Abstract
Zero valent iron (ZVI) is widely used in permeable reactive barriers (PRBs) for the remediation of contaminated groundwater. The hydraulic conductivity of ZVI can be reduced due to iron corrosion processes activated by water and its constituents including pollutants. To overcome this issue, ZVI particles can be mixed with granular materials that avoid a drastic reduction in the hydraulic conductivity over time. In light of the most recent studies concerning iron corrosion processes and recalling the basic principles of century-old chemistry of iron corrosion, we have revised the results of 24 long-term column tests investigating the hydraulic and reactive behavior of granular mixtures composed of ZVI and pumice or lapillus. From this analysis, we found a clear correlation between the reactive behavior, described by the retardation factor (i.e., the ratio between flow velocity and propagation velocity of the contamination front), and the hydraulic behavior, described by means of the permeability ratio of the reactive medium (i.e., the ratio between the final and initial value of hydraulic conductivity). In particular, the permeability ratio decreased with the increase in the retardation factor. Moreover, it was found that the retardation factor is a useful parameter to evaluate the influence of flow rate, contaminant concentration, and ZVI content on the reactive behavior of the granular medium.
1. Introduction
Groundwater contamination represents one of the current challenges the world has to face. In this context, the technology of permeable reactive barrier (PRB) plays an important role. Unlike other groundwater remediation technologies (e.g., pump and treat, air sparging, or bioventing), PRB can be considered as a sustainable remediation technology that does not require high operating costs or significant energy consumption, operates with no surrounding disturbances, and could also be effective in developing countries [1].
A PRB is installed downstream of the contamination source in order to intercept the contaminated plume and remove the contaminants dissolved in it [2]. Reactivity and hydraulic conductivity are the two main characteristics required in the long-term of the granular material constituting the barrier [3]. Therefore, an accurate study of the reactive and hydraulic behavior of the granular materials, especially for those whose hydraulic conductivity can reduce over time, is required for the correct design of the barrier.
A PRB is a versatile technology that fits different scenarios of contamination by choosing the most appropriate reactive medium [4]. Zero valent iron (ZVI) is a material that can be effective to remove different typologies of contaminants such as chlorinated solvents [5,6,7], nitrates [8,9], or heavy metals [10,11,12]. The removal capacity and the hydraulic performance of the ZVI are strictly linked to the corrosion process activated by water and its constituents including pollutants [13,14,15]. Due to the corrosion process, iron oxides and hydroxides and hydrogen gas are produced [16,17,18]. In particular, solid iron corrosion products (iron oxides and hydroxides) are involved in the removal of contaminants but reduce the initial porosity of the barrier [19,20,21,22]. It was found that these products occupy a volume larger than that occupied by corroded iron [21,23]. Depending on the level of oxidation and on environmental conditions, iron can expand up to six times its original volume, and this can be considered as the very first cause of hydraulic conductivity loss in PRB [22]. Based on the extent of the iron corrosion process, porosity reduction could lead to the clogging of the reactive medium and, as a consequence, barrier hydraulic conductivity decreases, reaching values not compatible with the PRB working. In some PRBs, hydraulic conductivity reduction occurred after a few years after installation [24,25], whereas in other cases, the PRB still effectively fulfils its function, as described by [26] after 21 years of operation for the treatment of chlorinated compounds.
The iron corrosion rate influences the hydraulic performance of the barrier and depends on different parameters such as the intrinsic reactivity of iron [13], its particle size [27], flow velocity, and solutes [14,28,29,30]. Therefore, understanding the evolution of hydraulic conductivity over time is quite complex and the time for the system to reach a possible clogging state is extremely variable. A known method to slow down the phenomenon of hydraulic conductivity reduction is mixing ZVI with another granular material (admixing agent) with a similar particle size and whose hydraulic conductivity does not change over time such as sand, pumice, and lapillus [31,32,33,34,35]. During the corrosion process, iron particles expand their volume and iron oxides (or hydroxides) and chemical species with low solubility precipitate, causing the filling of pores adjacent to ZVI particles [13]. Dispersing the ZVI particles in a greater volume means avoiding localizing the reduction of porosity in concentrated areas of the reactive medium (Figure 1) such as to not significantly affect the hydraulic conductivity of the barrier [36].
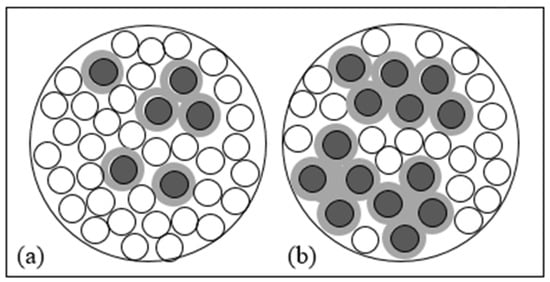
Figure 1.
Schematic representation of a cross-section area of a granular reactive mixture composed of ZVI particles (grey circles) including iron corrosion products (light grey circles) and admixing agent particles (white circles), considering a minor (a), or major (b) ZVI content per unit volume.
Choosing the right volumetric ratio between the ZVI and the admixing agent is not easy because the process of the generation of iron corrosion products depends on the iron corrosion rate that, as above-mentioned, changes over time with the ZVI particle size, flow velocity, and water chemical composition [22,37,38].
The aim of this study was to propose a method for the choice of the optimal composition of a granular mixture containing ZVI. To achieve this purpose, we reinterpreted the results of 24 long-term column tests carried out in our laboratory in the last decade, taking into account the most recent studies on iron corrosion processes and based on principles of the century-old chemistry of iron corrosion [13,14,15,16,21,38,39]. The long-term column tests were carried out by the authors and the results are described in detail in the pertinent scientific literature (Table 1). The materials used were ZVI and granular mixtures of ZVI and either pumice or lapillus at different weight ratios. The tests were performed using different values of flow velocity, and concentrations of heavy metals (i.e., copper, nickel and zinc) were present as single metal or in pluri-contaminated solutions.

Table 1.
Main data of the column tests.
2. Materials and Methods
The PRB operation was simulated in columns (polymethyl methacrylate (PMMA)—Plexiglas, internal diameter and height of 5 and 50 cm, respectively) filled with the granular reactive material. Columns were fed with the synthetic contaminated water by a peristaltic pump (Ismatec ISM 930 for column tests carried out using ZVI/pumice mixtures and Watson-Marlow 205S for those carried out with ZVI/lapillus mixtures). The contaminated aqueous solution was prepared by dissolving appropriate amounts of nickel(II) nitrate hexahydrate (purity 99.999), zinc(II) nitrate hexahydrate (purity 99.999), and copper(II) nitrate hydrate (purity 99.999), obtained from Sigma-Aldrich (Germany) in distilled water in order to obtain single or pluri-contaminated solutions. The measurement of the initial concentration of the contaminants in the pluri-contaminated solution entering the column made it possible to exclude any interference among them. Most of the column tests were performed using nickel as a contaminant as it was found to be the most difficult contaminant to remove after zinc and copper. The ZVI and pumice or lapillus used in the column tests had a similar and uniform grain size distribution, the mean grain size (d50) was 0.5 mm for ZVI, 0.3 for pumice, and 0.4 for lapillus. Pumice and lapillus are two volcanic rocks of large availability; they mainly consist of silica and oxides of various elements (e.g., Al2O3, Fe2O3-FeO, and K2O). Lapillus is derived from magma with a lower content of silica compared to pumice and is characterized by a higher content of oxides of potassium, iron magnesium, and calcium than pumice. Moreover, lapillus has shown a higher removal capacity toward heavy metals such as copper, nickel, and zinc than pumice [32].
During the column tests performed at room temperature (21 ± 2 °C), hydraulic conductivity was determined by using the falling-head or constant-head permeability tests. Heavy metal concentration in the aqueous samples, collected during column tests, was determined using ICP-OES (Perkin Elmer OPTIMA 8000).
The main data, referring to the column tests and to the typology of the reactive medium, contaminant concentration, flow rate (Q), reactive medium thickness (L), and test duration used in this study to find a correlation between the reactive and hydraulic behavior of granular mixtures composed of ZVI, are summarized in Table 1.
Column test results were analyzed in terms of two performance factors: the permeability ratio (Kr) and the retardation factor (Rf).
The permeability ratio, Kr, was defined as the ratio between the final (kf) and initial value (k0) of hydraulic conductivity.
The retardation factor, Rf, is defined through the following equation:
where the flow velocity (vct) is calculated by dividing the flow rate used in the test (Q) by the area of the column, while vcont is the average value of the propagation velocity of the contamination front through the reactive medium and is calculated as follows:
where n is the number of sampling ports where the breakthrough is observed and Li is the reactive medium thickness (distance of the i-th sampling from column inlet) where the breakthrough is observed and Tbi is the breakthrough time at the i-th sampling port. The breakthrough time, for each sampling port, is identified as the time where a rapid increase of the relative contaminant concentration (C/C0 with C concentration at time t and C0 initial concentration) above the Italian regulatory limits (equal to 1 mg/L for copper, 0.02 mg/L for nickel and 3 mg/L for zinc) is clearly observed, as shown, for example, in Figure 2.
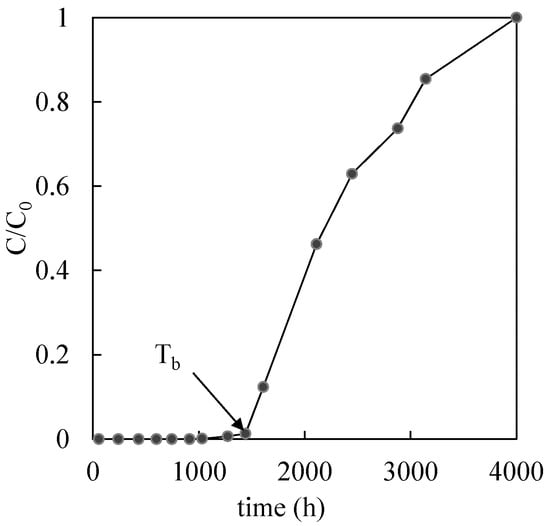
Figure 2.
Example of a breakthrough curve for a generic thickness (L) of the reactive medium.
3. Results and Discussion
In Figure 3, the curves Kr vs. Rf are shown for the reactive media permeated with solutions contaminated by zinc (Figure 3a) or nickel (Figure 3b) in single metal solutions or copper, nickel, and zinc in pluri-contaminated solutions (Figure 3c). For the pluri-contaminated solutions, the lowest value of the retardation factor calculated for each of the three contaminants, respectively, was used. The removal sequence observed in the three tests was Cu > Zn > Ni, only in one particular case did the removal sequence for the mixture ZVI/pumice change to Cu > Ni > Zn [40]. The values of the two performance factors are summarized in Table 2. From Figure 3 (or Table 2) it can be observed that with the increase in the retardation factor, the hydraulic conductivity of the system decreased.
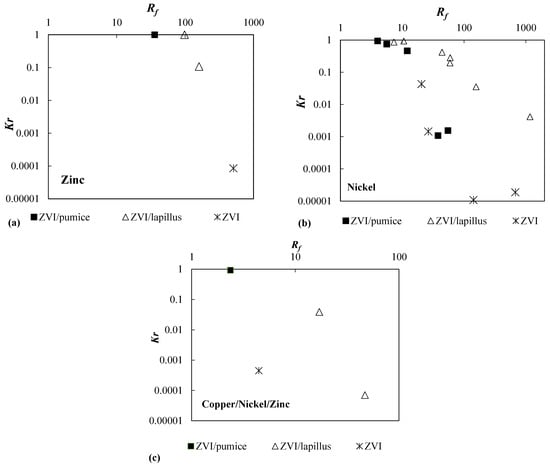
Figure 3.
Kr vs. Rf for granular reactive media permeated with (a) zinc, (b) nickel, and (c) copper/nickel/zinc.

Table 2.
Rf and Kr values calculated for each column test.
High values of Rf mean that the reactive medium efficiently removes the contaminant. Therefore, it is necessary to guarantee during the design life of the PRB high values of Rf and Kr values that in terms of barrier long-term hydraulic conductivity meet the permeability criteria of granular filters [42].
High values of Rf generally occur when the test adopts either (i) a high value of ZVI content per unit volume in the granular mixture; (ii) a low value of contaminant concentration; and (iii) a low value of flow velocity or a combination of these three parameters. Moreover, the retardation factor depends on the corrosion rate of the iron because the generation of iron oxyhydroxides (iron corrosion products) is the basis of contaminant removal [13,22].
When ZVI is immersed in an aqueous solution, a transfer of electrons from the Fe0 (solid state) to the Fe0/H2O interface occurs because the redox couple H+/H2 (E0 = 0.00 V) is higher than that of FeII/Fe0 (E0 = −0.44 V). The oxidative dissolution of Fe0 by H+ from water forms Fe2+ and Fe(OH)2. In the presence of dissolved oxygen, Fe2+ and Fe(OH)2 can be oxidized to the less soluble Fe(OH)3. Fe(OH)2 and Fe(OH)3 are polymerized and further transformed to various oxyhydroxides [22]. Therefore, ZVI is a generator of iron oxides (contaminant scavengers) and secondary reducing agents (e.g., Fe(II), Fe3O4, H2, green rust) [22]. Indeed, adsorbed FeII (or structural FeII) (E0 = −0.34 to −65 V) can be more powerful in reducing contaminants than Fe0 (for E < −0.44 V) [21]. In contrast to what has been argued for years, the indirect reduction of contaminants is more favored than the direct reduction from Fe0 [38,43].
For the mechanisms described above, contaminant removal is attributed to the ability of ZVI to generate iron corrosion products. This ability is linked to iron dissolution, which depends on the water composition (i.e., solution chemistry) and residence time, or contact time between the ZVI particles and water (i.e., flow velocity). Regarding the solution chemistry, solutes can accelerate iron dissolution by increasing the H+ concentration [13,39]. Regarding the flow velocity, the residence time affects the time necessary to generate oxides on Fe0 [21].
Being aware of the chemical processes just described, the reactive and hydraulic behavior of the granular mixtures was analyzed considering (i) the initial contaminant concentration; (ii) the flow rate; and (iii) the weight ratio between the ZVI and the admixing agent (pumice or lapillus). We found that the retardation factor is a parameter that well represents these variables (i.e., the contaminant concentration, the flow rate, and the ZVI content).
In Figure 4a and Figure 5a, the Rf values are shown as a function of the initial concentration of nickel in column tests carried out using the ZVI/pumice mixture (weigh ratio 30:70) and a flow rate of 2.5 mL/min (Figure 4a) or the ZVI/lapillus mixture (weigh ratio 30:70) and a flow velocity of 0.5 mL/min (Figure 5a). The laws that link the Rf with the initial concentration of the contaminant are shown in Figure 4a and Figure 5a for the ZVI/pumice mixture (Q = 2.5 mL/min) and for the ZVI/lapillus mixture (Q = 0.5 mL/min), respectively. The laws that link Kr with Rf are shown in Figure 4b and Figure 5b for the ZVI/pumice (Q = 2.5 mL/min) and the ZVI/lapillus mixture (Q = 0.5 mL/min), respectively. The optimal correlation was confirmed by correlation coefficient values (R2) greater than 0.97.
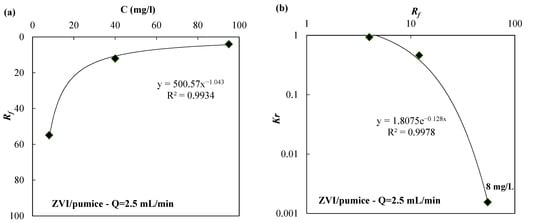
Figure 4.
(a) Rf vs. the initial nickel concentration (C) and (b) Kr vs. Rf for the ZVI/pumice granular mixture.
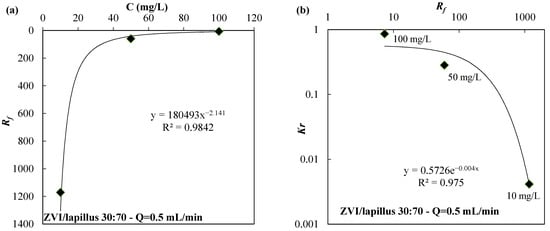
Figure 5.
(a) Rf vs. the initial nickel concentration (C) and (b) Kr vs. Rf for the ZVI/lapillus granular mixture.
When the tested variable is the contaminant concentration, a reduction in the retardation factor with the increase in contaminant concentration can be observed (Figure 4a and Figure 5a). Considering the removal mechanisms previously described, the different velocity at which the contaminant propagates confirms that the extent of corrosion is influenced by solution chemistry [21,38]. A slow propagation of the contaminated front (i.e., a high value of the retardation factor) means that the removal mechanisms are concentrated in the first part of the column (close to the inlet) and this phenomenon can cause a decrease in the hydraulic conductivity at the barrier entrance (Figure 4b and Figure 5b).
In Figure 6a, the Rf values are shown as a function of the flow rate (Q) in column tests carried out using the ZVI/lapillus mixture (weigh ratio 30:70) and nickel at initial concentration of 50 mg/L (Figure 6a). The same figure shows the law that links the Rf with Q values. Figure 6b shows the law that link Kr with Rf values for the same column tests. The two different laws well fit the data with R2 values higher than 0.97.
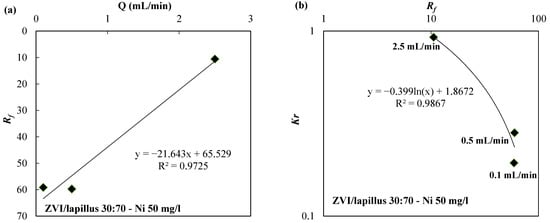
Figure 6.
(a) Rf vs. flow rate (Q) and (b) Kr vs. Rf for the ZVI/lapillus granular mixture.
As shown in Figure 6a, the retardation factor increased as the flow rate decreased from 2.5 mL/min to 0.1 or 0.5 mL/min. This behavior is linked to the removal mechanisms that vary with boundary conditions (i.e., contaminant concentration or water composition and flow rate). In particular, when mass transfer is accelerated, for example, by groundwater velocity, a delay in the formation of the oxide scale on ZVI can be assumed, in this case, the relevance of indirect reduction is minimized, while direct reduction could be favored [21].
Figure 7 summarizes, in a flow chart, the effect generally observed in the increase in flow velocity or contaminant concentration on the hydraulic and the reactive behavior of a granular mixture composed of ZVI (setting the ZVI content per unit volume). When the mass of contaminant flowing through the reactive medium is accelerated by the flow rate (the residence time is reduced) or the contaminant concentration increases, the thickness of the reactive medium necessary to reach the regulatory limit also increases.
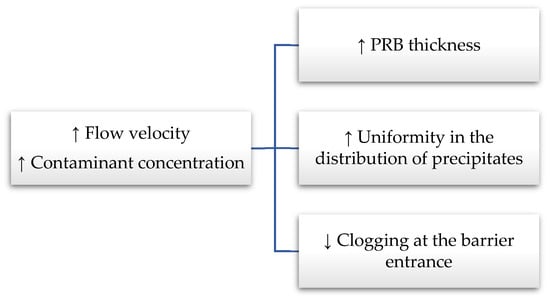
Figure 7.
Flow chart showing the effects, for a given granular mixture composed of ZVI, of increasing the flow velocity or the concentration of contaminants.
As shown for nickel [41], by increasing the flow rate or contaminant concentration, the mass of the removed contaminant is distributed over the entire thickness of the reactive medium (in this study 50 cm) and thus, it is likely to assume a uniform distribution of the ZVI corrosion products, which are responsible for contaminant removal. This uniform distribution of iron corrosion products (or mass of removed contaminant) suggests a uniform reduction in porosity along the entire thickness of the reactive medium that has no effect on hydraulic conductivity (no significant reduction). By reducing the flow rate, or contaminant concentration, nickel (the pollutant investigated by Madaffari et al., 2017 [41]) was quickly removed in the first centimeters of the reactive medium (i.e., 3 cm). In these cases, where ZVI has not been sufficiently dispersed, precipitates accumulated faster near the inlet of the reactive medium (corresponding to the in situ aquifer/PRB interface) and a reduction in the initial value of the hydraulic conductivity was observed.
Figure 8 shows the photos of the specimens inside the column (inlet section) taken at the end of some of the tests. The images were sorted in descending order with respect to Kr values. Thanks to the lighter color of pumice, the areas containing the corroded iron (greenish and orange/brown areas) were easily distinguished (Figure 8a). In this case, as suggested by the Kr value almost equal to unity (i.e., 0.932), these colored areas did not affect the hydraulic conductivity of the reactive medium.
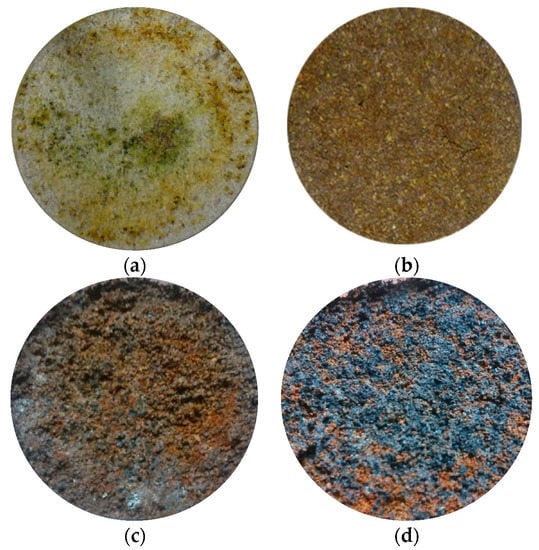
Figure 8.
Photos of the inlet section of the column at the end of the column tests for different test conditions. (a) ZVI/pumice (w.r. 30:70) Ni 95 mg/L Q = 2.5 mL/min Kr = 0.932. (b) ZVI/lapillus (w.r. 30:70) Ni 100 mg/L Q = 0.5 mL/min Kr = 0.857. (c) ZVI/lapillus (w.r. 50:50) Zn 50 mg/L Q = 0.5 mL/min Kr = 0.107. (d) ZVI/lapillus (w.r. 30:70) Ni 10 mg/L Q = 0.5 mL/min Kr = 0.0041.
When lapillus was used and the hydraulic conductivity of the mixtures was kept constant (Kr equal to 0.857), the areas containing corroded iron were not discernible (Figure 8b) because they were limited. When Kr decreased about one (Kr equal to 0.107 in Figure 8c) or two (Kr equal to 0.0041 in Figure 8d) orders of magnitude, the areas occupied by corroded iron (grey areas) were easily recognized. In the most severe case (Kr equal to 0.0041), these areas occupied almost the entire inlet section of the column (Figure 8d) and consequently severely affected the hydraulic conductivity of the reactive medium.
Finally, Figure 9 and Figure 10 show the link between Rf and either the ZVI content (ZVI % in weight contained in the mixture) or Kr for the ZVI/lapillus mixture permeated with a solution of nickel (Figure 9) or zinc (Figure 10). Additionally, for this variable (ZVI %) and for both solutions (nickel and zinc), it was possible to fit the data with R2 values greater than 0.91. According to this and previous research [31,41,44], when the flow rate and the contaminated solution are the same, the iron corrosion rate does not change in the three tests varying the ZVI content per unit volume. The strong relation between the Kr and Rf values (R2 value next to unity in both cases) confirmed that the clogging phenomena depend on the dispersion rate of the ZVI (or ZVI per unit volume). In a more dispersed configuration, the reduction in porosity near the iron particles affected, to a lesser extent, the hydraulic conductivity (Figure 1, Figure 3 and Figure 8). The interpretation of the tests according to Figure 9 or Figure 10 can help the designer choose the optimal granular mixture by fixing the flow velocity and water composition. In particular, the proposed procedure consists of the first step to determine the Rf value by choosing the minimum Kr value by means of the Kr versus Rf curve (Figure 9b or Figure 10b). The Kr value can be set, for example, so that the reduction in hydraulic conductivity was not greater than one order of magnitude (Kr equal to 0.1) compared to the initial value. The second step consists of establishing the degree of iron dispersion entering, with the Rf value in the graph showing the relation between % ZVI and Rf (Figure 9a or Figure 10a).
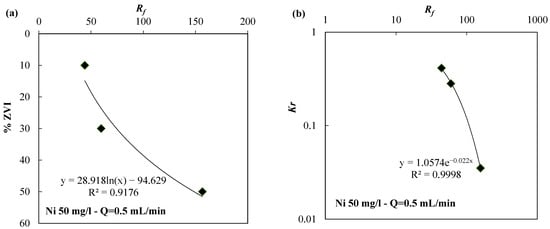
Figure 9.
(a) % ZVI vs. Rf and (b) Kr vs. Rf for the ZVI/lapillus granular mixture permeated with a solution of nickel 50 mg/L at a flow rate equal to 0.5 mL/min.
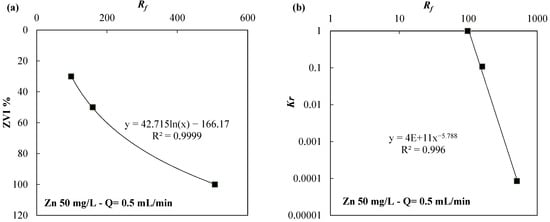
Figure 10.
(a) ZVI % vs. Rf and (b) Kr vs. Rf for the ZVI/lapillus granular mixture permeated with a solution of zinc 50 mg/L at a flow rate equal to 0.5 mL/min.
To use this procedure, it is necessary to carry out tests using (i) columns of suitable diameter and height (see Calabrò et al., 2021 [37]); (ii) the existing in situ boundary conditions (i.e., groundwater velocity and contaminant concentration); and (iii) considering a duration sufficient to observe the breakthrough in different sampling ports.
4. Conclusions
The iron corrosion rate affects the hydraulic and reactive behavior of a granular mixture containing ZVI and is used in PRB technology. The iron corrosion products (iron oxides) promote the removal of contaminants, especially heavy metals, but reduce the porosity in the vicinity of ZVI particles because these species occupy a volume larger than that occupied by corroded iron. By mixing ZVI particles with a granular material with a similar particle size whose hydraulic conductivity does not change over time, the ZVI particles are dispersed in a greater volume, which helps to preserve the long term hydraulic conductivity of the barrier. Understanding how iron corrodes over time, and consequently the evolution of the hydraulic and reactive performance of the barrier, is a complex challenge, as claimed in the recent scientific literature. In this paper, the authors reinterpreted 24 long-term column tests taking into account the most recent studies on iron corrosion processes, and provided a new performance parameter that can provide useful information about changes in the reactivity and hydraulic behavior of the barrier over time. This performance parameter is the retardation factor that can easily be found through column tests by knowing the breakthrough time determined at different thicknesses of the reactive medium.
The largest values of the retardation factor were found for the granular reactive media where the hydraulic conductivity was reduced by more than one order of magnitude. By reducing the flow rate or contaminant concentration, the pollutant was efficiently removed in the first part of the column (close to the inlet) of the reactive medium thickness. In those cases, where ZVI had not been sufficiently dispersed, precipitates and iron corrosion products accumulated faster near the inlet of the reactive medium (corresponding to the in situ aquifer/PRB interface), causing a clogging phenomena.
The strong relation found between the permeability ratio values (i.e., the ratio between the final and initial value of hydraulic conductivity) and the retardation factor for three granular mixtures at different iron content confirms that the clogging phenomena depend on the dispersion rate of the ZVI (for set flow rate and water composition). In a more dispersed configuration, the reduction of porosity near the iron particles affects, to a lesser extent, the hydraulic conductivity. The interpretation of the column tests using the new performance factors (i.e., the retardation factor and the permeability ratio) can help the designer choose the optimal granular mixture for the groundwater remediation, taking into account, the boundary conditions (i.e., flow rate and water chemical composition).
Author Contributions
Conceptualization, S.B., P.S.C. and N.M.; Methodology, S.B., P.S.C. and N.M.; Investigation, S.B.; Writing—review and editing, S.B., P.S.C. and N.M.; Visualization, S.B.; Supervision, P.S.C. and N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available within the article. For the availability of detailed data sets the corresponding authors can be contacted.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thakur, A.K.; Vithanage, M.; Das, D.B.; Kumar, M. A review on design, material selection, mechanism, and modelling of permeable reactive barrier for community-scale groundwater treatment. Environ. Technol. Innov. 2020, 19, 100917. [Google Scholar] [CrossRef]
- Elder, C.R.; Benson, C.H. Performance and economic comparison of PRB types in heterogeneous aquifers. Environ. Geotech. 2018, 6, 214–224. [Google Scholar] [CrossRef]
- Moraci, N.; Bilardi, S.; Calabrò, P.S. Critical aspects related to Fe0 and Fe0/pumice PRB design. Environ. Geotech. 2016, 3, 114–124. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Sulaymon, A.H.; Khaliefa, Q.M. A review of permeable reactive barrier as passive sustainable technology for groundwater remediation. Int. J. Environ. Sci. Technol. 2018, 15, 1123–1138. [Google Scholar] [CrossRef]
- Vogan, J.L.; Focht, R.M.; Clark, D.K.; Graham, S.L. Performance evaluation of a permeable reactive barrier for remediation of dissolved chlorinated solvents in groundwater. J. Hazard. Mater. 1999, 68, 97–108. [Google Scholar] [CrossRef]
- Phillips, D.H.; Van Nooten, T.; Bastiaens, L.; Russell, M.I.; Dickson, K.; Plant, S.; Ahad, J.M.E.; Newton, T.; Elliot, T.; Kalin, R.M. Ten Year Performance Evaluation of a Field-Scale Zero-Valent Iron Permeable Reactive Barrier Installed to Remediate Trichloroethene Contaminated Groundwater. Environ. Sci. Technol. 2010, 44, 3861–3869. [Google Scholar] [CrossRef]
- Gandhi, S.; Oh, B.-T.T.; Schnoor, J.L.; Alvarez, P.J.J. Degradation of TCE, Cr(VI), sulfate, and nitrate mixtures by granular iron in flow-through columns under different microbial conditions. Water Res. 2002, 36, 1973–1982. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J. Reduction of nitrate by zero valent iron (ZVI)-based materials: A review. Sci. Total Environ. 2019, 671, 388–403. [Google Scholar] [CrossRef]
- Guan, Q.; Li, F.; Chen, X.; Tian, C.; Liu, C.; Liu, D. Assessment of the use of a zero-valent iron permeable reactive barrier for nitrate removal from groundwater in the alluvial plain of the Dagu River, China. Environ. Earth Sci. 2019, 78, 244. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, Y.; Yoon, J.; Kamala-Kannan, S.; Park, S.M.; Oh, B.T. Assessment of zero-valent iron as a permeable reactive barrier for long-term removal of arsenic compounds from synthetic water. Environ. Technol. 2009, 30, 1425–1434. [Google Scholar] [CrossRef]
- Statham, T.M.; Stark, S.C.; Snape, I.; Stevens, G.W.; Mumford, K.A. A permeable reactive barrier (PRB) media sequence for the remediation of heavy metal and hydrocarbon contaminated water: A field assessment at Casey Station, Antarctica. Chemosphere 2016, 147, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, R.D.; Smyth, D.J.A.; Blowes, D.W.; Spink, L.E.; Wilkin, R.T.; Jewett, D.G.; Weisener, C.J. Treatment of Arsenic, Heavy Metals, and Acidity Using a Mixed ZVI-Compost PRB. Environ. Sci. Technol. 2009, 43, 1970–1976. [Google Scholar] [CrossRef] [PubMed]
- Cao, V.; Ndé-Tchoupé, A.I.; Hu, R.; Gwenzi, W.; Noubactep, C. The mechanism of contaminant removal in Fe(0)/H2O systems: The burden of a poor literature review. Chemosphere 2021, 280, 130614. [Google Scholar] [CrossRef]
- Hu, R.; Gwenzi, W.; Sipowo-Tala, V.R.; Noubactep, C. Water Treatment Using Metallic Iron: A Tutorial Review. Processes 2019, 7, 622. [Google Scholar] [CrossRef]
- Hu, R.; Noubactep, C. Redirecting Research on Fe0 for Environmental Remediation: The Search for Synergy. Int. J. Environ. Res. Public Health 2019, 16, 4465. [Google Scholar] [CrossRef]
- Hu, R.; Yang, H.; Tao, R.; Cui, X.; Xiao, M.; Amoah, B.K.; Cao, V.; Lufingo, M.; Soppa-Sangue, N.P.; Ndé-Tchoupé, A.I.; et al. Metallic Iron for Environmental Remediation: Starting an Overdue Progress in Knowledge. Water 2020, 12, 641. [Google Scholar] [CrossRef]
- Moraci, N.; Ielo, D.; Bilardi, S.; Calabrò, P.S. Modelling long-term hydraulic conductivity behaviour of zero valent iron column tests for permeable reactive barrier design. Can. Geotech. J. 2016, 53, 946–961. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Impact of Solids Formation and Gas Production on the Permeability of ZVI PRBs. J. Environ. Eng. 2011, 137, 689–696. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef]
- Noubactep, C. Relevant Reducing Agents in Remediation Fe0/H2O Systems. CLEAN—Soil Air Water 2013, 41, 493–502. [Google Scholar] [CrossRef]
- Hu, R.; Cui, X.; Gwenzi, W.; Wu, S.; Noubactep, C. Fe0/H2O Systems for Environmental Remediation: The Scientific History and Future Research Directions. Water 2018, 10, 1739. [Google Scholar] [CrossRef]
- Yang, H.; Hu, R.; Ruppert, H.; Noubactep, C. Modeling porosity loss in Fe0-based permeable reactive barriers with Faraday’s law. Sci. Rep. 2021, 11, 16998. [Google Scholar] [CrossRef] [PubMed]
- Bilardi, S.; Calabrò, P.S.; Caré, S.; Moraci, N.; Noubactep, C. Effect of pumice and sand on the sustainability of granular iron beds for the aqueous removal of CuII, NiII, and ZnII. Clean—Soil Air Water 2013, 41, 835–843. [Google Scholar] [CrossRef]
- Henderson, A.D.; Demond, A.H. Long-Term Performance of Zero-Valent Iron Permeable Reactive Barriers: A Critical Review. Environ. Eng. Sci. 2007, 24, 401–423. [Google Scholar] [CrossRef]
- Liang, L.; Moline, G.R.; Kamolpornwijit, W.; West, O.R. Influence of hydrogeochemical processes on zero-valent iron reactive barrier performance: A field investigation. J. Contam. Hydrol. 2005, 80, 71–91. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Lee, T.R.; Sexton, M.R.; Acree, S.D.; Puls, R.W.; Blowes, D.W.; Kalinowski, C.; Tilton, J.M.; Woods, L.L. Geochemical and Isotope Study of Trichloroethene Degradation in a Zero-Valent Iron Permeable Reactive Barrier: A Twenty-Two-Year Performance Evaluation. Environ. Sci. Technol. 2019, 53, 296–306. [Google Scholar] [CrossRef]
- Mackenzie, P.D.; Horney, D.P.; Sivavec, T.M. Mineral precipitation and porosity losses in granular iron columns. J. Hazard. Mater. 1999, 68, 1–17. [Google Scholar] [CrossRef]
- Jun, D.; Yongsheng, Z.; Weihong, Z.; Mei, H. Laboratory study on sequenced permeable reactive barrier remediation for landfill leachate-contaminated groundwater. J. Hazard. Mater. 2009, 161, 224–230. [Google Scholar] [CrossRef]
- Klausen, J.; Ranke, J.; Schwarzenbach, R.P. Influence of solution composition and column aging on the reduction of nitroaromatic compounds by zero-valent iron. Chemosphere 2001, 44, 511–517. [Google Scholar] [CrossRef]
- Liu, T.; Lo, I.M.C. Influences of Humic Acid on Cr(VI) Removal by Zero-Valent Iron From Groundwater with Various Constituents: Implication for Long-Term PRB Performance. Water Air Soil Pollut. 2011, 216, 473–483. [Google Scholar] [CrossRef]
- Moraci, N.; Bilardi, S.; Calabrò, P.S. Design of permeable reactive barriers for remediation of groundwater contaminated by heavy metals. Riv. Ital. di Geotec. 2015, 49, 59–86. [Google Scholar]
- Bilardi, S.; Calabrò, P.S.; Moraci, N.; Madaffari, M.G.; Ranjbar, E. A comparison between Fe0 /pumice and Fe0 /lapillus mixtures in permeable reactive barriers. Environ. Geotech. 2020, 7, 524–539. [Google Scholar] [CrossRef]
- Ruhl, A.S.; Jekel, M. Degassing, gas retention and release in Fe(0) permeable reactive barriers. J. Contam. Hydrol. 2014, 159, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Benson, C.H. Evaluation of five strategies to limit the impact of fouling in permeable reactive barriers. J. Hazard. Mater. 2010, 181, 170–180. [Google Scholar] [CrossRef]
- Bilardi, S.; Amos, R.T.; Blowes, D.W.; Calabrò, P.S.; Moraci, N. Reactive Transport Modeling of ZVI Column Experiments for Nickel Remediation. Groundw. Monit. Remediat. 2013, 33, 97–104. [Google Scholar] [CrossRef]
- Bilardi, S.; Calabrò, P.S.; Moraci, N. The removal efficiency and long-term hydraulic behaviour of zero valent iron/lapillus mixtures for the simultaneous removal of Cu2+, Ni2+ and Zn2+. Sci. Total Environ. 2019, 675, 490–500. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Bilardi, S.; Moraci, N. Advancements in the use of filtration materials for the removal of heavy metals from multicontaminated solutions. Curr. Opin. Environ. Sci. Health 2021, 20, 100241. [Google Scholar] [CrossRef]
- Noubactep, C. Should the term ‘metallic iron’ appear in the title of a research paper? Chemosphere 2022, 287, 132314. [Google Scholar] [CrossRef]
- Cao, V.; Yang, H.; Ndé-Tchoupé, A.I.; Hu, R.; Gwenzi, W.; Noubactep, C. Tracing the Scientific History of Fe0-Based Environmental Remediation Prior to the Advent of Permeable Reactive Barriers. Processes 2020, 8, 977. [Google Scholar] [CrossRef]
- Bilardi, S.; Calabró, P.S.; Moraci, N. Simultaneous removal of CU II, NI II and ZN II by a granular mixture of zero-valent iron and pumice in column systems. Desalin. Water Treat. 2015, 55, 767–776. [Google Scholar] [CrossRef]
- Madaffari, M.G.; Bilardi, S.; Calabrò, P.S.; Moraci, N. Nickel removal by zero valent iron/lapillus mixtures in column systems. Soils Found. 2017, 57, 745–759. [Google Scholar] [CrossRef]
- Moraci, N.; Bilardi, S.; Mandaglio, M.C. Factors affecting geotextile filter long-term behaviour and their relevance in design. Geosynth. Int. 2022, 29, 19–42. [Google Scholar] [CrossRef]
- Noubactep, C. Research on metallic iron for environmental remediation: Stopping growing sloppy science. Chemosphere 2016, 153, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Moraci, N.; Bilardi, S.; Calabrò, P.S. Fe0/pumice mixtures: From laboratory tests to permeable reactive barrier design. Environ. Geotech. 2017, 4, 245–256. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).