Abstract
The Kokshaal racerunner, Eremias kokshaaliensis Eremchenko et Panfilov, 1999, together with other central Asian racerunner species, is included in the Eremias multiocellata complex. In the present work, for the first time, the results of the analysis of historical mitochondrial DNA (barcode) are presented and the taxonomic status and preliminary phylogenetic relationships within the complex are specified. We present, for the first time, the results of the molecular analysis using historical DNA recovered from specimens of several species of this complex (paratypes of the Kokshaal racerunner and historical collections of the Kashgar racerunner E. buechneri from Kashgaria) using DNA barcoding.
1. Introduction
Modern phylogenetic methods allow a look into the past with the extraction of DNA from specimens collected a century and a half ago. Collections at the Zoological Institute in St. Petersburg and Moscow State University have herpetological samples stored in ethanol that have never been exposed to formalin that go back to the 1880s. This study accesses this unique resource to answer taxonomic questions in the lizard genus Eremias. Currently, the genus Eremias includes 40 species [1] of predominantly arid sand, steppe, and desert lizards distributed from Northern China, Korea, central and southwestern Asia to southeastern Europe [2,3,4]. The central Asian racerunners of the Eremias multiocellata—E. przewalskii complex belong to the taxonomically most complicated group of the genus Eremias [5,6,7,8], which includes 8–9 central Asian species: Tien Shan racerunner E. stummeri Wettstein, 1940 st. nov. Eremchenko et Panfilov, 1999; Szczerbak’s racerunner E. szczerbaki Eremchenko et Panfilov, 1999 status nov.; Yarkand racerunner E. yarkandensis Blanford, 1875; Kokshaal racerunner E. kokshaaliensis Eremchenko et Panfilov, 1999; Kashgar racerunner E. buechneri Bedriaga, 1907; Dzhungar racerunner E. dzhungarica Orlova et al., 2017; multi-ocellated racerunner E. multiocellata Günther, 1872; Przewalski’s racerunner E. przewalskii (Strauch, 1876); E. m. var. reticulata Bedriaga, 1912 (status questionable). The genetic variability and phylogenetic relationships between E. multiocellata, E. przewalskii and allied taxa remain insufficiently studied. Further progress in this area is hampered by complicated taxonomy and a lack of verified identifications and comparisons with type material. Until recently, the multi-ocellated racerunner, Eremias multiocellata, was considered as a single species with several subspecies, only one of which, E. m. yarkandensis, was recognized before 1992 in the territory of the former USSR [2,5]. Long before that, an interesting population of racerunners was reported from the Sary-Dzhaz syrts of Kyrgyzstan [9], which was later assigned to the Kashgar racerunner E. buechneri [10]. This population, however, differs markedly from the Kashgar racerunner both in habitus and in other characteristics of external morphology [11]. As a result of many years of studying the herpetofauna of Kyrgyzstan, by applying integrative analysis to taxonomically complex groups or using integrative analysis to identify taxonomically complex groups, it was shown that racerunners from the E. multiocellata complex are represented by several distinct subspecies [12].
Later on, E. stummeri Wettstein, 1940 inhabiting the Chu-Issyk-Kul Lake basin, E. szczerbaki Eremchenko et Panfilov, 1992 from the inner Tien Shan and the Naryn River basin, and E. yarkandensis Blanford, 1875 from the Tarim basin in China were recognized as distinct species [13]. The Yarkand racerunner from the Tarim basin in China has penetrated to the territory of Kyrgyzstan (eastern Alai). E. kokshaaliensis Eremchenko et Panfilov, 1999 is distributed in the extreme northeast of Kyrgyzstan (Sary-Jaz and Pokrovsky Syrts) and can be easily distinguished from all previous forms by a slender habitus, a miniature head and a speckled pattern of the dorsal side of the body. The validity of these taxa was confirmed by the authors from the results of laboratory hybridization and some craniological characteristics: the presence and size of pterygoid teeth, the texture of septomaxillae and the shape of the premaxillary process [13]. All species of this complex are allopatric. The species status of the Kyrgyz and central Asian racerunners was recently confirmed by the results of molecular analysis [14]. Unfortunately, no Kokshaal racerunner specimens from Sary-Jazz were used in the analysis carried out in the cited paper. However, the data on Eremias sp. from the southern slopes of the central Tien Shan (China: Xinjiang, Aksu District) were analyzed and discussed.
Museum collections, especially type specimens, represent a very important source for taxonomic studies, even in the field of molecular phylogenetics. DNA degrade in historical specimens, but modern methods and special facilities allow us to obtain data from such difficult material, including successful attempts to extract DNA from formalin-fixed museum specimens [15,16,17,18]. As the historical DNA, we consider DNA isolated from old museum samples (or historical samples) following the understanding of this term in the zoological literature [19,20,21,22]. The Laboratory of Historical DNA at the Zoological Museum of the Lomonosov Moscow State University focuses on the genotyping of museum collections, especially type specimens and the specimens from the type localities, which is of crucial importance in clarifying challenging taxonomic and nomenclature issues. Previously, we successfully obtained DNA sequences for historical reptile samples: Phrynocephalus rossicowi (Reptilia), Dipus saggita (Mammalia), Blarinella griselda (Mammalia), Tonkinodentus lestes (Chilopoda), etc. [23,24,25,26]. This paper attempts to use the possibilities of studying historical DNA for the clarification of unsolved problems in this taxonomically challenging complex.
The aim of the present paper is to analyze the taxonomic status and distribution of species of the E. multiocellata complex based on the molecular identification of museum collections: type specimens of E. kokshaaliensis and historical specimens of E. buechneri, as well as other collections of these Eremias species from China and Kyrgyzstan, including those previously used in molecular analysis [14]. We also study the uniformity of the type series, which consists of specimens collected from different localities.
2. Materials and Methods
The materials for this study were the following museum specimens in the herpetological collections of Zoological museum of Lomonosov Moscow State University, Moscow, Russia (ZMMU), Zoological Institute of Russian Academy of Science, St. Petersburg, Russia (ZISP) and Institute of Biology and Pedologyof the National Academy of Sciences, Bishkek, Kyrgyzstan (BSI):
E. kokshaaliensis: 4 topotypes: ZMMUR-3302 (3 males and 1 female): Kyrgyzstan, central Tien Shan, Sary-Dhaz River at the confluence with the Inylchek River, 05.08.1967, leg. S.L. Pereshkolnik.
E. kokshaaliensis: 15 paratypes: BSI R-000587–000591, 000596, 000599, 000601 (8 adults: 6 females, 2 males): Kyrgyzstan, central Tien Shan, Sary-Dhaz, vicinity of Engylchek (Enylchek), 08.1986, leg. V.K. Eremchenko.
E. kokshaaliensis: 5 paratypes, ZISP R-8277, males: China, Xinjiang, Kara-Teke, 1889. leg. M.V. Pevzov.
E. kokshaaliensis: paratype ZISP R-8289, female: China, Xinjiang, Taushkan –Darya, 1891, leg. M.V. Pevzov.
E. kokshaaliensis: paratype ZISP 8292, female: China, Xinjiang, southern Tien Shan, 1891, leg. M.V. Pevzov
E. kokshaaliensis: ZMMU R-14327, female: China, Xinjiang Uygur autonomous region, Aksu district, 35 km northeast of Aksu city, 1110 m a.s.l., spurs of Talyktau mountain ridge, 23.08.2014, leg. E.A. Dunayev.
E. kokshaaliensis: ZMMU R-14328 (1 male, 1 female, 1 juv.): China, Xinjiang Uygur autonomous region, Aksu district, 89 km northeast of Aksu city, 1364 m a.s.l., east spurs of Pobeda Peak Mountain, 24.08.2014, leg. E.A. Dunayev.
E. kokshaaliensis: ZMMU R-14329 (1 subadult male), 60 km northeast of Aksu city, 1435 m a.s.l., east spurs of Pobeda Peak Mountain, 24.08.2014, leg. E.A. Dunayev.
E. kokshaaliensis: ZMMU R-14330 (1 adult male, 1 subadult male and 2 juveniles: China, Xinjiang Uygur autonomous region, 75 km northeast of Aksu city 1927 (1922–2040) m a.s.l., 25–26.08.2014, leg. E.A. Dunayev.
E. buechneri: ZISP R-9131, 2 females: China, near Tolan-Khodjha River in Kashgaria, 1890, leg. V.I. Roborovsky.
In the molecular genetic analysis, seven specimens of lizards of this complex (OK624437-OK624443) were used (Table 1; Figure 1).

Table 1.
Samples used in molecular analyses.
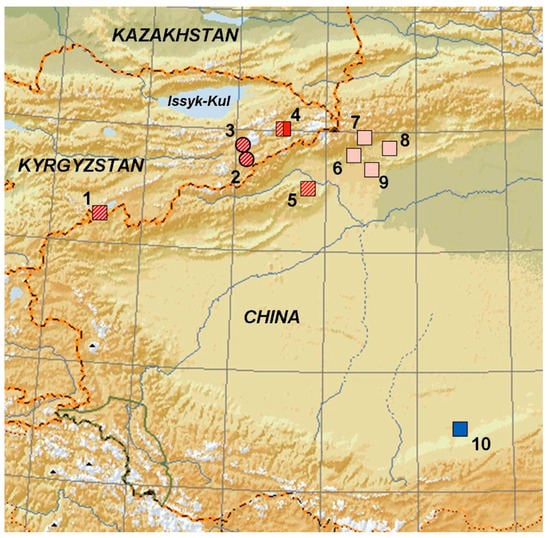
Figure 1.
Sites of examined specimen of Eremias kokshaaliensis (red) and E. buechneri (blue). Kyrgyzstan: 1—border with China, Xinjiang, Kara-Teke (ZISP 8277); 2—Bedel valley (ZISP 8291); 3—southern Tien Shan (ZISP 8292); 4—Sary-Dhaz, vicinity of Engylchek (Enylchek (BSI R000587–591, 599–601 and ZMMU R-3302; China: 5—Taushkan–Darya (ZISP 8289); 6–60 km northeast of Aksu city, east spurs of Pobeda Peak Mountain (ZMMU 14329); 7—75 km northeast of Aksu city (ZMMU 14330); 8—9 km northeast of Aksu city, east spurs of Pobeda Peak Mountain (ZMMU 14328); 9—5 km northeast of Aksu city, spurs of Talyktau mountain (ZMMU 14327); 10—near Tolan-Khodjha River in Kashgaria (ZISP 9131). Numbers in square correspond to location of the specimens used both in morphological and molecular genetic analysis: solid square; this study: dashed square [14]. Red square corresponds to specimens used in this study; pink square ([27], Figure 1 and Appendix II). Numbers in circle correspond to location of the specimens used in morphological analysis only.
MtDNA sequencing and analyses. Tissue samples of Eremias sp. were taken from seven specimens preserved in ethanol including five specimens of type series from collections of ZISP and BSI.
Molecular analysis (DNA extraction and PCR) was carried out in the ZMMU Laboratory of Historical DNA, equipped exclusively for the work with museum DNA specimens, where no previous work on fresh tissues had been performed. DNA was extracted and purified using the Qiagen QIAamp DNA MiniKit (Qiagen, Hilden, Germany) with some changes: overnight lysis step at 56 °C and two steps of elution with AE buffer, each with 50 μL of buffer and 5 min of incubation at room temperature. We amplified a fragment of the COI (cytochrome oxidase I subunit) gene of mitochondrial DNA. DNA was highly degraded, so only short fragments (100–200 bp) were obtained using a combination of internal primers designed for this study. Five primer pairs for five overlapping fragments were manually developed using Bioedit [28,29] and an alignment of Eremias multiocellata-complex sequences from GenBank (Table 2).

Table 2.
Samples used in molecular analyses.
Amplification was performed in 22 μL reaction volumes containing 2 μL DNA, 4 μL of Evrogen HS-Screen mix, and 1 μL of each primer (10 pmol/μL). All stages of the extraction process included a blank as a negative control run in parallel. PCR products were visualized using a 1% agarose gel. PCR products were sequenced at the Evrogen laboratory using an ABI PRISM 3500xl sequencer with BigDye Terminator Chemistry v. 3.1 (Applied Biosystems, Foster City, CA, USA) using PCR primers.
Short fragments were first aligned using Seqman v5.06 software [27], assembled into longer ones and then aligned using BioEdit Sequence Alignment Editor 7.1.3.0 [30] with default parameters. Subsequently, the alignment including all complete sequences of studied samples was reviewed and manually revised if necessary. Obtained consensus sequences of Eremias ssp. were deposited in GenBank under the following accession numbers: OK624437—OK624443. Other sequences of Eremias were downloaded from GenBank [14,27] (Table 1). Two sequences of E. multiocellata (KY366658 and KY366636) and Darevskia rudis (MN613693) were included as outgroups. The final alignment used for the phylogenetic analyses comprised 676 bp for 52 ingroup sequences of Eremias spp. Mean inter- and intraspecific uncorrected genetic p-distances and sequence characteristics were calculated using MEGA 6.1.
Phylogenetic trees were reconstructed by Bayesian inference criteria (BI), by maximum-likelihood (ML) and maximum-parsimony (MP) methods. The optimal partitioning schemes for Bayesian inference analysis were identified using PartitionFinder software [31] using greedy search algorithm under BIC criterion: HKY + I+G for all positions together. Bayesian inference (BI) was performed using Mr Bayes v3.1.2 software [32] with two simultaneous runs, each with four chains, for 5 million generations. We checked the convergence of the runs and that the effective sample sizes (ESSs) were all above 200 by exploring the likelihood plots using TRACER v1.5 software [33]. The initial 10% of trees were discarded as burn-in. Confidence in tree topology was assessed by posterior probability (PP) [34]. The ML trees were generated using IQ tree software [35] using ultrafast bootstrap = 10,000 [36], the model was selected using ModelFinder software [37]: TPM2u + F+G4. The unweighted MP analysis was conducted in PAUP 4.0b10 software [38] with 1000 bootstrap replications. Additionally, we calculated decay indices for parsimonious tree using PRAP2 [39] under non-ratchet parameters.
3. Results
3.1. Sequence Characteristics
We obtained from 329 to 672 bp of each historical specimen of Eremias. Of the 676 aligned sites, 540 were found to be conserved, 130 variable and 110 parsimony-informative; the transition–transversion bias was estimated at 4.11 (all data given for ingroups only). The nucleotide frequencies on the light strand were 24.5% (A), 28.5% (T/U), 29.2% (C), and 17.8% (G). Uncorrected p-distances are provided in Table 3. Between-group distances vary from 1.46% to 9.57%, and within-group distances vary from 0 to 1.41%. A histogram showing the distribution of pairwise genetic p-distances demonstrates two gaps: at 3.0 and 6–7%, respectively (Figure 1).

Table 3.
Uncorrected p-distances (%) for sequences of mtDNA COI gene for groups (below the diagonal). Values on the diagonal correspond to average uncorrected ingroup p-distances. Standard-error estimates are shown above the diagonal.
3.2. Molecular Phylogeny
The extensive type series (35 specimens) of Eremias kokschaaliensis, selected by the authors of the description [10], includes specimens collected at different times in China and Kyrgyzstan. It includes eight historical specimens collected by M.V. Pevtsov at the end of the 19th century from Xinjiang, China (ZISP 8277, ZISP 8289, and ZISP 8291-92). In addition, it includes more recently collected specimens from Kyrgyzstan: holotype BSI R 000580 and paratypes BSI (R 000581–R000586, R 002855, Sary-Dzhaz, Terekty Gorge, 07/11/1986; R 000587–R 000591, R 000599–R 000601, Sary-Dzhaz, near the village of Engilchek (= Enilchek), 07/09/1986; R 000592–R 000595, Sary-Dzhaz, near the village of Uch-Kel, 07.09.1986; R 002005–002009, near the Sary-Dzhaz River, 08.1986; R 002834, near the Sary-Dzhaz River, 16.09.1988). We succeeded in identifying seven historical specimens, including five type specimens (BSI R000589; ZISP 8277:1, 2, 4; ZISP 8289) and one topotype from the type locality (ZMMU R-3302), as well as one specimen of E. buechneri (ZISP No. 9131) from Kashgaria, China. The resulting phylogeny (Figure 2) mainly agrees with the one from [14]. All studied samples of Eremias, excluding outgroups, form a clade (PP/BS/MP = 1/64/56) and fall into four main groups: E. szczerbaki (PP/BS/MP = 1/99/100), E. stummeri (PP/BS/MP = 1/99/100), E. buechneri, and part of E. kokshaaliensis group A (PP/BS/MP = 1/99/93). The rest of the sequences form another group with low support (PP/BS/MP = 0.7/-/53), which consists of E. yarkandensis, E. dzhungarica, E. cf. buechneri and the rest of E. kokshaaliensis (Group B).
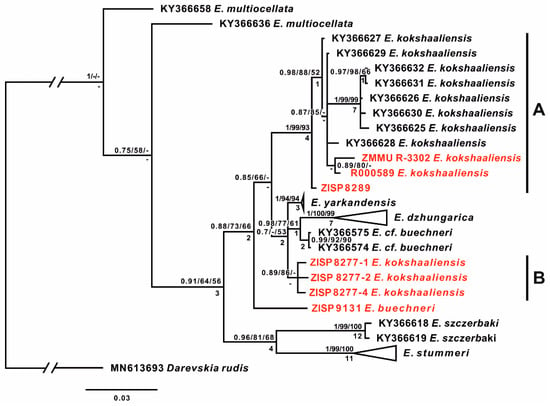
Figure 2.
The tree resulted from the Bayesian inference analysis of the studied samples of Eremias multiocellata complex. Values at nodes indicate posterior probabilities/bootstrap support for BI/ML analyses, respectively. The “-” sign shows that the node exists on the Bayesian tree, but the topology is different in the specified analysis. Numbers below the nodes represent decay indices for parsimony tree. Red labels highlight sequences from historical specimens.
4. Discussion
Our results showed that the studied samples of Eremias kokschaaliensis are divided into two groups. One group of E. kokschaaliensis A (paratype BSI no. R-000589, paratype no. ZISP R-8289, and one specimen no. R-3302 ZMMU from type territory) form a clade with Eremias specimens from Aksu (China) with high support (PP/BS/MP = 1/0.99/93); E. kokshaaliensis specimen no. R-8289 ZISP has a basal position within this group (PP/BS/MP = 0.98/0.88/52). The uncorrected within-group p-distance within this clade is 1.41%.
Three specimens of the type series E. kokshaaliensis, which are paratypes of E. kokschaaliensis (nos. ZISP-8277-1, ZISP 8277-2, ZISP 8277-3), form the second group: E. kokschaaliensis B (PP/BS/MP = 0.89/87/-). The resolution of their position is too low, and it would be impossible to assign them to any form until more molecular data have been obtained and studied.
E. kokshaaliensis from the type series of group A is easily recognizable by the characteristic of the mottled pattern of the dorsal surface of the body of ethanol-preserved specimens (Figure 3 and Figure 4). As for the type specimens belonging to group B, they differ in a darker dorsolateral stripe. There may be slight differences in the number of scales around the midbody and number of subdigital lamellae on the fourth toe of the hindlimb, which is higher in E. kokshaaliensis from the type series of group A. It should be noted that these preliminary conclusions are not based on statistically reliable data, due to the high variability of the pholidosis characteristics and a limited number of specimens in the sample of E. kokshaaliensis group B. New material from the type locality of the E. kokshaaliensis group B is required to study the morphological variability and differences of these cryptic forms in more detail.

Figure 3.
Paratypes of Eremias kokschaaliensis A (BSI R000587–591, 599–601) in preservative in dorsal view.

Figure 4.
Paratypes of Eremias kokschaaliensis B (ZISP 8277) in preservative in dorsal view.
The E. kokshaaliensis group A from the Sary-Dhaz River basin occurs mainly in rocky habitats with desert vegetation, along the rocky banks of rivers and slopes of the southern, southeastern and southwestern expositions. In the area of the village of Enylchek (2500 m above sea level), it occupies sandy terraces near the river and rough-stony lower slopes of the mountains. Here they are quite common, and in one hour of the tour, there can be from 18 to 30 individuals [13]. There is no available information about the habitat of E. kokshaaliensis group B from Kara-Teke.
The historical specimen of E. buechneri no. R-9131 ZISP (Figure 5) represents a separate lineage and derives from E. sczherbaki and E. stummeri, while Eremias cf. buechneri (as mentioned in [1] groups with E. dzhungarica as a sister lineage (PP/BS/MP = 0.98/77/61).

Figure 5.
Historical specimens of E. buechneri (ZISP 9131) in preservative in dorsal view.
It is currently not possible to discuss the phylogenetic relationships of E. kokshaaliensis and E. buechneri without newly collected specimens from Kashgaria and the results of DNA analysis.
Our DNA analysis confirmed the species designation of E. kokschaaliensis Eremchenko et Panfilov, 1999 and E. buechneri Bedriaga, 1907, which were previously described only on the basis of external morphology and partially on osteological and other characteristics. An important result is the revealing of the heterogeneity of the E. kokshaaliensis type series. The two phylogenetic groupings are geographically separated by about 300 km (Figure 1). The type of territory of the genotyped of E. kokshaaliensis group A coincides with the type of territory of the species, since the holotype: IBC BR 000580 originates from the same locality as the paratype BSI R000589: Kyrgyzstan, Settlement Sary-Dhaz. Thus, the phylogenetic line A corresponds to the species name given in the description, and the taxonomic status of the phylogenetic line B needs to be confirmed by new materials from the Kara Teke.
5. Conclusions
An important result of this study is our success in achieving the possibility of the molecular identification of historical specimens that were collected in 19th century (1880, 1881 and 1891). This novel DNA sequencing of historical specimens (ZISP) of E. kokschaaliensis reveals the heterogeneity of the type series and sheds light on the possibility of cryptic species within this group.
Author Contributions
All authors contributed to the manuscript. Conceptualization, V.F.O., E.N.S., E.A.D., N.B.A.; methodology, V.F.O., E.N.S., E.A.D., N.B.A.; software, V.F.O., E.N.S., E.A.D., N.B.A.; validation, V.F.O., E.N.S., E.A.D., N.B.A.; formal analysis, V.F.O., E.N.S., E.A.D., Ananjeva, N.B., investigation, V.F.O., E.N.S., E.A.D., N.B.A.; resources, V.F.O., E.N.S., E.A.D., N.B.A.; data curation, V.F.O., E.N.S., E.A.D., N.B.A.; writing—original draft preparation, V.F.O., E.N.S., E.A.D., N.B.A.; writing—review and editing, V.F.O., E.N.S., E.A.D., N.B.A.; visualization, V.F.O., E.N.S., E.A.D., N.B.A.; supervision, V.F.O., N.B.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was fulfilled in the frames of the State Themes of ZMMU 121032300105-0 and ZISP 122031100282-2 with support of Russian Science Foundation grant no. 22-14-00037 and Ministry of Science and Higher Education of the Russian Federation grant no. 075-15-2021-1069.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Zoological Institute, Russian Academy of Sciences (protocol #1-18/06-05-2022, 6 May 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Many thanks to the staff of Institute of Biology and Pedology of the National Academy of Sciences of Kyrgyzstan for the possibility to examine specimens deposited in the collections of their institution. We are sincerely grateful to anonymous reviewers for their kind help and useful comments, which helped us to improve the previous version of the manuscript, D.A. Gordeev, I.V.Doronin, D.A.Melnikov and E.Yu. Shepelya for their assistance in the manuscript preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uetz, P.; Freed, P.; Aguilar, R.; Hošek, J. The Reptile Database. Available online: https://reptile-database.reptarium.cz/ (accessed on 17 April 2022).
- Szczerbak, N.N. The Palearctic Desert Lizards (Eremias); Naukova Dumka: Kiev, Ukraine, 1974. (In Russian) [Google Scholar]
- Mayer, W.; Pavlicev, M. The Phylogeny of the Family Lacertidae (Reptilia) Based on Nuclear DNA Sequences: Convergent Adaptations to Arid Habitats within the Subfamily Eremiainae. Mol. Phylogenet. Evol. 2007, 44, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Sindaco, R.; Eremchenko, V.K. The Reptiles of the Western Palearctic. 1. Annotated Checklist and Distributional Atlas of the Turtles, Crocodiles, Amphisbaenians and Lizards of Europe, North Africa, Middle East and Central Asia. In Monografie della Societas Herpetologica Italica; Edizioni Belvedere: Latina, Italy, 2008. [Google Scholar]
- Bannikov, A.G.; Darevsky, I.S.; Ishchenko, V.G.; Rustamov, A.K.; Szczerbak, N.N. Guide to Amphibians and Reptiles of the USSR Fauna; Prosvetscheniye: Moscow, Russia, 1977. (In Russian) [Google Scholar]
- Guo, X.; Dai, X.; Chen, D.; Papenfuss, T.J.; Ananjeva, N.B.; Melnikov, D.A.; Wang, Y. Phylogeny and Divergence Times of Some Racerunner Lizards (Lacertidae: Eremias) Inferred from Mitochondrial 16S RRNA Gene Segments. Mol. Phylogenet. Evol. 2011, 61, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dujsebayeva, T.; Chirikova, M.; Song, Q.; Gong, X.; Guo, X. The Stummer’s Racerunner (Eremias stummeri Wettstein, 1940) Does Occur in Northwest China. Amphib. Reptil. 2021, 42, 93–105. [Google Scholar] [CrossRef]
- Liu, J.-L.; Dujsebayeva, T.N.; Chirikova, M.A.; Gong, X.; Li, D.-J.; Guo, X.-G. Does the Dzungarian Racerunner (Eremias dzungarica Orlova, Poyarkov, Chirikova, Nazarov, Munkhbaatar, Munkhbayar & Terbish, 2017) Occur in China? Species Delimitation and Identification with DNA Barcoding and Morphometric Analyses. Zool. Res. 2021, 42, 287–293. [Google Scholar] [CrossRef]
- Yakovleva, I.D. Reptiles of Kirgyzia; Academy of Sciences of Kirgyzia SSR: Frunze, Kyrgyzstan, 1964. (In Russian) [Google Scholar]
- Eremchenko, V.K.; Shukurov, E.D.; Szczerbak, N.N. A new species for the USSR fauna—Kashgar racerunner (Eremias buechneri, Sauria Reptilia). Vestn. Zool. 1985, 4, 79–80. (In Russian) [Google Scholar]
- Orlova, V.F.; Terbish, K. Materials on herpetofauna of Dzungarian Gobi. In Proceedings of the Gerpetologicheskiye Issledovaniya v; Mongolskoy Narodnoy Respublike: Moscow, Russia, 1986; pp. 91–108. (In Russian) [Google Scholar]
- Eremchenko, V.K.; Panfilov, A.M.; Tsarinenko, E.I. Synopsis of Research in Cytogenetic and Taxonomy of Some Asian Species of Scincidae and Lacertidae; Ilym: Bishkek, Kyrgyzstan, 1992. (In Russian) [Google Scholar]
- Eremchenko, V.K.; Panfilov, A.M. Taxonomic situation of multiocellated racerunner of the “multiocellata” complex of Kyrgyzstan and neighbor China (Sauria: Lacertidae: Eremias). Nauka I Novye Tekhnologii 1999, 4, 112–124. (In Russian) [Google Scholar]
- Orlova, V.F.; Poyarkov, N.A.; Chirikova, M.A.; Nazarov, R.A.; Munkhbaatar, M.; Munkhbayar, K.; Terbish, K. MtDNA Differentiation and Taxonomy of Central Asian Racerunners of Eremias multiocellata–E. przewalskii species complex (Squamata, Lacertidae). Zootaxa 2017, 1, 1–42. [Google Scholar] [CrossRef] [Green Version]
- Parham, J.F.; Stuart, B.L.; Danilov, I.G.; Ananjeva, N.B. A Genetic Characterization of CITES-Listed Iranian Tortoises (Testudo graeca) through the Sequencing of Topotypic Samples and a 19th Century Holotype. Herpetol. J. 2012, 22, 73–78. [Google Scholar]
- Hykin, S.M.; Bi, K.; McGuire, J.A. Fixing Formalin: A Method to Recover Genomic-Scale DNA Sequence Data from Formalin-Fixed Museum Specimens Using High-Throughput Sequencing. PLoS ONE 2015, 10, e0141579. [Google Scholar] [CrossRef] [Green Version]
- Mamani, L.; Cruz, R.; Mallqui, S.; Catenazzi, A. Molecular Phylogenetics and Comparative Examination of Voucher Museums Reveal Two New Species of Gymnophthalmid Lizards (Squamata, Gymnophthalmidae) from the Peruvian Andes, with Comments on Proctoporus guentheri (Boettger, 1891). Diversity 2022, 14, 215. [Google Scholar] [CrossRef]
- Mulcahy, D.G.; Ibáñez, R.; Jaramillo, C.A.; Crawford, A.J.; Ray, J.M.; Gotte, S.W.; Jacobs, J.F.; Wynn, A.H.; Gonzalez-Porter, G.P.; McDiarmid, R.W.; et al. DNA Barcoding of the National Museum of Natural History Reptile Tissue Holdings Raises Concerns about the Use of Natural History Collections and the Responsibilities of Scientists in the Molecular Age. PLoS ONE 2022, 17, e0264930. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, V.S.; Kovalskaya, Y.; Solovyeva, E.N.; Zemlemerova, E.D.; Bannikova, A.A.; Rusin, M.Y.; Matrosova, V.A. Molecular Systematics of the Sicista tianschanica Species Complex: A Contribution from Historical DNA Analysis. PeerJ 2021, 9, e10759. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, E.F., Jr.; Pavan, S.E.; Tsuchiya, M.T.N.; Wilson, D.E.; Percequillo, A.R.; Maldonado, J.E. Museomics of Tree Squirrels: A Dense Taxon Sampling of Mitogenomes Reveals Hidden Diversity, Phenotypic Convergence, and the Need of a Taxonomic Overhaul. BMC Evol. Biol. 2020, 20, 77. [Google Scholar] [CrossRef] [PubMed]
- Rancilhac, L.; Bruy, T.; Scherz, M.D.; Pereira, E.A.; Preick, M.; Straube, N.; Lyra, M.L.; Ohler, A.; Streicher, J.W.; Andreone, F.; et al. Target-Enriched DNA Sequencing from Historical Type Material Enables a Partial Revision of the Madagascar Giant Stream Frogs (Genus Mantidactylus). J. Nat. Hist. 2020, 54, 87–118. [Google Scholar] [CrossRef]
- Reyes-Velasco, J.; Goutte, S.; Freilich, X.; Boissinot, S. Mitogenomics of Historical Type Specimens Clarifies the Taxonomy of Ethiopian Ptychadena Boulenger, 1917 (Anura, Ptychadenidae). ZooKeys 2021, 1070, 135–149. [Google Scholar] [CrossRef]
- Solovyeva, E.N.; Lebedev, V.S.; Dunayev, E.A.; Nazarov, R.A.; Bannikova, A.A.; Che, J.; Murphy, R.W.; Poyarkov, N.A. Cenozoic Aridization in Central Eurasia Shaped Diversification of Toad-Headed Agamas (Phrynocephalus; Agamidae, Reptilia). PeerJ 2018, 6, e4543. [Google Scholar] [CrossRef] [Green Version]
- Lebedev, V.S.; Bannikova, A.A.; Lu, L.; Snytnikov, E.A.; Adiya, Y.; Solovyeva, E.N.; Abramov, A.V.; Surov, A.V.; Shenbrot, G.I. Phylogeographical Study Reveals High Genetic Diversity in a Widespread Desert Rodent, Dipus Sagitta (Dipodidae: Rodentia). Biol. J. Linn. Soc. 2018, 123, 445–462. [Google Scholar] [CrossRef]
- Bannikova, A.A.; Jenkins, P.D.; Solovyeva, E.N.; Pavlova, S.V.; Demidova, T.B.; Simanovsky, S.A.; Sheftel, B.I.; Lebedev, V.S.; Fang, Y.; Dalen, L.; et al. Who Are You, Griselda? A Replacement Name for a New Genus of the Asiatic Short-Tailed Shrews (Mammalia, Eulipotyphla, Soricidae): Molecular and Morphological Analyses with the Discussion of Tribal Affinities. ZooKeys 2019, 888, 133–158. [Google Scholar] [CrossRef]
- Schileyko, A.A.; Solovyeva, E.N. On the Taxonomic Position of the Enigmatic Genus Tonkinodentus Schileyko, 1992 (Chilopoda, Scolopendromorpha): The First Molecular Data. ZooKeys 2019, 840, 133–155. [Google Scholar] [CrossRef] [PubMed]
- GenBank. Available online: https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 13 May 2022).
- Hall, T. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Burland, T.G. DNASTAR’s Lasergene Sequence Analysis Software. Methods Mol. Biol. 2000, 132, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. Partitionfinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian Phylogenetic Inference under Mixed Models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [Green Version]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian Inference of Phylogenetic Trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.; Wong, T.K.F. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [Green Version]
- Swofford, D. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods); Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Müller, K. PRAP–Computation of Bremer Support for Large Data Sets. Mol. Phylogenet. Evol. 2004, 31, 780–782. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).