Abstract
Primordial germ cells (PGCs) are precursor cells of sperm and eggs. The fate decisions of chicken PGCs in terms of their development, integrity, and sex determination have unique features, thereby providing insights into evolutionary developmental biology. Additionally, fate decisions in the context of a self-renewal mechanism have been applied to establish culture protocols for chicken PGCs, enabling the production of genome-edited chickens and the conservation of genetic resources. Thus, studies on the fate decisions of chicken PGCs have significantly contributed to both academic and industrial development. Furthermore, studies on fate decisions have rapidly advanced owing to the recent development of essential research technologies, such as genome editing and RNA sequencing. Here, we reviewed the status of fate decisions of chicken PGCs and provided insight into other important research issues that require attention.
1. Introduction
The chicken (Gallus gallus) is a valuable species in terms of protein resources. Additionally, chickens have been used as a model organism for amniotes to elucidate embryonic developmental mechanisms [1]. Furthermore, chickens have been used as avian models in various areas of biology, thereby contributing to the understanding of vertebrate evolution. Thus, research using chickens greatly enhances both industrial development and developmental and evolutionary biology.
Primordial germ cells (PGCs) are the precursor cells of sperm and eggs. PGCs are the only cell lineage that can transmit genetic information to the next generation. Thus, elucidating the fate decision of PGCs, namely the mechanisms by which they develop, maintain integrity, differentiate into gametes of optimal sexes, and control their self-renewal, is a valuable research subject. Avian PGCs have characteristic developmental features, such as migration into the gonads using the vascular system [2]. Chicken PGCs have also shown a unique sex determination mechanism in which PGC-intrinsic factors may occur in a cell-autonomous manner [3,4,5]. In addition, several studies have revealed the self-renewal mechanism of chicken PGCs, resulting in the establishment of stable culture protocols for chicken PGCs [6,7]. Currently, culturing PGCs is a fundamental technique to establish genome-edited chickens and conserve avian genetic resources so that objective offspring can be produced via germline chimeras transplanted from cultured PGCs and directly incubated [8,9] or incubated with an ex ovo culture system [10]. Therefore, studies on the fate decisions of avian PGCs have demonstrated their unique features and have aided the development of avian biotechnologies.
With the development of essential technologies such as genome editing and RNA sequencing (RNA-seq) analysis, studies on chicken PGCs have shown rapid advancement over the last ten years. For example, the clustered regularly interspaced short palindromic repeats/CRISPR-associated protein (CRISPR/Cas) system [11] has been used not only to produce genome-edited chickens [12], but also to conduct functional analysis of PGC-intrinsic factors via application of this system to cultured PGCs [13]. Alternatively, RNA-seq technology enables us to predict the fate decision of chicken PGCs even at a single-cell resolution level [14,15]. Here, we review the fate decisions of avian PGCs, mainly focusing on chicken PGCs, with cutting-edge knowledge. Furthermore, we discuss the remaining issues that need to be addressed in future studies.
2. Early Development of Chicken PGCs
2.1. Origin and Identification of Chicken PGCs
In vertebrates, PGC formation is generally classified into two models: preformation and epigenesis [16]. The preformation model has been observed in zebrafish (Danio rerio) [17] and anuran amphibians (Xenopus laevis) [18]. In this model, germplasm, a maternal factor, acts as a determinant of germ cell formation. The germplasm is composed of maternally inherited RNAs and proteins and is partially asymmetrically partitioned to cells during cleavage divisions and development. As a result, PGCs arise from the partitioned cells. This mechanism is conserved in several non-vertebrate species, including Drosophila melanogaster [19] and Caenorhabditis elegans [20]. In contrast, the epigenesis model has been shown in urodele amphibians (Ambystoma mexicanum) [21] and mammals (Mus musculus) [22]. In these species, PGCs are induced in somatic cells via “epigenetic” regulation during embryonic development; thus, the germplasm is absent.
Several studies have been conducted to determine the origins of avian PGCs. In particular, studies focusing on the vasa gene have strongly supported the preformation model for avian PGC formation. VASA is a germ-cell-specific RNA helicase that is localized in germ cells as a germplasm component across various species, such as X. laevis and D. melanogaster. Tsunekawa et al. demonstrated the expression patterns of the chicken vasa homolog (CVH) [23]. The CVH is localized in cleavage furrows and asymmetrically distributed to limited cells. Additionally, the CVH is colocalized with the mitochondrial cloud, corresponding to the germplasm feature in D. melanogaster. Recent functional analyses have shown that the CVH significantly contributes to germ cell development in both males and females [24,25]. These studies suggest that chickens possess germplasm-like features and follow the preformation model for PGC formation.
Previous studies targeting deletion in the azoospermia-like (DAZL) protein also support the preformation model in chickens. DAZL is a germ-cell-specific RNA-binding protein localized in the germplasm in some vertebrates [26,27]. In chickens, DAZL is also localized in cleavage furrows as well as the CVH and is specifically expressed in germ cells [28]. Additionally, knockdown of DAZL in chicken PGCs causes apoptosis and aberrant expression patterns of germ-cell-characteristic genes, albeit under culture conditions [28]. Recently, whole transcriptome analysis predicted that chicken DAZL was co-expressed with its potential interacting genes according to zygotic genome activation, suggesting its central role in germ-cell specification [29].
Therefore, nowadays, avian germ cells are thought to be specified by the preformation model; however, the possibility that avian PGCs are formed via epigenetic regulation cannot be excluded [30]. Thus, while several studies have supported the preformation model in avian PGC formation, the origin of PGCs remains unknown.
2.2. Migration of Chicken PGCs into Embryonic Gonads
A chick embryo in an egg incubates for 0 h, corresponding to Eyal-Giladi and Kochav (EG & K) stage X, and consists of approximately 60,000 cells [31]. At this embryonic stage, approximately 30 CVH-positive cells, namely the origin of PGCs, are scattered at the center of the area pellucida in the blastoderm (Figure 1) [23]. The CVH-positive cells then start to migrate into the germinal crescent, an anterior extraembryonic region. At Hamburger–Hamilton (HH) stage 4 (chick embryos in eggs after 18–19 h of incubation), PGCs accumulated at a high density in the germinal crescent (Figure 1) [32]. Previously, it was thought that PGCs passively translocate into the germinal crescent via the morphogenetic movement of hypoblasts [33]. However, Kang et al. demonstrated that chick fibroblasts exogenously transplanted into the subgerminal cavity of the recipient could not settle in the germinal crescent, whereas transplanted PGCs could [34]. This indicates that PGC-intrinsic factors are also related to this migration. Recently, Huss et al. revealed that quail (Coturnix japonica) PGCs contribute to the extracellular matrix in the germinal crescent [35]. Although the molecular mechanism of PGC migration remains unclear, these previous studies showed their “active” role.
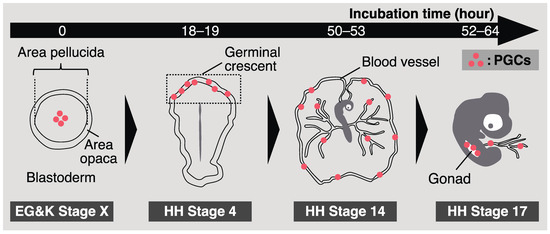
Figure 1.
A schematic illustration of chicken primordial germ cell (PGC) development.
After the settlement of PGCs into the germinal crescent, PGCs begin to migrate to the gonads. In many vertebrates, such as mice and zebrafish, PGCs migrate into gonads via “amoeboid migration” [36]. However, in birds (and reptiles), PGCs use the vascular system to migrate into the gonads. Several studies have attempted to elucidate this unique migration system in avian PGCs.
Around HH stage 6 (chick embryos in eggs incubated for 23–25 h), aggregations of endothelial cell progenitors and blood cell progenitors, called blood islands, appear in the extraembryonic mesoderm [37]. Murai et al. demonstrated that more than 60% of quail PGCs in an embryo were enveloped by differentiating endothelial cells forming blood islands in the germinal crescent [38]. Then, the PGCs flowed along with the heartbeat at HH stage 12 (after 48–49 h of incubation). This indicates that most avian PGCs were passively translocated into the vascular system.
Once avian PGCs are translocated into the blood vessels, they start circulating. The concentration of chicken PGCs in the bloodstream reaches a peak at HH stage 14 (after 50–53 h of incubation), and these settle in gonads from HH stage 15 (after 50–55 h of incubation) to HH stage 17 (after 52–64 h of incubation) (Figure 1) [39,40]. Recent studies have demonstrated the molecular mechanisms involved in gonadal migration of avian PGCs. The role of the interaction between chemotactic molecular stromal cell-derived factor 1 (SDF1) and its receptor C-X-C chemokine receptor type 4 (CXCR4) is a well-known system that directs PGCs to the gonads in vertebrates, whose PGCs utilize amoeboid migration [41,42,43,44,45]. In chickens, the expression of CXCR4 has also been observed in PGCs [46], and PGCs are attracted to ectopically expressed SDF1s [47]. Furthermore, the transplantation of CXCR4 knockout (KO) PGCs into recipient embryos resulted in a reduction in their capacity to migrate into gonads, suggesting a critical role of the CXCR4–SDF1 interaction in this migration [13]. In contrast, recent studies have proposed other factors for gonadal migration of avian PGCs. Saito et al. showed that circulating avian PGCs were stiffer than blood cells; thus, PGCs were efficiently occluded at the vascular plexus near presumptive gonads, resulting in their homing to developing gonads [48]. Huang et al. proposed that platelet-derived growth factor signaling could be involved in the migration of avian PGCs into gonads using RNA-seq analysis [49]. These molecular studies have advanced our understanding of how avian PGCs migrate to gonads. Notably, previous research focusing on the migration of avian PGCs has been conducted mainly using chickens and quails. Thus, whether the molecular mechanism of this migration is conserved across avian species remains unknown.
3. Integrity of Chicken PGCs
3.1. Epigenetic Regulation
Epigenetic regulation is essential for PGCs to maintain their properties for germinal transmission, namely, establishing their integrity. In mice, PGCs undergo genome-wide epigenetic reprogramming between embryonic day (E) 8.5 and E13.5, and these embryonic stages correspond to the migration and colonization of PGCs to the gonads [50]. Epigenetic reprogramming is required to establish germ cell specification and erase somatic epigenetic memory [51]. The importance of epigenetic modifications in the establishment of PGC integrity has also been observed across species [52].
Several studies have been conducted to reveal epigenetic modifications of chicken PGCs. Yu et al. demonstrated that chicken PGCs undergo global DNA demethylation via ten-eleven translocation 1 during HH stage 21 (after 3.5 d of incubation) to HH stage 28 (after 5.5 d of incubation) [53]. These embryonic stages correspond to states in which chicken PGCs migrate to the gonads and form colonies within the gonads. Rengaraj et al. investigated the expression patterns of the DNA methyltransferase (DNMT) families DNMT1, DNMT 3 α (DNMT3A), and DNMT 3 β (DNMT3B) in chicken PGCs during embryogenesis and suggested that DNMT3B-dependent de novo DNA methylation occurred after PGCs settled into the gonads [54]. Jang et al. showed the characteristic DNA methylation patterns of gonadal PGCs by comparing them with those of chicken embryonic fibroblasts [55]. Although the understanding of DNA methylation in PGCs is not yet complete, these analyses are beginning to elucidate the underlying mechanism.
Furthermore, several studies have been reported concerning the elucidation of histone modification in avian PGCs. In mice, after the transient loss of histone modifications during epigenetic reprogramming, PGCs regain both histone H3 lysine 9 (H3K9) and H3K27 trimethylation (me3) around E12.5 [56]. However, histone modification of chicken PGCs was based on H3K9me3 rather than H3K27me3, suggesting the existence of avian-specific epigenetic regulation in PGCs [57]. For other modifications, H3K4me2 activates signaling pathways essential for avian PGC formation, such as bone morphogenetic protein 4 (BMP4) signaling [58]. Additionally, H3K9 acetylation (H3K9ac) contributes to maintaining the integrity of avian PGCs via the regulation of NANOG, a key transcription factor for germ cell development [59] (described below). These analyses have revealed histone modifications in avian PGCs, including avian-specific H3K9me3-dominant gene expression regulation.
3.2. Key Molecules for the Integrity of Avian PGC
Key molecules for the integrity of PGCs, including transcription factors, are well-conserved in vertebrates. Recently, several researchers conducted functional analyses of these molecules in avian PGCs. Interestingly, these key molecules exhibited bird-like features. In this section, we describe the functions and characteristics of the molecules involved in the integrity of chicken PGCs, along with their differences from those in other model organisms.
3.2.1. PRDM14 and BLIMP1
PR-domain-containing protein 14 (Prdm14) and B lymphocyte-induced maturation protein 1 (Blimp1) are transcription factors essential for the specification of PGCs in mammals. In mice, PRDM14 contributes to epigenetic reprogramming, the reacquisition of potential pluripotency, and PGC-specific gene expression [60,61]. In contrast, BLIMP1 represses somatic gene expression in PGCs [62]. In chickens, both PRDM14 and BLIMP1 facilitate PGC development. Okuzaki et al. demonstrated that PRDM14 and BLIMP1 were expressed in blastodermal cells and PGCs, and the knockdown of each gene in chicken embryos decreased the number of PGCs [63]. Interestingly, the lack of PRDM14 causes embryonic lethality in chickens, even though Prdm14 KO mice are viable [64]. This implies avian-specific functions of PRDM14 during early embryogenesis.
3.2.2. BMP4
Bone morphogenetic protein 4 (BMP4) directly induces the formation of mouse PGCs in an epigenetic model. In mice, BMP4 is emitted into the extra-embryonic ectoderm and induces Prdm14 and Blimp1 expression, resulting in the PGC fate [65,66]. In this fate decision, Wnt signaling is also involved in terms of inducing the Blimp1 expression [66,67]. In chickens, BMP4 is also essential for the integrity of PGCs. While the nucleotide sequence in the promoter region of chicken BMP4 is poorly conserved with mammalian Bmp4, the protein sequences in the coding region of BMP4 are highly conserved in birds and mammals [30]. Using chicken embryonic stem cells (ESCs) [68,69], Zuo et al. demonstrated that BMP4 contributes to the induction of PGC formation from the ESCs [70,71,72]. However, the molecular mechanisms underlying this induction may differ between chickens and mammals. In chickens, Wnt signaling activated by BMP4 directly regulates lin-28 homolog A (LIN28A), a significant factor for PGC formation [73]; this direct interaction between Wnt signaling and LIN28A has not yet been observed in the fate decisions of mammalian PGCs [72]. Additionally, a recent study showed that a long non-coding RNA, LncBMP4, has similar functions to chicken BMP4 and contributes to the fate decisions of PGCs [74]. Overall, studies on chicken BMP4 have contributed to both the elucidation of the fate decisions of chicken PGCs and the improvement in methods to induce PGCs from avian pluripotent stem cells.
3.2.3. NANOG
NANOG is a transcription factor involved in maintaining pluripotency in ESCs [75,76]. Additionally, NANOG plays a crucial role in the specification of mammalian PGCs via their epigenetic modification [77,78]. In chicken ESCs, NANOG also regulates pluripotency [79]. Because chicken NANOG can function as an alternative factor to mouse NANOG and contribute to the pluripotency of mouse cells [80], its function is conserved between mammals and birds, at least in terms of stem cell pluripotency. Interestingly, the oligomerization mechanism of NANOG proteins differs between mammals and chickens [81]. In addition, NANOG is essential for the somatic reprogramming of chicken and duck (Anas platyrhynchos) fibroblasts [82]. In chicken PGCs, NANOG expression has been observed, and its function in the self-renewal of PGCs has been suggested [63,83]. However, its expression level is downregulated during the migration of PGCs to the gonads, differing from the expression patterns observed in mouse PGCs [84]. Recent studies have revealed the expression regulation mechanisms of NANOG in chicken PGCs. Deacetylation of H3K9ac and several transcription factors, such as tumor protein p53 (TP53), POU domain class 5 transcription factor 3 (POU5F3), and SRY-box 2 (SOX2), control the expression of NANOG [59,85]. Nevertheless, alterations in the expression patterns of NANOG in chicken PGCs during embryogenesis remain unclear. Overall, although the function of chicken NANOG is similar to that of mammals, its expression pattern, regulatory mechanism, and oligomerization mechanism are different from those of mouse NANOG.
3.2.4. POU5F3
POU5F1 (also called octamer-binding transcription factor 4 (OCT4)) is an essential transcription factor for maintaining the pluripotency of ESCs and specification of the inner cell mass in mice [86,87,88]. Additionally, POU5F1 plays a critical role in establishing mammalian-induced pluripotent stem cells (iPSCs) [89]. Furthermore, POU5F1 is required for maintaining germ cell lineage in mice [90]. However, a previous study demonstrated that POU5F1 is evolutionarily lacking in chickens [91], which remained the prevailing theory until Lavial et al. successfully cloned the POU V gene as a homologous gene of Pou5f1 and identified its function in maintaining chicken ESCs [79]. Further studies have proposed that chicken POU V establishes synteny with the POU5F3 gene, which is conserved in several vertebrates, such as marsupials, monotremes, and teleost fishes [92,93,94]. Additionally, Nakanoh et al. recharacterized the nucleotide sequence of chicken POU V and showed its similarity with those from POU5F3 in other species [95]. In terms of maintaining the pluripotency of ESCs, the degree of functional conservation between POU5F1 and POU5F3 is species-dependent [94]. Therefore, it is thought that the relationship between chicken POU V (POU5F3) and mammalian Pou5f1 is a paralog rather than an ortholog.
In chicken PGCs, POU5F3 is expressed along with NANOG and SOX2 and may form a transcriptional network [84,96]. A recent study demonstrated that POU5F3 regulates globally active chromatin modification [97]. In addition, the knockdown of POU5F3 in chicken PGCs resulted in the severe impairment of their gonadal colonization [97]. These studies suggest an essential role of POU5F3 in chicken PGCs.
3.2.5. DND1
DND microRNA-mediated repression inhibitor 1 (DND1), an RNA-binding protein, maintains the fate of PGCs in vertebrates. In mice, DND1 inhibits apoptosis of germ cells by destabilizing target mRNAs [98,99]. In zebrafish, DND1 also regulates apoptosis [100]. Recently, Gross-Thebing et al. clarified that zebrafish DND1 mainly controls somatic differentiation of PGCs, and the lack of DND1 causes apoptosis, depending on the case [101]. In chickens, the DND1 homolog was cloned, and its germ-cell-specific expression pattern was characterized [102,103]. However, the function of the chicken DND1 homolog remains unclear. To reveal the molecular mechanisms that suppress somatic differentiation in chicken PGCs, functional analyses of the chicken DND1 homolog are needed to provide new insights.
3.2.6. Non-Coding RNA
Non-coding RNAs (ncRNAs) play key roles in germ cell development. PIWI-interacting RNAs (piRNAs) are a class of non-coding RNAs involved in germ cell development. piRNAs are non-coding RNAs of approximately 26–31 nucleotides in length strongly expressed in the gonads and control germ cell development by repressing transposons to maintain genomic integrity [104,105,106,107]. In chickens, piRNAs specifically expressed in germ cells have been identified by RNA-seq analyses [108,109]. Repression of piRNA pathways in chicken PGCs results in increased DNA double-strand breaks, indicating that piRNAs play a critical role in the integrity of PGCs [108].
Critical roles of other classes of non-coding RNAs, such as microRNAs (miRNAs) and lncRNAs, have also been reported with regard to the integrity of chicken PGCs. miRNAs include 18–23 nucleotides related to the post-transcriptional regulation of target mRNAs. Lee et al. identified miRNAs that are expressed explicitly in chicken PGCs [110]. They demonstrated that miR-181a* downregulates the expression of nuclear receptor subfamily 6 group A member 1 (NR6A1), and homeobox A1 (HOXA1), suggesting that miR-181a* regulates inappropriate meiosis and somatic differentiation of chicken PGCs in different pathways [110]. Other studies have also identified and characterized miRNAs involved in maintaining the integrity of chicken PGCs, such as the regulation of DNA methylation [54], glucose metabolism [111], and apoptosis [112]. In contrast, lncRNAs are non-coding RNAs composed of over 200 nucleotides and possess various functions, such as epigenetic, transcriptional, and translational regulation, via lncRNA–RNA or lncRNA–protein interactions [113]. Zuo et al. identified lncRNAs specifically expressed in chicken PGCs and demonstrated that lncRNA PGC transcript-1 (lncPGCAT-1) contributes to the formation of chicken PGCs by regulating CVH expression [114]. Other studies have also characterized several lncRNAs related to the development of chicken PGCs [115,116,117]. Overall, previous studies have demonstrated that non-coding RNAs possess various functions and contribute to chicken PGC integrity.
4. Sex Determination in Birds
4.1. Bird-Characteristic Sex Determination Mechanisms
Birds exhibit unique features of sex determination. The development of the urogenital system in birds resembles that in mammals. In contrast, birds naturally experience sex reversal, indicating the considerable role of sex hormones in avian sex determination, as well as that in fish. Indeed, Elbrecht and Smith experimentally demonstrated that the inhibition of aromatase, an enzyme that converts testosterone to estradiol, confers a male phenotype on genetically female chickens [118]. Nevertheless, naturally occurring gynandromorphous birds indicate that a cell-autonomous manner significantly determines the sexual identity of avian somatic cells [119,120]. Given the above, birds show characteristic features in their sex determination, but their comprehensive mechanisms remain unclear. Elucidating this mechanism is essential to understand vertebrate evolution in terms of sex determination.
In several vertebrates, the master transcription factors for gonadal sex determination, namely the fate decision of the testes or ovaries, have been determined. In mice, the sex-determining region Y (Sry) [121], which is located on the Y chromosome, acts as the master gene. In teleost fish medaka, a Y-chromosome-linked gene, the DM domain gene on the Y chromosome (DMY), determines gonadal sex [122]. Furthermore, in X. laevis, which uses a ZZ/ZW sex-determining system, the W-chromosome-linked DM domain gene (DM-W) was identified as the master gene [123].
A schematic image of gonadal sex determination in chickens is shown in Figure 2a. Birds also use the ZZ/ZW system; however, leading candidates for W-linked master transcription factors have not yet been identified. In contrast, significant effects of a Z-linked gene, doublesex and mab-3-related transcription factor 1 (DMRT1), for avian gonadal sex determination have been reported. Birds at least partially lack a compensation system for Z-chromosome-linked genes, which corresponds to mammalian X inactivation [124,125]. From HH stage 25 (after 4.5 d of incubation) onwards, higher DMRT1 expression in male (ZZ) chickens than in female (ZW) chickens has been observed in undifferentiated chicken gonads [126]. Subsequent functional analyses showed that DMRT1 significantly contributes to gonadal masculinization [127,128]. Interestingly, recent studies demonstrated that hetero KO of DMRT1 in ZZ chickens leads to incomplete female morphology, although ovarian development is induced [129,130]. This suggests that cell-autonomous sex identity is essential for sex determination in avian somatic cells.
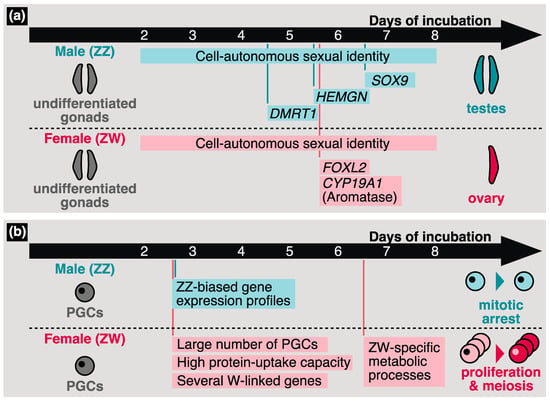
Figure 2.
Schematic images of chicken sex determination in undifferentiated gonads (a) and primordial germ cells (PGCs) (b). The timing of the expression of sex-determination-related genes and the sexual phenotypes observed or predicted in the previous studies are illustrated.
Other sex-determination-related genes have also been identified in birds. hemogen (HEMGN) is a chicken-specific gonadal-masculinization-related transcription factor. In mice, Hemgn is specifically expressed in hematopoietic tissues and cells [131]. However, in chickens, the upregulation of HEMGN is observed in undifferentiated male gonads from HH stage 28 (after 5.5 d of incubation) onwards, and this expression timing suggests that HEMGN operates downstream of DMRT1 [132]. Overexpression of HEMGN in ZW chicken embryos induces the expression of SRY-box 9 (SOX9), a testicular marker [132]. The essential role of the forkhead box L2 (FOXL2) gene in gonadal feminization in birds has also been characterized. FOXL2 expression is increased in undifferentiated ZW gonads from HH stage 28 to 29 (after 5.7 d of incubation) [133]. FOXL2 suppresses the expression of male pathway genes such as SOX9 and activates ovarian development [134]. Although the expression pattern of cytochrome P450 family 19 subfamily A member 1 (CYP19A1), which encodes for aromatase, resembles that of FOXL2, FOXL2 might not directly regulate CYP19A1 (Aromatase) expression [133,134]. Thereafter, the undifferentiated gonads of the ZZ and ZW embryos develop symmetrically and asymmetrically, respectively, and both the right and left undifferentiated gonads of the ZZ embryo develop functional testes, while only the left gonad becomes a functional ovary in the ZW embryo.
4.2. Sexual Identity of Avian PGCs
In several vertebrates, the sex of germ cells is affected by gonadal somatic cells following the gonadal migration of PGCs. However, in birds, several researchers have observed the cell-autonomous sexual identity of PGCs (Figure 2b). First, the number of chicken PGCs migrating to undifferentiated gonads is greater in females than in males [40]. Second, chicken PGCs cultured and harvested from female embryos showed a lower tendency for self-renewal compared to those from male embryos [135]; therefore, the culturing of female chicken PGCs was difficult compared to that of male chicken PGCs. Thus, the culture protocol for chicken PGCs has been optimized for each sex [7]. Third, the capacity for protein uptake differs between male and female PGCs [4]. These reports suggest that the sexual identity of initial PGCs is constructed by PGC-intrinsic factors independent of gonadal sex determination.
In vertebrates, medaka forkhead box L3 (FOXL3) was first identified as a germ-cell-intrinsic sex determination factor. FOXL3 KO female medaka developed functional sperm in the ovary, indicating that the sex determination mechanism differs between PGCs and gonads [136,137]. Subsequently, in tilapia (Oreochromis niloticus), both FOXL3 and DMRT1 significantly contribute to the sex determination of PGCs [138]. Recently, the FOXL3-like gene was cloned and characterized in chickens [3]. Although the chicken FOXL3-like protein is specifically expressed in female embryonic germ cells, its expression timing is considerably late compared to gonadal sex differentiation, suggesting that the chicken FOXL3-like gene may not affect the sexual identity of PGCs [3]. In addition, chicken PGC-intrinsic DMRT1 may not affect the initial sexual identity of PGCs owing to its expression pattern; sex-biased DMRT1 expression is temporally observed in female germ cells around HH stage 42 (after 16 d of incubation), which corresponds to the meiotic stage [14]. Taken together, although the chicken FOXL3-like gene and DMRT1 may be essential for germ cell development, the molecular mechanism of initial sex determination in chicken PGCs remains unclear.
Recent studies have shown characteristic avian features in the sex determination of PGCs. Ichikawa et al. conducted RNA-seq analysis using male and female PGCs harvested from embryonic chicken blood at HH stage 17 (after 2.5 d of incubation) and gonads at HH stage 25–26 (after 4.5 d of incubation) and HH stage 30 (after 6.5 d of incubation) [5]. The gene expression profiles obtained in that study suggest that several W-chromosome-linked genes are expressed in a cell-autonomous manner in female PGCs before they settle into the gonads (Figure 2b) [5]. Furthermore, alterations in gene expression profiles were observed during gonadal sex determination in female PGCs prior to that in male PGCs, and female-biased genes were enriched in several metabolic processes (Figure 2b) [5]. In contrast, Rengaraj et al. showed male-biased gene expression in chicken PGCs collected from early embryos around HH stage 17–34 (after 2.5–8 d of incubation) (Figure 2b) [14]. These differences may be due to the methods used to collect the PGCs; while Ichikawa et al. purified the PGCs using FACS targeting stage-specific embryonic antigen-1 (SSEA-1), Rengaraj et al. used DAZL as the marker of PGCs. Although SSEA-1 has been widely used as a PGC marker [46,139,140,141], a recent study demonstrated the existence of SSEA-1-negative PGCs [142]. Note that SSEA-1-positive PGCs, at least, possess germline transmission [141]. In summary, although the timing and molecular mechanisms of sex determination in avian PGCs remain unknown, the gene expression profiles obtained from recent studies can significantly contribute to solving these issues.
Importantly, the biological significance of the initial sexual identity of avian PGCs remains unclear. Previously, it was thought that the sexual identity of PGCs is constructed when the PGCs migrate into the undifferentiated gonads owing to the results of transplantation experiments. While transplantation of the precursor cells of PGCs harvested from the blastodermal stage into recipients of the opposite sex could differentiate into functional gametes [143], the migrating PGCs could barely differentiate those [144,145]. However, a recent study demonstrated that transplanted chicken PGCs circulating through blood vessels could differentiate into gametes of opposite sexes using genetically infertile chickens as recipients [146]. These differences may reflect that initial sexual identity does not completely determine the sexual fate of PGCs.
4.3. Sex Differentiation of Avian Germ Cells
Remarkable phenotypic differences between male and female chicken PGCs were observed after gonadal sex determination (Figure 2b). The number of chicken female germ cells is significantly increased from HH stage 35 (after 9 d of incubation) onwards, while male PGCs undergo mitotic arrest until hatching [147,148]. At HH stage 38 (after 12.5 d of incubation), stimulated by retinoic acid 8 (STRA8) is expressed in chicken female germ cells and induced by retinoic acid (RA) [149,150]. Then, the female PGCs undergo meiosis at HH stage 41 (after 15.5 d of incubation). In the early embryonic stages, both major enzymes involved in RA synthesis and RA degradation, retinaldehyde dehydrogenase, type 2 (RALDH2), and cytochrome P450 family 26 subfamily B member 1 (CYP26B1), respectively, are expressed in the gonads of each sex. In females, the expression of CYB26B1 is downregulated after HH stage 36 (after 10.5 d of incubation), which may be related to female-specific entry into meiosis [149]. Given the above, typical sexual differentiation in chicken germ cells is characterized by differences in proliferation activity and female-specific meiosis.
5. Self-Renewal of Chicken PGCs
Among vertebrates, chicken is the only species in which PGCs can be cultured for a long time (Figure 3). Owing to the fact that abundant egg yolk yields surround one-cell fertilized chicken eggs, direct injection of genome editing reagents is difficult. Thus, genome-edited chickens have been produced via gene modification and transplantation of cultured PGCs [151]. Currently, genome-edited chickens are widely used for the study of avian developmental biology [24,64,129,130], production of hypoallergenic eggs [12,152,153], improvement in production efficiency [154], and establishment of bioreactors [155,156]. Furthermore, culture technology can contribute to the conservation of rare avian strains [157]. Therefore, culturing chicken PGCs is an effective technique for fundamental research and industrial applications.
Figure 3.
A photograph of cultured chicken primordial germ cells (PGCs). The PGCs were cultured according to a protocol established by Ezaki et al. (2020) [159]. Scale bar = 50 μm.
In 2006, van de Lavoir et al. reported a culture protocol for chicken PGCs using the fibroblast growth factor (FGF), stem cell factor (SCF), and mammalian feeder cells, for the first time [6]. Since this achievement, several researchers have attempted to improve their culture efficiency and have revealed essential factors for the self-renewal of cultured PGCs. Optimization of the culture protocol was conducted by Miyahara et al., who demonstrated that the membrane-bound form of the chicken SCF (SCF2) could aid in the proliferation of cultured PGCs [135,158]. Whyte et al. identified the minimal number of signaling pathways required for the self-renewal of cultured PGCs and reconstructed the culture protocol. They showed that FGF2, insulin, and activin significantly contribute to the culture of PGCs, which induce the activation of ERK1/2, Akt, and SMAD3, respectively [7]. In this minimal condition, the SCF is not required. Thus, Whyte et al. hypothesized that insulin might replace SCF function [7]. In addition, several small molecules, such as blebbistatin and CHIR99021, induce the activation of the self-renewal of cultured PGCs by reducing apoptosis [159,160]. Furthermore, Chen et al. established a three-dimensional culture system for chicken PGCs, enabling the efficient culture of chicken PGCs on a large scale [161].
The studies described above played a significant part in the development of a method for culturing chicken PGCs. However, current culture protocols cannot maintain PGCs derived from avian species other than chicken. For example, although researchers have attempted to culture PGCs derived from several avian species, such as quail, duck, and zebra finch (Taeniopygia guttata), a stable culture of PGCs, as shown in chicken PGCs, could not be achieved [162,163,164]. Furthermore, current culture protocols have been applied only to certain chicken strains. Woodcock et al. showed that difficulties in culturing chicken PGCs differ among strains [157]. Therefore, to promote avian biotechnology and biodiversity conservation, the current culture protocol for chicken PGCs should be further expanded to include a generic protocol for all avian PGCs.
While recent studies have reported strategies to produce genome-edited birds without cultured PGCs, the value in improving the current culture protocols remains undeniable. For example, a method involving intracytoplasmic injection of sperm has been established in quails, and Mizushima et al. successfully produced genome-edited quails via the direct injection of CRISPR/Cas components into fertilized quail eggs [165,166]. However, this strategy requires advanced technology, and the production of genome-edited birds using this strategy has not yet been achieved in birds other than quail. In addition, methods using adenoviral vectors [167] and sperm [168] as delivery systems to induce CRISPR/Cas components have also been reported. Furthermore, a recent study produced chicken iPSC-derived offspring [169], which could contribute to the production of genome-edited birds without using cultured PGCs. Despite the potential for application in all avian species, the efficiency of producing objective offspring remains low. Therefore, these methods are not widely used. In summary, improvements in the culture protocols of PGCs are needed to further develop avian molecular biology as well as to improve the advanced strategies described above.
6. Conclusions
Elucidating the mechanisms of avian PGC fate decisions has long been an essential research topic. Recently, this research subject has received further attention owing to the application of genome editing technology and RNA-seq analysis. Further studies on the early development, integrity, and sex determination of chicken PGCs will provide significant insights into evolutionary developmental biology. Additionally, studies on sex determination using avian PGCs will contribute to the control of the sex of offspring, an essential technology for both efficient poultry production and animal welfare. Moreover, further studies on the self-renewal of chicken PGCs may make it possible for all avian species to be genetically conserved and modified by improving PGC culture protocols. Nonetheless, as many challenges remain unaddressed, the investigation of fate decisions is an essential research subject for future studies.
Author Contributions
K.I. conducted the literature review, designed the manuscript, and wrote the paper. H.H. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Kieikai Research Foundation (FY2022) to Dr. Kennosuke Ichikawa.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sheng, G. Defining epithelial-mesenchymal transitions in animal development. Development 2021, 148, dev198036. [Google Scholar] [CrossRef] [PubMed]
- Swift, C.H. Origin and early history of the primordial germ-cells in the chick. Am. J. Anat. 1914, 15, 483–516. [Google Scholar] [CrossRef]
- Ichikawa, K.; Ezaki, R.; Furusawa, S.; Horiuchi, H. Comparison of sex determination mechanism of germ cells between birds and fish: Cloning and expression analyses of chicken forkhead box L3-like gene. Dev. Dyn. 2019, 248, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Soler, L.; Alves, S.; Brionne, A.; Jacques, A.; Guérin, V.; Cherif-Feildel, M.; Combes-Soia, L.; Fouchécourt, S.; Thélie, A.; Blesbois, E.; et al. Protein expression reveals a molecular sexual identity of avian primordial germ cells at pre-gonadal stages. Sci. Rep. 2021, 11, 19236. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Nakamura, Y.; Bono, H.; Ezaki, R.; Matsuzaki, M.; Horiuchi, H. Prediction of sex-determination mechanisms in avian primordial germ cells using RNA-seq analysis. Sci. Rep. 2022, 12, 13528. [Google Scholar] [CrossRef] [PubMed]
- Van de Lavoir, M.C.; Diamond, J.H.; Leighton, P.A.; Mather-Love, C.; Heyer, B.S.; Bradshaw, R.; Kerchner, A.; Hooi, L.T.; Gessaro, T.M.; Swanberg, S.E.; et al. Germline transmission of genetically modified primordial germ cells. Nature 2006, 441, 766–769. [Google Scholar] [CrossRef]
- Whyte, J.; Glover, J.D.; Woodcock, M.; Brzeszczynska, J.; Taylor, L.; Sherman, A.; Kaiser, P.; McGrew, M.J. FGF, insulin, and SMAD signaling cooperate for avian primordial germ cell self-renewal. Stem Cell Rep. 2015, 5, 1171–1182. [Google Scholar] [CrossRef]
- Lázár, B.; Molnár, M.; Sztán, N.; Végi, B.; Drobnyák, Á.; Tóth, R.; Tokodyné, S.N.; McGrew, M.J.; Gócza, E.; Patakiné, V.E. Successful cryopreservation and regeneration of a partridge colored Hungarian native chicken breed using primordial germ cells. Poult. Sci. 2021, 100, 101207. [Google Scholar] [CrossRef]
- Boes, J.; Boettcher, P.; Honkatukia, M. (Eds.) Innovations in Cryoconservation of Animal Genetic Resources—Practical Guide. In FAO Animal Production and Health Guidelines; FAO: Rome, Italy, 2023; No. 33. [Google Scholar] [CrossRef]
- Perry, M.M. A complete culture system for the chick embryo. Nature 1988, 331, 70–72. [Google Scholar] [CrossRef] [PubMed]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Oishi, I.; Yoshii, K.; Miyahara, D.; Kagami, H.; Tagami, T. Targeted mutagenesis in chicken using CRISPR/Cas9 system. Sci. Rep. 2016, 6, 23980. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, J.W.; Kim, S.W.; Park, J.; Park, T.S. C-X-C chemokine receptor type 4 (CXCR4) is a key receptor for chicken primordial germ cell migration. J. Reprod. Dev. 2017, 63, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, D.; Cha, D.G.; Lee, H.J.; Lee, K.Y.; Choi, Y.H.; Jung, K.M.; Kim, Y.M.; Choi, H.J.; Choi, H.J.; Yoo, E.; et al. Dissecting chicken germ cell dynamics by combining a germ cell tracing transgenic chicken model with single-cell RNA sequencing. Comput. Struct. Biotechnol. J. 2022, 20, 1654–1669. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, D.; Cha, D.G.; Park, K.J.; Lee, K.Y.; Woo, S.J.; Han, J.Y. Finer resolution analysis of transcriptional programming during the active migration of chicken primordial germ cells. Comput. Struct. Biotechnol. J. 2022, 20, 5911–5924. [Google Scholar] [CrossRef]
- Extavour, C.G.; Akam, M. Mechanisms of germ cell specification across the metazoans: Epigenesis and preformation. Development 2003, 130, 5869–5884. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.; Kawakami, K.; Hopkins, N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development 1997, 124, 3157–3165. [Google Scholar] [CrossRef]
- Houston, D.W.; Zhang, J.; Maines, J.Z.; Wasserman, S.A.; King, M.L. A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule. Development 1998, 125, 171–180. [Google Scholar] [CrossRef]
- Ephrussi, A.; Lehmann, R. Induction of germ cell formation by oskar. Nature 1992, 358, 387–392. [Google Scholar] [CrossRef]
- Seydoux, G.; Strome, S. Launching the germline in Caenorhabditis elegans: Regulation of gene expression in early germ cells. Development 1999, 126, 3275–3283. [Google Scholar] [CrossRef]
- Johnson, A.D.; Bachvarova, R.F.; Drum, M.; Masi, T. Expression of axolotl DAZL RNA, a marker of germ plasm: Widespread maternal RNA and onset of expression in germ cells approaching the gonad. Dev. Biol. 2001, 234, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Tam, P.P.; Zhou, S.X. The allocation of epiblast cells to ectodermal and germ-line lineages is influenced by the position of the cells in the gastrulating mouse embryo. Dev. Biol. 1996, 178, 124–132. [Google Scholar] [CrossRef]
- Tsunekawa, N.; Naito, M.; Sakai, Y.; Nishida, T.; Noce, T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 2000, 127, 2741–2750. [Google Scholar] [CrossRef]
- Taylor, L.; Carlson, D.F.; Nandi, S.; Sherman, A.; Fahrenkrug, S.C.; McGrew, M.J. Efficient TALEN-mediated gene targeting of chicken primordial germ cells. Development 2017, 144, 928–934. [Google Scholar] [CrossRef]
- Aduma, N.; Izumi, H.; Mizushima, S.; Kuroiwa, A. Knockdown of DEAD-box helicase 4 (DDX4) decreases the number of germ cells in male and female chicken embryonic gonads. Reprod. Fertil. Dev. 2019, 31, 847–854. [Google Scholar] [CrossRef]
- Maegawa, S.; Yasuda, K.; Inoue, K. Maternal mRNA localization of zebrafish DAZ-like gene. Mech. Dev. 1999, 81, 223–226. [Google Scholar] [CrossRef]
- Houston, D.W.; King, M.L. A critical role for Xdazl, a germ plasm-localized RNA, in the differentiation of primordial germ cells in Xenopus. Development 2000, 127, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Choi, H.J.; Lee, H.G.; Lim, J.M.; Ono, T.; Han, J.Y. DAZL expression explains origin and central formation of primordial germ cells in chickens. Stem Cells Dev. 2016, 25, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, D.; Won, S.; Han, J.W.; Yoo, D.; Kim, H.; Han, J.Y. Whole-transcriptome sequencing-based analysis of DAZL and its interacting genes during germ cells specification and zygotic genome activation in chickens. Int. J. Mol. Sci. 2020, 21, 8170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, T.; Zhang, M.; Chen, C.; Gao, X.; Zhang, C.; Hu, C.; Zuo, Q.; Chen, G.; Li, B. Epigenetic modification cooperates with Zeb1 transcription factor to regulate Bmp4 to promote chicken PGCs formation. Gene 2021, 794, 145760. [Google Scholar] [CrossRef] [PubMed]
- Eyal-Giladi, H.; Kochav, S. From cleavage to primitive streak formation: A complementary normal table and a new look at the first stages of the development of the chick: I. Dev. Biol. 1976, 49, 321–337. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef]
- Ginsburg, M.; Eyal-Giladi, H. Primordial germ cells of the young chick blastoderm originate from the central zone of the area pellucida irrespective of the embryo-forming process. Development 1987, 101, 209–219. [Google Scholar] [CrossRef]
- Kang, K.S.; Lee, H.C.; Kim, H.J.; Lee, H.G.; Kim, Y.M.; Lee, H.J.; Park, Y.H.; Yang, S.Y.; Rengaraj, D.; Park, T.S.; et al. Spatial and temporal action of chicken primordial germ cells during initial migration. Reproduction 2015, 149, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Huss, D.J.; Saias, S.; Hamamah, S.; Singh, J.M.; Wang, J.; Dave, M.; Kim, J.; Eberwine, J.; Lansford, R. Avian primordial germ cells contribute to and interact with the extracellular matrix during early migration. Front. Cell Dev. Biol. 2019, 7, 35. [Google Scholar] [CrossRef]
- Grimaldi, C.; Raz, E. Germ cell migration-Evolutionary issues and current understanding. Semin. Cell Dev. Biol. 2020, 100, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G. Primitive and definitive erythropoiesis in the yolk sac: A bird’s eye view. Int. J. Dev. Biol. 2010, 54, 1033–1043. [Google Scholar] [CrossRef]
- Murai, H.; Shibuya, M.; Kishita, R.; Sunase, C.; Tamura, K.; Saito, D. Envelopment by endothelial cells initiates translocation of avian primordial germ cell into vascular tissue. Dev. Dyn. 2021, 250, 1410–1419. [Google Scholar] [CrossRef]
- Tajima, A.; Hayashi, H.; Kamizumi, A.; Ogura, J.; Kuwana, T.; Chikamune, T. Study on the concentration of circulating primordial germ cells (cPGCs) in early chick embryos. J. Exp. Zool. 1999, 284, 759–764. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, Y.; Usui, F.; Mushika, T.; Ono, T.; Setioko, A.R.; Takeda, K.; Nirasawa, K.; Kagami, H.; Tagami, T. Migration and proliferation of primordial germ cells in the early chicken embryo. Poult. Sci. 2007, 86, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Doitsidou, M.; Reichman-Fried, M.; Stebler, J.; Köprunner, M.; Dörries, J.; Meyer, D.; Esguerra, C.V.; Leung, T.; Raz, E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell 2002, 111, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Knaut, H.; Werz, C.; Geisler, R.; Nüsslein-Volhard, C.; Tübingen 2000 Screen Consortium. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature 2003, 421, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Molyneaux, K.A.; Zinszner, H.l.n.; Kunwar, P.S.; Schaible, K.; Stebler, J.; Sunshine, M.J.; O’Brien, W.; Raz, E.; Littman, D.; Wylie, C.; et al. The chemokine SDF1/CXCL12 and its receptor CXCR4 regulate mouse germ cell migration and survival. Development 2003, 130, 4279–4286. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Tanigawa, Y.; Minamide, R.; Ikenishi, K.; Komiya, T. Analysis of SDF-1/CXCR4 signaling in primordial germ cell migration and survival or differentiation in Xenopus laevis. Mech. Dev. 2010, 127, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Amat-Fernandez, J.; Hammond, M.J.; Liang, D.; Wang, T.; Ventura, T.; Elizur, A.; Cummins, S.F. Molecular characterization of sdf1 and cxcr4 in the Mozambique tilapia, Oreochromis mossambicus. Anim. Reprod. Sci. 2017, 176, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Motono, M.; Ohashi, T.; Nishijima, K.; Iijima, S. Analysis of chicken primordial germ cells. Cytotechnology 2008, 57, 199–205. [Google Scholar] [CrossRef]
- Stebler, J.; Spieler, D.; Slanchev, K.; Molyneaux, K.A.; Richter, U.; Cojocaru, V.; Tarabykin, V.; Wylie, C.; Kessel, M.; Raz, E. Primordial germ cell migration in the chick and mouse embryo: The role of the chemokine SDF-1/CXCL12. Dev. Biol. 2004, 272, 351–361. [Google Scholar] [CrossRef]
- Saito, D.; Tadokoro, R.; Nagasaka, A.; Yoshino, D.; Teramoto, T.; Mizumoto, K.; Funamoto, K.; Kidokoro, H.; Miyata, T.; Tamura, K.; et al. Stiffness of primordial germ cells is required for their extravasation in avian embryos. iScience 2022, 25, 105629. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Meng, L.; Wang, S.; Man, Q.; Jiang, Y.; Zhu, G. Transcriptional dynamics of the circulating chicken primordial germ cells revealing key genes in cell adhesion and proliferation prior to gonad colonization. Mol. Reprod. Dev. 2022, 89, 214–226. [Google Scholar] [CrossRef]
- Seisenberger, S.; Andrews, S.; Krueger, F.; Arand, J.; Walter, J.; Santos, F.; Popp, C.; Thienpont, B.; Dean, W.; Reik, W. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol. Cell 2012, 48, 849–862. [Google Scholar] [CrossRef]
- Tang, W.W.C.; Kobayashi, T.; Irie, N.; Dietmann, S.; Surani, M.A. Specification and epigenetic programming of the human germ line. Nat. Rev. Genet. 2016, 17, 585–600. [Google Scholar] [CrossRef]
- Strome, S.; Updike, D. Specifying and protecting germ cell fate. Nat. Rev. Mol. Cell Biol. 2015, 16, 406–416. [Google Scholar] [CrossRef]
- Yu, M.; Li, D.; Cao, W.; Chen, X.; Du, W. Effects of ten–eleven translocation 1 (Tet1) on DNA methylation and gene expression in chicken primordial germ cells. Reprod. Fertil. Dev. 2019, 31, 509–520. [Google Scholar] [CrossRef]
- Rengaraj, D.; Lee, B.R.; Lee, S.I.; Seo, H.W.; Han, J.Y. Expression patterns and miRNA regulation of DNA methyltransferases in chicken primordial germ cells. PLoS ONE 2011, 6, e19524. [Google Scholar] [CrossRef]
- Jang, H.J.; Seo, H.W.; Lee, B.R.; Yoo, M.; Womack, J.E.; Han, J.Y. Gene expression and DNA methylation status of chicken primordial germ cells. Mol. Biotechnol. 2013, 54, 177–186. [Google Scholar] [CrossRef]
- Hajkova, P.; Ancelin, K.; Waldmann, T.; Lacoste, N.; Lange, U.C.; Cesari, F.; Lee, C.; Almouzni, G.; Schneider, R.; Surani, M.A. Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 2008, 452, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Kress, C.; Montillet, G.; Jean, C.; Fuet, A.; Pain, B. Chicken embryonic stem cells and primordial germ cells display different heterochromatic histone marks than their mammalian counterparts. Epigenetics Chromatin 2016, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zuo, Q.; Wang, M.; Chen, H.; He, N.; Jin, J.; Li, T.; Jiang, J.; Yuan, X.; Li, J.; et al. Narrow H3K4me2 is required for chicken PGC formation. J. Cell. Physiol. 2021, 236, 1391–1400. [Google Scholar] [CrossRef]
- Jung, H.G.; Hwang, Y.S.; Park, Y.H.; Cho, H.Y.; Rengaraj, D.; Han, J.Y. Role of epigenetic regulation by the REST/CoREST/HDAC corepressor complex of moderate NANOG expression in chicken primordial germ cells. Stem Cells Dev. 2018, 27, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, M.; Seki, Y.; Kurimoto, K.; Yabuta, Y.; Yuasa, M.; Shigeta, M.; Yamanaka, K.; Ohinata, Y.; Saitou, M. Critical function of Prdm14 for the establishment of the germ cell lineage in mice. Nat. Genet. 2008, 40, 1016–1022. [Google Scholar] [CrossRef]
- Grabole, N.; Tischler, J.; Hackett, J.A.; Kim, S.; Tang, F.; Leitch, H.G.; Magnúsdóttir, E.; Surani, M.A. Prdm14 promotes germline fate and naive pluripotency by repressing FGF signalling and DNA methylation. EMBO Rep. 2013, 14, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Ohinata, Y.; Payer, B.; O’Carroll, D.; Ancelin, K.; Ono, Y.; Sano, M.; Barton, S.C.; Obukhanych, T.; Nussenzweig, M.; Tarakhovsky, A.; et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 2005, 436, 207–213. [Google Scholar] [CrossRef]
- Okuzaki, Y.; Kaneoka, H.; Suzuki, T.; Hagihara, Y.; Nakayama, Y.; Murakami, S.; Murase, Y.; Kuroiwa, A.; Iijima, S.; Nishijima, K.I. PRDM14 and BLIMP1 control the development of chicken primordial germ cells. Dev. Biol. 2019, 455, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, Y.; Okuzaki, Y.; Matsubayashi, K.; Kaneoka, H.; Suzuki, T.; Iijima, S.; Nishijima, K.I. Primordial germ cell-specific expression of eGFP in transgenic chickens. Genesis 2020, 58, e23388. [Google Scholar] [CrossRef] [PubMed]
- Lawson, K.A.; Dunn, N.R.; Roelen, B.A.; Zeinstra, L.M.; Davis, A.M.; Wright, C.V.; Korving, J.P.; Hogan, B.L. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999, 13, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Yamaji, M. Primordial germ cells in mice. Cold Spring Harb. Perspect. Biol. 2012, 4, a008375. [Google Scholar] [CrossRef]
- Ohinata, Y.; Ohta, H.; Shigeta, M.; Yamanaka, K.; Wakayama, T.; Saitou, M. A signaling principle for the specification of the germ cell lineage in mice. Cell 2009, 137, 571–584. [Google Scholar] [CrossRef]
- Pain, B.; Clark, M.E.; Shen, M.; Nakazawa, H.; Sakurai, M.; Samarut, J.; Etches, R.J. Long-term in vitro culture and characterisation of avian embryonic stem cells with multiple morphogenetic potentialities. Development 1996, 122, 2339–2348. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, H.; Tategaki, A.; Yamashita, Y.; Hisamatsu, H.; Ogawa, M.; Noguchi, T.; Aosasa, M.; Kawashima, T.; Akita, S.; Nishimichi, N.; et al. Chicken leukemia inhibitory factor maintains chicken embryonic stem cells in the undifferentiated state. J. Biol. Chem. 2004, 279, 24514–24520. [Google Scholar] [CrossRef]
- Zuo, Q.; Jin, K.; Zhang, Y.; Song, J.; Li, B. Dynamic expression and regulatory mechanism of TGF-β signaling in chicken embryonic stem cells differentiating into spermatogonial stem cells. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef]
- Zuo, Q.; Jin, J.; Jin, K.; Sun, C.; Song, J.; Zhang, Y.; Chen, G.; Li, B. Distinct roles of retinoic acid and BMP4 pathways in the formation of chicken primordial germ cells and spermatogonial stem cells. Food Funct. 2019, 10, 7152–7163. [Google Scholar] [CrossRef]
- Zuo, Q.; Jin, K.; Wang, M.; Zhang, Y.; Chen, G.; Li, B. BMP4 activates the Wnt–Lin28A–Blimp1–Wnt pathway to promote primordial germ cell formation via altering H3K4me2. J. Cell Sci. 2021, 134, jcs249375. [Google Scholar] [CrossRef] [PubMed]
- West, J.A.; Viswanathan, S.R.; Yabuuchi, A.; Cunniff, K.; Takeuchi, A.; Park, I.H.; Sero, J.E.; Zhu, H.; Perez-Atayde, A.; Frazier, A.L.; et al. A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 2009, 460, 909–913. [Google Scholar] [CrossRef]
- Zuo, Q.; Jing, J.; Zhou, J.; Zhang, Y.; Wei, W.; Chen, G.; Li, B. Dual regulatory actions of LncBMP4 on BMP4 promote chicken primordial germ cell formation. EMBO Rep. 2022, 23, e52491. [Google Scholar] [CrossRef]
- Mitsui, K.; Tokuzawa, Y.; Itoh, H.; Segawa, K.; Murakami, M.; Takahashi, K.; Maruyama, M.; Maeda, M.; Yamanaka, S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003, 113, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Chambers, I.; Colby, D.; Robertson, M.; Nichols, J.; Lee, S.; Tweedie, S.; Smith, A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003, 113, 643–655. [Google Scholar] [CrossRef]
- Chambers, I.; Silva, J.; Colby, D.; Nichols, J.; Nijmeijer, B.; Robertson, M.; Vrana, J.; Jones, K.; Grotewold, L.; Smith, A. Nanog safeguards pluripotency and mediates germline development. Nature 2007, 450, 1230–1234. [Google Scholar] [CrossRef]
- Murakami, K.; Günesdogan, U.; Zylicz, J.J.; Tang, W.W.C.; SenGupta, R.; Kobayashi, T.; Kim, S.; Butler, R.; Dietmann, S.; Surani, M.A. NANOG alone induces germ cells in primed epiblast in vitro by activation of enhancers. Nature 2016, 529, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Lavial, F.; Acloque, H.; Bertocchini, F.; Macleod, D.J.; Boast, S.; Bachelard, E.; Montillet, G.; Thenot, S.; Sang, H.M.; Stern, C.D.; et al. The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development 2007, 134, 3549–3563. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, T.W.; Costa, Y.; Radzisheuskaya, A.; van Oosten, A.L.; Lavial, F.; Pain, B.; Castro, L.F.C.; Silva, J.C.R. Reprogramming capacity of Nanog is functionally conserved in vertebrates and resides in a unique homeodomain. Development 2011, 138, 4853–4865. [Google Scholar] [CrossRef]
- Choi, H.J.; Kim, I.; Lee, H.J.; Park, Y.H.; Suh, J.Y.; Han, J.Y. Chicken NANOG Self-associates via a novel folding-upon-binding mechanism. FASEB J. 2018, 32, 2563–2573. [Google Scholar] [CrossRef]
- Fuet, A.; Montillet, G.; Jean, C.; Aubel, P.; Kress, C.; Rival-Gervier, S.; Pain, B. NANOG is required for the long-term establishment of avian somatic reprogrammed cells. Stem Cell Rep. 2018, 11, 1272–1286. [Google Scholar] [CrossRef] [PubMed]
- Cañón, S.; Herranz, C.; Manzanares, M. Germ cell restricted expression of chick Nanog. Dev. Dyn. 2006, 235, 2889–2894. [Google Scholar] [CrossRef] [PubMed]
- Naeemipour, M.; Dehghani, H.; Bassami, M.; Bahrami, A. Expression dynamics of pluripotency genes in chicken primordial germ cells before and after colonization of the genital ridges. Mol. Reprod. Dev. 2013, 80, 849–861. [Google Scholar] [CrossRef]
- Choi, H.J.; Jin, S.D.; Rengaraj, D.; Kim, J.H.; Pain, B.; Han, J.Y. Differential transcriptional regulation of the NANOG gene in chicken primordial germ cells and embryonic stem cells. J. Anim. Sci. Biotechnol. 2021, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Zevnik, B.; Anastassiadis, K.; Niwa, H.; Klewe-Nebenius, D.; Chambers, I.; Schöler, H.; Smith, A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 1998, 95, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Niwa, H.; Miyazaki, J.; Smith, A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000, 24, 372–376. [Google Scholar] [CrossRef]
- Niwa, H.; Toyooka, Y.; Shimosato, D.; Strumpf, D.; Takahashi, K.; Yagi, R.; Rossant, J. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 2005, 123, 917–929. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Kehler, J.; Tolkunova, E.; Koschorz, B.; Pesce, M.; Gentile, L.; Boiani, M.; Lomelí, H.; Nagy, A.; McLaughlin, K.J.; Schöler, H.R.; et al. Oct4 is required for primordial germ cell survival. EMBO Rep. 2004, 5, 1078–1083. [Google Scholar] [CrossRef]
- Soodeen-Karamath, S.; Gibbins, A.M. Apparent absence of oct 3/4 from the chicken genome. Mol. Reprod. Dev. 2001, 58, 137–148. [Google Scholar] [CrossRef]
- Niwa, H.; Sekita, Y.; Tsend-Ayush, E.; Grützner, F. Platypus Pou5f1 reveals the first steps in the evolution of trophectoderm differentiation and pluripotency in mammals. Evol. Dev. 2008, 10, 671–682. [Google Scholar] [CrossRef]
- Frankenberg, S.; Pask, A.; Renfree, M.B. The evolution of class V POU domain transcription factors in vertebrates and their characterisation in a marsupial. Dev. Biol. 2010, 337, 162–170. [Google Scholar] [CrossRef]
- Frankenberg, S.R.; Frank, D.; Harland, R.; Johnson, A.D.; Nichols, J.; Niwa, H.; Schöler, H.R.; Tanaka, E.; Wylie, C.; Brickman, J.M. The POU-er of gene nomenclature. Development 2014, 141, 2921–2923. [Google Scholar] [CrossRef] [PubMed]
- Nakanoh, S.; Fuse, N.; Takahashi, Y.; Agata, K. Verification of chicken Nanog as an epiblast marker and identification of chicken PouV as Pou5f3 by newly raised antibodies. Dev. Growth Differ. 2015, 57, 251–263. [Google Scholar] [CrossRef]
- Rengaraj, D.; Won, S.; Jung, K.M.; Woo, S.J.; Lee, H.; Kim, Y.M.; Kim, H.; Han, J.Y. Chicken blastoderms and primordial germ cells possess a higher expression of DNA repair genes and lower expression of apoptosis genes to preserve their genome stability. Sci. Rep. 2022, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Wang, S.; Jiang, H.; Hua, Y.; Yin, B.; Huang, X.; Man, Q.; Wang, H.; Zhu, G. Oct4 dependent chromatin activation is required for chicken primordial germ cell migration. Stem Cell Rev. Rep. 2022, 18, 2535–2546. [Google Scholar] [CrossRef] [PubMed]
- Youngren, K.K.; Coveney, D.; Peng, X.; Bhattacharya, C.; Schmidt, L.S.; Nickerson, M.L.; Lamb, B.T.; Deng, J.M.; Behringer, R.R.; Capel, B.; et al. The Ter mutation in the dead end gene causes germ cell loss and testicular germ cell tumours. Nature 2005, 435, 360–364. [Google Scholar] [CrossRef]
- Yamaji, M.; Jishage, M.; Meyer, C.; Suryawanshi, H.; Der, E.; Yamaji, M.; Garzia, A.; Morozov, P.; Manickavel, S.; McFarland, H.L.; et al. DND1 maintains germline stem cells via recruitment of the CCR4–NOT complex to target mRNAs. Nature 2017, 543, 568–572. [Google Scholar] [CrossRef]
- Weidinger, G.; Stebler, J.; Slanchev, K.; Dumstrei, K.; Wise, C.; Lovell-Badge, R.; Thisse, C.; Thisse, B.; Raz, E. Dead end, a novel vertebrate germ plasm component, is required for zebrafish primordial germ cell migration and survival. Curr. Biol. 2003, 13, 1429–1434. [Google Scholar] [CrossRef]
- Gross-Thebing, T.; Yigit, S.; Pfeiffer, J.; Reichman-Fried, M.; Bandemer, J.; Ruckert, C.; Rathmer, C.; Goudarzi, M.; Stehling, M.; Tarbashevich, K.; et al. The vertebrate protein dead end maintains primordial germ cell fate by inhibiting somatic differentiation. Dev. Cell 2017, 43, 704–715.e5. [Google Scholar] [CrossRef]
- Aramaki, S.; Sato, F.; Kato, T.; Soh, T.; Kato, Y.; Hattori, M.A. Molecular cloning and expression of dead end homologue in chicken primordial germ cells. Cell Tissue Res. 2007, 330, 45–52. [Google Scholar] [CrossRef]
- Aramaki, S.; Kubota, K.; Soh, T.; Yamauchi, N.; Hattori, M.A. Chicken dead end homologue protein is a nucleoprotein of germ cells including primordial germ cells. J. Reprod. Dev. 2009, 55, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Aravin, A.; Gaidatzis, D.; Pfeffer, S.; Lagos-Quintana, M.; Landgraf, P.; Iovino, N.; Morris, P.; Brownstein, M.J.; Kuramochi-Miyagawa, S.; Nakano, T.; et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 2006, 442, 203–207. [Google Scholar] [CrossRef]
- Grivna, S.T.; Beyret, E.; Wang, Z.; Lin, H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006, 20, 1709–1714. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.C.; Seto, A.G.; Kim, J.; Kuramochi-Miyagawa, S.; Nakano, T.; Bartel, D.P.; Kingston, R.E. Characterization of the piRNA complex from rat testes. Science 2006, 313, 363–367. [Google Scholar] [CrossRef]
- Hirakata, S.; Siomi, M.C. piRNA biogenesis in the germline: From transcription of piRNA genomic sources to piRNA maturation. Biochim. Biophys. Acta 2016, 1859, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, D.; Lee, S.I.; Park, T.S.; Lee, H.J.; Kim, Y.M.; Sohn, Y.A.; Jung, M.; Noh, S.J.; Jung, H.; Han, J.Y. Small non-coding RNA profiling and the role of piRNA pathway genes in the protection of chicken primordial germ cells. BMC Genom. 2014, 15, 757. [Google Scholar] [CrossRef]
- Xu, L.; Qiu, L.; Chang, G.; Guo, Q.; Liu, X.; Bi, Y.; Zhang, Y.; Wang, H.; Li, Z.; Guo, X.; et al. Discovery of piRNAs pathway associated with early-stage spermatogenesis in chicken. PLoS ONE 2016, 11, e0151780. [Google Scholar] [CrossRef]
- Lee, S.I.; Lee, B.R.; Hwang, Y.S.; Lee, H.C.; Rengaraj, D.; Song, G.; Park, T.S.; Han, J.Y. MicroRNA-mediated posttranscriptional regulation is required for maintaining undifferentiated properties of blastoderm and primordial germ cells in chickens. Proc. Natl. Acad. Sci. USA 2011, 108, 10426–10431. [Google Scholar] [CrossRef]
- Rengaraj, D.; Park, T.S.; Lee, S.I.; Lee, B.R.; Han, B.K.; Song, G.; Han, J.Y. Regulation of glucose phosphate isomerase by the 3′ UTR-specific miRNAs miR-302b and miR-17-5p in chicken primordial germ cells. Biol. Reprod. 2013, 89, 33. [Google Scholar] [CrossRef]
- Lázár, B.; Szabadi, N.T.; Anand, M.; Tóth, R.; Ecker, A.; Urbán, M.; Aponte, M.T.S.; Stepanova, G.; Hegyi, Z.; Homolya, L.; et al. Effect of miR-302b microRNA inhibition on chicken primordial germ cell proliferation and apoptosis rate. Genes 2021, 13, 82. [Google Scholar] [CrossRef]
- Hirose, T.; Mishima, Y.; Tomari, Y. Elements and machinery of non-coding RNAs: Toward their taxonomy. EMBO Rep. 2014, 15, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Q.; Jin, J.; Jin, K.; Zhou, J.; Sun, C.; Song, J.; Chen, G.; Zhang, Y.; Li, B. P53 and H3K4me2 activate N6-methylated LncPGCAT-1 to regulate primordial germ cell formation via MAPK signaling. J. Cell. Physiol. 2020, 235, 9895–9909. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, C.; Jin, K.; Zhang, Y.; Zuo, Q.; Li, B. Analysis of lncRNA expression profile during the formation of male germ cells in chickens. Animals 2020, 10, 1850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zuo, Q.; Gao, X.; Hu, C.; Zhou, S.; Chen, C.; Zou, Y.; Zhao, J.; Zhang, Y.; Li, B. H3K4me2 promotes the activation of lncCPSET1 by jun in the chicken PGC formation. Animals 2021, 11, 1572. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Chen, C.; Cheng, S.; Yuan, X.; Jin, J.; Zhang, C.; Sun, X.; Song, J.; Zuo, Q.; Zhang, Y.; et al. Long noncoding RNA LncPGCR mediated by TCF7L2 regulates primordial germ cell formation in chickens. Animals 2021, 11, 292. [Google Scholar] [CrossRef] [PubMed]
- Elbrecht, A.; Smith, R.G. Aromatase enzyme activity and sex determination in chickens. Science 1992, 255, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; McBride, D.; Nandi, S.; McQueen, H.A.; McGrew, M.J.; Hocking, P.M.; Lewis, P.D.; Sang, H.M.; Clinton, M. Somatic sex identity is cell autonomous in the chicken. Nature 2010, 464, 237–242. [Google Scholar] [CrossRef]
- Clinton, M.; Zhao, D.; Nandi, S.; McBride, D. Evidence for avian cell autonomous sex identity (CASI) and implications for the sex-determination process? Chromosome Res. 2012, 20, 177–190. [Google Scholar] [CrossRef]
- Koopman, P.; Gubbay, J.; Vivian, N.; Goodfellow, P.; Lovell-Badge, R. Male development of chromosomally female mice transgenic for Sry. Nature 1991, 351, 117–121. [Google Scholar] [CrossRef]
- Matsuda, M.; Shinomiya, A.; Kinoshita, M.; Suzuki, A.; Kobayashi, T.; Paul-Prasanth, B.; Lau, E.L.; Hamaguchi, S.; Sakaizumi, M.; Nagahama, Y. DMY gene induces male development in genetically female (XX) medaka fish. Proc. Natl. Acad. Sci. USA 2007, 104, 3865–3870. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Okada, E.; Umemoto, H.; Tamura, K.; Uno, Y.; Nishida-Umehara, C.; Matsuda, Y.; Takamatsu, N.; Shiba, T.; Ito, M.A. W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc. Natl. Acad. Sci. USA 2008, 105, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- McQueen, H.A.; McBride, D.; Miele, G.; Bird, A.P.; Clinton, M. Dosage compensation in birds. Curr. Biol. 2001, 11, 253–257. [Google Scholar] [CrossRef]
- Itoh, Y.; Melamed, E.; Yang, X.; Kampf, K.; Wang, S.; Yehya, N.; Van Nas, A.; Replogle, K.; Band, M.R.; Clayton, D.F.; et al. Dosage compensation is less effective in birds than in mammals. J. Biol. 2007, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; McClive, P.J.; Western, P.S.; Reed, K.J.; Sinclair, A.H. Conservation of a sex-determining gene. Nature 1999, 402, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; Roeszler, K.N.; Ohnesorg, T.; Cummins, D.M.; Farlie, P.G.; Doran, T.J.; Sinclair, A.H. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 2009, 461, 267–271. [Google Scholar] [CrossRef]
- Lambeth, L.S.; Raymond, C.S.; Roeszler, K.N.; Kuroiwa, A.; Nakata, T.; Zarkower, D.; Smith, C.A. Over-expression of DMRT1 induces the male pathway in embryonic chicken gonads. Dev. Biol. 2014, 389, 160–172. [Google Scholar] [CrossRef]
- Ioannidis, J.; Taylor, G.; Zhao, D.; Liu, L.; Idoko-Akoh, A.; Gong, D.; Lovell-Badge, R.; Guioli, S.; McGrew, M.J.; Clinton, M. Primary sex determination in birds depends on DMRT1 dosage, but gonadal sex does not determine adult secondary sex characteristics. Proc. Natl. Acad. Sci. USA 2021, 118, e2020909118. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, M.; Choi, H.J.; Rengaraj, D.; Jung, K.M.; Park, J.S.; Lee, K.Y.; Kim, Y.M.; Park, K.J.; Han, S.T.; et al. DMRT1 gene disruption alone induces incomplete gonad feminization in chicken. FASEB J. 2021, 35, e21876. [Google Scholar] [CrossRef]
- Yang, L.V.; Nicholson, R.H.; Kaplan, J.; Galy, A.; Li, L. Hemogen is a novel nuclear factor specifically expressed in mouse hematopoietic development and its human homologue EDAG maps to chromosome 9q22, a region containing breakpoints of hematological neoplasms. Mech. Dev. 2001, 104, 105–111. [Google Scholar] [CrossRef]
- Nakata, T.; Ishiguro, M.; Aduma, N.; Izumi, H.; Kuroiwa, A. Chicken hemogen homolog is involved in the chicken-specific sex-determining mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, 3417–3422. [Google Scholar] [CrossRef]
- Govoroun, M.S.; Pannetier, M.; Pailhoux, E.; Cocquet, J.; Brillard, J.P.; Couty, I.; Batellier, F.; Cotinot, C. Isolation of chicken homolog of the FOXL2 gene and comparison of its expression patterns with those of aromatase during ovarian development. Dev. Dyn. 2004, 231, 859–870. [Google Scholar] [CrossRef]
- Major, A.T.; Ayers, K.; Chue, J.; Roeszler, K.; Smith, C. FOXL2 antagonises the male developmental pathway in embryonic chicken gonads. J. Endocrinol. 2019, 243, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, D.; Mori, T.; Makino, R.; Nakamura, Y.; Oishi, I.; Ono, T.; Nirasawa, K.; Tagami, T.; Kagami, H. Culture conditions for maintain propagation, long-term survival and germline transmission of chicken primordial germ cell-like cells. J. Poult. Sci. 2014, 51, 87–95. [Google Scholar] [CrossRef]
- Nishimura, T.; Sato, T.; Yamamoto, Y.; Watakabe, I.; Ohkawa, Y.; Suyama, M.; Kobayashi, S.; Tanaka, M. Sex determination. foxl3 is a germ cell-intrinsic factor involved in sperm-egg fate decision in medaka. Science 2015, 349, 328–331. [Google Scholar] [CrossRef]
- Tanaka, M. Germline stem cells are critical for sexual fate decision of germ cells. BioEssays 2016, 38, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Qi, S.; Wei, X.; Liu, X.; Li, Y.; Zhou, X.; Xiao, H.; Lu, B.; Wang, D.; Li, M. Germline sexual fate is determined by the antagonistic action of dmrt1 and foxl3/foxl2 in tilapia. Development 2021, 148, dev199380. [Google Scholar] [CrossRef]
- Karagenç, L.; Cinnamon, Y.; Ginsburg, M.; Petitte, J.N. Origin of primordial germ cells in the prestreak chick embryo. Dev. Genet. 1996, 19, 290–301. [Google Scholar] [CrossRef]
- Mozdziak, P.E.; Angerman-Stewart, J.; Rushton, B.; Pardue, S.L.; Petitte, J.N. Isolation of chicken primordial germ cells using fluorescence-activated cell sorting. Poult. Sci. 2005, 84, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Mozdziak, P.E.; Wysocki, R.; Angerman-Stewart, J.; Pardue, S.L.; Petitte, J.N. Production of chick germline chimeras from fluorescence-activated cell-sorted gonocytes. Poult. Sci. 2006, 85, 1764–1768. [Google Scholar] [CrossRef]
- De Melo Bernardo, A.; Sprenkels, K.; Rodrigues, G.; Noce, T.; Chuva De Sousa Lopes, S.M. Chicken primordial germ cells use the anterior vitelline veins to enter the embryonic circulation. Biol. Open 2012, 1, 1146–1152. [Google Scholar] [CrossRef] [PubMed]
- Kagami, H.; Tagami, T.; Matsubara, Y.; Harumi, T.; Hanada, H.; Maruyama, K.; Sakurai, M.; Kuwana, T.; Naito, M. The developmental origin of primordial germ cells and the transmission of the donor-derived gametes in mixed-sex germline chimeras to the offspring in the chicken. Mol. Reprod. Dev. 1997, 48, 501–510. [Google Scholar] [CrossRef]
- Naito, M.; Matsubara, Y.; Harumi, T.; Tagami, T.; Kagami, H.; Sakurai, M.; Kuwana, T. Differentiation of donor primordial germ cells into functional gametes in the gonads of mixed-sex germline chimaeric chickens produced by transfer of primordial germ cells isolated from embryonic blood. J. Reprod. Fertil. 1999, 117, 291–298. [Google Scholar] [CrossRef]
- Tagami, T.; Kagami, H.; Matsubara, Y.; Harumi, T.; Naito, M.; Takeda, K.; Hanada, H.; Nirasawa, K. Differentiation of female primordial germ cells in the male testes of chicken (Gallus gallus domesticus). Mol. Reprod. Dev. 2007, 74, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, M.; Taylor, L.; Hu, T.; Meunier, D.; Nandi, S.; Sherman, A.; Flack, B.; Henshall, J.M.; Hawken, R.J.; McGrew, M.J. Avian primordial germ cells are bipotent for male or female gametogenesis. Front. Cell Dev. Biol. 2021, 9, 726827. [Google Scholar] [CrossRef]
- Hughes, G.C. The population of germ cells in the developing female chick. J. Embryol. Exp. Morphol. 1963, 11, 513–536. [Google Scholar] [CrossRef] [PubMed]
- Méndez, C.; Carrasco, E.; Pedernera, E. Adenohypophysis regulates cell proliferation in the gonads of the developing chick embryo. J. Exp. Zool. A Comp. Exp. Biol. 2005, 303, 179–185. [Google Scholar] [CrossRef]
- Smith, C.A.; Roeszler, K.N.; Bowles, J.; Koopman, P.; Sinclair, A.H. Onset of meiosis in the chicken embryo; evidence of a role for retinoic acid. BMC Dev. Biol. 2008, 8, 85. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yu, P.; Leghari, I.H.; Ge, C.; Mi, Y.; Zhang, C. RALDH2, the enzyme for retinoic acid synthesis, mediates meiosis initiation in germ cells of the female embryonic chickens. Amino Acids 2013, 44, 405–412. [Google Scholar] [CrossRef]
- Ichikawa, K.; Matsuzaki, M.; Ezaki, R.; Horiuchi, H. Genome editing in chickens. Gene Genome Ed. 2022, 3–4, 100015. [Google Scholar] [CrossRef]
- Park, T.S.; Lee, H.J.; Kim, K.H.; Kim, J.S.; Han, J.Y. Targeted gene knockout in chickens mediated by TALENs. Proc. Natl. Acad. Sci. USA 2014, 111, 12716–12721. [Google Scholar] [CrossRef] [PubMed]
- Mukae, T.; Yoshii, K.; Watanobe, T.; Tagami, T.; Oishi, I. Production and characterization of eggs from hens with ovomucoid gene mutation. Poult. Sci. 2021, 100, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.D.; Lee, J.H.; Song, S.; Kim, S.W.; Han, J.S.; Shin, S.P.; Park, B.C.; Park, T.S. Generation of myostatin-knockout chickens mediated by D10A-Cas9 nickase. FASEB J. 2020, 34, 5688–5696. [Google Scholar] [CrossRef] [PubMed]
- Oishi, I.; Yoshii, K.; Miyahara, D.; Tagami, T. Efficient production of human interferon β in the white of eggs from ovalbumin gene-targeted hens. Sci. Rep. 2018, 8, 10203. [Google Scholar] [CrossRef] [PubMed]
- Mukae, T.; Okumura, S.; Watanobe, T.; Yoshii, K.; Tagami, T.; Oishi, I. Production of recombinant monoclonal antibodies in the egg white of gene-targeted transgenic chickens. Genes 2020, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, M.E.; Gheyas, A.A.; Mason, A.S.; Nandi, S.; Taylor, L.; Sherman, A.; Smith, J.; Burt, D.W.; Hawken, R.; McGrew, M.J. Reviving rare chicken breeds using genetically engineered sterility in surrogate host birds. Proc. Natl. Acad. Sci. USA 2019, 116, 20930–20937. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, D.; Oishi, I.; Makino, R.; Kurumisawa, N.; Nakaya, R.; Ono, T.; Kagami, H.; Tagami, T. Chicken stem cell factor enhances primordial germ cell proliferation cooperatively with fibroblast growth factor 2. J. Reprod. Dev. 2016, 62, 143–149. [Google Scholar] [CrossRef]
- Ezaki, R.; Hirose, F.; Furusawa, S.; Horiuchi, H. An improved protocol for stable and efficient culturing of chicken primordial germ cells using small-molecule inhibitors. Cytotechnology 2020, 72, 397–405. [Google Scholar] [CrossRef]
- Chen, D.; Yang, M.; Xie, L.; Lu, Z.; Mo, L.; Yang, W.; Sun, J.; Xu, H.; Lu, K.; Liao, Y.; et al. GSK-3 signaling is involved in proliferation of chicken primordial germ cells. Theriogenology 2020, 141, 62–67. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chang, W.C.; Lin, S.P.; Minami, M.; Jean, C.; Hayashi, H.; Rival-Gervier, S.; Kanaki, T.; Wu, S.C.; Pain, B. Three-dimensional culture of chicken primordial germ cells (cPGCs) in defined media containing the functional polymer FP003. PLoS ONE 2018, 13, e0200515. [Google Scholar] [CrossRef]
- Park, T.S.; Kim, M.A.; Lim, J.M.; Han, J.Y. Production of quail (Coturnix japonica) germline chimeras derived from in vitro-cultured gonadal primordial germ cells. Mol. Reprod. Dev. 2008, 75, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Lin, S.P.; Chang, Y.Y.; Chang, W.P.; Wei, L.Y.; Liu, H.C.; Huang, J.F.; Pain, B.; Wu, S.C. In vitro culture and characterization of duck primordial germ cells. Poult. Sci. 2019, 98, 1820–1832. [Google Scholar] [CrossRef]
- Jung, K.M.; Kim, Y.M.; Keyte, A.L.; Biegler, M.T.; Rengaraj, D.; Lee, H.J.; Mello, C.V.; Velho, T.A.F.; Fedrigo, O.; Haase, B.; et al. Identification and characterization of primordial germ cells in a vocal learning Neoaves species, the zebra finch. FASEB J. 2019, 33, 13825–13836. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, S.; Hiyama, G.; Shiba, K.; Inaba, K.; Dohra, H.; Ono, T.; Shimada, K.; Sasanami, T. The birth of quail chicks after intracytoplasmic sperm injection. Development 2014, 141, 3799–3806. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, S.; Sasanami, T.; Ono, T.; Matsuzaki, M.; Kansaku, N.; Kuroiwa, A. Cyclin D1 gene expression is essential for cell cycle progression from the maternal-to-zygotic transition during blastoderm development in Japanese quail. Dev. Biol. 2021, 476, 249–258. [Google Scholar] [CrossRef]
- Lee, J.; Ma, J.; Lee, K. Direct delivery of adenoviral CRISPR/Cas9 vector into the blastoderm for generation of targeted gene knockout in quail. Proc. Natl. Acad. Sci. USA 2019, 116, 13288–13292. [Google Scholar] [CrossRef]
- Cooper, C.A.; Challagulla, A.; Jenkins, K.A.; Wise, T.G.; O’Neil, T.E.; Morris, K.R.; Tizard, M.L.; Doran, T.J. Generation of gene edited birds in one generation using sperm transfection assisted gene editing (STAGE). Transgenic Res. 2017, 26, 331–347. [Google Scholar] [CrossRef]
- Zhao, R.; Zuo, Q.; Yuan, X.; Jin, K.; Jin, J.; Ding, Y.; Zhang, C.; Li, T.; Jiang, J.; Li, J.; et al. Production of viable chicken by allogeneic transplantation of primordial germ cells induced from somatic cells. Nat. Commun. 2021, 12, 2989. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).