Abstract
The tea plant (Camellia sinensis (L.) O. Ktze) is an important cash crop grown worldwide. It is often subjected to environmental stresses that influence the quality and yield of its leaves. Acetylserotonin-O-methyltransferase (ASMT) is a key enzyme in melatonin biosynthesis, and it plays a critical role in plant stress responses. In this paper, a total of 20 ASMT genes were identified in tea plants and classified into three subfamilies based on a phylogenetic clustering analysis. The genes were unevenly distributed on seven chromosomes; two pairs of genes showed fragment duplication. A gene sequence analysis showed that the structures of the ASMT genes in the tea plants were highly conserved and that the gene structures and motif distributions slightly differed among the different subfamily members. A transcriptome analysis showed that most CsASMT genes did not respond to drought and cold stresses, and a qRT-PCR analysis showed that CsASMT08, CsASMT09, CsASMT10, and CsASMT20 significantly responded to drought and low-temperature stresses; in particular, CsASMT08 and CsASMT10 were highly expressed under low-temperature stress and negatively regulated in response to drought stress. A combined analysis revealed that CsASMT08 and CsASMT10 were highly expressed and that their expressions differed before and after treatment, which indicates that they are potential regulators of abiotic stress resistance in the tea plant. Our results can facilitate further studies on the functional properties of CsASMT genes in melatonin synthesis and abiotic stress in the tea plant.
1. Introduction
Melatonin (N-acetyl-5-methoxytryptamine), a molecule with multiple functions in plant physiological responses, plays an important role in the regulation of stress tolerance in various plant species, including wheat and tobacco [1,2]. N-acetyl-5-hydroxytryptamine-methyltransferase (ASMT) is a key enzyme in melatonin biosynthesis, and it not only affects melatonin synthesis but also plays a crucial role in antioxidation [3,4,5,6] and abiotic stress responses [7,8,9,10]. For example, melatonin has been found to significantly enhance drought tolerance in plants by regulating the mitochondrial synthesis of melatonin [11,12]. Low doses of melatonin in rice have also been found to increase its antioxidant enzyme activity and non-enzymatic antioxidant levels, thus alleviating cold-stress-induced photosynthesis [13]. Melatonin is widely present in various plants, animals, and fungi; it is involved in various biological activities, and it is important for hormonal signaling and emergency responses [14,15].
Melatonin synthesis in plants involves four enzymes: tryptophan decarboxylase (TDC), 5-hydroxytryptamine-N-acetyltransferase (SNAT), tryptamine 5-hydroxylase (T5H), and acetylserotonin-O-methyltransferase (ASMT) [16,17]. Not only is ASMT a key enzyme in melatonin biosynthesis essential for regulating plant melatonin content, but it also plays a crucial role in the oxidative and abiotic stress response [3,4,5,6] and abiotic stress response [7,8,9,10], which is closely related to the regulation of melatonin levels by ASMT. Therefore, the systematic identification and analysis of the ASMT gene family members in tea plants are essential for understanding how the members of this family participate in abiotic stress regulation.
Plants live in complex and variable environments, and abiotic stresses, such as drought and cold stresses, cause damage to plant cells, destroy the cell structure, and reduce enzyme activation, as well as affecting their growth and development and influencing their spatial distribution and yield, thus fundamentally threatening production security [18]. In plants, melatonin acts as a protective agent and plays an important role in regulating plant tolerance to abiotic stresses. To date, the whole-genome identification of ASMT genes has been carried out in various plants, such as tomato [17] pepper [19], apple [20], walnut [21], and wild mulberry [22], and related genes have been shown to play important roles in abiotic stress responses, pathogen induction, and the melatonin synthesis pathway. In addition, previous studies have demonstrated that melatonin is involved in plant abiotic stress responses and that acetylserotonin-O-methyltransferase can methylate N-ethyl-5-hydroxytryptamine to form melatonin [23]. Many plants are deficient in ASMT homologs, which leads to impaired melatonin synthesis and reduced plant resilience to stress; thus, ASMT is essential for the regulation of stress tolerance. Few studies have been carried out on the functional validation of ASMT related genes in plants, and these have only investigated individual plants, such as rice and apple. The overexpression of ASMT1, ASMT2, and ASMT3 in rice can enhance its enzymatic activity and drought tolerance [24], whereas the cloning and overexpression of the apple MzASMT gene in Arabidopsis thaliana lead to increases in melatonin levels and drought tolerance [25]. Thus, these studies suggest that ASMT genes are essential for the regulation of plant growth and stress responses.
Tea is a nutritious aromatic beverage and an economically valuable product. The quality and yield of tea plants decrease considerably under environmental stress. As ASMT genes play non-negligible roles in plant stress resistance, in the present study, we aimed to improve our understanding of the relevant functions of the ASMT genes in tea plants under abiotic stresses. We identified and classified the ASMT gene family members from the whole genome of tea plants based on bioinformatics approaches and chromosomal locations, gene duplication events, phylogenetic relationships, gene structures, conserved structural domains, and cis-elements. In addition, we investigated their expression profiles in different tissues under environmental stresses (cold and drought). Our findings provide a theoretical basis for further studies on the function of the ASMT gene family, as well as for understanding the molecular mechanism by which melatonin regulates stress tolerance in tea plants.
2. Materials and Methods
2.1. Material Treatment
The experimental materials originated from the teaching practice tea garden of Xinyang Agriculture and Forestry College, and two-year-old ‘Fuding Dabaicha’ tea plants from the same seedbed (free of pests and diseases and about 20 cm in height) were selected and transplanted into pots (Danish peat soil). The artificial climate chamber growth conditions were set as follows: a temperature of 22 ± 2 °C, humidity of 65%, untreated plants as control (CK), simulated drought (20% PEG 6000), and low-temperature (4 °C) treatment. The tea plants were watered with 20% PEG-6000 (500 mL), and one bud and three leaves were collected after drought treatment (0, 0.5, 1, 2, 4, and 8 d) and low-temperature treatment (0, 0.5, 1, 4, and 8 d). Three biological replicates were maintained for each treatment, and the samples were stored at −80 °C after quick freezing in liquid nitrogen.
2.2. Genome-Wide Identification of ASMT Proteins in C. sinensis
The ASMT structural protein (AK069308) model was downloaded from the Rice Protein Structure Database (http://structure.rice.dna.affrc.go.jp/ (accessed on 9 January 2022)) for a BLASTP search against the tea plant genome using an E-value < 1.0 × 10−10. Rice-related acetylserotonin-O-methyltransferase structural proteins, such as LOC_Os09g17560, LOC_Os10g02880, and LOC_Os10g02840 [26], were also used in the sequence query of CSS (cv. Shuchazao), with an E-value < 1.0 × 10−10. The ASMT Hidden Markov Model (HMM) profile (PF00891) was downloaded from the Pfam database to search the C. sinensis genome with an E-value < 1.0 × 10−10. To confirm that the results were error-free when screening out the proteins that did not belong to the target gene family, the protein sequences of these genes were also uploaded to PfamScan (https://www.ebi.ac.uk/Tools/pfa/pfamscan/ (accessed on 15 January 2022)) and SMART (http://smart.embl-heidelberg.de/ (accessed on 16 January 2022)), and an E-value < 1.0 × 10−10 was used for searching to ensure that any putative genes belonging to the ASMT gene family were not excluded from the analysis. Based on previous studies [25,27], the tea plant ASMT homologs with an amino acid homology higher than 31% were considered gene family members. In total, we obtained 20 candidate genes, and their chromosomal location information was used for numbering. The molecular weights and physicochemical properties of the tea plant ASMT proteins were analyzed using the online portal ExPASy (https://www.expasy.org/ (accessed on 19 January 2022)).
2.3. Phylogenetic and Structural Analyses of C. sinensis ASMT Genes
To determine the phylogenetic relationships of the tea plant ASMT genes, a phylogenetic tree was constructed using the ASMT protein sequences, implementing the neighbor-joining method in MEGA X software, along with bootstrap testing (n = 1000). Evolutionary trees were constructed using the Evolview online tool (https://evolgenius.info//evolview-v2 (accessed on 1 December 2022)).
2.4. Analysis of C. sinensis ASMT Motifs and Promoter Cis-Acting Elements
The genome information and gene structure annotation files of the tea plant were analyzed using TBtools 1.09876 [28], and the information files of the 20 candidate genes of the ASMT gene family were obtained and used to visualize the gene structure and to identify the number and arrangement of introns and exons. A motif analysis of the ASMT genes was performed using the MEME Suite online tool (http://meme-suite.org/index.html (accessed on 1 February 2022)) with the following parameters: the number of motifs = 10, occurrence per motif = 0 or 1, optimal motif width ranging from 6 to 50 amino acid residues, and maximum mismatch = 10. The 2.0 kb promoter sequence upstream of the ASMT proteins was extracted using TBtools and submitted to PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 1 February 2022)) in order to identify the active elements [29]. The results were mapped using TBtools software.
2.5. Chromosome Localization and Collinearity Analysis
The tea plant genome and chromosome annotation information were downloaded from the C. sinensis genome database (http://tpia.teaplant.org/index.html (accessed on 2 January 2022)), and the chromosomes were mapped using the GFF file configuration information. A collinearity analysis of the tea plant ASMT genes was performed using TBtools, while the Ka, Ks, and Ka/Ks ratios of the duplication events [30] were calculated using DNASP 6.0 software to further analyze the duration of duplication occurrences [31].
2.6. Expression Profiling of ASMT Family Genes in C. sinensis
The RNA-seq data were obtained from the Anhui Agricultural University C. sinensis genome database (http://tpia.teaplant.org/index.html (accessed on 3 February 2022)), and the gene expression levels were reported in fragment per kilobase of transcript per million mapped reads (FPKM). Expression profile mapping was performed using TBtools.
2.7. Quantitative Real-Time Fluorescence Analysis
A qRT-PCR analysis was performed on the cDNA of samples from different nodes of the experimental treatment using the Bio-Rad CFX96 (Bio-Rad, Hercules, CA, USA) fluorescence quantification system. Gene primers were first designed using Primer 5.0 software, with primer lengths of 150–250 bp (Table S1), using the 1 μL cDNA template, 10 μL SYBR Premix ExTaq (Takara, Kyoto, Japan), 2 μL specific primers, and 7 μL ddH2O. The PCR thermal cycling parameters were as follows: 95 °C for 5 min, 45 cycles at 95 °C for 20 s, 60 °C for 20 s, and 72 °C for 10 s, 60 °C for 20 s, and 72 °C for 10 s for 45 cycles. CsPTB-RT is a specific primer for tea tree actin, and it was used as an internal control to normalize gene expressions. The relative expressions of genes were determined using the 2−ΔΔCT method for analyses, and three biological replicates were performed for each sample.
3. Results
3.1. Genome-Wide Identification of ASMT Genes in C. sinensis
A total of 20 ASMT gene family members were identified in the tea plant genome via the bioinformatics analysis; they were sequentially named CsASMT01–CsASMT20 according to their position on the chromosomes. The gene name, gene ID, chromosomal position, open-reading frame (ORF), amino acid (AA), molecular weight (MW), and isoelectric point (pI) corresponding to each gene are listed in Table 1. The sequence analysis revealed that the ASMT gene family members had different corresponding ORFs, AAs, MWs, and pIs. The length of the ORFs ranged from 888 bp (CsASMT09) to 1182 bp (CsASMT18), and the length of the ASMT proteins ranged from 295 AAs (CsASMT11) to 393 AAs (CsASMT19). The MW of the proteins ranged from 32.28 (CsASMT11) to 43.80 kDa (CsASMT19), with a theoretical isoelectric point (pI) varying from 4.94 (CsASMT05) to 6.06 (CsASMT19). The isoelectric point analysis revealed that all the ASMT proteins were acidic.

Table 1.
Physical and chemical properties of ASMT proteins in C. sinensis.
3.2. Systematic Analysis and Conserved Motifs of C. sinensis ASMT Genes
For an in-depth understanding of the evolutionary relationships among the ASMT gene family members, a phylogenetic tree was constructed based on the 20 amino acid sequences of the tea plant (Figure 1). The results show that these ASMT proteins could be divided into three subfamilies, which were classified as subfamilies I, II, and III. The largest subfamily, subfamily III, contained nine ASMT members, followed by subfamilies I and II, which contained seven and four ASMT members, respectively. Members of the same subfamily showed a high level of homology.
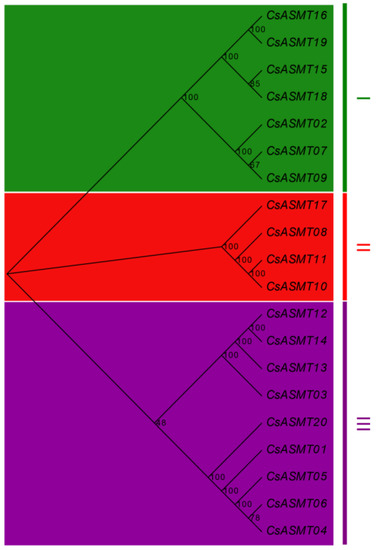
Figure 1.
Phylogenetic analysis of ASMT genes in C. sinensis. The phylogenetic tree was constructed based on the sequences of CsASMT proteins using the neighbor-joining method with bootstrap analysis (1000 replicates) in MEGA X.
To better characterize the diversity of the CsASMT proteins, the AA sequences of the 20 genes were analyzed using the MEME online tool, revealing a total of 10 different motifs, namely, motifs 1–10 (Figure 2), whose corresponding protein sequences ranged from 15 to 50 AAs in length. The distribution of most of the gene motifs was relatively concentrated. Motifs 1, 2, and 4 were present in all genes (Figure 2), indicating that these proteins were highly conserved during the evolution of the tea plant. However, CsASMT11 was unique because it did not contain motifs 5, 7, or 9, which were present in most genes. Similarly, individual genes showed minor differences in motif distribution when comparing subclade members or members of the same subclade branch, which indicates that the ASMT genes in tea plants underwent different selection pressures and evolutionary processes. Our gene structure analysis showed that all CsASMT genes contained introns; 20% of them contained three introns, while the rest contained one or two introns. The comparison also revealed that the genes with a higher number of introns had a relatively lower motif number, which confirmed the previous conclusion that the number and length of introns may be related to the loss of conserved motifs as a result of evolutionary processes.
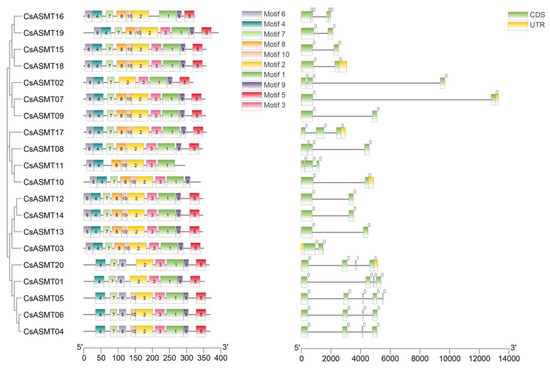
Figure 2.
Conserved motifs and gene structure analysis of C. sinensis ASMT gene family. Different colored boxes with numbers on the left side represent different types of motifs. UTRs are represented by green boxes on the right side of the figure, CDS sequences are represented by yellow rectangles and introns are represented by grey lines.
3.3. Chromosome Distribution and Collinearity Analysis
The chromosomal localization analysis of the ASMT gene family members revealed that the 20 genes were located on seven chromosomes (Figure 3). Five CsASMT genes were distributed on chromosomes 5 and 7, whereas only one was detected on chromosomes 1 and 2. The collinearity analysis showed that only two pairs of the 20 ASMT genes, namely, CsASMT03/CsASMT13 and CsASMT10/CsASMT17, had a linear relationship (Figure 4). Thus, we concluded that the ASMT gene family expansion occurred via fragment replication. In the analysis of the replication event time, the Ka/Ks ratios of the two gene pairs were lower than 1, and these genes suffered from an evolutionary negative purifying selection over 19.78 to 34.66 million years (Table 2).
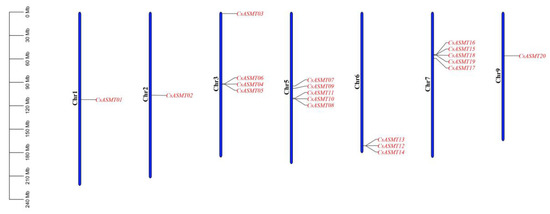
Figure 3.
Chromosome localization analysis of C. sinensis ASMT gene family.
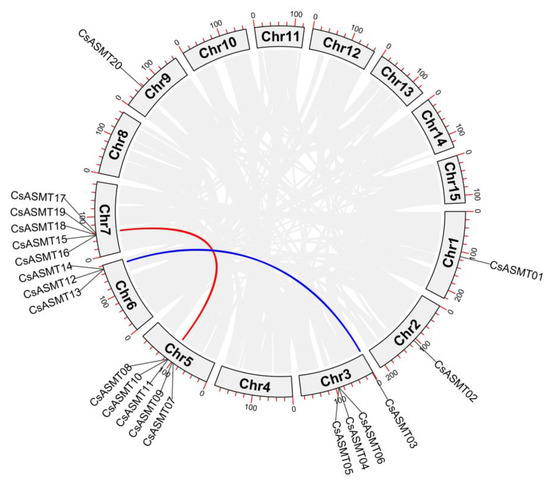
Figure 4.
Collinearity analysis of C. sinensis ASMT gene family. The lines between chromosomes indicate a fragmentary replication relationship between ASMT genes in tea plant.

Table 2.
The parameters and data of the duplication evens in the CsASMTs.
3.4. Analysis of Cis-Acting Elements of the C. sinensis ASMT Promoter
An analysis of cis-acting elements is important for predicting the regulation of gene expressions and for understanding the possible involvement of genes in the related regulation pathways. In the present study, by investigating the 2000 bp promoter region upstream of the ASMT gene family members, three types of cis-acting elements were found: those associated with stress responses, those associated with hormone responses, and those associated with plant development. Based on the predicted results, the promoters were classified into 21 main types of promoter cis-acting elements, including those associated with cell cycle regulation, light responses, the MYBHv1 binding site, low-temperature responses, gibberellin responses, abscisic acid responses, and drought induction (Figure 5). Moreover, based on a functional classification, antioxidant cis-acting elements (ARE) were found in most of the promoter regions of the CsASMT genes. In addition, stress-response elements, including low-temperature-response elements, defense-response elements (TC-rich repeats), and drought-response elements (MBS), were distributed in the gene promoters. Most genes contained abscisic-acid-responsive elements (ABRE) and MeJA-responsive elements (TGACG-motif and CGTCA-motif), which are hormone-responsive elements, and these cis-acting elements were widely distributed and numerous. Among the cis-elements associated with plant development, most genes contained cis-acting elements related to light responses and soluble protein metabolism, such as Box 4, O2-site, and G-Box (Figure 6). Therefore, ASMT genes may play important roles in the response of tea plants to environmental stresses.
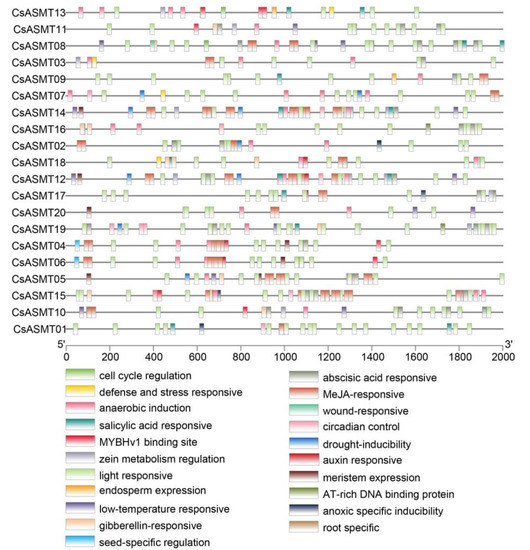
Figure 5.
Cis-element analysis of C. sinensis ASMT gene promoters. The binding sites of promoter regions are indicated by the different colored boxes.
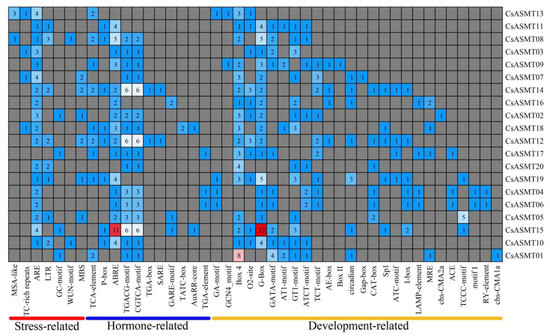
Figure 6.
Distribution of the number of cis-acting elements in the C. sinensis ASMT gene promoter.
3.5. Expression Profiles of CsASMT Genes under Different Types of Stress
The FPKM values obtained from the RNA-seq data were used to construct two heat maps of the 20 CsASMT genes under cold and drought stresses in order to explore the expression patterns of the ASMT gene family in tea plants under different stress conditions. The results show that eight genes were not expressed before or after treatment under cold stress conditions. Moreover, according to the RNA-seq analysis results, most of the expressed CsASMT genes showed differential expression levels after 6 h of treatment, and their expression levels were not high (Figure 7). We identified seven differentially expressed genes, namely, CsASMT01, CsASMT08, CsASMT09, CsASMT10, CsASMT18, CsASMT19, and CsASMT20, by combining the expression results after 7 d of treatment. The recovery culture showed high expression levels of these genes and relatively stable replicate results. Among them, the expression levels of CsASMT01 and CsASMT20 were consistently maintained, and these genes were differentially expressed before and after stress treatment. The expressions of CsASMT01 and CsASMT18 showed significant increases under cold stress, whereas the expression of CsASMT20 decreased under cold stress; however, it returned to a normal level after the recovery culture. The expressions of all other genes decreased after treatment, and the recovery culture expression did not reach a normal level. These results suggest that the seven CsASMT genes may mediate cold damage in tea plants. Similarly, in the construction of the heat map of the 20 CsASMT genes under drought stress, we found that the expressions of CsASMT08, CsASMT10, CsASMT18, CsASMT19, and CsASMT20 decreased significantly after drought treatment and during each of its three stages (Figure 8); CsASMT01 and CsASMT20 were highly expressed before and after treatment, and the expression of CsASMT01 increased after drought treatment.
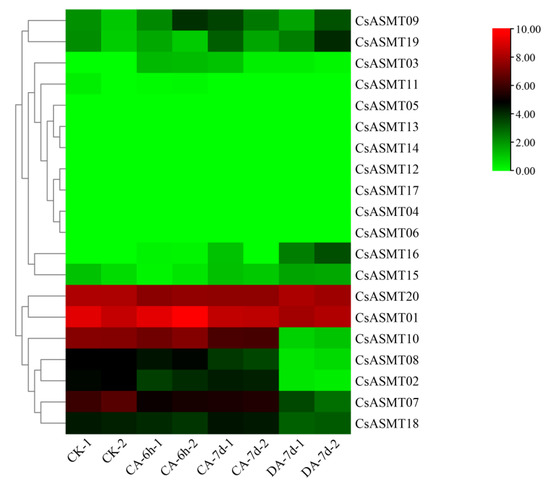
Figure 7.
Heat map of FPKM expressions of C. sinensis ASMT genes under cold stress based on RNA-Seq data. FPKM values were all log2-processed, and heat map clusters were constructed. The color scale representing relative expression values is shown on the left, CA: 10 °C treatment, DA: room-temperature recovery.
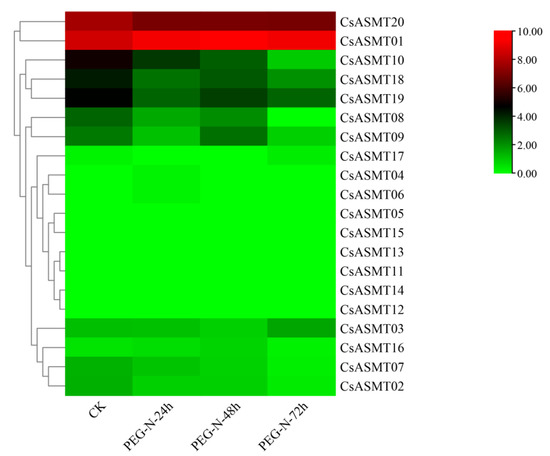
Figure 8.
Heat map of FPKM expressions of C. sinensis ASMT genes under drought stress based on RNA-Seq data. FPKM values were all log2-processed, and heat map clusters were constructed. The color scale representing relative expression values is shown on the left.
To verify the RNA-seq results and to further analyze which of these genes had a mediating effect under drought treatment versus low-temperature treatment, we further validated the seven selected differentially expressed genes using a qRT-PCR analysis (Table S2). Under drought treatment, CsASMT08, CsASMT09, and CsASMT10 were negatively regulated at different treatment time points, and CsASMT18 and CsASMT20 were upregulated at 0.5 d and 1 d of treatment, respectively; the other genes were not significantly different before and after treatment. Under low-temperature treatment, CsASMT08 and CsASMT10 were significantly upregulated at different time points of treatment; CsASMT20 was also significantly upregulated at 1 d and 4 d of treatment (Figure 9); CsASMT01, CsASM09, and CsASMT19 showed negative regulation at different treatment time points; and CsASMT18 showed no significant difference before and after treatment. These results also indicate that CsASMT08, CsASMT09, CsASMT10, and CsASMT20 may play vital roles in responses to environmental stresses in the tea plant.
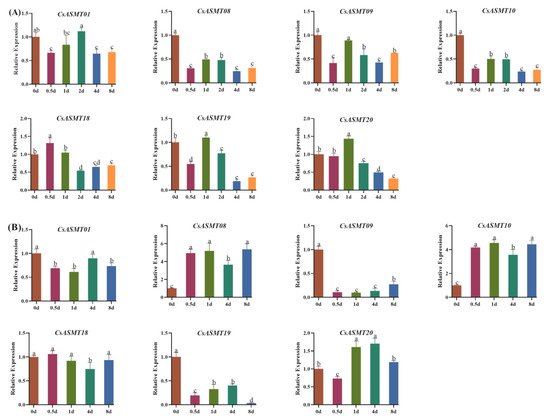
Figure 9.
(A) qRT-PCR of 7 selected C. sinensis ASMT genes in response to drought stress; (B) qRT-PCR of 7 selected C. sinensis ASMT genes in response to cold stress. Bars represent the mean ± standard deviation of three replicates, different letters indicate significant differences at different time points, and all data were subjected to one-way ANOVA and LSD test (p < 0.05).
4. Discussion
Melatonin is widely known as a multifunctional molecule, and it plays an important role in oxidative stress tolerance in plants [32]. Therefore, melatonin-related studies are a crucial part of plant adversity research, and studying the function of ASMT is important, as it is one of the key enzymes participating in melatonin synthesis in plants [23]. Previous studies have shown that exogenous melatonin applications can improve the ability of plants to cope with abiotic stresses, and the functional validation of the related genes has been demonstrated in rice and A. thaliana [24,25]. In the present study, 30 candidate ASMT genes were initially identified through an analysis of the tea plant genome. Considering that multiple putative ASMT gene family members have been identified in rice, 20 ASMT genes were identified with a rice ASMT homology greater than 31% with structural proteins, while 10 gene family members with a low level of homology (less than 31%) were excluded. The ASMT gene family in tea plant is larger than that in tomato (14) and pepper (16); however, it contains fewer ASMT genes than the gene families of mulberry (20) and apple (37), indicating that it evolved differently in different species.
To explain the evolutionary relationships between the ASMT family genes in the tea plant, we constructed a phylogenetic tree using aligned protein sequences. Our cluster analysis showed that the 20 tea plant ASMT genes could be classified into three subfamilies with six homologous gene pairs (CsASMT16/CsASMT19, CsASMT15/CsASMT18, CsASMT07/CsASMT09, CsASMT10/CsASMT11, CsASMT12/CsASMT14, and CsASMT04/06), which were relatively conserved and perhaps functionally similar. To further investigate the structural functions of these ASMT genes, we performed a motif analysis and found that the ASMT members in the same subfamily or cluster had similar motif compositions, further supporting the subclade classification identified in the phylogenetic analysis. The tea plant ASMT gene family structure was also found to be highly conserved according to the motif distribution. An exon–intron organization analysis showed that all the genes contained introns, which were relatively long and interrupted the gene coding sequence. If a gene has fewer introns, it may respond to external stress regulation more rapidly; however, highly expressed plant genes generally contain more introns than lowly expressed genes [33], which indicates that ASMT genes may play roles in abiotic stress responses.
Gene duplication is the basis of gene diversity, leading to genetic novelty; therefore, it is the main source of species evolution and adaptation [34,35]. It is divided into tandem and fragment duplications; it is also one of the main factors in the evolution of gene families, and it contributes to differences in gene size and distribution [36,37]. Our analysis revealed that only two pairs of tea plant ASMT genes underwent fragment duplication and that none underwent tandem duplication; this low frequency of duplication may be the underlying reason for the similar lengths of the ASMT genes. Furthermore, the occurrence of duplication events indirectly indicated that genetic information was being transmitted, which also suggests that fragment duplication may have played an important role in the evolution of the tea plant ASMT genes. The 20 tea plant ASMT genes were unevenly distributed on seven chromosomes and were mostly located in the middle of the chromosome; this may explain why most of them were not involved in the duplication events. Similarly, tandem repeats were found in the ASMT genes of the pepper plant.
The gene expression patterns of plants under various stresses can provide important clues for gene function analyses, and cis-acting element analyses can help predict the network of regulatory mechanisms in which genes may be involved. In the present study, we analyzed the expression profiles of the ASMT genes in the tea plants under drought and low-temperature stress conditions using RNA-seq and qRT-PCR. The results of the RNA-seq analysis showed that eight genes were not expressed before or after treatment under cold stress conditions, and the expression analysis comparing 6 h of treatment, 7 d of treatment, and after the recovery culture revealed that CsASMT01 CsASMT08, CsASMT09, CsASMT10, CsASMT18, CsASMT19, and CsASMT20 responded to cold stress and drought stress. To further identify which of these CsASMT genes are most important in mediating cold and drought stresses, these seven differentially expressed CsASMT genes were further validated using qRT-PCR. The results show that CsASMT08, CsASMT09, and CsASMT10 were negatively regulated to drought and that CsASMT01 and CsASMT20 were positively regulated to drought; CsASMT08, CsASMT10, and CsASMT20 positively responded to low-temperature stress, and CsASM09 negatively fed back to low-temperature stress, which indicates that these genes may have different response mechanisms to drought and low temperatures and that they may play central roles in abiotic stress processes. Similarly, in grapes, it has been found that the overexpression of VvASMT1 enhances tolerance to salt and osmotic stresses in Nicotiana benthamiana [38]. These results suggest that ASMT genes play important roles in abiotic stress processes in plants. It is well-known that cis-acting elements, ABREs, MBSs, G-boxes, AREs, and TC-rich repeats play important roles in drought stress responses and the regulation of downstream gene expressions [39,40,41]. The cis-acting element analysis revealed that CsASMT08, CsASMT09, CsASMT10, and CsASMT20 contained one to five stress-like cis-acting elements; for example, CsASMT08 contained one ARE, and CsASMT10 contained one ARE and four ABREs, indicating that all these genes may mediate the stress response pathways.
5. Conclusions
In our study, we identified 20 ASMT genes by bioinformatics methods and analyzed them in terms of phylogeny, gene structure, and cis-acting elements to provide a preliminary resolution and better understanding of the ASMT gene family in tea plant. We showed that the expressions of CsASMT08, CsASMT09, CsASMT10, and CsASMT20 were differentially induced under drought and low-temperature treatments, indicating that they may regulate abiotic stress resistance in the tea plant. These results lay the foundation for further studies on the functions of ASMT genes, as well as for identifying candidate genes for abiotic stress resistance regulation in tea plants.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/genes14020409/s1. Table S1. Primers for qRT-PCR used in this study; Table S2. Expression levels for selected tea plant ASMT genes under abiotic stress.
Author Contributions
Data curation, Formal analysis, F.X.; Writing—original draft, F.X.; Writing—review & editing, F.X. and M.F.; Validation, W.L.; Methodology, H.W.; Software, P.A.; Funding acquisition, W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Scientific Research Project of Henan Province Colleges and Universities 23B22001 and the Xinyang Agriculture and Forestry University School Youth Fund Project QN2021013.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available within the article or its Supplementary Materials. The authors confirm that the data supporting the findings of this study are available within the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kobylińska, A.; Borek, S.; Posmyk, M.M. Melatonin redirects carbohydrates metabolism during sugar starvation in plant cells. J. Pineal Res. 2018, 64, e12466. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Brestic, M.; Tan, D.X.; Zivcak, M.; Zhu, X.; Liu, S.; Liu, F. Melatonin alleviates low PS I-limited carbon assimilation under elevated CO2 and enhances the cold tolerance of offspring in chlorophyll b-deficient mutant wheat. J. Pineal Res. 2018, 64, e12453. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Alcaraz, O.; Arnao, M.B. Free radical-scavenging activity of indolic compounds in aqueous and ethanolic media. Anal. Bioanal. Chem. 2003, 376, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Helton, P.; Reiter, R.J. Phytoremediative Capacity of Plants Enriched with Melatonin. Plant Signal. Behav. 2007, 2, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Reiter, R.J.; Qi, W.-B.; Karbownik, M.; Calvo, J.R. Significance of Melatonin in Antioxidative Defense System: Reactions and Products. Neurosignals 2000, 9, 137–159. [Google Scholar] [CrossRef]
- Van Tassel, D.L.; O’Neill, S.D. Putative regulatory molecules in plants: Evaluating melatonin. J. Pineal Res. 2001, 31, 1–7. [Google Scholar] [CrossRef]
- Lee, H.Y.; Byeon, Y.; Back, K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 2014, 57, 262–268. [Google Scholar] [CrossRef]
- Shi, H.; Tan, D.-X.; Reiter, R.J.; Ye, T.; Yang, F.; Chan, Z. Melatonin induces class A1 heat-shock factors (HSFA1s) and their possible involvement of thermotolerance in Arabidopsis. J. Pineal Res. 2015, 58, 335–342. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Zhang, N.; Yang, R.-C.; Wang, L.; Sun, Q.-Q.; Li, D.-B.; Cao, Y.-Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, L.; Su, T.; Jiang, Y.; Hu, L.; Ma, F. Melatonin regulates carbohydrate metabolism and defenses against Pseudomonas syringae pv. tomato DC 3000 infection in Arabidopsis thaliana. J. Pineal Res. 2015, 59, 109–119. [Google Scholar] [CrossRef]
- Lee, K.; Choi, G.H.; Back, K. Cadmium-induced melatonin synthesis in rice requires light, hydrogen peroxide, and nitric oxide: Key regulatory roles for tryptophan decarboxylase and caffeic acid O-methyltransferase. J. Pineal Res. 2017, 63, e12441. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, S.; et al. Effects of Melatonin on Anti-oxidative Systems and Photosystem II in Cold-Stressed Rice Seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A Multifunctional Factor in Plants. Int. J. Mol. Sci. 2018, 19, 1528. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, H.Y.; Back, K. Rice histone deacetylase 10 and Arabidopsis histone deacetylase 14 genes encode N-acetylserotonin deacetylase, which catalyzes conversion of N-acetylserotonin into serotonin, a reverse reaction for melatonin biosynthesis in plants. J. Pineal Res. 2018, 64, e12460. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, D.; Zheng, C.; Chen, C.; Peng, X.; Cheng, Y.; Wan, H. Genomic analysis of the ASMT gene family in Solanum lycopersicum. Molecules 2017, 22, 1984. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Pan, L.; Zheng, J.; Liu, J.; Guo, J.; Liu, F.; Liu, L.; Wan, H. Analysis of the ASMT gene family in pepper (Capsicum annuum L.): Identification, phylogeny, and expression profiles. Int. J. Genom. 2019, 2019, 7241096. [Google Scholar] [CrossRef]
- Wang, H.; Song, C.; Fang, S.; Wang, Z.; Song, S.; Jiao, J.; Wang, M.; Zheng, X.; Bai, T. Genome-wide identification and expression analysis of the ASMT gene family reveals their role in abiotic stress tolerance in apple. Sci. Hortic. 2022, 293, 110683. [Google Scholar] [CrossRef]
- Ma, K.; Xu, R.; Zhao, Y.; Han, L.; Xu, Y.; Li, L.; Wang, J.; Li, N. Walnut N-Acetylserotonin Methyltransferase Gene Family Genome-Wide Identification and Diverse Functions Characterization during Flower Bud Development. Front. Plant Sci. 2022, 13, 1131. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Zhu, Y.; Liu, C.; Fan, W.; Xiang, Z.; Zhao, A. Genome-wide identification and characterization of genes involved in melatonin biosynthesis in Morus notabilis (wild mulberry). Phytochemistry 2021, 189, 112819. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Kong, K.; Park, S.; Natsagdorj, U.; Kim, Y.S.; Back, K. Molecular cloning of a plant N-acetylserotonin methyltransferase and its expression characteristics in rice. J. Pineal Res. 2011, 50, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Byeon, Y.; Back, K. Functional analyses of three ASMT gene family members in rice plants. J. Pineal Res. 2013, 55, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Zuo, B.; Zheng, X.; He, P.; Wang, L.; Lei, Q.; Feng, C.; Zhou, J.; Li, Q.; Han, Z.; Kong, J. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J. Pineal Res. 2014, 57, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.R.; Kim, Y.-J.; Lim, Y.J.; Duan, S.; Eom, S.H.; Jung, K.-H. Key Genes in the Melatonin Biosynthesis Pathway with Circadian Rhythm Are Associated with Various Abiotic Stresses. Plants 2021, 10, 129. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.J.; Lee, H.Y.; Back, K. Back Cloning and functional characterization of the Arabidopsis N-acetylserotonin O-methyltransferase responsible for melatonin synthesis. J. Pineal Res. 2016, 60, 65–73. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Zhao, X.Q.; Wang, J.; Wong, G.K.S.; Yu, J. KaKs_Calculator: Calculating Ka and Ks through model selection and model averaging. Genom. Proteom. Bioinform. 2006, 4, 259–263. [Google Scholar] [CrossRef]
- Blanc, G.; Wolfe, K.H. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell 2004, 16, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Howlader, P. Melatonin plays multifunctional role in horticultural crops against environmental stresses: A review. Environ. Exp. Bot. 2020, 176, 104063. [Google Scholar] [CrossRef]
- Parra, G.; Bradnam, K.; Rose, A.B.; Korf, I. Comparative and functional analysis of intron-mediated enhancement signals reveals conserved features among plants. Nucleic Acids Res. 2011, 39, 5328–5337. [Google Scholar] [CrossRef] [PubMed]
- Sémon, M.; Wolfe, K.H. Consequences of genome duplication. Curr. Opin. Genet. Dev. 2007, 17, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Evolution by gene duplication: An update. Trends Ecol. Evol. 2003, 18, 292–298. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Moore, R.C.; Purugganan, M.D. The early stages of duplicate gene evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 15682–15687. [Google Scholar] [CrossRef]
- Yu, Y.; Ni, Y.; Qiao, T.; Ji, X.; Xu, J.; Li, B.; Sun, Q. Overexpression of VvASMT1 from grapevine enhanced salt and osmotic stress tolerance in Nicotiana benthamiana. PLoS ONE 2022, 17, e0269028. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, S.H.; Kim, S.R. Drought inducible OsDhn1 promoter is activated by OsDREB1A and OsDREB1D. J. Plant Biol. 2013, 56, 115–121. [Google Scholar] [CrossRef]
- Nakashima, K.; Jan, A.; Todaka, D.; Maruyama, K.; Goto, S.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Comparative functional analysis of six drought-responsive promoters in transgenic rice. Planta 2014, 239, 47–60. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, L.; An, H.; Wu, C.; Guo, X. GhMPK16, a novel stress-responsive group D MAPK gene from cotton, is involved in disease resistance and drought sensitivity. BMC Mol. Biol. 2011, 12, 22. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).