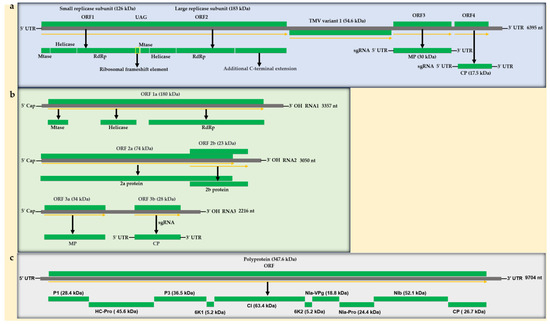
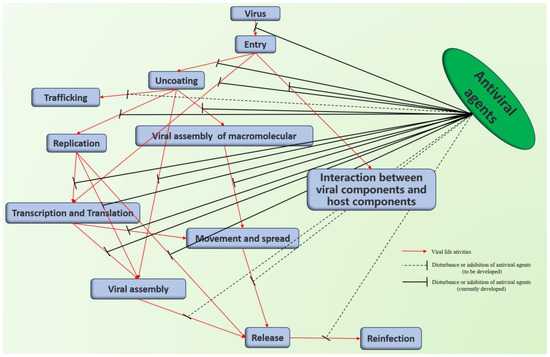
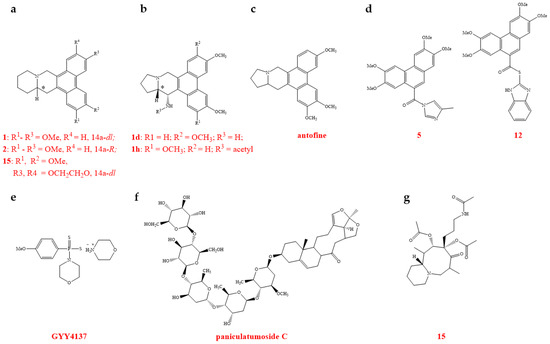
Abstract
Research into the biology of plant viruses, their mechanisms of pathogenicity, and the induction of host resistance has laid a solid foundation for the discovery of antiviral agents and their targets and the development of effective control technologies. Additionally, recent advancements in fields such as chemical biology, cheminformatics, bioinformatics, and synthetic biology have provided valuable methods and tools for the design of antiviral drugs, the synthesis of drug molecules, assessment of their activity, and investigation of their modes of action. Compared with drug development for human viral diseases, the control of plant viral diseases presents greater challenges, including the cost-benefit of agents, simplification of control technologies, and the effectiveness of treatments. Therefore, in the current context of complex outbreaks and severe damage caused by plant viral diseases, it is crucial to delve deeper into the research and development of antiviral agents. This review provides a detailed overview of the biological characteristics of current targets for antiviral agents, the mode of interaction between plant virus targets and antivirals, and insights for future drug development. We believe this review will not only facilitate the in-depth analysis of the development of antivirals for crops but also offer valuable perspectives for the development of antiviral agents for use in human and veterinary medicine.
1. Introduction
Anti-plant virus agents encounter difficulties in exerting their effects due to the obligately parasitic nature of plant viruses and the specific tissue and cellular structures of plants, such as the waxy epidermal layer on leaf surfaces. To date, in contrast to fungicides, insecticides, and herbicides, the variety of antiviral agents against plant viruses developed both domestically and internationally remains relatively limited, with a narrow range of types and a limited number of viral targets and target viruses [1]. In recent years, changes in farming patterns have led to large-scale monoculture of crops, as well as the emergence of viruses that spread through seed transmission, mechanical transmission, and other means. Additionally, global warming and the long-term irrational use of pesticides—resulting in increased pest resistance and difficulties in pest (and virus) control—have contributed to a year-on-year escalation in the incidence of crop viral diseases, causing substantial economic losses to agricultural production [1,2]. Therefore, the development of effective antiviral agents is urgently required to address the challenges of viral disease control in agriculture [1]. There are numerous types of plant virus diseases, diverse transmission pathways, and complex patterns of prevalence [2]. The majority of viral diseases are latent and have no obvious symptoms in the early stages, but they cause significant harm in the later stages [2,3]. Due to the challenges in preventing and controlling viral diseases, plant viral disease is often referred to as “plant cancer” in agriculture. Farmers mainly employ strategies such as selecting disease-resistant varieties, improving cultivation methods, utilizing insecticides to control vector insects, and other approaches [2,3]. Anti-plant virus agents can be categorized into three types based on their mode of action: viral inactivation agents (which destroy the viral particle), virus curative agents (which inhibit the virus), and plant activators against the virus (which enhance host resistance) [1,4,5]. The methods for applying these three types of antiviral agents differ in agricultural practice. In the early stages of viral disease or during the seedling phase of crops, the primary objectives are to enhance host resistance, alleviate plant symptoms and damage, and reduce the viral load within the host using plant activators. During the infection period, viral inactivation agents are primarily employed to destroy the virus and mitigate the risk of further infection. When significant viral proliferation occurs, the focus shifts to using viral curative agents to inhibit the replication and spread of the virus [1,2,3].
In recent years, China has gradually emerged as the world’s largest producer of crop protection chemicals. In response to the challenging issue of controlling plant virus diseases, the country has initiated research and development efforts focused on antiviral agents for plants. Since the beginning of this century, significant progress has been made in the study of anti-plant virus agents. Notable advancements include research into the targets of plant viruses and the mechanisms for inducing disease resistance in hosts [6], the design of molecules based on these viral targets [7], and the discovery of new natural products with antivirus activity derived from plants [8,9,10], animals [6], microbes [11], and marine organisms [12].
However, the creation of antiviral agents for use on plants is quite challenging due to several factors, including the cost of the agent, their environmental risk, field application technology, control effectiveness, crop type, and field application contexts [1,3]. The development of antiviral agents for use on plants differs fundamentally from that of medicinal and veterinary drugs; thus, it cannot simply rely on the research and development approaches used in those fields. It is essential to summarize the innovative concepts surrounding the development of antiviral agents for plants in China over the past 20 years. This includes the discovery and rational evaluation of targets, design principles for antiviral agents for plants, and lead molecules. Systematic thinking is crucial in integrating these elements to advance research and development in this area. This paper focuses on several key aspects: the druggability of plant virus targets, the interaction modes between targets and active substances, the effects of anti-plant virus substances, the research models for anti-plant virus targets, and the screening methods for anti-plant virus agents. Although plant activators play a significant role in combating plant viruses, this paper will not review work in this field due to space limitations. For more information, please refer to our review on plant activators [5].
4. Conclusions and Future Prospects
In the 25 years from the beginning of this century, China has made significant progress in the development of anti-plant virus agents, including the discovery of lead compounds targeting plant viruses and research into the mechanisms of action of these agents. Simultaneously, Chinese scientists have identified numerous lead compounds with anti-plant viral activity from natural sources and have developed technologies for the modification and synthesis of natural product derivatives [2,3,162]. They have also discovered numerous highly active compounds with anti-plant virus properties and developed crop protection chemicals, such as dufulin and amino oligosaccharins, which offer innovative solutions for controlling viral diseases. These efforts have successfully addressed the issue of viral diseases affecting vegetables, rice, and other crops in China (Figure 11). However, we must also acknowledge the challenges that have arisen in recent years regarding the research and development of antiviral agents. These challenges are as follows:
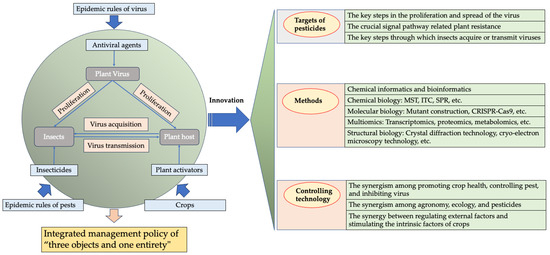
Figure 11.
Development of plant virus agents, strategies for controlling plant viral diseases, and future research directions.
- (1)
- Limited source, mode of action of antiviral agent: The active substances are derived from a limited range of sources, predominantly plants, marine organisms, and other living organisms. This search for natural compounds has nearly reached its limits, indicating that new approaches are required. Additionally, the research focus has been rather narrow, with most studies centered on TMV, which does not reflect the realities of crop viral disease. Furthermore, the research methods employed are quite similar, primarily relying on the analysis of binding interactions between CPs and candidate drug molecules, along with visualization by electron microscopy.
- (2)
- Limited availability of antiviral agents for farmers: Currently, the limitations of the strategies used mean that there are few anti-plant virus products available, principally dufulin, amino oligosaccharides, and NNM. The main issues are that the efficacy of these antiviral agents in the field control of crop viruses is not particularly impressive, the control costs are high, and their adoption by farmers is challenging. With few products available, it is difficult to meet the demands of agricultural production.
- (3)
- Focus is needed on novel targets, novel methods, and novel controlling technology. Firstly, it is essential to consider the key targets involved in the viral decapsidation, replication, movement, and assembly in the plant hosts and vector insects, as well as the mechanisms of interaction between these viral and host biological macromolecules. Secondly, the research methods and approaches for studying the interaction between targets and active molecules need further expansion. This includes areas such as chemical informatics, bioinformatics, chemical biology, molecular biology, multiomics, and structural biology, which should be actively developed and applied. In addition, there is a need for extensive collection and preparation of transgenic or mutant materials from plants, insects, and other organisms for target research. Thirdly, the approach to the prevention and control of plant viruses should shift from merely inhibiting viral proliferation to regulating it, with a focus on transmission and reducing symptoms. This shift should minimize the incidence of viral diseases, resulting in fewer symptoms and less overall damage to the crop. Finally, it is important to consider the various transmission routes to prevent virus spread by vector insects, examine the effective targeting of these vectors, and investigate agents for preventing insect-mediated transmission. Additionally, coordinating this research avenue with plant activators is crucial to maximizing the effectiveness of treatments. Therefore, we propose the model for plant virus disease control as “three objectives and one overall strategy” (Figure 11).
Author Contributions
Z.C. completed the conceptualization; Z.C. wrote the original draft manuscript; Z.C. and Y.Y. reviewed and edited the manuscript; Y.Y. and Z.C. made the figures and tables; Z.C., Y.Y., L.H., T.C., D.W. and L.Z. conducted supervision; Z.C. and D.W. completed the funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research Development Program of China (2022YFD1700504), the National Natural Science Foundation of China (No. 32472621), the China Agriculture Research System of MOF and MARA (CARS-23-C07), and the Central Government Guides Local Science and Technology Development Fund Projects (Qiankehezhongyindi (2024) 007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support this study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jin, J.; Shen, T.; Shu, L.; Huang, Y.; Deng, Y.; Li, B.; Jin, Z.; Li, X.; Wu, J. Recent achievements in antiviral agent development for plant protection. J. Agric. Food Chem. 2023, 71, 1291–1309. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Li, F.; Zhang, X.; Ye, J.; Wei, T.; Li, Z.; Tao, X.; Cui, F.; Wang, X.; et al. Plant virology in the 21st century in China: Recent advances and future directions. J. Integr. Plant Biol. 2024, 66, 579–622. [Google Scholar] [CrossRef]
- Chen, Z.; Li, X.Y.; Yu, L.; Song, B.A. The development and application of pesticides against the disease caused by Southern rice black-streaked dwarf virus. J. Plant Prot. Res. 2017, 44, 905–918. [Google Scholar] [CrossRef]
- Yang, B.X.; Li, Z.X.; Liu, S.S.; Yang, J.; Wang, P.Y.; Liu, H.W.; Zhou, X.; Liu, L.W.; Wu, Z.B.; Yang, S. Novel cinnamic acid derivatives as a versatile tool for developing agrochemicals for controlling plant virus and bacterial diseases by enhancing plant defense responses. Pest Manag. Sci. 2023, 79, 2556–2570. [Google Scholar] [CrossRef]
- Naz, M.; Zhang, D.; Liao, K.; Chen, X.; Ahmed, N.; Wang, D.; Zhou, J.; Chen, Z. The past, present, and future of plant activators targeting the salicylic acid signaling pathway. Genes 2024, 15, 1237. [Google Scholar] [CrossRef]
- Chen, Z.; Zeng, M.; Song, B.; Hou, C.; Hu, D.; Li, X.; Wang, Z.; Fan, H.; Bi, L.; Liu, J.; et al. Dufulin activates HrBP1 to produce antiviral responses in tobacco. PLoS ONE 2012, 7, e37944. [Google Scholar] [CrossRef]
- Li, Z.; Yang, B.; Ding, Y.; Meng, J.; Hu, J.; Zhou, X.; Liu, L.; Wu, Z.; Yang, S. Insights into a class of natural eugenol and its optimized derivatives as potential tobacco mosaic virus helicase inhibitors by structure-based virtual screening. Int. J. Biol. Macromol. 2023, 248, 125892. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Wei, Z.; Shen, J.; Wang, L.; Ma, Z.; Zhang, X. Antiphytoviral toxins of Actinidia chinensis root bark (ACRB) extract: Laboratory and semi-field trials. Pest Manag. Sci. 2018, 74, 1630–1636. [Google Scholar] [CrossRef]
- Guo, W.; Lu, X.; Liu, B.; Yan, H.; Feng, J. Anti-TMV activity and mode of action of three alkaloids isolated from Chelidonium majus. Pest Manag. Sci. 2021, 77, 510–517. [Google Scholar] [CrossRef]
- Spak, J.; Votruba, I.; Pavingerová, D.; Holý, A.; Spaková, V.; Petrzik, K. Antiviral activity of tenofovir against cauliflower mosaic virus and its metabolism in Brassica pekinensis plants. Antivir. Res. 2011, 92, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Gao, Y.; Zhang, M.; Ding, X.; Wang, Z.; Ma, D.; Wang, Q. Streptindole and its derivatives as novel antiviral and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2020, 68, 7839–7849. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hao, Y.; Ji, X.; Wang, Z.; Liu, Y.; Ma, D.; Li, Y.; Pang, H.; Ni, J.; Wang, Q. Optimization, structure-activity relationship, and mode of action of nortopsentin analogues containing thiazole and oxazole moieties. J. Agric. Food Chem. 2019, 67, 10018–10031. [Google Scholar] [CrossRef]
- Mayer, A. Über die Mosaikkrankheit des Tabaks. Landwirtsch. Vers. Stn. 1886, 32, 451–467. [Google Scholar]
- Culver, J.N.; Lehto, K.; Close, S.M.; Hilf, M.E.; Dawson, W.O. Genomic position affects the expression of tobacco mosaic virus movement and coat protein genes. Proc. Natl. Acad. Sci. USA 1993, 90, 2055–2059. [Google Scholar] [CrossRef] [PubMed]
- Lumata, J.L.; Ball, D.; Shahrivarkevishahi, A.; Luzuriaga, M.A.; Herbert, F.C.; Brohlin, O.; Lee, H.; Hagge, L.M.; D’Arcy, S.; Gassensmith, J.J. Identification and physical characterization of a spontaneous mutation of the tobacco mosaic virus in the laboratory environment. Sci. Rep. 2021, 11, 15109. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Zhou, Z.H. Hydrogen-bonding networks and RNA bases revealed by cryo electron microscopy suggest a triggering mechanism for calcium switches. Proc. Natl. Acad. Sci. USA 2011, 108, 9637–9642. [Google Scholar] [CrossRef]
- Bendahmane, M.; Chen, I.; Asurmendi, S.; Bazzini, A.A.; Szecsi, J.; Beachy, R.N. Coat protein-mediated resistance to TMV infection of Nicotiana tabacum involves multiple modes of interference by coat protein. Virology 2007, 366, 107–116. [Google Scholar] [CrossRef]
- Buck, K.W. Replication of tobacco mosaic virus RNA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999, 354, 613–627. [Google Scholar] [CrossRef]
- Zerbini, F.M.; Kitajima, E.W. From Contagium vivum fluidum to Riboviria: A tobacco mosaic virus-centric history of virus taxonomy. Biomolecules 2022, 12, 1363. [Google Scholar] [CrossRef]
- Caspar, D.L.D.; Namba, K. Switching in the self-assembly of tobacco mosaic virus. Adv. Biophys. 1990, 26, 157–185. [Google Scholar] [CrossRef]
- Kadaré, G.; Haenni, A.L. Virus-encoded RNA helicases. J. Virol. 1997, 71, 2583–2590. [Google Scholar] [CrossRef] [PubMed]
- Rozanov, M.N.; Koonin, E.V.; Gorbalenya, A.E. Conservation of the putative methyltransferase domain: A hallmark of the ‘Sindbis-like’ supergroup of positive-strand RNA viruses. J. Gen. Virol. 1992, 73, 2129–2134. [Google Scholar] [CrossRef]
- Ishihama, A.; Barbier, P. Molecular anatomy of viral RNA-directed RNA polymerases. Arch. Virol. 1994, 134, 235–258. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, E.K.; Kao, C.C. Analysis of RNA-dependent RNA polymerase structure and function as guided by known polymerase structures and computer predictions of secondary structure. Virology 1998, 252, 287–303. [Google Scholar] [CrossRef]
- Béclin, C.; Berthomé, R.; Palauqui, J.C.; Tepfer, M.; Vaucheret, H. Infection of tobacco or Arabidopsis plants by CMV counteracts systemic post-transcriptional silencing of nonviral (trans) genes. Virology 1998, 252, 313–317. [Google Scholar] [CrossRef]
- Hamera, S.; Song, X.; Su, L.; Chen, X.; Fang, R. Cucumber mosaic virus suppressor 2b binds to AGO4-related small RNAs and impairs AGO4 activities. Plant J. 2012, 69, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yuan, Y.R.; Pei, Y.; Lin, S.S.; Tuschl, T.; Patel, D.J.; Chua, N.H. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 2006, 20, 3255–3268. [Google Scholar] [CrossRef]
- Canto, T.; Prior, D.A.M.; Hellwald, K.H.; Oparka, K.J.; Palukaitis, P. Characterization of cucumber mosaic virus IV. Movement protein and coat protein are both essential for cell-to-cell movement of cucumber mosaic virus. Virology 1997, 237, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.; Boer, S.D.; Lorenzen, J.; Karasev, A.; Whitworth, J.; Nolte, P.; Singh, R.; Boucher, A.; Xu, H. Potato virus Y: An evolving concern for potato crops in the United States and Canada. Plant Dis. 2010, 94, 1384–1397. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Laín, S.; García, J.A. Highlights and prospects of potyvirus molecular biology. J. Gen. Virol. 1992, 73, 1–16. [Google Scholar] [CrossRef]
- Nichol, S.T.; Beaty, B.J.; Elliott, R.M.; Goldbach, R.; Plyusnin, A.; Schmaljohn, C.S.; Tesh, R.B. VIIIth report of the International Committee on Taxonomy of Viruses; Elsevier: Amsterdam, The Netherlands, 2005; pp. 695–716. [Google Scholar]
- Chung, B.Y.; Miller, W.A.; Atkins, J.F.; Firth, A.E. An overlapping essential gene in the Potyviridae. Proc. Natl. Acad. Sci. USA 2008, 105, 5897–5902. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.L.; Shen, J.G.; Shi, F.Y.; Fang, Z.G.; Xie, L.H.; Zhan, J.S. Detection and molecular variation of Potato virus Y CP gene in China. Sci. Agric. Sin. 2013, 46, 3125–3133. [Google Scholar] [CrossRef]
- Hu, X.; Nie, X.; He, C.; Xiong, X. Differential pathogenicity of two different recombinant PVY(NTN) isolates in Physalis floridana is likely determined by the coat protein gene. Virol. J. 2011, 8, 207. [Google Scholar] [CrossRef]
- Ogawa, T.; Tomitaka, Y.; Nakagawa, A.; Ohshima, K. Genetic structure of a population of Potato virus Y inducing potato tuber necrotic ringspot disease in Japan; comparison with North American and European populations. Virus Res. 2008, 131, 199–212. [Google Scholar] [CrossRef]
- Kežar, A.; Kavčič, L.; Polák, M.; Nováček, J.; Gutiérrez-Aguirre, I.; Žnidarič, M.T.; Coll, A.; Stare, K.; Gruden, K.; Ravnikar, M.; et al. Structural basis for the multitasking nature of the potato virus Y coat protein. Sci. Adv. 2019, 5, eaaw3808. [Google Scholar] [CrossRef]
- Ivanov, K.; Eskelin, K.; Lohmus, A.; Makinen, K. Molecular and cellular mechanisms underlying potyvirus infection. J. Gen. Virol. 2014, 95, 1415–1429. [Google Scholar] [CrossRef]
- Cronin, S.; Verchot, J.; Haldeman-Cahill, R.; Schaad, M.C.; Carrington, J.C. Long-distance movement factor: A transport function of the Potyvirus helper component proteinase. Plant Cell 1995, 7, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.R.; Zerbini, F.M.; Allison, R.F.; Gilbertson, R.L.; Lucas, W.J. Capsid protein and helper component-proteinase function as potyvirus cell-to-cell movement proteins. Virology 1997, 237, 283–295. [Google Scholar] [CrossRef]
- Anandalakshmi, R.; Pruss, G.J.; Ge, X.; Marathe, R.; Mallory, A.C.; Smith, T.H.; Vance, V.B. A viral suppressor of gene silencing in plants. Proc. Natl. Acad. Sci. USA 1998, 95, 13079–13084. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Cui, X.; Chen, H.; Renaud, J.B.; Yu, K.; Chen, X.; Wang, A. Dynamin-like proteins of endocytosis in plants are coopted by potyviruses to enhance virus infection. J. Virol. 2018, 92, e01320-18. [Google Scholar] [CrossRef]
- Schaad, M.C.; Lellis, A.D.; Carrington, J.C. VPg of tobacco etch potyvirus is a host genotype-specific determinant for long-distance movement. J. Virol. 1997, 71, 8624–8631. [Google Scholar] [CrossRef]
- Wylie, S.J.; Adams, M.; Chalam, C.; Kreuze, J.; López-Moya, J.J.; Ohshima, K.; Praveen, S.; Rabenstein, F.; Stenger, D.; Wang, A.; et al. ICTV Virus Taxonomy Profile: Potyviridae. J. Gen. Virol. 2017, 98, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus taxonomy: The database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Lian, Q.; Lin, H.H.; Chen, L.; Lu, Y.Z.; Zhai, Y.Y.; Han, X.; Du, Z.G.; Gao, F.L.; Wu, Z.J. A clade of telosma mosaic virus from Thailand is undergoing geographical expansion and genetic differentiation in passionfruit of Vietnam and China. Phytopathol. Res. 2021, 3, 24. [Google Scholar] [CrossRef]
- Gou, B.; Dai, Z.; Qin, L.; Wang, Y.; Liu, H.; Wang, L.; Liu, P.; Ran, M.; Fang, C.; Zhou, T.; et al. A zinc finger motif in the P1 N terminus, highly conserved in a subset of potyviruses, is associated with the host range and fitness of telosma mosaic virus. J. Virol. 2023, 97, e0144422. [Google Scholar] [CrossRef]
- Robaglia, C.; Caranta, C. Translation initiation factors: A weak link in plant RNA virus infection. Trends Plant Sci. 2006, 11, 40–45. [Google Scholar] [CrossRef]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Changes to taxonomy and the international code of virus classification and nomenclature ratified by the international committee on taxonomy of viruses. Arch. Virol. 2017, 162, 2505–2538. [Google Scholar] [CrossRef] [PubMed]
- Scholthof, K.B.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahiquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef]
- Oliver, J.E.; Whitfield, A.E. The genus tospovirus: Emerging bunyaviruses that threaten food security. Annu. Rev. Virol. 2016, 3, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Turina, M.; Kormelink, R.; Resende, R.O. Resistance to tospoviruses in vegetable crops: Epidemiological and molecular aspects. Annu. Rev. Phytopathol. 2016, 54, 347–371. [Google Scholar] [CrossRef]
- Rotenberg, D.; Jacobson, A.L.; Schneweis, D.J.; Whitfield, A.E. Thrips transmission of tospoviruses. Curr. Opin. Virol. 2015, 15, 80–89. [Google Scholar] [CrossRef]
- Takeda, A.; Sugiyama, K.; Nagano, H.; Mori, M.; Kaido, M.; Mise, K.; Tsuda, S.; Okuno, T. Identification of a novel RNA silencing suppressor, NSs protein of Tomato spotted wilt virus. FEBS Lett. 2002, 532, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; Billecocq, A.; Crance, J.M.; Prins, M.; Garin, D.; Bouloy, M. Viral suppressors of RNA interference impair RNA silencing induced by a Semliki forest virus replicon in tick cells. J. Gen. Virol. 2006, 87, 1985–1989. [Google Scholar] [CrossRef]
- Li, J.; Feng, Z.K.; Wu, J.Y.; Huang, Y.; Lu, G.; Zhu, M.; Wang, B.; Mao, X.; Tao, X.R. Structure and function analysis of nucleocapsid protein of tomato spotted wilt virus interacting with RNA using homology modeling. J. Biol. Chem. 2015, 290, 3950–3961. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, B.C.; Ding, Z.Z.; Li, G.B.; Liu, M.Z.; Zhu, D.T.; Sun, Y.N.; Dong, S.S.; Lou, Z.Y. Distinct mechanism for the formation of the ribonucleoprotein complex of tomato spotted wilt virus. J. Virol. 2017, 91, e00892-17. [Google Scholar] [CrossRef]
- Uhrig, J.F.; Soellick, T.R.; Minke, C.J.; Philipp, C.; Kellmann, J.W.; Schreier, P.H. Homotypic interaction and multimerization of nucleocapsid protein of tomato spotted wilt tospovirus: Identification and characterization of two interacting domains. Proc. Natl. Acad. Sci. USA 1999, 96, 55–60. [Google Scholar] [CrossRef]
- Ribeiro, D.; Jung, M.; Moling, S.; Borst, J.W.; Goldbach, R.; Kormelink, R. The cytosolic nucleoprotein of the plant-infecting bunyavirus tomato spot-ted wilt recruits endoplasmic reticulum-resident proteins to endoplasmic re-ticulum export sites. Plant Cell 2013, 25, 3602–3614. [Google Scholar] [CrossRef]
- Ribeiro, D.; Borst, J.W.; Goldbach, R.; Kormelink, R. Tomato spotted wilt virus nucleocapsid protein interacts with both viral glycoproteins Gn and Gc in planta. Virology 2009, 383, 121–130. [Google Scholar] [CrossRef]
- Zhou, G.H.; Wen, J.J.; Cai, D.J.; Li, P.; Xu, D.L.; Zhang, S.G. Southern rice black-streaked dwarf virus: A new proposed Fijivirus species in the family Reoviridae. Chin. Sci. Bull. 2008, 53, 3677–3685. [Google Scholar] [CrossRef]
- Hoang, A.T.; Zhang, H.M.; Yang, J.; Chen, J.P.; Hébrard, E.; Zhou, G.H.; Vinh, V.N.; Cheng, J.A. Identification, characterization, and distribution of southern rice black-streaked dwarf virus in Vietnam. Plant Dis. 2011, 95, 1063–1069. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, D.; Xu, D.; Zhang, M. Southern rice black-streaked dwarf virus: A white-backed planthopper-transmitted Fijivirus threatening rice production in Asia. Front. Microbiol. 2013, 4, 270. [Google Scholar] [CrossRef]
- Chen, Z.; Yin, C.; Liu, J.; Zeng, M.; Wang, Z.; Yu, D.; Bi, L.; Jin, L.; Yang, S.; Song, B. Methodology for antibody preparation and detection of southern rice black-streaked dwarf virus. Arch. Virol. 2012, 157, 2327–2333. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, L.; Jin, L.; Wang, W.; Zhao, Q.; Ran, L.; Li, X.; Chen, Z.; Guo, R.; Wei, Y.; et al. Evaluation of rice resistance to southern rice black-streaked dwarf virus and rice ragged stunt virus through combined field tests, quantitative real-time PCR, and proteome analysis. Viruses 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, J.; Zhou, G.H.; Zhang, H.M.; Chen, J.P.; Adams, M.J. The complete genome sequence of two isolates of Southern rice black-streaked dwarf virus, a new member of the genus Fijivirus. J. Phytopathol. 2010, 158, 733–737. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Ding, Y.; Wang, Z.; Wu, Z.; Yu, L.; Hu, D.; Li, P.; Song, B. Characterization of the importance of terminal residues for southern rice black-streaked dwarf virus P9-1 viroplasm formations. Protein Expr. Purif. 2015, 111, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.F.; Xie, L.; Wang, H.F.; Wang, H.D.; Chen, J.P.; Zhang, H.M. Biology of Southern rice black-streaked dwarf virus: A novel fijivirus emerging in East Asia. Plant Pathol. 2017, 66, 515–521. [Google Scholar] [CrossRef]
- Zhang, H.M.; Yang, J.; Chen, J.P.; Adams, M.J. A black-streaked dwarf disease on rice in China is caused by a novel Fijivirus. Arch. Virol. 2008, 153, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Ding, Y.; Luo, L.; Gan, X.; Li, X.; Chen, Y.; Hu, D.; Song, B. Interaction research on an antiviral molecule that targets the coat protein of Southern rice black-streaked dwarf virus. Int. J. Biol. Macromol. 2017, 103, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, A.; Cui, M.; Liu, Y.; Wang, L.; Wang, Q. First discovery and stucture-activity relationship study of phenanthroquinolizidines as novel antiviral agents against tobacco mosaic virus (TMV). PLoS ONE 2012, 7, e52933. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Ma, S.; Liu, Y.; Wang, L.; Wang, Q. Design, synthesis, antiviral activity, and SARs of 14-aminophenanthroindolizidines. J. Agric. Food Chem. 2012, 60, 5825–5831. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Zhang, R.Y.; Yu, Z.H.; Ouyang, D. Selective interaction between tylophorine B and bulged DNA. Bioorg. Med. Chem. Lett. 2005, 15, 2673–2677. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Zhang, R.; Yu, Z.; Ouyang, D. The interaction between tylophorine B and TMV RNA. Bioorg. Med. Chem. Lett. 2006, 16, 4300–4304. [Google Scholar] [CrossRef]
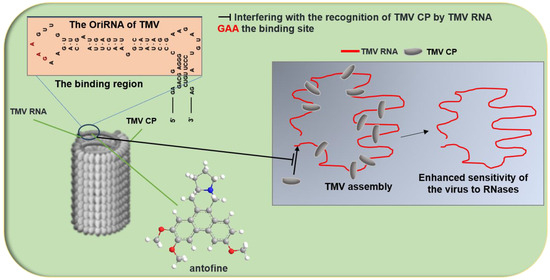
- Gao, S.; Zhang, R.; Yu, Z.; Xi, Z. Antofine analogues can inhibit tobacco mosaic virus assembly through small-molecule-RNA interactions. Chembiochem 2012, 13, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wei, P.; Wang, Z.; Liu, Y.; Wang, L.; Wang, Q. Design, synthesis, antiviral activity and mode of action of phenanthrene-containing N-heterocyclic compounds inspired by the phenanthroindolizidine alkaloid antofine. Pest Manag. Sci. 2016, 72, 371–378. [Google Scholar] [CrossRef]
- Pang, Z.; Ye, H.; Ma, D.; Tu, X.; Yi, L.; Xi, Z. A H2 S-specific ultrasensitive fluorogenic probe reveals TMV-Induced H2 S production to limit virus replication. Chembiochem 2021, 22, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Wang, L.H.; Li, S.L.; Li, S.L.; Chen, X.Y.; Shen, Y.M.; Zhang, Z.K.; He, H.P.; Xu, W.B.; Shu, Y.L.; et al. Seco-pregnane steroids target the subgenomic RNA of alphavirus-like RNA viruses. Proc. Natl. Acad. Sci. USA 2007, 104, 8083–8088. [Google Scholar] [CrossRef]
- Hu, Z.X.; Zhang, J.; Zhang, T.; Tian, C.Y.; An, Q.; Yi, P.; Yuan, C.M.; Zhang, Z.K.; Zhao, L.H.; Hao, X.J. Aloperine-type alkaloids with antiviral and antifungal activities from the seeds of Sophora alopecuroides L. J. Agric. Food Chem. 2024, 72, 8225–8236. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Xia, R.; Chen, M.; He, J.; Su, S.; Liu, L.; Li, X.; Xue, W. Biological activity evaluation and action mechanism of chalcone derivatives containing thiophene sulfonate. RSC Adv. 2019, 9, 24942–24950. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Xing, L.; He, B.; Deng, T.; Qin, Y.; Hu, Y.; An, Y.; Xue, W. Antiviral activity evaluation and action mechanism of chalcone derivatives containing phenoxypyridine. Mol. Divers. 2024; advance online publication. [Google Scholar] [CrossRef]
- Liao, A.; Li, L.; Wang, T.; Lu, A.; Wang, Z.; Wang, Q. Discovery of phytoalexin camalexin and its derivatives as novel antiviral and antiphytopathogenic-fungus agents. J. Agric. Food Chem. 2022, 70, 2554–2563. [Google Scholar] [CrossRef]
- He, H.W.; Wang, F.Y.; Zhang, D.; Chen, C.Y.; Xu, D.; Zhou, H.; Liu, X.; Xu, G. Discovery of novel α-methylene-γ-butyrolactone derivatives containing vanillin moieties as antiviral and antifungal agents. J. Agric. Food Chem. 2022, 70, 10316–10325. [Google Scholar] [CrossRef] [PubMed]
- Hanson, A.D.; Ditz, K.M.; Singletary, G.W.; Leland, T.J. Gramine accumulation in leaves of barley grown under high-temperature stress. Plant Physiol. 1983, 71, 896–904. [Google Scholar] [CrossRef]
- Lu, A.; Wang, T.; Hui, H.; Wei, X.; Cui, W.; Zhou, C.; Li, H.; Wang, Z.; Guo, J.; Ma, D.; et al. Natural products for drug discovery: Discovery of gramines as novel agents against a plant virus. J. Agric. Food Chem. 2019, 67, 2148–2156. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Li, X.; Yin, L.; Jiang, D.; Hu, D. Design, synthesis, antiviral bioactivity, and mechanism of the ferulic acid ester-containing sulfonamide moiety. ACS Omega 2020, 5, 19721–19726. [Google Scholar] [CrossRef]
- Tai, G.; Zhang, Q.; He, J.; Li, X.; Gan, X. Ferulic acid dimers as potential antiviral agents by inhibiting TMV self-assembly. J. Agric. Food Chem. 2024, 72, 14610–14619. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Peng, F.; Cao, X.; Liu, F.; Wang, Q.; Liu, L.; Xue, W. Design, synthesis, antibacterial activity, antiviral activity, and mechanism of myricetin derivatives containing a quinazolinone moiety. ACS Omega 2021, 6, 30826–30833. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Ma, Z.Z.; Hano, Y.; Chen, Y.J. Two new pyrroloquinazolinoquinoline alkaloids from Peganum nigellastrum. Heterocycles 1997, 46, 541–546. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, K.; Wang, Z.; Liu, Y.; Ma, D.; Wang, Q. Luotonin A and its derivatives as novel antiviral and antiphytopathogenic fungus agents. J. Agric. Food Chem. 2020, 68, 8764–8773. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Yang, Z.; Sun, W.; Tian, K.; Sun, P.; Wu, J. TMV-CP based rational design and discovery of α-Amide phosphate derivatives as anti plant viral agents. Bioorg. Chem. 2024, 147, 107415. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Zhang, G.; Ding, Y.; Ran, L.; Luo, L.; Wu, J.; Hu, D.; Song, B. Binding interactions between enantiomeric α-aminophosphonate derivatives and tobacco mosaic virus coat protein. Int. J. Biol. Macromol. 2017, 94, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ding, X.; Kang, J.; Gao, Y.; Wang, Z.; Wang, Q. Marine natural product for pesticide candidate: Pulmonarin alkaloids as novel antiviral and anti-phytopathogenic-fungus agents. J. Agric. Food Chem. 2020, 68, 11350–11357. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.N.; Yang, S.; Shi, S.Y.; Yuan, W.Y.; Chen, J.X.; Duan, Z.Y.; Lu, A.D.; Wang, Z.W.; Wang, Q.M. Pityriacitrin marine alkaloids as novel antiviral and anti-phytopathogenic-fungus agents. Pest Manag. Sci. 2021, 77, 4691–4700. [Google Scholar] [CrossRef]
- Ding, X.; Xu, Y.; Yan, L.; Chen, L.; Lu, Z.; Ge, C.; Zhao, X.; Wang, Z.; Lu, A.; Wang, Q. Marine sesquiterpenes for plant protection: Discovery of laurene sesquiterpenes and their derivatives as novel antiviral and antiphytopathogenic fungal agents. J. Agric. Food Chem. 2022, 70, 6006–6014. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Tian, Z.; Yin, X.; Yuan, X.; Gao, J.; Yuan, W.; Lu, A.; Wang, Z.; Li, L.; Wang, Q. Structural optimization of the natural product: Discovery of almazoles C-D and their derivatives as novel antiviral and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2022, 70, 15693–15702. [Google Scholar] [CrossRef]
- Yan, L.; Gao, Y.; Li, T.; Wang, X.; Xie, R.; Liu, Y.; Xie, Y.; Wang, Z.; Lu, A.; Wang, Q. Design, synthesis, antiviral and fungicidal activities of novel polycarpine simplified analogues. Bioorg. Chem. 2023, 135, 106508. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.P.; Rahman, M.T.; Cook, J.M. Bisindole alkaloids from the Alstonia species: Recent isolation, bioactivity, biosynthesis, and synthesis. Molecules 2021, 26, 3459. [Google Scholar] [CrossRef]
- Gao, Y.; He, X.; Yan, L.; Zhang, H.; Liu, S.; Ma, Q.; Zhang, P.; Zhang, Y.; Zhang, Z.; Wang, Z.; et al. Discovery of barakacin and its derivatives as novel antiviral and fungicidal agents. Molecules 2023, 28, 3032. [Google Scholar] [CrossRef]
- Yang, S.; Wang, T.; Lu, A.; Wang, Q. Discovery of chiral diamine derivatives containing 1,2-diphenylethylenediamine as novel antiviral and fungicidal agents. J. Agric. Food Chem. 2023, 71, 10989–11000. [Google Scholar] [CrossRef]
- El-Gendy, M.M.A.; Shaaban, M.; Shaaban, K.A.; El-Bondkly, A.M.; Laatsch, H. Essramycin: A first triazolopyrimidine antibiotic isolatedfrom nature. J. Antibiot. 2008, 61, 149–157. [Google Scholar] [CrossRef]
- Battaglia, U.; Moody, C.J. A short synthesis of the triazolopyrimidine antibiotic essramycin. J. Nat. Prod. 2010, 73, 1938–1939. [Google Scholar] [CrossRef] [PubMed]
- Tee, E.; Karoli, T.; Ramu, S.; Huang, J.; Butler, M.; Cooper, M. Synthesis of essramycin and comparison of its antibacterial activity. J. Nat. Prod. 2010, 73, 1940–1942. [Google Scholar] [CrossRef]
- Wang, T.; Yang, S.; Li, H.; Lu, A.; Wang, Z.; Yao, Y.; Wang, Q. Discovery, structural optimization, and mode of action of essramycin alkaloid and its derivatives as anti-tobacco mosaic virus and anti-phytopathogenic fungus agents. J. Agric. Food Chem. 2020, 68, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, L.; Zhou, Y.; Lu, A.; Li, H.; Chen, J.; Duan, Z.; Wang, Q. Structural simplification of marine natural products: Discovery of hamacanthin derivatives containing indole and piperazinone as novel antiviral and anti-phytopathogenic-fungus agents. J. Agric. Food Chem. 2021, 69, 10093–10103. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Luo, Y.; Qin, S.; Xi, L.; Wan, B.; Du, L. Induction of systemic resistance against tobacco mosaic virus by Ningnanmycin in tobacco. Pestic. Biochem. Phy. 2014, 111, 14–18. [Google Scholar] [CrossRef]
- Yang, R.; Jiang, S.L.; Wen, X.D.; Song, X.C.; Wang, X.; Li, D.X.; Yin, Q.X.; Wu, X.; Wang, D.L.; Chen, Z. Antifungal activity and possible mode of action of Ningnanmycin against tea gray blight disease pathogen Pseudopestalotiopsis camelliae-sinensis. Phytopathology 2021, 111, 1735–1742. [Google Scholar] [CrossRef]
- Li, X.; Hao, G.; Wang, Q.; Chen, Z.; Ding, Y.; Yu, L.; Hu, D.; Song, B. Ningnanmycin inhibits tobacco mosaic virus virulence by binding directly to its coat protein discs. Oncotarget 2017, 8, 82446–82458. [Google Scholar] [CrossRef]
- Osawa, T.; Namiki, M. Structure elucidation of streptindole, a novel genotoxic metabolite isolated from intestinal bacteria. Tetrahedron Lett. 1983, 24, 4719–4722. [Google Scholar] [CrossRef]
- Ouyang, G.; Chen, Z.; Cai, X.J.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activity of novel pyrazole derivatives containing oxime esters group. Bioorgan. Med. Chem. 2008, 16, 9699–9707. [Google Scholar] [CrossRef]
- Chen, M.; Hu, D.; Li, X.; Yang, S.; Zhang, W.; Li, P.; Song, B. Antiviral activity and interaction mechanisms study of novel glucopyranoside derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 3840–3844. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Xu, F.Z.; Zhu, Y.Y.; Song, B.; Luo, D.; Yu, G.; Chen, S.; Xue, W.; Wu, J. Pyrazolo [3,4-d]pyrimidine derivatives containing a Schiff base moiety as potential antiviral agents. Bioorg. Med. Chem. Lett. 2018, 28, 2979–2984. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Tang, X.; Xia, R.; Guo, T.; Zhang, C.; Li, X.; Xue, W. Design, synthesis, antiviral bioactivities and interaction mechanisms of penta-1,4-diene-3-one oxime ether derivatives containing a quinazolin-4(3H)-one scaffold. BMC Chem. 2019, 13, 34. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Li, X.; He, F.; Wu, R.; Hu, D.; Song, B. Discovery of dithioacetal derivatives containing sulfonamide moiety of novel antiviral agents by TMV coat protein as a potential target. ACS Omega 2020, 5, 22596–22602. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, W.; Hou, S.; Xie, D.; Yang, J.; Liu, L.; Yang, S. In vivo antiviral activity and disassembly mechanism of novel 1-phenyl-5-amine-4-pyrazole thioether derivatives against tobacco mosaic virus. Pestic. Biochem. Phys. 2021, 173, 104771. [Google Scholar] [CrossRef]
- Shao, W.B.; Liao, Y.M.; Luo, R.S.; Ji, J.; Xiao, W.L.; Zhou, X.; Liu, L.W.; Yang, S. Discovery of novel phenothiazine derivatives as new agrochemical alternatives for treating plant viral diseases. Pest Manag. Sci. 2023, 79, 4231–4243. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Ding, Y.; Zhang, T.H.; Hu, J.H.; Luo, R.S.; Zhou, X.; Liu, L.W.; Yang, S. Identification of novel bisamide-decorated benzotriazole derivatives as anti-phytopathogenic virus agents: Bioactivity evaluation and computational simulation. J. Agric. Food Chem. 2024, 72, 6900–6912. [Google Scholar] [CrossRef]
- He, B.; Hu, Y.; Qin, Y.; Zhang, Y.; Luo, X.; Wang, Z.; Xue, W. Design, synthesis and antiviral activity of indole derivatives containing quinoline moiety. Mol. Divers. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Gong, C.; Meng, K.; Sun, Z.; Zeng, W.; An, Y.; Zou, H.; Qiu, Y.; Liu, D.; Xue, W. Flavonol derivatives containing a quinazolinone moiety: Design, synthesis, and antiviral activity. Chem. Biodivers. 2024, 21, e202301737. [Google Scholar] [CrossRef]
- Wang, Q.; Xing, L.; Zhang, Y.; Gong, C.; Zhou, Y.; Zhang, N.; He, B.; Xue, W. Antiviral activity evaluation and action mechanism of myricetin derivatives containing thioether quinoline moiety. Mol. Divers. 2024, 28, 1039–1055. [Google Scholar] [CrossRef]
- Yuan, C.; Tian, J.; Zhou, Q.; Xin, H.; Liu, Y.; Deng, T.; Zeng, W.; Sun, Z.; Xue, W. Myricetin derivatives containing the benzoxazinone moiety discovered as potential anti-tobacco mosaic virus agents. Fitoterapia 2024, 173, 105812. [Google Scholar] [CrossRef]
- Sasaki, T.; Ohtani, I.I.; Tanaka, J.; Higa, T. Iheyamines, new cytotoxic bisindole pigments from a colonial ascidian, Polycitorella sp. Tetrahedron Lett. 1999, 40, 303–306. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, P.; Chen, M.; Li, T.; Hou, C.; Que, X.; Xu, L.; Zhou, Z.; Wang, Q.; Wang, Z. Synthesis, structural modification, and biological activity of a novel bisindole alkaloid iheyamine A. Bioorg. Chem. 2024, 153, 107757. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Guo, J.; Wang, Z.; Liu, Y.; Li, Y.; Ma, D.; Wang, Q. Discovery of tryptanthrins as novel antiviral and anti-phytopathogenic-fungus agents. J. Agric. Food Chem. 2020, 68, 5586–5595. [Google Scholar] [CrossRef]
- Li, Y.; Ye, S.; Hu, Z.; Hao, N.; Bo, X.; Liang, H.; Tian, X. Identification of anti-TMV active flavonoid glycosides and their mode of action on virus particles from Clematis lasiandra Maxim. Pest Manag. Sci. 2021, 77, 5268–5277. [Google Scholar] [CrossRef]
- Tian, Z.; Liao, A.; Kang, J.; Gao, Y.; Lu, A.; Wang, Z.; Wang, Q. Toad alkaloid for pesticide discovery: Dehydrobufotenine derivatives as novel agents against plant virus and fungi. J. Agric. Food Chem. 2021, 69, 9754–9763. [Google Scholar] [CrossRef]
- Shih, Y.T.; Chen, P.S.; Wu, C.H.; Tseng, Y.T.; Wu, Y.C.; Lo, Y.C. Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system. Free Radic. Biol. Med. 2010, 49, 1471–1479. [Google Scholar] [CrossRef]
- Li, T.; Liu, S.; Guo, X.; He, X.; Lu, A.; Wang, Q.; Wang, Z. Design, synthesis, and biological activities of arecoline derivatives containing 1,3,4-oxadiazole structure. Bioorg. Chem. 2024, 151, 107708. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Wu, Q.F.; Fan, Z.J.; Huo, J.Q.; Zhang, J.L.; Zhao, B.; Lai, C.; Qian, X.L.; Ma, D.J.; Wang, D.W. Synthesis, bioactivity and mode of action of 5A 5B 6C tricyclic spirolactones as novel antiviral lead compounds. Pest Manag. Sci. 2019, 75, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Yasunari, Y.; Suzuki, T.; Imai, N.; Kurosawa, E.; Masamune, T. A new sesquiterpene hydrocarbro from Laurbficia glaivdulif. Tetrahedron Lett. 1965, 40, 3619–3624. [Google Scholar] [CrossRef]
- Xu, Y.; Guo, X.; Li, T.; Li, T.; Ding, X.; Wang, Z.; Lu, A.; Wang, Q. Design, synthesis, and biological evaluation of novel derivatives of the marine natural product laurene. J. Agric. Food Chem. 2023, 71, 14483–14492. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, W.L.; Zeng, S.; Chen, Z.; Yang, A.M.; Shi, J.; Zhao, X.Z.; Song, B.A. Label-free quantitative proteomics analysis of Cytosinpeptidemycin responses in southern rice black-streaked dwarf virus-infected rice. Pestic. Biochem. Physiol. 2018, 147, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Liu, H.; Xia, Z.H.; Zhao, X.X.; Wu, Y.H.; An, M.N. Purification and structural analysis of the effective anti-TMV compound ε-poly-l-lysine produced by Streptomyces ahygroscopicus. Molecules 2019, 24, 1156. [Google Scholar] [CrossRef] [PubMed]
- An, M.N.; Zhao, X.X.; Zhou, T.; Wang, G.Z.; Xia, Z.H.; Wu, Y.H. A novel biological agent Cytosinpeptidemycin inhibited the pathogenesis of tobacco mosaic virus by inducing host resistance and stress response. J. Agric. Food Chem. 2019, 67, 7738–7747. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, K.; Gao, D.; Wang, D.; Huang, M.; Zhu, H.; Kang, J. Binding studies between cytosinpeptidemycin and the superfamily 1 helicase protein of tobacco mosaic virus. RSC Adv. 2018, 8, 18952–18958. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, G.; Zhang, S.; Zhao, K.; Li, X. Study on the binding of ningnanmycin to the helicase of Tobamovirus virus. Pestic. Biochem. Phy. 2023, 194, 105494. [Google Scholar] [CrossRef]
- Wang, D.; Huang, M.; Gao, D.; Chen, K.; Xie, X.; Xu, W.; Li, X. Screening anti-TMV agents targeting tobacco mosaic virus helicase protein. Pestic. Biochem. Phy. 2020, 166, 104449. [Google Scholar] [CrossRef]
- Li, Z.; Yang, B.; Liu, H.; Ding, Y.; Fang, Z.; Shao, W.; Qi, P.; Zhou, X.; Liu, L.; Yang, S. The discovery of novel ferulic acid derivatives incorporating substituted isopropanolamine moieties as potential tobacco mosaic virus helicase inhibitors. Int. J. Biol. Macromol. 2022, 23, 13991. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Liu, H.; Guo, L.; Zhou, T.; Shan, Y.; Xia, Z.; Li, X.; An, M.; Wu, Y. Antiviral modes of action of the novel compound GLY-15 containing pyrimidine heterocycle and moroxydine skeleton against tobacco mosaic virus. Pest Manag. Sci. 2022, 78, 5259–5270. [Google Scholar] [CrossRef]
- Li, Z.; Yang, B.; Ding, Y.; Zhou, X.; Fang, Z.; Liu, S.; Yang, J.; Yang, S. Discovery of phosphonate derivatives containing different substituted 1,2,3-triazole motif as promising tobacco mosaic virus (TMV) helicase inhibitors for controlling plant viral diseases. Pest Manag. Sci. 2023, 79, 3979–3992. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.N.; Verma, M.; Clercq, E.D. Synthesis of some heterocycle containing urea derivatives and their anti-viral activity. Heterocycles 2006, 68, 11–12. [Google Scholar] [CrossRef]
- Regan, J.; Breitfelder, S.; Cirillo, P.; Gilmore, T.; Graham, A.G.; Hickey, E.; Klaus, B.; Madwed, J.; Moriak, M.; Moss, N.; et al. Pyrazole urea-based inhibitors of p38 MAP kinase: From lead compound to clinical candidate. J. Med. Chem. 2002, 45, 2994–3008. [Google Scholar] [CrossRef] [PubMed]
- Bai, Q.; Jiang, J.; Luo, D.; Huang, Y.; Huang, M.; Zhao, G.; Wang, Z.; Li, X. Cysteine protease domain of potato virus Y: The potential target for urea derivatives. Pestic. Biochem. Phys. 2023, 189, 105309. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Chen, K.; Gao, D.; Wang, D.; Xue, W. Cucumber mosaic virus coat protein: The potential target of 1, 4-pentadien-3-one derivatives. Pestic. Biochem. Phys. 2019, 155, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yan, Y.; Huang, M.; Ma, G.; Wang, L.; Xie, X.; Xue, W.; Li, X. Myricetin derivative LP11 targets cucumber mosaic virus 2b protein to achieve in vivo antiviral activity in plants. J. Agric. Food Chem. 2022, 70, 15360–15370. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.L.; Zhao, C.N.; Li, J.; Li, C.Y.; Song, B.A.; Song, R.J. Innovative arylimidazole-fused phytovirucides via carbene-catalyzed [3+4] cycloaddition: Locking viral cell-to-cell movement by out-competing virus capsid-host interactions. Adv. Sci. 2024, 11, e2309343. [Google Scholar] [CrossRef]
- Jin, J.; Mou, C.; Zou, J.; Xie, X.; Wang, C.; Shen, T.; Deng, Y.; Li, B.; Jin, Z.; Li, X.; et al. Development of axially chiral urazole scaffolds for antiplant virus applications against potato virus Y. Pest Manag. Sci. 2023, 79, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Lv, Y.; Chen, Z.; Huang, Y.; Zhang, M.; Jin, Z.; Li, T.; Chi, Y.R. Design, synthesis and anti-PVY activity of planar chiral thiourea derivatives incorporated with [2.2]Paracyclophane. Pest Manag. Sci. 2024, 80, 4450–4458. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, C.; Huang, Y.; Tao, N.; Wang, T.; Cai, X.; Wang, Z.; Li, X. Tryptanthrin derivative B1 binds viral genome-linked protein (VPg) of potato virus Y. J. Agric. Food Chem. 2024, 72, 5699–5709. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, W.; Fu, X.; Xie, X.; Bai, S.; Li, X. Synthesis and screening of chemical agents targeting viral protein genome-linked protein of telosma mosaic virus. J. Agric. Food Chem. 2023, 71, 13645–13653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Luo, Y.Q.; Hu, D.Y.; Song, B.A. Design, synthesis, anti-tomato spotted wilt virus activity, and mechanism of action of thienopyrimidine-containing dithioacetal derivatives. J. Agric. Food Chem. 2022, 70, 6015–6025. [Google Scholar] [CrossRef] [PubMed]
- Zan, N.N.; Li, J.; He, H.F.; Hu, D.Y.; Song, B.A. Discovery of novel chromone derivatives as potential anti-TSWV agents. J. Agric. Food Chem. 2021, 69, 10819–10829. [Google Scholar] [CrossRef]
- Jiang, D.H.; Zhang, J.; He, H.F.; Li, J.; Hu, D.Y.; Song, B.A. Discovery of novel chromone derivatives containing a sulfonamide moiety as potential anti-TSWV agents. Bioorg. Med. Chem. Lett. 2021, 53, 128431. [Google Scholar] [CrossRef]
- Zhang, P.; An, Q.; Yi, P.; Cui, Y.; Zou, J.B.; Yuan, C.M.; Zhang, Y.; Gu, W.; Huang, L.J.; Zhao, L.H.; et al. Thermlanseedlines A-G, seven thermopsine-based alkaloids with antiviral and insecticidal activities from the seeds of Thermopsis lanceolata R. Br. Fitoterapia 2022, 158, 105140. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; An, Q.; Wang, J.; Yi, P.; Yuan, C.M.; Zhang, Z.K.; Zhao, L.H.; Hu, Z.X.; Hao, X.J. Matrine-Type alkaloids with anti-tomato spotted wilt virus activity from the root of Sophora tonkinensis Gagnep. J. Agric. Food Chem. 2023, 71, 4394–4407. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, X.; Wang, W.; Zhang, W.; Yu, L.; Hu, D.; Song, B. Interaction research on the antiviral molecule dufulin targeting on southern rice black streaked dwarf virus p9-1 nonstructural protein. Viruses 2015, 7, 1454–1473. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Huang, M.; Wang, L.; Xue, W.; Xie, X.; Li, X. Insights into a rapid screening method for anti-cucumber mosaic virus compounds. J. Virol. Methods 2022, 301, 114402. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.M.; Kwon, J.; Seo, Y.H.; Song, E.G.; Hong, S.S.; Kim, B.S.; Hong, J.S.; Ryu, K.H.; Lee, D. Quassinoids isolated from Brucea javanica inhibit pepper mottle virus in pepper. Virus Res. 2017, 227, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Song, Y.S.; Ryu, K.H. Development of infectious transcripts from full-length and GFP-tagged cDNA clones of pepper mottle virus and stable systemic expression of GFP in tobacco and pepper. Virus Res. 2011, 155, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; He, H.; Hu, D.; Song, B. Defense mechanism of Capsicum annuum L. infected with pepper mild mottle virus induced by vanisulfane. J. Agric. Food Chem. 2022, 70, 3618–3632. [Google Scholar] [CrossRef]
- Li, X.; Song, B.; Chen, X.; Wang, Z.; Zeng, M.; Yu, D.; Hu, D.; Chen, Z.; Jin, L.; Yang, S.; et al. Crystal structure of a four-layer aggregate of engineered TMV CP implies the importance of terminal residues for oligomer assembly. PLoS ONE 2013, 8, e77717. [Google Scholar] [CrossRef]
- Li, X.; Song, B.; Hu, D.; Wang, Z.; Zeng, M.; Yu, D.; Chen, Z.; Jin, L.; Yang, S. The development and application of new crystallization method for tobacco mosaic virus coat protein. Virol. J. 2012, 9, 279. [Google Scholar] [CrossRef]
- Chen, J.; Luo, X.; Chen, Y.; Wang, Y.; Peng, J.; Xing, Z. Recent research progress: Discovery of anti-plant virus agents based on natural scaffold. Front. Chem. 2022, 10, 926202. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).