Elimination of photodamage factor | Karuppanapandian, et al. (2011) [86] Tamaki et al. (2021) [87] | |
| Environmental Conditions | Protein | Carbohydrates | Fat | Cell Size | Growth Rate |
|---|---|---|---|---|---|
| Phosphorus limitation | ↑ | ↑ | ↑ | ↓ | |
| Nitrogen limitation | ↓ | ↓ | ↑ | ↓ | ↓ |
| High temperature | ↑ | ↑ | ↓ | ||
| Low temperature | ↓ | ↓ | ↓ | ↓ | |
| Silicon Restriction | ↑ | ↓ | ↓ | ||
| Suitable light | ↑ | ↑ | ↓ | ↑ |
| Carbon Nutrition Strategy | Habitat Conditions | Monitoring Methods | Impact on Carbon Fixation | |
|---|---|---|---|---|
| Carbon Capture | CCM mechanism | Low CO2 concentration | CO2 monitoring, isotope monitoring | Driver of carbon fixation by algal species [7,28] |
| Ingestion | Light-limited, nutrient-limited | Isotope monitoring, fluorescence | Organic carbon uptake [14,19] | |
| Active movement | Practical observation | Improves carbon fixation efficiency [17,76] | ||
| Passive motion | Practical observation | None | ||
| Carbon metabolism | Photosynthetic pigment regulation | Changes in light intensity | Pigment Monitoring | Improves carbon fixation efficiency [8,120] |
| Photoprotection | Excessive light exposure | Chlorophyll fluorescence and photosynthetic electron transport | Reduces carbon fixation efficiency [15,110] | |
| Carbon flow distribution | Changing nutrient conditions | Biomolecular monitoring | Determines carbon fixation efficiency [36,137] | |
| Carbon excretion | Extracellular polymers | Biomolecular monitoring, chemical monitoring | Determines distribution of carbon fixation [29,94] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yin, Z.; Chen, J.; Liu, J. RETRACTED: Phytoplankton Carbon Utilization Strategies and Effects on Carbon Fixation. Water 2023, 15, 2137. https://doi.org/10.3390/w15112137
Wang X, Yin Z, Chen J, Liu J. RETRACTED: Phytoplankton Carbon Utilization Strategies and Effects on Carbon Fixation. Water. 2023; 15(11):2137. https://doi.org/10.3390/w15112137
Chicago/Turabian StyleWang, Xin, Zhuo Yin, Jielai Chen, and Jing Liu. 2023. "RETRACTED: Phytoplankton Carbon Utilization Strategies and Effects on Carbon Fixation" Water 15, no. 11: 2137. https://doi.org/10.3390/w15112137
APA StyleWang, X., Yin, Z., Chen, J., & Liu, J. (2023). RETRACTED: Phytoplankton Carbon Utilization Strategies and Effects on Carbon Fixation. Water, 15(11), 2137. https://doi.org/10.3390/w15112137







