Abstract
Exposure to even low concentrations of heavy metals can be toxic to aquatic organisms, especially during embryonic development. Thus, this study aimed to investigate the toxicity of nickel and cadmium in zebrafish (Danio rerio) embryos exposed to environmentally relevant concentrations of each metal alone or in combination from 4 h through to 72 h postfertilization. Neither metal altered survival, but individual and combined exposures decreased hatching rate. Whereas cadmium did not affect total body length, trunk area, eye diameter, or eye area, nickel alone and in combination with cadmium decreased each morphological parameter. Yolk sac area, an index of metabolic rate, was not affected by nickel, but was larger in embryos exposed to high cadmium concentrations or nickel and cadmium combined at high concentrations. Nickel decreased spontaneous movement, whereas cadmium alone or nickel and cadmium combined had no effect. Neither metal altered elicited movement, but nickel and cadmium combined decreased elicited movement. Myosin protein expression in skeletal muscle was not altered by cadmium exposure. However, exposure to nickel at low concentrations and combined exposure to nickel and cadmium decreased myosin expression. Overall, nickel was more toxic than cadmium. In conclusion, we observed that combined exposures had a greater effect on movement than gross morphology, and no significant additive or synergistic interactions were present. These results imply that nickel and cadmium are toxic to developing embryos, even at very low exposure concentrations, and that these metals act via different mechanisms.
1. Introduction
Environmental heavy metal contamination is an increasing global concern. Both natural events and human activities contribute to environmental heavy metal contamination [1,2]. Nickel and cadmium are transition metals that easily form salts and readily dissolve in water [2,3]. Nickel and cadmium content in plants appears to be linear with respect to metal contamination of ground soil, which can easily contaminate aquatic systems due to runoff [4,5,6]. Heavy metal contamination is typically higher near industrial plants, mining sites, and regions with high vehicular traffic [7]. Millions of tons of electronic waste containing a range of metals, including cadmium and nickel, have been deposited in the environment [8,9]. Subsequent leakage of industrial waste and sewage into groundwater or the application of sewage sludge onto soil and mobilization of metals from sediments during floods further increase the environmental heavy metal contamination of aquatic systems [10]. Heavy metal contamination of fresh and salt waters is of growing concern and even in trace amounts, metals such as mercury, lead, cadmium, and nickel are considered to be a severe threat to the aquatic environment. Sublethal heavy metal exposure can result in a range of adverse effects on activity, growth, metabolism, and reproduction [11,12,13,14].
Many toxicants have more deleterious effects during embryonic development than during adult stages [15]. It is established that lead and mercury are highly toxic to the developing embryo, but increasing evidence indicates that cadmium, nickel, and other heavy metals are developmental toxicants. Cadmium exposure in early embryos decreases blastocyst formation [16] and elicits degeneration in embryonic blastocysts due to apoptosis and loss of normal cell adhesion properties [17]. In later developmental stages, cadmium induces a wide range of embryonic abnormalities, including neurological, cardiovascular, gastrointestinal, genitourinary, and limb anomalies in placental mammals and axial and somite abnormalities in fish [17].
Most research conducted to elucidate the adverse effects of toxicants has been relegated to studying different components individually, though organisms are typically exposed to combinations of toxic agents. Little is known regarding the developmental effects of exposure to mixtures of heavy metals. An additional concern with mixture exposure is possible amplification of toxicity from exposure to toxicant mixtures [18]. Amplification of toxicity is predicted for compounds with common modes of action that could act in an additive or synergistic manner, but little evidence has yet been published to confirm this prediction.
Early toxicology research used primarily rodents as animal models, but the use of zebrafish (Danio rerio) in toxicology research is increasingly common. Zebrafish have been a model organism for studying developmental processes for more than 30 years [19]. Early embryological processes, such as initial cleavage stages and organ formation, are strikingly similar in zebrafish and mammals. Furthermore, using zebrafish as an animal model offers several practical advantages. Their small size allows large numbers to be housed inexpensively in the laboratory. Fertilization is external, facilitating toxicant exposure at defined concentrations for durations that can begin at the point of fertilization. Females produce up to 300 eggs every 7–10 days, providing large sample sizes, and eggs and early embryos are transparent; thus, development, which is rapid, can be readily observed [20].
Increasingly, fish embryos are being used in aquatic toxicity testing [21]. Numerous studies have examined the effects of a range of heavy metals in developing fish, including mercury, lead, iron, cadmium, chromium, and manganese [22,23,24,25]. For example, cadmium exposure in zebrafish embryos inhibited the commitment of neural progenitor cells during brain development [26], but it increased spontaneous tail movements in 24-h postfertilization zebrafish embryos [25]. Cadmium exposure also was shown to disrupt growth hormone expression in rainbow trout embryos [27], delay hatching in carp embryos [28], retard development in zebrafish embryos [29,30], and inhibit pigmentation development in embryonic African catfish [31].
While considerable study of the effects of cadmium alone or in combination with other compounds has been published, less information is known about the effects of nickel on fish development. In one study, the exposure of zebrafish embryos to nickel and cadmium was demonstrated to negatively impact visually guided larval behavior but did not alter the gross morphology of exposed embryos [32]. Scheil et al. reported that exposure to nickel delayed hatching in zebrafish embryos [33]. Kim et al. indicated that exposure to nickel plus vanadium resulted in heart developmental toxicity in exposed zebrafish embryos [34]. However, the majority of published studies only studied exposures to one toxicant at a time and not to combinations, or they have investigated combinations of many toxicants, making it difficult to elucidate the underlying specific mechanisms of action.
Therefore, in this study, we exposed zebrafish embryos to a series of low concentrations of nickel and cadmium individually but more importantly, we also exposed zebrafish embryos to combinations of these two metals. We hypothesized that individual exposure to low levels of nickel and cadmium would adversely affect zebrafish development. We also hypothesized that combined nickel and cadmium exposure would have a synergistic, adverse effect on embryonic development. We assessed different developmental parameters, such as mortality rate, hatching rate, spontaneous and elicited movement, as well as the morphometric endpoints of body length, eye area, and diameter, yolk sac area, trunk area, and tail area. We also assessed the expression of muscle myosin protein by immunohistochemistry. Exposure to cadmium and nickel had the most dramatic effects on hatching and myosin protein expression.
2. Materials and Methods
2.1. Zebrafish Husbandry and Embryo Collection
Adult wildtype zebrafish (AB strain) were maintained in a circulating freshwater aquarium system in the Biology Department at Texas A&M University under standard laboratory conditions at an average ambient temperature of 28.5 °C [35]. In the late afternoon, one male and two female adult zebrafish were placed in breeding tanks and separated by a transparent barrier. The next morning, the barrier was removed to initiate spawning [22]. At 2 h postfertilization (hpf), zebrafish embryos (ZFE) were gathered into 50-mL sterile PE tubes containing aquarium water. ZFE were transported to the laboratory where they were transferred to the wells of 24-well, flat-bottom plates with low-evaporation lids (BD Biosciences, San Jose, CA, USA). Each well contained 3–4 embryos and 2 mL of embryo medium. The plates were incubated at 28.5 °C until the start of the metal exposure, which began at approximately 4 hpf. For the pre-equilibration period and duration of all experimental treatments, ZFE were maintained at a constant temperature of 28.5 °C (Thelco Incubator; Cole-Palmer Instruments, Vernon Hills, IL, USA). Fresh embryo medium was prepared for each experiment and consisted of ultrapure water with (in mM) 13.7 NaCl, 0.54 KCl, 0.025 Na2PO4, 0.44 KH2PO4, 1.3 CaCl2, 1.0 MgSO4, and 4.3 NaHCO3 and pH adjusted to 7.20 as per Westerfield [35]. This study was conducted under an animal use protocol approved by the Texas A&M University institutional animal use committee. Zebrafish were maintained and used according to protocols consistent with the Information Resources on Zebrafish, Animal Welfare Information Center Resource Series, No. 46, August 2010, USDA (https://pubs.nal.usda.gov/sites/pubs.nal.usda.gov/files/Zebrafish.pdf, compiled by D. Scholfield).
2.2. Heavy Metal Exposure
Separate stock solutions of cadmium and nickel were prepared from dichlorocadmium hydrate (CdCl2.H2O) and nickel chloride hexahydrate (NiCl2.6H2O) (VWR Laboratory Supplies, Radnor, PA, USA). Stock solutions were prepared at 1.0 g of cadmium or nickel per liter (g/L) using sterile water and stored at 4 °C, and then, diluted with fresh embryo medium to prepare treatment solutions. The effects of nickel alone were examined in ZFE incubated in embryo medium with 0.1, 0.5, 2.5, and 5 mg/L (0.4, 2.1, 10.5, and 21.0 µM, respectively) nickel, and the effects of cadmium alone were examined in ZFE incubated in embryo medium with 0.015, 0.15, 0.705, and 1.5 mg/L (0.07, 0.7, 3.1, and 6.6 µM, respectively) cadmium. For combined exposure to nickel and cadmium, ZFE were exposed simultaneously to 0.5 mg/L (2.1 µM) nickel and 0.15 mg/L (0.7 µM) cadmium (low concentrations) or 2.5 mg/L (10.5 µM) nickel and 0.7 mg/L (3.1 µM) cadmium (high concentrations). These concentrations were chosen based on reports of heavy metal contamination in different water sources around the globe. For example, cadmium groundwater contamination in Pakistan was as high as 0.18 mg/L and nickel was as high as 1.0 mg/L [36]. Cadmium contamination in the Caspian Sea, Iran, has been measured at 120 µg/L [37]. The highest level of nickel contamination in the ocean near the Philippines was 0.6 mg/L [38]. Thus, the ranges of concentrations used in this investigation were environmentally relevant for highly contaminated water sources around the world. We note that these metal concentrations were nominal concentrations due to the possibility that some absorption of metal to the plastic may have occurred. Therefore, the actual concentrations to which the ZFEs were exposed may have been somewhat lower.
At 4 hpf, the preincubation medium was gently removed from each well and replaced with 2 mL of treatment medium containing nickel, cadmium, or both nickel and cadmium at the prescribed concentrations. For the control condition, the preincubation medium was replaced with 2 mL fresh embryo medium with no added metals. ZFE were incubated at 28.5 °C and removed briefly from the incubator for developmental and behavioral assessments at 24, 48, and 72 hpf.
2.3. Survival and Developmental Assessments
For each assessment, ZFE were viewed at room temperature with a SZ-40 binocular dissecting microscope (Olympus, Center Valley, PA, USA). Survival and hatch rate were assessed at 24, 48, and 72 hpf; numbers of alive versus dead ZFE and numbers of hatched versus unhatched ZFE were counted for the entire set of ZFE in each exposure and control group. Spontaneous movement was evaluated in individual 24-hpf embryos, each of which was still enveloped within the transparent chorion. At 24 hpf, the number of spontaneous movements was evaluated in 30 randomly selected ZFE from each control and experimental exposure group; movements or “twitches” by an individual embryo were measured by counting the number of movements in 60 s. At 72 hpf, elicited movement was assessed in 40–80 randomly selected ZFE in the control and each experimental exposure group. For this assay, an individual ZFE was placed in a Petri dish filled with embryo medium at the center of a 6 × 6 cm grid demarcated into 5 × 5 mm squares, and the distance an embryo moved in response to a single stimulus, i.e., one gentle touching of the tail, was recorded. We used Chi-square analysis to assess elicited movement; data were categorized into two groups (0 to 25 mm swim distance and 26 to 50 mm distance). After each set of survival or developmental assessments at 24, 48, or 72 hpf, ZFE were euthanized by immersion in a solution of MS-222 (20 mg/100 mL embryo medium [39]; Sigma-Aldrich, St. Louis, MO, USA) for a minimum of 20 min and rinsed in distilled water. All embryos were then immersed in 10% neutral buffered formalin (NBF) for 24 h at 4 °C. The embryos were transferred to phosphate-buffered saline (PBS; pH 7.2) and stored at 4 °C. The 48 and 72 hpf embryos were subsequently processed for morphometric and immunohistochemical analyses.
2.4. Morphometric Measurements
Morphological parameters were measured in fixed ZFE exposed to heavy metals and age-matched control ZFE. Images of whole fixed 48 and 72 hpf embryos were obtained using an Eclipse E400 upright light microscope and DS-Ril digital camera (Nikon Instruments, Melville, NY, USA). Using the NIH Image J program, total body length and eye diameter were measured to the nearest ±0.1 mm; eye area, yolk sac area, trunk area, and tail area were measured to the nearest ±0.1 mm2.
2.5. Immunohistochemical Analysis of Myosin Expression
Fixed ZFE were embedded in paraffin, sectioned at 5 µm thickness, and mounted on glass slides (Veterinary Integrative Biosciences, Texas A&M University). ZFE sections were stained for heavy chain myosin following a standard immunohistochemistry protocol [40]. Sections were deparaffinized, permeabilized in PBS with 0.3% Triton X-100 (RT, 30 min), and blocked in PBS with 5% normal horse serum (RT, 1 h) before incubation (4 °C, 24 h) with primary antibody (MF-20) (1:500; Developmental Studies Hybridoma Bank, Iowa City, IA, USA). After thorough rinsing, sections were incubated (4 °C, 24 h) in PBS with biotinylated horse anti-mouse secondary antibody (1:400; Vector Laboratories, Burlingame, CA, USA). Sections were rinsed and incubated in PBS (4 °C, 24 h) with horseradish peroxidase-conjugated streptavidin (1:5000, Kirkegaard & Perry Laboratories, Inc. Gaithersburg, MD, USA). After rinses in PBS and one rinse in 0.05 M Tris-HCl (pH 7.6), immunoreactivity was visualized with 0.06% diaminobenzidine (DAB, Sigma-Aldrich, St. Louis, MO, USA), 0.6% nickel ammonium sulfate, and 0.02% hydrogen peroxide in 0.05 M Tris-HCl. The reaction was terminated with 0.05 M Tris-HCl; stained sections were rinsed, dehydrated, and mounted with coverslips using Omimount (Electron Microscopy Sciences, Hatfield, PA, USA). Representative ZFE sections processed in parallel without primary antibody served as negative controls for non-specific DAB staining. Images of stained sections were obtained with an Eclipse E400 microscope fitted with 20× and 40× objectives, a DXM1200 digital camera, and ACTI imaging software (Nikon Instruments, Melville, NY, USA). Fixed embryos were immunostained for myosin heavy chain protein; staining intensity in experimental embryos was expressed as a percent of staining in control embryos. The myosin-positive muscle in the tail region of the embryos was measured using NIH ImageJ [41]. The data were reported as optical density measurements based on the initial pixel values in gray level units from 0 to 255 and calibrated to optical density.
2.6. Statistical Analyses
Embryos were collected on a weekly basis and assigned to control and experimental metal exposures. Due to the large number of experimental groups utilized in this study, not all metals and combinations of metals could be assessed in a single week. Thus, for each concentration of cadmium, nickel, or combination of cadmium and nickel, the exposures were repeated a minimum of three times to ensure that a sufficient number of embryos were included in this study. Each week, we tested a complete series of concentration exposures for each metal or combination of metals. As described, each control and experimental treatment well contained 3–4 embryos. When embryos were sampled for the different assays, only one embryo was taken from a well for sampling of a given parameter, even though there were three or four embryos undergoing treatment in each well.
The Chi-square test was used to assess spontaneous movement, comparing exposed embryos to control embryos. One-way analysis of variance (ANOVA) was used to determine whether differences existed among the remaining responses to the different concentrations of heavy metals. The D’Agostino–Pearson test for homogeneity of variances was applied to data analyzed using Kruskal–Wallis ANOVA. Results are expressed as percentages (for mortality and hatching) or as mean ± standard error of the mean (SEM). For movement assessment, a minimum of 30 embryos per group were assessed. For immunohistochemical staining of myosin, 8 to 12 ZFE were assessed for each heavy metal, age, and concentration analyzed. For all morphometric measurements, a minimum of 20 embryos were measured for each concentration of heavy metal analyzed. Comparisons between means for each treatment were made using Student’s t-test, and the Bonferroni correction was applied. Significance for all analyses was set at p ≤ 0.05.
3. Results
3.1. Survival and Hatching Success
Numbers of live embryos were recorded at 24, 48, and 72 hpf for each experimental group. No significant differences in survival were observed in any of the experimental exposure groups at all three time points or for any of the three experimental conditions—exposure to nickel only, exposure to cadmium only, and exposure to combinations of nickel and cadmium (data not shown). Zebrafish embryos (ZFE) typically hatch at 48–96 hpf, with the majority hatching by 72 hpf [35]; we assessed the hatching rate at 48 and 72 hpf (Figure 1). Even though nickel and cadmium exposure did not adversely affect survival, individual or combined exposure to nickel and cadmium significantly reduced hatching rates at 48 hpf (Figure 1A–C). At 48 hpf, all four concentrations of nickel decreased hatching as compared to controls (ANOVA; p < 0.00001), but only the highest cadmium concentration significantly decreased hatching (ANOVA; p = 0.01). Both combinations of nickel and cadmium significantly decreased hatching at 48 hpf (ANOVA; p = 0.0001). At 72 hpf (Figure 1D–F), the hatching rate in embryos exposed to the two lowest nickel concentrations and embryos exposed to all cadmium concentrations were comparable to that of age-matched controls (ANOVA; nickel, p < 0.00001; cadmium, p = 0.46). However, at 72 hpf, embryos exposed to the two highest nickel concentrations hatched at significantly reduced rates; similarly, at 72 hpf, embryos exposed to both combinations of nickel and cadmium still hatched at significantly reduced rates (ANOVA; p = 0.00000004).
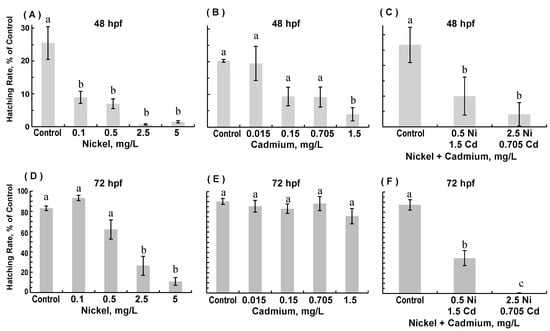
Figure 1.
Hatching rate at 48 hpf (A–C) and 72 hpf (D–F) zebrafish embryos (survivors) reared in medium containing 0.1–5 mg/L nickel (Ni), 0.15–1.5 mg/L cadmium (Cd), and combinations of 0.5 mg/L Ni + 0.15 Cd or 2.5 mg/L Ni + 0.705 mg/L Cd. Note: In all figures, within a given panel, statistical similarity is denoted with the same letter (p < 0.05). (Data are means ± SEM for N = 50–80).
3.2. Spontaneous and Elicited Movements
In developing ZFE, spontaneous movements of tail musculature are initiated at approximately 17 hpf, peak at 19 hpf, and continue until hatching [42]. Spontaneous tail movements or “twitches” are attributed to the establishment of motor neural circuits between the spinal cord and developing skeletal muscles [42]. We assessed spontaneous movement of ZFE at 24 hpf in controls and all experimental groups (Table 1). Data were evaluated by Chi-square analysis. Because the embryos moved so infrequently in the 60-s period (i.e., less than five times), movements were categorized as no movement observed in 60 s or movement observed in 60 s. Embryos exposed to nickel alone showed significantly less movement (p = 0.02), while those exposed to cadmium only or the combination of nickel and cadmium showed no differences in spontaneous movement (p = 0.18 and p = 0.5, respectively; data not shown).

Table 1.
Comparison of spontaneous movement in 24 hpf zebrafish embryos reared in medium containing 0.1–5 mg/L nickel.
The elicited movement was assessed at 72 hpf in hatched embryos (Table 2). A single slight touch of the embryo’s tail initiates swimming, presumably an escape reflex. The proportion of control embryos that initiate swimming in response to tail-touch should approach 100% by 60 hpf [42]. Data were categorized into two groups (0–25 mm swim distance and 26–50 mm distance) and subjected to Chi-square analysis. Exposure to either nickel or cadmium alone did not significantly affect elicited movement at 72 hpf (ANOVA; p = 0.1 and p = 0.06, respectively, data not shown). However, combined exposure to lower concentrations of nickel and cadmium (0.5 mg/L nickel; 0.15 mg/L cadmium) significantly decreased elicited movement (p = 0.04), indicating a synergistic effect. Because so few ZFE hatched when exposed to the combined higher concentrations of nickel and cadmium (2.5 mg/L nickel; 0.7 mg/L cadmium), we could not assess elicited movement in that group.

Table 2.
Comparison of elicited movement in 72 hpf hatched zebrafish embryos reared in control medium and medium containing 0.5 mg/L nickel + 0.15 mg/L cadmium.
3.3. Morphological Assessments
Several morphological parameters were evaluated in fixed 48 and 72 hpf ZFE. Total body length was decreased in several conditions (Figure 2). At 48 hpf, exposure to nickel did not alter body length, whereas at 72 hpf, ZFE exposed to the two highest nickel concentrations were significantly shorter than controls and shorter than ZFE exposed to the two lower nickel concentrations. Exposure to cadmium alone did not significantly alter total body length (data not shown). The combined exposure to nickel and cadmium did not decrease body length at 48 hpf, but the highest combination exposure decreased total body length at 72 hpf (ANOVA, p < 0.0001).
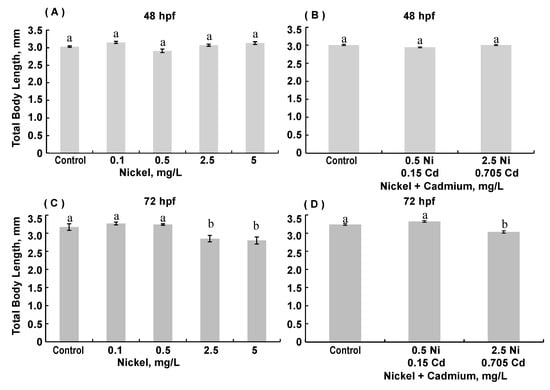
Figure 2.
Total body length in 48 hpf (A,B) and 72 hpf (C,D) zebrafish embryos reared in medium containing 0.1–5 mg/L Ni and combinations of either 0.5 mg/L Ni + 0.15 Cd or 2.5 mg/L Ni + 0.705 mg/L Cd. (A): 48 hpf with Ni exposure, ANOVA p < 0.0001. (B): 48 hpf with exposure to combinations of Ni and Cd, ANOVA p = 0.0107. (C): 72 hpf with Ni exposure, ANOVA p < 0.0001. (D): 72 hpf with combined exposure to Ni and Cd, ANOVA p < 0.0001. (Data are means ± SEM for N = 50–80 surviving embryos).
With respect to eye development, exposure to 2.5 mg/L nickel (ANOVA, p = 0.0019) decreased eye diameter at 48 hpf (p = 0.006, Figure 3A). Nevertheless, by 72 hpf, eye diameters in ZFE exposed to nickel were comparable to those in controls (Figure 3B). Exposure to cadmium did not affect eye development significantly (data not shown). Exposure to combinations of nickel and cadmium did not affect eye diameter (data not shown). Combined exposure to nickel and cadmium did not alter eye area at 48 hpf. However, in 72 hpf embryos exposed to the combination of nickel and cadmium at higher concentrations, eye area was decreased as compared to age-matched controls (ANOVA, p < 0.0001, Figure 3D).
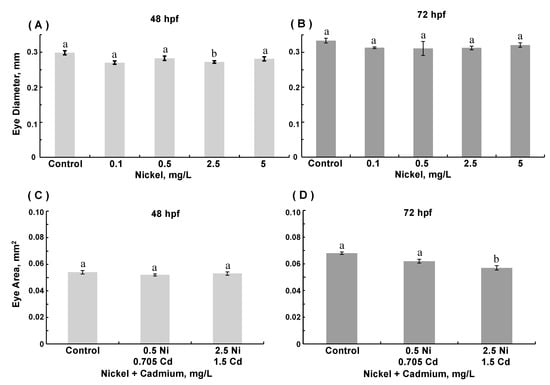
Figure 3.
Eye diameter in 48 hpf (A) and 72 hpf (B) zebrafish embryos reared in medium containing 0.1–5 mg/L Ni, and eye area in 48 hpf (C) and 72 hpf (D) embryos reared in medium containing combinations of 0.5 mg/L Ni + 0.15 mg/L Cd or 2.5 mg/L Ni + 0.705 mg/L Cd. (A): Eye diameter at 48 hpf with Ni exposure, ANOVA p = 0.0019. (D): Eye area at 72 hpf with combined metal exposure, ANOVA p < 0.0001. (Data are means ± SEM for N = 50–80 surviving embryos).
Few differences in the trunk area were observed (Figure 4). In 48 hpf embryos (but not 72 hpf embryos), exposure to 0.5 mg/L nickel modestly decreased trunk area (ANOVA, p = 0.0187) compared to age-matched controls. The tail area was decreased in 72 hpf embryos exposed to the highest combined nickel and cadmium concentrations (ANOVA, p =0.002; Figure 5). The yolk sac area was greater in 72 hpf ZFE exposed to the highest concentration of cadmium alone (ANOVA, p = 0.002; Figure 6A) compared to controls or in ZFE exposed to all other cadmium concentrations (ANOVA, p < 0.0001); exposure to nickel alone had no significant effect on yolk sac area (data not shown). Lastly, yolk sac areas were significantly greater in 72 hpf ZFE exposed to the highest combined concentrations of nickel and cadmium as compared to age-matched controls (ANOVA, p < 0.0001; Figure 6B).
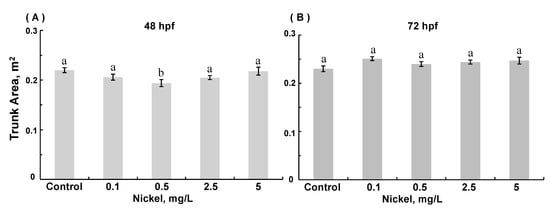
Figure 4.
Trunk area in 48 hpf (A) and 72 hpf (B) zebrafish embryos reared in medium containing 0.1–5 mg/L Ni. (A): 48 hpf with Ni exposure, ANOVA p = 0.0187. (Data are means ± SEM for N = 50–80 surviving embryos).
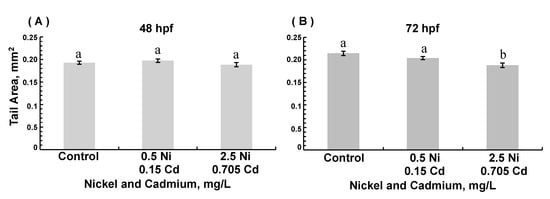
Figure 5.
Tail area in 48 hpf (A) and 72 hpf (B) zebrafish embryos reared in medium containing combinations of 0.5 mg/L Ni + 0.15 mg/L Cd or 2.5 mg/L Ni + 0.705 mg/L Cd. (B): 72 hpf with combined metal exposure, ANOVA p = 0.0030. (Data are means ± SEM for N = 50–80 surviving embryos).
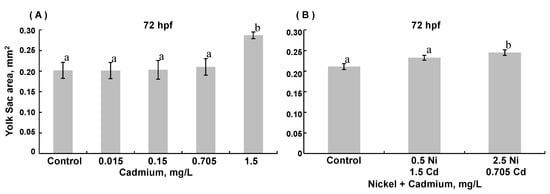
Figure 6.
Yolk sac area in 72 hpf zebrafish embryos reared in medium containing (A) 0.15–1.5 mg/L Cd and (B) combinations of either 0.5 mg/L Ni + 0.15 mg/L Cd or 2.5 mg/L Ni + 0.705 mg/L Cd. (A): 72 hpf with Cd exposure, ANOVA p < 0.0001. (B): 72 hpf with combined metal exposure, ANOVA p < 0.0001. (Data are means ± SEM for N = 50–80 surviving embryos).
3.4. Myosin Expression in Skeletal Muscle
The changes observed in body length and area and movement could be due to inadequate or delayed skeletal muscle development. Therefore, we examined myosin expression by immunohistochemical staining of the myosin heavy chain subunit in skeletal muscle (Figure 7). The staining intensity of myosin heavy chain protein in experimental embryos was expressed as a percent of staining in control embryos. Only a few differences were noted in 48 hpf ZFEs. No significant differences were observed in 48 hpf ZFEs exposed to cadmium (ANOVA, p = 0.094), though there was a trend towards decreased myosin expression in 48 hpf ZFE exposed to cadmium at the two highest concentrations (data not shown). In 48 hpf ZFE exposed to nickel alone, myosin expression was decreased (ANOVA, p < 0.0001); myosin expression significantly decreased in ZFE exposed to 2.5 and 5.0 mg/L nickel (p < 0.001; Figure 7A). In contrast, combined exposure to nickel and cadmium significantly increased myosin expression in 48 hpf ZFE as compared to the controls (ANOVA, p = 0.0003), with exposure to the lower combined concentrations of Ni and Cd having a slightly more pronounced effect (p = 0.0141; Figure 7B). However, in 72 hpf ZFE exposed to nickel at 0.1 and 0.5 mg/L, myosin expression was significantly decreased as compared to the controls (ANOVA, p = 0.0052; Figure 7C). In 72 hpf ZFE exposed to cadmium alone, there were minor changes in myosin expression (ANOVA, p = 0.0067), and ZFE exposed to 0.705 mg/L cadmium exhibited significantly lower myosin expression compared to ZFE exposed 1.5 mg/L cadmium (p = 0.0216; Figure 7D). However, in 72 hpf ZFE exposed to the lower combination of nickel and cadmium, myosin expression was significantly decreased compared to controls (Welch’s unpaired t-test, p < 0.0001; Figure 7E). The hatch rate was too low in 72 hpf embryos exposed to the higher combination of nickel (2.5 mg/L) and cadmium (0.705 mg/L) to assess myosin expression.
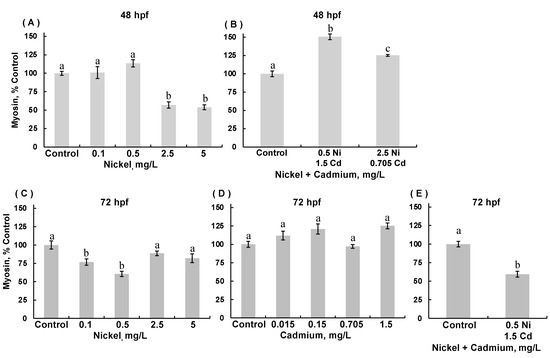
Figure 7.
Modified myosin protein expression in 48 hpf (A,B) and 72 hpf (C–E) zebrafish embryos reared in medium containing 0.1–5 mg/L Ni, 0.15–1.5 mg/L Cd, and combinations of either 0.5 mg/L Ni + 0.15 mg/L Cd or 2.5 mg/L Ni + 0.705 mg/L Cd. Fixed embryos were immunostained for myosin heavy chain protein; staining intensity in metal-exposed embryos was expressed as a percent of staining in control embryos. (A): 48 hpf with Ni exposure, ANOVA p < 0.0001. (B): 48 hpf with combined exposure to Ni + Cd, ANOVA p = 0.0003. (C): 72 hpf with Ni exposure, ANOVA p = 0.0052. (D) 72 hpf with Cd exposure, ANOVA p = 0.0067. (E): Too few embryos survived exposure to 2.5 mg/L Ni + 0.705 mg/L Cd through 72 hpf to permit valid comparison with controls. (Data are means ± SEM for N = 50–80 surviving embryos).
4. Discussion
Understanding the embryonic toxicity of heavy metals is imperative as environmental contamination increases. In this study, zebrafish embryos (ZFE) were exposed to nickel and cadmium before hatching from the protective chorion. Although most metals bind to the chorion [43,44], metals can breach the chorion and accumulate in fish embryos [45]. Thus, even small amounts of metal can pass through the chorion and adversely affect early embryonic development. For example, a substantial effect of metal exposure has been observed in fish embryos immediately after fertilization during swelling of the zygote due to water absorption in the perivitelline space, which contains proteins secreted by the vitelline membrane [46]. Metal ions also can alter chorion structure and permeability [45].
Similar to other studies [22,47], we did not observe significant increases in embryonic death at any point of the 72-h exposure to heavy metals. However, others have reported that exposure of ZFE to higher cadmium concentrations (e.g., 24 μM) can result in significant mortality [48,49]. Studies have indicated that heavy metals interfere with hatching in ZFE [50]. In our study, nickel and cadmium individually and in combination markedly reduced ZFE hatching rates at 48 hpf. Exposure to nickel alone and combined exposure to nickel and cadmium continued to decrease hatching rates through to 72 hpf. Similarly, Peng et al. reported that exposure to various heavy metals significantly diminished ZFE hatching [51]. Kienle et al. exposed ZFE to several concentrations of nickel from 0.25 to 15 mg/L and observed delayed hatching at concentrations higher than 10 mg/L, which indicated the severity of toxicity depended on concentration and exposure duration [52]. Hagenmaier described the presence of a metalloprotease called hatching protease or chorionase in teleost embryos (Salmo gairdneri) [53]. Reduced hatching success of embryos exposed to nickel was reported previously for zebrafish [51] and other teleosts, including rainbow trout (Oncorhynchus mykiss) [54]. Scheil et al. reported a reduced hatching rate in zebrafish exposed to nickel and suggested that nickel possibly decreased the activity of chorionase [33]. Those investigators also found that low-dose exposure to the pesticide chlorpyrifos did not affect ZFE hatching. However, when chlorpyrifos was combined with nickel, the hatching rate was similar to that with nickel alone, indicating the combination of these specific toxicants produced no synergistic or antagonistic effects [33]. Likewise, we did not observe synergistic or antagonistic effects of combined exposures to nickel and cadmium on ZFE hatching success. Interestingly, in adult male rats, combined exposure to nickel and cadmium was less toxic than exposure to cadmium alone [55].
Heavy metal exposures elicited relatively few gross morphologic changes in this study. Nickel exposure alone decreased trunk area and eye diameter at 48 hpf and body length at 72 hpf. In contrast, combined exposure to higher concentrations of nickel and cadmium affected body length, tail area, and eye area at 72 hpf. Exposure to the highest cadmium concentration and combined exposure to higher concentrations of nickel and cadmium resulted in increased yolk sac area, which was likely due to the decreased metabolism of yolk by the embryo. We did not observe any antagonistic or synergistic effects of combined exposures to cadmium and nickel on the gross morphology of ZFE. Others have reported multiple effects of heavy metals on embryonic morphology in zebrafish and other teleosts. Dang et al. reported that cadmium exposure decreased body length in zebrafish larvae [56]. Cadmium was shown to cause dose-dependent decreases in body length and other body malformations in embryos of various fish species [57,58]. The differences in our results versus those of other studies are likely attributed to the lower heavy metal concentrations used in our study.
Nickel exposure alone decreased spontaneous movement in ZFE at 24 hpf, whereas cadmium exposure alone and combined exposures to nickel and cadmium did not. It was noted that not many spontaneous movements were observed for any of the ZFE at 24 hpf. The range of movements was zero to three or four for most groups. Thus, we analyzed the data by determining movement or no movement during the assessment time. It is possible that this approach limited our ability to discern more subtle differences in spontaneous movements. Nevertheless, these results suggest simultaneous exposure to cadmium and nickel attenuated the adverse effect of nickel on spontaneous movement in 24 hpf ZFE. Other studies support our finding that cadmium exposure had little adverse effect on spontaneous embryonic movements, but nickel [22] or nickel combined with chromium [59] are relatively more toxic than cadmium. Spontaneous movements are thought to be important in the hatching process [59]. Thus, the reduced hatching rate we noted primarily in nickel-exposed and combination-exposed embryos could be due in part to the adverse effects of nickel on spontaneous movement.
Only combined exposure to nickel and cadmium reduced elicited movement in 72 hpf ZFE. We analyzed only the effects of combined exposure to nickel and cadmium at lower concentrations on elicited movement, due to the low hatching rate in embryos exposed to the higher combination of the metals. Because exposure to nickel or cadmium individually did not affect elicited movement, our results suggest a synergistic adverse effect when embryos were exposed simultaneously to these metals. Combined exposure to nickel and chromium was shown to reduce swimming distances in six-day-old zebrafish larvae [59].
Having observed changes in movement, we assessed skeletal muscle development by immunohistochemical staining for heavy chain myosin, a motor protein of muscle thick filaments. Exposure to lower concentrations of nickel significantly decreased myosin protein expression at both 48 and 72 hpf. In contrast, cadmium exposure did not affect myosin protein expression. Interestingly, combined exposure to nickel and cadmium significantly increased myosin expression at 48 hpf, but decreased myosin protein expression at 72 hpf. Decreased myosin heavy chain protein expression can be correlated with delayed muscle fiber development [60]. Delayed muscle development could be one explanation for the subtle but significant decreases in spontaneous and elicited movements observed in this study. However, if any delay in muscle development did occur, it was not enough to have a major effect on the rate of hatching. The reason for decreased myosin expression is not known, nor is the reason for increased myosin expression in 48 hpf ZFE exposed to combined nickel and cadmium but decreased expression in 72 hpf embryos exposed to combined nickel and cadmium. Myosin mRNA expression in the masseter muscle during postnatal development in mice was shown to both increase and decrease and correlated with the use or lack of use of the muscle [61,62]. Perhaps increased or decreased skeletal muscle use in ZFE in our study was related to the observed myosin expression.
We did not directly assess the mechanism(s) by which nickel and cadmium might affect muscle development and function. However, several published reports have suggested possible mechanisms. Nickel exposure can increase free radical formation and lead to altered calcium and sulfhydryl homeostasis, disrupt the mitochondrial membrane potential, and decrease mtDNA content [11,63,64]. Any one or combination of these alterations could result in abnormal muscle function. Another point to consider is the adverse effects of heavy metal exposure on neuromuscular junction development and motor neuron function, which also can affect locomotion. Others have shown that cadmium exposure can delay or inhibit neuron differentiation and axonogenesis in ZFE [26]. No reported study has directly examined the effects of nickel or cadmium on neuromuscular junction development. However, in ZFE, exposure to bisphenol A can result in motor neuron degeneration, reduced neuromuscular junction integrity, and adverse motor function. This suggests that adverse effects on motor neurons and/or the neuromuscular junction could be a possible mechanism by which heavy metal exposure adversely affects locomotion [65].
5. Conclusions
In this study, ZFE were exposed to several different concentrations of nickel, cadmium, and combinations of these two metals during the first three days of development. The concentrations used were relatively low and comparable to levels of environmental contamination to which some humans and animals are exposed globally. Minor gross morphological alterations were observed, and adverse effects were observed with respect to hatching success, movement, and myosin protein expression. In general, nickel exposure alone was more toxic than cadmium exposure alone. The combination of nickel and cadmium presented a modest synergistic adverse effect on elicited movement but not on spontaneous movement. Combined exposure to nickel and cadmium had no apparent synergistic effect on gross morphology. Lack of significant antagonistic effects and only minimal synergistic effects could be due to several reasons. The toxicity of the two heavy metals may be mediated via different mechanisms, making synergism less likely. Thus, these results indicate that additional study of the toxicity of exposure of developing embryos to mixtures of low concentrations of heavy metals is warranted.
Author Contributions
Conceptualization, L.C.A., S.J.A., A.R.V.; methodology, L.C.A., S.J.A., Z.R.E., D.J.G., M.D.L., N.M.Q.-A., A.R.V.; formal analysis, L.C.A., S.J.A., A.R.V.; supervision, L.C.A., S.J.A., A.R.V.; writing, review, and editing, L.C.A., S.J.A., A.R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Bruce Riley for graciously providing access to adult wildtype zebrafish to produce the zebrafish embryos used in this study. We also thank Jennifer Dong for her expert help with breeding the adult zebrafish and obtaining the zebrafish embryos. We are grateful for the expertise of Chaitali Mukherjee, MS, HT (ASCP), and Lin Bustamante, HT (ASCP), in providing the histology sections used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Health Risks of Heavy Metals from Long-Range Transboundary Air Pollution. Copenhagen, World Health Organization Regional Office for Europe 2007. Available online: http://www.euro.who.int/document/E91044.pdf (accessed on 30 June 2019).
- Rathor, G.; Chopra, N.; Adhikari, T. Nickel as a pollutant and its management. Int. Res. J. Environ. Sci. 2014, 3, 94–98. [Google Scholar]
- WHO. Exposure to Cadmium: A Major Public Health Concern. Geneva, World Health Organization Regional Office for Europe 2010. Available online: http://www.who.int/ipcs/features/cadmium.pdf (accessed on 30 June 2019).
- Boluda, R.; Errecalde, F.; Lagarda, M.J. Environmental cadmium, lead and nickel contamination: Possible relationship between soil and vegetable content. Anal. Bioanal. Chem. 1991, 339, 654–657. [Google Scholar] [CrossRef]
- Gil, C.; Boluda, R.; Miras, J.J.R.; Gil, C. Determination and evaluation of cadmium, lead and nickel in greenhouse soils of Almería (Spain). Chemosphere 2004, 55, 1027–1034. [Google Scholar] [CrossRef]
- Olsson, I.-M.; Bensryd, I.; Lundh, T.; Ottosson, H.; Skerfving, S.; Oskarsson, A. Cadmium in blood and urine—Impact of sex, age, dietary intake, iron status, and former smoking—Association of renal effects. Environ. Health Perspect. 2002, 110, 1185–1190. [Google Scholar] [CrossRef]
- Yu, C.; Ling, Q.; Yan, S.; Li, J.; Chen, Z.; Peng, Z. Cadmium contamination in various environmental materials in an industrial area, Hangzhou, China. Chem. Speciat. Bioavailab. 2010, 22, 35–42. [Google Scholar] [CrossRef]
- Abudayyak, M.; Guzel, E.; Özhan, G. Nickel oxide nanoparticles are highly toxic to SH-SY5Y neuronal cells. Neurochem. Int. 2017, 108, 7–14. [Google Scholar] [CrossRef]
- Robinson, B.H. E-waste: An assessment of global production and environmental impacts. Sci. Total. Environ. 2009, 408, 183–191. [Google Scholar] [CrossRef]
- Redelstein, R.; Zielke, H.; Spira, D.; Feiler, U.; Erdinger, L.; Zimmer, H.; Wiseman, S.; Hecker, M.; Giesy, J.P.; Seiler, T.-B.; et al. Bioaccumulation and molecular effects of sediment-bound metals in zebrafish embryos. Environ. Sci. Pollut. Res. 2015, 22, 16290–16304. [Google Scholar] [CrossRef]
- Das, K.K.; Das, S.N.; Dhundasi, S.A. Nickel: Molecular Diversity, Application, Essentiality and Toxicity in Human Health. Biometals: Molecular Structures, Binding Properties and Applications; Nova Science Publishers: New York, NY, USA, 2010; pp. 33–58. [Google Scholar]
- Jacobo-Estrada, T.; Santoyo-Sánchez, M.; Thévenod, F.; Barbier, O. Cadmium Handling, Toxicity and Molecular Targets Involved during Pregnancy: Lessons from Experimental Models. Int. J. Mol. Sci. 2017, 18, 1590. [Google Scholar] [CrossRef]
- Jijie, R.; Solcan, G.; Nicoara, M.; Micu, D.; Strungaru, S. Antagonistic effects in zebrafish (Danio rerio) behavior and oxidative stress induced by toxic metals and deltamethrin acute exposure. Sci. Total. Environ. 2020, 698, 134299. [Google Scholar] [CrossRef] [PubMed]
- Sonnack, L.; Klawonn, T.; Kriehuber, R.; Hollert, H.; Schäfers, C.; Fenske, M. Comparative analysis of the transcriptome responses of zebrafish embryos after exposure to low concentrations of cadmium, cobalt and copper. Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 25, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Lahnsteiner, F. The sensitivity and reproducibility of the zebrafish (Danio rerio) embryo test for the screening of waste water quality and for testing the toxicity of chemicals. Altern. Lab. Anim. 2008, 36, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-L.; Ru, Y.-F.; Liu, M.; Tang, J.-N.; Zheng, J.-F.; Wu, B.; Gu, Y.-H.; Shi, H.-J. Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS ONE 2017, 12, e0186727. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Bannigan, J. Cadmium: Toxic effects on the reproductive system and the embryo. Reprod. Toxicol. 2008, 25, 304–315. [Google Scholar] [CrossRef] [PubMed]
- National Research Council; Safe Drinking Water Committee. Drinking Water and Health, Volume 9; National Academies Press: Washington, DC, USA, 1989.
- Nagel, R. DarT: The embryo test with the Zebrafish Danio rerio—A general model in ecotoxicology and toxicology. Altex 2002, 19, 38–48. [Google Scholar] [PubMed]
- Dubińska-Magiera, M.; Daczewska, M.; Lewicka, A.; Migocka-Patrzałek, M.; Niedbalska-Tarnowska, J.; Jagla, K. Zebrafish: A Model for the Study of Toxicants Affecting Muscle Development and Function. Int. J. Mol. Sci. 2016, 17, 1941. [Google Scholar] [CrossRef]
- Krzykwa, J.C.; Saeid, A.; Jeffries, M.K.S. Identifying sublethal endpoints for evaluating neurotoxic compounds utilizing the fish embryo toxicity test. Ecotoxicol. Environ. Saf. 2019, 170, 521–529. [Google Scholar] [CrossRef]
- Fraysse, B.; Mons, R.; Garric, J. Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals. Ecotoxicol. Environ. Saf. 2006, 63, 253–267. [Google Scholar] [CrossRef]
- Weil, M.; Meißner, T.; Busch, W.; Springer, A.; Kühnel, D.; Schulz, R.; Duis, K. The oxidized state of the nanocomposite Carbo-Iron® causes no adverse effects on growth, survival and differential gene expression in zebrafish. Sci. Total. Environ. 2015, 198–208. [Google Scholar] [CrossRef]
- Ribeiro, R.X.; Brito, R.D.S.; Pereira, A.C.; Monteiro, K.B.E.S.; Gonçalves, B.B.; Rocha, T.L. Ecotoxicological assessment of effluents from Brazilian wastewater treatment plants using zebrafish embryotoxicity test: A multi-biomarker approach. Sci. Total Environ. 2020, 735, 139036. [Google Scholar] [CrossRef]
- Zindler, F.; Beedgen, F.; Brandt, D.; Steiner, M.; Stengel, D.; Baumann, L.; Braunbeck, T. Analysis of tail coiling activity of zebrafish (Danio rerio) embryos allows for the differentiation of neurotoxicants with different modes of action. Ecotoxicol. Environ. Saf. 2019, 186, 109754. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.S.H.; Hui, M.N.Y.; Lin, C.C.; Cheng, S.H. Cadmium inhibits neurogenesis in zebrafish embryonic brain development. Aquat. Toxicol. 2008, 87, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.; Kille, P.; Sweeney, G. Cadmium delays growth hormone expression during rainbow trout development. J. Fish Biol. 2005, 59, 1015–1022. [Google Scholar] [CrossRef]
- Witeska, M.; Jezierska, B.; Chaber, J. The influence of cadmium on common carp embryos and larvae. Aquaculture 1995, 129, 129–132. [Google Scholar] [CrossRef]
- Wu, S.M.; Tsai, P.J.; Chou, M.Y.; Wang, W.-D. Effects of Maternal Cadmium Exposure on Female Reproductive Functions, Gamete Quality, and Offspring Development in Zebrafish (Danio rerio). Arch. Environ. Contam. Toxicol. 2013, 65, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, Z.; Liu, F.; Ye, Y.; Peng, T.; Ota, T. Embryonic exposure to cadmium (II) and chromium (VI) induce behavioral alterations, oxidative stress and immunotoxicity in zebrafish (Danio rerio). Neurotoxicology Teratol. 2015, 48, 9–17. [Google Scholar] [CrossRef]
- Nguyen, C.R.J.L.T.H.; Nguyen, L.T.H.; Janssen, C.R. Embryo-Larval Toxicity Tests with the African Catfish (Clarias gariepinus): Comparative Sensitivity of Endpoints. Arch. Environ. Contam. Toxicol. 2002, 42, 256–262. [Google Scholar] [CrossRef]
- Lefauve, M.K.; Connaughton, V.P. Developmental exposure to heavy metals alters visually-guided behaviors in zebrafish. Curr. Zool. 2017, 63, 221–227. [Google Scholar] [CrossRef]
- Scheil, V.; Zürn, A.; Triebskorn, R.; Köhler, H.-R. Embryo development, stress protein (Hsp70) responses, and histopathology in zebrafish (Danio rerio) following exposure to nickel chloride, chlorpyrifos, and binary mixtures of them. Environ. Toxicol. 2009, 25, 83–93. [Google Scholar] [CrossRef]
- Kim, K.; Wang, C.-H.; Ok, Y.S.; Lee, S.-E. Heart developmental toxicity by carbon black waste generated from oil refinery on zebrafish embryos (Danio rerio): Combined toxicity on heart function by nickel and vanadium. J. Hazard. Mater. 2019, 363, 127–137. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio Rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Khalid, S.; Shahid, M.; Shah, A.H.; Saeed, F.; Ali, M.; Qaisrani, S.A.; Dumat, C. Heavy metal contamination and exposure risk assessment via drinking groundwater in Vehari, Pakistan. Environ. Sci. Pollut. Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Saravi, S.S.; Karami, B.; Karami, S.; Shokrzadeh, M. Evaluation of Metal Pollution in Fish and Water Collected from Gorgan Coast of the Caspian Sea, Iran. Bull. Environ. Contam. Toxicol. 2012, 89, 419–423. [Google Scholar] [CrossRef]
- Corales-Ultra, O.G.; Peja, R.P.; Casas, E.V. Baseline study on the levels of heavy metals in seawater and macroalgae near an abandoned mine in Manicani, Guiuan, Eastern Samar, Philippines. Mar. Pollut. Bull. 2019, 149, 110549. [Google Scholar] [CrossRef] [PubMed]
- Kline, T.L.; Sussman, C.R.; Irazabal, M.V.; Mishra, P.K.; Pearson, E.A.; Torres, V.E.; Macura, S.I. Three-dimensional NMR microscopy of zebrafish specimens. NMR Biomed. 2018, 32, e4031. [Google Scholar] [CrossRef] [PubMed]
- Abbott, L.; Jacobowitz, D. Development of calretinin-immunoreactive unipolar brush-like cells and an afferent pathway to the embryonic and early postnatal mouse cerebellum. Brain Struct. Funct. 1995, 191, 541–599. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhaes, P.J.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Saint-Amant, L.; Drapeau, P. Time course of the development of motor behaviors in the zebrafish embryo. J. Neurobiol. 1998, 37, 622–632. [Google Scholar] [CrossRef]
- Beattie, J.H.; Pascoe, D. Cadmium uptake by rainbow trout, Salmo gairdneri eggs and alevins. J. Fish Biol. 1978, 13, 631–637. [Google Scholar] [CrossRef]
- Micihibata, H. Uptake and distribution of cadmium in the egg of the teleost, Oryzias latipes. J. Fish Biol. 1981, 19, 691–696. [Google Scholar] [CrossRef]
- Jezierska, B.; Ługowska, K.; Witeska, M. The effects of heavy metals on embryonic development of fish (a review). Fish Physiol. Biochem. 2008, 35, 625–640. [Google Scholar] [CrossRef]
- Peterson, R.H.; Martin-Robichaud, D.J. Perivitelline and Vitelline Potentials in Teleost Eggs as Influenced by Ambient Ionic Strength, Natal Salinity, and Electrode Electrolyte; and the Influence of these Potentials on Cadmium Dynamics within the Egg. Can. J. Fish. Aquat. Sci. 1986, 43, 1445–1450. [Google Scholar] [CrossRef]
- Liu, L.; Yan, Y.; Wang, J.; Wu, W.; Xu, L. Generation ofmt:egfptransgenic zebrafish biosensor for the detection of aquatic zinc and cadmium. Environ. Toxicol. Chem. 2016, 35, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Consigli, V.; Guarienti, M.; Bilo, F.; Benassi, L.; Depero, L.E.; Bontempi, E.; Presta, M. Evaluation of the Biotoxicity of Tree Wood Ashes in Zebrafish Embryos. Zebrafish 2016, 13, 449–455. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, S.-J.; Bae, M.A.; Cho, K.-H.; Kim, J.-R. Cadmium exposure exacerbates severe hyperlipidemia and fatty liver changes in zebrafish via impairment of high-density lipoproteins functionality. Toxicol. Vitr. 2018, 47, 249–258. [Google Scholar] [CrossRef]
- Xiu, R. Toxicity of mercury, copper, nickel, lead, and cobalt to embryos and larvae of zebrafish, Brachydanio rerio. Arch. Environ. Contam. Toxicol. 1991, 21, 126–134. [Google Scholar] [CrossRef]
- Peng, G.; He, Y.; Zhao, M.; Yu, T.; Qin, Y.; Lin, S. Differential effects of metal oxide nanoparticles on zebrafish embryos and developing larvae. Environ. Sci. Nano 2018, 5, 1200–1207. [Google Scholar] [CrossRef]
- Kienle, C.; Kohler, H.-R.; Filser, J.; Gerhardt, A. Effects of nickel chloride and oxygen depletion on behaviour and vitality of zebrafish (Danio rerio, Hamilton, 1822) (Pisces, Cypriniformes) embryos and larvae. Environ. Pollut. 2008, 152, 612–620. [Google Scholar] [CrossRef]
- Hagenmaier, H.E. The hatching process in fish embryos. Dev. Genes Evol. 1974, 175, 157–162. [Google Scholar] [CrossRef]
- Nebeker, A.V.; Savonen, C.; Stevens, D.G. Sensitivity of rainbow trout early stages to nickel chloride. Environ. Toxicol. Chem. Int. J. 1985, 2, 233–239. [Google Scholar] [CrossRef]
- Novelli, E.; Hernandes, R.; Filho, J.N.; Barbosa, L. Differential/combined effect of water contamination with cadmium and nickel on tissues of rats. Environ. Pollut. 1998, 103, 295–300. [Google Scholar] [CrossRef]
- Dang, Y.; Wang, F.; Liu, C. Real-time PCR array to study the effects of chemicals on the growth hormone/insulin-like growth factors (GH/IGFs) axis of zebrafish embryos/larvae. Chemosphere 2018, 207, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, H.; Meng, Y.; Jin, G.; Zhu, M. The toxicity of cadmium (Cd2+) towards embryos and pro-larva of soldatov’s catfish (Silurus soldatovi). Ecotoxicol. Environ. Saf. 2012, 80, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liu, L.; Li, D.-L.; Ling, F.; Wang, G. Developmental toxicity in rare minnow (Gobiocypris rarus) embryos exposed to Cu, Zn and Cd. Ecotoxicol. Environ. Saf. 2014, 104, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Si, J.; Wei, Y.; Li, S.; Jiang, Y.; Zhou, R.; Liu, B.; Zhang, H. Toxicity of porcelain-fused-to-metal substrate to zebrafish (Danio rerio) embryos and larvae. Life Sci. 2018, 203, 66–71. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Patterson, J.; Kimmel, R.O. The development and behavioral characteristics of the startle response in the zebra fish. Dev. Psychobiol. 1974, 7, 47–60. [Google Scholar] [CrossRef]
- Katayama, R.; Yamane, A.; Fukui, T. Changes in the Expression of Myosins During Postnatal Development of Masseter Muscle in the Microphthalmic Mouse. Open Dent. J. 2010, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, R.J.; Acosta, W.; Garland, T. Myosin heavy chain isoform expression in adult and juvenile mini-muscle mice bred for high-voluntary wheel running. Mech. Dev. 2014, 134, 16–30. [Google Scholar] [CrossRef]
- He, M.-D.; Xu, S.-C.; Lu, Y.-H.; Li, L.; Zhong, M.; Zhang, Y.-W.; Wang, Y.; Li, M.; Yang, J.; Zhang, G.-B.; et al. L-carnitine protects against nickel-induced neurotoxicity by maintaining mitochondrial function in Neuro-2a cells. Toxicol. Appl. Pharmacol. 2011, 253, 38–44. [Google Scholar] [CrossRef]
- Xu, S.; He, M.; Zhong, M.; Li, L.; Lu, Y.; Zhang, Y.; Zhang, L.; Yu, Z.; Zhou, Z. The neuroprotective effects of taurine against nickel by reducing oxidative stress and maintaining mitochondrial function in cortical neurons. Neurosci. Lett. 2015, 590, 52–57. [Google Scholar] [CrossRef]
- Morrice, J.R.; Gregory-Evans, C.Y.; Shaw, C. Modeling Environmentally-Induced Motor Neuron Degeneration in Zebrafish. Sci. Rep. 2018, 8, 4890. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).