Abstract
(1) Background: obesity is a global public health problem; various factors have been associated with this disease, and genetic factors play a very important role. Previous studies in multiple populations have associated a gene with fat mass and obesity (FTO). Thus, the present work aims to identify and determine associations between genetic variants of FTO with indicators of overweight and obesity in the Mexican population. (2) Methods: a total of 638 subjects were evaluated to compile data on body mass index (BMI), the percentage of body fat (%BF), the waist circumference (WC), the serum levels of triglycerides (TG), and food consumption. A total of 175 genetic variants in the FTO gene were sampled by a microarray in the evaluated population, followed by association statistical analyses and comparisons of means. (3) Results: a total of 34 genetic variants were associated with any of the 6 indicators of overweight and obesity, but only 15 showed mean differences using the recessive model after the Bonferroni correction. The present study shows a wide evaluation of FTO genetic variants associated with a classic indicator of overweight and obesity, which highlights the importance of genetic analyses in the study of obesity.
1. Introduction
Obesity is a global health problem [1]. Studies in various populations have shown the importance of the genetic component in obesity. Studies in twins have revealed that 80% of variations in the body mass index (BMI) are related to a genetic component [2]; other studies have reported that adopted children have more BMI alterations compared to those shown by their biological parents, indicating that 63% of these alterations resulted from a hereditary component and 31% from environmental factors. Furthermore, studies based on single nucleotide polymorphisms (SNP) have only been able to attribute 3% of BMI variation to a genetic effect [3,4]. Available data suggest a susceptibility of some populations to have higher figures of obesity, as in the case of the Latin American population, where obesity statistics have increased alarmingly and Mexico is considered the country with the highest rate of obesity. Both adult and child populations [5,6] are susceptible to being diagnosed as obese in the same proportion. Several independent studies have shown an association between FTO SNPs and fat mass and obesity [7,8,9].
FTO has been studied for several years and is known to encode for an enzyme nucleic acid methylase, dependent on α-ketoglutarate and iron (Fe II), which is ubiquitous in human tissues. Understanding the exact mechanism by which it is associated with obesity has been difficult [10,11]. However, a loss of function in homozygous FTO carriers has been observed to cause growth retardation and central nervous system disorders. Likewise, there are thin and obese heterozygous individuals, which indicates that the loss or gain of the FTO function is not a condition for the development of obesity but rather specific modifications in their activity. Such modifications can be due to subtle changes in the gene sequence, such as SNPs [11].
In a study investigating adipocytes derived from human adipose tissue, the researchers observed that the presence of the risk allele of SNP rs1421085 promoted a greater darkening of fat cells [12]. On the other hand, in studies looking directly at humans, the association of various SNPs has been observed in different populations in recent decades. Such is the case for SNPs rs9939609, rs6499640, rs8050136, and rs1558902 in the Chinese population [13]. A positive association was also observed with high BMI in the Korean population for SNPs rs1421085 and rs17817449 [13]. Furthermore, rs1421085, rs17817449, and rs9939609 have been associated with obesity in European populations, while these relationships differed in Melanesian, Micronesian, and Polynesian populations [14].
In the Mexican population, some associations of FTO SNPs with obesity of the SNPs rs1121980, rs17817449, rs3751812, and rs9930506 have been observed in the mestizo population [7], and rs9939609 and rs1421085 were associated with obesity in the Mayan population [15].
As these previous studies have not been able to analyze a greater number of genetic variants associated with phenotypic obesity markers, the present work focuses on the evaluation of 175 FTO SNPs filtered from a microarray to investigate their potential associations with common indicators of obesity phenotypes.
2. Materials and Methods
2.1. Subjects and Genetic Sampling
A total of 638 subjects were included in this study from the SUSALUD-UAQ, an initiative that seeks to determine the risk factors of the main non-communicable diseases in the young population. Participants who met the following criteria were included: men and women with an age range of 18 to 22 years, who agreed to sign the informed consent letter and who had a complete evaluation. Likewise, those with previously diagnosed chronic diseases such as cancer, diabetes, cardiovascular disease, women with polycystic ovarian syndrome, pregnant or lactating women, and those who had thyroid problems were excluded from the study; those who did not have complete evaluation information were eliminated.
From this evaluation, the anthropometric parameters of height (m) and weight (Kg) were selected for the calculation of the BMI (kg/m2) and waist circumference (cm) in the same way as the percentage of body fat, determined by 4-pole multifrequency bioelectrical impedance, using the mBCA Mod. 514 equipment (SECA, Hamburg, Germany). Likewise, biochemical parameters of glucose, TG, cholesterol, and HDL were determined from a blood sample extracted by venipuncture, enzymatic methods (SPINREACT, Girona, Spain), and using the Chemistry Analyzer Mod. BS 120 automated equipment (Mindray, Shenzhen, China).
Since there is no accurate diagnosis of obesity, this study took as markers of obesity those that have been found to be the best predictors of obesity and its comorbidities: body mass index (BMI), waist circumference (WC), and body fat percentage (BF%) [16], as well as elevated triglyceride levels [17] and high energy intake [18]. The following values were used as obesity parameters: body mass index > 25.0 kg/m2; WC in women > 80 cm and in men > 90 cm; percentage of body fat in women > 35% and in men > 20%; TG > 150 mg/dL; and energy intake > population median (>2400 Kcal). Fasting glucose < 100 mg/dL was used to rule out diabetes mellitus.
2.2. Analysis of Genetic Material
Subjects’ DNA samples were obtained from whole blood, using the QIAamp 96 DNA blood kit (QIAGEN, Illumina, CA, USA) and following the supplier’s specifications. The Illumina Infinium HTS Automated protocol, along with the Beadchip Global Screening Array microarray (GSA-24 v1.0), were used for human genotyping [19,20] in the Código 46 Genetics Laboratories. Data from 216 genetic variants on the FTO gene were initially recovered from the whole 669,672 variants on the Illumina microarray. We applied two data filters using PLINK, the percentage of missing variants per sample below 0.05, and the quality per individual with a call rate above 0.95 [21], which resulted in 175 variants on FTO fulfilling these filters. Genotypic and allelic frequencies were determined with GenAlEx 6.51 [22]. Null alleles were excluded from the dataset prior to further analyses; all markers were analyzed for the Hardy–Weinberg equilibrium (HWE) (Table S1).
2.3. Statistical Analysis
Statistical and descriptive analyses were performed to determine the general characteristics of the population. For the present study, the genotypes were grouped according to the additive model. For the recessive model, the alternative homozygous (xx) and reference homozygous plus heterozygous (XX + Xx) models were used, while for the dominant model, the reference homozygote (XX) and the set of heterozygote and alternative homozygotes were used. Binary logistic regressions were performed to determine significant associations (p ≤ 0.05) between genetic variants and indicators of obesity. Student’s t-tests (p ≤ 0.05) were performed to compare the means of the indicators of obesity for each of the models. One-way ANOVAs were performed to describe the mean differences between the homozygous reference, heterozygous, and homozygous risk populations, followed by the Bonferroni adjustment (p ≤ 0.05). All statistical analyses were performed using the Statistical Package for the Social Sciences (IBM SPSS Statistics for Macintosh, Version 23.0., Armonk, NY, USA: IBM Corp) [23].
3. Results
3.1. Description of the Population
From the studied population, 307 were men (48.27%) and 329 were women (51.73%); the mean age of the population was 19.34 years: 19.58 years for men and 19.12 years for women. Data from the variables related to obesity showed that the mean WC for men was 84.03 ± 11.46 cm and for women 77.88 ± 11.53 cm; the mean BMI for men was 24.03 ± 4.09 Kg/m2, and for women it was 23.41 ± 4.38 Kg/m2. The BF% data presented a mean of 21.13 ± 7.94% in men and 31.21 ± 7.31% in women; the average value of TG in serum was 114.34 ± 72.45 mg/dL in men and 96.67 ± 9.16 mg/dL in women; the calorie consumption values for men were 2584.6 ± 952.97 Kcal/day and 2227.6 ± 815.60 Kcal/day for women (Table 1).

Table 1.
General characteristics of the population.
Likewise, a prevalence of high waist circumference was observed in the population of 30.8%, being 25.6% for men and 35.6% for women; according to the BMI > 25 Kg/m2, 33.23% of the population was overweight or obese, being higher in men than in women. On the other hand, the percentage of high body fat was shown in 48.55% of the population, and the energy consumption was greater than the population average (2400 Kcal/day) was observed high in 57.5% of men and 40.18% of women. Hyperglycemia was observed in 3.1% of the population, while hypercholesterolemia was observed in 7.89%. Hypertriglyceridemia (20.3%) and low HDL (36.02) were the most prevalent biochemical markers in the studied population (Figure 1).
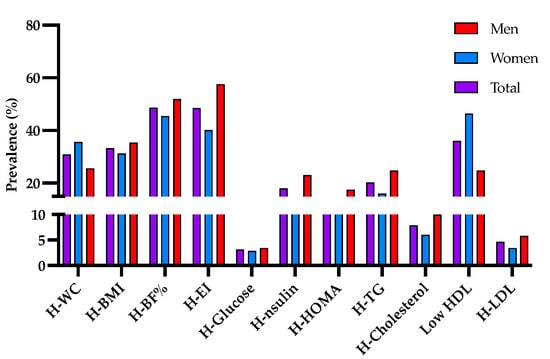
Figure 1.
Prevalence of clinical markers associated with indicators of overweight and obesity. Total prevalence (purple bar); of women (blue bar) and men (red bar) with high waist circumference (H-WC); body mass index greater than 25 kg/m2 (H-BMI); high body fat percentage (H-BF%); energy intake greater than 2400 Kcal/day (H-EI); hyperglycemia (H-Glucose); hyperinsulinemia (H-Insulin); elevated HOMA index (H-HOMA); hypertriglyceridemia (H-TG); hypercholesterolemia (H-Cholesterol); low levels of HDL (Low-HDL); and elevated levels of LDL (H-LDL).
3.2. Associations of FTO SNPs with Indicators of Obesity
According to the used model (xx − (Xx + XX)), significant associations (p = 0.05) between 34 genetic variants were associated with the risk (OR > 1) to 6 obesity indicators (WC, BMI > 25 Kg/m2, BMI > 30 Kg/m2, BF%, TG, and energy intake > 2400 Kcal). The genetic variants rs17219983, rs1966435, and rs12051261 showed a protective effect (OR < 1) for BMI > 25 Kg/m2, as well as the rs2111650, rs1966435, and rs12051261 to the BF%. This was the same with rs3751813 for serum TG, but energy consumption (>2400 Kcal) was negatively associated with the SNPs rs1075440 and rs7191566. Significant risk associations (OR > 1) were also observed between high WC and rs17817964 and rs6499662. Similarly, significant risk associations were found for between BMI > 25 Kg/m2 and SNPs rs8043785, rs35510800, rs6499662, and rs12931859. On the contrary, BMI > 30 Kg/m2 was positively associated with the genetic variants rs16945088, rs17817449, rs8043757, rs12931859, and rs7194243, and rs4389136, rs8043785, rs12232391, rs7194243, and energy consumption greater than 2400 Kcal/Day were associated with high BF%, as well as rs9939973, rs9940128, rs1421085, rs3751812, rs9936385, rs11075990, rs9939609, rs7202116, rs7185735, rs9941349, rs17817964, rs9922619, rs12149832, rs12149832, rs12149832, and rs9929152 (Table 2).

Table 2.
Association of FTO SNPs with indicators of overweight and obesity, recessive model.
On the other hand, according to the analysis of the dominant model (XX − (Xx + xx)), rs1421091, rs4389136, rs12232391, rs7200972, rs12931859, and rs7194243 resulted in significant associations for risk at a high percentage of fat, while rs10852523, rs61743972, rs3826169, and rs7203572 were associated with obesity (BMI >30 Kg/m2) and rs4389136 with hypertriglyceridemia. Protective effects were also observed of rs7205986, rs7203521, rs6499640, rs2111650, rs17819033, rs17219983, rs9934504, rs56335873, rs12933996, rs35090620, and rs16952686 for high fat percentage, and rs9934504, rs12933996, rs16952686, and rs1966435 for BMI > 25 Kg/m2; while rs9934504 turned out to be a protective factor for high waist circumference, and rs74018195, rs74449711, rs7191566, rs17820328, and rs16952657 for calorie intake greater than the median of the studied population (Table 3).

Table 3.
Association of FTO SNPs with indicators of overweight and obesity, dominant model.
3.3. Comparison of Means BMI, %BF, WC, TG, and Energy Consumption with Additive Models and Genotype
Mean comparisons by Student’s t-test showed statistically significant differences (p ≤ 0.05) only among WC, BMI, and BF% and 16 genetic variants. Specifically, WC with rs12232391; BMI with rs12232391 and rs12051261; and percentage of body fat with rs12232391 and rs12051261; rs9939973, rs9940128, rs1421085, rs17817449, rs3751812, rs9936385, rs11075990, rs9939609, rs7206629, rs7202116, rs7185735, rs9941349, rs17817964, and rs12051261 (Table 4).

Table 4.
Comparison of indicators of overweight and obesity means in recessive model.
According to the results of Student’s t-test for the dominant model (XX − (Xx + xx)), differences can be observed in the means of waist circumference in the SNPs rs1421091, rs4389136, and rs16952686, as well as in the BMI means in the variants rs9934504, rs56335873, rs12232391, and rs16952686. Likewise, significant statistical differences for the percentage of fat were observed in rs7191566, rs1421085, rs3751812, rs17817964, rs2111650, rs11642841, rs9934504, rs56335873, rs12232391, and rs7200972; in the same way as for triglyceride levels, the variants rs17817449, rs8043757, rs9936385, rs11075990, rs9939609, rs7202116, and rs7185735 affected the means of the studied population; likewise, the average energy consumption was affected in this model by the variants rs74449711, rs16952657, and rs35510800 (Table 5).

Table 5.
Comparison of indicators of overweight and obesity means in dominant model.
The results showed significant statistical differences (p ≤ 0.05, ANOVA) between the means of 12 of the determined genetic variants for WC of the SNPs rs12232391 and rs17817449, while for BMI, the variants that showed differences were rs17817449 and rs12232391. For the percentage of body fat, the differences were observed with rs7191566, rs1421085, rs17817449, rs3751812, rs17817964, rs2111650, and rs12232391. On the other hand, energy consumption also showed significant statistical differences (p ≤ 0.05) in the variants rs9936385, rs11075990, rs9939609, rs7202116, and rs7185735 (Table 6).

Table 6.
Association of the genotype with indicators of overweight and obesity.
It is important to mention that only four variants passed the Bonferroni adjustment: rs12232391 for WC, rs17817449 and rs12232391 for BMI, and rs17817449, rs3751812, rs2111650, and rs12232391 for %BF. None of the variants associated with energy consumption passed the Bonferroni adjustment (Table 6, highlighted in bold).
4. Discussion
Recent studies have shown the importance of the FTO gene in the development of the organism. Studies in experimental mice showed that suppression of FTO leads to reduced body weight and body mass, while overexpression promotes an increase in body mass, fat mass, and food consumption [24]. Therefore, FTO and downstream genes regulated from non-coding regions, mainly IRX3 and IRX5, which are genes related to neural development in areas associated with food consumption and may be valuable therapeutic targets for obesity [25,26] The effects of SNPs have been observed to be differential between populations; such is the case of rs9930506, which was observed to have risk associations with BMI in the European population but not in the Asian population [27].
The present work analyzed 175 genetic variants of FTO, of which only 34 were associated with any of the indicators of overweight and obesity, while only 16 of these variants showed differences in means according to the recessive model of the minor allele and 12 differences in the average of the indicators of overweight and obesity according to the genotype present, whereas only 4 passed the Bonferroni adjustment. From these last variants, rs12232391 showed differences between the population means in WC, BMI, and BF%; however, it has not been reported in association with any condition.
The variant rs17817449 has been extensively studied, and its effects have been observed in different populations, such as in the case of a study in an Iranian population with type 2 diabetes mellitus [28] and obesity [29]. This has also been replicated in Chinese women [30]. In the current study, the association of this marker with BMI >30 Kg/m2 and with higher energy consumption was observed, while differences were observed in the means of WC, BMI, and percentage of body fat. The genetic variant rs7191566 was observed in a population study in Mexico but not included in further analyses because it was not in Hardy-Weinberg equilibrium [7]. Interestingly, in the current work, this marker showed a different genotype when compared with the mean percentage of fat in the population.
In our results, variant rs1421085 was found to be associated with high consumption of Kcal, and mean differences were observed in the percentage of body fat. This marker has been extensively studied, and recent studies have found higher allelic frequencies in people with obesity as well as its associations with higher triglyceride and cholesterol levels in Turkish children [31]. Likewise, rs1421085 was previously reported with higher allelic frequencies in the Iranian population, showing its association with obesity markers [32]. In the adult Mexican obese population, rs1421085 has also been considered a genetic marker of risk [15]. On the other hand, rs3751812 has been associated with obesity in the Taiwanese population, as well as the reduction of its effects by increasing the physical activity for this population [33], while in a population of Greek adults, when analyzing the same SNP, it was not found in Hardy–Weinberg equilibrium, explaining why it had to be discarded from the study [34]. Similarly, in a study in the Polish population, it was observed that people carrying this polymorphism tended to have higher levels in the blood lipid profile [35].
Our findings show a positive association between energy consumption greater than 2400 Kcal and the said marker, in addition to differences in the means of body fat, both in the additive model and in the complete genotype. Similarly, rs17817964 in this study was associated with WC and energy consumption greater than the population median. It should be noted that this genetic marker was also associated with obesity in African-American women over 18 years of age with low birth weight [36], and it was generally observed as associated with obesity in people with African-American ancestry [37]. The variant rs2111650 was associated with the percentage of body fat and showed differences in the means of %BF related to its polymorphism; however, it has not been identified as a risk or protective variant in any other population. Furthermore, rs2111650 has been associated in various genome-wide association studies, mainly with obesity markers [38,39].
The rs9936385, rs7202116, and rs11075990 did not pass the Bonferroni adjustment and associations with obesity markers have not been observed in other studies. Interestingly, rs9939609 is one of the variants of the FTO gene that has been most researched for its relationship with clinical obesity markers in various populations, including the Mexican child and adult populations [40,41]. It was also associated with hyperglycemia in women from southern Mexico with metabolic syndrome [42]. In the current study, rs9939609 showed mean differences in the percentage of body fat according to the recessive model and with a higher energy intake, without exceeding the Bonferroni adjustment.
Finally, although FTO is a possible genetic marker for obesity as we have discussed throughout the previous lines when comparing the results of various studies in different populations, further population studies are required to corroborate it as a genetic marker for obesity risk.
5. Conclusions
Some genetic variants of FTO showed a strong relationship with indicators of obesity in the studied population, opening the possibility for specific studies on a population previously diagnosed as obese to confirm the specific influence of the genetic variants identified in this study, since the results obtained were carried out in a young population of mestizo Mexicans without diagnosed diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes14010159/s1, Table S1: Genetic variants analyzed. Genotypes, reference and alternative alleles show their respective frequencies. HWE p-values are also included.
Author Contributions
A.C.-A.: both authors completed the total analysis of the data and participated in the draft writing; K.L.F.-V.: participated in obtaining the clinical data; J.L.C.-S. participated in revising the manuscript, J.A.C.-A. participated in data analysis, A.A.-G.: participated in obtaining the clinical data and writing the manuscript; W.G.-M.: participated in the genetic analysis of the samples and data curation; L.H.-T.: participated in the genetic analysis of the samples; M.d.L.A.-C.: participated in the genetic analysis of the samples; C.V.-S.: participated in the genotyping of the samples; M.A.A.-L.: participated in obtaining clinical data and revising the manuscript; T.G.-G.: participated in obtaining the data and samples and in data analysis; V.M.R.-G. and U.M.-C.: participated in obtaining the samples and data, data analysis, and direction of the entire work. All authors participated in the writing, discussion, and structuring of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially financed by funds provided by the Universidad Autónoma de Querétaro, and the genetic analysis of the samples was carried out by CÓDIGO 46 S.A. of C.V.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Facultad de Ciencias Naturales, Universidad Autónoma de Querétaro (protocol number 58FCN2020 and date of approval 1 October 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data is not publicly available as the overall project continues to analyze the results.
Acknowledgments
To the UAQ Health System (SUSALUD-UAQ), to the “Carlos Alcocer Cuarón” FCN-UAQ Nutrition Clinic, Human Nutrition Laboratory (FCN-UAQ), to the program “Summer of science in the central region, Mexico 2022”. To the academic group of Biomedical Research and Functional Foods (UAQ-CA-140) and CÓDIGO 46 S.A. de C.V., for their support with the technological facilities for the development of this project; and to Mark Arcuri for reviewing the manuscript language.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 July 2022).
- Elks, C.E.; Den Hoed, M.; Zhao, J.H.; Sharp, S.J.; Wareham, N.J.; Loos, R.J.F.; Ong, K.K. Variability in the heritability of body mass index: A systematic review and meta-regression. Front. Endocrinol. 2012, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Tavalire, H.F.; Budd, E.L.; Natsuaki, M.N.; Neiderhiser, J.M.; Reiss, D.; Shaw, D.S.; Ganiban, J.M.; Leve, L.D. Using a sibling-adoption design to parse genetic and environmental influences on children’s body mass index (BMI). PLoS ONE 2020, 15, e0236261. [Google Scholar] [CrossRef]
- Rohde, K.; Keller, M.; la Cour Poulsen, L.; Blüher, M.; Kovacs, P.; Böttcher, Y. Genetics and epigenetics in obesity. Metabolism 2019, 92, 37–50. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Cominato, L.; Finardi, G.; Biagio, D.; Lellis, D.; Franco, R.R.; Mancini, M.C.; Melo, M.E. De Obesity Prevention: Strategies and Challenges in Latin America. Curr. Obes. Rep. 2018, 7, 97–104. [Google Scholar] [CrossRef]
- Saldaña-Alvarez, Y.; Salas-Martínez, M.G.; García-Ortiz, H.; Luckie-Duque, A.; García-Cárdenas, G.; Vicenteño-Ayala, H.; Cordova, E.J.; Esparza-Aguilar, M.; Contreras-Cubas, C.; Carnevale, A.; et al. Gender-dependent association of FTO polymorphisms with body mass index in Mexicans. PLoS ONE 2016, 11, e0145984. [Google Scholar] [CrossRef]
- González-Herrera, L.; Zavala-Castro, J.; Ayala-Cáceres, C.; Pérez-Mendoza, G.; López-González, M.J.; Pinto-Escalante, D.; Canto-Cetina, T.; García-Escalante, M.G.; Rubi-Castellanos, R.; Contreras-Capetillo, S.; et al. Genetic variation of FTO: rs1421085 T>C, rs8057044 G>A, rs9939609 T>A, and copy number (CNV) in Mexican Mayan school-aged children with obesity/overweight and with normal weight. Am. J. Hum. Biol. 2019, 31, e23192. [Google Scholar] [CrossRef] [PubMed]
- Zermeño-Rivera, J.J.; Astocondor-Pérez, J.P.; Valle, Y.; Padilla-Gutiérrez, J.R.; Orozco-Castellanos, R.; Figuera, L.E.; Gutiérrez-Amavizca, B.E. Association of the FTO gene SNP rs17817449 with body fat distribution in Mexican women. Genet. Mol. Res. 2014, 13, 8561–8567. [Google Scholar] [CrossRef]
- Speakman, J.R. The “Fat Mass and Obesity Related” (FTO) gene: Mechanisms of Impact on Obesity and Energy Balance. Curr. Obes. Rep. 2015, 4, 73–91. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, Y.; Sun, B.F.; Zhao, Y.L.; Yang, Y.G. FTO and obesity: Mechanisms of association. Curr. Diab. Rep. 2014, 14, 486. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.B.; Arianti, R.; Shaw, A.; Vámos, A.; Veréb, Z.; Póliska, S.; Győry, F.; Bacso, Z.; Fésüs, L.; Kristóf, E. FTO Intronic SNP Strongly Influences Human Neck Adipocyte Browning Determined by Tissue and PPARγ Specific Regulation: A Transcriptome Analysis. Cells 2020, 9, 987. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.W.; Choi, S.M.; Kim, K.S.; Park, B.L.; Kim, J.R.; Kim, J.Y.; Shin, H.D. Replication of genetic effects of FTO polymorphisms on BMI in a Korean population. Obesity 2008, 16, 2187–2189. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, J.; Naka, I.; Kimura, R.; Natsuhara, K.; Yamauchi, T.; Furusawa, T.; Nakazawa, M.; Ataka, Y.; Patarapotikul, J.; Nuchnoi, P.; et al. FTO polymorphisms in oceanic populations. J. Hum. Genet. 2007, 52, 1031–1035. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Villalobos-Comparán, M.; Teresa Flores-Dorantes, M.; Teresa Villarreal-Molina, M.; Rodríguez-Cruz, M.; García-Ulloa, A.C.; Robles, L.; Huertas-Vázquez, A.; Saucedo-Villarreal, N.; López-Alarcón, M.; Sánchez-Muñoz, F.; et al. The FTO gene is associated with adulthood obesity in the Mexican population. Obesity 2008, 16, 2296–2301. [Google Scholar] [CrossRef]
- O’Neill, D. Measuring obesity in the absence of a gold standard. Econ. Hum. Biol. 2015, 17, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Abbasalizad Farhangi, M.; Nikniaz, L.; Nikniaz, Z. Higher dietary acid load potentially increases serum triglyceride and obesity prevalence in adults: An updated systematic review and meta-analysis. PLoS ONE 2019, 14, e0216547. [Google Scholar] [CrossRef]
- Fernández-Verdejo, R.; Marlatt, K.L.; Ravussin, E.; Galgani, J.E. Contribution of brown adipose tissue to human energy metabolism. Mol. Asp. Med. 2019, 68, 82–89. [Google Scholar] [CrossRef]
- Ilumina Inc. (Ed.) Infinium HTS Assay: Reference Guide; Illumina Way: San Diego, CA, USA, 2019; Volume 2, pp. 1–83. ISBN 1000000074604. [Google Scholar]
- Cadena-López, R.O.; Hernández-Rodríguez, L.V.; Aguilar-Galarza, A.; García-Muñoz, W.; Haddad-Talancón, L.; Anzures-Cortes, M.d.L.; Velázquez-Sánchez, C.; Flores-Viveros, K.L.; Anaya-Loyola, M.A.; García-Gasca, T.; et al. Association between SNPs in Leptin Pathway Genes and Anthropometric, Biochemical, and Dietary Markers Related to Obesity. Genes 2022, 13, 945. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; De Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research--an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; IBM: Armonk, NY, USA, 2015. [Google Scholar]
- Chang, J.Y.; Park, J.H.; Park, S.E.; Shon, J.; Park, Y.J. The Fat Mass- and Obesity-Associated (FTO) Gene to Obesity: Lessons from Mouse Models. Obesity 2018, 26, 1674–1686. [Google Scholar] [CrossRef] [PubMed]
- Chauhdary, Z.; Rehman, K.; Akash, M.S.H. The composite alliance of FTO locus with obesity-related genetic variants. Clin. Exp. Pharmacol. Physiol. 2021, 48, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Littleton, S.H.; Berkowitz, R.I.; Grant, S.F.A. Genetic Determinants of Childhood Obesity. Mol. Diagn. Ther. 2020, 24, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Doaei, S.; Mosavi Jarrahi, S.A.; Sanjari Moghadam, A.; Akbari, M.E.; Javadi Kooshesh, S.; Badeli, M.; Azizi Tabesh, G.; Abbas Torki, S.; Gholamalizadeh, M.; Zhu, Z.H.; et al. The effect of rs9930506 FTO gene polymorphism on obesity risk: A meta-analysis. Biomol. Concepts 2020, 10, 237–242. [Google Scholar] [CrossRef]
- Younus, L.A.; Algenabi, A.H.A.; Abdul-Zhara, M.S.; Hussein, M.K. FTO gene polymorphisms (rs9939609 and rs17817449) as predictors of Type 2 Diabetes Mellitus in obese Iraqi population. Gene 2017, 627, 79–84. [Google Scholar] [CrossRef]
- Hosseini-Esfahani, F.; Koochakpoor, G.; Mirmiran, P.; Daneshpour, M.S.; Azizi, F. Dietary patterns modify the association between fat mass and obesity-associated genetic variants and changes in obesity phenotypes. Br. J. Nutr. 2019, 121, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, Z.; Chen, J.; Ji, J.; Shen, J.; Xu, Y.; Zhao, Y.; Liu, D.; Shen, Y.; Zhang, W.; et al. Association of fat mass and obesity-associated and retinitis pigmentosa guanosine triphosphatase (GTpase) regulator-interacting protein-1 like polymorphisms with body mass index in Chinese women. Endocr. J. 2018, 65, 783–791. [Google Scholar] [CrossRef]
- Inandiklioğlu, N.; Yaşar, A. Association between rs1421085 and rs9939609 Polymorphisms of Fat Mass and Obesity-Associated Gene with High-Density Lipoprotein Cholesterol and Triglyceride in Obese Turkish Children and Adolescents. J. Pediatr. Genet. 2021, 10, 009–015. [Google Scholar] [CrossRef]
- Sedaghati-khayat, B.; Barzin, M.; Akbarzadeh, M.; Guity, K.; Fallah, M.S.; Pourhassan, H.; Azizi, F.; Daneshpour, M.S. Lack of association between FTO gene variations and metabolic healthy obese (MHO) phenotype: Tehran Cardio-metabolic Genetic Study (TCGS). Eat. Weight Disord. 2020, 25, 25–35. [Google Scholar] [CrossRef]
- Liaw, Y.C.; Liaw, Y.P.; Lan, T.H. Physical activity might reduce the adverse impacts of the FTO gene variant rs3751812 on the body mass index of adults in Taiwan. Genes 2019, 10, 354. [Google Scholar] [CrossRef]
- Goutzelas, Y.; Kotsa, K.; Vasilopoulos, Y.; Tsekmekidou, X.; Stamatis, C.; Yovos, J.G.; Sarafidou, T.; Mamuris, Z. Association analysis of FTO gene polymorphisms with obesity in Greek adults. Gene 2017, 613, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, P.; Adamska-Patruno, E.; Bauer, W.; Krasowska, U.; Fiedorczuk, J.; Moroz, M.; Gorska, M.; Kretowski, A. Dietary fiber intake may influence the impact of FTO genetic variants on obesity parameters and lipid profile—A cohort study of a caucasian population of Polish origin. Antioxidants 2021, 10, 1793. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Narváez, E.A.; Haddad, S.A.; Rosenberg, L.; Palmer, J.R. Birth weight modifies the association between central nervous system gene variation and adult body mass index. J. Hum. Genet. 2016, 61, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Monda, K.L.; Chen, G.K.; Taylor, K.C.; Palmer, C.; Edwards, T.L.; Lange, L.A.; Ng, M.C.Y.; Adeyemo, A.A.; Allison, M.A.; Bielak, L.F.; et al. A meta-analysis identifies new loci associated with body mass index in individuals of African ancestry. Nat. Genet. 2013, 45, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.J.; Zhu, H.; He, H.; Wu, K.H.; Li, J.; Chen, X.D.; Zhang, J.G.; Shen, H.; Tian, Q.; Krousel-Wood, M.; et al. Replication of 6 obesity genes in a meta-analysis of genome-wide association studies from diverse ancestries. PLoS ONE 2014, 9, e96149. [Google Scholar] [CrossRef]
- Paternoster, L.; Evans, D.M.; Aagaard Nohr, E.; Holst, C.; Gaborieau, V.; Brennan, P.; Prior Gjesing, A.; Grarup, N.; Witte, D.R.; Jørgensen, T.; et al. Genome-wide population-based association study of extremely overweight young adults-the GOYA study. PLoS ONE 2011, 6, e24303. [Google Scholar] [CrossRef]
- López-Rodríguez, G.; Estrada-Neria, A.; Suárez-Diéguez, T.; Tejero, M.E.; Fernández, J.C.; Galván, M. Common polymorphisms in MC4R and FTO genes are associated with BMI and metabolic indicators in Mexican children: Differences by sex and genetic ancestry. Gene 2020, 754, 144840. [Google Scholar] [CrossRef]
- León-Mimila, P.; Villamil-Ramírez, H.; Villalobos-Comparán, M.; Villarreal-Molina, T.; Romero-Hidalgo, S.; López-Contreras, B.; Gutiérrez-Vidal, R.; Vega-Badillo, J.; Jacobo-Albavera, L.; Posadas-Romeros, C.; et al. Contribution of Common Genetic Variants to Obesity and Obesity-Related Traits in Mexican Children and Adults. PLoS ONE 2013, 8, e70640. [Google Scholar] [CrossRef]
- Ortega, P.E.N.; Meneses, M.E.; Delgado-Enciso, I.; Irecta-Nájera, C.A.; Castro-Quezada, I.; Solís-Hernández, R.; Flores-Guillén, E.; García-Miranda, R.; Valladares-Salgado, A.; Locia-Morales, D.; et al. Association of rs9939609-FTO with metabolic syndrome components among women from Mayan communities of Chiapas, Mexico. J. Physiol. Anthropol. 2021, 40, 11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).