Abstract
A recently discovered haplotype—CYP2C:TG—determines the ultrarapid metabolism of several CYP2C19 substrates. The platelet inhibitor clopidogrel requires CYP2C19-mediated activation: the risk of ischemic events is increased in patients with a poor (PM) or intermediate (IM) CYP2C19 metabolizer phenotype (vs. normal, NM; rapid, RM; or ultrarapid, UM). We investigated whether the CYP2C:TG haplotype affected efficacy/bleeding risk in clopidogrel-treated patients. Adults (n = 283) treated with clopidogrel over 3–6 months were classified by CYP2C19 phenotype based on the CYP2C19*2*17 genotype, and based on the CYP2C19/CYP2C cluster genotype, and regarding carriage of the CYP2:TG haplotype, and were balanced on a number of covariates across the levels of phenotypes/haplotype carriage. Overall, 45 (15.9%) patients experienced ischemic events, and 49 (17.3%) experienced bleedings. By either classification, the incidence of ischemic events was similarly numerically higher in PM/IM patients (21.6%, 21.8%, respectively) than in mutually similar NM, RM, and UM patients (13.2–14.8%), whereas the incidence of bleeding events was numerically lower (13.1% vs. 16.6–20.5%). The incidence of ischemic events was similar in CYP2C:TG carries and non-carries (14.1% vs. 16.1%), whereas the incidence of bleedings appeared mildly lower in the former (14.9% vs. 20.1%). We observed no signal to suggest a major effect of the CYP2C19/CYP2C cluster genotype or CYP2C:TG haplotype on the clinical efficacy/safety of clopidogrel.
1. Introduction
The cytochrome P-450 isoenzyme CYP2C19 (CYP2C19) is encoded by a highly polymorphic CYP2C19 gene: more than 39 star (*) allele haplotypes have been defined by the Pharmacogene Variation Consortium [1,2]. Alleles greatly reflect on the enzyme activity, and are categorized into functional groups: normal function alleles (e.g., CYP2C19*1), decreased function alleles (e.g., CYP2C19*9), no function alleles (e.g., CYP2C19*2 and *3), and increased function alleles (e.g., CYP2C19*17) [2,3]. Based on the genotype, five CYP2C19 metabolizing phenotypes can be distinguished: normal metabolizers (NM) carry two normal function alleles (e.g., CYP2C19*1/*1); intermediate metabolizers (IM) carry one normal or one increased function allele and one no function allele (e.g., CYP2C19*1/*2, CYP2C19*2/*17); poor metabolizers (PM) carry two no function alleles (e.g., CYP2C19*2/*3); rapid metabolizers (RM) carry one normal and one increased function allele (i.e., CYP2C19*1/*17), whereas carriers of two increased function alleles (i.e., CYP2C19*17/*17) are categorized as ultrarapid metabolizers (UM) [4,5].
Clopidogrel is the oldest and most extensively studied of the second-generation thienopyridines—platelet aggregation inhibitors of the P2Y12 receptor antagonist class. It is indicated for the prevention of occlusive arterial incidents (ischemic incidents) in patients at increased risk. As a monotherapy, or combined with aspirin, clopidogrel is indicated in patients who underwent percutaneous coronary intervention (PCI), embolization of cerebrovascular aneurisms or arterio-venous malformations (AVM), cerebrovascular stenting, in patients who have sustained acute myocardial infarction/acute coronary syndromes, transitory ischemic attack (TIA) or ischemic stroke, and in those with verified peripheral artery diseases (PAD) [6,7,8]. Regarding clopidogrel, CYP2C19 activity has a specific role: clopidogrel is a prodrug, and after oral administration, around 85% is inactivated in the first pass by the hepatic carboxylesterase 1 (CES1), whereas the remaining 15% requires enzymatic biotransformation into an active moiety, a reversible P2Y12 antagonist. While several hepatic CYP450 enzymes are involved, CYP2C19 is the most important one. This is a delicate role, since inadequate clopidogrel activation results in recurrent ischemic events, whereas excessive activation might, at least theoretically, increase the risk of bleeding [9,10,11,12,13,14]. The situation is further complicated by the fact that antiplatelet-treated patients commonly receive gastroprotective treatments, typically proton pump inhibitors, all of which are CYP2C19 substrates, and some (omeprazole, esomeprazole) are rather strong enzyme inhibitors [15]. The platelet response to clopidogrel shows considerable interindividual variability, and some 4–30% of patients fail to achieve adequate platelet inhibition [16,17]. After more than a decade of research, PM and IM CYP2C19 phenotypes have been extensively shown to result in lower active metabolite levels, and higher (preserved) platelet reactivity than NM (and, likely, RM and UM) phenotype, and are associated with an increased risk of major ischemic events (reviewed in, e.g., [4,12,18,19]). On the other hand, RM and UM patients display the highest active metabolite levels, but it is unclear whether this means greater efficacy and/or greater bleeding risk vs. NM patients—some studies have suggested that both could be the case [20,21,22,23,24], whereas others have suggested no association between the *17 allele and clopidogrel pharmacokinetics, pharmacodynamics, and ischemic or bleeding outcomes (i.e., no relevant difference between NM, RM, and UM subjects) after accounting for the *2 allele [25,26,27,28,29]. It has been postulated that the observed relationship between the *17 allele and clopidogrel antiplatelet effects may be due to absence of the no function *2 allele rather than presence of the increased function *17 allele [12,28]. The clinical utility of CYP2C19 genotype-guided strategy for selection of P2Y12 inhibitors has been evaluated in multicenter randomized clinical trials [18]: PHARMCLO [30], POPular-Genetics [31], and TAILOR-PCI [32,33]. Overall, genotyping for the CYP2C19 no function alleles and identification of PM and IM subjects to guide P2Y12-targeted antiplatelet therapy has a reasonable clinical utility, whereas the identification of RM/UM subjects is not considered relevant [34].
Recently, a novel haplotype composed of two non-coding variants in the CYP2C gene cluster, CYP2C18 NM_000772.3:c.*31T (rs2860840) and NM_000772.2:c.819+2182G (rs11188059), has been identified, and is reported to be associated with the ultrarapid metabolism of CYP2C19 substrates escitalopram and sertraline [35,36]. The haplotype is referred to as “CYP2C:TG”. It has been reported to be associated with exposure to and efficacy of a proton pump inhibitor omeprazole [37] and the azole antifungal voriconazole [38]. Another study found no association between the CYP2C:TG haplotype and in vivo exposure to CYP2C19 substrates citalopram, sertraline, voriconazole, omeprazole, pantoprazole, and rabeprazole in healthy volunteers, and no association between this haplotype and CYP2C18/2C19 abundance in human liver sample or activity in vitro [39].
Considering that the role of RM/UM phenotypes in bioactivation of clopidogrel is still doubtful, we aimed to explore whether accounting for the CYP2C:TG might add information about the risk of ischemic and bleeding events in patients treated with clopidogrel over 3–6 months. For this purpose, the incidence of events was analyzed with respect to the “classically” defined CYP2C19 phenotypes, that is, based on the CYP2C19*2*17 genotypes, phenotypes defined with respect to the CYP2C cluster genotype, that is combined CYP2C19*2*17 and CYP2C rs11188059 and rs2860840, and with respect to the presence of the CYP2C:TG haplotype.
2. Materials and Methods
2.1. Study Outline
The present data are preliminary results from a larger prospective study (“Pharmacogenomics in Prediction of Cardiovascular Drugs Adverse Reactions”) that started on 1 January 2022, and will last 60 months and include 1200 subjects. The study (ClinicalTrials.gov, NCT05307718) is conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committees of the School of Medicine, University of Zagreb, (reg. number 380-59-10106-20-111/125; class 641-01/20-02/01) and the University Hospital Centre Zagreb (class 8.1-20/142-2; number 02/21 AG), Croatia. All subjects provided written informed consent for genotyping the pharmacogenes of interest, and for publishing anonymized data for scientific purposes. The present analysis included consecutive adults (≥18 years of age), unrelated Caucasians of European (Slavic) descent, who started clopidogrel due to percutaneous coronary intervention (PCI), embolization of cerebrovascular aneurisms (AVM), or cerebrovascular stenting requiring >3 months of therapy, and were followed up over at least 3 and to a maximum of 6 months (all patients with no events were observed over 6 months) between 1 January 2022 and 1 March 2024. The decision to prescribe clopidogrel was at the discretion of attending cardiologist/stroke neurologist, and was in line with the approved prescribing information including recommendations to avoid it in patients who need to be treated with strong CYP2C19 inducers or inhibitors. Patients were genotyped for CPY2C19*2, CYP2C19*17, CYP2C rs11188059G>A, and rs2860840C>T. Phenotypes (PM, IM, NM, UM, RM) were determined based on the CYP2C19*2,*17 genotype, and on combined CYP2C19*2,*17 and CYP2C18 rs11188059 and CYP2C18 rs2860840 genotypes (cluster genotype). They were also classified as CYP2C:TG carriers and non-carriers. Phenotypes/haplotype carriage were considered as “exposures” indicating genetically determined enzyme activity, and incidence of ischemic and bleeding events were recorded. Levels of exposure were then balanced on a number of relevant covariates, and their effects on outcomes of interest were estimated.
2.2. Patients
HIV patients were not included in this study. Patients undergoing PCI were not included if older than 80 years of age, were treated with continuous post-interventional glycoprotein IIb/IIIa inhibitors, had thrombocytopenia (<150 × 109/L), more pronounced renal failure (creatinine > 200 µmol/L), anemia (hematocrit < 30%), hemorrhagic diathesis, history of major surgery within previous 6 weeks, or hemorrhagic stroke within previous 6 months. No particular exclusion criteria were implemented for neurovascular patients (i.e., undergoing cerebrovascular stenting or embolization of cerebrovascular aneurisms/arterio-venous malformations).
2.3. Genotyping Procedures
Genomic DNA was extracted from whole blood samples using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Genotyping was performed using TaqMan® Drug Metabolism Genotyping Assays or TaqMan® SNP Genotyping Assays (Applied Biosystems, Carlsbad, CA, USA) for CYP2C19*2 (c.681G>A, rs4244285; assay ID: C__25986767_70), CYP2C19*17 (c.-806C>T, rs12248560; assay ID: C____469857_10), CYP2C18 c.*31C>T (rs2860840; assay ID: C__11201742_10) and CYP2C18 c.819+2182G>A (rs11188059; C__31983321_10), and TaqMan®Universal PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA) by real-time PCR genotyping on the 7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA), according to the manufacturer’s instructions. Genotyping of CYP2C19*2 and *17 was performed with methods validated for routine pharmacogenetic testing in the clinical laboratory included in external quality assessment schemes, while rs11188059 and rs2860840 variants were validated by frequency for Caucasian population. Genotyping of CYP2C19*2 and *17 was performed with methods implemented and validated for routine pharmacogenetic testing in the clinical laboratory that regularly participates in external quality assessment schemes (European Molecular Genetics Quality Network—EMQN; Reference Institute for Bioanalytics—RfB).
CYP2C:TG haplotype refers to the combination of T at CYP2C18 c.*31C>T (rs2860840) and G at c.819+2182G>A (rs11188059).
2.4. Genotype-Predicted Phenotypes and CYP2C:TG Haplotype
Phenotypes based on CYP2C19*2,*17 genotype were defined as follows: (i) poor metabolizer (PM) = *2/*2; (ii) intermediate metabolizer (IM) = *1/*2, *2/*17; (iii) normal metabolizer (NM) = *1/*1; (iv) rapid metabolizer (RM) = *1/*17; (v) ultrarapid metabolizer (UM) = *17/*17. Phenotypes based on the CYP2C19/CYP2C cluster genotype were defined as follows: (i) PM = CYP2C19 Null/CYP2C19 Null; (ii) IM = CYP2C19*17/CYP2C19 Null, CYP2C:TG/CYP2C19 Null or CYP2C19*1/CYP2C19 Null; (iii) NM = CYP2C19*1/CYP2C19*1; (iv) RM = CYP2C19*1/CYP2C19*17 or CYP2C19*1/CYP2C:TG; (v) UM = CYP2C19*17/CYP2C19*17, CYP2C:TG/CYP2C:TG or CYP2C:TG/CYP2C19*17. Finally, CYP2C:TG haplotype carriers were defined as those with CYP2C cluster diplotype TG/TG or “other”/TG (vs. “other”/“other”).
2.5. Outcomes of Interest
The following ischemic events were recorded: PCI target lesion, stent thrombosis, transitory ischemic attack (TIA), ischemic stroke (CVI), (re)hospitalization for myocardial ischemia, acute myocardial infarction/acute coronary syndrome, cardiovascular death, retinal ischemia, ischemia of the optic nerve, amaurosis fugax.
The following bleeding events were recorded: (i) all intracranial bleedings (e.g., nontraumatic intraparenchymal hematoma, subarachnoid, intraventricular, or subdural hemorrhage [40]), except for microhemorrhages and minor asymptomatic sulcal subarachnoid hemorrhages; (ii) any extracranial bleeding that required at least non-surgical medical intervention; (iii) any intraspinal or intraocular bleeding that compromised vision; (iv) fatal bleeding of any location or cause.
No grading based on severity of either ischemic or bleeding events was employed.
2.6. Data Analysis
All ischemic events and all bleeding events were considered jointly, regardless of severity or location. To estimate the effects of CYP2C19 phenotypes and of CYP2C:TG haplotype, different phenotypes (levels of exposure) and CYP2C:TG carriers and non-carriers were mutually balanced on several covariates that could have affected the outcome of interest: age, sex, indication for clopidogrel treatment (3 levels: percutaneous coronary intervention, embolism of cerebral aneurism, cerebrovascular stenting), chronic kidney, heart or liver failure (jointly), hypertension, diabetes or dyslipidemia, concurrent use of aspirin, concurrent use of anticoagulants, concurrent use of gastroprotective treatments (yes or no), and concurrent use of proton pump inhibitors (yes or no). For this purpose, we used entropy balancing [41] implemented in package WeightIt (Version 1.1.0) [42] in R [43]. Entropy balancing is a data preprocessing method that (where this is possible) guarantees covariate balance via a reweighting scheme that assigns a scalar weight to each sample unit such that the reweighted groups satisfy a set of balance constraints that are imposed on the sample moments of covariate distributions [41]. Standardized mean differences (d) < 0.1 between pairs of exposure (phenotype, haplotype) levels were considered to indicate adequate balance. Balanced data were analyzed by fitting weighted log-binomial models with robust variance estimation to binary outcomes to generate adjusted proportions. Since both the sample and number of events were limited, confidence intervals around relative risks would have been rather wide. Instead, we present adjusted proportions, their confidence intervals, and Cochran–Armitage test of trend in adjusted (weighted) proportions, where appropriate. We used SAS 9.4 for Windows (SAS Inc., Cary, NC, USA).
3. Results
3.1. Patient Characteristics
The present report addresses 283 patients (60.8% women) aged 22–85 years, who mostly (56.5%) underwent cerebral aneurism embolization and, less commonly, cerebrovascular stenting (27.9%) and PCI (15.6%) (Table 1). A history of ischemic cerebrovascular incidents (33.2%) and peripheral artery disease (38.2%) were common, and the most common comorbidities were hypertension (69.3%) and dyslipidemia (41.7%) (Table 1). Along clopidogrel, most patients were using aspirin (82.3%), whereas 13.1% were co-treated with anticoagulants (mainly direct oral anticoagulants) (Table 1). Gastroprotection was administered in 70.3% patients, typically proton pump inhibitors (mostly pantoprazole) (Table 1). The predominant phenotype based on the CYP2C19*2,*17 genotype was “rapid metabolizer” (RM) (41.0%), followed by the “normal metabolizer” (NM) (31.1%) and “intermediate metabolizer” (IM) (18.7%). “Ultrarapid” (UM) and “poor metabolizer” (PM) phenotypes were rare/sporadic (6.7% and 2.5%, respectively) (Table 1). Most patients (73.8%) were wild-type homozygotes at CYP2C rs11188959G>A, whereas the prevalence of wild-type homozygotes (41.7%) and heterozygotes (48.1%) at CYP2C rs2860840C>T was similar (Table 1). There were 162 (57.2%) CYP2C:TG haplotype carriers (Table 1). When the phenotype was determined based on the combined *2*17, rs11188059G>A, and rs2869840C>T genotypes, the prevalence of PM and IM remained unchanged, the prevalence of NM was greatly reduced to 23 (8.1%), and the prevalence of UM greatly increased to 95 (33.6%), while the prevalence of RM was 37.1% (Table 1). A total of 45 patients (15.9%) experienced ischemic events, and 49 (17.3%) experienced bleeding events, practically exclusively extracerebral (Table 1).

Table 1.
Patient characteristics. Values are n (percent) or mean ± SD (minimum–maximum) for age.
3.2. Outcomes across CYP2C19 Phenotypes—Raw Data
Although some phenotypes were uncommon (e.g., PM, UM by *2,*17 classification, NM by cluster-based classification), a tendency of a numerically higher incidence of ischemic events and a lower incidence of bleeding events was apparent for PM/IM vs. NM, RM and UM phenotypes by either classification (Figure 1A,B). The tendency was more clearly visible when phenotype levels was collapsed to 3 (based on a biological rationale) (Figure 1C) for the combined PM/IM phenotype (n = 60), NM (n = 88 based on *2,*17, n = 23 based on the cluster-genotype), and RM/UM phenotypes (n = 135 based on *2,*17, n = 200 based on cluster-genotype) (Figure 1C). For the cluster-based phenotype, the tendency was apparent also when phenotypes were collapsed to PM/IM, combined NM and RM (n = 128), and UM (n = 95) (Figure 1C). This might be biologically more plausible, since a large prevalence of UM subjects in this phenotype classification is based predominantly on a “switch” of many of the NM and some of the RM subjects to the UM phenotype, indicating that there is a greater similarity between NM and RM subjects than between RM and UM subjects.
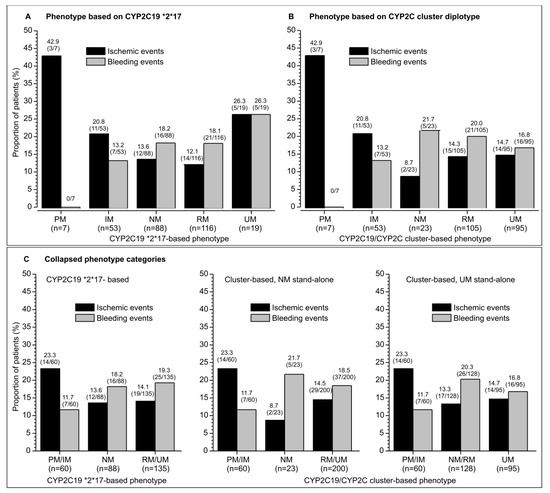
Figure 1.
Raw incidence of ischemic and bleeding events in clopidogrel-treated patients with different CYP2C19 phenotypes: poor (PM), intermediate (IM), normal (NM), rapid (RM), and ultrarapid (UM) metabolizer. Depicted are percentages and number of patients with events/total number in brackets. (A) Incidence of events across phenotypes defined based on the CPY2C19*2,*17 genotype. (B) Incidence of events across phenotypes defined based on the CYP2C19/CYP2C cluster genotype. (C) Incidence of events across collapsed phenotype categories.
3.3. Outcomes across Phenotypes—Balanced Data
Patients across the collapsed levels of CYP2C19 phenotypes (PM/IM combined, NM, and RM/UM combined based on *2,*17; and PM/IM, NM, and RM combined, and UM based on the CYP2C19/CYP2C cluster) differed largely with respect to most of the covariates (Table 2).

Table 2.
Patient characteristics across the CYP2C19 phenotypes (poor, intermediate, normal, rapid, ultrarapid metabolizers [PM, IM, NM, RM, UM, respectively]) defined based on the CYP2C19*2/*17 genotype or based on the CPY2C19/CYP2C cluster genotype. Data are mean ± SD or count (percent). Shown are maximum standardize mean differences (Max d) between any two of the three phenotype levels.
After entropy balancing, all subsets were mutually closely similarly with an adequate balance (all d < 0.1) (Table 3). The adjusted proportions showed similar trends as the raw data: numerically higher proportion of ischemic events and lower proportion of bleeding events in PM/IM patients vs. NM and combined RM/UM patients, or vs. the combined NM/RM and UM patients (Figure 2).

Table 3.
Patient characteristics across the CYP2C19 phenotypes (PM, IM, NM, RM, UM) defined based on the CYP2C19*2*17 genotype or on the CPY2C19/CYP2C cluster genotype after entropy balancing 1. Data are mean ± SD or percent patients with a characteristic in the weighted pseudopopulation. Shown are maximum standardize mean differences (Max d) between any two of the three phenotype levels.
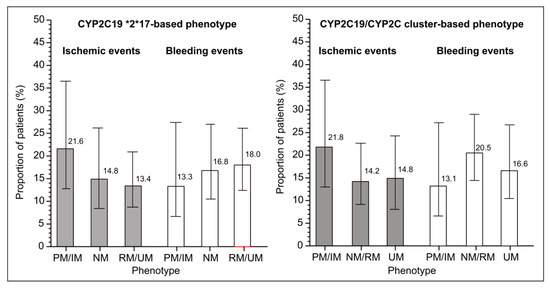
Figure 2.
Adjusted incidence (based on the fully balanced data in Table 3) of ischemic and bleeding events in clopidogrel-treated patients with different CYP2C19 phenotypes: poor (PM), intermediate (IM), normal (NM), rapid (RM), or ultrarapid (UM) metabolizer. Depicted are percentages from the weighted pseudopopulations.
The sample was modest in size and the number of events was low, so formal tests of trend in proportions for all outcomes yielded relatively low z-values. However, despite this lack of precision of the estimates, numerical trends appeared obvious and were similar for both types of phenotype definitions (Figure 2).
3.4. Outcomes Regarding the CYP2C:TG Haplotype
The CYP2C:TG carriers (n = 162) and non-carriers (n = 121) mildly differed regarding the covariates, and the prevalence of ischemic events (14.8% vs. 17.4%) was generally similar between them (Table 4). The prevalence of bleeding events appeared to be slightly lower in haplotype carriers (14.8% vs. 20.7%, d = −0.153). After entropy balancing, virtually identical values for all covariates were achieved for haplotype carriers and non-carriers: the incidence of ischemic events remained closely similar (14.1% vs. 16.1%) in CYP2C:TG carriers and non-carriers, whereas the difference in bleeding events was minimal (14.9% vs. 20.1%) (Table 4).

Table 4.
Patient characteristics in CYP2C:TG carriers (TG-other, TG-TG) and non-carriers in the CYP2C cluster diplotype and ischemic and bleeding events before and after entropy balancing. Data are mean ± SD for age, and count (percent) for raw data, or percent in the entropy-balanced data 1. Depicted are standardized mean differences (d; values < 0.1 indicate fully adequate balance). Shown are also outcomes of interest, i.e., incidence of ischemic and bleeding events: for raw data (before entropy balancing of covariates), simple proportions are provided; for adjusted (balanced) data, incidence is given with 95% confidence intervals.
4. Discussion
As a result of extensive investigations—including genome-wide association studies and candidate-gene analyses of observational data and data from large randomized trials—the current guidelines by the Clinical Pharmacogenetic Implementation Consortium on the CYP2C19 genotype (genotype-predicted phenotype) in clopidogrel-treated patients strongly recommend that in cardiovascular indications, clopidogrel should be avoided in PM/IM CYP2C19 metabolizers (preference towards the non-CYP2C19-dependent P2Y12 antagonists like prasugrel or ticagrelor) [4]. A moderate recommendation for clopidogrel avoidance in PM/IM patients refers to cerebrovascular indications [4]. No recommendation pertains to RM or UM patients in either indication, implying that differentiation between NM, RM, and UM subjects appears to be of no clinical utility [4]. The considerable inter-subject variability in response to clopidogrel is considered largely genetically determined [44], and only partly explained by identification of the loss-of-function CYP2C19 alleles [45,46,47].
Polymorphisms in several other genes (e.g., CYP2B6, CES1, SCOS5P1, CDC42BPA, CTRAC1, ABCB1, G4GALT2, P2RY12, PON1) have been suggested as relevant for the antiplatelet effects of clopidogrel, although their impact on “hard” clinical outcomes is yet to be clarified [27,28,48,49]. It is reasonable to envisage a risk-grading system encompassing multiple genetic variants and non-genetic factors that would be more predictive and reliable than any individual indicator alone, regarding the clinical efficacy/safety of clopidogrel in cardiovascular and neurovascular indications [27,50].
The newly discovered CYP2C:TG haplotype has been shown associated with accelerated metabolism (by around 25%) of CYP2C19 substrates escitalopram and sertraline [35,36], and it has been suggested that it could affect the exposure to/effects of other CYP2C19 substrates such as omeprazole [37] and voriconazole [38]. However, opposing observations (not indicating effects of CYP2C:TG) have been reported, as well [39]. Currently, it is considered that the CYP2C:TG haplotype provides useful additional information to predict enhanced CYP2C19 activity, an effect that might be ethnically specific, but further investigations are needed before it could be routinely used in clinical settings [51].
Considering that in the case of CYP2C19 phenotypes and clopidogrel efficacy/safety, “rapid” or “ultrarapid” designation has not been thus far considered practically relevant (i.e., no distinction has been made vs. normal metabolizers), we considered it reasonable to explore a concept that “additional information” about enhanced activity conveyed by the CYP2C:TG haplotype might also additionally inform about efficacy and bleeding risks in clopidogrel-treated patients. For this purpose, we assessed the incidence of ischemic and bleeding events with respect to PM/IM, NM, and RM/UM phenotype based on the “classical” criteria of the CYP2C19*2*17 genotype, PM/IM, NM/RM, and UM phenotype informed by the combined CYP2C19*2*17 genotype and rs2860840 and rs11188059 (cluster CYP2C19/CYP2C genotype), and with respect to the presence of the CYP2C:TG haplotype. Due to the limited sample size and limited number of events, the present risk estimates are imprecise (wide confidence intervals), but numerical trends are obvious: higher incidence of ischemic events in PM/IM patients vs. mutually similar NM/RM/UM patients by either classification; lower incidence of bleeding events in PM/IM patients vs. NM/RM/UM (also mutually similar) by either classification; a similar incidence of ischemic events in CYP2C:TG carriers and non-carriers, and a mildly lower incidence of bleeding events in the former than in the latter. Despite this imprecision, however, the patient subsets were not extremely small (between 60 and 135 across the collapsed phenotype categories, 162 vs. 121 regarding CYP2C:TG), and the observed proportions do not appear critically fragile: indeed, one or two events more or less could have occurred in any subset by shear chance, but it is unlikely that this would have substantially changed the observed numerical trends. It could be objected that mixing cardiovascular and neurovascular patients/indications was not appropriate, but we deemed it a feasible option since clopidogrel is indicated in both settings, where it is expected to convey similar effects on ischemic/bleeding events through the same mechanism of action. Also, some residual confounding should be attributed to the fact that we did not consider factors that could have influenced effects of the commonly co-administered aspirin, or other already mentioned genetic factors that have been suggested to affect the antiplatelet effect of clopidogrel. On the other hand, we did account for a number of factors known to have an impact on the risk of ischemic or bleeding events in clopidogrel-treated patients: (i) in line with the prescribing recommendations, clopidogrel was avoided in patients using strong CYP2C19 inducers or inhibitors, and in HIV-positive patients; (ii) exposed and control subjects were balanced according to age, chronic kidney disease, hypertension, dyslipidemia, diabetes, serious liver disease, known history of coronary, cerebral or peripheral artery disease (and, by virtue of this, also indirectly regarding treatments used in such patients); (iii) concomitant aspirin or anticoagulant use [27,50], the use of gastroprotection (which may directly reflect on incidence of gastro-intestinal bleeding), and, specifically, the use of proton pump inhibitors (PPIs). This latter factor is of two-fold relevance: (i) all PPIs are CYP2C19 substrates that could compete with clopidogrel or inhibit it [15] and thus affect clopidogrel bioactivation and its effects [52,53]. Of note, the effects of pantoprazole and rabeprazole used in the present sample of patients are minimal, if any, both in vitro and in vivo [15,52,53]; (ii) CYP2C:TG could affect their clearance/exposure and, thus, efficacy in gastroprotection. In this regard, it is of note that no gastro-intestinal bleedings were recorded during the observed period. By using the entropy balancing method, all contrasted “exposures” (different phenotypes, haplotypes) were perfectly balanced with respect to all these factors. Finally, certain validity to the present estimates is conveyed by the fact that In PM/IM, NM, and RM/UM patients classified based on “classical” criteria of the CYP2C19*2*17 genotype, we observed the incidence of ischemic and bleeding events in line with the theoretical expectations [4], and the observations based on the CYP2C19/CYP2C cluster genotype-based phenotypes were closely similar. Under these circumstances, it appears justified to state that no clear-cut, strong signal has been detected in the present exploration that would indicate a major role of the CYP2C:TG haplotype for informing the risk of treatment failure or bleeding events in clopidogrel-treated patients.
5. Conclusions
In the present analysis, we explored the possibility that the awareness about the newly discovered haplotype CYP2C:TG might convey useful information to anticipate the risk of treatment failure (ischemic events) or the risk of bleeding in clopidogrel-treated Caucasians of European (Slavic) descent. With the limitations of a modest sample size, and possible residual confounding, we failed to observe clear-cut strong signals to support such possibilities. However, having in mind racial/ethnic differences in patient responsiveness to P2Y12 inhibitors in general, and specifically to clopidogrel [54], the situation in other populations might be substantially different.
Author Contributions
Conceptualization, L.G., K.S. and T.B.; Methodology, J.P., K.S. and L.Š.; Validation, L.G. and Z.P.; Formal analysis, J.P., V.T., L.Š. and Z.P.; Investigation, K.S., N.B., M.L.-B. and T.B.; Resources, K.S. and M.L.-B.; Data curation, L.G., V.T., M.L.-B. and Z.P.; Writing—original draft, L.G., J.P., V.T., K.S. and Z.P.; Writing—review & editing, L.G., V.T., L.Š., N.B., M.L.-B. and T.B.; Visualization, V.T.; Supervision, N.B. and T.B.; Project administration, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Croatian Science Foundation. Project name and grant number: Pharmacogenomics in Prediction of Cardiovascular Drugs Adverse Reaction (PGx-CardioDrug), HRZZ-UIP-2020-02-8189.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committees of the University of Zagreb School of Medicine (reg. number 380-59-10106-20-111/125; class 641-01/20-02/01) and the University Hospital Centre Zagreb (class 8.1-20/142-2; number 02/21 AG), Zagreb, Croatia.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Botton, M.R.; Whirl-Carrillo, M.; Del Tredici, A.L.; Sangkuhl, K.; Cavallari, L.H.; Agúndez, J.A.G.; Duconge, J.; Lee, M.T.M.; Woodahl, E.L.; Claudio-Campos, K.; et al. PharmVar GeneFocus: CYP2C19. Clin. Pharmacol. Ther. 2021, 109, 352–366. [Google Scholar] [CrossRef] [PubMed]
- PharmVar. PharmVar CYP2C19 Gene. Available online: https://www.pharmvar.org/gene/CYP2C19 (accessed on 13 April 2024).
- Pratt, V.M.; Del Tredici, A.L.; Hachad, H.; Ji, Y.; Kalman, L.V.; Scott, S.A.; Weck, K.E. Recommendations for Clinical CYP2C19 Genotyping Allele Selection. J. Mol. Diagn. 2018, 20, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Luzum, J.A.; Sangkuhl, K.; Gammal, R.S.; Sabatine, M.S.; Stein, C.M.; Kisor, D.F.; Limdi, N.A.; Lee, Y.M.; Scott, S.A.; et al. Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2C19 Genotype and Clopidogrel Therapy: 2022 Update. Clin. Pharmacol. Ther. 2022, 112, 959–967. [Google Scholar] [CrossRef] [PubMed]
- PharmGKB. Gene-Specific Information Tables for CYP2C19. Available online: https://www.pharmgkb.org/page/cyp2c19RefMaterials (accessed on 13 April 2024).
- Johnston, S.C.; Easton, J.D.; Farrant, M.; Barsan, W.; Conwit, R.A.; Elm, J.J.; Kim, A.S.; Lindblad, A.S.; Palesch, Y.Y.; Neurological Emergencies Treatment Trials Network; et al. Clopidogrel and Aspirin in Acute Ischemic Stroke and High-Risk TIA. N. Engl. J. Med. 2018, 379, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Levine, G.N.; Bates, E.R.; Bittl, J.A.; Brindis, R.G.; Fihn, S.D.; Fleisher, L.A.; Granger, C.B.; Lange, R.A.; Mack, M.J.; Mauri, L.; et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2016, 68, 1082–1115. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhao, X.; Liu, L.; Wang, D.; Wang, C.; Wang, C.; Li, H.; Meng, X.; Cui, L.; et al. Clopidogrel with Aspirin in Acute Minor Stroke or Transient Ischemic Attack. N. Engl. J. Med. 2013, 369, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kazui, M.; Nishiya, Y.; Ishizuka, T.; Hagihara, K.; Farid, N.A.; Okazaki, O.; Ikeda, T.; Kurihara, A. Identification of the Human Cytochrome P450 Enzymes Involved in the Two Oxidative Steps in the Bioactivation of Clopidogrel to Its Pharmacologically Active Metabolite. Drug Metab. Dispos. 2010, 38, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Mega, J.L.; Simon, T.; Collet, J.-P.; Anderson, J.L.; Antman, E.M.; Bliden, K.; Cannon, C.P.; Danchin, N.; Giusti, B.; Gurbel, P.; et al. Reduced-Function CYP2C19 Genotype and Risk of Adverse Clinical Outcomes among Patients Treated with Clopidogrel Predominantly for PCI: A Meta-Analysis. JAMA 2010, 304, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, W.; Xu, Y.; Yi, X.; Han, Y.; Yang, Q.; Li, X.; Huang, L.; Johnston, S.C.; Zhao, X.; et al. Genetic Polymorphisms and Clopidogrel Efficacy for Acute Ischemic Stroke or Transient Ischemic Attack. Circulation 2017, 135, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.A.; Sangkuhl, K.; Stein, C.M.; Hulot, J.-S.; Mega, J.L.; Roden, D.M.; Klein, T.E.; Sabatine, M.S.; Johnson, J.A.; Shuldiner, A.R. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C19 Genotype and Clopidogrel Therapy: 2013 Update. Clin. Pharmacol. Ther. 2013, 94, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Lin, J.; Li, H.; Johnston, S.C.; Lin, Y.; Pan, Y.; Liu, L.; Wang, D.; Wang, C.; et al. Association between CYP2C19 Loss-of-Function Allele Status and Efficacy of Clopidogrel for Risk Reduction among Patients with Minor Stroke or Transient Ischemic Attack. JAMA 2016, 316, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.-G.; Zou, J.-J.; Hu, Z.-Y.; Zhang, J.-J.; Ye, F.; Chen, S.-L. Individual Variability in the Disposition of and Response to Clopidogrel: Pharmacogenomics and Beyond. Pharmacol. Ther. 2011, 129, 267–289. [Google Scholar] [CrossRef]
- Zvyaga, T.; Chang, S.-Y.; Chen, C.; Yang, Z.; Vuppugalla, R.; Hurley, J.; Thorndike, D.; Wagner, A.; Chimalakonda, A.; Rodrigues, A.D. Evaluation of Six Proton Pump Inhibitors as Inhibitors of Various Human Cytochromes P450: Focus on Cytochrome P450 2C19. Drug Metab. Dispos. Biol. Fate Chem. 2012, 40, 1698–1711. [Google Scholar] [CrossRef] [PubMed]
- Mejin, M.; Tiong, W.N.; Lai, L.Y.H.; Tiong, L.L.; Bujang, A.M.; Hwang, S.S.; Ong, T.K.; Fong, A.Y.Y. CYP2C19 Genotypes and Their Impact on Clopidogrel Responsiveness in Percutaneous Coronary Intervention. Int. J. Clin. Pharm. 2013, 35, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Lin, J.; Zhou, Q.; Wu, L.; Cheng, W.; Wang, C. Clopidogrel Resistance Increases Rate of Recurrent Stroke and Other Vascular Events in Chinese Population. J. Stroke Cerebrovasc. Dis. 2016, 25, 1222–1228. [Google Scholar] [CrossRef]
- Gower, M.N.; Ratner, L.R.; Williams, A.K.; Rossi, J.S.; Stouffer, G.A.; Lee, C.R. Clinical Utility of CYP2C19 Genotype-Guided Antiplatelet Therapy in Patients at Risk of Adverse Cardiovascular and Cerebrovascular Events: A Review of Emerging Evidence. Pharmacogenomics Pers. Med. 2020, 13, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-Y.; Wang, Y.-L.; Fan, Z.-X.; Sun, X.-P.; Wang, S.; Liu, Z. Effect of Cytochrome P450 2C19 (CYP2C19) Gene Polymorphism and Clopidogrel Reactivity on Long Term Prognosis of Patients with Coronary Heart Disease after PCI. J. Geriatr. Cardiol. JGC 2024, 21, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Gross, L.; Trenk, D.; Jacobshagen, C.; Krieg, A.; Gawaz, M.; Massberg, S.; Baylacher, M.; Aradi, D.; Stimpfle, F.; Hromek, J.; et al. Genotype-Phenotype Association and Impact on Outcomes Following Guided De-Escalation of Anti-Platelet Treatment in Acute Coronary Syndrome Patients: The TROPICAL-ACS Genotyping Substudy. Thromb. Haemost. 2018, 118, 1656–1667. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Rodríguez, M.; Romero-Palacián, D.; Villalobos-Vilda, C.; Caniego, J.L.; Belmonte, C.; Koller, D.; Bárcena, E.; Talegón, M.; Abad-Santos, F. Influence of CYP2C19 Phenotype on the Effect of Clopidogrel in Patients Undergoing a Percutaneous Neurointervention Procedure. Clin. Pharmacol. Ther. 2019, 105, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Sibbing, D.; Koch, W.; Gebhard, D.; Schuster, T.; Braun, S.; Stegherr, J.; Morath, T.; Schömig, A.; von Beckerath, N.; Kastrati, A. Cytochrome 2C19*17 Allelic Variant, Platelet Aggregation, Bleeding Events, and Stent Thrombosis in Clopidogrel-Treated Patients with Coronary Stent Placement. Circulation 2010, 121, 512–518. [Google Scholar] [CrossRef]
- Sibbing, D.; Gebhard, D.; Koch, W.; Braun, S.; Stegherr, J.; Morath, T.; Von Beckerath, N.; Mehilli, J.; Schömig, A.; Schuster, T.; et al. Isolated and Interactive Impact of Common CYP2C19 Genetic Variants on the Antiplatelet Effect of Chronic Clopidogrel Therapy. J. Thromb. Haemost. 2010, 8, 1685–1693. [Google Scholar] [CrossRef]
- Tiroch, K.A.; Sibbing, D.; Koch, W.; Roosen-Runge, T.; Mehilli, J.; Schömig, A.; Kastrati, A. Protective Effect of the CYP2C19 *17 Polymorphism with Increased Activation of Clopidogrel on Cardiovascular Events. Am. Heart J. 2010, 160, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Danielak, D.; Karaźniewicz-Łada, M.; Komosa, A.; Burchardt, P.; Lesiak, M.; Kruszyna, Ł.; Graczyk-Szuster, A.; Główka, F. Influence of Genetic Co-Factors on the Population Pharmacokinetic Model for Clopidogrel and Its Active Thiol Metabolite. Eur. J. Clin. Pharmacol. 2017, 73, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Thomas, C.D.; Beitelshees, A.L.; Tuteja, S.; Empey, P.E.; Lee, J.C.; Limdi, N.A.; Duarte, J.D.; Skaar, T.C.; Chen, Y.; et al. Impact of the CYP2C19*17 Allele on Outcomes in Patients Receiving Genotype-Guided Antiplatelet Therapy after Percutaneous Coronary Intervention. Clin. Pharmacol. Ther. 2021, 109, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.P.; Backman, J.D.; Reny, J.-L.; Bergmeijer, T.O.; Mitchell, B.D.; Ritchie, M.D.; Déry, J.-P.; Pakyz, R.E.; Gong, L.; Ryan, K.; et al. Pharmacogenomic Polygenic Response Score Predicts Ischaemic Events and Cardiovascular Mortality in Clopidogrel-Treated Patients. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.P.; Stephens, S.H.; Horenstein, R.B.; O’Connell, J.R.; Ryan, K.; Peer, C.J.; Figg, W.D.; Spencer, S.D.; Pacanowski, M.A.; Mitchell, B.D.; et al. The CYP2C19 *17 Variant Is Not Independently Associated with Clopidogrel Response. J. Thromb. Haemost. 2013, 11, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; James, S.; Storey, R.F.; Armstrong, M.; Barratt, B.J.; Horrow, J.; Husted, S.; Katus, H.; Steg, P.G.; Shah, S.H.; et al. Effect of CYP2C19 and ABCB1 Single Nucleotide Polymorphisms on Outcomes of Treatment with Ticagrelor versus Clopidogrel for Acute Coronary Syndromes: A Genetic Substudy of the PLATO Trial. Lancet 2010, 376, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, F.M.; Maglietta, G.; Bevilacqua, P.; Cereda, M.; Merlini, P.A.; Villani, G.Q.; Moruzzi, P.; Patrizi, G.; Malagoli Tagliazucchi, G.; Crocamo, A.; et al. Pharmacogenomic Approach to Selecting Antiplatelet Therapy in Patients with Acute Coronary Syndromes: The PHARMCLO Trial. J. Am. Coll. Cardiol. 2018, 71, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Claassens, D.M.F.; Vos, G.J.A.; Bergmeijer, T.O.; Hermanides, R.S.; van ‘t Hof, A.W.J.; van der Harst, P.; Barbato, E.; Morisco, C.; Tjon Joe Gin, R.M.; Asselbergs, F.W.; et al. A Genotype-Guided Strategy for Oral P2Y12 Inhibitors in Primary PCI. N. Engl. J. Med. 2019, 381, 1621–1631. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.L.; Farkouh, M.E.; So, D.; Lennon, R.; Geller, N.; Mathew, V.; Bell, M.; Bae, J.-H.; Jeong, M.H.; Chavez, I.; et al. Effect of Genotype-Guided Oral P2Y12 Inhibitor Selection vs Conventional Clopidogrel Therapy on Ischemic Outcomes after Percutaneous Coronary Intervention: The TAILOR-PCI Randomized Clinical Trial. JAMA 2020, 324, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.L.; Rihal, C.S.; So, D.; Rosenberg, Y.; Lennon, R.J.; Mathew, V.; Goodman, S.; Weinshilboum, R.M.; Wang, L.; Baudhuin, L.M.; et al. Clopidogrel Pharmacogenetics: State of the Art Review and the TAILOR-PCI Study. Circ. Cardiovasc. Interv. 2019, 12, e007811. [Google Scholar] [CrossRef] [PubMed]
- Massmann, A.; Christensen, K.D.; Van Heukelom, J.; Schultz, A.; Shaukat, M.H.S.; Hajek, C.; Weaver, M.; Green, R.C.; Wu, A.C.; Hickingbotham, M.R.; et al. Clinical Impact of Preemptive Pharmacogenomic Testing on Antiplatelet Therapy in a Real-World Setting. Eur. J. Hum. Genet. 2024; ahead of print. [Google Scholar] [CrossRef]
- Bråten, L.S.; Haslemo, T.; Jukic, M.M.; Ivanov, M.; Ingelman-Sundberg, M.; Molden, E.; Kringen, M.K. A Novel CYP2C-Haplotype Associated with Ultrarapid Metabolism of Escitalopram. Clin. Pharmacol. Ther. 2021, 110, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Bråten, L.S.; Ingelman-Sundberg, M.; Jukic, M.M.; Molden, E.; Kringen, M.K. Impact of the Novel CYP2C:TG Haplotype and CYP2B6 Variants on Sertraline Exposure in a Large Patient Population. Clin. Transl. Sci. 2022, 15, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Kee, P.S.; Maggo, S.D.S.; Kennedy, M.A.; Barclay, M.L.; Miller, A.L.; Lehnert, K.; Curtis, M.A.; Faull, R.L.M.; Parker, R.; Chin, P.K.L. Omeprazole Treatment Failure in Gastroesophageal Reflux Disease and Genetic Variation at the CYP2C Locus. Front. Genet. 2022, 13, 869160. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.N.; Robinson, M.; Morris, S.A.; Jandrisevits, E.; Lopes, K.E.; Hamilton, A.; Steuerwald, N.; Druhan, L.J.; Avalos, B.; Copelan, E.; et al. Pharmacogenetic and Clinical Predictors of Voriconazole Concentration in Hematopoietic Stem Cell Transplant Recipients Receiving CYP2C19-Guided Dosing. Pharmacogenomics J. 2023, 23, 201–209. [Google Scholar] [CrossRef]
- Zubiaur, P.; Soria-Chacartegui, P.; Boone, E.C.; Prasad, B.; Dinh, J.; Wang, W.Y.; Zugbi, S.; Rodríguez-Lopez, A.; González-Iglesias, E.; Leeder, J.S.; et al. Impact of CYP2C:TG Haplotype on CYP2C19 Substrates Clearance In Vivo, Protein Content, and In Vitro Activity. Clin. Pharmacol. Ther. 2023, 114, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- von Kummer, R.; Broderick, J.P.; Campbell, B.C.V.; Demchuk, A.; Goyal, M.; Hill, M.D.; Treurniet, K.M.; Majoie, C.B.L.M.; Marquering, H.A.; Mazya, M.V.; et al. The Heidelberg Bleeding Classification. Stroke 2015, 46, 2981–2986. [Google Scholar] [CrossRef] [PubMed]
- Hainmueller, J. Entropy Balancing for Causal Effects: A Multivariate Reweighting Method to Produce Balanced Samples in Observational Studies. Polit. Anal. 2012, 20, 25–46. [Google Scholar] [CrossRef]
- Greifer, N. WeightIt: Weighting for Covariate Balance in Observational Studies. R Package Version 1.0.0. Available online: https://ngreifer.github.io/WeightIt/ (accessed on 11 April 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.r-project.org/ (accessed on 13 April 2024).
- Shuldiner, A.R.; O’Connell, J.R.; Bliden, K.P.; Gandhi, A.; Ryan, K.; Horenstein, R.B.; Damcott, C.M.; Pakyz, R.; Tantry, U.S.; Gibson, Q.; et al. Association of Cytochrome P450 2C19 Genotype with the Antiplatelet Effect and Clinical Efficacy of Clopidogrel Therapy. JAMA 2009, 302, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Bouman, H.J.; Harmsze, A.M.; van Werkum, J.W.; Breet, N.J.; Bergmeijer, T.O.; ten Cate, H.; Hackeng, C.M.; Deneer, V.H.M.; ten Berg, J.M. Variability in On-Treatment Platelet Reactivity Explained by CYP2C19*2 Genotype Is Modest in Clopidogrel Pretreated Patients Undergoing Coronary Stenting. Heart 2011, 97, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Fontana, P.; James, R.; Barazer, I.; Berdagué, P.; Schved, J.-F.; Rebsamen, M.; Vuilleumier, N.; Reny, J.-L. Relationship between Paraoxonase-1 Activity, Its Q192R Genetic Variant and Clopidogrel Responsiveness in the ADRIE Study. J. Thromb. Haemost. 2011, 9, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Hochholzer, W.; Trenk, D.; Fromm, M.F.; Valina, C.M.; Stratz, C.; Bestehorn, H.-P.; Büttner, H.J.; Neumann, F.-J. Impact of Cytochrome P450 2C19 Loss-of-Function Polymorphism and of Major Demographic Characteristics on Residual Platelet Function After Loading and Maintenance Treatment with Clopidogrel in Patients Undergoing Elective Coronary Stent Placement. J. Am. Coll. Cardiol. 2010, 55, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Scott, S.; Collet, J.-P.; Baber, U.; Yang, Y.; Peter, I.; Linderman, M.; Sload, J.; Qiao, W.; Kini, A.; Sharma, S.; et al. Exome Sequencing of Extreme Clopidogrel Response Phenotypes Identifies B4GALT2 as a Determinant of On-Treatment Platelet Reactivity. Clin. Pharmacol. Ther. 2016, 100, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.S.; Bergmeijer, T.O.; Gong, L.; Reny, J.-L.; Lewis, J.P.; Mitchell, B.D.; Alexopoulos, D.; Aradi, D.; Altman, R.B.; Bliden, K.; et al. Genomewide Association Study of Platelet Reactivity and Cardiovascular Response in Patients Treated with Clopidogrel: A Study by the International Clopidogrel Pharmacogenomics Consortium. Clin. Pharmacol. Ther. 2020, 108, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Franchi, F.; Rossi, J.S.; Keeley, E.C.; Anderson, R.D.; Beitelshees, A.L.; Duarte, J.D.; Ortega-Paz, L.; Gong, Y.; Kerensky, R.A.; et al. Effectiveness of Clopidogrel vs Alternative P2Y12 Inhibitors Based on the ABCD-GENE Score. J. Am. Coll. Cardiol. 2024, 83, 1370–1381. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M.; Jukic, M.; Bråten, L.S.; Kringen, M.K.; Molden, E. What Is the Current Clinical Impact of the CYP2CTG Haplotype? Clin. Pharmacol. Ther. 2024, 115, 183. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chou, Y.-C.; Chang, J.-Y.; Sun, C.-A. Effects of Treatment with Clopidogrel with or without Proton Pump Inhibitor Omeprazole on the Risk of Ischemic Stroke: A Nationwide Cohort Study. Sci. Rep. 2024, 14, 1686. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, J.S.; Kim, B.J.; Shin, S.Y.; Kim, D.B.; Ahn, H.S. Influence of Individual Proton Pump Inhibitors on Clinical Outcomes in Patients Receiving Clopidogrel Following Percutaneous Coronary Intervention. Medicine 2021, 100, e27411. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Kaski, J.C.; Kimura, T.; Barton, J.C.; Yamamoto, K.; Komiyama, M.; Drexel, H.; Lewis, B.S.; Agewall, S.; Hasegawa, K. Racial and ethnic differences in pharmacotherapy to prevent coronary artery disease and thrombotic events. Eur. Heart J. Cardiovasc. Pharmacol. 2022, 8, 738–751. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).