Abstract
Pilumnopeus makianus is a crab that belongs to Pilumnidae, Brachyura. Although many recent studies have focused on the phylogeny of Brachyura, the internal relationships in this clade are far from settled. In this study, the complete mitogenome of P. makianus was sequenced and annotated for the first time. The length of the mitogenome is 15,863 bp, and includes 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNA), and 2 ribosomal RNA genes (rRNA). The mitogenome exhibits a high AT content (72.26%), with a negative AT-skew (−0.01) and a GC-skew (−0.256). In the mitogenome of P. makianus, all the tRNA genes are folded into the typical cloverleaf secondary structure, except trnS1 (TCT). A comparison with the ancestors of Brachyura reveals that gene rearrangement occurred in P. makianus. In addition, phylogenetic analyses based on thirteen PCGs indicated that P. makianus, Pilumnus vespertilio, and Echinoecus nipponicus clustered into a well-supported clade that supports the monophyly of the family Pilumnidae. These findings enabled a better understanding of phylogenetic relationships within Brachyura.
1. Introduction
The “true” crabs in the infraorder Brachyura [1] belong to the Arthropoda, Malacostraca, Decapoda. It is one of the most diverse groups among the decapods [2]. The infraorder Brachyura contains more than 7250 known species in 104 families [3], which are widely distributed from shallow coral reefs to hydrothermal vents in the ocean, as well as freshwater and terrestrial habitats [4]. The current classification of Brachyura was established by Guinot [5] based on gonopore position morphology, and is currently divided into three sections (i.e., Podotremata, Heterotremata, and Thoracotremata) [5]. A high incidence of derived characteristics has given rise to many controversies concerning brachyuran phylogenetic relationships. Pinnotheroidea, Hymenosomatoidea, and Hexapodidae were originally assigned to the Thoracotremata [5]. However, Guinot and Forges [6] argued that these three superfamilies should be assigned to the Heterotremata based on the gonopore location and ultrastructure of the male reproductive system. Von Sternberg et al. [7] re-established a phylogenetic relationship between Potamoidea and other crabs by considering morphological features. They assigned Potamoidea to Heterotremata and further identified a sister-group relationship with Thoracotremata, thereby embedding Potamoidea within Thoracotremata [7].
The superfamily Pilumnidea is widely distributed along the western Pacific coast, and consists of three families (i.e., Pilumnidae, Galenidae, and Tanaochelidae). At present, only two complete mitogenomes of Pilumnidea have been published in the NCBI database (i.e., P. vespertilio (MF457402) and E. nipponicus (NC_039618)). The crab P. makianus belongs to Pilumnidae, which inhabits the rocky and muddy shores of tropical and subtropical seas. Some previous studies classified the Pilumnidae as a subfamily of Xanthidae [8]. This classification was challenged by Guinot [5], who elevated the Pilumninae to Pilumnidae due to their exhibition of characteristics more advanced than those found in other Xanthoidea families. Guinot [5] also elevated the Xanthidae to Xanthoidea based on the position of the male genital foramen. Subsequently, with the rise of comparative morphology research, the classification of Pilumnidea has been revised again. Due to the special genital characteristics of males (i.e., a slender and curved first abdominal limb, and a prominent, short, “S”-shaped second abdominal limb), Pilumnidae was elevated into Pilumnidea [9,10]. However, the morphology-based phylogenetic analysis presents problems [11,12], and the whole process of identification by morphology is still controversial. The analysis of the mitogenomic sequence data might provide a useful approach for identifying new brachyuran species and analyzing brachyuran phylogenetics.
The mitochondrial genome in Brachyura is usually a closed loop constituted of 13 protein-coding genes (PCGs), 22 transfer RNA genes (tRNAs), 2 ribosomal RNA genes (12S and 16S), and an AT-rich region (also called a control region, or CR) [13]. In phylogenetic studies, PCGs are commonly used to construct phylogenetic trees. The protein-coding genes include three cytochrome oxidase subunits (COX1-3), one cytochrome dehydrogenase subunit (CYTB), two ATP synthase subunits (ATP6 and ATP8), and seven NADH dehydrogenase subunits (ND1-6 and ND4L) [14]. Due to the long evolutionary history of symbiosis between mitochondria and eukaryotes, their genomes contain key messages reflecting their evolution as species [15]. Their mitochondrial genomes were characterized by fast evolution, maternal genetics, simple molecular structure, and relatively easy acquisition [16]. In general, mitochondrial rearrangement is common in invertebrates, including Cephalopoda [17], Bivalvia [18], and Brachyura [19]. Therefore, a complete mitogenome would be useful for analyzing gene rearrangements and identifying evolutionary relationships.
In this study, we sequenced the complete mitogenome of P. makianus. We first obtained the mitogenome map of P. makianus to determine the location of genes and confirm its nucleotide composition. The gene rearrangement and phylogeny were analyzed to improve the level of classification and phylogenetic within Brachyura.
2. Materials and Methods
2.1. Sample Collection and DNA Extraction
We collected one specimen of P. makianus from Changzhi Island, Zhoushan, Zhejiang Province, China (122°14′ N, 29°97′ E) on 27 September 2020. We identified the specimen by morphology, then excised the epaxial musculature and saved tissue in absolute ethyl alcohol for the purpose of DNA extraction. The total genomic DNA was extracted by the salt-extraction procedure with a minor modification [20], then stored in 1 × TAE buffer at 4 °C after being extracted immediately. We then used 1.5% agarose gel electrophoresis to identify the extracted DNA and then stored it at −20 °C.
2.2. Sequence Assembly, Annotation, and Analysis
The P. makianus mitogenome was sequenced by Origin gene Co. Ltd., Shanghai, China, on the Illumina HiSeq X Ten platform (Illumina, CA, USA). Each library generated about 10 Gb of raw data with inserts of 300–500 bp size from genomic DNA. The reads and adapters of low-quality, sequences with high “N” ratios and fragments with lengths less than 25 bp were moved away. We aggregated clean reads using NOVOPlasty software [21]. To validate the sequences, we compared the aggregated mitochondrial genes to the other Pilumnidea species and checked the COX1 sequence in NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 1 March 2022)) to verify the mitogenomic sequences [22]. We determined aberrant start and stop codons by comparison with similar codons in other invertebrate species. We then used the de novo assembly program to rebuild the reads. The software Sequin 16.0 (https://www.ncbi.nlm.nih.gov/sra (accessed on 1 March 2022)) was used to annotate the complete mtDNA, and the MITOS web server [22] was used to verify the correctness of transfer RNA genes and their secondary structures. Then CGView [23] was used to obtain the mitogenome map of P. makianus (Figure 1). We analyzed base composition by DAMBE [24] and obtained relative synonymous codon usage (RSCU) using MEGA XI [25]. To calculate strand asymmetry, we used the following formulae: AT-skew = (A − T) / (A + T); and GC-skew = (G − C)/(G + C) [26].
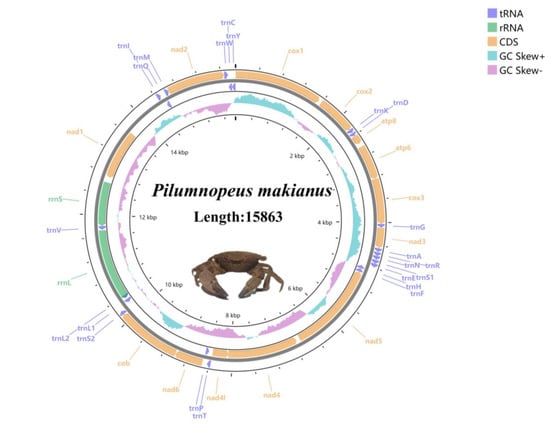
Figure 1.
Mitogenome map of the P. makianus. Lavender arrows represent tRNA, green arrows represent rRNA, and yellow arrows represent CDS. Blue represents GC skew + and pink represents GC skew.
2.3. Phylogenetic Analysis
We downloaded 76 complete mitogenome sequences, including outgroups Pagurus nigrofascia and Pagurus gracilipes, from NCBI (Table S1). We determined the phylogenetic relationship of Brachyura using the mitogenome sequences of the 76 species and P. makianus. Thirteen PCGs from the mitogenome sequences of all species were extracted from the GenBank files using DAMBE [24]. We then aligned the genes in MEGA XI [25] and constructed a single alignment file that connected all sequences, and then carried out a format conversion to create nexus format files and paste files that we used for our phylogenetic analyses. We used DAMBE [24] to detect the saturation of protein-coding genes and to remove supersaturated genes. The maximum likelihood (ML) and Bayesian inference (BI) were employed to construct a phylogenetic tree. Based on the Bayesian information criterion (BIC), we selected the best-fit model GTR + F + R6 for each subarea, and then used IQ-TREE [27] with 1000 replicates for ML analysis. For BI analysis, after applying the Akaike information criterion (AIC) in MrModelTest 2.3 [28], we confirmed best-fit evolutionary models (GTR + I + G) in MrMTgui [29]. The PAUP, ModelTest, and MrModelTest were associated through MrMTgui across platforms. We then ran the BI analysis using 3×106 Markov Chain Monte Carlo (MCMC) sampling and estimated the posterior distribution. We sampled every 1000 generations and discarded burn-in for 25% of the generations. To guarantee stationarity, we set the average standard deviation of split frequencies below 0.01. Finally, we used Figure Tree v1.4.3 software [30] to visualize the phylogenetic trees. In this research, we used Baeza’s [31] workflow to guide our detailed analysis, which includes the de novo assembled, annotated, manually curated, and characterized of mitochondrial genomes.
3. Results and Discussion
3.1. Base Structure and Composition of the Mitogenome
The complete mitogenome sequence of P. makianus is 15,863 bp in length. It has been uploaded to GenBank under accession number OM461360, and the raw reads were deposited in GenBank with the SRA number SRR21765684. The mitogenome content of P. makianus is a closed-circular molecule that includes 13 PCGs, 22 tRNAs, 2 rRNAs (rrnl and rrns), and a small non-coding region (Table 1).

Table 1.
Features of the mitochondrial genome of P. makianus.
The mitogenome sequence of P. makianus shares common features with other Brachyura whose genes have been identified and published. A total of four PCGs (ND5, ND4, ND4L, and ND1), eight tRNAs (tRNA-His, Phe, Pro, Tyr, Leu, Val, Gln, Cys), and two rRNAs are located in the light (L–) strand; the remainder of the 37 genes are located in the heavy (H–) strand (Table 1). The nucleotide composition of the P. makianus complete mitogenome is as follows: 35.76% A, 36.5% T, 10.32% G, and 17.42% C (Table 2). In addition, the P. makianus mitogenome has a high AT bias (72.26%). While its GC-skew and AT-skew values are negative, being −0.256 and −0.01, respectively, indicating that Cs and Ts exceed Gs and As (Table 2). In short, for P. makianus, the nucleotide composition, the GC-skew and AT-skew of the total mitogenomes, and the gene sequence length are all similar to the other Pilumnidea species (Table 3).

Table 2.
Composition and skewness of P. makianus mitogenome.

Table 3.
Nucleotide composition of the mitogenomes of three Pilumnidae species.
Brachyura mitochondrial genomes are compact in structure, and include some overlaps between adjacent coding genes. However, gene spacers are also present. The P. makianus mitogenome possessed 21 intergenic spacers and seven overlapping regions. The seven overlaps range from 1 to 56 bp, including three representative overlaps in protein-coding genes (1 bp between ATP6 and COX3, 5 bp between ND4 and ND4L, 1 bp between ND6 and CYTB), which are typically found in other crabs.
3.2. PCGs and Codon Usage
The total length of PCGs in the P. makianus mitogenome is 11,066 bp, including seven NADH dehydrogenases (ND1-6 and ND4L), two ATPases (ATP6 and ATP8), three cytochrome c oxidases (COX1-3), and one cytochrome b (CYTB). All thirteen PCGs begin with the start codon ATN (ATA, ATG, ATC, and ATT). The majority of the 13 PCGs end with TAA or TAG, while the stop codon of CYTB is a single T (Table 1). Incomplete stop codons are a common phenomenon in the mitochondrial genes of vertebrates and invertebrates [32]. Figure 2 demonstrates the relative synonymous codon usage (RSCU) and the amino acid compositions of the P. makianus mitochondrial genome. The most frequently occurring codon is UUA-Leu (2.23) (Figure 2a), and the most common amino acids are Ile (7.0%), Asn (8.0%), Phe (8.9%), and Ile (11.0%). The least common amino acids are Glu (1.3%), Arg (1.3%), Asp (2%), and Cys (2%) (Figure 2b).
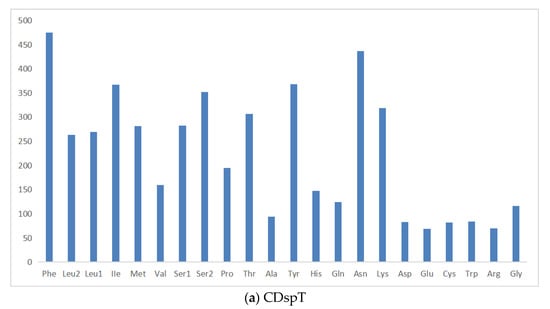
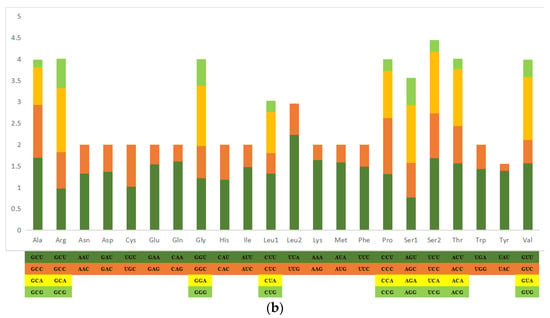
Figure 2.
Amino acid composition in P. makianus mitogenome. Codons are expressed in thousands and the x-axis indicates the codon families (a) and the relative synonymous codon usage (b).
3.3. Transfer and Ribosomal RNAs
In common with other crabs, the mitogenome of P. makianus contains 22 tRNA genes. The length of these ranges from 63 bp (trnC, trnA) to 72 bp (trnV) of nucleotides (Table 1). The overall A + T content of tRNA genes is 73.11%; in addition, they exhibit a positive AT skew (0.023) and GC skew (0.156) (Table 2). As trnS1 lacks the dihydrouridine (DHU) arm, only trnS1 (TCT) cannot be folded to a representative cloverleaf secondary structure (Figure 3). This phenomenon is common in metazoans [33].
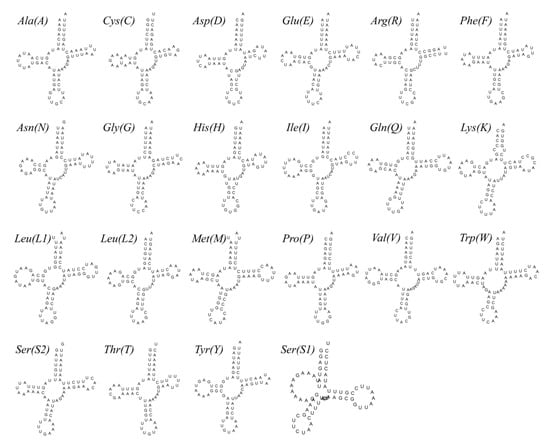
Figure 3.
Secondary structures of tRNAs of the P. makianus mitogenome.
In the P. makianus genome, rrnS and rrnL have lengths of 831 bp and 1355 bp and are separated by trnV. The 12S rRNA gene is followed by trnV, and the 16S rRNA gene is situated between trnL2 (TAA) and trnV. The A + T content of rRNAs is 76.2%, AT-skew (0.030) and GC-skew (0.322) are both positive (Table 2), indicating that As and Gs exceed Ts and Cs.
3.4. Gene Rearrangement
In general, the mitochondrial gene orders (MGOs) of invertebrates exhibit gene rearrangements on different scales [34,35]. This phenomenon has been demonstrated in the MGOs of P. makianus by means of comparison with the ancestor of Brachyura and subsequent analysis. To identify gene rearrangement, previous researchers have used four different models: the tandem duplication-random loss model (TDRL) [36], which is the most commonly used means of explaining the mechanism of mitochondrial genome rearrangement, the tandem duplication-nonrandom loss model (TDNL) [37], recombination [38], and tRNA miss-priming model [39].
We compared the MGOs of Pilumnidae and other Heterotremata species with ancestral Brachyura (Figure 4). We found the MGOs of the families Leucosiidae, Matutidae, Portunidae, and Oregoniidae were identical to ancestral Brachyura. However, the families Pilumnidae, Xanthidae, and Majidae have undergone gene rearrangements. Compared with the ancestral Brachyura, P. makianus exhibited translocation and duplication-random loss (Figure 4). In P. makianus mitogenome, trnL2 has moved to a position between trnL1 and 16S and changed from heavy (H–) strand to light (L–) strand, resulting in a new gene order trnL1-trnL2-16S-trnV-12S-ND1, which can be explained by the TDRL model (Figure 4). In most families, MGOs of individual species within the family were largely consistent [40,41]. However, the MGOs of the three Pilumnidae species exhibited considerable differences. For instance, compared with P. makianus, the trnL1 and trnL2 were inverted in P. vespertilio; and in E. nipponicus, the ND1 and trnL1 moved to the back of CR (Figure 4). Within Pilumnidae, Chen et al. [42] have reported the study of Heteropanope glabra, and we found the MGOs of H. glabra studied by Chen et al. [42] are the same as those of P. makianus.
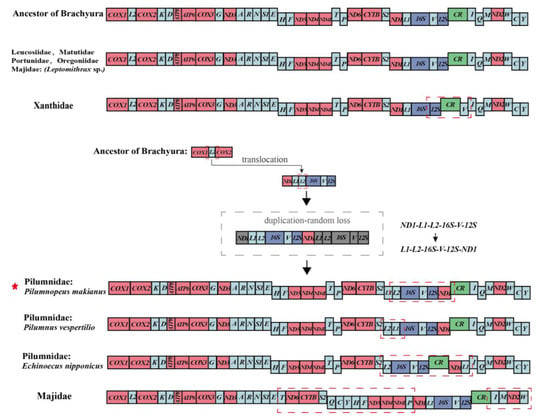
Figure 4.
Linear portrayal of gene arrangements of the ancestor Brachyura, P. makianus, and six families in Heterotremata. The single-letter amino acid codes represent corresponding tRNA, and the 16S and 12S are the large and small ribosomal RNA subunits, respectively. The rearranged gene blocks are signed by red gridlines and compared with the gene arrangement of ancestral Brachyura.
Mitochondrial gene orders provide compelling phylogenetic information. Previous studies suggested that Pilumnidae and Xanthidae were closely related and might even belong to the same family [8]. In this study, by assessing the MGOs of Heterotremata, we found that gene rearrangement had taken place in both Pilumnidae and Xanthidae, and the MGOs of other species within Heterotremata were consistent with ancestral Brachyura, except for the more distant Majidae families. In addition, we found the families Pilumnidae and Xanthidae have undergone different rearrangements, which provided key evidence of their evolutionary history. The difference in the MGOs of Xanthidae [43] and Pilumnidae also provided valuable independent support for the separation of Pilumnidae from Xanthidae, but the low number of published Pilumnidae mitochondrial studies made it difficult to explore the ancestral sequence of this family.
3.5. Phylogenetic Relationships
In this study, we used the sequences of 13 PCGs in mitochondrial genomes to establish a phylogenetic relationship of Brachyura. We analyzed P. makianus and 74 other brachyuran species, with P. nigrofascia and P. gracilipes as outgroups (Table S1). Numbers above the branches indicate posterior probabilities from BI and bootstrap percentages from ML, respectively. For all species, ML and BI methods generated the same topological structure for each dataset, and the BI tree was demonstrated for high supporting values on the whole. Support values obtained through ML and BI methods were shown on the BI tree, and P. makianus was marked with a red dot. It can be observed that P. vespertilio and E. nipponicus form a sister clade with high support values (BI posterior probabilities PP = 1, ML bootstrap BP = 92%). P. makianus, along with those two species, formed a Pilumnidae group with high support values, with maximum values for both BI posterior probabilities and ML bootstrap percentages (Figure 5).
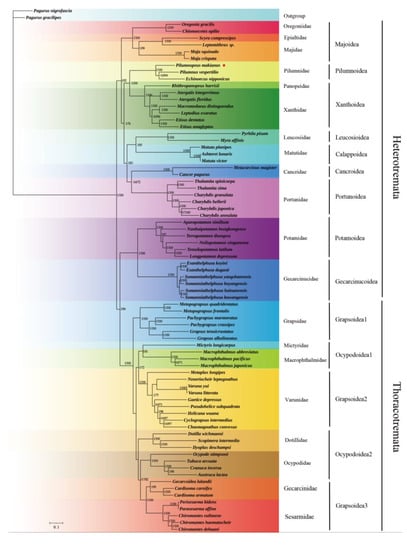
Figure 5.
The phylogenetic tree constructed using the 13 PCGs, with BI and ML methods. Numbers above branches are posterior probabilities from BI (left) and bootstrap percentages from ML (right), respectively.
There are 20 families in this phylogeny. Heterotremata was divided into two groups (Majoidea + ((Pilumnoidea+Xanthoidea) + ((Leucosioidea + Calappoidea) + (Cancroidea + Portunoidea)))) + (Potamoidea + Gecarcinucoidea). The species in Majoidea are one of the oldest lineages in brachyuran crabs and are deemed to be the nearest branch to the base of a brachyuran tree, based on the spermatozoal ultrastructure [44]. Our phylogenetic tree confirmed this. Pilumnidae was clustered with Xanthidae and Panopeidae, and formed a sisterhood with another group which includes Portunidae, Matutidae, Leucosiidae, and Cancridae [45]. Previously, Pilumnidae were considered a subfamily of Xanthidae; however, Tsang et al. [11] constructed a Brachyura molecular phylogeny based on six nucleoprotein coding genes and two rRNA genes and verified the Pilumnidea species formed a separate branch, independent of Xanthidea. Our mitochondrial genome confirmed the reliability of this result and further explained the separation of Pilumnidae from Xanthidae, so that the former is now considered a separate family. The specific classification status of P. makianus has also been verified from the perspective of morphology and molecular biology [46].
The superfamilies Gecarcinucoidea and Potamoidea are freshwater crabs that belong to Heterotremata, but they were found to have a close relationship with Thoracotremata. Von Sternberg and Cumberlidge [47] suggested that most families of freshwater crabs, including Potamidae and Gecarcinucidae, should be placed in the Thoracotremata. The Gecarcinucoidea, Potamoidea, and Thoracotremata clustered into a branch, supporting the conclusion that Heterotremata is polyphyletic. Brosing et al. [48] confirmed the polyphyletism of Heterotremata through morphological identification. Ahyong et al. [49] used a partial sequence of 18s rDNA to reconstruct the phylogeny of Brachyura, and found that the two representative classes of Thoracotremata were completely nested in Heterotremata, thus confirming the paraphyly of Heterotremata. This conclusion was in line with the findings of Ahyong et al. [49] and Bracken et al. [48]. Our tree demonstrates that Thoracotremata is divided into Grapsoidea and Ocypodoidea, and Grapsoidea is further divided into three clades (i.e., (Grapsidea + Varunidae + (Gecarcinidae + Sesarmidae))) and the Ocypodoidea was divided into two clades (i.e., ((Mictyridae + Macrophthalmidae) + (Dotillidae + Ocypodoidae))) (Figure 5). These findings are also consistent with those of previous studies [48,49,50]. Chen et al. [51] found that Gecarcinucoidea and Potamoidea are more connected with thoracotreme crabs, and Thoracotremata is nested within Heterotremata clades. These results are also in accordance with our findings.
4. Conclusions
In this study, the complete mitogenome of P. makianus was sequenced and described for the first time. The 15863 bp mitogenome of P. makianus includes 37 genes and a AT-rich region, in common with the metazoan mitogenome. The genome composition exhibits a high A + T biased (72.26%) and demonstrates a negative AT-skew (–0.01) and GC-skew (–0.256). Compared with the ancestor of Brachyura, there has been a genetic rearrangement in P. makianus mitogenomes, which can be explained by the TDRL model. The phylogenetic tree was structured by 77 species, and was divided into two branches. The P. makianus exhibited the closest relationship with P. vespertilio and E. nipponicus, and these three species formed the Pilumnidae cluster. We also explained the reason why genetic scientists separated Pilumnidae from Xanthidae. Our research results enable a better understanding of taxonomy and phylogenetic relationships within Brachyura.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13111943/s1: Table S1. List of Brachyuran species with their GenBank accession numbers.
Author Contributions
Methodology, K.X., X.D. (Xinbing Duan) and X.D. (Xiangli Dong); software, J.L. (Jiji Li); data analysis and sample processing, J.L. (Jiayin Lü); writing—original draft preparation and visualization, X.D. (Xinbing Duan) and X.D. (Xiangli Dong); writing—review and editing, B.G., J.L. (Jiji Li) and Y.Y.; supervision and funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key R&D Program of China (2019YFD0901204), NSFC Projects of International Cooperation and Exchanges (42020104009), and the National Natural Science Foundation of China (42107301).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The mitochondrial genome was deposited at NCBI, with accession number OM461360. The data that support the finding of this study are openly available in Microsoft OneDrive at https://1drv.ms/u/s!Agslj0zkcUG8gRAZPuvOxsXtz3aQ?e=2CxF5k(accessed on 17 January 2022). The raw genome sequencing datasets generated during the current study have been submitted to the NCBI Sequence Read Archive (SRA) and are available online at https://www.ncbi.nlm.nih.gov/bioproject/PRJNA885758 (accessed on 30 September 2022).
Acknowledgments
We acknowledge all the financial support in the paper. We thank Chen Jian, a taxonomist from the Marine Biological Museum of Zhejiang Ocean University, for the accurate morphological identification of this specie before sequencing. The authors would like to express their gratitude for permits for sampling and observational fields.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rice, A.L. Crab zoeal morphology and its bearing on the classification of the Brachyura. Trans. Zool. Soc. Lond. 1980, 35, 271–372. [Google Scholar] [CrossRef]
- Grave, S.D.; Pentcheff, N.D.; Ahyong, S.T.; Chan, T.Y.; Wetzer, R. A classification of living and fossil genera of decapod crustaceans. Raffles. B. Zool. 2009, 21, 1–109. [Google Scholar]
- Wang, Q.; Wang, J.; Wu, Q.; Xu, X.; Wang, P.; Wang, Z. Insights into the evolution of Brachyura (Crustacea: Decapoda) from mitochondrial sequences and gene order rearrangements. Int. J. Biol. Macromol. 2021, 170, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.Y.; Qin, J.; Lin, C.-W.; Chan, T.-Y.; Ng, P.K.L.; Chu, K.H.; Tsang, L.M. Phylogenomic analyses of brachyuran crabs support early divergence of primary freshwater crabs. Mol. Phylogenet. Evol. 2019, 135, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Guinot, D. Principes d’une classification évolutive des Crustacés Decapodes Brachyoures. Bull. Biol. Fr. Belg. 1978, 119, 7–20. [Google Scholar]
- Guinot, D.; Richer de Forges, B. Affinités entre les Hymenosomatidae macLeay, 1838 et les Inachoididae dana, 1851 (Crustacea Decapoda Brachyura). Zoosystema 1997, 19, 453–502. [Google Scholar]
- Von Sternberg, R.; Cumberlidge, N.; Rodriguez, G. On the marine sister groups of the freshwater crabs (Crustacea: Decapoda: Brachyura). J. Zool. Syst. Evol. Res. 1999, 37, 19–38. [Google Scholar] [CrossRef]
- Takeda, M. Studies on the Crustacea Brachyura of the Palau Islands: Ⅲ. Xanthidae (1). Res. Crustasea 1976, 7, 69–99c. [Google Scholar] [CrossRef][Green Version]
- Clark, P.F.; Ng, P.K.L.; Noho, H.; Shokita, S. The first-stage zoeas of Carpilius convexus (Forskål, 1775) and Carpilius maculatus (Linnaeus, 1758) (Crustacea: Decapoda: Brachyura: Xanthoidea: Carpiliidae): An example of heterochrony. J. Plankton Res. 2005, 27, 211–219. [Google Scholar] [CrossRef][Green Version]
- Clark, P.F.; Ng, P. The larval development of Actumnus setifer (de Haan, 1835) (Brachyura: Xanthoidea: Pilumnidae) described from laboratory reared material. Crustacean Res. 2004, 33, 27–50. [Google Scholar] [CrossRef][Green Version]
- Tsang, L.M.; Schubart, C.D.; Ahyong, S.T.; Lai, J.C.Y.; Au, E.Y.C.; Chan, T.-Y.; Ng, P.K.L.; Chu, K.H. Evolutionary history of true crabs (Crustacea: Decapoda: Brachyura) and the origin of freshwater crabs. Mol. Biol. Evol. 2014, 31, 1173–1187. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.K.L.; Rahayu, D.L. A new genus and species of pilumnid crab (Decapoda: Brachyura: Pilumnidae) symbiotic with the sponge Callyspongia Duchassaing & Michelotti, 1864 (Porifera: Demospongiae: Callyspongiidae) from Lombok, Indonesia; the identity of Pseudactumnus pestae Balss, 1933; and a review of symbiosis in the Pilumnidae. J. Crustacean Biol. 2020, 40, 918–932. [Google Scholar]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Boore, J.L.; Lavrov, D.V.; Brown, W.M. Gene translocation links insects and crustaceans. Nature 1998, 392, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhu, C.C.; Nie, Z.H.; Zou, J.X.; Wang, Y.; Zhou, X.M. Introduction and comparative analysis of Brachyura mitochondrial genomes research. Genom. Appl. Biol. 2016, 35, 2627–2639. [Google Scholar]
- Lan, D.; Hu, Y.D.; Zhu, Q.; Liu, Y.P. Mitochondrial DNA study in domestic chicken. Mitochondrial DNA A 2017, 28, 25–29. [Google Scholar] [CrossRef]
- Jiang, L.H.; Kang, L.S.; Wu, C.W.; Chen, M.; Lu, Z.M. A comprehensive description and evolutionary analysis of 9 Loliginidae mitochondrial genomes. Hydrobiologia 2018, 808, 115–124. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Li, L.; Xu, X.; Xia, J.; Yu, Z. New features of Asian Crassostrea oyster mitochondrial genomes: A novel alloacceptor tRNA gene recruitment and two novel ORFs. Gene 2012, 507, 112–118. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Q.W.; Zou, J.X.; Zhu, C.C.; Bai, J.; Shih, H.T.; Cheng, S.L.; Zhou, X.M. The complete mitochondrial genome of freshwater crab Sinopotamon xiushuiense (Decapoda: Brachyura: Potamoidea). Mitochondrial DNA B 2016, 1, 750–752. [Google Scholar] [CrossRef]
- Aljanabi, S.M.; Martinez, I. Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 1997, 25, 4692–4693. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. Mitos: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Grant, J.R.; Stothard, P. The CGView Server: A comparative genomics tool for circular genomes. Nucleic Acids Res. 2008, 36, W181–W184. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Ma, X. Study on Complete Mitochondrial Genome of Cypridopsis vidua and Molecular Phylogeny of Ostracoda. Ph.D. Thesis, East China Normal University, Shanghai, China, 2016. [Google Scholar]
- Rambaut, A. FigTree, A Graphical Viewer of Phylogenetic Trees. Available online: http://tree.bio.ed.ac.uk/software/pathogen/ (accessed on 1 September 2022).
- Baeza, J.A. An introduction to the special section on crustacean mitochondrial genomics: Improving the assembly, annotation, and characterization of mitochondrial genomes using user-friendly and open-access bioinformatics tools, with decapod crustaceans as an example. J. Crustacean Biol. 2022, 42, 1–4. [Google Scholar] [CrossRef]
- Hamasaki, K.; Iizuka, C.; Sanda, T.; Imai, H.; Kitada, S. Phylogeny and phylogeography of the land hermit crab Coenobita purpureus (Decapoda: Anomura: Coenobitidae) in the Northwestern Pacific region. Mar. Ecol. 2017, 38, e12369. [Google Scholar] [CrossRef]
- Yamauchi, M.M.; Miya, M.U.; Nishida, M. Complete mitochondrial DNA sequence of the swimming crab, Portunus trituberculatus (Crustacea: Decapoda: Brachyura). Gene 2003, 311, 129–135. [Google Scholar] [CrossRef]
- Temple, M.; Makaroff, C.A.; Mutschler, M.A.; Earle, E.D. Novel mitochondrial genomes in Brassica napus somatic hybrids. Curr. Genet. 1992, 22, 243–249. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Hanson, D.K.; Fox, T.D.; Leaver, C.J. Mitochondrial genome rearrangement leads to extension and relocation of the cytochrome c oxidase subunit I gene in sorghum. Cell 1986, 47, 567–576. [Google Scholar] [CrossRef]
- Moritz, C.; Brown, W.M. Tandem duplications in animal mitochondrial DNAs: Variation in incidence and gene content among lizards. Proc. Natl. Acad. Sci. USA 1987, 84, 7183–7187. [Google Scholar] [CrossRef] [PubMed]
- Lavrov, D.V.; Boore, J.L.; Brown, W.M. Complete mtDNA sequences of two millipedes suggest a new model for mitochondrial gene rearrangements: Duplication and nonrandom loss. Mol. Biol. Evol. 2002, 19, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Rokas, A.; Ladoukakis, E.; Zouros, E. Animal mitochondrial DNA recombination revisited. Trends Ecol. Evol. 2003, 18, 411–417. [Google Scholar] [CrossRef]
- Cantatore, P.; Gadaleta, M.N.; Roberti, M.; Saccone, C.; Wilson, A.C. Duplication and remoulding of tRNA genes during the evolutionary rearrangement of mitochondrial genomes. Nature 1987, 329, 853–855. [Google Scholar] [CrossRef]
- Tang, B.-P.; Liu, Y.; Xin, Z.-Z.; Zhang, D.-Z.; Wang, Z.-F.; Zhu, X.-Y.; Wang, Y.; Zhang, H.-B.; Zhou, C.-L.; Chai, X.-Y.; et al. Characterisation of the complete mitochondrial genome of Helice wuana (Grapsoidea: Varunidae) and comparison with other Brachyuran crabs. Genomics 2018, 110, 221–230. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Shi, X.; Wu, Q.; Tao, Y.; Guo, H.; Ji, C.; Bai, Y. Complete mitochondrial genome of Parasesarma affine (Brachyura: Sesarmidae): Gene rearrangements in Sesarmidae and phylogenetic analysis of the Brachyura. Int. J. Biol. Macromol. 2018, 118, 31–40. [Google Scholar] [CrossRef]
- Chen, J.Q.; Zhang, Z.H.; Xing, Y.H.; Yao, W.J.; Zhang, C.L.; You, L. The complete mitochondrial genome of Heteropanope glabra and implications in phylogenetic research. J. Nanjing Norm. Univ. (Nat. Sci. Edit.) 2018, 41, 108–114. [Google Scholar]
- Xu, X.; Wang, Q.; Wu, Q.; Xu, J.; Wang, J.; Wang, Z. The entire mitochondrial genome of Macrophthalmus abbreviatus reveals insights into the phylogeny and gene rearrangements of Brachyura. Biochem. Genet. 2021, 59, 617–636. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, B.G.M. Phylogeny of the Brachyura with particular reference to the Podotremata: Evidence from a review of spermatozoal ultrastructure (Crustacea, Decapoda). Philos. Trans. R. Soc. London Ser. B Biol. Sci. 1994, 345, 373–393. [Google Scholar]
- Spiridonov, V.A.; Neretina, T.V.; Schepetov, D. Morphological characterization and molecular phylogeny of Portunoidea rafinesque, 1815 (Crustacea Brachyura): Implications for understanding evolution of swimming capacity and revision of the family-level classification. Zool. Anz. 2014, 253, 404–429. [Google Scholar] [CrossRef]
- Fahimi, N.; Zolgharnein, H.; Keykhosravi, A.; Naderloo, R. Molecular phylogeny and taxonomy of the genus Pilumnus leach, 1815 (Eucrustacea: Brachyura: Pilumnidae) in the Persian Gulf and Gulf of Oman. Zool. Anz. 2021, 291, 7–22. [Google Scholar] [CrossRef]
- Von Sternberg, R.; Cumberlidge, N. On the heterotreme-thoracotreme distinction in the Eubrachyura de Saint Laurent, 1980 (Decapoda, Brachyura). Crustaceana 2001, 74, 321–338. [Google Scholar] [CrossRef]
- Brösing, A.; Richter, S.; Scholtz, G. Phylogenetic analysis of the Brachyura (Crustacea, Decapoda) based on characters of the foregut with establishment of a new taxon. J. Zool. Syst. Evol. Res. 2007, 45, 20–32. [Google Scholar] [CrossRef]
- Ahyong, S.T.; Lai, J.C.Y.; Sharkey, D.; Colgan, D.J.; Ng, P.K.L. Phylogenetics of the brachyuran crabs (Crustacea: Decapoda): The status of Podotremata based on small subunit nuclear ribosomal RNA. Mol. Phylogenet. Evol. 2007, 45, 576–586. [Google Scholar] [CrossRef]
- Kitaura, J.; Nishida, W.M. Molecular phylogeny of grapsoid and ocypodoid crabs with special reference to the genera Metaplax and Macrophthalmus. J. Crustacean Biol. 2002, 22, 682–693. [Google Scholar] [CrossRef]
- Chen, J.; Xing, Y.; Yao, W.; Zhang, C.; Zhang, Z.; Jiang, G.; Ding, Z. Characterization of four new mitogenomes from Ocypodoidea & Grapsoidea, and phylomitogenomic insights into thoracotreme evolution. Gene 2018, 675, 27–35. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).