Abstract
Gaseous air pollutants emitted primarily by anthropogenic sources form secondary products through photochemical reactions, complicating the regulatory analysis of anthropogenic emissions in the atmosphere. We used an environmental chassis dynamometer and a photochemical smog chamber to conduct a parameter sensitivity experiment to investigate the formation of secondary products from a gasoline passenger car. To simulate the mitigation of ammonia emissions from gasoline vehicle exhausts assuming future emission controls and to allow photochemical oxidation and aging of the vehicle exhaust, ammonia was selectively removed by a series of five denuders installed between the vehicle and photochemical smog chamber. Overall, there were no differences in the formation of secondary organic aerosols and ozone with or without ammonia mitigation. However, the potential for ammonium nitrate particle formation was significantly reduced with ammonia mitigation. In addition, ammonia mitigation resulted in increased aerosol acidity due to nitric acid in the gas phase not being neutralized by ammonia and condensing onto the liquid particle phase, indicating a potentially important secondary effect associated with ammonia mitigation. Thus, we provide new insights into the effects of ammonia mitigation on secondary emissions from gasoline vehicle exhaust and into a potentially useful experimental approach for determining primary and secondary emissions.
1. Introduction
Vehicle exhaust emissions are a major source of gaseous and particulate matter (PM) in the urban atmosphere. The recent regulatory landscape and electrification of vehicles have led to decreases in primary PM emissions released directly into the atmosphere via vehicle exhaust, such as elemental carbon (EC), primary organic aerosols (POAs), volatile organic compounds (VOCs), and nitrogen oxides (NOX) (e.g., [1]). However, there are still concerns about high ozone concentrations in susceptible areas due to transboundary pollution and localized high ozone (O3) concentration formation due to the reaction of VOCs and NOX (e.g., [2]). Additionally, several studies have shown that secondary PM emissions, such as secondary organic aerosols (SOAs), are formed in the atmosphere by photochemical reactions of VOCs and NOX contained in vehicle exhaust [3,4]. Because SOAs produced from vehicle exhaust can be more toxic than SOAs produced from VOCs from other sources or from POAs produced from vehicle exhaust [5,6], a better understanding of the properties of SOAs produced from vehicle exhaust is needed.
Studies using radiocarbon (14C) isotope measurements to investigate the origin of SOA, secondary organic carbon (SOC) in PM2.5 at 25 sites around the world, have shown that SOC from fossil fuel contributes 12–54% to the total SOC emitted to the atmosphere (e.g., [7]). However, such approaches using field-based atmospheric observations are limited in their ability to accurately estimate origin-based contributions compared to model-based calculations. Indeed, although SOAs formed from vehicle exhaust are an important fossil fuel-derived atmospheric pollutant, their relative contribution to the total SOAs emitted varies considerably among studies. In modeling studies, gasoline vehicles are reported to account for only 56–79% of SOAs formed in the United States as a whole [8] but ≥90% in Southern California [9,10]. The relative contributions of diesel and gasoline vehicles to total vehicle SOAs are also unclear. For example, diesel vehicles are reported to contribute significantly to SOAs in the atmosphere in the United States (around 90% of the total vehicle SOA) [11] and in London [12]. However, in a laboratory-based study, gasoline vehicles produced SOA, but diesel vehicles equipped with diesel particulate filters did not, suggesting that gasoline passenger cars make the major contribution to the SOAs produced by vehicles in urban areas [13]. Thus, to be able to obtain accurate estimates of the contribution to environmental pollution of different sources of secondary products such as SOA, a deeper understanding of the characteristics of the secondary products from vehicle exhaust is needed.
The photochemical smog chamber is a useful tool for examining the formation and evolution of air pollutants under controlled conditions, as well as for parameterizing atmospheric processes and revealing their underlying mechanisms. However, generating realistic vehicle emissions under controlled and reproducible conditions also requires specialized test equipment (e.g., chassis dynamometers) that is usually not available in large stationary smog chambers. To overcome these facility limitations, mobile smog chambers have been used to evaluate automotive emissions and the photochemical reactions they undergo in the atmosphere [13,14,15,16,17]. However, it is clear that the potential for secondary particle formation varies greatly, depending on the experimental parameters, including vehicle type (gasoline or diesel, year) [13,14,15,18,19,20], the use of emission control technologies [21,22], driving conditions [23,24], and fuel type [25,26,27,28]. In addition, these studies have focused on elucidating the mechanisms underlying SOA formation and have not evaluated the impacts of the formation of high concentrations of O3 from the reaction of VOCs and NOX, which is a recent issue in the field of transboundary pollution (e.g., [2]). A further limitation of these studies has been that nitrous acid was used as the source of OH radicals in the experiments (e.g., [15,16]), which precluded evaluation of the formation of nitrate-derived particles.
Evaluating the impacts of vehicle emissions on air quality requires consideration not only of photochemical reactions involving VOCs and NOX but also those involving ammonia (NH3), which is a highly reactive alkaline inorganic gas that can negatively impact the environment [29,30,31]. Therefore, quantification of NH3 emissions from vehicle exhaust is crucial for assessing air quality impacts and developing effective control strategies. The National Emission Ceilings Directive 2001/81/EC, the Gothenburg Protocol under the United Nations Convention on Long-Range Transboundary Air Pollution, and the Integrated Pollution Prevention and Control Directive (2008/1/EC) all aim to reduce emissions of NH3 [30]. As the only alkaline inorganic gas with high reactivity, NH3 is known to contribute to the formation of ammonium nitrate (NH4NO3) particles in automotive emissions [17,19,22,25,26,28]. However, since there is an overall paucity of knowledge regarding the role of NH3 in the formation of SOAS in automotive emissions, more detailed investigations of its potential impacts are needed.
As already mentioned, secondary pollution is caused by the formation of secondary particles and O3. Previous studies of vehicle emissions based on photochemical smog chamber experiments have been limited in their assessment of both secondary particles and O3. Therefore, studies are needed to evaluate the O3 formation for modern vehicles in this study compared to previous studies. Here, we used an environmental chassis dynamometer and a photochemical smog chamber to conduct a parameter sensitivity experiment to investigate the formation of secondary pollutants, both secondary particles and ozone, in exhaust from a direct-injection gasoline passenger car with or without NH3 emissions mitigation.
2. Materials and Methods
2.1. Vehicle Chassis Dynamometer Experiments
Vehicle tests were performed in an environmental chamber with controlled temperature and with or without humidity (23 °C with 50% relative humidity control, 0 °C without relative humidity control, or −7 °C without relative humidity control) at the Japan Automobile Research Institute (JARI). The temperature and humidity settings were based on existing protocols that are widely used in vehicle exhaust testing to ensure that vehicle testing with realistic ambient temperature conditions can be performed. The ambient temperatures of 23 °C and −7 °C were taken from the Worldwide Light Duty Test Cycle (WLTC) with cold engine start. In the WLTC, humidity control is used only at 23 °C. The ambient temperature of 0 °C corresponds to the upper threshold of the Real Driving Emission measurement of the Euro 6 regulation.
The chassis dynamometer test facility within JARI complies with the World harmonized Light duty vehicle Test Procedure (WLTP) for type approval testing. The chamber contained a four-wheel drive chassis dynamometer for environmental-type experimental testing (Meiden, Tokyo, Japan) on which the test vehicle was placed. The exhaust pipe of the test vehicle was connected to a dilution tunnel (DLS-ONE-D; Horiba, Ltd., Kyoto, Japan) and a constant volume sampler (12 m3 min−1; CVC-ONE-MV-HE(ESU); Horiba, Ltd., Kyoto, Japan). Exhaust gases were diluted an average of 24 times with high-efficiency particulate air (HEPA). Background air was filtered through an activated carbon, and a HEPA filter was maintained at 298 K and 50% relative humidity. Carbon monoxide, carbon dioxide, NOX, total hydrocarbons, and non-methane hydrocarbons (NMHC) were sampled near the end of the dilution tunnel, and their concentrations were measured with an exhaust gas analyzer (MEXA7200LE; Horiba, Ltd., Kyoto, Japan). EC and primary organic carbon (OC) were collected from the dilution tunnel onto a quartz filter (Pallflex, 2500QAT-UP, 47 φ; Pall Corp., Port Washington, NY, USA). The EC and OC were determined by a thermal–optical carbon analyzer (model 2001; Desert Research Institute, Reno, NV, USA) using the IMPROVE protocol [32].
A small, direct-injection, two-wheel drive, gasoline passenger car of a size that is currently popular in Japan was tested (vehicle mass: 1340 kg, displacement: approximately 1.5 L, mileage: 23,192 km, model year: 2020, regulatory compliance year: 2018). The average life expectancy of Japanese passenger cars in 2022 is reported to be 13.84 years, i.e., passenger cars driven in the 2020 model year and reflecting the modern market [33]. Cold-start operational tests were performed based on the WLTC of operation. For these cold-start experiments, the vehicle was warmed up by the same test cycle prior to the experiment and then left for approximately 23.5 h. The cold-start experiment was performed once per day.
2.2. Photochemical Smog Chamber
2.2.1. Facility
A mobile photochemical smog chamber (e.g., [13,14,15,16,17,34]), designed for the evaluation of secondary particle formation potential from automotive exhaust gases, was installed next to the environmental chassis dynamometer. The photochemical smog chamber comprised a fixed frame (2.08 m height × 2.08 m width × 2.66 m depth) fitted with casters, a reactor bag, and a light source. The reactor bag was made from a transparent, chemically inert, ultraviolet light (UV)–permeable, 54-μm thick, fluorinated ethylene propylene (FEP) Teflon film. When fully filled with sample gas, the volume of the bag was 7.5 m3. The bag was fixed inside its own aluminum frame (1.5 m height × 2 m width × 2 m depth), and the frame was attached to the fixed frame of the smog chamber with a polytetrafluoroethylene gasket between the two frames such that the reactive gas contact area was not in contact with the aluminum frame. A reflector made of treated stainless steel (SUS304) was placed on the underside of the fixed frame, and 80 UV lamps (40 W, UVA340+; Q-Lab Crop., Westlake, OH, USA) were installed on the reflector with a sheet of FEP Teflon placed between the lamps and the reactor bag to prevent direct contact. The light transmission through FEP at wavelengths in the range 290–800 nm is reported to be >90% [35,36]. The UV lamps were cooled by push–pull ventilation of the room air through 16 fans, with the air being exhausted by 16 other fans.
2.2.2. Light Source
To trigger photolysis within the vehicle exhaust, particularly that of NO2, which occurs at wavelengths <420 nm, the lights installed under the photochemical smog chamber were used to irradiate the reactor bag. A portable light spectrometer (USB2000 UV-VIS; Ocean Optics, Inc., Orlando, FL, USA) was used to characterize the irradiance spectrum reaching the inside of the reactor bag (Figure 1). The irradiance peaked at 350 nm, which was within the range of peak UV irradiances used in previously reported indoor chamber-based studies (340–370 nm). Compared to the sunlight spectrum observed during the summer season in Tokyo (Figure 1), the installed lamps were found to be reproducing mainly UV radiation between 295 and 320 nm. Furthermore, UV light sources have a different visible light spectrum at wavelengths >380 nm compared with sunlight (Figure 1), which significantly reduces the rate of photolysis reactions affected by longer wavelength light, such as photolysis of NO3 radicals [37]. Therefore, ideally, a light that produces a realistic spectrum that reproduces daytime environmental conditions should be used, but if not, the spectral difference between natural sunlight and the light used in the experiment should be taken into account when interpreting the results. Consequently, the reaction in a mobile photochemical smog chamber is generally evaluated in terms of photochemical reaction performance based on the photolysis rate constant of NO2, which is related to OH radical generation, as described in the next paragraph.

Figure 1.
Relative intensities of the solar spectrum observed in the summer in Tokyo (N 35°41′45″, E 139°45′8″), Japan, the irradiation spectra of a representative UVA340+ lamp, and the wavelength range of NO2 photolysis.
The photolysis rate constant of NO2 can be used to characterize irradiation intensity. In previous studies [37,38,39,40], the photolysis rate constant has often been calculated from the photolysis rate () of NO2 and steady-state concentrations of NOX and O3 [37,38,40]. However, in the present study, the of NO2 was measured, and the photolysis rates of several species important in atmospheric photochemistry were calculated; the JNO2 values were obtained by NOX and O3 analyzers connected to the photochemical smog chamber while under UV illumination from steady-state NOX-O3 concentrations. Using the NO2 photolysis intensity determined from the zenith angle [37,38], our light source at 23 °C (photolysis rate: 0.43 min−1) was comparable to an average morning during the summer solstice in Tokyo (0.46 min−1, N 35°41′45″, E 139°45′8″) and at 0 °C (photolysis rate: 0.31 min−1) to the transit time when the sun is due south at the winter solstice in Tokyo (0.33 min−1). Maximum values in previous studies typically ranged from 0.12 to 0.54 min−1 [15,37,38], with the present values being close to the median value of the other studies. At −7 °C (photolysis rate: 0.2 min−1), our light source was comparable to an average morning during the summer solstice in Sapporo, a city in the far north of Japan (0.16 min−1, N 43°3’51”, E 141°30′49″).
OH exposure is a factor that affects the concentration of SOAs and SOA carbon/oxygen ratios (e.g., [19]). OH exposure was determined by using Equation (1):
where OH exposure (molecules cm−3 h−1) was calculated based on the average decay rate of toluene as measured by a gas chromatograph equipped with a flame ionization detector (GC-NMHC analyzer, GL Sciences Inc., Saitama, Japan). The average OH reactivity rate coefficient (kOH) of toluene (5.63 × 10−12 cm3 molecules−1 s−1 at 298 K (25 °C) [41]) and measurement errors in toluene were potential sources of uncertainty in the estimation of OH exposure.
2.2.3. Instrumentation
A series of instruments was used to characterize the gas- and particle-phase emissions in the reactor bag. Particle number distribution was measured with a scanning mobility particle sizer (classifier model 3080 and condensation particle counter model 3750; TSI, Inc., Shoreview, MO, USA). Particle mass was measured as a 1-h value with a beta-ray absorption PM densitometer (PM712; Kimoto, Osaka, Japan). Secondary particle formation was monitored either by a soot-particle time-of-flight aerosol mass spectrometer or an aerosol chemical speciation monitor (Aerodyne, Inc., Billerica, MA, USA). Secondary particles were quantified by collecting a volume of 1 m3 aerosol sample in the reaction bag on a quartz filter (Pallflex, 2500QAT-UP, 47 φ; Pall Corp., Port Washington, NY, USA) after the 5-h reaction. The concentrations of nitrate and ammonium ions collected on the quartz filter were determined by ion chromatography (Dionex Integrion; Thermo Fisher Scientific Inc., Waltham, MA, USA). The concentration of organic carbon (OC) and EC collected on the quartz filters were determined by a thermal–optical carbon analyzer (model 2001; Desert Research Institute, Reno, NV, USA) using the IMPROVE protocol.
Gas-phase organic species in a reaction bag were monitored using a proton transfer reaction mass spectrometer (PTR-TOF 8000; Ionicon, Innsbruck, Austria). The gas-phase organic species were collected into a 5-L vinyl alcohol polymer bag (Smart Bag PA-AAK-5; GL Sciences Inc., Saitama, Japan) both before and after the photochemical reaction, and the concentrations of non-methane hydrocarbons with carbon numbers between 2 and 12 were determined by a gas chromatograph equipped with a flame ionization detector (model GC-NMHC, GL Sciences Inc., Tokyo, Japan). Gas monitors were used to determine the concentrations of CO2, NOX-NH3, and O3 (Models 410i, 17i, and 49i, respectively; Thermo Fisher Scientific Inc., Waltham, MA, USA); the monitors were zeroed daily and calibrated at least weekly.
The NOx-NH3 gas analyzer used in this study uses a chemiluminescence detector for NO to make measurements. The air sample enters the reaction chamber and reacts with O3 produced by a generator inside the instrument; the reaction of NO with O3 produces luminescent radiation that is directly proportional to the NO concentration. Once inside the instrument, the air sample is passed first through a molybdenum converter heated to 325 °C to convert NO2 to NO and then through a stainless-steel converter heated to 750 °C to convert NH3 to NO. The detector measures NO, then NO and NO2, and finally NO, NO2, and NH3. The conversion efficiency of NO2 and NH3 through the converter is corrected by a calibration procedure. Molybdenum converters are also known to convert peroxyacetyl nitrate, nitrous acid (HONO), nitric acid (HNO3), and organic nitrates formed after the reaction of VOCs with NO [42,43,44]. NO2 interferences are generally lower in concentration than NO2 and photochemical smog chamber experiments can obtain effective NO2 concentrations under pre-reaction conditions [45]. The NOX-NH3 gas analyzer used in this study has been widely used to measure NH3 concentrations in the atmosphere [45]. The results of the present NOX and NH3 measurements are described in detail in Section 3.1., Primary Gas and Particle Emissions, where the ratios of NO to NOX were 82 ± 3%, 87 ± 5%, and 95 ± 2% at 23 °C, 0 °C, and −7 °C, respectively, and the ratio of NO2 (approximately equal to NOX minus NO) to NOX was small; NO2 measurements can provide valid NO2 concentrations under conditions prior to photochemical reactions. Since the measurement of NO2 in emissions is beyond the scope of this study, the nitrogen oxide types are not distinguished and are expressed as the NOX concentrations in this study. Regarding NH3, no NOX was detected when NH3 was removed by the denuder, which provides a reasonable basis upon which the present study can show the effect of NH3 reduction prior to the photochemical reaction.
The concentration of HNO3 gas and the aerosol acidity (H+(aq)), i.e., the concentration of free hydrogen ions in the aqueous liquid phase of the particles, were obtained by calculation due to facility limitations. The concentration of HNO3 gas was approximated from O3 and HNO3 concentration curves for the photochemical smog chamber obtained by using the Statewide Air Pollution Research Center chemical reaction model [46]. Aerosol acidity (H+(aq)) was determined by using the ISORROPIA thermodynamic equilibrium model [47].
2.2.4. Experimental Procedures
Figure 2 shows a summary of the process of injecting the exhaust gas into the photochemical smog chamber, performing photochemical oxidation and aging, measuring the sample gas, and cleaning the chamber after the experiment.
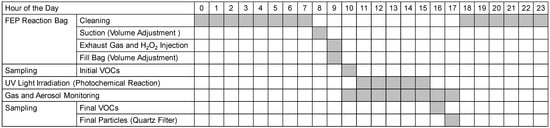
Figure 2.
Timing of the various components of the smog chamber experiment.
Figure 3a shows a photograph of the experimental chamber with the test vehicle placed on the environmental chassis dynamometer. The vehicle exhaust was connected to the photochemical smog chamber via an ejector diluter without (base scenario) or with a denuder line connected between the ejector diluter and photochemical smog chamber. The exhaust gas was injected by the ejector diluter (dilution ratio: 12 times; DI-1000; Dekati Ltd., Kangasala, Finland) into the reactor bag of the portable smog chamber at a rate of 4.2 L min−1. To supply the dilution gas with a constant flow rate into the reactor bag, clean air was supplied to the ejector diluter at 50 L min−1 under the control of a mass flow controller (MQV0050; Azbil Corp., Tokyo, Japan). During a 30-min WLTC, 1.625 m3 of dilution gas was introduced into the reactor bag; therefore, before injecting the exhaust gas into the photochemical smog chamber, suction was used to reduce the volume of the reactor bag by approximately 2 m3. As an additional hydroxyl radical (OH) source when adding the exhaust, H2O2 (0.25 mL, 30% v/v, 5 ppm equivalent in the reactor in our experiments) was also injected into the reactor bag via the make-up air supplied at 5 L min−1 [17,22,48]. The air supplied to the reactor bag was dry, but the exhaust gas contained moisture; at 23 °C, the relative humidity inside the reactor bag was <13%, at 0 °C <34%, and at −7 °C <43%.
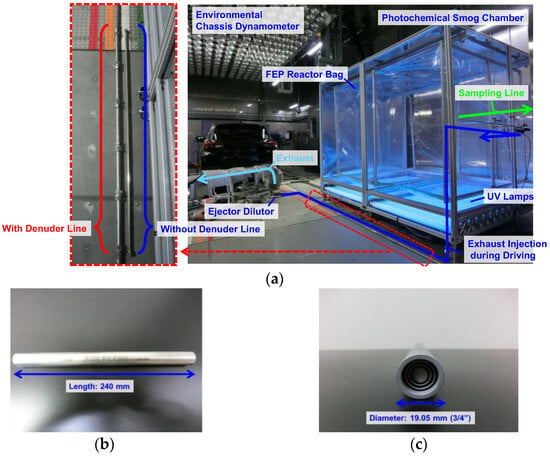
Figure 3.
Photographs of the experimental setup. (a) Flow diagram and a close-up of the series of five ammonia denuders. (b,c) Specifications of a single ammonia denuder. FEP: fluorinated ethylene propylene.
Clean air was generated by an oil-free scroll compressor equipped with a membrane air dryer (SLP-221CD; Anest-Iwata Corp., Kanagawa, Japan); the dehumidified, compressed air was passed through a manual air dryer (model 4001; CKD, Aichi, Japan), an oxidation catalyst heated to 350 °C, Purafil chemical adsorbents (Purafil and Purafil Puracarb AM; Purafil Inc., Doraville, GA, USA) [37], activated carbon, a molecular sieve, and finally through a HEPA filter.
The difference between the pressure in the reactor bag and atmospheric pressure was monitored by a differential pressure gauge (GC62; Nagano Keiki Co. Ltd., Tokyo, Japan), and the mass flow controller automatically stopped the air supply when the pressure was ≥5 Pa, thus ensuring a constant reactor bag volume. After receiving the exhaust gas, the reactor bag was allowed to stand for 15 min to allow mixing. To allow determination of the gas concentrations in the exhaust prior to the photochemical reaction, 5 L of sample gas was collected in a Smart Bag PA and an aldehyde cartridge (InertSep mini AERO DNPH-HR; GL Sciences Inc., Saitama, Japan) over a 20-min period.
To obtain NH3-free exhaust gas, NH3 was selectively removed by installing a series of five stainless steel concentric tube denuders (DN-315; Sunset Laboratory Inc., Portland, OR, USA) (Figure 3) between the ejector dilutor and the photochemical smog chamber. The inner wall of the denuder was impregnated with 10% malic acid in ethanol, which was allowed to dry before use.
UV light irradiation was started at about 2 h after the exhaust gas from one WLTC run was started to be injected into the photochemical smog chamber. The irradiation duration was 5 h, which corresponds to the average daily solar irradiation duration in Japan. During the UV irradiation, the instruments described in Section 2.2.3 collected samples of gas from the reactor bag and continuously measured their concentrations. The volume of the reactor bag was maintained by introducing clean air as a make-up gas at 5 L min−1; 25% dilution was achieved during the 5-h photochemical reaction.
Immediately after the end of the photoreaction period, 5 L of sample gas was collected in a Smart Bag PA and an aldehyde cartridge over a 20-min period. The sample gas was collected in the Smart Bag PA while mixing at 20 mL min−1 with 10 ppm nitric oxide and in the aldehyde cartridge with a potassium iodide cartridge in series in front to avoid reactions with the O3 collection device and analyzer, respectively. Particles in the reaction bag after the 5-h photochemical reaction were collected on a quartz filter with a volume of 1.00 m3 at a flow rate of 50 L min−1 by a pump with mass flow control, and secondary particles were quantified by measuring the concentrations of ionic components (NO3−, NH4+), OC, and EC.
2.2.5. Data Analysis
Emission factors (EFc) for the aerosols and gases detected in the reactor bag were calculated using the following equation and are reported as mass per mass of fuel burned (mg kg-fuel−1):
where [C] is the background- and dilution-corrected concentration of the gas or aerosol compositions in mg m−3, [CO2] is the background- and dilution-corrected concentration of CO2 in the chamber in g m−3; and EFCO2 is the emission factor of CO2 measured by a gas analyzer with the dilution tunnel shown in Section 2.1 in g kg-fuel−1. The emission factors for SOAs (secondary aerosol), NH4NO3, and NH3 (primary gas) were determined from the concentrations in the smog chamber. The emission factors for EC and primary organic carbon (POC) (primary aerosols) and for NOx and NMHC (primary gases) were determined from the concentrations in the dilution tunnel.
To quantify O3 and HNO3 gas formation in the smog chamber, we corrected the rate of dilution air introduced into the smog chamber (0.04 h−1). To quantify secondary organic aerosol and NH4NO3 particle formation in the smog chamber, we corrected the loss of aerosol particles to the reactor bag walls. Briefly, aerosol particle loss to the bag walls was treated as a first-order process with rate constants determined from decay measurements of inert tracer species (black carbon or sulfate seed) [49]. An aerosol particle wall loss rate constant without dilution was calculated using black carbon that is not lost because of reaction but only decays in concentration through dilution or wall loss, as measured on a microAeth black carbon monitor (model MA350; AethLabs, San Francisco, CA, USA) [50]. Smog chambers with nearly spherical surface-to-volume ratios have the lowest aerosol particle wall loss rate constants [51], and compared to previous studies (e.g., 0.46 to 0.66 h−1, e.g., [52]), the aerosol particle wall loss rate constant in the present study (0.12 h−1) was smaller.
For the quantification of SOA, the most common measurement, secondary organic carbon (SOC), was quantified as organic carbon (OC) [53,54,55], which was collected on a quartz fiber filter and quantified by a thermal–optical carbon analyzer. The conversion of OC, where only carbon was quantified, to organic aerosol, which also contains carbon, hydrogen, and oxygen, was performed by multiplying OC by a constant conversion factor, the organic mass-to-organic carbon (OM/OC) ratio, to estimate the total amount of SOAs and POAs [53] in Equation (3):
where OM/OCSOA_T is the OM/OC ratio of OA containing mainly SOAs and some POAs in the chamber after the photochemical reaction, as observed by soot-particle time-of-flight aerosol mass spectrometry, which is a standard high-resolution time-of-flight aerosol mass spectrometer (HR-ToF-AMS) used to analyze elements in organic compounds [54,55,56], coupled to a diode-pumped, Nd:YAG, intracavity, 1064-nm infrared laser vaporizer. The OM/OC ratios obtained were 1.8 at −7 °C, 2.0 at 0 °C, and 2.2 at 23 °C. OM/OCPOA_T is the OM/OC ratio including only POA, also observed by HR-ToF-AMS, and the value was 1.2 at all three temperatures. Our OM/OC ratio values are consistent with the OM/OC ratio of 1.2 for POAs reported for gasoline vehicles (1.2) [55], 2.0 reported from smog chamber experiments [55], and the range of 1.7 ± 0.5 for the Southern California atmosphere [56]. Further investigations are needed to assess the suitability of the OM/OC ratios used in our experiment, and this study only uses it as an empirical coefficient.
3. Results and Discussion
3.1. Primary Gas and Particle Emissions
Table 1 shows a comparison of emission results from the present study versus previously published data. For the gasoline vehicle tested under the WLTC in the present study, the emission factors at 23 °C for NMHC (161–187 mg kg-fuel−1) and NOX (97–116 mg kg-fuel−1) were generally lower than those reported from previous studies; NHMC and NOX emissions in previous studies were in rather large ranges of 4–34,980 mg kg-fuel−1 and 34–21,970 mg kg-fuel−1, respectively [15,16,25,28,57].

Table 1.
Comparison of emission results from the present and previous studies, showing formation potentials for primary gases (NMHC, NOX, NH3) and particles (EC and POA), a secondary gas (O3) and particles (SOAs and NH4NO3), and OH radical exposure.
In general, gasoline vehicle emissions contribute more to final pollutant emissions during cold starts in cold environments than at room temperature [16,22,23]. This is because the engine and drive-related systems take time to stabilize, and the after-treatment system needs time to reach its optimum activation temperature. Several previous studies have highlighted the need to consider the effects of low-temperature environments when evaluating emissions and air pollution [16,22]. The present emission factors for the low-temperature environments were 521–562 mg kg-fuel−1 for NMHC and, 107–119 mg kg-fuel−1 for NOX at 0 °C, and 763–1210 mg kg-fuel−1 for NMHC and 89–97 mg kg-fuel−1 for NOX at −7 °C. At these low temperatures, the NMHC emissions exceeded those detected under the 23 °C environment, whereas there was no marked change in NOX emissions. These emission levels were similar to those observed for gasoline vehicle emissions in a previous study where NMHC emissions were in the range of 1676–2064 mg kg-fuel−1 at 22 °C and 5362–5778 mg kg-fuel−1 at −7 °C, and NOX emissions were in the range of 123–131 mg kg-fuel−1 at 22 °C and 384–408 mg kg-fuel−1 at −7 °C [25].
Secondary pollution is suggested to occur when NH3 reacts with nitrogen oxides in the atmosphere to form NH4NO3 [22,25,26,28,57]. For the gasoline vehicle used in the present study, the emission factor for NH3 was in the range 25–28, 25–40, and 23–73 mg kg-fuel−1 at 23 °C, 0 °C, and −7 °C, respectively; thus, NH3 emissions tended to be greatest at −7 °C. Previous studies have reported NH3 emissions with considerable variation, ranging from 4 to 223 mg kg-fuel−1 at 23 °C [25,28]. The NH3 in gasoline vehicle exhaust is produced by a mechanism involving NO and H2; H2 is produced by the water–gas shift reaction between CO and water or by fluid reforming of hydrocarbons [30]. Consequently, the NH3 emissions in gasoline-vehicle emissions typically increase as tailpipe CO concentrations increase [30]. Regarding the emission factors of NH3 and CO in our study, the average NH3 emission factors (n = 2) were 26.4, 32.6, and 48.3 mg kg-fuel−1 at 23 °C, 0 °C, and −7 °C, respectively. The mean emission factors for CO (n = 2) were 10.3, 15.7, and 27.8 mg kg-fuel−1 at 23 °C, 0 °C, and −7 °C, respectively. There was a strong correlation between the emission factors of NH3 and CO at these three temperatures in our study (correlation coefficient r = 0.9997, n = 3). This suggests that the differences in NH3 emissions between the previous studies and ours may be due to the difference in CO emissions occurring in the three-way catalyst in the tailpipe.
In interpreting these results, it is important to consider the causes of the large variations in the reported values. Emission factors contributing to these large variations include regulatory age [13,14,15,18], engine maintenance history, and the type of after-treatment equipment installed on the vehicle [21,22]; thus, it is important to note the likelihood of individual vehicle effects when interpreting the results.
3.2. Primary and Photochemical Reacted Exhaust
Figure 4 shows the PM composition (sum of primary emissions and secondary formation potentials as emissions) in the diluted exhaust gas after it was subjected to UV irradiation for 5 h. The graph also summarizes the primary emissions of NMHC, NOX, and NH3 (taken from Table 1) and the secondary formation potentials of SOA, NH4NO3, aerosol acidity, and O3, in order to allow comparison of overall emissions, including primary emissions and secondary formation potentials.
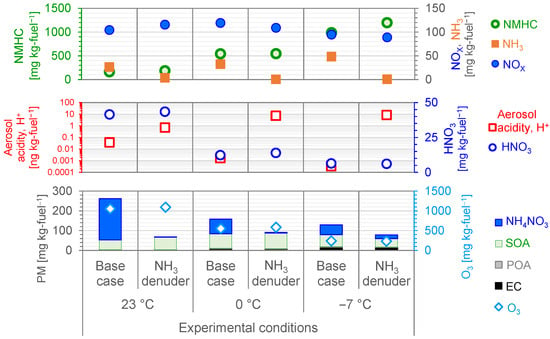
Figure 4.
Summary of primary gas and particle emissions in the dilution tunnel and the particulate matter (PM) composition (sum of primary emission and secondary formation potentials as emissions) after the exhaust was photochemically reacted for 5 h.
The repeatability of the particle mass composition measurements (n = 2) at 23 °C was 9.3% for EC, 5.6% for POA, 11.0% for SOA, 13.4% for NH4NO3, and 5.1% for O3; at 0 °C it was 37.7% for EC, 72.0% for POA, 7.6% for SOA, 24.9% for NH4NO3, and 30.0% for O3; and at −7 °C it was 42.8% for EC, 9.9% for POA, 58.2% for SOA, 7.7% for NH4NO3, and 14.1% for O3.
The emission percentages of the particle mass composition varied slightly with temperature, with the secondary particles—NH4NO3 (79.6% at 23 °C, 58.8% at 0 °C, and 38.3% at −7 °C) and SOAs (19.2% at 23 °C, 35.7% at 0 °C, and 47.5% at −7 °C)—comprising 79.6%, 58.8%, and 38.3% of the particles, respectively. The emission percentages of the total primary particles—EC, POA, and POA—were 0.8%, 4.0%, and 12.3% at 23 °C, 0 °C, and −7 °C, respectively, and 0.4%, 1.5%, and 1.9% at 23 °C, 0 °C, and −7 °C, respectively.
The PM composition after UV irradiation varied with temperature. Among the primary particles, EC accounted for 0.8%, 5.4%, and 12.3% of the total PM composition at 23 °C, 0 °C, and −7 °C, respectively, and POAs accounted for 0.4%, 1.3%, and 1.9%, respectively. Among the secondary particles, NH4NO3 accounted for 79.6%, 46.7%, and 38.3% of the total PM composition at the three temperatures, and SOAs accounted for 19.2%, 46.6%, and 47.5%, respectively. Thus, the majority of aerosols remaining after the photochemical reaction were secondary aerosols of NH4NO3 and SOA. Overall, the primary particles accounted for only 1.2%, 6.7%, and 14.2% of the total PM at 23 °C, 0 °C, and −7 °C, respectively.
Recent emission controls targeting primary particles have generally been based on worst-case scenarios, such as under conditions of very low temperature (e.g., −7 °C, e.g., [58]). However, the present findings show that when secondary particles formed by atmospheric photochemical reactions are considered, the worst-case scenario for total PM emissions is actually at 23 °C and that under this scenario, the PM emissions are dominated by secondary particles. In addition, our results shown in Figure 4 indicate that NH3 mitigation resulted in significant aerosol acidity formation in the low-temperature environment. Further studies are needed to elucidate the impacts of vehicle type and humidity conditions on the secondary particle composition of vehicle exhaust.
3.3. Effects of Ammonia Mitigation
3.3.1. NH4NO3 Particle Formation
As shown in Figure 4, NH3 mitigation by using a series of five NH3 denuders had a marked effect on the amount of secondary NH4NO3 particles formed during UV irradiation. Without NH3 mitigation, NH4NO3 particles were emitted at 208 ± 28, 74 ± 12, and 49 ± 4 mg kg-fuel−1 at 23 °C, 0 °C, and −7 °C, respectively, whereas with NH3 mitigation the emissions were 2, 3, and 19 mg kg-fuel−1, respectively, which was a considerable reduction. Thus, the NH3 removal efficiency of the series of denuders was 85% at 23 °C, 98% at 0 °C, and 98% at −7 °C, and the reduction of NH4NO3 particle formation was 99%, 96%, and 61% at 23 °C, 0 °C, and −7 °C, respectively. These findings show that although NH3 mitigation was effective at reducing NH4NO3 particle formation, the reduction was not necessarily linear with environmental temperature. In addition, although we do not yet have a complete explanation for the discrepancy between the collection efficiency of the denuders and the reduction rate of NH4NO3 particle formation, we consider that it is likely due to the rather complicated chemical equilibrium of NH4NO3 particle formation, as described below.
NH4NO3 is formed when nitric acid (produced by the oxidation of NOX) reacts homogeneously with gaseous NH3. It has been noted that the secondary formation of NH4NO3 from gasoline vehicle emissions is due to the presence of NH3 and NOX in the emissions [59]. It is also known that nitrate radicals (NO3) and dinitrogen pentoxide (N2O5) are involved in the formation of nitric acid (HNO3) gas from NOX [60]. The rate of photolysis of NO3 radicals by visible light (wavelength 420–690 nm) is about 10 times that of nitrogen dioxide; therefore, the atmospheric NO3 radical concentration is very low during the daytime (i.e., under conditions involving visible light) [61].
As demonstrated in Figure 1, the light spectrum of the mobile photochemical smog chamber differs from that of sunlight in that there is less of the spectrum above the long daytime wavelength >380 nm, resulting in slower photolysis of NO3 radicals. Recently, however, it has been found that marked amounts of nitrate are present during the evening and morning twilight hours and occasionally during the day when light levels are low [62]. Studies assessing the importance of this nitrate radical generation during the day have also been published [63,64]. During the night, NO3 radical is produced by the reaction of NO2 with O3; NO3 radical reacts with NO2 to produce N2O5, and the produced N2O5 then reacts with liquid water droplets on aerosol particles to produce HNO3:
When HNO3 is present in the atmosphere, it tends to react with basic species such as NH3 gas. The neutralization reaction between NH3(g) and HNO3 gas (HNO3(g)) to form NH4NO3 particles (NH4NO3(p)) is reversible and is considered the main source of particulate nitric acid aerosols (NH4NO3(p)) in urban air [65]. The formation of NH4NO3 particles was confirmed in this study (Figure 4), implying that HNO3 gas is produced according to Equation (6). Consequently, the mobile photochemical smog chamber used in the present study, which uses UV light sources, reproduces not only the daytime response but also that during the twilight hours from evening to morning and the daytime when light levels are low. The present approach, therefore, will be useful for the evaluation of the worst-case scenario of secondary pollutant formation that includes both mechanisms. The reaction for the formation of NH4NO3 is as follows:
The equilibrium constant for the reaction in Equation (7) depends on the gas concentration, relative humidity, and temperature [66,67,68]. The formation of particulate NH4NO3 is enhanced under conditions of high gas concentration, high relative humidity, and low temperature [67]. Aqueous ammonium nitrate exhibits temperature dependence, and the amount of particulate ammonium nitrate is determined from the amount above the equilibrium concentration of HNO3 and available NH3. Aqueous NH4NO3 also exhibits a temperature dependence, and the amount of particulate NH4NO3 is determined from the concentration of HNO3 and NH3 above the equilibrium of Equation (7). As shown in Figure 4, only NH4NO3 particles tended to be reduced by selective NH3 mitigation; the reason is that the equilibrium reaction is not established due to the elimination of NH3 gas on the left side of Equation (7).
Figure 5 shows contour plots of the emission factors of NH4NO3 particles formed in the equilibrium reaction in Equation (7) versus the emission factors of HNO3 and NH3 gases, as determined in the present study. The contour plots were calculated in ISORROPIA [47] using relative humidity and the concentrations of HNO3 and NH3 precursor gases in the reaction bag. The NH3 gas was the initial concentration before the photochemical reaction, and the HNO3 gas was the calculated concentration after the reaction (see Section 2.2.5). The concentrations of NH4NO3 particles, NH3 gas, and HNO3 gas were each converted to an emission factor, which was then used to create the contour plots shown in Figure 5. The contour plots do not show significant differences, indicating that the influence of water on the process in the presence of NH3 did not play a very important role in the wide range of NH3 and HNO3 concentrations obtained in our study with respect to changes in humidity and temperature. The deliquescence relative humidity (DRH) of NH4NO3 particles is 61.8% at 298 K and increases exponentially with decreasing temperature [69]. The relative humidities listed in the present experimental procedures (see Section 2.2.4) are lower than the DRH of NH4NO3 particles. The implication here is that the process of NH3 gas uptake into the deliquescent droplet particles does not contribute markedly to the gas-to-particle equilibrium in Equation (7).

Figure 5.
Contour plots of NH4NO3 particles formed from NH3 and HNO3 gases at (a) 23 °C and <13% relative humidity, (b) 0 °C and <34% relative humidity, and (c) −7 °C and <43% relative humidity. The contour plots were calculated by ISORROPIA [47], and the plots are values observed in the photochemical smog chamber.
The equilibrium constant for the reaction in Equation (6) shows that the formation of NH4NO3 particles is less temperature-dependent when the emissions of HNO3 and NH3 are sufficiently high. In the present study, when NH3 mitigation was used, the concentration of NH3 was reduced (i.e., the plots in Figure 5 were shifted toward the y-axis), and thus the formation of NH4NO3 particles was reduced at all three temperature conditions (i.e., 23 °C, 0 °C, and −7 °C) compared to the base scenario without NH3 mitigation. Compared to results at 23 °C, the HNO3 concentration produced from NOX emissions at 0 °C and −7 °C tended to decrease (i.e., the plots in Figure 5 were shifted toward the x-axis) because of a slowing of the photochemical reaction.
In the measurement concentrations of NH4NO3 particles, a denuder is sometimes used in filter sampling to reduce interference from measurement artifacts such as adsorption or evaporation of ammonia or nitric acid gas. In the present study, although evaporation of NH4NO3 particles was a concern (see Equation (7)), we did not perform any special sampling. This was because the purpose of the study was to examine the relative reduction of NH4NO3 particles with the reduction of NH3 gas, not to obtain exact measurements of NH4NO3 particle production. In Figure 5, the measured value of the NH4NO3 particles agrees with the calculated value if the color of the plot is the same as the background contour plots. Not all plots matched the calculated values, tending to be slightly overestimated at 23 °C and slightly underestimated at 0 °C and −7 °C. In this study, the concentration of HNO3 gas was not directly measured, and NH3 gas is generally quite difficult to accurately measure due to its sticky nature. Therefore, the consistency between the calculated and observed NH4NO3 values may be attributed to experiments based on limited resources for measuring the precursor gas NH3 and calculating HNO3. Further research should clarify the consistency with the calculated and observed NH4NO3 values based on highly sensitive and accurate measurements of the precursor gases. Regardless, our experiments indicated that selective NH3 mitigation using NH3 denuders tended to reduce NH4NO3 particles relative to those without NH3 mitigation.
Since the HNO3 concentration was lower at lower temperatures, the trend of increasing aerosol acidity due to NH3 removal also tended to be less at lower temperatures (Figure 4). Given that the aerosol formation potential was evaluated under dry conditions in the present study, further studies are needed to evaluate changes in the NH4NO3 particle formation potential and aerosol acidity due to NH3 removal in relation to humidity.
It has been reported that the increase in NH4NO3 mass after the photochemical reaction of gasoline vehicle exhaust is due more to the presence of NH3 than to a reduction in NOX emissions [22,59]. Gasoline particulate filters (GPFs) with catalysts, an after-treatment device for gasoline vehicles intended to reduce primary PM emissions, are reported to reduce NOX emissions from the tailpipe by as little as 16.6% [22] or as much as 87.6% [59], and it has been reported previously that more NH4NO3 was produced in a photochemical smog chamber experiment with GPFs than without [22]. Together, these previous studies suggest that NH3 emissions may contribute significantly to the formation of secondary inorganic aerosols, primarily in the form of NH4NO3. NH3 can also be produced in three-way catalysts from NOX emitted from the engine, and H2 is produced through the water–gas shift reaction and steam reforming of hydrocarbons [70,71]. Such NH3 is known to pass through GPF systems or be oxidized to N2O, NOX, or N2 [70,71]. To address this, three-way catalysts are usually coated with precious metals such as Pt, Rh, or Pd on a ceramic or metal substrate. In general, Rh reduces NOX, whereas Pd or Pt oxidizes CO and CH4 emissions [72]. The composition of the catalytically active metal, the air/fuel ratio, and the operating temperature all play important roles in the formation of NH3, which itself is a factor in the secondary formation of NH4NO3 particles and of N2O, a global warming potential. Also, catalysts with Pd/Rh or Pt/Rh as active metals produce NH3 [73,74] and N2O [75]. In the present study, we did not evaluate the differences in the formation potential of NH4NO3 particles relative to differences in NH3 emissions. However, it is reasonable to assume that the differences in the ratio of NH4NO3 particles to overall PM between the previous studies [22,59] and our study were most likely the cause of the difference in the amount of NOX and NH3 in the tailpipe detected in the present study.
3.3.2. Aerosol Acidity
Fewer total PM emissions (sum of primary and secondary particles) were observed with NH3 mitigation compared to without (Figure 4). These lower total PM emissions are attributed to the fact that with NH3 mitigation, the nitric acid gas produced by the oxidation of NOX reacts uniformly with the NH3 gas to neutralize it and produce fewer NH4NO3 particles; with NH3 removal, the nitric acid gas condenses onto aerosol particles (aq: particles in the liquid phase), increasing acidity (i.e., increasing the net of Equations (8) and (9)):
The aerosol acidity showed an increasing trend with NH3 mitigation (red circle plot in the middle of Figure 4), but no change in nitrate gas formation was obtained (blue circle plot in the middle of Figure 4). The decrease of NH4NO3 and the increase of aerosol acidity confirmed that no neutralization reaction between nitrate and NH3 gases occurred. Less HNO3 gas was produced with decreasing temperature due to a decrease of OH exposure (6.1 × 107 at 23 °C, 3.3 × 107 at 0 °C, and 1.9 × 107 molecules cm−3 h−1 at −7 °C), indicating a slowdown in the progress of the atmospheric oxidation reaction. Although there was a small amount of NH3 gas remaining at 23 °C with 85% NH3 gas removal, it was neutralized such that there was no marked increase in aerosol acidity. These findings suggest that a small amount of residual NH3 gas can be neutralized but that excessive NH3 mitigation promotes acidification. Further studies are needed to assess the human health effects of such increased aerosol acidity, as well as the effects on air quality, rainfall, soil, and vegetation.
3.3.3. SOA Formation
No marked difference in SOA formation potential was observed with NH3 mitigation (Figure 4). Without NH3 mitigation, SOAs were emitted at 50.2 ± 5, 73.8 ± 1, and 61.1 ± 36 mg kg-fuel−1 at 23 °C, 0 °C, and −7 °C, respectively, whereas with NH3 mitigation it was emitted at 63.3, 78.9, and 42.5 mg kg-fuel−1, respectively. The effect of NH3 on SOA formation has been demonstrated by previous photochemical smog chamber experiments and model analyses, which have shown that ammonium salts formed by the reaction of NH3 with organic acids in SOAs derived from styrene and α-pinene cause an increase of SOA formation [76] and that NH3 competes with aldehydes to reduce the yield of secondary ozonides, which decreases SOA formation [77,78]. Although the organic acids and ozonide in photochemically reacted gasoline vehicle exhaust were not quantified in the present study, it is unlikely that they would have an effect on a reaction dependent on the presence of NH3. It is reasonable to assume that the differences in the values of SOA formation due to NH3 mitigation obtained in this study were due to experimental variability, given the limited number of experiments.
The trend of SOA formation can be quantified in terms of effective SOA yields (Y), defined as the measured SOA mass divided by the mass of SOA precursors reacted. Since SOA yields vary widely among VOC components [79,80], only a portion of NMHC emissions are SOA precursors. Because of the limited number of laboratory studies available in the literature, SOA production data are not available for all precursors. Although SOA yields remain a subject of debate [79,80], presenting the data as SOA yields accounts for differences in SOA production across experiments. Our estimates of SOA yields (0.372–0.866) shown in Table 1 and Figure 6a varied but were comparable to previously reported values (0.07–0.9, e.g., [25]). SOA yields (Y) have been shown to be a function of SOA concentration (Mo) according to a classical model [81,82,83], and the relationship is described as follows:
where Kom,i and i are the mass-based gas-particle equilibrium partition coefficient and stoichiometric coefficient of product i, respectively, and Mo is the total mass concentration of organic matter (μg m−3). “Mo” is a common notation in previous studies, and it was obtained in this study by multiplying the dilution- and particle loss-corrected OC concentrations observed in the reaction bag after 5 h of photochemical reaction by the OM/OCSOA_T ratio used in Equation (3). Our effective SOA yield estimates shown in Figure 6a varied considerably, but they plotted in Figure 6 roughly backward and forwards on the SOA yield curves for each environmental temperature (23 °C, 0 °C, and −7 °C). The higher SOA yields at higher OA concentrations reflect the partitioning of the semi-volatile oxidation products in the SOAs into the particulate phase at higher OA concentrations.

Figure 6.
(a) Relationship between the effective yield of secondary organic aerosol (SOA) and SOA concentration in the present photochemical smog chamber. The gray dashed line represents a one-product fit (α1 = 0.311 and K1 = 0.043) to the apparent mass yield taken from reference [14]. (b) Scatter plots comparing the emission factor of the SOA production potential vs. the emission factor of the VOCs consumed in the reaction (i.e., the change in non-methane hydrocarbon emissions; ΔNMHC). (c) Relationship between SOA emission factor and non-methane hydrocarbon (NMHC) emission factor. (d) Scatter plots of SOA yield vs. OH exposure. The white plots in all panels show values obtained under NH3 mitigation conditions.
The scatter plots in Figure 6b compare the emission factor of the SOA production potential with that of the VOCs consumed in the reaction (ΔNMHC). The straight lines in Figure 6b are the regression lines of SOA vs. ΔNMHC and, therefore, indicate the expected SOA yield. At 23 °C and −7 °C, the plots lie mostly on the regression lines, indicating that reasonable experimental results for SOA yield were obtained; at 0 °C, the plots deviate from the straight line, indicating that SOAs and NMHC were overestimated and underestimated, respectively. At 0 °C, the relative standard deviation (i.e., the scatter) of the three experiments, including NH3 reduction, was 4% for SOAs and 15% for NHHC, suggesting that the measurements were not significantly different, and the experiments were well reproduced. Figure 6c, which shows a scatter plot of the emission factors of the SOA production potential for the NMHC emission factors, also shows that the scatter at 0 °C was minimal. Comparing SOA yields with OH exposure (Figure 6d), the SOA yields at 0 °C showed some variation, while the OH exposure remained somewhat constant. At 23 °C and −7 °C, the OH exposure, and SOA yields were less variable than at 0 °C; at −7 °C, the OH exposure was more variable than the SOA yield, whereas at 23 °C the OH exposure and SOA yield were both variable to the same degree. Although the reproducibility of the SOA measurements at 0 °C was good, the results suggest that SOA yields varied due to measurement error, although this is not relevant to understanding the rationale for the SOA yields obtained; further systematic, large-scale studies will be needed to settle the SOA yield debate.
With NH3 mitigation, the obtained SOA yields may be judged as deviating somewhat from the SOA yield curve obtained for the 0 °C condition; however, based on the relationship between the NMHC and SOA formation potentials (Figure 6c), it is reasonable to interpret this as simply the variation obtained from the series of experiments. The relationship between SOA yield and temperature remains a subject of debate, with reports of both higher [13] and lower [25] SOA yields under low-temperature conditions for SOAs produced from gasoline vehicle exhaust. Considering a gas–particle equilibrium based on the concept of effective evaporation enthalpy of a liquid becoming a gas (e.g., [84,85]), the higher SOA yield at low temperatures is considered to be a natural phenomenon in which less volatile gases condense into the particle phase, leading to more particle formation.
Our estimated yield of SOAs at 23 °C was higher than previously reported values (lower SOA yields of 0.07–0.7 with lower OH exposure of 0.1–1.5 × 107 molecules cm−3 h−1 in Table 1). We attribute this difference to a higher OH exposure (higher SOA yields of 0.731–0.866 with higher OH exposure of 6.0–6.3 × 107 molecules cm−3 h−1), and many studies support a higher SOA yield with higher OH exposure (e.g., [21]). In the present study, we found that the lower the temperature, the lower the OH exposure. We believe that the lower SOA yield at lower temperatures is due to the lower SOA formation as a result of the slowing of the oxidation process (Figure 6d). Consequently, our data shown in Figure 6c indicate that the higher NMHC emissions at low temperatures (0 °C and −7 °C), which are often used as worst-case scenarios for atmospheric environmental policymaking, did not lead to the greatest SOA formation potential. They also suggest that the reduction of NH3 did not lead to a reduction in SOA formation. However, we would like to emphasize that the actual experimental data suggest that the yield of SOAs formed from gasoline vehicle emissions is also highly dependent on environmental temperature conditions.
3.3.4. O3 Formation
No marked differences in O3 emissions were observed with NH3 mitigation (Figure 4). Without NH3 mitigation, O3 was emitted at 1053 ± 53, 553 ± 48, and 239 ± 34 mg kg-fuel−1 at 23 °C, 0 °C, and −7 °C, respectively, whereas with NH3 mitigation it was emitted at 1093, 585, and 234 mg kg-fuel−1, respectively. The effect of NH3 on O3 formation can be evaluated through compound-limited photochemical smog chamber experiments; however, few such studies exist in the literature. In one study, a photochemical smog chamber was used to investigate the effect of NH3 on secondary aerosol formation by photooxidation of toluene and NOX under different O3 formation regimes, and the study showed that although NH3 concentration does not affect O3 formation, it does affect secondary particle formation and composition [86]. Thus, our results indicate that O3 is only formed by the reaction cycle between VOCs and NOX, and NH3 concentration has no marked effect on O3.
The O3 formation potential (OFP) index, which quantifies the relative impact of individual VOCs on O3 formation, has been widely used to help develop cost-effective ground-level ozone pollution control strategies [87,88]. For a given VOC or VOC mixture, OFP is determined by using the maximum incremental reactivity (MIR) index [87,88,89,90,91,92,93,94,95,96,97,98]. The MIR index is defined as the gram of change in O3 per gram of VOC. The index was developed using the Statewide Air Pollution Research Center (SAPRC) chemical reaction model, which was built on a semi-explicit chemical mechanism [89,90,97,98]. Generally, the calculation of the OFP index uses the MIR index developed under high NOX conditions, thus limiting the O3-forming region to conditions of limited VOC concentration or at least conditions where VOC and NOX mixing is limited [91]. For most studies of gasoline vapor emissions and gasoline vehicle emissions with low NOX emissions (e.g., [88,92,93,94,95,96]), however, the OFP index has been derived by multiplying the MIR index by observed VOC emission factors. Thus, the OFP index (mg-O3 kg-fuel−1) of emitted NMHC can be calculated using the MIR index [97,98] and Equation (11):
where MIRi is the maximum incremental reactivity of VOC composition i (mg-O3 mg-VOC−1), and Ci is the emission factor [mg kg-fuel−1] of VOC composition i (VOC type: alkanes, alkenes, aromatics, aldehydes).
Figure 7 shows a comparison of the OFP index (calculated using the VOC concentration in the reaction bag) and O3 formation potentials as emissions (measured in the reaction bag); the percentage contributions of alkenes and aromatics to the OFP are also indicated. In general, alkenes, aromatics, and aldehydes contribute more to a higher MIR index [96]. In the present study, alkenes contributed 31%, 35%, and 33% to the OFP index at 23 °C, 0 °C, and −7 °C, respectively; aromatics contributed 40%, 43%, and 47%, respectively; and aldehydes contributed 12%, 4.3%, and 1.7%, respectively. The distribution of the alkenes and aromatics did not change significantly with ambient temperature. There was almost no change in the contribution of the VOC categories to the OFP index due to the removal of NH3. The calculated OFP index results are interpreted as being representative of the relative O3 formation potential as emissions from different fuel compositions. They do not suggest the possibility of changing ozone concentrations in urban areas [94]. In the present study, the OFP index did not agree with the O3 formation potential; assessment by the OFP index generally requires the use of detailed atmospheric chemistry models that account for many important additional factors (such as local meteorology and all sources of ozone precursors) [94]. Because the present study was too small in scope (i.e., single vehicle and fuel type), we could not conclude that the observations conclusively explained the performance of the technologies considered. However, the present observations contribute to our understanding of the potential for changes in the composition of vehicle emissions to have a positive effect on the suppression of atmospheric ozone formation. That is, our findings emphasize that the ratios of VOCs contributing to the OFP index are largely independent of ambient temperature and the presence of NH3 mitigation.
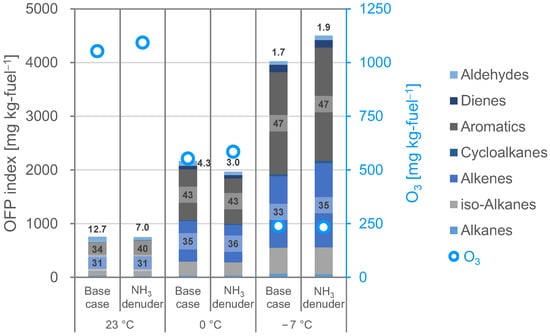
Figure 7.
Comparison of O3 formation potential measured by photochemical smog chamber experiments and O3 formation potential (OFP) index calculated by using the concentrations of volatile organic compounds in the reaction bag and Equation (11) for gasoline vehicle emissions with or without ammonia mitigation at the indicated ambient temperatures.
4. Conclusions
The formation potentials of secondary particles and O3 from gasoline vehicle exhaust were examined at different temperatures with or without NH3 mitigation. Among the total PM emitted, which included that produced by photochemical oxidation reactions, POAs, and EC accounted for only a small fraction, whereas the contribution of the secondary particles NH4NO3 and SOAs was dominant. The yield of SOAs was lower at lower temperatures. In a parameter sensitivity analysis, using a denuder to selectively reduce the concentration of NH3 gas in the vehicle exhaust was found to have a marked effect on reducing the formation of NH4NO3 but not of SOAs or O3. Increased aerosol acidity was also observed with NH3 mitigation. Overall, the present study highlights the importance of using photochemical smog chamber experiments to gain an informed understanding of the potential toxic effects and atmospheric and environmental impacts of vehicle emissions when implementing source control measures such as NH3 emission limits.
Author Contributions
Conceptualization, H.H.; methodology, H.H.; validation, H.H.; investigation, H.H.; resources, H.H.; data curation, H.H. and R.U.; writing—original draft preparation, H.H.; writing—review and editing, H.H. and R.U.; visualization, H.H.; supervision, H.H.; project administration, H.H.; funding acquisition, H.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study is a JARI internally funded Photochemical Smog Chamber Study for the Automotive Emissions Measurement Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are not publicly available due to a confidentiality agreement with the part providers. However, all data presented in this study are available upon reasonable request to the corresponding author.
Acknowledgments
The authors would like to thank the co-workers who supported the set-up and operation of the dynamometer and the measurements. In addition, the authors would like to thank Akiyoshi Ito for his support in proofreading during the preparation of the draft manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fujitani, Y.; Takahashi, K.; Saitoh, K.; Fushimi, A.; Hasegawa, S.; Kondo, Y.; Tanabe, K.; Takami, A.; Kobayashi, S. Contribution of industrial and traffic emissions to ultrafine, fine, coarse particles in the vicinity of industrial areas in Japan. Environ. Adv. 2021, 5, 100101. [Google Scholar] [CrossRef]
- Achebak, H.; Garatachea, R.; Pay, M.T.; Jorba, O.; Guevara, M.; García-Pando, C.P.; Ballester, J. Geographic sources of ozone air pollution and mortality burden in Europe. Nat. Med. 2024, 30, 1732–1738. [Google Scholar] [CrossRef]
- Fujitani, Y.; Furuyama, A.; Tanabe, K.; Hirano, S. Comparison of oxidative abilities of PM2.5 collected at traffic and residential sites in Japan. Contribution of transition metals and primary and secondary aerosols. Aerosol Air Qual. Res. 2017, 17, 574–587. [Google Scholar] [CrossRef]
- Shiraiwa, M.; Ueda, K.; Pozzer, A.; Lammel, G.; Kampf, C.J.; Fushimi, A.; Enami, S.; Arangio, A.M.; Frohlich-Nowoisky, J.; Fujitani, Y.; et al. Aerosol health effects from molecular to global scales. Environ. Sci. Technol. 2017, 51, 13545–13567. [Google Scholar] [CrossRef]
- Künzi, L.; Krapf, M.; Daher, N.; Dommen, J.; Jeannet, N.; Schneider, S.; Platt, S.; Slowik, J.G.; Baumlin, N.; Salathe, M.; et al. Toxicity of aged gasoline exhaust particles to normal and diseased airway epithelia. Sci. Rep. 2015, 5, 11801. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.S.; Poon, H.Y.; Organ, B.; Chuang, H.C.; Chan, M.-N.; Guo, H.; Ho, S.S.; Ho, K.-F. Toxicological effects of fresh and aged gasoline exhaust particles in Hong Kong. J. Hazard Mater. 2023, 441, 129846. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Favez, O.; Perraudin, E.; Villenave, E.; Albinet, A. Comparison of Measurement-Based Methodologies to Apportion Secondary Organic Carbon (SOC) in PM2.5: A Review of Recent Studies. Atmosphere 2018, 9, 452. [Google Scholar] [CrossRef]
- Hayes, P.L.; Carlton, A.G.; Baker, K.R.; Ahmadov, R.; Washenfelder, R.A.; Alvarez, S.; Rappenglück, B.; Gilman, J.B.; Kuster, W.C.; de Gouw, J.A.; et al. Modeling the formation and aging of secondary organic aerosols in Los Angeles during CalNex 2010. Atmos. Chem. Phys. 2015, 15, 5773–5801. [Google Scholar] [CrossRef]
- Jathar, S.H.; Gordon, T.D.; Hennigan, C.J.; Pye, H.O.; Pouliot, G.; Adams, P.J.; Donahue, N.M.; Robinson, A.L. Unspeciated organic emissions from combustion sources and their influence on the secondary organic aerosol budget in the United States. Proc. Natl. Acad. Sci. USA 2014, 111, 10473–10478. [Google Scholar] [CrossRef]
- Jathar, S.H.; Woody, M.; Pye, H.O.T.; Baker, K.R.; Robinson, A.L. Chemical transport model simulations of organic aerosol in southern California: Model evaluation and gasoline and diesel source contributions. Atmos. Chem. Phys. 2017, 17, 4305–4318. [Google Scholar] [CrossRef]
- Gentner, D.R.; Jathar, S.H.; Gordon, T.D.; Bahreini, R.; Day, D.A.; Haddad, I.E.; Haynes, P.L.; Pieber, S.M.; Platt, S.M.; De Gouw, J.; et al. Review of urban secondary organic aerosol formation from gasoline and diesel motor vehicle emissions. Environ. Sci. Technol. 2017, 51, 1074–1093. [Google Scholar] [CrossRef] [PubMed]
- Dunmore, R.E.; Hopkins, J.R.; Lidster, R.T.; Lee, J.D.; Evans, M.J.; Rickard, A.R.; Lewis, A.C.; Hamilton, J.F. Diesel-related hydrocarbons can dominate gas phase reactive carbon in megacities. Atmos. Chem. Phys. 2015, 15, 9983–9996. [Google Scholar] [CrossRef]
- Platt, S.M.; El Haddad, I.; Pieber, M.; Zardini, A.A.; Suarez-Bertoa, R.; Clairotte, M.; Daellenbach, K.R.; Huang, R.J.; Slowwik, J.G.; Hellebust, S.; et al. Gasoline cars produce more carbonaceous particulate matter than modern filter-equipped diesel cars. Sci. Rep. 2017, 7, 4926. [Google Scholar] [CrossRef] [PubMed]
- Nordin, E.Z.; Eriksson, A.C.; Roldin, P.; Nilsson, P.T.; Carlsson, J.E.; Kajos, M.K.; Hellén, H.; Wittbom, C.; Rissler, J.; Löndahl, J.; et al. Secondary organic aerosol formation from idling gasoline passenger vehicle emissions investigated in a smog chamber. Atmos. Chem. Phys. 2013, 13, 6101–6116. [Google Scholar] [CrossRef]
- Platt, S.M.; El Haddad, I.; Zardini, A.A.; Clairotte, M.; Astorga, C.; Wolf, R.; Slowik, J.G.; Temime-Roussel, B.; Marchand, N.; Ježek, I.; et al. Secondary organic aerosol formation from gasoline vehicle emissions in a new mobile environmental reaction chamber. Atmos. Chem. Phys. 2013, 13, 9141–9158. [Google Scholar] [CrossRef]
- Gordon, T.D.; Presto, A.A.; May, A.A.; Nguyen, N.T.; Lipsky, E.M.; Donahue, N.M.; Gutierrez, A.; Zhang, M.; Maddox, C.; Rieger, P.; et al. Secondary organic aerosol formation exceeds primary particulate matter emissions for light-duty gasoline vehicles. Atmos. Chem. Phys. 2014, 14, 4661–4678. [Google Scholar] [CrossRef]
- Vu, D.; Roth, P.; Berte, T.; Yang, J.; Cocker, D.; Durbin, T.D.; Karavalakis, G.; Asa-Awuku, A. Using a new Mobile Atmospheric Chamber (MACh) to investigate the formation of secondary aerosols from mobile sources: The case of gasoline direct injection vehicles. J. Aerosol Sci. 2019, 133, 1–11. [Google Scholar] [CrossRef]
- Zhao, Y.; Saleh, R.; Saliba, G.; Presto, A.A.; Gordon, T.D.; Drozd, G.T.; Goldstein, A.H.; Donahue, N.M.; Robinson, A.L. Reducing secondary organic aerosol formation from gasoline vehicle exhaust. Proc. Natl. Acad. Sci. USA 2017, 114, 6984–6989. [Google Scholar] [CrossRef]
- Morino, Y.; Li, Y.; Fujitani, Y.; Sato, K.; Inomata, S.; Tanabe, K.; Jathar, S.H.; Kondo, Y.; Nakayama, T.; Fushimi, A.; et al. Secondary organic aerosol formation from gasoline and diesel vehicle exhaust under light and dark conditions. Environ. Sci. Atmos. 2022, 2, 46–64. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Deng, W.; Hu, Q.; Ding, X.; Zhang, Y.; He, Q.; Zhang, Z.; Lü, S.; Bi, X.; et al. Secondary organic aerosol formation from photochemical aging of light-duty gasoline vehicle exhausts in a smog chamber. Atmos. Chem. Phys. 2015, 15, 9049–9062. [Google Scholar] [CrossRef]
- Pieber, S.M.; Kumar, N.K.; Klein, F.; Comte, P.; Bhattu, D.; Dommen, J.; Bruns, E.A.; Kılıç, D.; El Haddad, I.; Keller, A.; et al. Gas-phase composition and secondary organic aerosol formation from standard and particle filter-retrofitted gasoline direct injection vehicles investigated in a batch and flow reactor. Atmos. Chem. Phys. 2018, 18, 9929–9954. [Google Scholar] [CrossRef]
- Roth, P.; Yang, J.; Fofie, E.; Cocker, D.R.; Durbin, T.D.; Brezny, R.; Geller, M.; Asa-Awuku, A.; Karavalakis, G. Catalyzed gasoline particulate filters reduce secondary organic aerosol production from gasoline direct injection vehicles. Environ. Sci. Technol. 2019, 53, 3037–3047. [Google Scholar] [CrossRef] [PubMed]
- Drozd, G.T.; Zhao, Y.; Saliba, G.; Frodin, B.; Maddox, C.; Oliver Chang, M.C.; Maldonado, H.; Sardar, S.; Weber, R.J.; Robinson, A.L.; et al. Detailed speciation of intermediate volatility and semivolatile organic compound emissions from gasoline vehicles: Effects of cold-starts and implications for secondary organic aerosol formation. Environ. Sci. Technol. 2019, 53, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, P.; Timonen, H.; Saukko, E.; Kuuluvainen, H.; Saarikoski, S.; Aakko-Saksa, P.; Murtonen, T.; Bloss, M.; Dal Maso, M.; Simonen, P.; et al. Time-resolved characterization of primary particle emissions and secondary particle formation from a modern gasoline passenger car. Atmos. Chem. Phys. 2016, 16, 8559–8570. [Google Scholar] [CrossRef]
- Suarez-Bertoa, R.; Zardini, A.A.; Platt, S.M.; Hellebust, S.; Pieber, S.M.; El Haddad, I.; Temime-Roussel, B.; Baltensperger, U.; Marchand, N.; Prévôt, A.S.H.; et al. Primary Emissions and Secondary Organic Aerosol Formation from the Exhaust of a Flex-Fuel (Ethanol) Vehicle. Atmos. Environ. 2015, 117, 200–211. [Google Scholar] [CrossRef]
- Roth, P.; Yang, J.; Peng, W.; Cocker III, D.R.; Durbin, T.D.; Asa-Awuku, A.; Karavalakis, G. Intermediate and high ethanol blends reduce secondary organic aerosol formation from gasoline direct injection vehicles. Atmos. Environ. 2020, 220, 117064. [Google Scholar] [CrossRef]
- Wang, H.; Guo, S.; Yu, Y.; Shen, R.; Zhu, W.; Tang, R.; Tan, R.; Liu, K.; Song, K.; Zhang, W.; et al. Secondary aerosol formation from a Chinese gasoline vehicle: Impacts of fuel (E10, gasoline) and driving conditions (idling, cruising). Sci. Total Environ. 2021, 795, 148809. [Google Scholar] [CrossRef]
- Hartikainen, A.; Ihalainen, M.; Yli-Pirila, P.; Hao, L.; Kortelainen, M.; Pieber, S.; Sippula, O. Photochemical Transformation and Secondary Aerosol Formation Potential of Euro6 Gasoline and Diesel Passenger Car Exhaust Emissions. J. Aerosol Sci. 2023, 171, 106159. [Google Scholar] [CrossRef]
- Farren, N.J.; Davison, J.; Rose, R.A.; Wagner, R.L.; Carslaw, D.C. Underestimated ammonia emissions from road vehicles. Environ. Sci. Technol. 2020, 54, 15689–15697. [Google Scholar] [CrossRef]
- Suarez-Bertoa, R.; Zardini, A.A.; Astorga, C. Ammonia exhaust emissions from spark ignition vehicles over the New European Driving Cycle. Atmos. Environ. 2014, 97, 43–53. [Google Scholar] [CrossRef]
- Bajwa, A.; Shankar, V.; Leach, F. Ammonia Emissions from Combustion in Gasoline Engines; 2023-01-1655; SAE Technical Paper: Pittsburgh, PA, USA, 2023. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Crow, D.; Lowenthal, D.H.; Merrifield, T. Comparison of IMPROVE and NIOSH Carbon Measurements. Aerosol Sci. Technol. 2001, 34, 23–34. [Google Scholar] [CrossRef]
- Japan Automobile Manufacturers Association, Inc. The Motor Industry of Japan. 2023. Available online: https://www.jama.or.jp/english/reports/docs/MIoJ2023_e.pdf (accessed on 16 August 2024).
- Kaltsonoudis, C.; Jorga, S.D.; Louvaris, E.; Florou, K.; Pandis, S.N. A portable dual-smog-chamber system for atmospheric aerosol field studies. Atmos. Meas. Tech. 2019, 12, 2733–2743. [Google Scholar] [CrossRef]
- Kelly, N.A. Characterization of Fluorocarbon-Film Bags as Smog Chambers. Environ. Sci. Technol. 1982, 16, 763–770. [Google Scholar] [CrossRef]
- Paulsen, D.; Dommen, J.; Kalberer, M.; Prevot, A.S.H.; Richter, R.; Sax, M.; Steinbacher, M.; Weingartner, E.; Baltensperger, U. Secondary organic aerosol formation by irradiation of 1,3,5- trimethylbenzene-NOx-H2O in a new reaction chamber for atmospheric chemistry and physics. Environ. Sci. Technol. 2005, 39, 2668–2678. [Google Scholar] [CrossRef]
- Carter, W.P.; Cockeriii, D.R., III; Fitz, D.R.; Malkina, I.L.; Bumiller, K.; Sauer, C.G.; Pisano, J.; Bufalino, C.; Song, C. A new environmental chamber for evaluation of gas-phase chemical mechanisms and secondary aerosol formation. Atmos. Environ. 2005, 39, 7768–7788. [Google Scholar] [CrossRef]
- Wang, X.; Liu, T.; Bernard, F.; Ding, X.; Wen, S.; Zhang, Y.; Zhang, Z.; He, Q.; Lü, S.; Chen, J.; et al. Design and characterization of a smog chamber for studying gas-phase chemical mechanisms and aerosol formation. Atmos. Meas. Tech. 2014, 7, 301–313. [Google Scholar] [CrossRef]
- Peng, J.; Hu, M.; Du, Z.; Wang, Y.; Zheng, J.; Zhang, W.; Yang, Y.; Qin, Y.; Zheng, R.; Xiao, Y.; et al. Gasoline aromatics: A critical determinant of urban secondary organic aerosol formation. Atmos. Chem. Phys. 2017, 17, 10743–10752. [Google Scholar] [CrossRef]
- Atkinson, R.; Baulch, D.L.; Cox, R.A.; Crowley, J.N.; Hampson, R.F.; Hynes, R.G.; Jenkin, M.E.; Rossi, M.J.; Troe, J. Evaluated kinetic and photochemical data for atmospheric chemistry: Volume I—gas phase reactions of Ox, HOx, NOx and SOx species. Atmos. Chem. Phys. 2004, 4, 1461–1738. [Google Scholar] [CrossRef]
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef]
- Villena, G.; Bejan, I.; Kurtenbach, R.; Wiesen, P.; Kleffmann, J. Interferences of commercial NO2 instruments in the urban atmosphere and in a smog chamber. Atmos. Meas. Tech. 2012, 5, 145–159. [Google Scholar] [CrossRef]
- Alam, M.S.; Crilley, L.R.; Lee, J.D.; Kramer, L.J.; Pfrang, C.; Vázquez-Moreno, M.; Ródenas, M.; Muñoz, A.; Bloss, W.J. Interference from alkenes in chemiluminescent NOX measurements. Atmos. Meas. Tech. 2020, 13, 5977–5991. [Google Scholar] [CrossRef]
- Jordan, N.; Garner, N.M.; Matchett, L.C.; Tokarek, T.W.; Osthoff, H.D.; Odame-Ankrah, C.A.; Rosentreter, B.W. Potential interferences in photolytic nitrogen dioxide converters for ambient air monitoring: Evaluation of a prototype. J. Air Waste Manage. Assoc. 2020, 70, 753–764. [Google Scholar] [CrossRef]
- Delon, C.; Galy-Lacaux, C.; Serça, D.; Loubet, B.; Camara, N.; Gardrat, E.; Saneh, I.; Fensholt, R.; Tagesson, T.; Le Dantec, V.; et al. Soil and vegetation-atmosphere exchange of NO, NH3, and N2O from field measurements in a semi arid grazed ecosystem in Senegal. Atmos. Environ. 2017, 156, 36–51. [Google Scholar] [CrossRef]
- William, P.L. Carter. Development of the SAPRC-07 chemical mechanism. Atmos. Environ. 2010, 44, 5324–5335. [Google Scholar] [CrossRef]
- Nenes, A.; Pandis, S.N.; Pilinis, C. ISORROPIA: A new thermodynamic equilibrium model for multiphase multicomponent inorganic aerosols. Aquat. Geoch. 1998, 4, 123–152. [Google Scholar] [CrossRef]
- Xing, J.; Shao, L.; Zhang, W.; Peng, J.; Wang, W.; Shuai, S.; Hu, M.; Zhang, D. Morphology and size of the particles emitted from a gasoline-direct-injection-engine vehicle and their ageing in an environmental chamber. Atmos. Chem. Phys. 2020, 20, 2781–2794. [Google Scholar] [CrossRef]
- McMurry, P.H.; Grosjean, D. Gas and Aerosol Wall Losses in Teflon Film Smog Chambers. Environ. Sci. Technol. 1985, 19, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, D.L.; Hill, L.D.; Blair, J.; Crosman, E.T. A Long-Term Comparison between the AethLabs MA350 and Aerosol Magee Scientific AE33 Black Carbon Monitors in the Greater Salt Lake City Metropolitan Area. Sensors 2024, 24, 965. [Google Scholar] [CrossRef]
- Cocker, D.; Flagan, R.; Seinfeld, J. State-of-the-art chamber facility for studying atmospheric aerosol chemistry. Environ. Sci. Technol. 2001, 35, 2594–2601. [Google Scholar] [CrossRef]
- Gordon, T.D.; Presto, A.A.; Nguyen, N.T.; Robertson, W.H.; Na, K.; Sahay, K.N.; Zhang, M.; Maddox, C.; Rieger, P.; Chattopadhyay, S.; et al. Secondary Organic Aerosol Production from Diesel Vehicle Exhaust: Impact of Aftertreatment, Fuel Chemistry and Driving Cycle. Atmos. Meas. Tech. 2014, 14, 4643–4659. [Google Scholar] [CrossRef]
- Pang, Y.; Turpin, B.J.; Gundel, L.A. On the Importance of Organic Oxygen for Understanding Organic Aerosol Particles. Aerosol Sci. Technol. 2006, 40, 128–133. [Google Scholar] [CrossRef]
- Aiken, A.C.; DeCarlo, P.F.; Jimenez, J.L. Elemental Analysis of Organic Species with Electron Ionization High-Resolution Mass Spectrometry. Anal. Chem. 2007, 79, 8350–8358. [Google Scholar] [CrossRef]
- Aiken, A.C.; DeCarlo, P.F.; Kroll, J.H.; Worsnop, D.R.; Huffman, J.A.; Docherty, K.S.; Ulbrich, I.M.; Mohr, C.; Kimmel, J.R.; Sueper, D.; et al. O/C and OM/OC Ratios of Primary, Secondary, and Ambient Organic Aerosols with High-Resolution Time-of-Flight Aerosol Mass Spectrometry. Environ. Sci. Technol. 2008, 42, 4478–4485. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.L.; Ortega, A.M.; Cubison, M.J.; Froyd, K.D.; Zhao, Y.; Cliff, S.S.; Hu, W.W.; Toohey, D.W.; Flynn, J.H.; Lefer, B.L.; et al. Organic Aerosol Composition and Sources in Pasadena, California, during the 2010 CalNex Campaign. J. Geophys. Res. Atmos. 2013, 118, 9233–9257. [Google Scholar] [CrossRef]
- Park, G.; Kim, K.; Park, T.; Kang, S.; Ban, J.; Choi, S.; Yu, D.G.; Lee, S.; Lim, Y.; Kim, S.; et al. Primary and Secondary Aerosols in Small Passenger Vehicle Emissions: Evaluation of Engine Technology, Driving Conditions, and Regulatory Standards. Environ. Pollut. 2021, 286, 117195. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Dardiotis, C.; Kandlhofer, C.; Arndt, M. Challenges related to the measurement of particle emissions of gasoline direct injection engines under cold-start and low-temperature conditions. Int. J. Auto. Eng. 2019, 10, 332–339. [Google Scholar] [CrossRef][Green Version]
- Yang, J.; Roth, P.; Durbin, T.D.; Johnson, K.C.; Cocker, D.R., III; Asa-Awuku, A.; Brezny, R.; Geller, M.; Karavalakis, G. Gasoline particulate filters as an effective tool to reduce particulate and PAH emissions from GDI vehicles: A case study with two GDI vehicles. Environ. Sci. Technol. 2018, 52, 3275–3284. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.G.; Stockwell, W.R. Acid generation in the troposphere by gas-phase chemistry. Environ. Sci. Technol. 1983, 17, 428A–443A. [Google Scholar] [CrossRef]
- Stockwell, W.R.; Kirchner, F.; Kuhn, M.; Seefeld, S. A new mechanism for regional atmospheric chemistry modeling. J. Geophys. Res. Atmos. 1997, 102, 25847–25879. [Google Scholar] [CrossRef]
- Geyer, A.; Alicke, B.; Ackermann, R.; Martinez, M.; Harder, H.; Brune, W.; di Carlo, P.; Williams, E.; Jobson, T.; Hall, S.; et al. Direct observations of daytime NO3. Implications for urban boundary layer chemistry. J. Geophys. Res. Atmos. 2003, 108, 4368. [Google Scholar] [CrossRef]
- Brown, S.S.; Osthoff, H.D.; Stark, H.; Dubé, W.P.; Ryerson, T.B.; Warneke, C.; de Gouw, J.A.; Wollny, A.G.; Parrish, D.D.; Fehsenfeld, F.C.; et al. Aircraft observations of daytime NO3 and N2O5 and their implications for tropospheric chemistry. J. Photochem. Photobiol. A Chem. 2005, 176, 270–278. [Google Scholar] [CrossRef]
- Osthoff, H.D.; Sommariva, R.; Baynard, T.; Pettersson, A.; Williams, E.J.; Lerner, B.M.; Roberts, J.M.; Stark, H.; Goldan, P.D.; Kuster, W.C.; et al. Observation of daytime N2O5 in the marine boundary layer during New England Air Quality Study–Intercontinental Transport and Chemical Transformation 2004. J. Geophys. Res. Atmos. 2006, 111, D23S14. [Google Scholar] [CrossRef]
- Meng, Z.; Dabdub, D.; Seinfeld, J.H. Chemical coupling between atmospheric ozone and particulate matter. Science 1997, 277, 116–119. [Google Scholar] [CrossRef]
- Stelson, A.W.; Seinfeld, J.H. Thermodynamic prediction of the water activity, NH4HO3 dissociation constant, density and refractive index for the NH4NO3–(NH4)2SO4H2O system at 25 °C. Atmos. Environ. 1982, 16, 2507–2514. [Google Scholar] [CrossRef]
- Stelson, A.W.; Seinfeld, J.H. Relative humidity and temperature dependence of the ammonium nitrate dissociation constant. Atmos. Environ. 1982, 16, 983–992. [Google Scholar] [CrossRef]
- Stelson, A.W.; Seinfeld, J.H. Relative humidity and pH dependence of the vapor pressure of ammonium nitrate–nitric acid solutions at 25 °C. Atmos. Environ. 1982, 16, 993–1000. [Google Scholar] [CrossRef]
- Peng, C.; Chen, L.; Tang, M. A database for deliquescence and efflorescence relative humidities of compounds with atmospheric relevance. Fundam. Res. 2022, 2, 578–587. [Google Scholar] [CrossRef]
- Gong, J.; Rutland, C. Three Way Catalyst Modeling with Ammonia and Nitrous Oxide Kinetics for A Lean Burn Spark Ignition Direct Injection (Sidi) Gasoline Engine; 2013−01−1572; SAE Technical Paper: Pittsburgh, PA, USA, 2013. [Google Scholar] [CrossRef]
- Heeb, N.V.; Forss, A.M.; Bruhlmann, S.; Luscher, R.; Saxer, C.J.; Hug, P. Three-way catalyst-induced formation of ammonia-velocity and acceleration-dependent emission factors. Atmos. Environ. 2006, 40, 5986–5997. [Google Scholar] [CrossRef]
- Gandhi, H.S.; Graham, G.W.; McCabe, R.W. Automotive exhaust catalysis. J. Catal. 2003, 216, 433–442. [Google Scholar] [CrossRef]
- Schlatter, J.C.; Taylor, K.C. Platinum and palladium addition to supported rhodium catalysts for automotive emission control. J. Catal. 1977, 49, 42–50. [Google Scholar] [CrossRef]
- Kobylinski, T.P.; Taylor, B.W. The catalytic chemistry of nitric oxide: II. Reduction of nitric oxide over noble metal catalysts. J. Catal. 1974, 33, 376–384. [Google Scholar] [CrossRef]
- Renème, Y.; Dhainaut, F.; Granger, P. Kinetics of the NO/H2/O2 reactions on natural gas vehicle catalysts—Influence of Rh addition to Pd. Appl. Catal. B Environ. 2012, 111–112, 424–432. [Google Scholar] [CrossRef]
- Na, K.; Song, C.; Switzer, C.; Cocker, D.R. Effect of ammonia on secondary organic aerosol formation from α-pinene ozonolysis in dry and humid conditions. Environ. Sci. Technol. 2007, 41, 6096–6102. [Google Scholar] [CrossRef] [PubMed]
- Na, K.; Song, C.; Cocker, D.R. Formation of secondary organic aerosol from the reaction of styrene with ozone in the presence and absence of ammonia and water. Atmos. Environ. 2006, 40, 1889–1900. [Google Scholar] [CrossRef]
- Qiao, M.; Xiaoxiao, L.; Chengqiang, Y.; Bo, L.; Yanbo, G.; Weijun, Z. The influences of ammonia on aerosol formation in the ozonolysis of styrene: Roles of Criegee intermediate reactions. R. Soc. Open Sci. 2018, 5, 5172171. [Google Scholar] [CrossRef]
- McDonald, B.C.; de Gouw, J.A.; Gilman, J.B.; Jathar, S.H.; Akherati, A.; Cappa, C.D.; Jimenez, J.L.; Lee-Taylor, J.; Hayes, P.L.; McKeen, S.A.; et al. Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 2018, 359, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Vu, T.V.; Tong, S.; Shi, Z.; Harrison, R.M. Formation of secondary organic aerosols from anthropogenic precursors in laboratory studies. NPJ Clim. Atmos. Sci. 2022, 5, 22. [Google Scholar] [CrossRef]
- Pankow, J.F. An absorption-model of gas-particle partitioning of organic compounds in the atmosphere. Atmos. Environ. 1994, 28, 185–188. [Google Scholar] [CrossRef]
- Pankow, J.F. An absorption-model of the gas aerosol partitioning involved in the formation of secondary organic aerosol. Atmos. Environ. 1994, 28, 189–193. [Google Scholar] [CrossRef]
- Odum, J.R.; Jungkamp, T.P.W.; Griffin, R.J.; Forstner, H.J.L.; Flagan, R.C.; Seinfeld, J.H. Aromatics, reformulated gasoline, and atmospheric organic aerosol formation. Environ. Sci. Technol. 1997, 31, 1890–1897. [Google Scholar] [CrossRef]
- Takekawa, H.; Minoura, H.; Yamazaki, S. Temperature Dependence of Secondary Organic Aerosol Formation by Photo-Oxidation of Hydrocarbons. Atmos Environ. 2003, 37, 3413–3424. [Google Scholar] [CrossRef]
- Svendby, T.M.; Lazaridis, M.; Tørseth, K. Temperature dependent secondary organic aerosol formation from terpenes and aromatics. J. Atmos. Chem. 2008, 59, 25–46. [Google Scholar] [CrossRef]
- Bao, Z.E.; Xu, H.F.; Li, K.W.; Chen, L.H.; Zhang, X.; Wu, X.C.; Gao, X.; Azzi, M.; Cen, K.F. Effects of NH3 on secondary aerosol formation from toluene/NOx photo-oxidation in different O3 formation regimes. Atmos. Environ. 2021, 261, 11. [Google Scholar] [CrossRef]
- Carter, W.P.L.; Atkinson, R. Computer modeling study of incremental hydrocarbon reactivity. Environ. Sci. Technol. 1989, 23, 864–880. [Google Scholar] [CrossRef]
- Chang, T.Y.; Rudy, S.J. Ozone-forming potential of organic emissions from alternative-fueled vehicles. Atmos. Environ. 1990, 24, 2421–2430. [Google Scholar] [CrossRef]
- Carter, W.P.L. Development of ozone reactivity scales for volatile organic compounds. J. Air Waste Manage. 1994, 44, 881–899. [Google Scholar] [CrossRef]
- Venecek, M.A.; Carter, W.P.L.; Kleeman, M.J. Updating the SAPRC maximum incremental reactivity (MIR) scale for the United States from 1988 to 2010. J. Air Waste Manage. 2018, 68, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, L.; Carter, W.P.L.; Pei, C.; Chen, T.; Mu, J.; Wang, Y.; Zhang, Q.; Wang, W. Development of ozone reactivity scales for volatile organic compounds in a Chinese megacity. Atmos. Chem. Phys. 2021, 21, 11053–11068. [Google Scholar] [CrossRef]
- Yamada, H.; Inomata, S.; Tanimoto, H. Refueling emissions from cars in Japan: Compositions, temperature dependence and effect of vapor liquefied collection system. Atmos. Environ. 2015, 120, 455–462. [Google Scholar] [CrossRef]
- Yamada, H.; Inomata, S.; Tanimoto, H.; Hata, H.; Tonokura, K. Estimation of refueling emissions based on theoretical model and effects of E10 fuel on refueling and evaporative emissions from gasoline cars. Sci. Total Environ. 2018, 622, 467–473. [Google Scholar] [CrossRef]
- Black, F.; Tejada, S.; Gurevich, M. Alternative fuel motor vehicle tailpipe and evaporative emissions composition and ozone potential. J. Air Waste Manag. Assoc. 1998, 48, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Kajima, K.; Hirota, T.; Yakushiji, K.; Iwakiri, Y.; Oda, K.; Akutsu, Y. Effect of reformulated gasoline and methanol on exhaust emissions. SAE Tech. Pap. 1991, 100, 912431. [Google Scholar] [CrossRef]
- Hata, H.; Okada, M.; Funakubo, C.; Hoshi, J. Tailpipe VOC Emissions from late model gasoline passenger vehicles in the Japanese market. Atmosphere 2019, 10, 621. [Google Scholar] [CrossRef]
- Carter, W.P.L.; Heo, G. Development of revised SAPRC aromatics mechanisms. Report to the California Air Resources Board Contracts No. 07-730 and 08-326. 12 April 2012. Available online: https://intra.engr.ucr.edu/~carter/SAPRC/scales11.xls (accessed on 29 August 2024).
- Carter, W.P.L.; Heo, G. Development of revised SAPRC aromatic mechanisms. Atmos. Environ. 2013, 77, 404–414. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).