Abstract
Circadian rhythms are 24-h oscillations driven by a hypothalamic master oscillator that entrains peripheral clocks in almost all cells, tissues and organs. Circadian misalignment, triggered by industrialization and modern lifestyles, has been linked to several pathological conditions, with possible impairment of the quality or even the very existence of life. Living organisms are continuously exposed to air pollutants, and among them, ozone or particulate matters (PMs) are considered to be among the most toxic to human health. In particular, exposure to environmental stressors may result not only in pulmonary and cardiovascular diseases, but, as it has been demonstrated in the last two decades, the skin can also be affected by pollution. In this context, we hypothesize that chronodistruption can exacerbate cell vulnerability to exogenous damaging agents, and we suggest a possible common mechanism of action in deregulation of the homeostasis of the pulmonary, cardiovascular and cutaneous tissues and in its involvement in the development of pathological conditions.
1. Introduction
The circadian machinery governs many human physiological functions that exhibit rhythmic variations over the course of the day, with the adaptive purpose of providing the optimum temporal organization of physiological processes in relation to the environment [1,2,3]. These changes are regulated by a central oscillator located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus [4]. In humans, the central circadian pacemaker coordinates autonomous peripheral oscillators located in almost all tissues and organs, including the lung, heart and skin [5,6,7].
At a cellular level, the molecular circadian system consists of a transcriptional and translational feedback loop based on the CLOCK (Circadian Locomotor Output Cycles Kaput):BMAL (Brain and Muscle Arnt-Like protein 1) heterodimer, which activates the transcription of period (PER) and cryptochrome (CRY). In turn, the PER and CRY heterodimeric complex inhibits its own transcription by suppressing the CLOCK:BMAL transcriptional function [8]. The CLOCK:BMAL complex regulates Rev-Erb and Ror transcriptions, which modulate the timing and amplitude of Bma1 expression, forming a second stabilizing feedback loop [9]. In addition to the core clock components, the molecular oscillator also dictates the timing of tissues and cell-specific genes, referred to as clock-controlled genes (CCGs) [10]. These specific tissue and cell clock-controlled transcriptomes ensure the temporal regulation of physiological processes.
Circadian clocks have to be plastic, because most organisms, including humans, face different adaptive challenges every day. They have to cope with cyclical variations of the physical properties of their environment, such as light intensity and temperature. A synchronized clock improves cellular homeostasis; in fact, evidence suggests that circadian arrhythmicity has detrimental consequences on human health [11,12].
Loss of synchronization between biological clock and external stimuli can be induced by the alteration of metabolic pathways as occurs in many diseases, such as cancers and age-dependent disorders [13,14]. Irregular schedules of life activity, such as work schedules that include long-term night shifts, rotating shift work, late-night activity or jet lag [15,16,17] induced by transcontinental travel, are an important disruptor of functional circadian synchronization. These changes in the day/night lifestyle are a consequence of the increase in modernization and industrialization that is characterized by world economic growth.
Starting from 1700, industrialization heavily affected the environment, with harmful effects on air quality. Global air quality results from the concentration of gaseous pollutants and particulate matters. As defined by Jacobson, “air pollution occurs when gases or aerosol particles emitted anthropogenically, build up in concentrations sufficiently high to cause direct or indirect damage to plants, animals, other lifeforms, ecosystems, structures, or works of art” [18].
The effects of short-term and chronic exposure to airborne stressors due to intensified industrialization and urbanization are well documented [19,20,21].
Even though the complicated mechanisms of the harmful health effects of airborne pollution are not fully clear, many epidemiological and clinical studies have demonstrated a correlation between exposure to pollutants and pathological conditions in tissues, such as lung dysfunction, cardiovascular diseases, skin conditions and, more generally, detrimental multi-organ effects [22,23,24,25]. Although pollution is defined by a single word, its meaning includes a wide range of compounds that have been recently classified by the EPA into six different groups: particulate matters (PMs), nitrogen oxides (NO), sulphur oxide (SO), carbon monoxide (CO), heavy metals and tropospheric (ground-level) ozone (O3) [26]. Among them, PMs and O3 have been considered among the most relevant to our modern time, as their tropospheric concentration is in constant increase. PMs emitted from vehicles, construction sites, industries and burning of fossil fuels include different inhalable suspended particles, which, based on their size, are divided into PM10 (with an aerodynamic diameter < 10 μm), PM2.5 (with an aerodynamic diameter < 2.5 μm) and UFP (ultrafine or nanoparticles, ≤0.1 µm) [27]. In a recent work conducted in 652 different cities of the six different continents, a significant correlation between PMs exposure and all-cause mortality has been demonstrated in countries with a high annual mean temperature and a limited management of the environment [28].
As mentioned above, another relevant air pollution compound is the tropospheric O3, defined as a secondary pollutant, since it is formed through chemical reactions from precursors (volatile organic compounds, nitrogen oxides, carbon monoxide) in the presence of sunlight [29]. Over the last ten years, the World Health Organization (WHO) has reviewed many studies on the effects of acute O3 exposure on human health [30], ranging from the respiratory to cardiovascular [31] systems, including the central nervous system [32]. In particular, it is well known that acute O3 exposure affects pulmonary function, inducing throat irritation and consequently, coughing, and worsening the health conditions of asthmatic people [33]. Moreover, a clear deterioration of the olfactory function as a consequence of acute exposure to 0.2 ppm O3 in healthy subjects has been recently documented [34]. Concerning the chronic effects of O3 [35], it has been demonstrated that long-term exposure to O3 is significantly associated with increased mortality for cardiovascular or ischemic heart disease, respiratory pathologies or chronic obstructive pulmonary disease.
Since PMs and O3 exposure mainly occur by inhalation, the deleterious effects of both environmental stressors have been widely studied, especially on the pulmonary and cardiovascular systems. However, researchers have recently focused their attention also on the impact of airborne pollution on skin conditions not limited to premature aging, but also to proinflammatory diseases such as dermatitis, eczema, psoriasis and rash [36,37,38].
Despite the different mechanisms underlying air pollutant-induced diseases, oxidative stress has been recognized as a unifying feature of their toxic effects.
Based on this evidence, this review intends to focus on the synergic connection of circadian clock desynchronization and environmental pollutant toxicity. In this regard, this paper summarizes a panel of data concerning the deleterious effects of both exposure to environmental stressors, and chronodistruption on three body regions which are considered the principal targets of air pollution: the pulmonary, cardiovascular and cutaneous systems. Finally, we identify the antioxidant defense system of the tissue as a possible link between these aspects. Until now, to the best of our knowledge, research has focused on how environmental pollution affects circadian rhythm, and how circadian rhythm affects the toxicity of environmental stressors. Here, we explore the topic using a different perspective, since we hypothesize that circadian deregulation exacerbates the detrimental effects of airborne pollution as a result of less efficient defensive redox machinery.
A comprehensive literature review identified 141 papers published (between 2000 and 2021, except [29,39,40]) in peer-reviewed scientific journals, books, or available on scientific internet sites. This work was centered on a systematic search in the PubMed and Google Scholar databases by the following specific keywords or combinations of them: circadian clock; pollution; oxidative stress; circadian clock deregulation; oxidative stress and cardiovascular system; oxidative stress and pulmonary system; oxidative stress and skin; oxidative stress and circadian clock; pollution and circadian clock; antioxidant system and pollution; circadian system and antioxidant defenses.
2. Detrimental Health Effects of Circadian Desynchronization in the Pulmonary System
Since the concentration and composition of air pollution show strong correlations to the time of the day, with the ozone peak later in the noon hours [41,42], and also diurnal trends in the concentration and composition of PMs [41,43], a defense system able to anticipate the diurnal variation of environmental pollutants provides fitness advantages to organisms. In this light, the circadian system plays a critical role in lung tissue defense against outdoor stressors [39,44,45,46].
Air pollutants, allergens, pollens, cigarette smoke, as well as respiratory viral and bacterial infections can lead to the onset of acute diseases, and in parallel, to the worsening of chronic pathologies like COPD (Chronic Obstructive Pulmonary Disease) and asthma [40,47,48,49]. In physiological conditions, the circadian system modulates several lung functions, such as airway caliber and resistance, respiratory symptoms, mucus secretion and immune-inflammatory responses; however, circadian disruption has been associated with several chronic and acute airway conditions [50,51]. Moreover, circadian desynchronization can boost lung tumorigenesis, as demonstrated in a recent work by Papagiannakopoulos et al. [52] where the tumor-suppressive role of the core clock genes Per2 and Bmal1 was established. Clock dysregulation is also responsible for alterations in various cellular and molecular lung functions during the pathogenesis of chronic airway disease. As mentioned above, a link between the circadian timing system and the worsening of lung function, observed in both COPD and asthma, has been widely demonstrated [5,46,50,53,54,55]. Particularly, a growing number of studies show the role of clock perturbation in pulmonary physiology and pathology in response to pollutants. In rodents, alteration of pulmonary clock gene expression induced by air pollutants and proinflammatory agents like LPS and TNF-α has also been shown [56].
The interconnection of chronic inflammatory lung diseases, circadian function and oxidative stress is established by the link between the molecular clock and NF-κB and Nrf2 pathways, particularly in the case of pulmonary responses to environmental pollutants, viruses and bacteria [5]. The circadian system may act under normal circumstances as a modulator of lung inflammo-immune responses that, when challenged (as in response to a pollutant), can also act to increase the amplitude and extent of proinflammatory impact on pulmonary function [5].
Understanding the mechanism whereby the arrhythmic circadian clock influences lung pathophysiological functions could lead to new and effective ways for treatment based on chronopharmacological agents. This knowledge highlights the need for the development of novel strategies that act on the circadian molecular clock for the treatment of pulmonary disorders, and that also account for the rhythmic variation of lung function across the 24-h day.
3. Detrimental Health Effects of Circadian Desynchronization on the Cardiovascular System
In humans, most cardiovascular (CV) functions exhibit circadian variations, including heartbeat dynamics [57], cardiac vagal modulation [58], platelet aggregability [59] and plasminogen activator inhibitor 1 (PAI-1) [60,61].
These rhythmic and predictable-in-time oscillations of cardiovascular functions are guaranteed by a fine-tuned regulation of the circadian system. Thus, circadian variation in the physiology of the cardiovascular system leads to daily rhythmicity in the susceptibility and occurrence of pathological events. Indeed, epidemiological studies report a morning peak in cardiovascular failures, largely attributable to the fact that the circadian system influences CV risk factors [62].
As synchronized circadian rhythms contribute to CV well-being [63], circadian misalignment may influence CV risk. Chronodisruption leads to physiological alterations that are linked with CV disease, such as an increase in both systolic and diastolic blood pressure, alteration of daily rhythmicity of heart rate, disruptions to the rhythmic urinary epinephrine and norepinephrine excretion rates, and increased serum levels of inflammatory markers, including interleukin-6, high-sensitivity C-reactive protein, resistin and TNFα [62]. These alterations have negative consequences on hemodynamic function [64,65,66], arterial stiffness [67] and thrombogenic pathways [66]. Moreover, circadian desynchronization has directly been related to cardiomyopathy [65,68,69], coronary artery disease [70] and stroke [71]. An association between shift work and CV disease has been proven [72], further supporting the hypothesis that circadian misalignment (typical in night-shift workers) negatively alters CV risk factors.
Recent investigations focus on the role of circadian machinery in the modulation of oxidative stress pathways, which are often involved in heart disease outcomes. In particular, Khaper et al. [73] suggested a key novel relationship between the circadian system and oxidative stress after myocardial infarction (MI). It is well established that circadian rhythms regulate healing after MI, and circadian desynchronization contributes to adverse cardiac remodeling post-MI [74]. Moreover, melatonin seems to have a cardioprotective role beside its free radical scavenging properties [75].
This evidence supports the hypothesis that the circadian clock plays an important role in regulating oxidative stress pathways that can potentially contribute to cardioprotection. Furthermore, considering the ability of pollution to affect tissue redox homeostasis, it is possible that pollution affects cardiovascular conditions also via the deregulation of the circadian clock.
4. Detrimental Health Effects of Circadian Desynchronization on Skin
The largest organ of the human body, the skin, is the first barrier of protection against external stressors. Different skin functions have been shown to follow a strong circadian rhythm, optimized for more protection during daylight and for repair during night-time [76]. Synchronization to environmental time cues is crucial for optimal benefits to the skin. A recent work emphasized the importance of the circadian clock in regulating skin functions [77]: predictable daily periodicity has been reported in humans in skin cell proliferation rates, cellular senescence, epidermal barrier function, hydration and transepidermal water loss, capillary blood flow, sebum production, temperature, surface pH and the appearance of wrinkles [76,78,79]. Regulated circadian activities allow the skin to adapt its functions to variations in environmental conditions.
The impact of a deregulated circadian clock on the development or, at least, the progression of several pathologies has been shown [11,12]. Studies have suggested the role of clock synchronization in protecting the skin from exogenous stressors [7]. In particular, several proinflammatory cutaneous conditions (such as psoriasis, eczema, cutaneous rashes, and atopic dermatitis) have been attributed to environmental factors, such as O3 [80,81]. In a recent study, our group has suggested that a synchronized circadian clock not only facilitates the protective role of the defensive pathway in terms of a faster and more efficient antioxidant response against environmental insults, but also moderates the cellular damage resulting from chronic inflammation [82].
Moreover, the data showed a link between the circadian clock and skin aging: arrhythmic Bmal1 mutant mice exhibit accelerated skin aging, probably related to oxidative stress that generated an unbalanced redox homeostasis [83,84]. An epidemiological study by Li et al. [85] on the association of shift work with increased risk of psoriasis suggests the possibility that lifestyle changes and consequent circadian disruption could be involved in many skin diseases.
5. Effects of Pollution on the Respiratory Tract
Several experimental data correlate both acute and long-term exposure to air pollutants with changes in lung function. Environmental stressors are associated worldwide with hospitalization for respiratory disease and, in particular, close links with particulate matter, PM2.5, PM10, and O3 have been demonstrated [86,87]. During pulmonary ventilation, these pollutants could induce an increase in respiratory dysfunction, such as asthma, COPD and lung cancer [88]. In more detail, older people, infants or subjects with comorbidities [89,90,91] seem to be more vulnerable to the harmful effects of airborne pollution.
The correlation between COPD and increased levels of PM10 and PM2.5 in the air is well documented. In the last decade, Gan et al. demonstrated that exposure to high levels of air pollutants resulted in a noticeable increase in COPD hospitalizations and mortality in a Canadian population sample [92]. These data confirmed previous studies conducted in several US cities [86,93]. There is also evidence concerning the European population; in particular, different studies associate respiratory infections during early childhood to particulate matter concentration [90,94,95,96].
The noxious action of tropospheric O3, due to its low solubility in water, is carried out in the inner layer of the respiratory tract, where it reacts with epithelial lining fluid which protects the alveolar structures from external stressors and pathogens [97,98].
Just in the last year, several human studies have recognized significant associations between both short-term and chronic exposure to O3 and hospitalizations for decrease in lung function due to increased airway inflammation, respiratory illness, aggravation of asthma and even for COPD [99,100,101], but the data concerning the susceptibility of populations are still unsatisfying.
In a meta-analysis study conducted by Bell and colleagues [102], a direct association between short-term O3 exposure and age has been suggested, and elderly populations seem to be more sensitive to this specific airborne stressor. On the other hand, it was demonstrated that children are more vulnerable to O3 and, especially among children who live in industrial zones, O3 exposure is significantly associated with wheezing and allergic rhinitis [103,104].
Concerning the mechanism by which external pollutants trigger or exacerbate pulmonary system diseases, in vitro and in vivo evidence suggests that they have the intrinsic ability to induce oxidative stress [97,105]. In particular, the toxic effects of pollutants result both from increased exposure to oxidant components (i.e., ROS), and from impairment of the antioxidant defense system, which initiates a number of redox-sensitive signaling pathways that play a key role in pulmonary inflammatory response [106].
6. Effects of Pollution on the Cardiovascular System
Due to the close connection between the respiratory and cardiovascular systems, airborne pollutants are not only associated with altered lung functions, but also with cardiovascular morbidity. Evidence has been accumulated indicating that environmental stressors negatively affected cardiovascular function, in terms of increases in heart failure events, arrhythmias, myocardial infarction, stroke and reduced survival after stroke [107].
Some evidence indicates that particulate matters, in particular PM2.5, unsafely affect the cardiovascular system, inducing events occurring within a short or long time after exposure [108]. Exposure to PMs can induce acute cardiovascular events, such as heart arrhythmias, stroke or myocardial infarction, [109] while long-term exposure amplifies the risk of heart failure [110].
Concerning O3, there is a large amount of epidemiological evidence on the burden of short-term exposure on cardiovascular functionality and associated mortality. In a very recent work conducted on 67 volunteers, it was demonstrated that O3 exposure altered blood pressure and heart rate, or more generally, affected autonomic function. In the same study, the authors established that short-term exposure to O3 also induces systemic inflammation and oxidative stress, and alters hemostasis in terms of activation of fibrinolysis [111].
With reference to the chronic effects of O3 exposure and cardiovascular outcomes, the evidence is instead limited and controversial, although a substantial alteration in circulating biomarkers of oxidative stress, inflammation and coagulation has been demonstrated [112].
In a recent prospective US cohort study, with an extensive follow-up period of 17 years [35], long-term exposure to O3 was significantly associated with increased cardiovascular disease mortality or ischemic heart disease.
As mentioned for the respiratory tract, there is increasing evidence that indicates reactive oxygen species (ROS)-dependent pathways as common threads in the mechanism of action of external stressors on cardiovascular tissues [113,114,115,116].
Davel and colleagues [117] demonstrated that in adult Wistar rats, PM2.5 induced vascular oxidative stress and augmented the expression level of two pivotal antioxidant enzymes in vascular tissue: Cu/Zn- and Mn-superoxide dismutase. The authors suggested that the enhanced expression of these two isoforms of superoxide dismutase represents a protective cellular mechanism induced by PM2.5 exposure.
In an interesting study by Kodavanti and colleagues using a murine model, it has been demonstrated that episodic exposure to external stressors induced vasoconstriction in the aorta associated with a marked mRNA upregulation of biomarkers of oxidative stress [118]. They also observed that cardiac mitochondrial phospholipid fatty acids diminished after exposure to injurious particles, resulting in oxidative modifications.
7. Effects of Pollution on Skin
Besides the pulmonary and cardiovascular systems, the skin, due to its critical location, is one of the most susceptible tissues to the deleterious effect of airborne pollutants [119,120].
External pollutants can clearly affect skin homeostasis by acting in different ways. Concerning O3, as discussed by Pecorelli et al. [37], it cannot penetrate cutaneous tissue, but its toxicity is a consequence of the interaction with the lipids present in stratum corneum (the outermost layer of the epidermis), leading to the generation of bioactive molecules (i.e., lipid peroxidation) that can then affect the deeper layer of the skin [121,122,123]. Conversely, PMs can move (although still under debate) through the different layers of the skin, inducing nuclear translocation of the transcription factor NFκB, increasing levels of HNE and promoting DNA damage [124,125,126].
Besides these differences, we can identify a common denominator in their mechanism of action, which is represented by an altered oxidative homeostasis and peroxidation that can lead to inflammation and exacerbation of pre-existing cutaneous diseases and/or premature skin aging. Although the skin is prepared for this injury with solid and valid antioxidant systems (vitamin C, lipoic acid, vitamin E, carotenoids, or enzymatic antioxidants, such as catalase, dismutase, superoxide dismutase, glutathione peroxidase), their efficiency decreases with age, making the skin more susceptible to damage induced by exposure to airborne pollutants [127].
As a consequence, outdoor stressors are strictly associated with several skin conditions, including cancer [128]. One of the first epidemiological studies conducted by Vierkotter and colleagues in the SALIA (Study on the influence of Air pollution on Lung function, Inflammation and Ageing) cohort demonstrated that the danger of forming age spots or coarse wrinkles increased, in particular, by exposure to high levels of background traffic-related PMs. Moreover, the authors concluded that skin ageing is more evident in women [129].
The link between PMs and inflammatory skin diseases was also described, although the impact of external pollution on the development of atopic skin pathologies is controversial [130]. It is now clear that exposure to air pollutants exacerbates the manifestations of inflammatory skin pathologies, such as psoriasis, dermatitis or acne in both childhood and adults [119].
A large amount of evidence demonstrated that the harmful effect of O3 exposure on skin tissue correlated with increasing emergency-room visits for eczema, urticaria, contact dermatitis, nonspecific eruption and other skin disorders [80].
Finally, the mechanism involved in the correlation between O3 exposure and inflammatory cutaneous disease has been described recently [131]: O3 can stimulate the activation of the well-known inflammasome in a redox-dependent manner, proposing a conceivable role of this pathway in O3-induced inflammatory skin diseases [132].
To date, a number of biological mechanisms and intracellular pathways could be associated with adverse human outcomes as a result of exposure to airborne pollutants; indeed, great progress has been made in establishing that exposure to external stressors induces oxidative stress and inflammation.
8. Conclusions
Disturbances to the circadian rhythm and worsening of air quality are an integral part of environmental responses to technological progress and industrialization [133,134]. Despite this, the majority of environmental studies have not taken into account the impact of exposure to air pollution on human health, in a condition where the rhythmic circadian clock is dysregulated—today a very frequent situation as a result of modern lifestyles.
At present, studies on pollution-induced tissue damage correlated to circadian biology focus on two different aspects: how exposure to air pollutants disturbs the molecular clock [5,135], and how circadian rhythms influence susceptibility to external stressors throughout the day [136].
To fill the gap in knowledge on this topic, our review investigates whether circadian desynchronization increases susceptibility to ozone and particulate matter, two of the principal air pollutants affecting pulmonary, cardiovascular and cutaneous systems, which are among the principal targets of pollution-induced damage.
We believe that the intersection of the circadian clock and environmental pollution susceptibility could be traced back to the efficiency of the antioxidant system in free radical detoxification processes.
It has been shown that O3 and PMs, even if they are two different injurious agents, can trigger the formation of reactive oxygen species (ROS), leading to deleterious effects on different human tissues [23,27,31]. In particular, overexposure to airborne pollutants causes a cellular redox imbalance between ROS generation and nonenzymatic and enzymatic antioxidant systems, with a consequent disruption of tissue homeostasis [137].
Desynchronization of the circadian clock has previously been associated with augmented sensitivity to air pollution exposure in the cardiovascular tissue of mice. In particular, the sensitivity to inhaled nanoparticles in Bmal1−/− mice increased, compared to Bmal1+/+ mice. After exposure to air pollutants, the antioxidant cellular defense system is less efficient in clock-deficient mice [138].
Moreover, the role of clock synchronization in protecting human tissues from environmental injury has been properly described. In particular, it has been shown that in clock-entrained human keratinocytes exposed to oxidative stressors, the activation of NRF2, the key master of the antioxidant pathway, is faster and more efficient compared to arrhythmic cells [7,82].
Starting from this evidence, we speculate that chronodistruption triggered by industrialization and modern lifestyles can exacerbate cell vulnerability to exogenous damaging agents, reducing the performance of the antioxidant system, and contributing to a worsened health state.
To conclude, the link between balanced redox homeostasis and the deleterious effects of airborne pollutants on the circadian system underlines the importance of a sustainable development and proper management of air quality standards to ensure healthy lives and to promote human well-being (Figure 1).
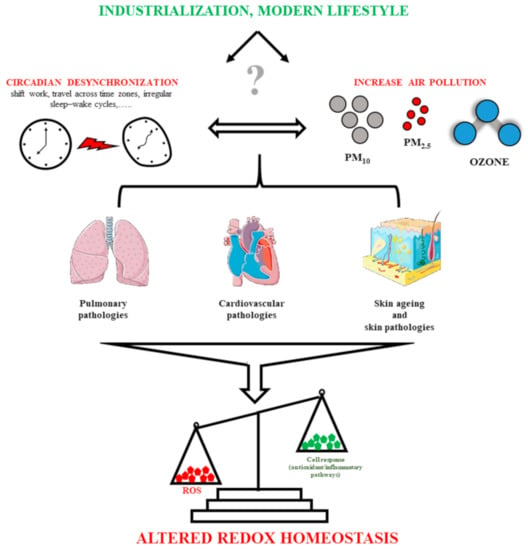
Figure 1.
Schematic summary of the conclusion section. In this review, we summarize the negative impact of the environmental stressors (in particular, ozone and particulate matters) and desynchronization of the circadian system on pulmonary, cardiovascular and cutaneous systems. We hypothesize that circadian misalignment can exacerbate cell susceptibility to airborne pollutants, reducing the efficiency of the antioxidant defense system, and worsening the state of health.
Author Contributions
Writing—original draft preparation, M.B. and E.F.; writing—review and editing, G.V. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the University of Ferrara (Italy) to MB (FAR2020), CB (FAR2020), and GV (FAR2020).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We are grateful to the reviewers for their professional and constructive suggestions in forming this review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pando, M.P.; Pinchak, A.B.; Cermakian, N.; Sassone-Corsi, P. A cell-based system that recapitulates the dynamic light-dependent regulation of the vertebrate clock. Proc. Natl. Acad. Sci. USA 2001, 98, 10178–10183. [Google Scholar] [CrossRef] [PubMed]
- Rensing, L.; Ruoff, P. Temperature effect on entrainment, phase shifting, and amplitude of circadian clocks and its molecular bases. Chronobiol. Int. 2002, 19, 807–864. [Google Scholar] [CrossRef] [PubMed]
- Mistlberger, R.E.; Antle, M.C. Entainment of circadian clocks in mammals by arousal and food. Essays Biochem. 2011, 49, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Rouyer, F. Clock genes: From Drosophila to humans. Bull. Acad. Natl. Med. 2015, 7, 1115–1131. [Google Scholar]
- Sundar, I.K.; Yao, H.; Sellix, M.T.; Rahman, I. Circadian molecular clock in lung pathophysiology. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L1056–L1075. [Google Scholar] [CrossRef]
- Martino, T.A.; Young, M.E. Influence of the cardiomyocyte circadian clock on cardiac physiology and pathophysiology. J. Biol. Rhythm. 2015, 30, 183–205. [Google Scholar] [CrossRef]
- Benedusi, M.; Frigato, E.; Beltramello, M.; Bertolucci, C.; Valacchi, G. Circadian clock as possible protective mechanism to pollution induced keratinocytes damage. Mech. Ageing Dev. 2018, 172, 13–20. [Google Scholar] [CrossRef]
- Shi, G.; Xie, P.; Qu, Z.; Zhang, Z.; Dong, Z.; An, Y.; Xing, L.; Liu, Z.; Dong, Y.; Xu, G.; et al. Distinct Roles of HDAC3 in the Core Circadian Negative Feedback Loop Are Critical for Clock Function. Cell Rep. 2016, 14, 823–834. [Google Scholar] [CrossRef]
- Akashi, M.; Takumi, T. The orphan nuclear receptor RORα regulates circadian transcription of the mammalian core-clock Bmal1. Nat. Struct. Mol. Biol. 2005, 12, 441–448. [Google Scholar] [CrossRef]
- Robinson, I.; Reddy, A.B. Molecular mechanisms of the circadian clockwork in mammals. FEBS Lett. 2014, 588, 2477–2483. [Google Scholar] [CrossRef]
- Gamble, K.L.; Resuehr, D.; Johnson, C.H. Shift work and circadian dysregulation of reproduction. Front. Endocrinol. 2013, 4, 92. [Google Scholar] [CrossRef] [PubMed]
- Touitou, Y.; Reinberg, A.; Touitou, D. Association between light at night, melatonin secretion, sleep deprivation, and the internal clock: Health impacts and mechanisms of circadian disruption. Life Sci. 2017, 173, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian rhythm connections to oxidative stress: Implications for human health. Antioxid. Redox Signal. 2013, 19, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Scheer, F.A.J.L. Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends Endocrinol. Metab. 2016, 27, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.C. Negative impacts of shiftwork and long work hours. Rehabil. Nurs. 2014, 39, 16–25. [Google Scholar] [CrossRef]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017, 32, 246–256. [Google Scholar] [CrossRef]
- Cheng, P.; Drake, C. Shift Work Disorder. Neurol. Clin. 2019, 37, 563–577. [Google Scholar] [CrossRef]
- Jacobson, M. Basic and history of discovery of atmospheric chemicals. In Atmospheric Pollution: History, Science, and Regulation; Cambridge University Press: Cambridge, UK, 2002; ISBN 9780521010443. [Google Scholar]
- Rückerl, R.; Schneider, A.; Breitner, S.; Cyrys, J.; Peters, A. Health effects of particulate air pollution: A review of epidemiological evidence. Inhal. Toxicol. 2011, 23, 555–592. [Google Scholar] [CrossRef]
- Orru, H.; Ebi, K.L.; Forsberg, B. The Interplay of Climate Change and Air Pollution on Health. Curr. Environ. Health Rep. 2017, 4, 504–513. [Google Scholar] [CrossRef]
- Yang, D.; Yang, X.; Deng, F.; Guo, X. Ambient Air Pollution and Biomarkers of Health Effect. Adv. Exp. Med. Biol. 2017, 1017, 59–102. [Google Scholar] [CrossRef]
- Brunekreef, B.; Beelen, R.; Hoek, G.; Schouten, L.; Bausch-Goldbohm, S.; Fischer, P.; Armstrong, B.; Hughes, E.; Jerrett, M.; Van den Brandt, P. Effects of long-term exposure to traffic-related air pollution on respiratory and cardiovascular mortality in the Netherlands: The NLCS-AIR study. Res. Rep. Health Eff. Inst. 2009, 139, 5–89. [Google Scholar]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.E. Airborne Particulate Matter: Human Exposure and Health Effects. J. Occup. Environ. Med. 2018, 60, 392–423. [Google Scholar] [CrossRef] [PubMed]
- Kinney, P.L. Interactions of Climate Change, Air Pollution, and Human Health. Curr. Environ. Health Rep. 2018, 5, 179–186. [Google Scholar] [CrossRef]
- Vallero, D. Air Pollution. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons Incorporated: New York, NY, USA, 2015; pp. 1–48. [Google Scholar] [CrossRef]
- Pope, C.A.; Dockery, D.W. Health effects of fine particulate air pollution: Lines that connect. J. Air Waste Manag. Assoc. 2006, 56, 709–742. [Google Scholar] [CrossRef]
- Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Guo, Y.; Tong, S.; Coelho, M.; Saldiva, P.; Lavigne, E.; Matus, P.; et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N. Engl. J. Med. 2019, 381, 705–715. [Google Scholar] [CrossRef]
- Sillman, S. The relation between ozone, NOx and hydrocarbons in urban and polluted rural environments. Atmos. Environ. 1999, 33, 1821–1845. [Google Scholar] [CrossRef]
- World Health Organization. Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease. World Health Organization. 2016. Available online: https://apps.who.int/iris/handle/10665/250141 (accessed on 14 January 2021).
- WHO Regional Office for Europe. Review of Evidence on Health Aspects of air Pollution—REVIHAAP Project: Technical Report. Copenhagen: WHO Regional Office for Europe. 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK361805/U.S (accessed on 14 January 2021).
- Environmental Protection Agency (US EPA). Integrated Science Assessment (ISA) of Ozone and Related Photochemical Oxidants (Final Report, Feb 2013); EPA/600/R-10/076F; Environmental Protection Agency: Washington, DC, USA. Available online: https://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=247492 (accessed on 27 October 2017).
- Gent, J.F.; Triche, E.W.; Holford, T.R.; Belanger, K.; Bracken, M.B.; Beckett, W.S.; Leaderer, B.P. Association of low-level ozone and fine particles with respiratory symptoms in children with asthma. JAMA 2003, 290, 1859–1867. [Google Scholar] [CrossRef]
- Muttray, A.; Gosepath, J.; Schmall, F.; Brieger, J.; Mayer-Popken, O.; Melia, M.; Letzel, S. An acute exposure to ozone impairs human olfactory functioning. Environ. Res. 2018, 167, 42–50. [Google Scholar] [CrossRef]
- Lim, C.C.; Hayes, R.B.; Ahn, J.; Shao, Y.; Silverman, D.T.; Jones, R.R.; Garcia, C.; Bell, M.L.; Thurston, G.D. Long-Term Exposure to Ozone and Cause-Specific Mortality Risk in the United States. Am. J. Respir. Crit. Care Med. 2019, 200, 1022–1031. [Google Scholar] [CrossRef]
- McDaniel, D.; Farris, P.; Valacchi, G. Atmospheric skin aging-Contributors and inhibitors. J. Cosmet. Dermatol. 2018, 17, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Woodby, B.; Prieux, R.; Valacchi, G. Involvement of 4-hydroxy-2-nonenal in pollution-induced skin damage. Biofactors 2019, 45, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, F.; Benedusi, M.; Manarini, F.; Woodby, B.; Russo, M.; Valacchi, G.; Pietrogrande, M.C. Proinflammatory properties and oxidative effects of atmospheric particle components in human keratinocytes. Chemosphere 2020, 240, 124746. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Sakamoto, K.; Okada, T.; Nagase, T.; Ishida, N. Antiphase circadian expression between BMAL1 and period homologue mRNA in the suprachiasmatic nucleus and peripheral tissues of rats. Biochem. Biophys. Res. Commun. 1998, 253, 199–203. [Google Scholar] [CrossRef]
- Petty, T.L. Circadian variations in chronic asthma and chronic obstructive pulmonary disease. Am. J. Med. 1988, 85, 21–23. [Google Scholar] [CrossRef]
- Masiol, M.; Squizzato, S.; Formenton, G.; Harrison, R.M.; Agostinelli, C. Air quality across a European hotspot: Spatial gradients, seasonality, diurnal cycles and trends in the Veneto region, NE Italy. Sci. Total Environ. 2017, 576, 210–224. [Google Scholar] [CrossRef]
- Wei, W.; Lv, Z.; Cheng, S.; Wang, L.; Ji, D.; Zhou, Y.; Han, L.; Wang, L. Characterizing ozone pollution in a petrochemical industrial area in Beijing, China: A case study using a chemical reaction model. Environ. Monit. Assess. 2015, 187, 377. [Google Scholar] [CrossRef]
- Hasheminassab, S.; Pakbin, P.; Delfino, R.J.; Schauer, J.J.; Sioutas, C. Diurnal and seasonal trends in the apparent density of ambient fine and coarse particles in Los Angeles. Environ. Pollut. 2014, 187, 1–9. [Google Scholar] [CrossRef][Green Version]
- Levi, F.; Schibler, U. Circadian rhythms: Mechanisms and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 593–628. [Google Scholar] [CrossRef]
- Reilly, D.F.; Westgate, E.J.; FitzGerald, G.A. Peripheral circadian clocks in the vasculature. Arter. Thromb. Vasc. Biol. 2007, 27, 1694–1705. [Google Scholar] [CrossRef]
- Gibbs, J.E.; Beesley, S.; Plumb, J.; Singh, D.; Farrow, S.; Ray, D.W.; Loudon, A.S. Circadian timing in the lung; a specific role for bronchiolar epithelial cells. Endocrinology 2009, 150, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Brenner, B.E.; Camargo, C.A. Circadian-rhythm differences among emergency department patients with chronic obstructive pulmonary disease exacerbation. Chronobiol. Int. 2007, 24, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Traves, S.L.; Proud, D. Viral-associated exacerbations of asthma and COPD. Curr. Opin. Pharmacol. 2007, 7, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Traylor, Z.P.; Aeffner, F.; Davis, I.C. Influenza A H1N1 induces declines in alveolar gas exchange in mice consistent with rapid post-infection progression from acute lung injury to ARDS. Influenza Other Respir. Viruses 2013, 7, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Davidson, A.J. Health consequences of circadian disruption in humans and animal models. Prog. Mol. Biol. 2013, 119, 283–323. [Google Scholar] [CrossRef]
- Marcheva, B.; Ramsey, K.M.; Peek, C.B.; Affinati, A.; Maury, E.; Bass, J. Circadian clocks and metabolism. Hand Exp. Pharmacol. 2013, 217, 127–155. [Google Scholar] [CrossRef]
- Papagiannakopoulos, T.; Bauer, M.R.; Davidson, S.M.; Heimann, M.; Subbaraj, L.; Bhutkar, A.; Bartlebaugh, J.; Vander Heiden, M.G.; Jacks, T. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab. 2016, 24, 324–331. [Google Scholar] [CrossRef]
- Hadden, H.; Soldin, S.J.; Massaro, D. Circadian disruption alters mouse lung clock gene expression and lung mechanics. J. Appl. Physiol. 2012, 113, 385–392. [Google Scholar] [CrossRef]
- Hwang, J.W.; Sundar, I.K.; Yao, H.; Sellix, M.T.; Rahman, I. Circadian clock function is disrupted by environmental tobacco/cigarette smoke, leading to lung inflammation and injury via a SIRT1-BMAL1 pathway. FASEB J. 2014, 28, 176–194. [Google Scholar] [CrossRef]
- Pekovic-Vaughan, V.; Gibbs, J.; Yoshitane, H.; Yang, N.; Pathiranage, D.; Guo, B.; Sagami, A.; Taguchi, K.; Bechtold, D.; Loudon, A.; et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014, 28, 548–560. [Google Scholar] [CrossRef]
- Gebel, S.; Gerstmayer, B.; Kuhl, P.; Borlak, J.; Meurrens, K.; Muller, T. The kinetics of transcriptomic changes induced by cigarette smoke in rat lungs reveals a specific program of defense, inflammation, and circadian clock gene expression. Toxicol. Sci. 2006, 93, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Ivanov, P.; Hilton, M.F.; Chen, Z.; Ayers, R.T.; Stanley, H.E.; Shea, S.A. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc. Natl. Acad. Sci. USA 2004, 101, 18223–18227. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Scheer, F.A.; Laker, M.; Smales, C.; Shea, S.A. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation 2011, 123, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Michelson, A.D.; Frelinger, A.L.; Evoniuk, H.; Kelly, E.E.; McCarthy, M.; Doamekpor, L.A.; Barnard, M.R.; Shea, S.A. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS ONE 2011, 6, e24549. [Google Scholar] [CrossRef]
- Pinotti, M.; Bertolucci, C.; Portaluppi, F.; Colognesi, I.; Frigato, E.; Foà, A.; Bernardi, F. Daily and circadian rhythms of tissue factor pathway inhibitor and factor VII activity. Arter. Thromb. Vasc. Biol. 2005, 25, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Shea, S.A. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 2014, 123, 590–593. [Google Scholar] [CrossRef]
- Chellappa, S.L.; Vujovic, N.; Williams, J.S.; Scheer, F.A. Impact of Circadian Disruption on Cardiovascular Function and Disease. Trends Endocrinol. Metab. 2019, 30, 767–779. [Google Scholar] [CrossRef]
- Thosar, S.S.; Butler, M.P.; Shea, S.A. Role of the circadian system in cardiovascular disease. J. Clin. Investig. 2018, 128, 2157–2167. [Google Scholar] [CrossRef]
- Jeyaraj, D.; Haldar, S.M.; Wan, X.; McCauley, M.D.; Ripperger, J.A.; Hu, K.; Lu, Y.; Eapen, B.L.; Sharma, N.; Ficker, E.; et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 2012, 483, 96–99. [Google Scholar] [CrossRef]
- Young, M.E.; Brewer, R.A.; Peliciari-Garcia, R.A.; Collins, H.E.; He, L.; Birky, T.L.; Peden, B.W.; Thompson, E.G.; Ammons, B.J.; Bray, M.S.; et al. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J. Biol. Rhythm 2014, 29, 257–276. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Hu, K.; Scheer, F.A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E1402–E1411. [Google Scholar] [CrossRef] [PubMed]
- Jankowiak, S.; Backé, E.; Liebers, F.; Schulz, A.; Hegewald, J.; Garthus-Niegel, S.; Nübling, M.; Blankenberg, S.; Pfeiffer, N.; Lackner, K.J.; et al. Current and cumulative night shift work and subclinical atherosclerosis: Results of the Gutenberg Health Study. Int. Arch. Occup. Environ. Health 2016, 89, 1169–1182. [Google Scholar] [CrossRef] [PubMed]
- Martino, T.A.; Oudit, G.Y.; Herzenberg, A.M.; Tata, N.; Koletar, M.M.; Kabir, G.M.; Belsham, D.D.; Backx, P.H.; Ralph, M.R.; Sole, M.J. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1675–R1683. [Google Scholar] [CrossRef] [PubMed]
- McGinnis, G.R.; Tang, Y.; Brewer, R.A.; Brahma, M.K.; Stanley, H.L.; Shanmugam, G.; Rajasekaran, N.S.; Rowe, G.C.; Frank, S.J.; Wende, A.R.; et al. Genetic disruption of the cardiomyocyte circadian clock differentially influences insulin-mediated processes in the heart. J. Mol. Cell Cardiol. 2017, 110, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Havakuk, O.; Zukerman, N.; Flint, N.; Sadeh, B.; Margolis, G.; Konigstein, M.; Keren, G.; Aviram, G.; Shmilovich, H. Shift Work and the Risk of Coronary Artery Disease: A Cardiac Computed Tomography Angiography Study. Cardiology 2018, 139, 11–16. [Google Scholar] [CrossRef]
- Durgan, D.J.; Pulinilkunnil, T.; Villegas-Montoya, C.; Garvey, M.E.; Frangogiannis, N.G.; Michael, L.H.; Chow, C.W.; Dyck, J.R.; Young, M.E. Short communication: Ischemia/reperfusion tolerance is time-of-day-dependent: Mediation by the cardiomyocyte circadian clock. Circ. Res. 2010, 106, 546–550. [Google Scholar] [CrossRef]
- Vetter, C.; Devore, E.E.; Wegrzyn, L.R.; Massa, J.; Speizer, F.E.; Kawachi, I.; Rosner, B.; Stampfer, M.J.; Schernhammer, E.S. Association between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA 2016, 315, 1726–1734. [Google Scholar] [CrossRef]
- Khaper, N.; Bailey, C.D.C.; Ghugre, N.R.; Reitz, C.; Awosanmi, Z.; Waines, R.; Martino, T.A. Implications of disturbances in circadian rhythms for cardiovascular health: A new frontier in free radical biology. Free Radic. Biol. Med. 2018, 119, 85–92. [Google Scholar] [CrossRef]
- Mousa, T.M.; Schiller, A.M.; Zucker, I.H. Disruption of cardiovascular circadian rhythms in mice post myocardial infarction: Relationship with central angiotensin II receptor expression. Physiol. Rep. 2014, 2, e12210. [Google Scholar] [CrossRef]
- Hardeland, R.; Coto-Montes, A.; Poeggeler, B. Circadian rhythms, oxidative stress, and antioxidative defense mechanisms. Chronobiol. Int. 2003, 20, 921–962. [Google Scholar] [CrossRef]
- Le Fur, I.; Reinberg, A.; Lopez, S.; Morizot, F.; Mechkouri, M.; Tschachler, E. Analysis of circadian and ultradian rhythms of skin surface properties of face and forearm of healthy women. J. Investig. Derm. 2001, 117, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Van Spyk, E.N.; Pham, K.; Geyfman, M.; Kumar, V.; Takahashi, J.S.; Andersen, B. The circadian clock in skin: Implications for adult stem cells, tissue regeneration, cancer, aging, and immunity. J. Biol. Rhythms 2015, 30, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Van Someren, E.J. Mechanisms and functions of coupling between sleep and temperature rhythms. Prog. Brain Res. 2006, 153, 309–324. [Google Scholar] [PubMed]
- Geyfman, M.; Kumar, V.; Liu, Q.; Ruiz, R.; Gordon, W.; Espitia, F.; Cam, E.; Millar, S.E.; Smyth, P.; Ihler, A.; et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc. Natl. Acad. Sci. USA 2012, 109, 11758–11763. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yan, S.; Wu, M.; Li, F.; Xu, X.; Song, W.; Zhao, J.; Xu, J.; Kan, H. Ambient ozone pollution as a risk factor for skin disorders. Br. J. Dermatol. 2011, 165, 224–225. [Google Scholar] [CrossRef]
- Valacchi, G.; Porada, E.; Rowe, B.H. Ambient ozone and bacterium Streptococcus: A link between cellulitis and pharyngitis. Int. J. Occup. Med. Environ. Health 2015, 28, 771–774. [Google Scholar] [CrossRef]
- Frigato, E.; Benedusi, M.; Guiotto, A.; Bertolucci, C.; Valacchi, G. Circadian Clock and OxInflammation: Functional Crosstalk in Cutaneous Homeostasis. Oxid. Med. Cell Longev. 2020, 2020, 2309437. [Google Scholar] [CrossRef]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006, 15, 1868–1873. [Google Scholar] [CrossRef]
- Janich, P.; Pascual, G.; Merlos-Suárez, A.; Batlle, E.; Ripperger, J.; Albrecht, U.; Cheng, H.Y.; Obrietan, K.; Di Croce, L.; Benitah, S.A. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature 2011, 9, 209–214. [Google Scholar] [CrossRef]
- Li, W.Q.; Qureshi, A.A.; Schernhammer, E.S.; Han, J. Rotating night-shift work and risk of psoriasis in US women. J. Investig. Dermatol. 2013, 133, 565–567. [Google Scholar] [CrossRef]
- Zanobetti, A.; Schwartz, J.; Dockery, D.W. Airborne particles are a risk factor for hospital admissions for heart and lung disease. Environ. Health Perspect. 2000, 108, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.; Dominici, F.; Zanobetti, A.; Schwartz, J.; Wang, Y.; Di, Q.; Balmes, J.; Christiani, D.C. Impact of Long-Term Exposures to Ambient PM2.5 and Ozone on ARDS Risk for Older Adults in the United States. Chest 2019, 156, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Carliste, C.; Melen, E. Air pollution, genetics, and allergy: An update. Curr. Opin. Clin. Immunol. 2012, 12, 455–460. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, E.A.; Gehring, U.; Mölter, A.; Fuertes, E.; Klümper, C.; Krämer, U.; Quass, U.; Hoffmann, B.; Gascon, M.; Brunekreef, B.; et al. Air pollution and respiratory infections during early childhood: An analysis of 10 European birth cohorts within the ESCAPE Project. Environ. Health Perspect. 2014, 122, 107–113. [Google Scholar] [CrossRef]
- Leyva, E.W.A.; Beaman, A.; Davidson, P.M. Health Impact of Climate Change in Older People: An Integrative Review and Implications for Nursing. J. Nurs. Sch. 2017, 49, 670–678. [Google Scholar] [CrossRef]
- Gan, W.Q.; FitzGerald, J.M.; Carlsten, C.; Sadatsafavi, M.; Brauer, M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am. J. Respir. Crit. Care Med. 2013, 187, 721–727. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 8, 1127–1134. [Google Scholar] [CrossRef]
- Khreis, H.; Cirach, M.; Mueller, N.; De Hoogh, K.; Hoek, G.; Nieuwenhuijsen, M.J.; Rojas-Rueda, D. Outdoor air pollution and the burden of childhood asthma across Europe. Eur. Respir. J. 2019, 54, 1802194. [Google Scholar] [CrossRef]
- Romieu, I.; Samet, J.M.; Smith, K.R.; Bruce, N. Outdoor air pollution and acute respiratory infections among children in developing countries. J. Occup. Environ. Med. 2002, 44, 640–649. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Mathioudakis, A.G.; Bartel, S.; Vijverberg, S.J.H.; Fuertes, E.; Comberiati, P.; Cai, Y.S.; Tomazic, P.V.; Diamant, Z.; Vestbo, J.; et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy 2020, 75, 2170–2184. [Google Scholar] [CrossRef] [PubMed]
- Canella, R.; Benedusi, M.; Martini, M.; Cervellati, F.; Cavicchio, C.; Valacchi, G. Role of Nrf2 in preventing oxidative stress induced chloride current alteration in human lung cells. J. Cell Physiol. 2018, 233, 6018–6027. [Google Scholar] [CrossRef]
- Cross, C.E.; Valacchi, G.; Schock, B.; Wilson, M.; Weber, S.; Eiserich, J.; Van der Vliet, A. Environmental oxidant pollutant effects on biologic systems: A focus on micronutrient antioxidant-oxidant interactions. Am. J. Respir. Crit. Care Med. 2002, 166, S44–S50. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.; Gasior, M.; Jastrzebski, D.; Desperak, A.; Ziora, D. Influence of Gaseous Pollutants on COPD Exacerbations in Patients with Cardiovascular Comorbidities. Adv. Exp. Med. Biol. 2018, 1114, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.Z.; Jalaludin, B.B.; Antó, J.M.; Hess, J.J.; Huang, C.R. Climate change, air pollution, and allergic respiratory diseases: A call to action for health professionals. Chin. Med. J. 2020, 133, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, K.; Au, W.W.; Zhao, W.; Xia, Z.L. A Systematic Review and Meta-Analysis of Short-Term Ambient Ozone Exposure and COPD Hospitalizations. Int. J. Environ. Res. Public Health 2020, 17, 2130. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Zanobetti, A.; Dominici, F. Who is more affected by ozone pollution? A systematic review and meta-analysis. Am. J. Epidemiol. 2014, 180, 15–28. [Google Scholar] [CrossRef]
- Kim, B.J.; Seo, J.H.; Jung, Y.H.; Kim, H.Y.; Kwon, J.W.; Kim, H.B.; Lee, S.Y.; Park, K.S.; Yu, J.; Kim, H.C.; et al. Air pollution interacts with past episodes of bronchiolitis in the development of asthma. Allergy 2013, 68, 517–523. [Google Scholar] [CrossRef]
- Kim, B.J.; Kwon, J.W.; Seo, J.H.; Kim, H.B.; Lee, S.Y.; Park, K.S.; Yu, J.; Kim, H.C.; Leem, J.H.; Sakong, J.; et al. Association of ozone exposure with asthma, allergic rhinitis, and allergic sensitization. Ann. Allergy Asthma Immunol. 2011, 107, 214–219. [Google Scholar] [CrossRef]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef]
- Kelly, F.J. Oxidative stress: Its role in air pollution and adverse health effects. Occup. Environ. Med. 2003, 60, 612–616. [Google Scholar] [CrossRef]
- Giorgini, P.; Di Giosia, P.; Petrarca, M.; Lattanzio, F.; Stamerra, C.A.; Ferri, C. Climate Changes and Human Health: A Review of the Effect of Environmental Stressors on Cardiovascular Diseases Across Epidemiology and Biological Mechanisms. Curr. Pharm. Des. 2017, 23, 3247–3261. [Google Scholar] [CrossRef]
- Du, Y.; Xu, X.; Chu, M.; Guo, Y.; Wang, J. Air particulate matter and cardiovascular disease: The epidemiological, biomedical and clinical evidence. J. Thorac. Dis. 2016, 8, E8–E19. [Google Scholar] [CrossRef]
- Kowalska, M.; Kocot, K. Short-term exposure to ambient fine particulate matter (PM2,5 and PM10) and the risk of heart rhythm abnormalities and stroke. Postepy Hig. Med. Dosw. 2016, 70, 1017–1025. [Google Scholar] [CrossRef]
- Bourdrel, T.; Bind, M.A.; Béjot, Y.; Morel, O.; Argacha, J.F. Cardiovascular effects of air pollution. Arch. Cardiovasc. Dis. 2017, 110, 634–642. [Google Scholar] [CrossRef]
- Song, J.; Zhu, J.; Tian, G.; Li, H.; Li, H.; An, Z.; Jiang, J.; Fan, W.; Wang, G.; Zhang, Y.; et al. Short time exposure to ambient ozone and associated cardiovascular effects: A panel study of healthy young adults. Environ. Int. 2020, 137, 105579. [Google Scholar] [CrossRef]
- Goodman, J.E.; Prueitt, R.L.; Sax, S.N.; Pizzurro, D.M.; Lynch, H.N.; Zu, K.; Venditti, F.J. Ozone exposure and systemic biomarkers: Evaluation of evidence for adverse cardiovascular health impacts. Crit. Rev. Toxicol. 2015, 45, 412–452. [Google Scholar] [CrossRef]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef]
- Gurgueira, S.A.; Lawrence, J.; Coull, B.; Murthy, G.G.; González-Flecha, B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ. Health Perspect 2002, 110, 749–755. [Google Scholar] [CrossRef]
- Nemmar, A.; Hoet, P.H.; Vanquickenborne, B.; Dinsdale, D.; Thomeer, M.; Hoylaerts, M.F.; Vanbilloen, H.; Mortelmans, L.; Nemery, B. Passage of inhaled particles into the blood circulation in humans. Circulation 2002, 105, 411–414. [Google Scholar] [CrossRef]
- Urkiewicz, T.R.; Porter, D.W.; Barger, M.; Millecchia, L.; Rao, K.M.; Marvar, P.J.; Hubbs, A.F.; Castranova, V.; Boegehold, M.A. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ. Health Perspect 2006, 114, 412–419. [Google Scholar] [CrossRef]
- Davel, A.P.; Lemos, M.; Pastro, L.M.; Pedro, S.C.; De André, P.A.; Hebeda, C.; Farsky, S.H.; Saldiva, P.H.; Rossoni, L.V. Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology 2012, 295, 39–46. [Google Scholar] [CrossRef]
- Kodavanti, U.P.; Thomas, R.; Ledbetter, A.D.; Schladweiler, M.C.; Shannahan, J.H.; Wallenborn, J.G.; Lund, A.K.; Campen, M.J.; Butler, E.O.; Gottipolu, R.R.; et al. Vascular and cardiac impairments in rats inhaling ozone and diesel exhaust particles. Environ. Health Perspect. 2011, 119, 312–318. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Pecorelli, A.; Cervellati, F.; Cervellati, C.; Maioli, E. Cutaneous responses to environmental stressors. Ann. N. Y. Acad. Sci. 2012, 1271, 75–81. [Google Scholar] [CrossRef]
- Fuks, K.B.; Woodby, B.; Valacchi, G. Hautschäden durch troposphärisches Ozon [Skin damage by tropospheric ozone]. Hautarzt 2019, 70, 163–168. [Google Scholar] [CrossRef]
- Valacchi, G.; Sticozzi, C.; Belmonte, G.; Cervellati, F.; Demaude, F.; Chen, N.; Krol, Y.; Oresajo, C. Vitamin C compound mixtures prevent ozone-induced oxidative damage in human keratinocytes as initial assessment of pollution protection. PLoS ONE 2015, 10, e0131097. [Google Scholar] [CrossRef]
- Valacchi, G.; Muresan, X.M.; Sticozzi, C.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Demaude, J.; Krol, Y.; Oresajo, C. Ozone-induced damage in 3D-Skin Model is prevented by topical vitamin C and vitamin E compound mixtures application. J. Dermatol. Sci. 2016, 82, 209–212. [Google Scholar] [CrossRef]
- Packer, L.; Valacchi, G. Antioxidants and the response of skin to oxidative stress: Vitamin E as a key indicator. Skin Pharmacol. Appl. Skin Physiol. 2002, 15, 282–290. [Google Scholar] [CrossRef]
- Magnani, N.D.; Muresan, X.M.; Belmonte, G.; Cervellati, F.; Sticozzi, C.; Pecorelli, A.; Miracco, C.; Marchini, T.; Evelson, P.; Valacchi, G. Skin Damage Mechanisms Related to Airborne Particulate Matter Exposure. Toxicol. Sci. 2016, 149, 227–236. [Google Scholar] [CrossRef]
- Valacchi, G.; Pagnin, E.; Okamoto, T.; Corbacho, A.M.; Olano, E.; Davis, P.A.; Van der Vliet, A.; Packer, L.; Cross, C.E. Induction of stress proteins and MMP-9 by 0.8 ppm of ozone in murine skin. Biochem. Biophys. Res. Commun. 2003, 305, 741–746. [Google Scholar] [CrossRef]
- Valacchi, G.; Pagnin, E.; Corbacho, A.M.; Olano, E.; Davis, P.A.; Packer, L.; Cross, C.E. In vivo ozone exposure induces antioxidant/stress-related responses in murine lung and skin. Free Radic. Biol. Med. 2004, 36, 673–681. [Google Scholar] [CrossRef]
- Oresajo, C.; Pillai, S.; Yatskayer, M.; Puccetti, G.; McDaniel, D. Antioxidants and Skin aging: A review. Cosmet. Dermatol. 2009, 22, 563–568. [Google Scholar]
- Burke, K.E. Mechanisms of aging and development—A new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech. Ageing Dev. 2018, 172, 123–130. [Google Scholar] [CrossRef]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef]
- Araviiskaia, E.; Berardesca, E.; Bieber, T.; Gontijo, G.; Sanchez Viera, M.; Marrot, L.; Chuberre, B.; Dreno, B. The impact of airborne pollution on skin. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1496–1505. [Google Scholar] [CrossRef]
- Ferrara, F.; Pambianchi, E.; Pecorelli, A.; Woodby, B.; Messano, N.; Therrien, J.P.; Lila, M.A.; Valacchi, G. Redox regulation of cutaneous inflammasome by ozone exposure. Free Radic. Biol. Med. 2020, 20, 561–570. [Google Scholar] [CrossRef]
- Ferrara, F.; Prieux, R.; Woodby, B.; Valacchi, G. Inflammasome Activation in Pollution-Induced Skin Conditions. Plast Reconstr. Surg. 2021, 147, 15S–24S. [Google Scholar] [CrossRef]
- Davis, S.; Mirick, D.K. Circadian disruption, shift work and the risk of cancer: A summary of the evidence and studies in Seattle. Cancer Causes Control. 2006, 17, 539–545. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Li, H.; Kilgallen, A.B.; Münzel, T.; Wolf, E.; Lecour, S.; Schulz, R.; Daiber, A.; Van Laake, L.W. Influence of mental stress and environmental toxins on circadian clocks: Implications for redox regulation of the heart and cardioprotection. Br. J. Pharmacol. 2020, 177, 5393–5412. [Google Scholar] [CrossRef]
- Patel, S.A.; Velingkaar, N.S.; Kondratov, R.V. Transcriptional control of antioxidant defense by the circadian clock. Antioxid. Redox Signal. 2014, 20, 2997–3006. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Luyts, K.; Smulders, S.; Napierska, D.; Van Kerckhoven, S.; Poels, K.; Scheers, H.; Hemmeryckx, B.; Nemery, B.; Hoylaerts, M.F.; Hoet, P.H. Pulmonary and hemostatic toxicity of multi-walled carbon nanotubes and zinc oxide nanoparticles after pulmonary exposure in Bmal1 knockout mice. Part. Fibre Toxicol. 2014, 11, 61. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).