Abstract
Anaerobic ammonia-oxidizing bacteria have a more comprehensive metabolism than expected - there may be other electron acceptors that oxidize ammonium nitrogen under anaerobic conditions, in addition to the well-known nitrite nitrogen, one of which is sulfate in the sulfammox process. Sulfate-containing compounds are part of the medium for the anammox process, but their concentrations are not particularly high (0.2 g MgSO4 ∙ 7H2O/dm3 and 0.00625 g FeSO4/dm3). They can react to some extent with influent ammonium nitrogen. In this work, tests were carried out in two sequencing batch reactors with granular sludge. The first reactor (R1) operated in a 6 h cycle, and the concentration of the inflowing sulfate was kept at 44 mg/dm3∙d. The second reactor (R2) was operated until the 36th day in a 6 h cycle; the influencing concentration was 180 mg SO42−/dm3∙d from the 37th to 64th day in a 3 h cycle, with an influencing concentration of 360 mg SO42−/dm3∙d; and from the 65th to 90th day, the reactor was operated again in a 6 h cycle with an influencing concentration of 180 mg SO42−/dm3∙d. Along with the increased share of sulfate, both the ammonium utilization rate and specific anammox activity showed an increasing trend. As soon as the sulfate dosage was reduced, the ammonium utilization rate and specific anammox activity values dropped. Therefore, it can be concluded that sulfate-containing compounds contribute to the efficiency and rate of the anammox process.
1. Introduction
Several industrial processes such as fermentation, tanning, landfill leachate production, paper production, pharmaceutical production and food processes produce wastewater containing high concentrations of sulfate (SO42−) and ammonium nitrogen (NH4-N) [1]. Such sewage requires treatment before discharge to the environment, as it is harmful to human life [2].
SO42− is conventionally removed by anaerobic processes by sulfate-reducing bacteria (SRB) [3,4], where SO42− is the final electron acceptor and organic carbon is the electron donor [5]. In contrast, the combined nitrification–denitrification processes are the main pathway responsible for the transformation of nitrogen (N) compounds in wastewater treatment systems in which ammonia-oxidizing bacteria (AOB), nitrogen-oxidizing bacteria (NOB) and heterotrophic bacteria are involved. The discovery of the anammox process shed new light on the nitrogen cycle. This biological process involves oxidizing ammonium nitrogen (NH4-N) under anoxic conditions to gaseous nitrogen (N2), using nitrite nitrogen (NO2-N) as the electron acceptor, via anaerobic ammonia-oxidizing bacteria (AAOB). Accordingly, the removal of SO42− and NH4-N generally takes place in separate processes, as each purification step requires different bacterial groups and environmental conditions. This is associated with high costs due to the necessity of aeration, external carbon sources and excess sludge disposal [6]. However, to date, little is known about the ability of AAOB to use SO42− as an electron acceptor [6].
Fdz-Polanco et al. [7] described the reaction of the autotrophic anaerobic oxidation of NH4-N and deoxidation of SO42− in three Equations (1)–(3):
3SO42− + 4NH4+ → 4NO2− + 3S2− + 4H2O + 8H+
3S2− + 2NO2− + 8H+ → N2 + 3S0 + 4H2O
2NO2− + 2NH4+ → 2N2 + 4H2O
At first, NH4-N is partially oxidized and deoxygenated by SO42− to produce NO2-N and sulfides (S2−) (see reaction 1). Then, some of the NO2-N is reduced by S2− in the sulfur-dependent autotrophic denitrification process and converted into N2 and elemental sulfur (S0) (see reaction 2). Ultimately, the conventional anammox process follows (see reaction 3).
It turns out that AAOB’s metabolism is more comprehensive than expected [8,9] and, in addition to the commonly known electron acceptor in the form of NO2-N, there may be other electron acceptors that oxidize NH4-N under anaerobic conditions [10]. The process described in reactions 1–3 is called the sulfammox process (i.e., sulfate-reducing ammonium oxidation (SRAO)) [11]. The sulfammox process is a promising resource for wastewater treatment systems, because wastewater contains high amounts of sulfur compounds [12]. It can be represented in one reaction as follows [13] (4):
SO42− + 2NH4+ → S0 + N2 + 4H2O
Producing N2 and elemental sulfur (S0) is desirable in wastewater treatment and for the recovery of resources. Moreover, the simultaneous removal of SO42− and NH4-N is more beneficial in terms of reducing costs than the separate removal of these pollutants [14]. The discovery of the sulfammox process suggests that the interrelationships between the N and S biochemical cycles is far more complex than previously assumed.
It is worth noting that the process of sulfur-dependent autotrophic denitrification has been described as a component of sulfammox. It is an autotrophic process in which chemotrophic sulfur-oxidizing bacteria (SOB) oxidize reduced sulfur compounds such as S2−, S0, sulfite (SO32−) or thiosulfate (S2O32−) as electron donors with NO3-N or NO2-N as electron acceptors [15,16,17,18]. Then, SO42− or S0 is formed depending on the sulfur-to-nitrogen ratio [2]. S2− produced by sulfate-reducing bacteria can also be used as an electron donor for sulfur denitrification [19].
Due to the complex transformations of sulfur and nitrogen in anaerobic conditions, it is worth considering the effect of SO42− on anaerobic NH4-N oxidation. The sulfammox process can run independently without the addition of NO2-N or in combination with the conventional (NO2-N based) annamox process. Research on the sulfammox process was carried out in various configurations. At the beginning of the research, SO42− was used as an electron acceptor without the addition of NO2-N [9,11,20,21,22,23]. Other studies started with a conventional anammox, with NO2-N as an electron acceptor, and replaced NO2-N with a new SO42− electron acceptor [11,12,24]. There are also reports in which SO42− was used simultaneously with NO2-N as an electron acceptor [25,26]. For example, Zhang et al. [25] and Wu et al. [26] showed a high degree of simultaneous removal of NH4-N and SO42−, in the range of 92–99% and 53–60%, respectively, when anammox and sulfammox reactions occurred simultaneously. Therefore, the research shows that combining the two processes can achieve an increase in the overall nitrogen removal efficiency.
To date, research work has focused mainly on the effect of increased proportions of NH4-N and N/S ratio in relation to the sulfammox process [10,20,21]. The influence of increased proportions of SO42− on anaerobic NH4-N oxidation in the presence of NO2-N due to the reduced cycle time has yet to be described. The purpose of this study is to compare the operation of two sequencing batch reactors (SBR) with granular sludge: one operates under a constant load of SO42− and constant duration of the process cycle, and the other operates with an increased and variable load of SO42− in a variable cycle time. The process efficiency was compared by calculating the ammonia utilization rate (AUR) and the specific anammox activity (SAA). It is suspected that SO42− will increase the AUR and SAA as it will act as an additional electron acceptor in the anaerobic oxidation of NH4-N.
2. Materials and Methods
2.1. Laboratory-Scale Bioreactor
The inoculated biomass originated from a full-scale side-stream deammonification system in Plettenberg, Germany.
The laboratory scale system used in this study consisted of two 4 dm3 sequencing batch reactors (SBRs) laid out according to the scheme in Figure 1. The system was equipped with a thermostatic jacket maintaining a constant temperature in the range of −35 to + 200 °C, with an accuracy of ± 0.1 °C. Each reactor was equipped with an electric stirrer with variable speed. In the main reactor, probes were placed to measure pH (Endress + Hauser EH CPS 471D-7211, Switzerland) and to measure dissolved oxygen (DO) (Endress + Hauser COS22D-10P3/O, Germany).
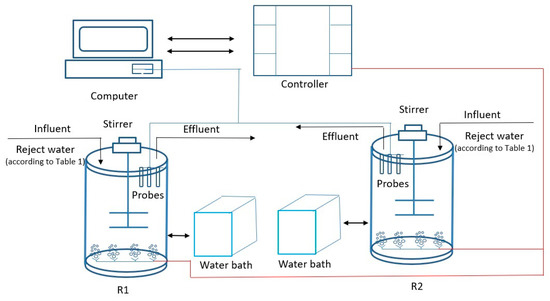
Figure 1.
Laboratory-scale system for the anammox process.
All measured data were transmitted to the programmable logic controller (PLC) and used for control and regulation. Measurement data for archival and further use were sent to an application called Intouch’a.
2.2. Operational Conditions of the Laboratory-Scale SBRs
The tests were carried out continuously for 90 days. During the entire test period, the SBRs operated at a constant temperature of 30 (± 1) °C. The pH was maintained in the range of 7.5–7.8 through the automatic addition of 4 M sodium hydroxide (NaOH). The DO concentration in unventilated SBRs did not exceed 0.2 mg/dm3, and SBRs were fed with synthetic substrate according to the method of Dapena-Mora et al. [27] and Table 1.

Table 1.
Number of cycles and concentrations of compounds in R1 and R2.
In each cycle, 2 dm3 of supernatant water was withdrawn from both reactors and replaced with a new portion of the synthetic substrate. The most important ingredients—i.e., nitrite, ammonium and sulfate—were supplied in the form of NH4Cl, NaNO2 and MgSO4, respectively.
2.3. Analytical Methods
The concentration of NO3-N, NO2-N and NH4-N compounds was determined using a DR 3900 spectrophotometer using cuvette tests from Hach Lange GmbH (Dusseldorf, Germany) for analysis. The biomass concentrations were determined as a volatile suspended solids (VSS) fraction of the total suspended solids (TSS) in accordance with the standard methods [28]. The biomass-specific AUR, SAA and nitrate production rate (NPR) were determined based on the maximum slope of NH4-N consumption, NH4-N combined with NO2-N consumption and NO3-N production in the reaction phase divided into mixed liquor volatile suspended solids (MLVSS) concentrations, respectively. Throughout the operation period, the MLVSS value was 1750 (±50) mg/dm3 in R1 and 1900 (±50) mg/dm3 in R2. AUR, SAA and NPR are given in units of mg N/g VSS∙h to represent these rates in relation to the indicated MLVSS.
3. Results and Discussion
The efficiency of NH4-N oxidation in anaerobic conditions is influenced by anammox, sulfammox, heterotrophic and autotrophic (full and partial) denitrification processes. On the other hand, under aerobic conditions, the oxidation of NH4-N takes place in the process of nitrification or partial nitrification. In our studies, SBR controlled DO at a low level (<0.2 mg/dm3), and the lack of an added external carbon source prevented the occurrence of heterotrophic conditions. Accordingly, the only possible pathways for NH4-N oxidation were through anammox, sulfammox and sulfur-dependent autotrophic denitrification.
Previous studies describe the complete efficiency of NH4-N and SO42− removal as a combination of anammox, sulfammox, nitrification and denitrification [10,11,20,26] or a result of anaerobic processes only [21,29] or of the sulfammox reaction only [30,31] (see Table 2). Moreover, it is worth noting that a few studies on the anaerobic oxidation of NH4-N in the presence of SO42− have been carried out with NO2-N [10,26]. Some of them consisted of only replacing NO2-N with a new electron acceptor in the form of SO42− [11,31], yet the vast majority of the oxidation took place without NO2-N [9,11,20,21,23,29,30].

Table 2.
Concentrations of influent NH4-N and SO42− and the efficiency of their removal under anaerobic conditions. SRAO: sulfate-reducing ammonium oxidation; SRB: sulfate-reducing bacteria.
A study by Zhang et al. [10] investigated the effect of NO2-N on the anaerobic oxidation of NH4-N. They showed that, with a combined decrease in concentration of SO42− from 216 to 100 mg/dm3, NH4-N from 183 to 80 mg/dm3 and NO2-N from 34 to 28 mg/dm3, the efficiency of NH4-N removal increased from 55% to 100%. However, this study does not clearly show the influence of SO42− itself on the process. In our study, we decided to keep the NH4-N and NO2-N inflow to the reactors unchanged in order to determine the influence of SO42− on the process.
In R1, where the influent SO42− concentration was constant at 22 mg SO42−/dm3, a gradual increase in the rates of AUR and SAA could be observed as well as their stabilization from day 49, as shown in Figure 2a. Comparing these values with the values in R2 in Figure 2b, it can be seen that, despite the approximately four-fold higher SO42− concentration in the effluents in R2 (90 mg SO42−/dm3 for R2), the AUR and SAA showed similar values from the beginning of the test to day 29. The AUR increased from 1.3 mg N/g VSS∙h to 2.1 mg N/g VSS∙h (R1) and from 1.1 mg N/g VSS∙h to 2.1 mg N/g VSS∙h (R2), while the SAA increased from 4 mg N/g VSS∙h to 5.6 mg N/g VSS∙h (R1) and 3.7 mg N/g VSS∙h to 5.3 mg N/g VSS∙h (R2).
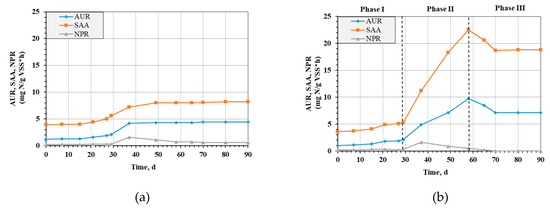
Figure 2.
Ammonia utilization rate (AUR), specific annamox activity (SAA) and nitrate production rate (NPR) in R1 (a) and in R2 (b).
On day 37, there was a clear increase in AUR, SAA and NPR in R1. This showed that the efficiency of the anammox process was greatly improved as more NH4-N was oxidized with NO2-N. The increase in NPR also confirmed that more NH4-N was oxidized as approximately 11% was converted to NO3-N in this process.
Near the end of the study, there was a stabilization of AUR values, SAA and a decrease in NPR in R1. AUR increased to a maximum of 4.4 mg N/g VSS∙h, and SAA increased to 8.1 mg N/g VSS∙h.
In R2, on day 37, the cycle time was reduced from 6 h to 3 h, which resulted in the concentration of SO42− being twice as high as in the previous period: −360 mg/dm3∙d and 180 mg/dm3∙d for phases II and I, respectively. This affected the AUR and SAA significantly, as can be seen in Figure 2b. This increase was evident throughout phase II. The AUR value at the end of this phase was 9.7 mg N/g VSS∙h, while SAA was 22.5 mg N/g VSS∙h. This confirmed the positive influence of SO42− on the course of the NH4-N oxidation process. SO42− seems to be an additional acceptor that improves the rate and efficiency of the process, increasing the efficiency of NH4-N removal as shown in Figure 3.
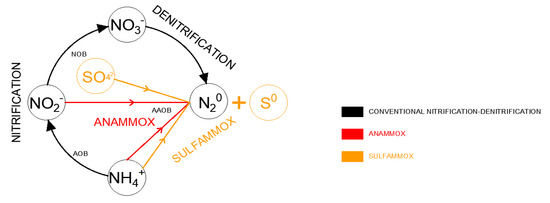
Figure 3.
Diagram showing the coexistence of anammox and sulfammox processes.
There are reports in the literature confirming that SO42− can increase the total removal of NH4-N. Liu et al. [9] noted in his research that the NH4-N removal rate was always higher than expected and the NH4-N/NO2-N consumption ratio was about 1.1:1, which was much higher than previously reported [32]. It was then concluded that, due to large amounts of (NH4)2SO4 in the feed, SO42− could be the source of the additional electron acceptor.
Moreover, Yang et al. [31] noted that as the concentration of NH4-N and SO42− increased, incrementally more of both were removed in their batch tests. When the NH4-N and SO42− concentrations in the inflow were approximately 28 and 76 mg/dm3, respectively, the removal efficiency was close to 0%. However, when the average NH4-N and SO42− concentrations in the inflow increased to 92 and 307 mg/dm3, the removed amount decreased to 40 and 130 mg/dm3, respectively. Thus, high concentrations of NH4-N and SO42− may promote the simultaneous removal of these compounds, as shown in our research.
Phase III in R2 showed a downward trend in AUR and anammox rates from 9.7 mg N/g VSS∙h to 7.1 mg N/g VSS∙h and from 22.5 mg N/g VSS∙h to 18.7 mg N/g VSS∙h, respectively. This was due to the reduction of the SO42− concentration flowing into the reactor. Again, fewer electron acceptors, in the form of SO42−, were present in the environment; therefore, the rate of NH4-N oxidation decreased because half as much SO42− flowed in per day. The tests were performed until the process stabilized, and constant values of AUR, SAA and NPR were achieved by the 90th day.
Moreover, Zhang et al. [20] noticed that, as the concentration of SO42− increased from about 90 mg/dm3 to about 170 mg/dm3 and NH4-N from about 50 mg/dm3 to about 120 mg/dm3, the efficiency of NH4-N removal increased from 40% to 90%. However, a further increase in the concentration of SO42− to about 360 mg/dm3 and NH4-N to about 180 mg/dm3 resulted in a decrease in NH4-N removal up to roughly 20%. Similarly, in an Expanded Granular Sludge Bed Reactor (EGSBR) [21] under chemical oxygen demand (COD) conditions, the NH4-N removal efficiency gradually improved from 40–58% to 40–70% when the inflow NH4-N concentrations increased from 166–666 mg N/dm3 to 1000–2000 mg N/dm3. Comparatively, after increasing the NH4-N concentration to >3000 mg N/dm3, the efficiency of NH4-N reduction decreased to approximately 10–25%. This was due to the inhibition of the anammox process with free ammonia. This proves that an increase in NH4-N and SO42− concentrations improves the process of anaerobic NH4-N oxidation only to a certain extent. In our study, there was no inhibition of the process due to excessively high concentrations of these compounds.
Wu et al. [26] noted that they had achieved an NH4-N removal efficiency of 98%, including 44% removed through sulfammox. Compounds containing SO42− can therefore effectively improve the efficiency of the anaerobic oxidation of NH4-N, but at the same time, anaerobic conditions favor the decomposition of SO42− to S0, which is less toxic to the environment. The sulfammox process has so far been studied mainly as an independent process (without NO2-N addition). Moreover, there has been more interest in the influence of NH4-N concentration on the sulfammox process [21] and the N/S ratio [20] rather than directly considering the effect of SO42− itself.
Bi et al. [11] challenged the sulfammox process and postulated that AAOBs did not have the ability to oxidize NH4-N using SO42− as an electron acceptor and that SRAO was a combination of aerobic ammonium oxidation, anammox and heterotrophic sulfate reduction processes. Moreover, the specification of the efficiency of NH4-N and SO42− removal in the sulfammox process does not reflect the course of the process as thoroughly as the AUR and the SAA, which the authors do not provide in their research.
4. Conclusions
In this study, it was shown that SO42− could be used as an additional electron acceptor in the anaerobic oxidation of NH4-N. Along with the increased share of SO42−, both AUR and SAA showed an increasing trend. In R1, where the concentration of SO42− in the inflow was constant at the level of 22 mg SO42−/dm3, there was a gradual increase in the AUR and SAA indicators from 1.2 mg N/g VSS∙h to 4.4 mg N/g VSS∙h and from 3.9 mg N/g VSS∙h to 8.2 mg N/g VSS∙h, respectively. In R2 in phase I, over a 6 h cycle, AUR and SAA increased from 1 mg N/g VSS∙h to 2.1 mg N/g VSS∙h and from 3.6 mg N/g VSS∙h to 5.3 mg N/g VSS∙h; in phase II, over a 3 h cycle, they increased to 9.7 mg N/g VSS∙h and 22.5 mg N/g VSS∙h; and in phase III, over a 3 h cycle, they dropped to 7.1 mg N/g VSS∙h and 18.8 mg N/g VSS∙h, respectively. It can therefore be concluded that SO42− contributes to the rate and efficiency of the anammox process. Further studies on the influence of the NH4-N/SO42− ratio on the process and identification of the bacteria responsible for sulfammox are suggested.
Author Contributions
Conceptualization, D.G. and J.M.; methodology, J.M.; software, J.M.; validation, J.M.; formal analysis, J.M.; investigation, D.G.; resources, J.M.; data curation, J.M.; writing—original draft preparation, D.G.; writing—review and editing, J.M.; visualization, D.G.; supervision, J.M.; project administration, D.G.; funding acquisition, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Narodowe Centrum Nauki grant number 2019/03/X/ST10/01127.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duyar, A.; Ozdemir, S.; Akman, D.; Akgul, V.; Sahinkaya, E.; Cirik, K. Optimization of sulfide-based autotrophic denitrification process in an anaerobic baffled reactor. J. Chem. Technol. Biotechnol. 2018, 93, 754–760. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, D.; Yan, L.; Wang, A.; Gu, Y.; Lee, D. Elemental sulfur formation and nitrogen removal from wastewaters by autotrophic denitrifiers and anammox bacteria. Bioresour. Technol. 2015, 191, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Sarti, A.; Pozzi, E.; Chinalia, F.A.; Ono, A.; Foresti, E. Microbial processes and bacterial populations associated to anaerobic treatment of sulfate-rich wastewater. Process Biochem. 2010, 45, 164–170. [Google Scholar] [CrossRef]
- Wei, C.; Wang, W.; Deng, Z.; Wu, C. Characteristics of high-sulfate wastewater treatment by two-phase anaerobic digestion process with Jet-loop anaerobic fluidized bed. J. Environ. Sci. 2007, 19, 264–270. [Google Scholar] [CrossRef]
- Sinbuathong, N.; Khaodhiar, S.; Liengcharernsit, W.; Sirirote, P.; Watts, D. Effect of sulfate on the methanogenic activity of a bacterial culture from a brewery wastewater during glucose degradation. J. Environ. Sci. 2007, 19, 1025–1027. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, L.; Zhu, H.; Madani, R.M.A.; Liang, J. Treatment performance and microbial community under ammonium sulphate wastewater in a sulphate reducing ammonium oxidation process. Environ. Technol. 2020. [Google Scholar] [CrossRef]
- Fdz-Polanco, F.; Fdz-Polanco, M.; Fernandez, N.; Urueña, M.A.; Garcia, P.A.; Villaverde, S. New process for simultaneous removal of nitrogen and sulphur under anaerobic conditions. Water Res. 2001, 35, 1111–1114. [Google Scholar] [CrossRef]
- Kartal, B.; van Niftrik, L.; Keltjens, J.T.; Op den Camp, H.J.M.; Jetten, M.S.M. Anammox-Growth Physiology, Cell Biology, and Metabolism. Adv. Microb. Physiol. 2012, 60, 211–262. [Google Scholar]
- Liu, S.; Yang, F.; Gong, Z.; Meng, F.; Chen, H.; Xue, Y.; Furukawa, K. Application of anaerobic ammonium-oxidizing consortium to achieve completely autotrophic ammonium and sulfate removal. Bioresour. Technol. 2008, 99, 6817–6825. [Google Scholar] [CrossRef] [PubMed]
- In ‘t Zandt, M.H.; de Jong, A.E.; Slomp, C.P.; Jetten, M.S. The hunt for the most-wanted chemolithoautotrophic spookmicrobes. FEMS Microbiol. Ecol. 2018, 94, fiy064. [Google Scholar] [CrossRef] [PubMed]
- Bi, Z.; Wanyan, D.; Li, X.; Huang, Y. Biological conversion pathways of sulfate reduction ammonium oxidation in anammox consortia. Front. Environ. Sci. Eng. 2020, 14, 38. [Google Scholar] [CrossRef]
- Rikmann, E.; Zekker, I.; Tomingas, M.; Tenno, T.; Loorits, L.; Vabamäe, P.; Mandel, A.; Raudkivi, M.; Daija, L.; Kroon, K.; et al. Sulfate-reducing anammox for sulfate and nitrogen containing wastewaters. Desalin. Water Treat. 2016, 57, 3132–3141. [Google Scholar] [CrossRef]
- Ali, M.; Okabe, S. Anammox-based technologies for nitrogen removal: Advances in process start-up and remaining issues. Chemosphere 2015, 141, 144–153. [Google Scholar] [CrossRef]
- Klein, K.; Kattel, E.; Goi, A.; Kivi, A.; Dulova, N.; Saluste, A.; Zekker, I.; Trapido, M.; Tenno, T. Combined treatment of pyrogenic wastewater from oil shale retorting. Oil Shale 2017, 34, 82–96. [Google Scholar] [CrossRef]
- Beller, H.R.; Chain, P.S.G.; Letain, T.E.; Chakicherla, A.; Larimer, F.W.; Richardson, P.M.; Coleman, M.A.; Wood, A.P.; Kelly, D.P. The genome sequence of the obligately chemolithoautotrophic, facultatively anaerobic bacterium Thiobacillus denitrificans. J. Bacteriol. 2006, 188, 1473–1488. [Google Scholar] [CrossRef]
- Yu, H.; Wang, A.; Chen, C. Structure and dynamics of microbial community in the denitrifying sulfide removal process. Huanjing Kexue 2013, 34, 1190–1195. [Google Scholar] [PubMed]
- Wang, X.; Sun, G.; Zhu, Y. Thermodynamic energy of anaerobic microbial redox reactions couples elemental biogeochemical cycles. J. Soils Sed. 2017, 17, 2831–2846. [Google Scholar] [CrossRef]
- Di Capua, F.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Electron donors for autotrophic denitrification. Chem. Eng. J. 2019, 362, 922–937. [Google Scholar] [CrossRef]
- Kosugi, Y.; Matsuura, N.; Liang, Q.; Yamamoto-Ikemoto, R. Nitrogen flow and microbial community in the anoxic reactor of “Sulfate Reduction, Denitrification/Anammox and Partial Nitrification” process. Biochem. Eng. J. 2019, 151, 107304. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, L.; Wang, H.; Liang, J. Study of sulfate-reducing ammonium oxidation process and its microbial community composition. Water Sci. Technol. 2019, 79, 137–144. [Google Scholar] [CrossRef]
- Wang, D.; Liu, B.; Ding, X.; Sun, X.; Liang, Z.; Sheng, S.; Du, L. Performance evaluation and microbial community analysis of the function and fate of ammonia in a sulfate-reducing EGSB reactor. Appl. Microbiol. Biotechnol. 2017, 101, 7729–7739. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, P.; He, Y.; Jin, R. Performance of sulfate-dependent anaerobic ammonium oxidation. Sci. China Ser. B 2009, 52, 86–92. [Google Scholar] [CrossRef]
- Zhao, Q.I.; Li, W.; You, S.J. Simultaneous removal of ammonium-nitrogen and sulphate from wastewaters with an anaerobic attached-growth bioreactor. Water Sci. Technol. 2006, 54, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Rikmann, E.; Zekker, I.; Tomingas, M.; Tenno, T.; Menert, A.; Loorits, L.; Tenno, T. Sulfate-reducing anaerobic ammonium oxidation as a potential treatment method for high nitrogen-content wastewater. Biodegradation 2012, 23, 509–524. [Google Scholar] [CrossRef]
- Zhang, D.; Cui, L.; Madani, R.M.A.; Wang, H.; Zhu, H.; Liang, J. Effect of nitrite and nitrate on sulfate reducing ammonium oxidation. Water Sci. Technol. 2019, 80, 634–643. [Google Scholar] [CrossRef]
- Wu, L.; Yan, Z.; Li, J.; Huang, S.; Li, Z.; Shen, M.; Peng, Y. Low temperature advanced nitrogen and sulfate removal from landfill leachate by nitrite-anammox and sulfate-anammox. Environ. Pollut. 2020, 259, 113763. [Google Scholar] [CrossRef]
- Dapena-Mora, A.; Arrojo, B.; Campos, J.L.; Mosquera-Corral, A.; Méndez, R. Improvement of the settling properties of Anammox sludge in an SBR. J. Chem. Technol. Biotechnol. 2004, 79, 1417–1420. [Google Scholar] [CrossRef]
- Greenberg, A.E.; Clesceri, L.S.; Eaton, A.D. APHA Standard Methods for the Examination of Water and Waste Water, 21st ed.; American Public Health Association, American Water Works Association, Water Pollution Control Federation: Washington, DC, USA, 2005. [Google Scholar]
- Prachakittikul, P.; Wantawin, C.; Noophan, P.; Boonapatcharoen, N. ANAMMOX-like performances for nitrogen removal from ammonium-sulfate-rich wastewater in an anaerobic sequencing batch reactor. J. Environ. Sci. Health 2016, 51, 220–228. [Google Scholar] [CrossRef]
- Cai, J.; Jiang, J.X.; Zheng, P. Isolation and identification of bacteria responsible for simultaneous anaerobic ammonium and sulfate removal. Sci. China Chem. 2010, 53, 645–650. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, S.; Sun, Y. Start-up of simultaneous removal of ammonium and sulfate from an anaerobic ammonium oxidation (anammox) process in an anaerobic up-flow bioreactor. J. Hazard. Mater. 2009, 169, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Van De Graaf, A.A.; De Bruijn, P.; Robertson, L.A.; Jetten, M.S.M.; Kuenen, J.G. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 1996, 142, 2187–2196. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).