Abstract
The petrochemical industry is regarded as the main source of anthropogenic VOCs emissions in China. As one of the main sources of unorganized emissions, circulating water is scarcely studied and reported. In this research, six circulating water systems (LC2X, HGLY, YJ, XJ, LC4X and LC5X) of a typical petrochemical enterprise were selected as targets to characterize VOCs emitted from such unorganized emissions. The results showed that there was a great difference in the VOCs disorganized emissions from the six circulating water systems, among which the main VOCs of HG2X, HGLY and YJ were oxygen-containing VOCs (OVOCs), accounting for about 48.0–81.2%. The main compounds of XJ, LC4X and LC5X were alkynes (89.1%), aromatic hydrocarbons (69.7%) and alkane (50.1%), respectively. TVOCs ranged from 276.0 to 23,009.6 µg·m−3. Based on POC test results, VOCs emissions of the circulating water system were 1237.5 tons, indicating further control was needed. As for their ambient impact, XJ had higher OFP contribution, and the OFP values of the six systems ranged from 823.3 to 145,739.0 µg·m−3, among which the major contributors were aromatic hydrocarbons (0.2–85.1%), OVOCs (0.1–77.2%) and alkynes (1.7–97.6%). In addition, aromatic hydrocarbons showed the largest contribution of the potential of SOA generation, which was more than 88.0%. As far as control was concerned, the replacement of an open cooling tower to closed cooling tower combined with regular POC detection will be an efficient way to control VOCs from such sources.
1. Introduction
Volatile organic compounds (VOCs), the collective term for organic compounds with a boiling point lower than 250 °C under standard atmospheric pressure, are important participants in atmospheric chemical reactions and major precursors for the formation of PM2.5 and O3 [1,2,3,4]. In addition, VOCs also have a certain impact on human health [5,6,7]. In recent years, China has successively issued relevant plans to put forward relevant requirements for VOCs management, put forward comprehensive rectification plans for key VOCs emission industries, such as petrochemicals, and pointed out the strengthening of fugitive emission control [8,9,10]. As a pillar industry of the national economy, the petrochemical industry is also the main anthropogenic VOCs emission source and has become the largest anthropogenic emission sub-source in China [11,12,13,14]. In view of this, researchers have paid more attention to VOCs pollution emissions of petrochemical enterprises. At present, the research mainly focuses on the emission characteristics of VOCs in the ambient air, inside and outside the factory area of petrochemical enterprises [15,16,17,18,19,20]. In addition, studies have shown that the unorganized VOCs emissions of petrochemical enterprises are as high as 14.4% of the anthropogenic emissions [21], and the emitted VOCs have the characteristics of high concentration, large emissions and strong activity, which easily produce O3 and secondary aerosol pollution and are harmful to human health [22,23]. Among those organized and unorganized sources, a circulating cooling water system is regarded as one of the most important sources contributing to VOCs emission amounts in the petrochemical industry. However, VOCs characterizations, including species, releasing behaviors and impact on environment from circulating cooling water system, are scarcely reported [19].
Based on those mentioned above, about six circulating cooling water systems were selected in a typical petrochemical enterprise in North China as the targets. VOCs were sampled at the cooling tower sites and then analyzed for the component determination. In addition, the releasing processes, impact on surrounding environment and control measures were also discussed with the purpose to understand more about VOCs from such sources for better control.
2. Materials and Methods
2.1. Sampling
VOCs from six circulating cooling water systems related to refining processes (HG2X, LC4X, LC5X), chemical product transportation (HGLY), organic product manufacture (YJ) and rubber manufacture (XJ) were passively collected by negative pressure through the Summa containers, and the inner wall was polished and silanized. The sampling sites were downwind of the wastewater treatment facility and the sample collection time was about 10 min. During the sampling period, a restrictor valve was used to ensure a uniform sampling speed. In addition, water samples were also collected. Two bottles of water samples were collected at the inlet and outlet of the cooling tower of each circulating cooling water system. The water samples were stored at 3–4 °C and sent to the testing unit for POC (purgeable organic carbon) testing.
2.2. Analysis Methods
The samples in the Summa containers were analyzed according to the TO-15 method recommended by the US EPA, and the qualitative and quantitative VOCs were analyzed by a three-stage cold-trap preconcentration two-dimensional GC-MS/FID system. The VOCs samples were first passed through an automatic pre-concentrator (Entech 7100) for pretreatment. After removing water and CO2, VOCs were trapped in the third-stage cold trap. The system was heated up rapidly to vaporize the components enriched in the cold trap and VOCs were frequently brought into the GC-MS/FID system (Agilent 7890A/5975C) for separation and quantification. The carrier gas was high-purity helium (purity > 99.999%). The standard gases included TO-15 mixed custom standard gas containing 63 compounds (Scott Gases, Philadelphia, PA, USA), mixed standard gas containing 56 ozone precursors (PAMS) (Spectra gases, Branchburg, NJ, USA) and internal standard gas containing (bromochloromethane,1,4-difluorobenzene, D5-chlorobenzene,1-bromo-4-fluorobenzene) (Spectra gases, Branchburg, NJ, USA). High-concentration samples were diluted by using Summa container pressure combined with dilution injection to avoid possible contamination. More information about the analysis can be seen elsewhere [24]. Meanwhile, for the POC analysis, they were thawed at room temperature and placed in a glass dish. Then the samples were dried at low temperature and wrapped with tin foil after removing the inorganic carbon with HCl (1 mol·L−1). Finally, Perkin Elmer Elemental analyzer was employed for the POC determination.
2.3. Emission Amount Calculation
In order to further study the current VOCs emission amount in this research, the formula cited from the “Guidelines for the Investigation of VOCs Pollution Sources in the Petrochemical Industry” issued by the Ministry of Environment is shown as follows.
where Eww is the amount of volatile organic compounds emitted by the wastewater collection or treatment facility, kg·a−1; Qi is the wastewater flow of the ith collection or treatment facility, m3·h−1; EVOCsini is the fugitive volatile organic compound concentration in the influent of the ith collection or treatment facility, mg·L−1; EVOCsouti is the concentration of fugitive volatile organic compounds in the effluent of the ith collection or treatment facility, mg·L−1; t is the annual operating time of each section of the wastewater collection and treatment system, h·a−1; n is the number of wastewater collection and treatment system facilities.
2.4. Environmental Impact Assessment
The effect of VOCs on atmospheric chemical reactivity can be measured by the Ozone Formation Potential (OFP) [25,26]. The Maximum Increment Reactivity (MIR) is used to measure the ozone generation potential (OFP) of VOCs, which reflect the ability of different components of VOCs to participate in atmospheric chemical reactions [27]. The formula is as follows:
where OFPi is the ozone formation potential of species i, MIRi is the ozone generation coefficient of species i in the maximum ozone increment reaction and VOCsi is the emission concentration of species i.
OFPi = MIRi × VOCsi
In addition, Secondary Organic Aerosol (SOA) is an important component of PM2.5. In order to study the contribution of VOCs emitted from circulating water systems to secondary organic aerosols, the aerosol formation coefficient method (FAC method) was used to estimate the secondary organic aerosol generation potential of VOCs released from the circulating water system. The calculation formula is as follows:
SOAp = VOCi × FACi/(1 − FVOCr)
In the formula, SOAp is the SOA formation potential of VOCs compounds, g·m−3; FACi is the formation coefficient of SOA, %; FVOCr is the fraction of VOCs compounds participating in the reaction, %, where FVOCr and FACi are obtained from relevant literature [28,29].
3. Results and Discussion
3.1. VOCs Emission Characteristics
VOCs is mainly caused by the leakage of VOCs containing materials into the circulating water during the heat-exchange process of the heat exchanger. Studies show that the corrosion of the circulating water heat exchanger is the most important factor that brings organic materials into the circulating water. When the circulating water containing VOCs entered the cooling tower, VOCs were released into the atmosphere under the stripping action of the cooling tower fan. The volatilization of VOCs in this process could be divided into three stages, which were the volatilization of static open surface, air stripping and falling system. In most cases, during the cooling process of the cooling tower, air entered from the bottom of the cooling tower at a high speed. During the falling and cooling process of the circulating water, the organic matter in the circulating water volatilized into the air and was taken away by the air in the process of surface flash evaporation and heat evaporation. During the cooling process of circulating water, the temperature difference of circulating water needs to be reduced by about 10 degrees and VOCs in the circulating water will be directly released through the air duct, which contributed to a large percentage of total VOCs emissions. While, in this research, the production process associated with each set of circulating water is different, the VOCs emission is also quite different. The results showed that the TVOCs emission concentrations of HG2X, HGLY and YJ were relatively low, which were 660.4 µg·m−3, 377.9 µg·m−3 and 276.0 µg·m−3, respectively. The TVOCs emission concentrations of LC4X and XJ were 1254.9 µg·m−3 and 1942.7 µg·m−3, respectively, 4–7-times that of YJ. The TVOCs emission concentration of LC5X was the highest, reaching 23,009.6 µg·m−3, which was 83-times that of YJ emission concentration.
As shown in Figure 1, OVOCs were the major emission types with the largest proportion of HG2X, HGLY and YJ, accounting for 48.0%, 69.2% and 82.1%, respectively. The VOCs emission composition of XJ was the same, mainly alkenes, accounting for 89.1%. Aromatic hydrocarbons were the main compounds in LC4X, accounting for nearly 70%, followed by alkanes and oxygen-containing VOCs, accounting for 11.6% and 11.8%, respectively. The VOCs emission composition of LC5X was mainly composed of alkanes and alkenes, of which the proportions were relatively close, accounting for 50.1% and 48.4%, respectively.
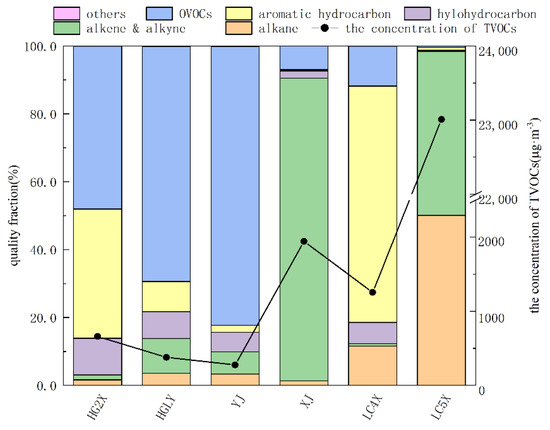
Figure 1.
VOCs components of six circulating cooling water systems.
The main components of VOCs emitted by each circulating water system were also different. The main VOCs components of HG2X were butyraldehyde, p/m−xylene, o−xylene, ethanol and 1,2−dichloromethane. Ethane accounted for 67.8% of the total emissions; ethanol was the primary pollutant of HGLY, accounting for 27.3%, followed by butyraldehyde, acetone, etc.; for YJ, the VOCs components are mainly vinyl acetate and ethanol, accounting for 28.7% and 22.2%; 1,3−butadiene and styrene were the primary pollutants of XJ and LC4X, accounting for 88.1% and 67.7%, respectively, and the remaining components accounted for less than 7.0%; isobutane, 1−Butene, trans−2−butene, butane and cis−2−butene were the main VOCs components of LC5X, accounting for 98.1% of the total emissions. The reason for the difference is mainly due to the different production processes at the front end of the circulating water connection and the different materials of the heat exchangers in each circulating water field, resulting in different VOCs components discharged from the circulating cooling water system. In addition, compared with the studies in other industries, VOCs were also different in terms of concentration and components [30,31,32,33].
As for the VOCs emission amount, the consumption of total circulating cooling water and the POC values shown in Table 1 were used for calculation according to Formula (1). Based on the investigation, the total flow of the six circulating cooling water systems was about 27,485.4 m3·h−1 and the average annual operating time of each system was 8040 h. As a result, the total amount of VOCs emitted from the circulating cooling water systems was 1237.5 tons, which accounted for about 43% of the total emission amount of the petrochemical enterprise. Though these circulating cooling water systems show high contributions, they were often ignored or underestimated [34]. In addition, according to the “Guidelines for the Investigation of VOCs Pollution Sources in the Petrochemical Industry”, if the relative difference (RD) ≥ 10%, there might be an obvious leakage from the organic materials into the circulating cooling water system. As shown in Table 1, the RD values of HG2X, YJ and LC5X all exceeded 10% and LC5X showed the highest value 45.5%, indicating there might be a serious leakage related to the refining processes, but the specific leakage units still need to be identified.

Table 1.
POC test results of circulating water system.
3.2. Environmental Impact Assessment
The ozone formation potential of VOCs in the circulating water system is shown in Figure 2. The OFP value of LC5X was the largest, which was 145,739 µg·m−3; the second is XJ, whose OFP value was 21,603 µg·m−3; the OFP values of HG2X, LC4X and HGLY ranged from 1176 to around 3175 µg·m−3; YJ showed the smallest OFP value relatively, which was just 823.3 µg·m−3.
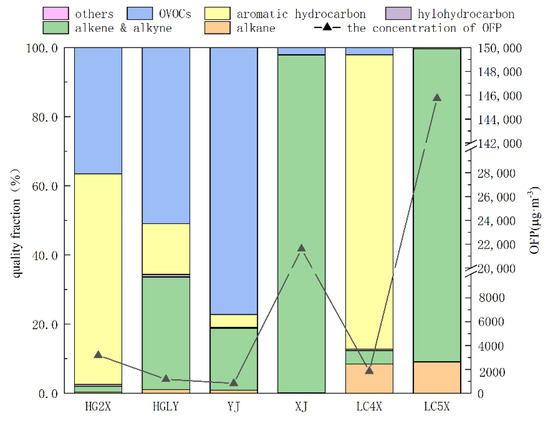
Figure 2.
OFP values of VOCs from six circulating cooling water systems.
The contribution ratio of VOCs species in the six circulating water systems is shown in Figure 2. Aromatic hydrocarbons were the compounds that contributed the most to O3 formation in HG2X, with a contribution rate exceeding 60.0%, followed by oxygen containing VOCs (36.5%), in which the high contribution rate components were mostly p/m−xylene (33.9%), butyraldehyde (26.6%) and o−xylene (18.9%). For HGLY, oxgen containing VOCs yielded the largest proportion of OFP and alkenes took the second place, with proportions of 50.9% and 32.6%, respectively. Propylene, butyraldehyde and ethanol were the components with high contribution rates of O3 formation, accounting for 27.1%, 19.2% and 12.7%, respectively. For YJ, the oxygen-containing VOCs showed that the largest proportions of OFP (30.0%), butyraldehyde (28.6%) and ethanol (10.8%) were also the key active components in YJ. For XJ and LC5X, olefins showed the largest contribution to the formation of O3, with a contribution rate more than 90.0%. The proportion of highly reactive olefins, such as 1,3−butadiene, 1−butene, trans−2−butene and cis−2−butene, was relatively high, in which 1,3−butadiene (96.7%) yielded the highest contribution rate in XJ. Meanwhile, LC5X was mainly composed of 1−butene (36.9%), trans−2−butene (33.4%) and cis−2−butene (20.0%), which was similar to HG2X.The compounds that contributed the most to O3 formation in LC4X were aromatic hydrocarbons (85.1%) followed by styrene with a proportion up to 76.0%, which was the key factor of active ingredients in LC4X. Generally, for the control of O3 formation, the main compounds to be controlled were aromatic hydrocarbons in HG2X and LC4X, oxygen-containing VOCs in HGLY and YJ and olefins in XJ and LC5X. In addition, compared with the studies in other industries, OFPs were also different, which were rather larger than others reported [30,31,32,33].
The SOA generation potential of VOCs in the circulating cooling water system is shown in Figure 3. The results showed that the SOA generation potential of HG2X was the highest, followed by LC5X, HGLY, LC4X, XJ and YJ. The SOA values of LC5X, HGLY, LC4X, XJ and YJ were 229.1, 216.9, 179.4, 42.9 and 31.0 µg·m−3, respectively. The SOA value of HG2X was 1467.9 µg·m−3, which was about 6~47 times of the other values in the remaining five circulating cooling water systems. Aromatic hydrocarbons were the components with the largest contribution rate to SOA generation in the six circulating cooling water systems, and the contribution rate to SOA in HG2X, HGLY, YJ, XJ and LC5X all exceeded 90.0%, except LC4X, which was just about 88%. For HG2X, the VOCs species with a higher contribution rate to SOA generation were mainly o−xylene (37.1%), p/m−xylene (36.8%) and ethylbenzene (13.2%). For HGLY, cumene (25.7%), −,3,5−trimethyl −benzene (21.1%) and m−ethyltoluene (20.3%) were the major species with higher contri−bution rates. The species with higher contribution rates to SOA generation were mainly m-ethyl toluene and 1,3,5−trimethylbenzene in YJ and XJ, with contribution rates higher than 25.0%.P/m−xylene (11.9~13.6%) and o−xylene (11.8~12.2%) were the species with higher contribution rates in LC4X and LC5X, and 1,2,3−trimethylbenzene (15.4%) was the species with the highest contribution rate in LC4X. Generally, for SOA generation control, the main compounds that need to be controlled in the six circulating water systems were mainly aromatic hydrocarbons.
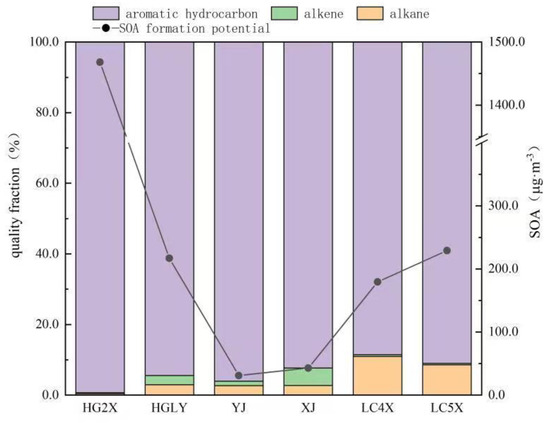
Figure 3.
SOA values of VOCs from six circulating cooling water systems.
3.3. Control Measures for VOCs
As mentioned above, VOCs in the circulating cooling water system mainly originated from the leakage of organic materials caused by corrosion of relevant heat exchangers and entered into the circulating water system, released into the air in the cooling tower unit. According to the emission amount calculation, VOCs emission from the circulating water cooling tower cannot be underestimated, and control measures must be taken for better control. Generally, the heat exchanger unit should be overhauled regularly to reduce corrosion, so as to reduce organic materials entering the circulating cooling water system. This approach is the most economical and feasible. In addition, POC detection can also be carried out regularly at the water inlet and outlet of the cooling tower to determine whether the RD value is greater than 10% as the basis for regular leak detection. Meanwhile, most of the existing petrochemical enterprises’ circulating cooling water systems are equipped with open cooling towers, which are characterized by economic operation, but the disadvantage is that direct contact of circulating water with air would lead to the release of VOCs into the atmosphere. Therefore, changing the open cooling tower to a closed cooling tower could also be a possible way, in which the direct contact of circulating water with air is banned and the escape of VOCs is prevented. However, it should be pointed out that although VOCs escape is eliminated, the leakage of VOCs from organic materials to circulating cooling water systems cannot be prevented. As far as this study is concerned, HG2X, YJ and LC5X with serious leakage should be checked one by one in the short term to find specific leakage units and repair them. In the medium term, POC detection of these six circulating water systems should be carried out regularly to find potential leakage units in time and repair them. In the long term, the replacement of open cooling mode to closed cooling mode could be considered, and POC detection could be carried out regularly at the inlet and outlet of the cooling tower to detect and repair the leakage in time for the purpose of completely eliminating VOCs from the circulating cooling water system.
4. Conclusions
In this research, VOCs from circulating cooling water systems in a typical petrochemical enterprise were firstly studied. The results indicated that VOCs varied in a rather wide range in terms of concentration and components. Oxygen containing VOCs, alkenes and alkanes were dominated, which might be related to the detailed refining, transportation, chemical and organic product manufacture processes. During the cooling process of circulating water, VOCs in the circulating water will be directly released through the air duct, which contributed to a large percentage of total VOCs emission. In addition, combined with the POC test results, it was calculated that the VOCs emissions of the circulating water system from the enterprise were 1237.5 tons, contributing to 40% of total emissions, which should not be underestimated. As for their impact on the environment, high OFP and SOAp values suggested a significant contribution to O3 and SOA formation. As a result, the replacement of the open cooling tower to be a closed cooling tower combined with regular POC detection will be an efficient way for VOCs control from such sources.
Author Contributions
Conceptualization, L.F. and H.W.; methodology, H.W.; formal analysis, R.H.; investigation, R.H. and H.W.; data curation, X.X. and G.L.; writing—original draft preparation, L.F.; writing—review and editing, L.F. and H.W.; visualization, X.X. and G.L.; supervision, H.W.; project administration, H.W.; funding acquisition, H.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (No. 2019YFC1806105) and Municipal Research Institute of Environmental Protection (NO. Y2020-011).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Q.; Su, G.; Li, C.; Liu, P.; Zhao, X.; Zhang, C.; Sun, X.; Mu, Y.; Wu, M.; Wang, Q.; et al. An investigation into the role of VOCs in SOA and ozone production in Beijing, China. Sci. Total Environ. 2020, 720, 137536. [Google Scholar] [CrossRef]
- Li, K.; Jacob, D.J.; Liao, H.; Shen, L.; Zhang, Q.; Bates, K.H. Anthropogenic drivers of 2013~2017 trends in summer surface ozone in China. Proc. Natl. Acad. Sci. USA 2019, 116, 422–427. [Google Scholar] [CrossRef]
- Yang, L.F.; Luo, H.H.; Yuan, Z.B.; Zheng, J.Y.; Huang, Z.J.; Li, C.; Lin, X.H.; Louie, P.K.K.; Chen, D.H.; Bian, Y.H. Quantitative impacts of meteorology and precursor emission changes on the long-term trend of ambient ozone over the Pearl River Delta, China, and implications for ozone control strategy. Atmos. Chem. Phys. 2019, 19, 12901–12916. [Google Scholar] [CrossRef]
- Liu, Y.H.; Wang, H.L.; Jing, S.G.; Peng, Y.R.; Gao, Y.Q.; Yan, R.S.; Wang, Q.; Lou, S.R.; Cheng, T.T.; Huang, C. Strong regional transport of volatile organic compounds (VOCs) during wintertime in Shanghai megacity of China. Atmos. Environ. 2021, 244, 117940. [Google Scholar] [CrossRef]
- Li, Y.D.; Yin, S.S.; Yu, S.J.; Yuan, M.H.; Dong, Z.; Zhang, D.; Yang, L.M.; Zhang, R.Q. Characteristics, source apportionment and health risks of ambient VOCs during high ozone period at an urban site in central plain, China. Chemosphere 2020, 250, 126283. [Google Scholar] [CrossRef]
- Swaen, G.M.H.; Scheffers, T.; Cock, J.D.; Slangen, J.; Drooge, H. Leukemia risk in caprolactam workers exposed to benzene. Ann. Epidemiol. 2005, 15, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sekizawa, J.; Ohtawa, H.; Yamamoto, H.; Okada, Y.; Nakano, T.; Hirai, H.; Yamamoto, S.; Yasuno, K. Evaluation of human health risks from exposures to four air pollutants in the indoor and the outdoor environments in tokushima, and communication of the outcomes to the local people. J. Risk Res. 2007, 10, 841–851. [Google Scholar] [CrossRef]
- State Council of the PRC. Three-Year Action Plan to Fight Air Pollution. 2018. Available online: http://www.gov.cn/zhengce/content/2018-07/03/content_5303158.htm (accessed on 14 November 2022).
- Ministry of Ecology and Environment of the People’s Republic of China. Comprehensive Treatment Plan of Volatile Organic Compounds in Key Industries. 2018. Available online: http://www.mee.gov.cn/zhengce/zhengceku/2019-11/25/content_5455387.html (accessed on 14 November 2022).
- Ministry of Ecology and Environment of the People’s Republic of China. Governance Solution of Volatile Organic Compounds in 2020. 2020. Available online: http://www.mee.gov.cn/xxgk2018/xxgk/xxgk03/202006/t20200624_785827.html (accessed on 14 November 2022).
- Simayi, M.; Hao, Y.F.; Li, J.; Wu, R.G.; Shi, Y.Q.; Xi, Z.Y.; Zhou, Y.; Xie, S.D. Establishment of county-level emission inventory for industrial NMVOCs in China and spatialtemporalcharacteristics for 2010–2016. Atmos. Environ. 2019, 211, 194–203. [Google Scholar] [CrossRef]
- Han, D.M.; Gao, S.; Fu, Q.Y.; Cheng, J.P.; Chen, X.J.; Xu, H.; Liang, S.; Zhou, Y.; Ma, Y.N. Do volatile organic compounds (VOCs) emitted from petrochemical industries affect regional PM2.5. Atmos. Res. 2018, 209, 123–130. [Google Scholar] [CrossRef]
- Lyu, X.P.; Chen, N.; Guo, H.; Zhang, W.H.; Wang, N.; Wang, Y.; Liu, M. Ambient volatile organic compounds and their effect on ozone production in Wuhan, central China. Sci. Total Environ. 2016, 54, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.R.; Xie, S.D. Spatial distribution of ozone formation in Chinaderived from emissions of speciated volatile organic compounds. Environ. Sci. Technol. 2017, 51, 2574–2583. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Li, W.Z.; Wei, W.; Li, G.H.; Wang, H.Y.; Jiang, C.Z.; Zhou, Y. Refinery VOCs Seasonal Composition and Analysis of Ozone Formation Potential. J. Beijing Univ. Technol. 2013, 39, 438–444, 465. [Google Scholar]
- Hu, T.P.; Li, G.; Mao, Y.; Zheng, H.; Qin, S.B.; Min, Y.; Zhang, J.Q.; Xing, X.L.; Qi, S.H. Characteristics and Source Apportionment of VOCs of a Petrochemical Industrial Park During Autumn in China. Environ. Sci. 2018, 39, 517–524. [Google Scholar]
- Mao, Y.; Li, G.; Hu, T.P.; Zheng, H.; An, Y.W.; Min, Y.; Xing, X.L.; Qi, S.H. Characteristics of VOCs Pollution in the Winter Atmosphere of a Typical Petrochemical Industry Park. Environ. Sci. 2018, 39, 525–532. [Google Scholar]
- Feng, Y.X.; Jia, R.Z.; Xiao, A.S.; Tian, S.B.; Shi, N.; Zhu, L. Source profiles and tracing of volatile organic compounds in refineries. China Pet. Process. Pe. 2020, 51, 92–96. [Google Scholar]
- Hou, X.H.; Luan, J.Y.; Zhang, J.H.; Xu, S.H.; Sun, J. Composition spectrum of VOCs in circulating water from petrochemical enterprises. Environ. Protec. Chem. Ind. 2019, 39, 349–353. [Google Scholar]
- Han, X.; Ma, S.T.; Wan, W.; Song, C.X.; Liu, Y.R. Progress in the study of volatile organic compound composition of petrochemical industry in China. China Pet. Process. Pe. 2022, 4, 9–16. [Google Scholar]
- Wei, W.; Wang, S.X.; Chatani, S.; Klimont, Z.; Cofala, J.; Hao, J.M. Emission and speciation of non-methane volatile organic compounds from anthropogenic sources in China. Atmos. Environ. 2008, 42, 4976–4988. [Google Scholar] [CrossRef]
- Liu, Y.; Shao, M.; Fu, L.L.; Lu, S.H.; Zeng, L.M.; Tang, D.G. Source profiles of volatile organic compounds (VOCs) measured in China: Part I. Atmos. Environ. 2008, 42, 6247–6260. [Google Scholar] [CrossRef]
- Zheng, J.; Shao, M.; Che, W.W.; Zhang, L.J.; Zhong, L.J.; Zhang, Y.H.; Streets, D. Speciated VOC emission inventory and spatial patterns of ozone formation potential in the Pearl River Delta, China. Envirom. Sci. Technol. 2009, 43, 8580–8586. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Hao, R.; Fang, L.; Nie, L.; Zhang, Z.S.; Hao, Z.P. Study on emissions of volatile organic compounds from a typical coking plant in China. Sci. Total Environ. 2021, 752, 141927. [Google Scholar] [CrossRef] [PubMed]
- Carter, W.P.L. Development of ozone reactivity scales for volatileorganic compounds. J. Air Waste Manag. 1994, 44, 881–889. [Google Scholar] [CrossRef]
- Carter, W.P.L. Developmentof the SAPRC-07 chemical mechanism. Atmos. Environ. 2010, 44, 5324–5335. [Google Scholar] [CrossRef]
- Venecek, M.A.; Carter, W.P.L.; Kleeman, M.J. Updating the SAPRC maximum incremental reactivity (MIR) scale for the united states from 1988 to 2010. J. Air Waste Manag. 2018, 68, 1301–1316. [Google Scholar] [CrossRef]
- Grosjean, D. In situ organic aerosol formation during a smog episode estimated production and chemical functionality. Atmos. Environ. 1992, 26, 953–963. [Google Scholar] [CrossRef]
- Grosjean, D.; Seinfeld, J.H. Parameterization of the formation potential of secondary organic aerosols. Atmos. Environ. 1989, 23, 1733–1747. [Google Scholar] [CrossRef]
- Wang, H.L.; Nie, L.; Li, J.; Wang, Y.F.; Wang, G.; Wang, J.H.; Hao, Z.P. Characterization and assessment of volatile organic compounds emissions from typical industries. Chin. Sci. Bull. 2013, 58, 724–730. [Google Scholar] [CrossRef]
- Wang, H.L.; Xue, S.; Hao, R.; Fang, L.; Nie, L. Emission characteristics and ozone formation potential analysis of VOCs from typical metal packaging plant. Atmosphere 2022, 13, 57. [Google Scholar] [CrossRef]
- Fang, L.; Liu, J.Y.; Nie, L.; He, L.J.; Wang, H.L. VOCs emission characteristics and ozone impact analysis of typical automobile repair enterpirses in beijign. Environ. Eng. 2020, 38, 146–155. [Google Scholar]
- Hao, R.; Xue, S.; Sun, H.; Yang, T.; Wang, H.L. Emission characteristics and environment impact of VOCs from typical FRP manufacture industry. Atmosphere 2022, 13, 1274. [Google Scholar] [CrossRef]
- Liu, T. Research on VOCs Accounting and Traceability of Circulating Water System in Petrochemical Enterprises. Master’s Thesis, China University of Petroleum (East China), Qingdao, China, 2017. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).