Abstract
Background: Testicular dysgenesis syndrome (TDS) is a complex disorder of the male reproductive system related to disfunction of the fetal testis. The clinical features of TDS may be evident at birth or infancy (cryptorchidism, hypospadias and/or reduced anogenital distance) or occur later in adulthood (testis cancer, infertility). Genetic background seems to be important for genetic predisposition, with new genes being associated with components of the syndrome in last years. Interestingly, the incidence of clinical manifestations of TDS has been increasing in many countries in recent decades, suggesting that genetic predisposition alone cannot explain this trend. Consequently, the hypothesis of multifactorial etiopathogenesis is becoming increasingly accepted nowadays, with environmental factors probably acting during early developmental stages in genetically predisposed individuals. Methods: In this narrative review, we aim to critically evaluate genetic and non-genetic factors involved in the pathogenesis of TDs. Results: Important associations with intrauterine growth disorders and maternal diseases (overweight/obesity and diabetes) as well as lifestyle factors (e.g., smoking and alcohol abuse) were found. In such context, endocrine disruptors probably play a major role. These substances are widely used in industry and can exert estrogenic and antiandrogenic effects, potentially interfering with the development of the fetal gonad. Conclusions: Considering their possible impact on male sexual health, more attention should be focused on maternal modifiable factors to confirm with prospective studies the mixed results of available evidence.
1. Introduction
Testicular dysgenesis syndrome (TDS) is characterized by dysfunction of the fetal male genitals leading to various clinical features that can manifest at birth or later in life [1]. The most common components are male infertility, hypospadias, cryptorchidism, and testicular germ cells tumors (GCTs) [2]. Recent evidence suggests that a shorter anogenital distance (AGD) could also be part of this syndromic picture [3]. Despite the association between cryptorchidism and testicular cancer being noted since the 1930s [4], a possible correlation with other conditions was only proposed after several years (Figure 1). Indeed, the first histological finding suggesting an association between male infertility and testicular cancer dates 1972 [5]. On the other hand, an increased risk of testicular cancer in patients affected by hypospadias was first observed in a Danish cohort study published in 1996 [6] and then confirmed in other large population-based studies [7,8]. Considering the co-occurrence of these conditions and the epidemiological overlap, in 2001, Skakkebaek et al. [9] suggested a common etiopathogenesis in the context of TDS. The concept of TDS is somewhat similar to that of disorders of sex development (DSDs), a group of congenital conditions involving atypical development of chromosomal, gonadal, or anatomical sex [10]. Analogies with subcategory 46, XY DSD, referring to XY karyotype patients, can also be found. A distinction from complete gonadal dysgenesis is easy to carry out considering the female phenotype. Instead, the clinical picture of partial gonadal dysgenesis, characterized by mild-to-severe penoscrotal hypospadias and reduced sperm production, can often mimic TDS. However, the phenotype of TDS is usually less pronounced than that of DSD, probably because of the stronger impact of genetics on the pathogenesis of DSDs [11,12]. In fact, even if nowadays the existence of TDS is strongly supported by the literature [2], its etiology remains unclear. Genetics play a central role, as suggested by the association between genetic alterations and all the components of TDS [13,14,15,16]. In more detail, genetics could be particularly relevant for male infertility, since many cases of idiopathic infertility may be linked to unknown genetic causes [17,18]. However, genetics does not seem to be the only factor contributing to the origin of TDS. Indeed, many studies have reported an increasing incidence of TDS cases, especially in developed countries [19]. Many chemical substances, so-called endocrine disruptors, have shown endocrine activities like estrogenic or antiandrogenic effects potentially interfering with the development of fetal male genitals [2,20,21,22]. Nowadays, there are more than 800 substances that can be classified as endocrine disruptors [23]. These substances are mainly found in plastics, building materials, combustion products, and pesticides, as well as in cosmetics and some foods [24,25,26,27,28,29].They are now practically ubiquitous, being commonly found in air and water [29,30]. Therefore, pregnant women may encounter these substances, which can then affect fetal development [31,32]. In addition, maternal lifestyle can represent an additional critical factor, since cigarette smoking, alcohol, obesity, and diabetes have been associated with the occurrence of TDS [33,34,35]. All these potential contributors to TDS should also be evaluated considering the declining trend in semen quality [36] and serum testosterone levels worldwide [37,38], with associated health issues [39]. Indeed, this decline could also be related to endocrine disruptors [40,41] and the increasing incidence of obesity and metabolic syndrome correlated with unhealthy eating habits [42,43,44].
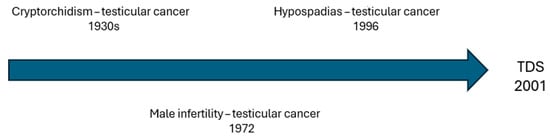
Figure 1.
Temporal trend of associations between components of testicular dysgenesis syndrome (TDS).
In this narrative review, we aim to critically evaluate genetic and non-genetic factors involved in the pathogenesis of TDS, and to assess possible future interventions to mitigate the increasing incidence of TDS.
2. Materials and Methods
An extensive literature search was conducted using Scopus and PubMed databases up to 30 June 2025. Only articles written in English were included, with no restrictions on publication date or study design.
The search terms included “testicular dysgenesis syndrome”, “testicular cancer”, “cryptorchidism”, “hypospadias”, “male infertility”, “couple infertility”, “anogenital distance”, “pregnancy”, “diabetes”, “overweight”, “obesity”, “cigarette”, “smoking”, “alcohol”, “coffee”, “diet”, “assisted reproductive techniques”, “drugs”, “genetics”, “epigenetics”, “endocrine disruptors”, “pollution”, “chemicals”, and “androgen receptor”. Boolean operators (AND/OR) were applied to create various combinations between search terms. References from retrieved studies were searched for further relevant literature.
To ensure transparency and consistency in our narrative review, we strictly adhered to the SANRA assessment scale (Scale for the Assessment of Non-Systematic Review Articles) [45], a 7-item tool that evaluates (1) justification of the article’s importance for the readership, (2) statement of concrete aims or formulation of questions, (3), description of the literature search, (4) referencing, (5) scientific reasoning, and (6) appropriate presentation of data.
3. Genetic and Epigenetic Causes of TDS
3.1. Cryptorchidism
Cryptorchidism is one of the earliest detectable signs of TDS [15], and it is defined as the failure of the testis to descend into the scrotal position [46]. Interestingly, the adult testicles of individuals affected by cryptorchidism show a characteristic organization of Leydig cells into micronodules, with a distribution of Reinke crystals within them that could correlate with the degree of testicular function impairment [47]. In addition, Sertoli cells of subjects with TDS show signs of immaturity similar to those of adults treated with high doses of estrogen and antiandrogens [48]. Testicular descent is a process occurring in two main phases: the transabdominal phase, primarily mediated by Insulin-like factor 3 (INSL3) secreted by fetal Leydig cells, and the inguino-scrotal phase, which is strongly dependent on androgen action and androgen receptor (AR) function. Mutations or polymorphisms in INSL3 and RXFP2 genes (encoding the INSL3 receptor) are associated with cryptorchidism [49,50]. However, pathogenic mutations of these genes are rare in isolated cryptorchidism cases, and findings are often inconsistent across different studies and populations [49,50]. Moreover, gene expression studies have shown that the exposure to endocrine-disrupting chemicals (EDCs) can induce INSL3 downregulation, further highlighting the gene–environment interplay [51]. Beyond INSL3 and RXFP2, other genes involved in testicular development and hormonal regulation—such as NR5A1 (Steroidogenic Factor 1), WT1 (Wilms tumor 1), SOX9 (SRY-Box Transcription Factor 9), DHH (Desert Hedgehog), and GATA4—are implicated. NR5A1 mutations are associated with a large spectrum of disorders of sex development (DSDs), including isolated cryptorchidism, male infertility, and hypospadias [52,53,54]. Nevertheless, identified variants often show high heterogeneity and are also detectable in phenotypically normal individuals. Furthermore, the AR gene was also studied, particularly in patients with bilateral cryptorchidism and associated hypospadias [55]. Point mutations and polymorphic repeats in the AR (e.g., CAG repeat length in the N-terminal domain) were associated with reduced androgen sensitivity during fetal life. However, available data remains inconclusive and often lacks replication in large-scale studies. KCTD13 (potassium-channel-tetramerization-domain-containing-13) is another candidate gene potentially involved in cryptorchidism and hypospadias. In mice, KCTD13 haploinsufficiency causes an increased incidence of cryptorchidism. Loss of KCTD13 in cell lines is associated with decreased intracellular levels of AR [56].
Moreover, epigenetic mechanisms may also contribute to the pathogenesis of cryptorchidism. Studies on testicular tissue of fetuses with undescended testes shown alterations in DNA methylation at regulatory regions of key genes involved in gonadal function [57], and the expression of regulatory microRNAs (miRNAs) involved in androgen signaling or Leydig cell differentiation appears disrupted in pathological samples [58].
In addition to these main genes implicated in testicular descent and differentiation, several other genes were identified through genome-wide association studies (GWAS), transcriptomic analyses, and functional studies in animal models. For instance, DMRT1 (Doublesex and Mab-3 Related Transcription Factor 1), a key regulator of testis development, was implicated in testicular dysgenesis and germ cell survival [59]. AXIN1, involved in Wnt/β-catenin signaling, may influence gonadal development through its role in cellular proliferation and differentiation [60]. Other genes such as ATRX (Alpha Thalassemia/Mental Retardation Syndrome X-linked), PIWIL1 (Piwi Like RNA-Mediated Gene Silencing 1), and CPEB1 (Cytoplasmic Polyadenylation Element Binding Protein 1) are involved in germ cell maturation and meiosis, processes that may be disrupted in the context of TDS [61,62].
A key factor in understanding the genetics of cryptorchidism is exploring its interaction with the intrauterine environment [63]. In fact, maternal exposure to phthalates, pesticides, cigarette smoke, or obesity has been linked to increased risk of cryptorchidism in male children, likely through interference with hormonal signaling and fetal gene expression [64].
Table 1 summarizes the current evidence available on the role of genetic alterations in the origin of TDS.

Table 1.
Summary of genes involved in cryptorchidism in TDS. INSL3 = Insulin-like factor 3, AR = androgen receptor, DSD: disorders of sex development, KCTD13 = potassium-channel-tetramerization-domain-containing-13, DMRT1 = Doublesex and Mab-3 Related Transcription Factor 1, SNP = Single Nucleotide Polimorphism.
3.2. Hypospadias
Hypospadias is a common congenital abnormality characterized by incomplete fusion of the urethral folds during embryological development of the penile urethra, leading to a proximally displaced urethral meatus, typically located on the ventral surface of the penis [65]. This condition is considered one of the cardinal features of TDS [66].
The prevalence of hypospadias varies across regions, with the highest rates reported in North America (1 in 140 male boys) [67], and lower frequencies in Europe (1 in ~500) [68].
Nowadays, hypospadias is widely recognized as a heterogeneous condition with multifactorial etiology, influenced by genetic susceptibility, environmental exposures, maternal-placental factors, and ethnic background [69,70].
This condition is thought to arise during a critical developmental window between 8 and 14 weeks of gestation, referred to as the male programming window (MPW). During this time, appropriate androgen exposure is essential for the correct differentiation and virilization of external male genitalia [71]. Inadequate androgen signaling during the MPW is hypothesized to lead to the characteristic phenotypic spectrum of TDS, including hypospadias. Despite its complexity, genes regulating testicular development, steroidogenesis, and androgen signaling—particularly those involved in androgen synthesis, metabolism, and receptor function—play a pivotal role in its pathogenesis, especially when linked to the TDS continuum [16,72].
Among these genes, the AR has been extensively studied. Polymorphic CAG repeats in exon 1 of the AR gene inversely affects receptor transactivation; longer CAG repeats reduce AR activity and were associated with reduced fertility and hypospadias in multiple studies [73,74]. For instance, AR CAG repeat length was significantly greater in 44 boys with isolated hypospadias compared to 79 controls (mean: 24.4 ± 2.8 vs. 22.7 ± 3.3; p < 0.05) [75]. Similarly, a meta-analysis of six case–control studies (444 cases vs. 727 controls) also reported a mean increase of +1.36 CAG repeats in hypospadias patients [76], an outcome replicated in a cohort of 211 Caucasian boys [77]. However, not all findings are consistent: one screening found no significant CAG repeat differences in 21 patients, suggesting that this polymorphism may not be universally relevant across all populations or hypospadias types [78].
In addition, other sequences may be involved. A large-scale whole-exome sequencing (WES) study revealed that approximately 27% of severe hypospadias cases carried rare damaging variants in genes critical to testosterone synthesis and signaling, including AR, NR5A1, and SRD5A2 [79]. Indeed, NR5A1 encodes Steroidogenic Factor-1 (SF-1), essential for steroidogenesis in Leydig cells. Loss-of-function mutations impair testosterone biosynthesis, resulting in under-virilization. NR5A1 mutations were reported in individuals with hypospadias, micropenis, and cryptorchidism, all clinical features that align closely with the TDS phenotype [79,80]. Whereas, SRD5A2 encodes 5α-reductase type 2, the enzyme responsible for converting testosterone to dihydrotestosterone (DHT), the key androgen for external genitalia development. Mutations in SRD5A2 result in variable degrees of hypospadias, sometimes with micropenis and undescended testes, still placing these cases within the TDS continuum [79]. Furthermore, rare AR variants were significantly enriched among patients with severe hypospadias in the WES cohort [79]. In addition to genes directly involved in AR structure and function, new candidate genes that regulate AR availability are emerging. Seth et al. identified KCTD13, a gene at the 16p11.2 locus, in a significant proportion of patients with genitourinary anomalies including hypospadias, micropenis, and cryptorchidism. KCTD13 modulates androgen receptor (AR) nuclear localization via ubiquitin ligase activity. In vitro knockdown of KCTD13 reduces nuclear AR levels without affecting AR expression. In the same way, in vivo KCTD13-null mice exhibited reduced nuclear AR levels, decreased expression of SOX9, smaller testis, cryptorchidism, micropenis, and subfertility. These findings suggest that KCTD13 plays a pivotal role in the intracellular handling of AR and its disruption may contribute to hypospadias occurrence by impairing androgen signaling during urogenital development [56].
Alongside these contributors, rare monogenic disorders such as WT1 mutations also produce TDS-like phenotypes [81]. Although TDS is often regarded as a multifactorial syndrome influenced by polygenic and environmental factors, WT1 mutations, typically linked to syndromic DSDs, result in gonadal dysgenesis and external genital anomalies, including penoscrotal hypospadias and cryptorchidism, reflecting features of testicular dysgenesis [81]. These cases, although syndromic, demonstrate how disruption of Sertoli and Leydig cell function by a single gene can mirror traits of the TDS spectrum [82,83]. A 2018 comprehensive review by Kalfa et al. emphasized that WT1, SRY, and members of key developmental pathways including HOX, SHH, FGF, and WNT gene families also regulate genital and urethral formation. These genes act upstream or in parallel to androgenic pathways, supporting the concept that early disruptions in gonadal programming, whether monogenic or polygenic, may underlie the clinical and anatomical features of TDS [84].
Beyond genetic variants, there is growing evidence that epigenetic dysregulation, including DNA methylation, histone modification, and non-coding RNA expression, plays a role in hypospadias pathogenesis and reflects a key mechanism in environmentally induced TDS [85].
In 2011, Vottero et al. found increased methylation of the AR promoter in preputial tissue from boys with hypospadias, along with higher levels of a specific DNA methyltransferase (DNMT3A) resulting in decreased gene expression. Crucially, in vitro exposure to dihydrotestosterone (DHT) or testosterone reversed these changes in a dose-dependent manner, suggesting that epigenetic silencing of AR may underlie hypospadias by suppressing androgen responsiveness during urethral differentiation [86].
Likewise, an epigenome-wide association study on preputial tissue identified 25 CpG sites differentially methylated in hypospadias patients. These changes affected genes involved in androgen metabolism (CYP4A11, EPHX1, KLK family), β-catenin/Wnt signaling (PKP2, TNKS) and developmental pathways (WDHD1, DNM1L). Notably, this suggests that epigenetic dysregulation may extend beyond AR itself, involving a broader network of genes, crucial for proper male genital development [87].
This idea is further supported by a 2023 pilot study that applied Genome-wide Methylated DNA sequencing (MeD-seq) in foreskin tissues from boys with proximal hypospadias. Although some differentially methylated regions (DMRs) were observed, most notably hypermethylation in LINC00665 and MAP3K1, the findings were variable and did not consistently map onto known DSD-related genes [88].
Adding functional insight, a recent pediatric study investigated both DNA methylation and gene expression in foreskin samples from hypospadias patients. The researchers reported decreased AR expression along with increased expression of ESR1, FGFR2, and BMP7, involved in estrogen signaling and genital development. Epigenetically, these changes were mirrored by hypermethylation at ESR1, FGFR2, and FGF8 loci as well as hypomethylation at AR, suggesting a coordinated yet dysregulated epigenetic program influencing multiple hormonal pathways [89].
3.3. Testicular Cancer
Testicular cancer is one of the most crucial elements in the definition of TDS originally proposed by Skakkebaek et al. [9] and it was also associated with poorer prognosis in that syndromic context [90,91]. Most of the testicular cancers (about 95%) are germ cells tumors (GCTs), about half of which are seminomas, mainly occurring in young adult men aged between 15 and 44 years [92]. Recently, a trend toward older age at diagnosis of GCT has been observed, with increasing percentage of seminomas over nonseminomas [93,94]. Cryptorchidism and a family history of testicular cancer are two well-known risk factors for testicular cancer [95], underscoring the strong link between this type of malignancy and genetic and/or congenital abnormalities. However, several acquired factors, such as occupational exposure to toxic substances, alcohol, smoking, and infections, can contribute to the onset of testicular cancer, justifying significant epidemiological variability between different geographical regions in developed and developing areas around the world [96]. In addition, it has been hypothesized that the upward trend in TDS may depend mainly on these acquired factors [2].
The recent revision of the World Health Organization (WHO) classification of testicular tumors introduced some relevant changes. GCTs are now divided into tumors derived from germ cell neoplasia in situ (GCNIS) and tumors unrelated to GCNIS [97] (Table 2).

Table 2.
WHO classification of testicular cancer. GCT = germ cells tumor, GCNIS = germ cell neoplasia in situ, NET = neuroendocrine tumor.
Currently, GNCIS is the preferred term for the testicular pre-invasive lesion first described by Skakkebaek in 1972 as “carcinoma-in situ” (CIS) [98] and renamed “intratubular germ cell neoplasia unclassified” (IGCNU) or “testicular intra-epithelial neoplasia” (TIN) in the 1980s [99]. In postpubertal subjects with diagnosis of testicular cancer, tubules with GCNIS are frequently found in proximity of invasive GCTs and in the contralateral testicle [100], whereas the occurrence of GCNIS before puberty has been described only in subjects with DSD after surgery or gonadectomy [101].
Hoei-Hansen et al. [102] first provided a systematic evaluation of differential gene expression in GCNIS, comparing pathological samples of GCNIS, invasive GCTs, and normal testicular tissue. Interestingly, they demonstrated an overexpression of several genes involved in testicular growth and development, such as Secreted frizzled-related protein 1 (SFRP1), Insulin-like growth factor binding protein-6 (IGFBP6), histone deacetylase 3 (HDAC3), and decorin (DCN). Similarly, Palmer et al. [103] showed an overexpression of microRNAs (miRNAs) miR-371~373 and miR-302 cluster, which are considered markers of embryonic stem cells, suggesting the persistence of an embryonic pattern of miRNA expression. This is in accordance with the hypothesis by Skakkebaek et al. [104], who, based on ultrastructural studies and histochemical findings showing common features of GCNIS cells and gonocytes, elaborated the following statements: GCNIS cells are malignant gonocytes, and their transformation into invasive tumors is hormone-dependent; GCNIS gonocytes may regress into totipotent embryonic cells, giving rise to all the types of non seminomatous GCTs; the ability to regress into embryonic cells decreases with age, and GCNIS that cannot regress can give raise to classical seminomas. More recent studies using genome-wide expression profiling confirmed the expression of a high number of genes linked to the stem cell phenotype in GCNIS cells, with 35–50% expressed genes overlapping between GCNIS and embryonic stem cells (ESCs) [105]. Comparing genetic expression profiles between GCNIS and immature germ cells, Sonne et al. confirmed a close resemblance between gonocytes and GCNIS. Interestingly, they also found that GCNIS showed neither overexpression of oncogenes nor under-expression of tumor suppressor genes, leading the authors to hypothesize that GCNIS cells are not malignant cells sensu stricto, but rather arrested gonocytes [106]. Afterwards, this was supported by the identification of a large range of stemness genes (POU5F1, NANOG, LIN28-A, DPPA4, DPPA5, KIT, and TFAP2C) expressed by GCNIS cells, suggesting the formation of GCNIS in utero, with subsequent transformation to malignant germ cells during the peri- and post-puberal period due to further genetic and epigenetic modifications [14]. Among genetic modifications, the gain of extra chromosome arm 12p material has been associated with malignant transformation of GCNIS cells, making them gradually independent of the intratubular environment, with consequent extratubular invasion [107].
To sum up, the majority of invasive GCTs derive from GCNIS cells of fetal origin. It has been hypothesized that GCNIS cells are arrested gonocytes whose maturation defect derives from a congenital dysfunction of Sertoli cells and Leydig cells [2]. A potential impairment of spermatogenesis and reduced testosterone production capacity consequently explains the infertility, cryptorchidism, and hypospadias observed in TDS [108]. There is an extreme lack of information on the relationship between TDS and stromal tumors of the sex cords, although there are sporadic reports of tumors of this type in patients with DSD [109] and gonadal dysgenesis such as Klinefelter syndrome [110]. Further studies on the genetics of sex cord stromal tumors could be therefore useful in clarifying their relationship with TDS.
3.4. Male Infertility
Infertility is another important component of TDS. Genetic causes of male infertility are frequent findings, and their evaluation is mandatory in cases of azoospermia or severe oligozoospermia [111]. The more common genetic causes of male infertility are Klinefelter syndrome and y chromosome azoospermia factor (AZF) microdeletions, but other important genetic diseases to consider in specific clinical contexts are cystic fibrosis and hypogonadotropic hypogonadism [112]. It is also possible that many idiopathic cases involve genetic alterations that are still unknown, and new genes potentially involved in fertility problems have been continuously discovered in recent years [17,18].
Considering this complex genetic context, separating genetic alterations related to infertility in TDS from the others is not simple. However, there are many genes related to infertility that are also involved in the pathogenesis of other TDS components. In detail, longer CAG repeat polymorphism of the AR is associated with worse semen parameters [113,114]. However, ethnicity may play a significant role, since the results found in a Russian population [114] were not confirmed in an Iranian population [115]. The impact of AR function on male infertility was also strengthened by a finding of decreased immunohistochemical AR expression in testicular tissue of men affected by non-obstructive azoospermia (NOA) [106]. AGD, defined as the distance from the anus to the genitalia, is a sexually dimorphic trait established during the fetal “masculinization programming window” (approximately gestational weeks 8–14 in humans), depending on adequate androgenic signaling [116]. Reduced AGD in males is considered a sensitive biomarker of impaired androgen action in utero. It was also associated with features of TDS, including cryptorchidism, hypospadias, impaired spermatogenesis, and increased risk of testicular cancer [3,117,118,119,120]. Similarly, the ratio of index to ring finger lengths (2D:4D) (a typical indicator of androgen exposure in utero) has been used to explore the relationship between androgenization and reproductive function, but no clear associations were observed [121].
Many studies, particularly in animal models, highlighted the significant role of genetics demonstrating the partial heritability of AGD and strengthening potential correlations with other aspects of TDS [122,123].
In humans, Sathyanarayana et al. (2012) investigated how polymorphisms in genes known to cause major genitourinary abnormalities could result in borderline TDS phenotypes also including reduced AGD [124]. This correlation was observed for ESR1 and ATF3 genes, whereas no clear association was found between reduced AGD and AR gene polymorphisms [124]. Eisenberg et al. further studies this topic, evaluating the association between AGD and CAG repeat length in AR. They noticed that men with a shorter AGD had a higher number of CAG repeats. However, a linear correlation was not established [13]. This finding was in line with the literature considering the indirect association between CAG repeat length and androgenic effects of the interaction between testosterone and its receptor [55,125].
Moreover, not only the length of CAG polymorphism, but also novel SNPs seem to be involved in fertility problems [126]. Genetic alterations of INSL3 and its receptor RXFP2 were correlated not only with cryptorchidism but also with azoospermia and crypto-/azoospermia [127,128,129]. In detail, biallelic RXFP2 variants were associated with histological findings of spermatogenic arrest at the spermatid stage [127]. Evidence in humans regarding the association between AGD and other genes involved in TDS (e.g., INSL3 or NR5A1 [79]) are lacking. However, animal models highlighted AGD reduction in association with NR5A1 mutations [122].
Mutations of NR5A1 were also found to be involved in male infertility [130]. SNPs seem to play a role in specific populations; however, this finding must be confirmed in different ethnicities [131]. FGF family genes were also associated with male infertility [132] and an interaction with CFTR in patients affected by azoospermia and cryptorchidism was proposed [133]. Many other genes involved in cryptorchidism, hypospadias, testicular cancer, or reduced AGD, like ATF3 [134], DHH [135], DMRT1 [136], EPHX1 [137], GATA4 [138], KIT [139,140], PIWIL1 [141], SOX9 [142,143], WNT [144], and WT1 [145,146], were associated with a lower level of evidence with various degrees of semen alterations and male fertility problems.
4. Maternal and Fetal Factors Associated with TDS
The association between TDS and environmental factors is well known [9] and many studies evaluated the role of maternal-fetal characteristics, also including exposure to toxins and drugs during pregnancy, in the pathogenesis of the disease.
4.1. Characteristics of Pregnancy
Many studies evaluated various maternal and perinatal factors in association with TDS. Zakaria et al. found five conditions associated with higher risk of cryptorchidism, namely, a gestational age of 37 weeks or less, birth weight ≤ 2.75 kg, cesarean delivery, steroid therapy, and twin pregnancy, but the only independent factor predicting cryptorchidism was low birth weight [147]. Maternal age appears to correlate with the risk of cryptorchidism, even though in the study by Stelios Mavrogenis et al. this trend did not reach statistical significance. In the same study, endometriosis in mothers was a risk factor for cryptorchidism in children [148]. In a 1997 study, maternal age over 30 years was associated with increased risk of cryptorchidism (odds ratio 1.9) and seminoma (odds ratio 2.0), especially in the first child. Birth weight, whether excessively low or high, appeared to be associated with increased risk of testicular cancer, particularly in cases of weight < 2.500 kg with an odds ratio of 2.6 [149]. A large Norwegian study in 2004 highlighted the association between hypospadias and several conditions like low parity, hypertension, bleeding during index pregnancy, pre-eclampsia, retained placenta, cesarean section, low birth weight, small for gestational age, presence of inguinal hernia, and prevalence among relatives of hypospadias cases [150]. Another important study involving more than 42 thousand subjects revealed an association between hypospadias and hypertensive disorders of pregnancy (HDP), primiparity, multiple births, hyperthyroidism, preterm delivery, low birth weight (LBW), and small for gestational age (SGA); after correction for potential confounders, some factors remained significant such as HDP, multiple births, and hyperthyroidism [151].
There is evidence that risk of testicular cancer changes in relation to parity. In fact, according to Westergaard et al., there is a reduction in risk of testicular cancer from the second child onwards, which is progressive but only significant when comparing the first child with subsequent children [152]. Similarly, in a large Swedish study, cryptorchidism was associated with being first born [153]. The association between testicular cancer and parity was also confirmed in a 2009 meta-analysis. There was a reduced risk of testicular cancer with increasing parity, with an odds ratio of 0.80 in fourth-born children compared to first-born children. The same article ruled out an association with maternal age, maternal nausea, maternal hypertension, maternal bleeding, pre-eclampsia, breech delivery, and cesarean section [154].
These data are interesting considering that increased parity is usually associated with increased maternal age, which in previous evidence seemed to be associated with higher risk of cryptorchidism and seminoma [149].
Studies on hyperemesis gravidarum were conducted by various authors and were subsequently summarized in the meta-analysis by Nijsten et al., which showed that hyperemesis gravidarum seemed to be associated with risk of various cancers in children, including testicular cancer, with an OR of 1.60 and a 95% confidence interval between 1.07 and 2.39 [155]. The pathophysiological mechanism is unknown, but a correlation with hyperestrogenism has been suggested.
4.2. Birth Weight
Birth weight is one of the most extensively studied aspect in relation to TDS, and there are various publications linking low birth weight with cryptorchidism [147,148], hypospadias [150], and testicular cancer [149], as partially mentioned above. In this regard, a retrospective case–control study involving more than 42,000 newborns was published in 2022, showing an association between low birth weight and small for gestational age newborns and hypospadias [151]. Similarly, in 2007, a systematic review found an association between low birth weight and cryptorchidism [156]. However, it is difficult to understand whether low birth weight is independently related to cryptorchidism or if there are common intrauterine factors that determine both conditions, as pointed out by Holmboe et al. [64].
4.3. Systemic Arterial Hypertension and Diabetes Mellitus
The literature also focused on maternal hypertension in relation to TDS. A systematic review with meta-analysis in 2009 did not find associations between hypertension or pre-eclampsia and testicular cancer in sons [154], while other papers showed a link between HDP and hypospadias as mentioned above [150,151].
Another interesting aspect is the role of pregestational and gestational diabetes in mothers of children affected by TDS. Pregestational diabetes appears to be associated with increased risk of congenital anomalies, probably due to prolonged exposure to hyperglycemia. It leads to increased reactive oxygen species (ROS) production and oxidative stress, compromising organogenesis and interfering with normal embryonic development [157]. The development of the male reproductive system is a process driven by androgenic production by the fetus in response to the placenta’s chorionic gonadotrophin (hCG) production. In diabetic women, placental abnormalities may develop, compromising its normal secretion and thus the masculinization process [158]. As observed by Arendt et al., pregestational diabetes, particularly type 1 diabetes mellitus, is associated with increased risk of hypospadias and cryptorchidism [158].
In 2006, Virtanen H.E. et al. showed that gestational diabetes was more common in mothers of patients with cryptorchidism, even in cases of mild gestational diabetes treated with diet alone. However, the authors themselves pointed out that the criteria used for the diagnosis of gestational diabetes were not described [159]. A 2020 study conducted in the US on data concerning more than twenty-nine million newborns showed an association between diabetes during pregnancy and hypospadias, with an adjusted risk ratio of 1.88 (95% CI 1.67–2.12) for pre-pregnancy diabetes and 1.29 (95% CI 1.21–1.36) for gestational diabetes [160]. Less-impressive data came from a systematic review with meta-analysis conducted in 2015 on thirteen studies including 15,373 cases, which highlighted that gestational diabetes was only marginally associated with increased risk of cryptorchidism with a trend towards statistical significance that was not, however, achieved (OR = 1.21, 95% CI: 1.00–1.46; p = 0.06) [161].
4.4. Maternal Weight and Physical Activity
Maternal weight was also studied in relation to TDS. Studies focused not only on pre-pregnancy weight but also on weight gain during pregnancy. Overweight and obesity can be associated with increased estrogens levels, as well as poorer nutritional status and glycemic control [162]. These alterations could interfere with genital development and testicular descent [35]. For example, sons of overweight or obese women had higher risk of developing hypospadias [163,164,165]. Similarly, a Swedish study on 1,055,705 infants highlighted an association between maternal overweight or obesity and cryptorchidism or hypospadias [35]. An interesting Chinese study analyzed the AGD of 556 male children, revealing that it was shorter in children whose mothers had gained more weight during pregnancy. In fact, AGD in male newborns decreased about 2.27 mm in the excessive-gestational-weight-gain group compared to the standard-gestational-weight-gain group [166].
Nutrition may also play a role in the development of defects in the male genital tract. A single study suggested that a vegetarian diet during pregnancy could be associated with increased risk of hypospadias, probably due to excessive intake of phytoestrogens [167]. However, this finding was not confirmed in more recent evidence [168].
Evenson et al. observed that maternal physical activity and reduced sedentary behavior were associated with lower risk of hypospadias [169], but no further studies are currently available on this topic. The result was probably related to better weight control and reduced risk of type 2 diabetes.
4.5. Smoking Habit
Various studies evaluated exposure to smoke during pregnancy and hypospadias in newborns. A recent meta-analysis of observational studies concluded that maternal active smoking was significantly associated with risk of hypospadias, whereas this was not true for paternal smoking nor maternal passive smoking [170]. Similarly, a large study based on nationwide birth certificate data revealed an increased risk of several malformations, including hypospadias, in children born from mothers who smoked during pregnancy [34]. The association between smoking during pregnancy and hypospadias has also been confirmed by large reviews and meta-analyses [161,171,172] and this phenomenon seems dose-dependent, in terms of number of cigarettes per day and nicotine content [173]. However, some studies showed opposite results, highlighting a reduced risk of hypospadias in children whose mothers smoked during pregnancy. In particular, the study by Daniel Lindbo et al. showed that this kind of exposure was associated with a lower risk of hypospadias with an adjusted hazard ratio of 0.82 and a 95% confidence interval between 0.58 and 1.18 [174]. Previously, a systematic review had found similar results, suggesting a reduction in risk of hypospadias considering 15 studies with 12,047 malformed cases and 1.5 million controls [171]. Moreover, there is evidence supporting a neutral effect of cigarette smoking during pregnancy on risk of hypospadias [175]. Finally, another study found that passive maternal exposure to tobacco smoke during pregnancy was associated with an 88% increased risk of hypospadias, but this result was not confirmed for active maternal smoking in the first trimester [176]. In conclusion, the variability of population examined and different study designs probably led to the different results previously described. For that reason, it is not possible to draw any definite conclusions regarding the impact of maternal smoking on hypospadias considering the available evidence.
Otherwise, the relationship between maternal smoking during pregnancy and cryptorchidism seems to be more consistent. In the study by Zakaria et al., maternal exposure to passive smoking was more common in patients with cryptorchidism than in controls, although statistical significance was not achieved [147]. In a Danish cohort study, authors demonstrated that sons of women who smoked 10–14 cigarettes/day had the highest hazard ratio for cryptorchidism (1.37; 95% CI: 1.06–1.76) and that smoking cessation was associated with a slightly higher HR of cryptorchidism compared to non-smokers [177]. The study by Lindbo et al. also demonstrated a dose-dependent relationship [174].
Finally, testicular cancer did not seem to be associated with smoking during pregnancy in a 2009 study, even with dividing the population into passive or active smokers or considering the number of cigarettes smoked daily [33,178].
4.6. Alcohol and Caffeine
The role of alcohol consumption in risk of TDS is under debate. According to Mongraw-Chaffin et al., mothers of sons with testicular cancer were more likely to drink alcohol than controls [33]. Other more recent studies did not find an association between maternal alcohol consumption and TDS components, in particular cryptorchidism [161,177]. Despite mixed results, it is interesting to consider that the first study described [33] had the advantage of being based on older data. At the time of data collection, alcohol consumption during pregnancy was not as stigmatized as today due to less knowledge of the negative effects of alcohol on the fetus at the time. For this reason, medical histories in this regard could be reasonably more reliable. However, the more-recent evidence has the strength of analyzing a larger population.
Regarding coffee consumption during pregnancy, no associations with increased risk of cryptorchidism were found [177]. It was noted that mothers of patients affected by testicular cancer were less likely to drink coffee than controls [33].
4.7. Assisted Reproductive Technology
There are several studies in the literature, which can be summarized by the meta-analysis by Zhang et al. [179], which showed that children born with Assisted Reproductive Technology (ART) had a 1.87 times higher risk of hypospadias than naturally conceived male offspring. The meta-analysis was affected by moderate heterogeneity. However, the risk was confirmed after exclusion of studies mainly implicated in the heterogeneity. The same meta-analysis showed no differences in risk of hypospadias or cryptorchidism when comparing In Vitro Fertilization (IVF) and Intracytoplasmic Sperm Injection (ICSI) techniques [179]. Other studies found ICSI to present higher risk of hypospadias and cryptorchidism than IVF. In any case, it has been hypothesized that the association between ART, cryptorchidism, and hypospadias is because newborns conceived through ART techniques are more frequently affected by low birth weight and prematurity [180].
4.8. Drugs
The Dorte Vesterholm Lind group evaluated the relationship between exposure to Non-Steroidal Anti-inflammatory Drugs (NSAIDs) and/or paracetamol (acetaminophen) and AGD. In the study, combined exposure to NSAIDs and paracetamol was associated with a statistically significant reduced AGD compared to non-exposed subjects (mean: 32.3 mm versus 36.2 mm, p = 0.03). The low sample size must be considered among the limitations of the study. This result was not confirmed considering exposure to paracetamol alone [181]. In fact, Navarro-Lafuente et al. did not find an association between exposure to paracetamol alone and AGD [182].
Allopurinol is not recommended during pregnancy, mainly due to a lack of data. In the prospective case series study conducted by Hoeltzenbein et al. on 31 unborn babies exposed to the drug in the first trimester, the overall rate of major malformations (3.7%) and spontaneous abortions (cumulative incidence 11%, 95% CI 3–40) were both within the normal range [183]. However, one fetus was described as having severe malformations (including microphthalmia, cleft lip and palate, renal hypoplasia, low-set ears, hearing deficit, bilateral cryptorchidism, and micropenis), which were like those described by Kozenko et al., leading to the suspicion that allopurinol may cause malformations related to TDS [184].
Antiepileptic drugs are statistically associated with fetal malformations when used during pregnancy. According to the meta-analysis by Veroniki et al., gabapentin (OR, 16.54; 95% CI, 2.50–121.70), primidone (OR, 5.92; 95% CI, 1.01–23.77), and valproic acid (OR, 2.58; 95% CI, 1.24–5.76) in monotherapy were associated with an increased risk of hypospadias, while the drugs least associated with malformations were levetiracetam and lamotrigine [185].
It is well known that the use of beta-blockers during pregnancy is associated with an increased risk of malformations. However, this increased risk does not appear applicable to hypospadias, as shown by two different meta-analyses of observational studies carried out several years apart [186,187].
Regarding exposure to macrolides, a systematic review with meta-analysis was published in 2023, which ruled out any association between hypospadias and use of macrolides during pregnancy. Considering two studies that specifically evaluated the use of erythromycin in the first trimester, the drug was significantly associated with a reduction in risk of hypospadias (OR 0.38; 0.18, 0.81), but given the small number of cases evaluated, the authors hypothesized that this was a chance finding [188].
4.9. Socioeconomic Status
Socioeconomic factors also appear to be involved in TDS and these aspects were already studied in the early 1990s. In 1996, a large Danish study was conducted, highlighting a weak association between testicular cancer and higher socioeconomic status, while cryptorchidism was associated with lower socioeconomic status. However, even at that time, the authors hypothesized that the study of socioeconomic factors is difficult for various reasons, including historical changes that, for example, may render past results no longer valid [189]. However, similar results were found in another study on a Hungarian population, revealing a lower socioeconomic status of mothers of cryptorchid newborns [148]. In any case, data on this topic is insufficient and no conclusions can be drawn.
5. Paternal Factors Associated with TDS
Studies mainly focused on maternal risk factors of TDS, considering the importance of gestational period on fetal organogenesis. However, there is also evidence regarding the impact of paternal risk factors. In 2017, the analysis of a population-based cohort of 1,056,058 males found that younger paternal age at birth was associated with increased risk of TGCT, especially seminoma, in sons. The association was confirmed even after adjustment for year of birth, years of education, height, history of cryptorchidism, and maternal age at birth [190]. Moreover, a direct correlation between older age of fathers and impaired sperm quality of children was observed [191]. Then, a case–control study found that paternal smoking (OR 2.0; 95% CI: 1.33–2.99), history of urological diseases (OR 2.31; 95% CI: 1.15–4.90), and occupational exposure to EDC (OR 3.90; 95% CI: 2.41–6.48) were risk factors for hypospadias and cryptorchidism in sons, whereas higher paternal educational level seemed to be a protective factor (OR 0.63; 95% CI: 0.42–0.93) [192]. An interesting study on 376,362 male births also highlighted the impact of paternal metabolic health on the risk of genital malformations in children. Indeed, sons of men affected by two or more components of metabolic syndrome were more likely to be diagnosed with hypospadias (OR 1.27 95% CI: 1.10–1.47) [193]. Considering the available evidence, similar risk factors on both maternal and paternal sides seem to impact on the risk of TDS in sons.
6. Endocrine-Disrupting Chemicals
Endocrine-disrupting chemicals (EDCs) are exogenous compounds that interfere with the endocrine system by mimicking, antagonizing, or altering synthesis, metabolism, and signaling of hormones. In the 1990s, researchers first noticed a connection between growing pollution and progressive decline in male fertility and rising incidence of male genitalia abnormalities, including testicular tumors (a cluster later defined as TDS). Evidence then confirmed this connection [36,194]. Different definitions of EDCs have been proposed since then, and currently the Endocrine Society describes EDCs as exogenous substances, or mixtures of chemicals, that interfere with any aspect of hormone function [195]. From a regulatory perspective, these substances are sorted by European Society into three groups—“Identified”, “Under Evaluation”, and “Considered by National Authority as EDCs”. Among more than 800 chemicals that might have potential endocrine action [23], researchers mainly focused on phthalates, phytoestrogens, pesticides, bisphenols, per- and polyfluoroalkyl substances (PFAS), and polychlorinated biphenyls (PCBs) because they are the more commonly used and more relevant in clinical practice.
6.1. Mechanism of Action
The main mechanism linking EDCs to TDS is the ability of these compounds to interfere with androgen and estrogen signaling. Indeed, they can bind hormonal receptors acting as agonists or antagonists. For example, Bisphenol A (BPA) can bind the main nuclear estrogen receptor (ER), exerting an agonistic effect even at low concentrations, possibly altering the physiologic hormonal balance. BPA can also bind other receptors of the estrogen pathway like estrogen-related receptor γ [196], G protein-coupled receptors, and GPR3 0 [197]. In this way, BPA could interfere with germ cell proliferation and differentiation and may also induce pro-apoptotic effects on spermatocytes [198]. Finally, BPA could potentially block or interfere with AR, the progesterone receptor, and their ligands [199]. Interestingly, several studies showed that BPA can induce changes in DNA methylation and consequently lead to epigenetic changes transmissible from exposed mothers to their offspring; however, further studies are needed to translate this mechanism into correlation with phenotypic outcomes [200]. Among pesticides, although it has been banned for many years, Dichlorodiphenyltrichloroethane (DDT), an organochlorine, represents a clear example of the harmful potential of EDCs. It is capable of binding with high affinity both the nuclear ER [201] and G-protein-coupled receptors [202], and this could translate into effects on spermatogenesis and gonadal development. PCBs are also compounds with estrogenic effects [203] and can cause alterations in the development of reproductive organs and impair the hypothalamus–pituitary–gonadal axis. Epigenetic modifications transmitted between generations were documented for PCBs, although a direct correlation between these modifications and components of TDS is still lacking [204]. Phthalates can directly affect steroid hormone production and increase ROS-derived damage both directly and indirectly, altering antioxidant enzyme activity [205]. Some compounds can act as agonists and others as antagonists on ER, but at AR level they are almost exclusively antagonists [206]. Animal evidence also showed a possible role in the apoptosis of testicular cells [207]. Particular concern regards the epigenetic alterations potentially associated with all the compounds mentioned above. They represent the mechanism by which EDCs modify gene expression without altering the DNA sequence. The main epigenetic changes highlighted so far are aberrant methylations, histone modifications, and alterations in the microRNA profile [208]. In summary, the main mechanisms by which EDCs exert their effects, potentially compromising male reproductive health, include receptor binding, oxidative stress, steroidogenic disruption, and epigenetic modifications. In addition, emerging evidence shows that reproductive adverse effects of EDCs may not be limited to the exposed generation of individuals but could be inherited by future generations, possibly impairing reproductive health and susceptibility to reproductive disorders [209].
6.2. Evidence Regarding TDS
In all experimental models, phthalates exposure, particularly di-2-ethylhexyl phthalate/dibutyl phthalate (DEHP/DBP), can reduce testosterone and INSL3 levels as well as AGD, and is associated with an elevated risk of cryptorchidism, with the risk being higher for mixtures of compounds [12]. However, in humans, maternal gestational urinary bisphenols and phthalates concentrations did not seem to be associated with increased risk of cryptorchidism in offspring [210]. BPA seems to reduce testosterone levels in experimental studies, but the reduction is relevant only in the absence of hormones stimulating gonadal function; in the presence of hCG or LH, the differences are irrelevant, suggesting a potential modulatory effect of the placenta on this outcome [211]. Evidence from epidemiological studies is scarce and association was found only for high levels of exposure. More recent and longitudinal studies did not find an association at low exposure levels, and results are very heterogeneous and difficult to interpret [212]. The most robust evidence exists for pesticides, which showed an augmented risk of cryptorchidism, but there is a lot of heterogeneity in the methods of studies carried out: this includes different matrices or indirect evaluation of exposures, small sample size, and diverse geographic origins of populations. Parabens, like BPA, were linked to cryptorchidism but only for high levels of exposure [64]. A clear association with PFAS is yet to be found, with only a few studies showing a possible link [43]. Similarly, only limited evidence exists for PCBs, with most studies showing no association [64]. In a recent retrospective cohort study on a group of 253 XY individuals who were exposed in utero to diethylstilbestrol (DES), a drug utilized for miscarriage and now ubiquitously banned, Gaspari et al. [213] described two cases of cryptorchidism. Interestingly, those individuals (and two other subjects) reported a female transgender identity later in life. A relationship between hormonal exposure in utero and gender identity has been suggested, and it is known that genes and gonadal hormones can have an effect on psychosexual development, shaping the sexual dimorphism of the brain and contributing to the formation of gender identity [214]. Accordingly, it is possible that endocrine disruptors may also play a role in this aspect, and the hypothesis is fascinating, but the current evidence is too scarce to be able to express a definitive opinion.
Evidence on hypospadias led to ambiguous results, and possible biases should be considered. In fact, prevalence and incidence of this condition are lower, and mild cases may go undiagnosed in early childhood. Initial evidence that altered estrogen balance could lead to hypospadias was derived from studies on DES, particularly if ingestion occurred during the MPW [215]. Regarding pesticides, results are mixed, with potential risk emerging from maternal exposure to elevated concentrations of dichlorodiphenyltrichloroethane (DDT) and its metabolites [216]. Atrazine showed in mice the potential to reduce and shorten AGD; however, human studies showed a non-linear and inconsistent association for hypospadias [217,218]. The mother’s exposure to insect repellents during the first trimester may also be associated with an increased risk for this outcome [219]. For PCBs, most evidence is against their role in the pathogenesis of hypospadias; only a single study found a weak association, but it was non-linear [220]. A study evaluating the combination of a fungicide and PCB showed a link with the fungicide only, with no correlation to PCB [221]. Evidence showed a correlation between hypospadias and phthalate concentrations in blood of children affected by this condition, but not with maternal phthalate levels [222]. Another study evaluating blood samples of children at birth showed an inverse correlation between low-weight phthalates and hypospadias [223]. Evaluation of amniotic fluid in the second trimester (an indication linked to maternal age) showed a weak association for DEHP and no statistically significant association for Diisononylphthalate (DINP) [224]. Even though most human studies are inconclusive, there is biological plausibility (mostly through antiandrogenic effects and more generally through alteration of the androgen–estrogen balance) and epidemiological evidence of pollutant impact from industrialized regions on both cryptorchidism and hypospadias [64,225].
Increasing incidence of testicular cancer has been observed in epidemiological studies that cannot be justified only by genetics, highlighting a possible role of environmental factors [226]. Evidence showed that TGCC has a fetal origin, with pre-cancerous lesions forming during gestation, and there is also a significant association between TGCC and both cryptorchidism and hypospadias, suggesting a possible common origin [227,228]. Studies in humans regarding the impact of EDCs on the pathogenesis of testicular cancer are mainly retrospective. Most of them evaluated persistent EDCs; only a few were conducted on more rapidly metabolized ones (i.e., phthalates and BPA) [229]. With regard to different classes of xenobiotics, pesticides, particularly organochlorines, present some evidence of association with testicular cancer; however, other studies did not find significant correlations [230,231]. A positive association exists for organ halogens, especially for prenatal/maternal exposure; however, some studies controversially showed that postnatal exposure decreases the overall risk of testicular tumors. These results are difficult to interpret, and no clear biological explanation is currently published [232]. Both organochlorines and organ halogens showed an associated increased risk of nonseminoma compared to seminoma, possibly reflecting differences in age at onset [232]. PCBs presented mixed evidence, with some studies showing associations between certain PCB congeners and seminoma patients, while others showed a significantly lower risk compared to controls [233,234]. Limited and mixed evidence of association exists for PFAS, phthalates, and BPA [229,235]. Overall, the data showed an increased risk for fetal windows of exposure, supporting the developmental origin of TGCC; in contrast, postnatal exposures do not appear linked to increased risk [236]. As for cryptorchidism and hypospadias, the plausibility of a link between TGCC and EDCs emerges from studies in the current literature, but methodological problems limit the quality of available evidence, particularly in humans.
The characteristics of the main ECDs are summarized in Table 3.

Table 3.
Main features of the most common EDCs.
8. Possible Mechanistic Models of Synergic Effect of Environmental Factors and Genetic Background on Pathogenesis of TDS
Experimental models to demonstrate the interaction of genetic variants with environmental disruptors were built (Figure 2). Many laboratories used cultures of human testis cell to evaluate the effects of EDCs and drugs on fetal testis. Phthalates exposure led to increased apoptosis of germ cells and bisphenols to reduced testosterone production and INSL3 transcription. Interestingly, paracetamol seemed also to reduce INSL3 production and gonocyte proliferation [12]. Studies using xenograft models of human fetal testis tissue were also carried out. Contrasting results were found, considering that exposure to DBP did not reduce the expression of genes involved in testosterone biosynthesis [242]. Instead, paracetamol exposure reduced testosterone production and lowered seminal vesicle weight in second-semester human fetal testis [12,243]. Mechanistic studies directly evaluating the effects of environmental factors on the presence of genetic variants in TDS models are missing. However, data deriving from mice affected by DSD, a conceptually similar condition, can be analyzed. Xie et al. found that the effects of exposure to different doses of DEHP were different in the presence of a specific genetic variant of the luteinizing hormone/choriogonadotropin receptor (LhcgrW495X/+). Indeed, low-dose DEHP did not exert significant effects on wild-type mouse offspring but caused DSD in LhcgrW495X/+ mice by suppressing the expression of genes involved in steroidogenesis. Moreover, even if high-dose DEHP caused DSD in both genotypes, LhcgrW495X/+ mice had more severe phenotypes [244]. It is possible that a similar mechanistic model could also be applicable to TDS considering the similar etiopathogenesis. Unfortunately, building a mechanistic model evaluating the effects of many maternal and fetal risks previously listed is quite difficult, even if it would surely add important information.
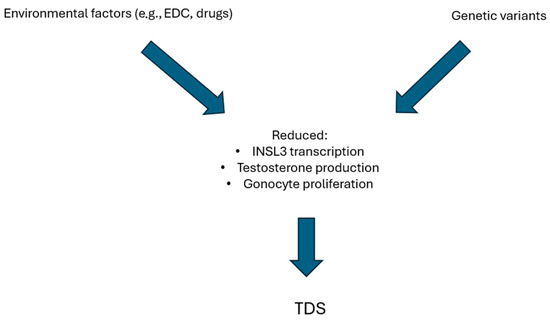
Figure 2.
Hypothesis of mechanistic models of synergic effect of environmental factors and genetic background on pathogenesis of TDS.
9. Conclusions
TDS is a complex syndrome. Concerning genetics, many genes correlated to various components of the syndrome, and others will probably be discovered in following years. Environmental factors and maternal habits are also associated with TDS and represent modifiable factors that should be identified and addressed. Prospective, multicentric, well-designed studies could add important information to this topic, leading to a significant impact on clinical practice as well as a better understanding of the pathogenesis of TDS.
Author Contributions
Conceptualization, A.C. and G.S.; writing—review and editing, all authors; supervision, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TDS | Testicular dysgenesis syndrome |
| GCTs | Germ cells tumors |
| AGD | Anogenital distance |
| INSL3 | Insulin-like factor 3 |
| AR | Androgen receptor |
| EDCs | Endocrine-disrupting chemicals |
| DSDs | Disorders of sex development |
| KCTD13 | Potassium-channel-tetramerization-domain-containing-13 |
| GWAS | Genome-wide association studies |
| SF1 | Steroidogenic Factor 1 |
| DMRT1 | Doublesex and Mab-3 Related Transcription Factor 1 |
| SNP | Single Nucleotide Polimorphism |
| MPW | Male programming window |
| WES | Whole-exome sequencing |
| DHT | Dihydrotestosterone |
| DMRs | Differentially methylated regions |
| WHO | World Health Organization |
| GCNIS | Germ cell neoplasia in situ |
| CIS | Carcinoma-in situ |
| IGCNU | Intratubular germ cell neoplasia unclassified |
| TIN | Testicular intra-epithelial neoplasia |
| ASEX | Arizona Sexual Experiences Scale |
| SFRP1 | Secreted frizzled-related protein 1 |
| IGFBP6 | Insulin-like growth factor binding protein-6 |
| HDAC3 | Histone deacetylase 3 |
| DCN | Decorin |
| ESCs | Embryonic stem cells |
| HDP | Hypertensive disorders of pregnancy |
| LBW | Low birth weight |
| SGA | Small for gestational age |
| ROS | Reactive oxygen species |
| hCG | Human Chorionic Gonadotrophin |
| ART | Assisted Reproductive Technology |
| IVF | In Vitro Fertilization |
| ICSI | Intracytoplasmic Sperm Injection |
| NSAIDs | Non-Steroidal Anti-inflammatory Drugs |
| PFAS | Per- and polyfluoroalkyl Substances |
| PCBs | Polychlorinated biphenyls |
| BPA | Bisphenol A |
| ER | Estrogen receptor |
| DDT | Dichlorodiphenyltrichloroethane |
| DEHP/DBP | Di-2-ethylhexyl phthalate/dibutyl phthalate |
| LH | Luteinizing hormone |
| DES | Diethylstilbestrol |
| DINP | Diisononyl phthalate |
| PFOA | Perfluorooctanoic acid |
| PFOS | Perfluorooctanesulfonic acid |
References
- Thorup, J.; McLachlan, R.; Cortes, D.; Nation, T.R.; Balic, A.; Southwell, B.R.; Hutson, J.M. What Is New in Cryptorchidism and Hypospadias—A Critical Review on the Testicular Dysgenesis Hypothesis. J. Pediatr. Surg. 2010, 45, 2074–2086. [Google Scholar] [CrossRef]
- Xing, J.-S.; Bai, Z.-M. Is Testicular Dysgenesis Syndrome a Genetic, Endocrine, or Environmental Disease, or an Unexplained Reproductive Disorder? Life Sci. 2018, 194, 120–129. [Google Scholar] [CrossRef]
- Hua, X.-G.; Hu, R.; Hu, C.-Y.; Li, F.-L.; Jiang, W.; Zhang, X.-J. Associations between Hypospadias, Cryptorchidism and Anogenital Distance: Systematic Review and Meta-Analysis. Andrologia 2018, 50, e13152. [Google Scholar] [CrossRef]
- Chavarriaga, J.; Nappi, L.; Papachristofilou, A.; Conduit, C.; Hamilton, R.J. Testicular Cancer. Lancet 2025, 406, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Skakkebæk, N.E. Abnormal Morphology of Germ Cells in Two Infertile Men. Acta Pathol. Microbiol. Scand. Sect. A Pathol. 1972, 80A, 374–378. [Google Scholar] [CrossRef]
- Prener, A.; Engholm, G.; Jensen, O.M. Genital Anomalies and Risk for Testicular Cancer in Danish Men. Epidemiology 1996, 7, 14–19. [Google Scholar] [CrossRef]
- Schnack, T.H.; Poulsen, G.; Myrup, C.; Wohlfahrt, J.; Melbye, M. Familial Coaggregation of Cryptorchidism, Hypospadias, and Testicular Germ Cell Cancer: A Nationwide Cohort Study. J. Natl. Cancer Inst. 2010, 102, 187–192. [Google Scholar] [CrossRef]
- Trabert, B.; Zugna, D.; Richiardi, L.; McGlynn, K.A.; Akre, O. Congenital Malformations and Testicular Germ Cell Tumors: Congenital Malformations and TGCT. Int. J. Cancer 2013, 133, 1900–1904. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.; De Meyts, E.R.; Main, K.M. Testicular Dysgenesis Syndrome: An Increasingly Common Developmental Disorder with Environmental Aspects. APMIS 2001, 109, S22–S30. [Google Scholar] [CrossRef]
- Hughes, I.A. Consensus Statement on Management of Intersex Disorders. Arch. Dis. Child. 2005, 91, 554–563. [Google Scholar] [CrossRef]
- Jacobson, J.D.; Willig, L.K.; Gatti, J.; Strickland, J.; Egan, A.; Saunders, C.; Farrow, E.; Heckert, L.L. High Molecular Diagnosis Rate in Undermasculinized Males with Differences in Sex Development Using a Stepwise Approach. Endocrinology 2020, 161, bqz015. [Google Scholar] [CrossRef]
- Jorgensen, A.; Svingen, T.; Miles, H.; Chetty, T.; Stukenborg, J.-B.; Mitchell, R.T. Environmental Impacts on Male Reproductive Development: Lessons from Experimental Models. Horm. Res. Paediatr. 2023, 96, 190–206. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Hsieh, T.-C.; Pastuszak, A.W.; McIntyre, M.G.; Walters, R.C.; Lamb, D.J.; Lipshultz, L.I. The Relationship between Anogenital Distance and the Androgen Receptor CAG Repeat Length. Asian J. Androl. 2013, 15, 286–289. [Google Scholar] [CrossRef]
- Lawaetz, A.C.; Almstrup, K. Involvement of Epigenetic Modifiers in the Pathogenesis of Testicular Dysgenesis and Germ Cell Cancer. Biomol. Concepts 2015, 6, 219–227. [Google Scholar] [CrossRef]
- Virtanen, H.; Rajpertdemeyts, E.; Main, K.; Skakkebaek, N.; Toppari, J. Testicular Dysgenesis Syndrome and the Development and Occurrence of Male Reproductive Disorders. Toxicol. Appl. Pharmacol. 2005, 207, 501–505. [Google Scholar] [CrossRef]
- Allali, S.; Muller, J.-B.; Brauner, R.; Lourenço, D.; Boudjenah, R.; Karageorgou, V.; Trivin, C.; Lottmann, H.; Lortat-Jacob, S.; Nihoul-Fékété, C.; et al. Mutation Analysis of NR5A1 Encoding Steroidogenic Factor 1 in 77 Patients with 46, XY Disorders of Sex Development (DSD) Including Hypospadias. PLoS ONE 2011, 6, e24117. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhang, J.; Xiong, J.; Ma, C.; Yang, B.; Li, H. New Insights into the Potential Mechanisms of Spermatogenic Failure in Patients with Idiopathic Azoospermia. Mol. Human Reprod. 2020, 26, 469–484. [Google Scholar] [CrossRef]
- Wyrwoll, M.J.; Van Der Heijden, G.W.; Krausz, C.; Aston, K.I.; Kliesch, S.; McLachlan, R.; Ramos, L.; Conrad, D.F.; O’Bryan, M.K.; Veltman, J.A.; et al. Improved Phenotypic Classification of Male Infertility to Promote Discovery of Genetic Causes. Nat. Rev. Urol. 2024, 21, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Toppari, J. Trends in the Incidence of Cryptorchidism and Hypospadias, and Methodological Limitations of Registry-Based Data. Hum. Reprod. Update 2001, 7, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, D.; Kaushik, A. Testicular Stem Cell Dysfunction Due to Environmental Insults Could Be Responsible for Deteriorating Reproductive Health of Men. Reprod. Sci. 2021, 28, 649–658. [Google Scholar] [CrossRef]
- Stewart, M.K.; Mattiske, D.M.; Pask, A.J. Exogenous Oestrogen Impacts Cell Fate Decision in the Developing Gonads: A Potential Cause of Declining Human Reproductive Health. Int. J. Mol. Sci. 2020, 21, 8377. [Google Scholar] [CrossRef]
- Calivarathan, L.; Mathur, P.P. Effect of Endocrine Disruptors on Testicular Function. In Molecular Male Reproductive Medicine; Advances in Experimental Medicine and Biology; Cheng, C.Y., Sun, F., Eds.; Springer Nature: Cham, Switzerland, 2025; Volume 1469, pp. 115–125. ISBN 978-3-031-82989-5. [Google Scholar]
- Bergman, Å.; Heindel, J.; Jobling, S.; Kidd, K.; Zoeller, R.T. State-of-the-science of endocrine disrupting chemicals. Toxicol. Lett. 2012, 211, S3. [Google Scholar] [CrossRef]
- Sarath Josh, M.K.; Pradeep, S.; Adarsh, V.K.; Vijayalekshmi Amma, K.S.; Sudha Devi, R.; Balachandran, S.; Sreejith, M.N.; Abdul Jaleel, U.C.; Benjamin, S. In Silico Evidences for the Binding of Phthalates onto Human Estrogen Receptor α, β Subtypes and Human Estrogen-Related Receptor γ. Mol. Simul. 2014, 40, 408–417. [Google Scholar] [CrossRef]
- Luo, Q.; Liu, Z.; Yin, H.; Dang, Z.; Wu, P.; Zhu, N.; Lin, Z.; Liu, Y. Global Review of Phthalates in Edible Oil: An Emerging and Nonnegligible Exposure Source to Human. Sci. Total Environ. 2020, 704, 135369. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, S.; Guo, X.; Ullah, S.; Ullah, S.; Nabi, G.; Wanghe, K. A Review of the Endocrine Disrupting Effects of Micro and Nano Plastic and Their Associated Chemicals in Mammals. Front. Endocrinol. 2023, 13, 1084236. [Google Scholar] [CrossRef]
- Lasaneya, A.; Saikia, Q.; Dutta, S.; Kalita, J.C. Impact of Endocrine Disruptors in Cosmetics on Reproductive Function in Males and Females. J. Environ. Sci. Health C 2025, 43, 184–207. [Google Scholar] [CrossRef] [PubMed]
- Nicolella, H.D.; De Assis, S. Epigenetic Inheritance: Intergenerational Effects of Pesticides and Other Endocrine Disruptors on Cancer Development. Int. J. Mol. Sci. 2022, 23, 4671. [Google Scholar] [CrossRef] [PubMed]
- Gonsioroski, A.; Mourikes, V.E.; Flaws, J.A. Endocrine Disruptors in Water and Their Effects on the Reproductive System. Int. J. Mol. Sci. 2020, 21, 1929. [Google Scholar] [CrossRef]
- Seli, D.A.; Taylor, H.S. The Impact of Air Pollution and Endocrine Disruptors on Reproduction and Assisted Reproduction. Curr. Opin. Obstet. Gynecol. 2023, 35, 210–215. [Google Scholar] [CrossRef]
- Li, X.; Wen, Z.; Wang, Y.; Mo, J.; Zhong, Y.; Ge, R.-S. Bisphenols and Leydig Cell Development and Function. Front. Endocrinol. 2020, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Romao, R.L.P.; Dodds, L.; Ashley-Martin, J.; Monnier, P.; Arbuckle, T.E. Prenatal Exposure to Phthalates and Male Reproductive System Development: Results from a Canadian Pregnancy Cohort Study. Reprod. Toxicol. 2020, 95, 11–18. [Google Scholar] [CrossRef]
- Mongraw-Chaffin, M.L.; Cohn, B.A.; Anglemyer, A.T.; Cohen, R.D.; Christianson, R.E. Maternal Smoking, Alcohol, and Coffee Use during Pregnancy and Son’s Risk of Testicular Cancer. Alcohol 2009, 43, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, H.; Yang, L.; Zhao, M.; Guo, Y.; Bovet, P.; Xi, B. Maternal Cigarette Smoking before or during Pregnancy Increases the Risk of Birth Congenital Anomalies: A Population-Based Retrospective Cohort Study of 12 Million Mother-Infant Pairs. BMC Med. 2022, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Arendt, L.H.; Ramlau-Hansen, C.H.; Lindhard, M.S.; Henriksen, T.B.; Olsen, J.; Yu, Y.; Cnattingius, S. Maternal Overweight and Obesity and Genital Anomalies in Male Offspring: A Population-Based Swedish Cohort Study. Paediatr. Perinat. Epidemiol. 2017, 31, 317–327. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Jolles, M.; Pinotti, R.; Swan, S.H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis of Samples Collected Globally in the 20th and 21st Centuries. Hum. Reprod. Update 2023, 29, 157–176. [Google Scholar] [CrossRef]
- Lokeshwar, S.D.; Patel, P.; Fantus, R.J.; Halpern, J.; Chang, C.; Kargi, A.Y.; Ramasamy, R. Decline in Serum Testosterone Levels Among Adolescent and Young Adult Men in the USA. Eur. Urol. Focus 2021, 7, 886–889. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Furini, C.; Griseta, V.; Zizzi, E.A.; Granata, A.R.M.; Simoni, M. Temporal Trends in Serum Testosterone and Luteinizing Hormone Levels Indicate an Ongoing Resetting of Hypothalamic-Pituitary-Gonadal Function in Healthy Men: A Systematic Review. J. Endocrinol. Investig. 2025, 48, 2721–2734. [Google Scholar] [CrossRef]
- Tanaka, T.; Kojo, K.; Suetomi, T.; Nagumo, Y.; Midorikawa, H.; Matsuda, T.; Nakazono, A.; Shimizu, T.; Fujimoto, S.; Ikeda, A.; et al. Distinct Clusters of Testosterone Levels, Symptoms, and Serum Trace Elements in Young Men: A Cross-Sectional Analysis. Nutrients 2025, 17, 867. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, N.; Wójtowicz, K.; Wdowiak-Filip, A.; Pucek, W.; Wróbel, A.; Wróbel, J.; Wdowiak, A. Environmental Factors as the Main Hormonal Disruptors of Male Fertility. J. Clin. Med. 2024, 13, 1986. [Google Scholar] [CrossRef]
- Woodruff, T.J. Health Effects of Fossil Fuel–Derived Endocrine Disruptors. N. Engl. J. Med. 2024, 390, 922–933. [Google Scholar] [CrossRef]
- Mambrini, S.P.; Menichetti, F.; Ravella, S.; Pellizzari, M.; De Amicis, R.; Foppiani, A.; Battezzati, A.; Bertoli, S.; Leone, A. Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies. Nutrients 2023, 15, 2583. [Google Scholar] [CrossRef]
- Petre, G.; Francini-Pesenti, F.; De Toni, L.; Di Nisio, A.; Mingardi, A.; Cosci, I.; Passerin, N.; Ferlin, A.; Garolla, A. Role of Mediterranean Diet and Ultra-Processed Foods on Sperm Parameters: Data from a Cross-Sectional Study. Nutrients 2025, 17, 2066. [Google Scholar] [CrossRef] [PubMed]
- Ceretti, E.; Bonaccio, M.; Iacoviello, L.; Di Castelnuovo, A.; Ruggiero, E.; Donato, F.; Lorenzetti, S.; Zani, D.; Montano, L. Consumption of Ultra-Processed Foods and Semen Quality in Healthy Young Men Living in Italy. Nutrients 2024, 16, 4129. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A Scale for the Quality Assessment of Narrative Review Articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Kolon, T.F.; Herndon, C.D.A.; Baker, L.A.; Baskin, L.S.; Baxter, C.G.; Cheng, E.Y.; Diaz, M.; Lee, P.A.; Seashore, C.J.; Tasian, G.E.; et al. Evaluation and Treatment of Cryptorchidism: AUA Guideline. J. Urol. 2014, 192, 337–345. [Google Scholar] [CrossRef]
- Soerensen, R.R.; Johannsen, T.H.; Skakkebaek, N.E.; Rajpert-De Meyts, E. Leydig Cell Clustering and Reinke Crystal Distribution in Relation to Hormonal Function in Adult Patients with Testicular Dysgenesis Syndrome (TDS) Including Cryptorchidism. Hormones 2016, 15, 518–526. [Google Scholar] [CrossRef]
- Nistal, M.; Gonzalez-Peramato, P.; De Miguel, M.P. Sertoli Cell Dedifferentiation in Human Cryptorchidism and Gender Reassignment Shows Similarities between Fetal Environmental and Adult Medical Treatment Estrogen and Antiandrogen Exposure. Reprod. Toxicol. 2013, 42, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Ivell, R.; Anand-Ivell, R. Biology of Insulin-like Factor 3 in Human Reproduction. Hum. Reprod. Update 2009, 15, 463–476. [Google Scholar] [CrossRef]
- Abou EL-Ella, S.S.; Tawfik, M.A.; Abd El-Aziz, T.F.; Shalaby, A.M.A.; Barseem, N.F. Correction to: The G178A Polymorphic Variant of INSL3 May Be Linked to Cryptorchidism among Egyptian Pediatric Cohort. Pediatr. Surg. Int. 2020, 36, 1395. [Google Scholar] [CrossRef]
- Fénichel, P.; Chevalier, N.; Lahlou, N.; Coquillard, P.; Wagner-Mahler, K.; Pugeat, M.; Panaïa-Ferrari, P.; Brucker-Davis, F. Endocrine Disrupting Chemicals Interfere with Leydig Cell Hormone Pathways During Testicular Descent in Idiopathic Cryptorchidism. Front. Endocrinol. 2018, 9, 786. [Google Scholar] [CrossRef]
- Ferlin, A.; Pepe, A.; Gianesello, L.; Garolla, A.; Feng, S.; Facciolli, A.; Morello, R.; Agoulnik, A.I.; Foresta, C. New Roles for INSL3 in Adults. Ann. N. Y. Acad. Sci. 2009, 1160, 215–218. [Google Scholar] [CrossRef]
- Jedidi, I.; Ouchari, M.; Yin, Q. Autosomal Single-Gene Disorders Involved in Human Infertility. Saudi J. Biol. Sci. 2018, 25, 881–887. [Google Scholar] [CrossRef]
- Seabra, C.M.; Quental, S.; Neto, A.P.; Carvalho, F.; Gonçalves, J.; Oliveira, J.P.; Fernandes, S.; Sousa, M.; Barros, A.; Amorim, A.; et al. A Novel Alu-Mediated Microdeletion at 11p13 Removes WT1 in a Patient with Cryptorchidism and Azoospermia. Reprod. Biomed. Online 2014, 29, 388–391. [Google Scholar] [CrossRef]
- Tirabassi, G.; Corona, G.; Falzetti, S.; Delli Muti, N.; Maggi, M.; Balercia, G. Influence of Androgen Receptor Gene CAG and GGC Polymorphisms on Male Sexual Function: A Cross-Sectional Study. Int. J. Endocrinol. 2016, 2016, 5083569. [Google Scholar] [CrossRef]
- Seth, A.; Rivera, A.; Chahdi, A.; Choi, I.; Medina-Martinez, O.; Lewis, S.; O’Neill, M.; Ridgeway, A.; Moore, J.; Jorgez, C.; et al. Gene Dosage Changes in KCTD13 Result in Penile and Testicular Anomalies via Diminished Androgen Receptor Function. FASEB J. 2022, 36, e22567. [Google Scholar] [CrossRef] [PubMed]
- Hadziselimovic, F.; Verkauskas, G.; Stadler, M.B. Epigenetics, Cryptorchidism, and Infertility. Basic Clin. Androl. 2023, 33, 24. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, X.; Xiao, X.; Ye, L.; Huang, Y.; Lu, C.; Su, Z. Identification and Functional Characterization of microRNAs in Rat Leydig Cells during Development from the Progenitor to the Adult Stage. Mol. Cell. Endocrinol. 2019, 493, 110453. [Google Scholar] [CrossRef] [PubMed]
- Dujardin, E.; André, M.; Dewaele, A.; Mandon-Pépin, B.; Poulat, F.; Frambourg, A.; Thépot, D.; Jouneau, L.; Jolivet, G.; Pailhoux, E.; et al. DMRT1 Is a Testis-Determining Gene in Rabbits and Is Also Essential for Female Fertility. Elife 2023, 12, RP89284. [Google Scholar] [CrossRef]
- Zhou, B.; Tang, T.; Chen, P.; Pu, Y.; Ma, M.; Zhang, D.; Li, L.; Zhang, P.; Song, Y.; Zhang, L. The Variations in the AXIN1 Gene and Susceptibility to Cryptorchidism. J. Pediatr. Urol. 2015, 11, 132.e1–132.e5. [Google Scholar] [CrossRef]
- Hadziselimovic, F.; Hadziselimovic, N.O.; Demougin, P.; Oakeley, E.J. Decreased Expression of Genes Associated with Memory and X-Linked Mental Retardation in Boys with Non-Syndromic Cryptorchidism and High Infertility Risk. Mol. Syndromol. 2014, 5, 76–80. [Google Scholar] [CrossRef]
- Docampo, M.J.; Hadziselimovic, F. Molecular Pathology of Cryptorchidism-Induced Infertility. Sex. Dev. 2015, 9, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, X.; Zhou, X.; Abulimiti, G.; Wang, Y.; Zhang, Q.; Cong, R.; Ji, C.; Luan, J.; Yao, L.; et al. Identification of Endocrine-Disrupting Chemicals Targeting the Genes and Pathways of Genital Anomalies in Males. Ecotoxicol. Environ. Saf. 2022, 247, 114241. [Google Scholar] [CrossRef]
- Holmboe, S.A.; Beck, A.L.; Andersson, A.-M.; Main, K.M.; Jørgensen, N.; Skakkebæk, N.E.; Priskorn, L. The Epidemiology of Cryptorchidism and Potential Risk Factors, Including Endocrine Disrupting Chemicals. Front. Endocrinol. 2024, 15, 1343887. [Google Scholar] [CrossRef] [PubMed]
- van der Horst, H.J.R.; de Wall, L.L. Hypospadias, All There Is to Know. Eur. J. Pediatr. 2017, 176, 435–441. [Google Scholar] [CrossRef]
- Dalgaard, M.D.; Weinhold, N.; Edsgärd, D.; Silver, J.D.; Pers, T.H.; Nielsen, J.E.; Jørgensen, N.; Juul, A.; Gerds, T.A.; Giwercman, A.; et al. A Genome-Wide Association Study of Men with Symptoms of Testicular Dysgenesis Syndrome and Its Network Biology Interpretation. J. Med. Genet. 2012, 49, 58–65. [Google Scholar] [CrossRef]
- Lavoie, C.; Chun, B.; Au, M.; Do, C.; Baker, Z.; Cortessis, V.; Sparks, S.S.; Syed, H.; Chang, A.Y. Comparing the Incidence of Hypospadias across the United States: A Contemporary Analysis. J. Pediatr. Urol. 2025, 21, 627–632. [Google Scholar] [CrossRef]
- Springer, A.; van den Heijkant, M.; Baumann, S. Worldwide Prevalence of Hypospadias. J. Pediatr. Urol. 2016, 12, 152.e1–152.e7. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.; Wilcox, D. Hypospadias: Lessons Learned. An Overview of Incidence, Epidemiology, Surgery, Research, Complications, and Outcomes. Int. J. Impot. Res. 2023, 35, 61–66. [Google Scholar] [CrossRef]
- Sheriff, F.R.; Lopez, A.; Lupo, P.J.; Seth, A.; Jorgez, C.; Agopian, A.J. Maternal Hypertension and Hypospadias in Offspring: A Systematic Review and Meta-Analysis. Birth Defects Res. 2019, 111, 9–15. [Google Scholar] [CrossRef]
- van den Driesche, S.; Kilcoyne, K.R.; Wagner, I.; Rebourcet, D.; Boyle, A.; Mitchell, R.; McKinnell, C.; Macpherson, S.; Donat, R.; Shukla, C.J.; et al. Experimentally Induced Testicular Dysgenesis Syndrome Originates in the Masculinization Programming Window. JCI Insight 2017, 2, e91204. [Google Scholar] [CrossRef]
- Sharpe, R.M. Androgens and the Masculinization Programming Window: Human-Rodent Differences. Biochem. Soc. Trans. 2020, 48, 1725–1735. [Google Scholar] [CrossRef]
- Giagulli, V.A.; Carbone, M.D.; De Pergola, G.; Guastamacchia, E.; Resta, F.; Licchelli, B.; Sabbà, C.; Triggiani, V. Could Androgen Receptor Gene CAG Tract Polymorphism Affect Spermatogenesis in Men with Idiopathic Infertility? J. Assist. Reprod. Genet. 2014, 31, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Lan, A.; Lin, Z.; Song, J.; Zhang, Y.; Li, J.; Gu, K.; Lv, B.; Zhao, D.; Zeng, S.; et al. Impact of CAG Repeat Length in the Androgen Receptor Gene on Male Infertility—A Meta-Analysis. Reprod. Biomed. Online 2016, 33, 39–49. [Google Scholar] [CrossRef]
- Parada-Bustamante, A.; Lardone, M.C.; Madariaga, M.; Johnson, M.C.; Codner, E.; Cassorla, F.; Castro, A. Androgen Receptor CAG and GGN Polymorphisms in Boys with Isolated Hypospadias. J. Pediatr. Endocrinol. Metab. 2012, 25, 157–162. [Google Scholar] [CrossRef]
- Huang, G.; Shan, W.; Zeng, L.; Huang, L. Androgen Receptor Gene CAG Repeat Polymorphism and Risk of Isolated Hypospadias: Results from a Meta-Analysis. Genet. Mol. Res. 2015, 14, 1580–1588. [Google Scholar] [CrossRef]
- Adamovic, T.; Nordenskjöld, A. The CAG Repeat Polymorphism in the Androgen Receptor Gene Modifies the Risk for Hypospadias in Caucasians. BMC Med. Genet. 2012, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.; Rajpert-De Meyts, E.; Main, K.M.; Skakkebaek, N.E. Testicular Dysgenesis Syndrome Comprises Some but Not All Cases of Hypospadias and Impaired Spermatogenesis. Int. J. Androl. 2010, 33, 298–303. [Google Scholar] [CrossRef]
- Luppino, G.; Wasniewska, M.; Coco, R.; Pepe, G.; Morabito, L.A.; Li Pomi, A.; Corica, D.; Aversa, T. Role of NR5A1 Gene Mutations in Disorders of Sex Development: Molecular and Clinical Features. Curr. Issues Mol. Biol. 2024, 46, 4519–4532. [Google Scholar] [CrossRef]
- Köhler, B.; Lin, L.; Mazen, I.; Cetindag, C.; Biebermann, H.; Akkurt, I.; Rossi, R.; Hiort, O.; Grüters, A.; Achermann, J.C. The Spectrum of Phenotypes Associated with Mutations in Steroidogenic Factor 1 (SF-1, NR5A1, Ad4BP) Includes Severe Penoscrotal Hypospadias in 46,XY Males without Adrenal Insufficiency. Eur. J. Endocrinol. 2009, 161, 237–242. [Google Scholar] [CrossRef]
- Joodi, M.; Amerizadeh, F.; Hassanian, S.M.; Erfani, M.; Ghayour-Mobarhan, M.; Ferns, G.A.; Khazaei, M.; Avan, A. The Genetic Factors Contributing to Hypospadias and Their Clinical Utility in Its Diagnosis. J. Cell. Physiol. 2019, 234, 5519–5523. [Google Scholar] [CrossRef]
- Köhler, B.; Biebermann, H.; Friedsam, V.; Gellermann, J.; Maier, R.F.; Pohl, M.; Wieacker, P.; Hiort, O.; Grüters, A.; Krude, H. Analysis of the Wilms’ Tumor Suppressor Gene (WT1) in Patients 46,XY Disorders of Sex Development. J. Clin. Endocrinol. Metab. 2011, 96, E1131–E1136. [Google Scholar] [CrossRef] [PubMed]
- Bouty, A.; Ayers, K.L.; Pask, A.; Heloury, Y.; Sinclair, A.H. The Genetic and Environmental Factors Underlying Hypospadias. Sex. Dev. 2015, 9, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Kalfa, N.; Gaspari, L.; Ollivier, M.; Philibert, P.; Bergougnoux, A.; Paris, F.; Sultan, C. Molecular Genetics of Hypospadias and Cryptorchidism Recent Developments. Clin. Genet. 2019, 95, 122–131. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Buck Louis, G.M.; Toppari, J.; Andersson, A.-M.; Eisenberg, M.L.; Jensen, T.K.; Jørgensen, N.; Swan, S.H.; Sapra, K.J.; et al. Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility. Physiol. Rev. 2016, 96, 55–97. [Google Scholar] [CrossRef]
- Vottero, A.; Minari, R.; Viani, I.; Tassi, F.; Bonatti, F.; Neri, T.M.; Bertolini, L.; Bernasconi, S.; Ghizzoni, L. Evidence for Epigenetic Abnormalities of the Androgen Receptor Gene in Foreskin from Children with Hypospadias. J. Clin. Endocrinol. Metab. 2011, 96, E1953–E1962. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.A.; Sok, P.; Canon, S.; Nembhard, W.N.; Brown, A.L.; Peckham-Gregory, E.C.; Ton, M.; Ehli, E.A.; Kallsen, N.A.; Peyton, S.A.; et al. Altered Mechanisms of Genital Development Identified through Integration of DNA Methylation and Genomic Measures in Hypospadias. Sci. Rep. 2020, 10, 12715. [Google Scholar] [CrossRef]
- van Bever, Y.; Boers, R.G.; Brüggenwirth, H.T.; van IJcken, W.F.; Magielsen, F.J.; de Klein, A.; Boers, J.B.; Looijenga, L.H.; Brosens, E.; Gribnau, J.; et al. Genome-Wide Methylation Analysis in Patients with Proximal Hypospadias—A Pilot Study and Review of the Literature. Epigenetics 2024, 19, 2392048. [Google Scholar] [CrossRef]
- Yıldız, S.; Inanç, I.; Zhuri, D.; Atlı, E.; Avlan, D. The Possible Role of Epigenetics in the Etiology of Hypospadias. J. Pediatr. Urol. 2024, 20, 877–883. [Google Scholar] [CrossRef]
- Selvi, I.; Ozturk, E.; Yikilmaz, T.N.; Sarikaya, S.; Basar, H. Effects of Testicular Dysgenesis Syndrome Components on Testicular Germ Cell Tumor Prognosis and Oncological Outcomes. Int. Braz. J. Urol. 2020, 46, 725–740. [Google Scholar] [CrossRef]
- Demirci, A.; Başar, H. Effects of Epidemiological Risk Factors on Prognosis in Testicular Cancer. Int. Urol. Nephrol. 2022, 55, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Znaor, A.; Skakkebæk, N.E.; Rajpert-De Meyts, E.; Laversanne, M.; Kuliš, T.; Gurney, J.; Sarfati, D.; McGlynn, K.A.; Bray, F. Testicular Cancer Incidence Predictions in Europe 2010–2035: A Rising Burden despite Population Ageing. Int. J. Cancer 2020, 147, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Ruf, C.G.; Isbarn, H.; Wagner, W.; Fisch, M.; Matthies, C.; Dieckmann, K.-P. Changes in Epidemiologic Features of Testicular Germ Cell Cancer: Age at Diagnosis and Relative Frequency of Seminoma Are Constantly and Significantly Increasing. Urol. Oncol. 2014, 32, 33.e1–33.e6. [Google Scholar] [CrossRef] [PubMed]
- Kojo, K.; Kawai, K.; Kawahara, T.; Kimura, T.; Kandori, S.; Nagumo, Y.; Nitta, S.; Kojima, T.; Okuyama, A.; Higashi, T.; et al. Recent Malignant Testicular Tumor Trend in Japan, a Country with an Aging Population: A Large-Scale Study of 2012–2015 Hospital-Based Cancer Registry Data. Jpn. J. Clin. Oncol. 2020, 50, 1201–1208. [Google Scholar] [CrossRef]
- Smith, Z.L.; Werntz, R.P.; Eggener, S.E. Testicular Cancer. Med. Clin. N. Am. 2018, 102, 251–264. [Google Scholar] [CrossRef]
- Tateo, V.; Thompson, Z.J.; Gilbert, S.M.; Cortessis, V.K.; Daneshmand, S.; Masterson, T.A.; Feldman, D.R.; Pierorazio, P.M.; Prakash, G.; Heidenreich, A.; et al. Epidemiology and Risk Factors for Testicular Cancer: A Systematic Review. Eur. Urol. 2025, 87, 427–441. [Google Scholar] [CrossRef]
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef]
- Skakkebæk, N. Possible Carcinoma-In-Situ of The Testis. Lancet 1972, 300, 516–517. [Google Scholar] [CrossRef]
- Berney, D.M.; Looijenga, L.H.J.; Idrees, M.; Oosterhuis, J.W.; Rajpert-De Meyts, E.; Ulbright, T.M.; Skakkebaek, N.E. Germ Cell Neoplasia in Situ (GCNIS): Evolution of the Current Nomenclature for Testicular Pre-invasive Germ Cell Malignancy. Histopathology 2016, 69, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.; Lindhardt Johansen, M.; Juul, A.; Skakkebaek, N.E.; Main, K.M.; Rajpert-De Meyts, E. Pathogenesis of Germ Cell Neoplasia in Testicular Dysgenesis and Disorders of Sex Development. Semin. Cell Dev. Biol. 2015, 45, 124–137. [Google Scholar] [CrossRef]
- Kathrins, M.; Kolon, T.F. Malignancy in Disorders of Sex Development. Transl. Androl. Urol. 2016, 5, 794–798. [Google Scholar] [CrossRef]
- Hoei-Hansen, C.E. Identification of Genes Differentially Expressed in Testes Containing Carcinoma in Situ. Mol. Hum. Reprod. 2004, 10, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.D.; Murray, M.J.; Saini, H.K.; Van Dongen, S.; Abreu-Goodger, C.; Muralidhar, B.; Pett, M.R.; Thornton, C.M.; Nicholson, J.C.; Enright, A.J.; et al. Malignant Germ Cell Tumors Display Common MicroRNA Profiles Resulting in Global Changes in Expression of Messenger RNA Targets. Cancer Res. 2010, 70, 2911–2923. [Google Scholar] [CrossRef]
- Skakkebæk, N.E.; Berthelsen, J.G.; Giwercman, A.; Müller, J. Carcinoma-in-situ of the Testis: Possible Origin from Gonocytes and Precursor of All Types of Germ Cell Tumours except Spermatocytoma. Int. J. Androl. 1987, 10, 19–28. [Google Scholar] [CrossRef]
- Almstrup, K.; Hoei-Hansen, C.E.; Wirkner, U.; Blake, J.; Schwager, C.; Ansorge, W.; Nielsen, J.E.; Skakkebæk, N.E.; Meyts, E.R.-D.; Leffers, H. Embryonic Stem Cell-Like Features of Testicular Carcinoma in Situ Revealed by Genome-Wide Gene Expression Profiling. Cancer Res. 2004, 64, 4736–4743. [Google Scholar] [CrossRef]
- Sonne, S.B.; Almstrup, K.; Dalgaard, M.; Juncker, A.S.; Edsgard, D.; Ruban, L.; Harrison, N.J.; Schwager, C.; Abdollahi, A.; Huber, P.E.; et al. Analysis of Gene Expression Profiles of Microdissected Cell Populations Indicates That Testicular Carcinoma In Situ Is an Arrested Gonocyte. Cancer Res. 2009, 69, 5241–5250. [Google Scholar] [CrossRef]
- Ottesen, A.M.; Skakkebæk, N.E.; Lundsteen, C.; Leffers, H.; Larsen, J.; Rajpert-De Meyts, E. High-resolution Comparative Genomic Hybridization Detects Extra Chromosome Arm 12p Material in Most Cases of Carcinoma in Situ Adjacent to Overt Germ Cell Tumors, but Not before the Invasive Tumor Development. Genes Chromosomes Cancer 2003, 38, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Hoei-Hansen, C.E.; Holm, M.; Rajpert-De Meyts, E.; Skakkebaek, N.E. Histological Evidence of Testicular Dysgenesis in Contralateral Biopsies from 218 Patients with Testicular Germ Cell Cancer. J. Pathol. 2003, 200, 370–374. [Google Scholar] [CrossRef]
- O’Dowd, J.; Gaffney, E.F.; Young, R.H. Malignant Sex Cord–Stromal Tumour in a Patient with the Androgen Insensitivity Syndrome. Histopathology 1990, 16, 279–282. [Google Scholar] [CrossRef]
- Slowikowska-Hilczer, J.; Szarras-Czapnik, M.; Duranteau, L.; Rapp, M.; Walczak-Jedrzejowska, R.; Marchlewska, K.; Oszukowska, E.; Nordenstrom, A. Risk of Gonadal Neoplasia in Patients with Disorders/Differences of Sex Development. Cancer Epidemiol. 2020, 69, 101800. [Google Scholar] [CrossRef]
- Ferlin, A.; Calogero, A.E.; Krausz, C.; Lombardo, F.; Paoli, D.; Rago, R.; Scarica, C.; Simoni, M.; Foresta, C.; Rochira, V.; et al. Management of Male Factor Infertility: Position Statement from the Italian Society of Andrology and Sexual Medicine (SIAMS): Endorsing Organization: Italian Society of Embryology, Reproduction, and Research (SIERR). J. Endocrinol. Invest. 2022, 45, 1085–1113. [Google Scholar] [CrossRef]
- Tüttelmann, F.; Wyrwoll, M.J.; Steingröver, J.; Wieacker, P. The Genetics of Female and Male Infertility. Dtsch. Ärzteblatt Int. 2025, 122, 115–120. [Google Scholar] [CrossRef]
- Metin Mahmutoglu, A.; Hurre Dirie, S.; Hekim, N.; Gunes, S.; Asci, R.; Henkel, R. Polymorphisms of Androgens-related Genes and Idiopathic Male Infertility in Turkish Men. Andrologia 2022, 54, e14270. [Google Scholar] [CrossRef]
- Osadchuk, L.; Vasiliev, G.; Kleshchev, M.; Osadchuk, A. Androgen Receptor Gene CAG Repeat Length Varies and Affects Semen Quality in an Ethnic-Specific Fashion in Young Men from Russia. Int. J. Mol. Sci. 2022, 23, 10594. [Google Scholar] [CrossRef]
- Sharestani, S.; Mehdi Kalantar, S.; Ghasemi, N.; Farashahi Yazd, E. CAG Repeat Polymorphism in Androgen Receptor and Infertility: A Case-Control Study. Int. J. Reprod. BioMed. 2021, 19, 845–850. [Google Scholar] [CrossRef]
- Welsh, M.; Suzuki, H.; Yamada, G. The Masculinization Programming Window. In Endocrine Development; Hiort, O., Ahmed, S.F., Eds.; S. Karger AG: Basel, Switzerland, 2014; Volume 27, pp. 17–27. ISBN 978-3-318-02558-3. [Google Scholar]
- Moreno-Mendoza, D.; Casamonti, E.; Riera-Escamilla, A.; Pietroforte, S.; Corona, G.; Ruiz-Castañe, E.; Krausz, C. Short Anogenital Distance Is Associated with Testicular Germ Cell Tumour Development. Andrology 2020, 8, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.L.; Shy, M.; Chanc Walters, R.; Lipshultz, L.I. The Relationship between Anogenital Distance and Azoospermia in Adult Men. Int. J. Androl. 2012, 35, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Tsutida, C.A.; Veiga, A.C.B.; Martino-Andrade, A.J.; Andrade, D.P.D.; Mello, R.G.; Müller, J.C. Association between Late Manifestations of Testicular Dysgenesis Syndrome and Anogenital Distance: A Systematic Review and Meta-Analysis. J. Hum. Reprod. Sci. 2023, 16, 174–184. [Google Scholar] [CrossRef]
- Priskorn, L.; Kreiberg, M.; Bandak, M.; Lauritsen, J.; Daugaard, G.; Petersen, J.H.; Aksglaede, L.; Juul, A.; Jørgensen, N. Testicular Cancer Survivors Have Shorter Anogenital Distance That Is Not Increased by 1 Year of Testosterone Replacement Therapy. Hum. Reprod. 2021, 36, 2443–2451. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Iwamoto, T.; Yamasaki, K.; Kariya, F.; Konishi, S. The Ratio of 2nd to 4th Digit Length and Reproductive Function of Infertile Male Patients. Reprod. Med. Biol. 2023, 22, e12500. [Google Scholar] [CrossRef]
- Stephen, M.A.; Burke, C.R.; Steele, N.; Pryce, J.E.; Meier, S.; Amer, P.R.; Phyn, C.V.C.; Garrick, D.J. Genome-Wide Association Study of Anogenital Distance and Its (Co)Variances with Fertility in Growing and Lactating Holstein-Friesian Dairy Cattle. J. Dairy Sci. 2023, 106, 7846–7860. [Google Scholar] [CrossRef]
- Souali-Crespo, S.; Condrea, D.; Vernet, N.; Féret, B.; Klopfenstein, M.; Grandgirard, E.; Alunni, V.; Cerciat, M.; Jung, M.; Mayere, C.; et al. Loss of NR5A1 in Mouse Sertoli Cells after Sex Determination Changes Cellular Identity and Induces Cell Death by Anoikis. Development 2023, 150, dev201710. [Google Scholar] [CrossRef]
- Sathyanarayana, S.; Swan, S.H.; Farin, F.M.; Wilkerson, H.-W.; Bamshad, M.; Grady, R.; Zhou, C.; Schwartz, S.M. A Pilot Study of the Association between Genetic Polymorphisms Involved in Estrogen Signaling and Infant Male Genital Phenotypes. Asian J. Androl. 2012, 14, 766–772. [Google Scholar] [CrossRef]
- Ciarloni, A.; Delli Muti, N.; Ambo, N.; Perrone, M.; Rossi, S.; Sacco, S.; Salvio, G.; Balercia, G. Contribution of Androgen Receptor CAG Repeat Polymorphism to Human Reproduction. DNA 2025, 5, 9. [Google Scholar] [CrossRef]
- Ghadirkhomi, E.; Abdolhamid Angaji, S.; Khosravi, M.; Reza Mashayekhi, M. Association Study of Novel Single Nucleotide Polymorphisms of Androgen Receptor and Estrogen Receptor-α Genes with Male Infertility in Northwest of Iran: A Case-Control Study. Int. J. Reprod. BioMed. 2022, 20, 501–510. [Google Scholar] [CrossRef]
- Syryn, H.; Van De Velde, J.; De Clercq, G.; Verdin, H.; Dheedene, A.; Peelman, F.; Sinclair, A.; Ayers, K.L.; Bathgate, R.A.D.; Cools, M.; et al. Biallelic RXFP2 Variants Lead to Congenital Bilateral Cryptorchidism and Male Infertility, Supporting a Role of RXFP2 in Spermatogenesis. Hum. Reprod. 2024, 39, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jia, J.; Sun, M.; Li, M.; Liu, N. Mutational Screening of the INSL3 Gene in Azoospermic Males with a History of Cryptorchidism. Andrologia 2016, 48, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Dicke, A.-K.; Albrethsen, J.; Hoare, B.L.; Wyrwoll, M.J.; Busch, A.S.; Fietz, D.; Pilatz, A.; Bühlmann, C.; Juul, A.; Kliesch, S.; et al. Bi-Allelic Variants in INSL3 and RXFP2 Cause Bilateral Cryptorchidism and Male Infertility. Hum. Reprod. 2023, 38, 1412–1423. [Google Scholar] [CrossRef]
- Fabbri-Scallet, H.; Sousa, L.M.; Maciel-Guerra, A.T.; Guerra-Júnior, G.; Mello, M.P. Mutation Update for the NR5A1 Gene Involved in DSD and Infertility. Hum. Mutat. 2020, 41, 58–68. [Google Scholar] [CrossRef]
- Behvarz, M.; Rahmani, S.A.; Siasi Torbati, E.; Danaei Mehrabad, S.; Bikhof Torbati, M. Correlación entre el polimorfismo de los genes LHCGR y NR5A1y el riesgo de infertilidad masculina. Actas Urológicas Españolas 2024, 48, 246–253. [Google Scholar] [CrossRef]
- Saucedo, L.; Rumpel, R.; Sobarzo, C.; Schreiner, D.; Brandes, G.; Lustig, L.; Vazquez-Levin, M.H.; Grothe, C.; Marín-Briggiler, C. Deficiency of Fibroblast Growth Factor 2 (FGF-2) Leads to Abnormal Spermatogenesis and Altered Sperm Physiology. J. Cell. Physiol. 2018, 233, 9640–9651. [Google Scholar] [CrossRef]
- Hadziselimovic, F.; Verkauskas, G.; Stadler, M. A Novel Role for CFTR Interaction with LH and FGF in Azoospermia and Epididymal Maldevelopment Caused by Cryptorchidism. Basic Clin. Androl. 2022, 32, 10. [Google Scholar] [CrossRef]
- McCallie, B.R.; Parks, J.C.; Griffin, D.K.; Schoolcraft, W.B.; Katz-Jaffe, M.G. Infertility Diagnosis Has a Significant Impact on the Transcriptome of Developing Blastocysts. MHR Basic Sci. Reprod. Med. 2017, 23, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Singh, P.; Gupta, N.J.; Sankhwar, S.N.; Chakravarty, B.; Thangaraj, K.; Rajender, S. Mutations in the Desert Hedgehog (DHH) Gene in the Disorders of Sexual Differentiation and Male Infertility. J. Assist. Reprod. Genet. 2021, 38, 1871–1878. [Google Scholar] [CrossRef]
- Zhang, M.-F.; Wan, S.-C.; Chen, W.-B.; Yang, D.-H.; Liu, W.-Q.; Li, B.-L.; Aierken, A.; Du, X.-M.; Li, Y.-X.; Wu, W.-P.; et al. Transcription Factor Dmrt1 Triggers the SPRY1-NF-κB Pathway to Maintain Testicular Immune Homeostasis and Male Fertility. Zool. Res. 2023, 44, 505–521. [Google Scholar] [CrossRef]
- Li, Z.; Zhuang, X.; Zeng, J.; Tzeng, C.-M. Integrated Analysis of DNA Methylation and mRNA Expression Profiles to Identify Key Genes in Severe Oligozoospermia. Front. Physiol. 2017, 8, 261. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, T.-J.; Liu, W.; Ning, Y.-N.; Bian, Y.-H.; Cao, Y.-Z.; Liu, H.-B.; Ma, J.-L.; Zhang, H.-B. Mutational Analysis of the GATA4 Gene in Chinese Men with Nonobstructive Azoospermia. Asian J. Androl. 2021, 23, 205–210. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, Y.; Wang, Z.-P.; Wang, X.-X.; Sun, T.-C.; Li, X.-Y.; Tang, J.-X.; Cheng, J.-M.; Li, J.; Chen, S.-R.; et al. EZH2 Deletion Promotes Spermatogonial Differentiation and Apoptosis. Reproduction 2017, 154, 615–625. [Google Scholar] [CrossRef]
- Cardoso, H.J.; Figueira, M.I.; Correia, S.; Vaz, C.V.; Socorro, S. The SCF/c-KIT System in the Male: Survival Strategies in Fertility and Cancer: SCF/c-KIT System in Male Fertility and Cancer. Mol. Reprod. Dev. 2014, 81, 1064–1079. [Google Scholar] [CrossRef]
- Wang, X.; Lin, D.-H.; Yan, Y.; Wang, A.-H.; Liao, J.; Meng, Q.; Yang, W.-Q.; Zuo, H.; Hua, M.-M.; Zhang, F.; et al. The PIWI-Specific Insertion Module Helps Load Longer piRNAs for Translational Activation Essential for Male Fertility. Sci. China Life Sci. 2023, 66, 1459–1481. [Google Scholar] [CrossRef]
- Alankarage, D.; Lavery, R.; Svingen, T.; Kelly, S.; Ludbrook, L.; Bagheri-Fam, S.; Koopman, P.; Harley, V. SOX9 Regulates Expression of the Male Fertility Gene Ets Variant Factor 5 (ETV5) during Mammalian Sex Development. Int. J. Biochem. Cell Biol. 2016, 79, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Roshan, M.M.; Azizi, H.; Majelan, M.A.; Tabar, A.N. Sox9 Downregulation in Non-Obstructive Azoospermia by UTF1 and Mediator Role of POU5F1. BMC Res. Notes 2024, 17, 77. [Google Scholar] [CrossRef]
- Basu, S.; Arya, S.P.; Usmani, A.; Pradhan, B.S.; Sarkar, R.K.; Ganguli, N.; Shukla, M.; Mandal, K.; Singh, S.; Sarda, K.; et al. Defective Wnt3 Expression by Testicular Sertoli Cells Compromise Male Fertility. Cell Tissue Res. 2018, 371, 351–363. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, L.; Yu, W.; Guo, H.; Zhang, H.; Wei, D.; Liang, L.; Feng, K.; Song, X.; Liu, Q.; et al. A Novel Functional Variant in Wilms’ Tumor 1 (WT1) Is Associated with Idiopathic Non-obstructive Azoospermia. Mol. Reprod. Dev. 2017, 84, 222–228. [Google Scholar] [CrossRef]
- Sharifi, N.; Boroujeni, P.B.; Haratian, K.; Sabbaghian, M.; Meybodi, A.M. WT1 Pathogenic Variant in Azoospermic Infertile Men with an Isolated Undescended Testis. Clin. Exp. Reprod. Med. 2025, 52, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.; Azab, S.; El Baz, M.; Fawaz, L.; Bahagat, A. Cryptorchidism in Egyptian Neonates. J. Pediatr. Urol. 2013, 9, 815–819. [Google Scholar] [CrossRef]
- Mavrogenis, S.; Urban, R.; Czeizel, A.E.; Ács, N. Possible Association of Maternal Factors with the Higher Risk of Isolated True Undescended Testis: A Population-based Case-control Study. Congenit. Anom. 2014, 54, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.; Skakkebæk, N.E. Testicular Cancer and Cryptorchidism in Relation to Prenatal Factors: Case-Control Studies in Denmark. Cancer Causes Control 1997, 8, 904–912. [Google Scholar] [CrossRef]
- Aschim, E.L.; Haugen, T.B.; Tretli, S.; Daltveit, A.K.; Grotmol, T. Risk Factors for Hypospadias in Norwegian Boys—Association with Testicular Dysgenesis Syndrome? Int. J. Androl. 2004, 27, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Yang, Z.; Chen, F.; Liu, Z.; Tang, Z. Association between Perinatal Factors and Hypospadias in Newborns: A Retrospective Case–Control Study of 42,244 Male Infants. BMC Pregnancy Childbirth 2022, 22, 579. [Google Scholar] [CrossRef]
- Westergaard, T.; Andersen, P.K.; Pedersen, J.B.; Frisch, M.; Olsen, J.H.; Melbye, M. Testicular Cancer Risk and Maternal Parity: A Population-Based Cohort Study. Br. J. Cancer 1998, 77, 1180–1185. [Google Scholar] [CrossRef]
- Hjertkvist, M.; Damber, J.E.; Bergh, A. Cryptorchidism: A Registry Based Study in Sweden on Some Factors of Possible Aetiological Importance. J. Epidemiol. Community Health 1989, 43, 324–329. [Google Scholar] [CrossRef]
- Cook, M.B.; Akre, O.; Forman, D.; Madigan, M.P.; Richiardi, L.; McGlynn, K.A. A Systematic Review and Meta-Analysis of Perinatal Variables in Relation to the Risk of Testicular Cancer—Experiences of the Mother. Int. J. Epidemiol. 2009, 38, 1532–1542. [Google Scholar] [CrossRef]
- Nijsten, K.; Jansen, L.A.W.; Limpens, J.; Finken, M.J.J.; Koot, M.H.; Grooten, I.J.; Roseboom, T.J.; Painter, R.C. Long-Term Health Outcomes of Children Born to Mothers with Hyperemesis Gravidarum: A Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2022, 227, 414–429.e17. [Google Scholar] [CrossRef] [PubMed]
- Sijstermans, K.; Hack, W.W.M.; Meijer, R.W.; Voort-Doedens, L.M.V.D. The Frequency of Undescended Testis from Birth to Adulthood: A Review. Int. J. Androl. 2008, 31, 1–11. [Google Scholar] [CrossRef]
- Zhang, T.-N.; Huang, X.-M.; Zhao, X.-Y.; Wang, W.; Wen, R.; Gao, S.-Y. Risks of Specific Congenital Anomalies in Offspring of Women with Diabetes: A Systematic Review and Meta-Analysis of Population-Based Studies Including over 80 Million Births. PLoS Med. 2022, 19, e1003900. [Google Scholar] [CrossRef]
- Arendt, L.H.; Lindhard, M.S.; Henriksen, T.B.; Olsen, J.; Cnattingius, S.; Petersson, G.; Parner, E.T.; Ramlau-Hansen, C.H. Maternal Diabetes Mellitus and Genital Anomalies in Male Offspring: A Nationwide Cohort Study in 2 Nordic Countries. Epidemiology 2018, 29, 280–289. [Google Scholar] [CrossRef]
- Virtanen, H.E.; Tapanainen, A.E.; Kaleva, M.M.; Suomi, A.-M.; Main, K.M.; Skakkebaek, N.E.; Toppari, J. Mild Gestational Diabetes as a Risk Factor for Congenital Cryptorchidism. J. Clin. Endocrinol. Metab. 2006, 91, 4862–4865. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, B.; Sun, Y.; Du, Y.; Santillan, M.K.; Santillan, D.A.; Snetselaar, L.G.; Bao, W. Association of Maternal Prepregnancy Diabetes and Gestational Diabetes Mellitus with Congenital Anomalies of the Newborn. Diabetes Care 2020, 43, 2983–2990. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.-H.; Zheng, X.-M.; Liu, T.-Z.; Zhang, W.-B.; Zheng, H.; Chen, M.-F. Maternal Gestational Smoking, Diabetes, Alcohol Drinking, Pre-Pregnancy Obesity and the Risk of Cryptorchidism: A Systematic Review and Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0119006. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.V.; Hastert, T.A.; Huang, Y.; Starr, J.R. No Association between Maternal Pre-Pregnancy Obesity and Risk of Hypospadias or Cryptorchidism in Male Newborns. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 241–248. [Google Scholar] [CrossRef]
- Waller, D.K. Prepregnancy Obesity as a Risk Factor for Structural Birth Defects. Arch. Pediatr. Adolesc. Med. 2007, 161, 745. [Google Scholar] [CrossRef]
- Indriasari, V.; Diposarosa, R.; Syukriani, Y.F.; Rachmadi, D. Nongenetic Risk Factors of Severe Hypospadias: A Case–Control Study. J. Indian Assoc. Pediatr. Surg. 2024, 29, 488–491. [Google Scholar] [CrossRef]
- Carmichael, S.L.; Rasmussen, S.A.; Shaw, G.M. Prepregnancy Obesity: A Complex Risk Factor for Selected Birth Defects. Birth Defects Res. 2010, 88, 804–810. [Google Scholar] [CrossRef]
- Wang, Z.; Niu, J.; Ji, H.; Miao, M.; Yang, L.; Chen, X.; Li, X.; Song, X.; Chen, A.; Liang, H.; et al. Association of Pre-Pregnancy Body Mass Index and Gestational Weight Gain with Neonatal Anogenital Distance in a Chinese Birth Cohort. Reprod. Health 2022, 19, 152. [Google Scholar] [CrossRef]
- North, K.; Golding, J. The Alspac Study Team a Maternal Vegetarian Diet in Pregnancy is Associated with Hypospadias. BJU Int. 2000, 85, 107–113. [Google Scholar] [CrossRef]
- Carmichael, S.L.; Ma, C.; Feldkamp, M.L.; Munger, R.G.; Olney, R.S.; Botto, L.D.; Shaw, G.M.; Correa, A. Nutritional Factors and Hypospadias Risks. Paediatr. Perinat. Epidemiol. 2012, 26, 353–360. [Google Scholar] [CrossRef]
- Evenson, K.R.; Mowla, S.; Olshan, A.F.; Shaw, G.M.; Ailes, E.C.; Reefhuis, J.; Joshi, N.; Desrosiers, T.A.; The National Birth Defects Prevention Study and Birth Defects Study to Evaluate Pregnancy exposureS. Maternal Physical Activity, Sitting, and Risk of Non-Cardiac Birth Defects. Pediatr. Res. 2024, 95, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.-H.; Chen, H.-S.; Zhang, Z.-C.; Wang, X.; Liu, X.; Wei, G.-H. Parental Smoking and Risk of Hypospadias: An Updated Meta-Analysis of Observational Studies. Front. Pediatr. 2023, 11, 1003037. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, A.; Rodeck, C.; Boniface, S. Maternal Smoking in Pregnancy and Birth Defects: A Systematic Review Based on 173 687 Malformed Cases and 11.7 Million Controls. Hum. Reprod. Update 2011, 17, 589–604. [Google Scholar] [CrossRef]
- Yu, C.; Wei, Y.; Tang, X.; Liu, B.; Shen, L.; Long, C.; Lin, T.; He, D.; Wu, S.; Wei, G. Correction to: Maternal Smoking during Pregnancy and Risk of Cryptorchidism: A Systematic Review and Meta-Analysis. Eur. J. Pediatr. 2019, 178, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.S.; Toft, G.; Thulstrup, A.M.; Bonde, J.P.; Olsen, J. Cryptorchidism According to Maternal Gestational Smoking. Epidemiology 2007, 18, 220–225. [Google Scholar] [CrossRef]
- Lindbo, D.; Arendt, L.H.; Ernst, A.; Lunddorf, L.L.H.; Brix, N.; Ramlau-Hansen, C.H. Maternal Cigarette Smoking During Pregnancy and Genital Anomalies in Boys: A Register-Based Cohort and Sibling-Matched Design Study. Clin. Epidemiol. 2022, 14, 901–910. [Google Scholar] [CrossRef]
- Carmichael, S.L.; Shaw, G.M.; Laurent, C.; Lammer, E.J.; Olney, R.S.; The National Birth Defects Prevention Study. Hypospadias and Maternal Exposures to Cigarette Smoke. Paediatr. Perinat. Epidemiol. 2005, 19, 406–412. [Google Scholar] [CrossRef]
- Akre, O.; Boyd, H.A.; Ahlgren, M.; Wilbrand, K.; Westergaard, T.; Hjalgrim, H.; Nordenskjöld, A.; Ekbom, A.; Melbye, M. Maternal and Gestational Risk Factors for Hypospadias. Environ. Health Perspect. 2008, 116, 1071–1076. [Google Scholar] [CrossRef]
- Kjersgaard, C.; Arendt, L.H.; Ernst, A.; Lindhard, M.S.; Olsen, J.; Henriksen, T.B.; Strandberg-Larsen, K.; Ramlau-Hansen, C.H. Lifestyle in Pregnancy and Cryptorchidism in Sons: A Study within Two Large Danish Birth Cohorts. Clin. Epidemiol. 2018, 10, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, J.; Holl, K.; Rantakokko, P.; Koskela, P.; Hallmans, G.; Wadell, G.; Stattin, P.; Dillner, J.; Ogmundsdottir, H.M.; Vartiainen, T.; et al. Maternal Smoking during Pregnancy and Testicular Cancer in the Sons: A Nested Case-Control Study and a Meta-Analysis. Eur. J. Cancer 2009, 45, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, X.; Wei, C.; Luo, J.; Shi, Y.; Lin, T.; He, D.; Wei, G. Assisted Reproductive Technologies and the Risk of Congenital Urogenital Tract Malformations: A Systematic Review and Meta-Analysis. J. Pediatr. Urol. 2021, 17, 9–20. [Google Scholar] [CrossRef]
- Bang, J.K.; Lyu, S.W.; Choi, J.; Lee, D.R.; Yoon, T.K.; Song, S.-H. Does Infertility Treatment Increase Male Reproductive Tract Disorder? Urology 2013, 81, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Lind, D.V.; Main, K.M.; Kyhl, H.B.; Kristensen, D.M.; Toppari, J.; Andersen, H.R.; Andersen, M.S.; Skakkebæk, N.E.; Jensen, T.K. Maternal Use of Mild Analgesics during Pregnancy Associated with Reduced Anogenital Distance in Sons: A Cohort Study of 1027 Mother-Child Pairs. Hum. Reprod. 2017, 32, 223–231. [Google Scholar] [CrossRef]
- Navarro-Lafuente, F.; Arense-Gonzalo, J.; Adoamnei, E.; Prieto-Sánchez, M.; Sánchez-Ferrer, M.; García-Marcos, L.; Morales, E.; Mendiola, J.; Torres-Cantero, A.; The NELA Study Group. Is Maternal Use of Paracetamol during Pregnancy Associated with Anogenital Distance in Male Newborns? The Results from the NELA Birth Cohort. Int. J. Environ. Res. Public Health 2021, 18, 6338. [Google Scholar] [CrossRef]
- Hoeltzenbein, M.; Stieler, K.; Panse, M.; Wacker, E.; Schaefer, C. Allopurinol Use during Pregnancy—Outcome of 31 Prospectively Ascertained Cases and a Phenotype Possibly Indicative for Teratogenicity. PLoS ONE 2013, 8, e66637. [Google Scholar] [CrossRef]
- Kozenko, M.; Grynspan, D.; Oluyomi-Obi, T.; Sitar, D.; Elliott, A.M.; Chodirker, B.N. Potential Teratogenic Effects of Allopurinol: A Case Report. Am. J. Med. Genet. A 2011, 155, 2247–2252. [Google Scholar] [CrossRef]
- Veroniki, A.A.; Cogo, E.; Rios, P.; Straus, S.E.; Finkelstein, Y.; Kealey, R.; Reynen, E.; Soobiah, C.; Thavorn, K.; Hutton, B.; et al. Comparative Safety of Anti-Epileptic Drugs during Pregnancy: A Systematic Review and Network Meta-Analysis of Congenital Malformations and Prenatal Outcomes. BMC Med. 2017, 15, 95. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, J.; Xu, L.; Chen, M.; Wan, L. Risk of Congenital Malformations in Offspring of Women Using Β-blockers during Early Pregnancy: An Updated Meta-analysis of Observational Studies. Br. J. Clin. Pharmacol. 2021, 87, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Yakoob, M.Y.; Bateman, B.T.; Ho, E.; Hernandez-Diaz, S.; Franklin, J.M.; Goodman, J.E.; Hoban, R.A. The Risk of Congenital Malformations Associated with Exposure to β-Blockers Early in Pregnancy: A Meta-Analysis. Hypertension 2013, 62, 375–381. [Google Scholar] [CrossRef]
- Keskin-Arslan, E.; Erol, H.; Uysal, N.; Karadas, B.; Temiz, T.; Kaplan, Y.C. Pregnancy Outcomes Following Maternal Macrolide Use: A Systematic Review and Meta-Analysis. Reprod. Toxicol. 2023, 115, 124–146. [Google Scholar] [CrossRef]
- Moller, H.; Skakkebæk, N.E. Risks of Testicular Cancer and Cryptorchidism in Relation to Socio-Economic Status and Related Factors: Case-Control Studies in Denmark. Int. J. Cancer 1996, 66, 287–293. [Google Scholar] [CrossRef]
- Levine, H.; Keinan-Boker, L.; Leiba, A.; Derazne, E.; Rais, A.; Kark, J.D. Paternal Age and Risk of Testicular Germ Cell Tumors: A Cohort Study of 1,000,000 Men. Andrology 2017, 5, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A.; Moustakli, E.; Zikopoulos, A.; Georgiou, I.; Dimitriadis, F.; Symeonidis, E.N.; Markou, E.; Michaelidis, T.M.; Tien, D.M.B.; Giannakis, I.; et al. Impact of Advanced Paternal Age on Fertility and Risks of Genetic Disorders in Offspring. Genes 2023, 14, 486. [Google Scholar] [CrossRef]
- Estors Sastre, B.; Campillo Artero, C.; González Ruiz, Y.; Fernández Atuan, R.L.; Bragagnini Rodríguez, P.; Frontera Juan, G.; Gracia Romero, J. Occupational Exposure to Endocrine-Disrupting Chemicals and Other Parental Risk Factors in Hypospadias and Cryptorchidism Development: A Case–Control Study. J. Pediatr. Urol. 2019, 15, 520.e1–520.e8. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, C.A.; Chen, T.; Mulloy, E.; Shaw, G.M.; Eisenberg, M.L. Congenital Male Genital Malformations and Paternal Health: An Analysis of the US Claims Data. Andrology 2023, 11, 1114–1120. [Google Scholar] [CrossRef]
- Sharpe, R.M.; Skakkebaek, N.E. Are Oestrogens Involved in Falling Sperm Counts and Disorders of the Male Reproductive Tract? Lancet 1993, 341, 1392–1396. [Google Scholar] [CrossRef]
- Hilz, E.N.; Gore, A.C. Endocrine-Disrupting Chemicals: Science and Policy. Policy Insights Behav. Brain Sci. 2023, 10, 142–150. [Google Scholar] [CrossRef]
- Matsushima, A.; Kakuta, Y.; Teramoto, T.; Koshiba, T.; Liu, X.; Okada, H.; Tokunaga, T.; Kawabata, S.-i.; Kimura, M.; Shimohigashi, Y. Structural Evidence for Endocrine Disruptor Bisphenol A Binding to Human Nuclear Receptor ERR. J. Biochem. 2007, 142, 517–524. [Google Scholar] [CrossRef]
- Bouskine, A.; Nebout, M.; Brücker-Davis, F.; Benahmed, M.; Fenichel, P. Low Doses of Bisphenol a Promote Human Seminoma Cell Proliferation by Activating PKA and PKG via a Membrane G-Protein–Coupled Estrogen Receptor. Environ. Health Perspect. 2009, 117, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.; Li, Q.; Zhang, T.; Deng, Z.; Lian, J.; Jia, D.; Li, R.; Zheng, T.; Ding, X.; et al. Low Concentration of BPA Induces Mice Spermatocytes Apoptosis via GPR30. Oncotarget 2017, 8, 49005–49015. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M.; Ahmad, E.; Sheikh, I.A.; Abuzenadah, A.M.; Damanhouri, G.A.; Bajouh, O.S.; AlBasri, S.F.; Assiri, M.M.; Beg, M.A. Androgen and Progesterone Receptors Are Targets for Bisphenol A (BPA), 4-Methyl-2,4-Bis-(P-Hydroxyphenyl)Pent-1-Ene—A Potent Metabolite of BPA, and 4-Tert-Octylphenol: A Computational Insight. PLoS ONE 2015, 10, e0138438. [Google Scholar] [CrossRef]
- Kundakovic, M.; Champagne, F.A. Epigenetic Perspective on the Developmental Effects of Bisphenol A. Brain Behav. Immun. 2011, 25, 1084–1093. [Google Scholar] [CrossRef]
- Zhuang, S.; Zhang, J.; Wen, Y.; Zhang, C.; Liu, W. Distinct Mechanisms of Endocrine Disruption of DDT-Related Pesticides toward Estrogen Receptor α and Estrogen-Related Receptor γ. Environ. Toxicol. Chem. 2012, 31, 2597–2605. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J. Effects of p,P′ -DDE Exposure on Gonadal Development and Gene Expression in Japanese Medaka (Oryzias latipes). J. Environ. Sci. 2008, 20, 347–352. [Google Scholar] [CrossRef]
- Li, X.; Ye, L.; Wang, X.; Wang, X.; Liu, H.; Qian, X.; Zhu, Y.; Yu, H. Molecular Docking, Molecular Dynamics Simulation, and Structure-Based 3D-QSAR Studies on Estrogenic Activity of Hydroxylated Polychlorinated Biphenyls. Sci. Total Environ. 2012, 441, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Mennigen, J.A.; Thompson, L.M.; Bell, M.; Tellez Santos, M.; Gore, A.C. Transgenerational Effects of Polychlorinated Biphenyls: 1. Development and Physiology across 3 Generations of Rats. Environ. Health 2018, 17, 18. [Google Scholar] [CrossRef]
- Oyovwi, M.O.; Oyelami, J.O.; Babawale, K.H.; Ben-Azu, B. Preventive Antioxidant Strategies to Reduce the Effects of Phthalate Exposure on Reproductive Health. Discov. Environ. 2025, 3, 76. [Google Scholar] [CrossRef]
- Engel, A.; Buhrke, T.; Imber, F.; Jessel, S.; Seidel, A.; Völkel, W.; Lampen, A. Agonistic and Antagonistic Effects of Phthalates and Their Urinary Metabolites on the Steroid Hormone Receptors ERα, ERβ, and AR. Toxicol. Lett. 2017, 277, 54–63. [Google Scholar] [CrossRef]
- Wang, S.-W.; Wang, S.S.-W.; Wu, D.-C.; Lin, Y.-C.; Ku, C.-C.; Wu, C.-C.; Chai, C.-Y.; Lee, J.-N.; Tsai, E.-M.; Lin, C.-L.; et al. Androgen Receptor-Mediated Apoptosis in Bovine Testicular Induced Pluripotent Stem Cells in Response to Phthalate Esters. Cell Death Dis. 2013, 4, e907. [Google Scholar] [CrossRef]
- Amir, S.; Shah, S.T.A.; Mamoulakis, C.; Docea, A.O.; Kalantzi, O.-I.; Zachariou, A.; Calina, D.; Carvalho, F.; Sofikitis, N.; Makrigiannakis, A.; et al. Endocrine Disruptors Acting on Estrogen and Androgen Pathways Cause Reproductive Disorders through Multiple Mechanisms: A Review. Int. J. Environ. Res. Public Health 2021, 18, 1464. [Google Scholar] [CrossRef]
- Schug, T.T.; Janesick, A.; Blumberg, B.; Heindel, J.J. Endocrine Disrupting Chemicals and Disease Susceptibility. J. Steroid Biochem. Mol. Biol. 2011, 127, 204–215. [Google Scholar] [CrossRef]
- Blaauwendraad, S.M.; Jaddoe, V.W.; Santos, S.; Kannan, K.; Dohle, G.R.; Trasande, L.; Gaillard, R. Associations of Maternal Urinary Bisphenol and Phthalate Concentrations with Offspring Reproductive Development. Environ. Pollut. 2022, 309, 119745. [Google Scholar] [CrossRef] [PubMed]
- Kilcoyne, K.R.; Mitchell, R.T. Effect of Environmental and Pharmaceutical Exposures on Fetal Testis Development and Function: A Systematic Review of Human Experimental Data. Hum. Reprod. Update 2019, 25, 397–421. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.F.; Arrebola, J.P.; Jiménez-Díaz, I.; Sáenz, J.M.; Molina-Molina, J.M.; Ballesteros, O.; Kortenkamp, A.; Olea, N. Bisphenol A and Other Phenols in Human Placenta from Children with Cryptorchidism or Hypospadias. Reprod. Toxicol. 2016, 59, 89–95. [Google Scholar] [CrossRef]
- Gaspari, L.; Soyer-Gobillard, M.O.; Kerlin, S.; Paris, F.; Sultan, C. Early Female Transgender Identity after Prenatal Exposure to Diethylstilbestrol: Report from a French National Diethylstilbestrol (DES) Cohort. J. Xenobiotics 2024, 14, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Ristori, J.; Cocchetti, C.; Romani, A.; Mazzoli, F.; Vignozzi, L.; Maggi, M.; Fisher, A.D. Brain Sex Differences Related to Gender Identity Development: Genes or Hormones? Int. J. Mol. Sci. 2020, 21, 2123. [Google Scholar] [CrossRef] [PubMed]
- Klip, H.; Verloop, J.; Van Gool, J.D.; Koster, M.E.; Burger, C.W.; Van Leeuwen, F.E. Hypospadias in Sons of Women Exposed to Diethylstilbestrol in Utero: A Cohort Study. Lancet 2002, 359, 1102–1107. [Google Scholar] [CrossRef]
- Giordano, F.; Abballe, A.; De Felip, E.; Di Domenico, A.; Ferro, F.; Grammatico, P.; Ingelido, A.M.; Marra, V.; Marrocco, G.; Vallasciani, S.; et al. Maternal Exposures to Endocrine Disrupting Chemicals and Hypospadias in Offspring. Birth Defects Res. 2010, 88, 241–250. [Google Scholar] [CrossRef]
- Agopian, A.J.; Lupo, P.J.; Canfield, M.A.; Langlois, P.H. Case–C Ontrol Study of Maternal Residential Atrazine Exposure and Male Genital Malformations. Am. J. Med. Genet. A 2013, 161, 977–982. [Google Scholar] [CrossRef]
- The National Birth Defects Prevention Study; Winston, J.J.; Emch, M.; Meyer, R.E.; Langlois, P.; Weyer, P.; Mosley, B.; Olshan, A.F.; Band, L.E.; Luben, T.J. Hypospadias and Maternal Exposure to Atrazine via Drinking Water in the National Birth Defects Prevention Study. Environ. Health 2016, 15, 76. [Google Scholar] [CrossRef] [PubMed]
- Dugas, J.; Nieuwenhuijsen, M.J.; Martinez, D.; Iszatt, N.; Nelson, P.; Elliott, P. Use of Biocides and Insect Repellents and Risk of Hypospadias. Occup. Environ. Med. 2010, 67, 196–200. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Guo, X.; Graubard, B.I.; Brock, J.W.; Klebanoff, M.A.; Longnecker, M.P. Maternal Pregnancy Levels of Polychlorinated Biphenyls and Risk of Hypospadias and Cryptorchidism in Male Offspring. Environ. Health Perspect. 2009, 117, 1472–1476. [Google Scholar] [CrossRef]
- Rignell-Hydbom, A.; Lindh, C.H.; Dillner, J.; Jönsson, B.A.G.; Rylander, L. A Nested Case-Control Study of Intrauterine Exposure to Persistent Organochlorine Pollutants and the Risk of Hypospadias. PLoS ONE 2012, 7, e44767. [Google Scholar] [CrossRef]
- Choi, H.; Kim, J.; Im, Y.; Lee, S.; Kim, Y. The Association between Some Endocrine Disruptors and Hypospadias in Biological Samples. J. Environ. Sci. Health A 2012, 47, 2173–2179. [Google Scholar] [CrossRef]
- Chevrier, C.; Petit, C.; Philippat, C.; Mortamais, M.; Slama, R.; Rouget, F.; Calafat, A.M.; Ye, X.; Silva, M.J.; Charles, M.-A.; et al. Maternal Urinary Phthalates and Phenols and Male Genital Anomalies. Epidemiology 2012, 23, 353–356. [Google Scholar] [CrossRef]
- Jensen, M.S.; Anand-Ivell, R.; Nørgaard-Pedersen, B.; Jönsson, B.A.G.; Bonde, J.P.; Hougaard, D.M.; Cohen, A.; Lindh, C.H.; Ivell, R.; Toft, G. Amniotic Fluid Phthalate Levels and Male Fetal Gonad Function. Epidemiology 2015, 26, 91–99. [Google Scholar] [CrossRef]
- Raghavan, R.; Romano, M.E.; Karagas, M.R.; Penna, F.J. Pharmacologic and Environmental Endocrine Disruptors in the Pathogenesis of Hypospadias: A Review. Curr. Environ. Health Rep. 2018, 5, 499–511. [Google Scholar] [CrossRef]
- Gurney, J.K.; Florio, A.A.; Znaor, A.; Ferlay, J.; Laversanne, M.; Sarfati, D.; Bray, F.; McGlynn, K.A. International Trends in the Incidence of Testicular Cancer: Lessons from 35 Years and 41 Countries. Eur. Urol. 2019, 76, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Elad, D.; Jaffa, A.J.; Grisaru, D.; Leibovitch, I. In Utero Programming of Testicular Cancer. J. Dev. Biol. 2021, 9, 35. [Google Scholar] [CrossRef]
- Fénichel, P.; Chevalier, N. Is Testicular Germ Cell Cancer Estrogen Dependent? The Role of Endocrine Disrupting Chemicals. Endocrinology 2019, 160, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Faja, F.; Esteves, S.; Pallotti, F.; Cicolani, G.; Di Chiano, S.; Delli Paoli, E.; Lenzi, A.; Lombardo, F.; Paoli, D. Environmental Disruptors and Testicular Cancer. Endocrine 2022, 78, 429–435. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Quraishi, S.M.; Graubard, B.I.; Weber, J.-P.; Rubertone, M.V.; Erickson, R.L. Persistent Organochlorine Pesticides and Risk of Testicular Germ Cell Tumors. J. Natl. Cancer Inst. 2008, 100, 663–671. [Google Scholar] [CrossRef]
- Uldbjerg, C.S.; Rantakokko, P.; Lim, Y.-H.; Petersen, J.H.; Sørensen, K.M.; Coull, B.A.; Lindh, C.; Hauser, R.; Bräuner, E.V.; Skakkebæk, N.E.; et al. Prenatal Exposure to Organochlorine Pesticides and Polychlorinated Biphenyls and Risk of Testicular Germ Cell Cancer Later in Life. Sci. Total Environ. 2025, 970, 179054. [Google Scholar] [CrossRef]
- Modica, R.; Benevento, E.; Colao, A. Endocrine-Disrupting Chemicals (EDCs) and Cancer: New Perspectives on an Old Relationship. J. Endocrinol. Invest. 2022, 46, 667–677. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, X.; Bassig, B.; Hauser, R.; Holford, T.R.; Zheng, E.; Shi, D.; Zhu, Y.; Schwartz, S.M.; Chen, C.; et al. Serum Polychlorinated Biphenyl (PCB) Levels and Risk of Testicular Germ Cell Tumors: A Population-Based Case-Control Study in Connecticut and Massachusetts. Environ. Pollut. 2021, 273, 116458. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, X.; Bassig, B.; Hauser, R.; Holford, T.R.; Zheng, E.; Shi, D.; Zhu, Y.; Schwartz, S.M.; Chen, C.; et al. Dataset of Testicular Germ Cell Tumors (TGCT) Risk Associated with Serum Polychlorinated Biphenyl (PCB) by Age at Diagnosis and Histologic Types. Data Brief 2021, 36, 107014. [Google Scholar] [CrossRef]
- Uldbjerg, C.S.; Lim, Y.-H.; Beck, A.L.; Petersen, J.H.; Sørensen, K.M.; Kristensen, D.M.; Rantakokko, P.; Coull, B.A.; Lindh, C.; Skakkebæk, N.E.; et al. Exposure to Per- and Polyfluoroalkyl Substances during Fetal Development and Risk of Testicular Germ Cell Cancer in Adulthood. Environ. Int. 2025, 203, 109762. [Google Scholar] [CrossRef]
- Bräuner, E.V.; Lim, Y.-H.; Koch, T.; Uldbjerg, C.S.; Gregersen, L.S.; Pedersen, M.K.; Frederiksen, H.; Petersen, J.H.; Coull, B.A.; Andersson, A.-M.; et al. Endocrine Disrupting Chemicals and Risk of Testicular Cancer: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2021, 106, e4834–e4860. [Google Scholar] [CrossRef]
- Wittassek, M.; Angerer, J. Phthalates: Metabolism and Exposure. Int. J. Androl. 2008, 31, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R.; Bolden, A.L. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ. Health Perspect. 2015, 123, 643–650. [Google Scholar] [CrossRef]
- Mnif, W.; Hassine, A.I.H.; Bouaziz, A.; Bartegi, A.; Thomas, O.; Roig, B. Effect of Endocrine Disruptor Pesticides: A Review. Int. J. Environ. Res. Public Health 2011, 8, 2265–2303. [Google Scholar] [CrossRef]
- Ritter, R.; Scheringer, M.; MacLeod, M.; Moeckel, C.; Jones, K.C.; Hungerbühler, K. Intrinsic Human Elimination Half-Lives of Polychlorinated Biphenyls Derived from the Temporal Evolution of Cross-Sectional Biomonitoring Data from the United Kingdom. Environ. Health Perspect. 2011, 119, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Heger, N.E.; Hall, S.J.; Sandrof, M.A.; McDonnell, E.V.; Hensley, J.B.; McDowell, E.N.; Martin, K.A.; Gaido, K.W.; Johnson, K.J.; Boekelheide, K. Human Fetal Testis Xenografts Are Resistant to Phthalate-Induced Endocrine Disruption. Environ. Health Perspect. 2012, 120, 1137–1143. [Google Scholar] [CrossRef]
- Van Den Driesche, S.; Macdonald, J.; Anderson, R.A.; Johnston, Z.C.; Chetty, T.; Smith, L.B.; McKinnell, C.; Dean, A.; Homer, N.Z.; Jorgensen, A.; et al. Prolonged Exposure to Acetaminophen Reduces Testosterone Production by the Human Fetal Testis in a Xenograft Model. Sci. Transl. Med. 2015, 7, 288ra80. [Google Scholar] [CrossRef] [PubMed]
- Qigen, X.; Kai, X.; Haiming, C.; Zhe, X.; Yong, G.; Chunhua, D. Genetic Variants (LhcgrW495X/+) and Environmental Toxicants (DEHP) Synergistically Induce DSD by Interfering with Steroidogenic Gene Expression. Biol. Sex Differ. 2025, 16, 70. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.