Abstract
Antimicrobial peptides (AMPs) are natural peptides possessing antimicrobial activities. These peptides are important components of the innate immune system. They are found in various organisms. AMP screening and identification by experimental techniques are laborious and time-consuming tasks. Alternatively, computational methods based on machine learning have been developed to screen potential AMP candidates prior to experimental verification. Although various AMP prediction programs are available, there is still a need for improvement to reduce false positives (FPs) and to increase the predictive accuracy. In this work, several well-known single and ensemble machine learning approaches have been explored and evaluated based on balanced training datasets and two large testing datasets. We have demonstrated that the developed program with various predictive models has high performance in differentiating between AMPs and non-AMPs. Thus, we describe the development of a program for the prediction and recognition of AMPs using MaxProbVote, which is an ensemble model. Moreover, to increase prediction efficiency, the ensemble model was integrated with a new hybrid feature based on logistic regression. The ensemble model integrated with the hybrid feature can effectively increase the prediction sensitivity of the developed program called Ensemble-AMPPred, resulting in overall improvements in terms of both sensitivity and specificity compared to those of currently available programs.
1. Introduction
Antimicrobial peptides (AMPs), a group of natural peptides, have a significant role in the immune system. There are various types of peptides with antimicrobial activities, such as antibacterial, antifungal, antiviral, and anticancer peptides. These peptides have been found to be effective against disease-causing pathogens. Due to the increase in antibiotic resistance becoming a major global health problem, novel anti-infective therapies are needed [1,2]. Basically, AMPs have abilities to kill microbes and other pathogens but do not cause drug resistance in bacteria and have received great attention as a promising/potential alternative to conventional antibiotics. [2,3,4] Action mechanisms of AMPs include various mechanisms, such as “barrel-stave”, “carpet”, or “toroidal-pore” mechanisms, to disrupt the cell membrane or intracellular functions of microbes. AMP characteristics, including amino acid composition, amphipathic structure, cationic charge, and size contribute in facilitating AMP interaction and the insertion into membranes of pathogens resulting in pore forming and membrane disruption [2,5,6]. AMPs can also stimulate the immune system to work together efficiently [2]. Therefore, research studies on AMPs have received much attention and have been widely investigated for use as another option, as a potential alternative or in conjunction with current antibiotic therapeutics [7].
Finding new AMPs from various organisms is currently receiving significant attention. However, large-scale identification through wet lab experiments is costly, time consuming, and resource intensive [1,8]. Therefore, developing a computation program for screening AMPs with high accuracy and high effectiveness can help such complicated tasks. An efficient computational machine learning predictive tool is required to screen antimicrobial candidate sequences prior to in vitro experimentation [1,7,9]. Several antimicrobial prediction tools have been designed and developed, as summarized in Table 1. These diverse prediction tools have been developed using different data features and different machine learning methods. Therefore, their performances differ depending on the nature of the training technique and data features. Most existing methods use single classifier models such as support vector machine (SVM), discriminant analysis, fuzzy K-nearest neighbors, and deep learning. Some methods use homologous ensemble, random forests, which is a committee of decision tree models. However, several other types of machine learning techniques and heterogeneous ensemble techniques have not been applied in this AMPs prediction problem. Using different machine learning techniques may provide a prediction result of AMP candidates that remain to be discovered. Therefore, other types of machine learning and heterogeneous ensembles techniques should be explored.

Table 1.
Summary of existing antimicrobial predictions using various machine learning techniques and different features.
Comparison of the performances of the available AMP prediction tools is difficult because different testing datasets are used for benchmarking these predictors [17]. Based on our preliminary study, we collected a benchmark AMP dataset S [13] composed of 920 AMPs and 920 non-AMPs and used it in testing current existing AMP prediction programs. From this preliminary review, we found that the false predictive answers of each program are different. This suggests that there is a different distribution of unpredictable answers due to the use of different models and features. Therefore, each program has gaps that should be considered for improvement, especially reducing false positives (FPs) and increasing predictive accuracy, in terms of both specificity and sensitivity. According to the issues mentioned above, we aim to (1) integrate different learning models using ensemble learning techniques to reduce FPs and to increase predictive accuracy and (2) use diverse informative features that contain sufficient discrimination information and are strongly related to AMP sequences.
Ensemble learning techniques are able to increase the accuracy and reduce the FPs of the prediction. The combination of predictions by different algorithms using different methods can reduce errors in bias or variance or otherwise reduce both bias and variance values found in a single algorithm through the voting of diverse algorithms. Moreover, for problems with complex decision boundaries, an appropriate combination of decision boundaries of various single models can learn the complex boundary of the problem [18]. The popular ensemble methods for incorporating individual classification models are bagging and boosting. There are different training procedures as follows. The bagging or bootstrap aggregating method [19] builds different classifiers from random bootstrapping of different training subsets. Therefore, individual models are different from each other, then reducing variance errors. Boosting [20] builds classifier models in incrementally sequential/linear combinations by adjusting the weight to improve the prediction values of the previous model and therefore can reduce the model bias [21].
Ensemble learning combines multiple points of view from different classifiers on the same problem domain to obtain a more accurate and robust (stable) prediction. In addition, this makes the ensemble model more generalizable with new data [22]. It also helps in reducing the overfitting problem found in single classification models [23], which makes it impossible to correctly predict new data. Moreover, the voting of heterogeneous methods can alleviate conflicting predictions found in single models.
The factors of a prediction method are a composite of good unbiased training data, a discriminative feature subset, and a suitable learning algorithm. To make the algorithm capable of learning patterns and distinguishing AMPs from other sequences, feature extraction, feature engineering, and feature selection became an important part of finding good representative features or informative features that can capture AMP patterns and increase the efficiency of predictions.
In this work, AMP prediction models based on ensemble methods, such as random forest (RF), max probability voting (MaxProbVote), majority voting, adaptive boosting (AdaBoost), and extreme gradient boosting (XGBoost), were built. In addition, we also compared various single models (support vector machine (SVM), naïve Bayes, logistic regression (LR), decision tree, multilayer perceptron (MLP), and K-nearest neighbor (KNN)). We collected and extracted various informative features related to AMP characteristics. The ability to train models relied substantially on a good representation of features that can detect the pattern of AMPs. Therefore, the extraction of data characteristics into data vectors was performed using a variety of 517 peptide features. Later, in addition to 517 features, we included a feature engineering process to explore the characteristics of AMPs and constructed a hybrid feature that combines four single preselected features based on the logistic regression equation. We observed that with the hybrid features integrated into the ensemble models, the sensitivity was between 93.39 and 97.51% for testing dataset 1, and the area under the receiver operating characteristic (ROC) curve (AUC) improved to between 0.917 and 0.946.
2. Materials and Methods
2.1. Workflow of Ensemble-AMPPred
The proposed predictive program was designed and built, as shown in Figure 1.
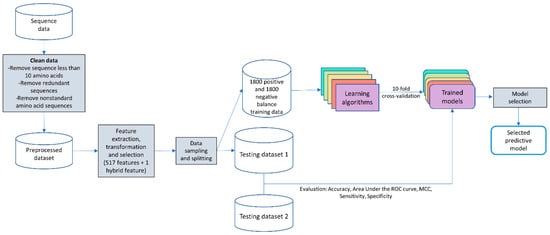
Figure 1.
Flowchart of building a predictive model.
2.2. Dataset Preparation
Data collection used in training and testing of models is shown in Figure 2.
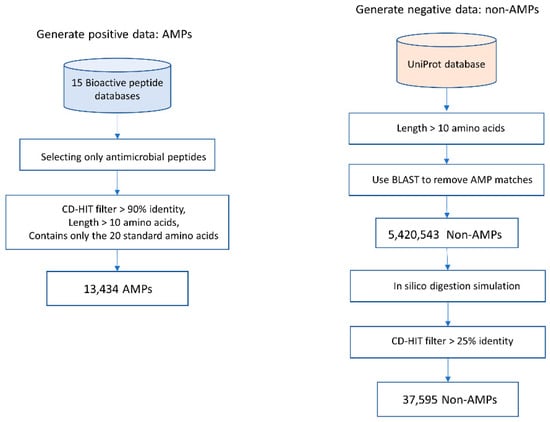
Figure 2.
Steps for data collection and preparation.
- AMP data were collected from 15 public bioactive peptide databases, as listed in Table 2. Only peptides that have description-matched antimicrobial activities were selected. Peptide sequences with lengths <10 amino acids were discarded. To reduce data redundancy, we applied the Cluster Database at High Identity with Tolerance (CD-HIT) program [24] with threshold of 0.9 (90% sequence similarity). A total of 13,434 peptides were used as positive sequence data. Notably, lower sequence similarity thresholds (less than 50%) might reduce the sequence homology bias and could improve the model reliability [25]. Since AMPs are highly heterogeneous substances and there are likely various novel subtypes of AMPs that have not been discovered, using a threshold of 0.9 is applicable to identifying a novel AMP sequence.
 Table 2. Public bioactive databases.
Table 2. Public bioactive databases. - Currently, there is no database of experimentally verified non-AMP available. Therefore, we build negative data using the approach described in [13,16]. Negative data or non-AMP data were collected from the UniProt [26] database (February 2020) by choosing only proteins that do not contain functional information related to antimicrobial activity and do not have a secretory signal peptide position. The basic local alignment search tool (BLAST) was used to filter out AMP matches. Peptide sequences with lengths <10 amino acids were discarded. Then, the in silico enzymatic digestion simulation [27] was performed to digest polypeptides into digested peptide sequences. Then, the CD-HIT program [24] was used to remove peptide sequences with >25% identity. Therefore, a total of 37,595 peptides were designated as negative sequence data.
- Balanced training data were created by proportionate stratified random sampling to select peptide sequences to represent the positive and negative data. The stratified sampling was conducted by similarity clustering of sequence data into homogenous strata based on the CD-HIT clustering tool. The proportional stratified random sampling was performed with the following steps. (i) The sequences were clustered by using CD-HIT with a similarity threshold of 0.3. (ii) Representative sequences were selected from each cluster to use as training data, while the other remaining sequences that were not representative of the cluster will be used as testing data (results are shown in Supplementary File S1 the representative sequences are denoted with the * symbol at the end of the line; the non-representative sequences are displayed with the percentage of sequence similarity to the representative sequence of that cluster). We balanced the number of representative sequences based on the cluster size, as shown in Figure 3. Note that there is no testing sequence presented in the cluster with one sequence member. For some clusters that contain only 1 sequence, the sequence in that cluster will be used as a training set, and the testing sequence is not presented in that cluster. A summary of the sequence similarities between training and testing sequences in all clusters is presented in Supplementary File S2. The sequence similarity between training and testing sequences falls between 30% and 89.47%, with an average of 47.29%. Finally, the training data consists of 1800 peptide sequences of the AMP dataset and 1800 sequences of the non-AMP dataset to make an evenly balanced training dataset in order to reduce the likelihood of generating a predictive model biased toward the majority class.
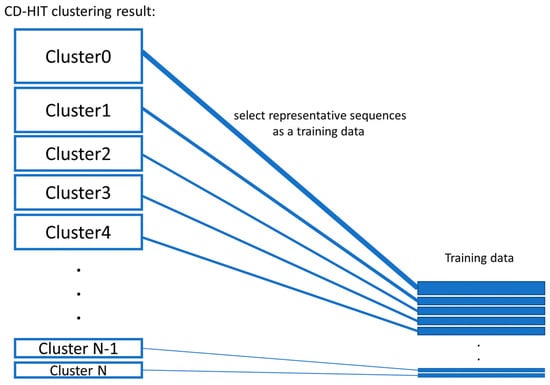 Figure 3. Proportionate stratified random sampling.
Figure 3. Proportionate stratified random sampling. - Two testing datasets (testing dataset 1 and testing dataset 2) were created. The first set of testing data was the remaining sequences of both positive data and negative data after training data preparation. Therefore, the first testing dataset consists of 11,634 positive sequence data or AMPs and 35,795 negative sequence data or non-AMPs. The second set of testing data was the benchmark dataset S from published works [13,16]. This dataset can be downloaded from the websites ([28,29]).
- Sequences having less than 10 amino acids were removed from further analysis. The benchmark dataset S1 contains 1461 AMPs (classified into five functional types: antibacterial peptides, anticancer/tumor peptides, antifungal peptides, anti-HIV peptides, and antiviral peptides) and 2404 non-AMPs, and the dataset S2 contains 917 AMP sequences and 828 non-AMP sequences.
2.3. Feature Extraction and Feature Engineering
Various numerical representation schemes of proteins and peptides from amino acid sequences of both AMP and non-AMP datasets were generated. Feature extraction of peptide characteristics that would be useful in predicting, namely, the amino acid composition, pseudo amino acid composition (PseAAC) in parallel and in series correlation, and the details of the secondary structure conformation, composition–transition–distribution (CTD), various physical–chemical properties, antimicrobial propensity scale (antimicrobial IC50 index derived from high-throughput values of different amino acids), and the percentage of different conformations in the peptide sequence were calculated using the protr R package [44,45], peptide R package [45], AMPA program [3,46], Tango program [47], and Pse-in-one program [48,49]. Various modes of Chou’s PseAAC descriptors were generated. Chou’s PseAAC has been widely used to convert complicated protein sequences with various lengths to fixed-length digital feature vectors while keeping considerable sequence-order information. [50]. Chou’s PseAAC can represent a protein sequence in a discrete model without completely losing its sequence-order information and hence has been widely applied for improving the prediction quality for various protein problems [51,52]. The description of features is described in Supplementary Table S3 (in Supplementary File S3). These feature characteristics are stored in the form of a vector of peptide data characteristics that consists of 517 numerical features. The flowchart of feature extraction of peptide data characteristics follows the steps shown in Figure 4.
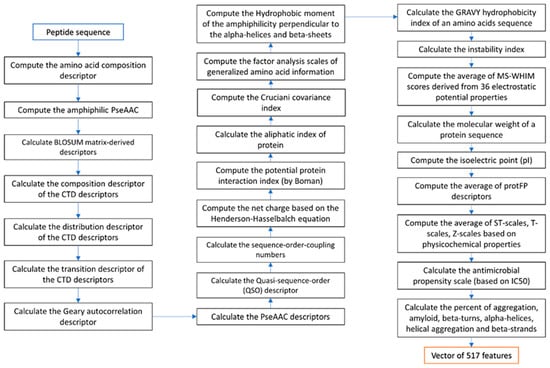
Figure 4.
Flowchart of feature extraction.
To improve the prediction with informative features, we proposed a hybrid feature generation by the fusion of various selected features using a logistic regression model. Logistic regression models were built by following the steps shown in Figure 5. Beginning with the preselection of features by using the wrapper feature selection method, feature subsets that specifically suit the logistic regression model were selected. The root-mean-square error (RMSE) and forward search method were performed in the wrapper method, providing 24 features that are most informative for the logistic regression model. To reduce the complexity of hybrid features and avoid the overfitting issues of the models, we used 4 features for creating a composite feature. A combination of 4 randomly selected features out of 24 preselected features generated a total of 10,626 sets of composite features. Next, logistic regression models were built using 10,626 sets of features for a total of 10,626 equations. Then, the performances of the logistic regression models were compared. The logistic regression model with the highest sensitivity would be selected for further study as a hybrid feature. The hybrid feature is defined as the following equation:
where β0 is the intercept, β1, β2, β3, and β4 represent the regression coefficients, APAAC1_5 is Amphiphilic Pseudo Amino Acid Composition of Cys (sequence-order-coupling mode along a protein sequence through a hydrophobicity correlation function), CTDD66 is the distribution descriptor of the first residue of the neutral charged amino acid found at the N terminus (Property 5 Group2 Residue 0), AMPA is the antimicrobial IC50 propensity index, and Tango4 is the percentage of aggregation conformation.
Hybrid feature = β0 + β1 APAAC1_5 + β2 CTDD66 + β3 AMPA + β4 Tango4
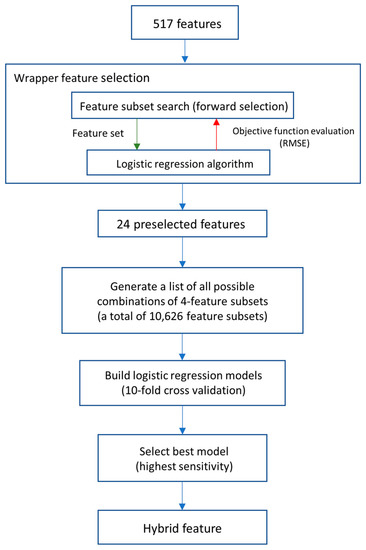
Figure 5.
Flowchart of building a hybrid feature.
2.4. Feature Selection
To select a discriminative feature subset, the feature selection (FS) method was used to select relevant and informative features that efficiently discriminate AMPs from non-AMPs. Various learning algorithms were used to compare the effectiveness of various feature selections, such as Infogain, ReliefF, and correlation-based feature selection (CFS). We used the area under the ROC curve (AUC) and sensitivity (Sn) values that were averaged over 10-fold cross-validation as measurements of the performance.
2.5. Prediction Models
We used a 10-fold cross-validation to examine the machine learning models. The performance of 10 results is averaged and reported as the performance of the classifier. Machine learning techniques, using both single predictive models and ensemble learning methods, were built and compared. The predictive models include neural networks using MLP, SVM, decision tree (DT), KNN, deep learning (DL), naïve Bayes (NB), linear discriminant analysis (LDA), radial basis function network (RBF), RF, max probability voting (MaxProbVote), majority voting, XGBoost, and AdaBoost. These models were built by using LibSVM [53], R programming [45], Waikato Environment for Knowledge Analysis (Weka) [54], and Python. Then, the models were fine-tuned and evaluated based on their performance and CPU processing requirements. The details of the models, hyperparameters, and parameter grid searches are described in the Supplementary File S3
In the algorithm selection for predictive program development, the model was selected based on the total efficiency of the program by evaluating the following metrics:
where ACC, Sn, Sp, and MCC are the accuracy, sensitivity, specificity, and Matthews coefficient correlation, respectively. These measurements were calculated based on the numbers of true positives (TPs), true negatives (TNs), FPs, and false negatives (FNs). The AUC was calculated to assess the tradeoff between the sensitivity and specificity performance of the different methods. The ROC is a plot of the TP rate vs. the FP rate at different thresholds. For a perfect predictor, the AUC is equal to 1.
3. Results and Discussion
3.1. Informative Features Extracted from Peptides Affect the Performance the Most
To detect hidden patterns, the feature extraction step is an important step to represent biological sequences with a fixed-length numerical form that can be further analyzed using a machine learning model to generalize to new unseen peptide data [51]. The proposed program consists of a module for extracting various features to represent peptides with 517 numerical features. This extraction module collects and extracts as many known peptide features as possible to have sufficient discrimination features in order to detect hidden patterns and to explain the characteristics of the peptide sequences that could be an active AMP.
The correlation between all 517 features was obtained by calculating the Pearson correlation coefficient. Then, Pearson correlation coefficients were plotted as shown in Figure S1. As shown in the correlation plot, there are some redundant and highly correlated features in the 517-dimensional feature set. Therefore, the feature selection step is needed to filter and select only informative and effective features. Feature selection is an important data analysis process to select a more effective feature subset, which can reduce the computation time and complexity, remove redundant features, and improve the understandability and simplicity of the model [55]. Therefore, different feature selection methods were compared. To select the discriminative feature subset, the empirical performance comparison of individual predictive models using different feature sets from various state-of-the-art feature selection methods, such as Infogain, ReliefF, CFS with a best-first search, and CFS with a genetics search, was performed. The CFS with a genetic search method consistently outperforms other feature selection methods in most classifiers while retaining fewer selected features on average. Compared to other feature selection methods, the CFS method can drastically reduce the dimensionality of datasets while maintaining or improving the performance of most learning algorithms (data not shown). The CFS method can remove redundant and irrelevant features based on the heuristic that “Good feature subsets contain features highly correlated with the target class, but uncorrelated with each other” [55]. Finally, based on comparison and the advantages of the CFS method, the 92 informative features (listed in Supplementary Table S2) that were selected by using the CFS method and genetics search were applied for further analysis in this work.
3.2. Performance Comparison of Various Predictive Models
Machine learning algorithms tend to be biased toward the majority class when the class distribution is imbalanced in order to yield high overall prediction accuracy. We also investigated the effect of balanced and imbalanced training sets in building models, as reported in Supplementary File S4. We compared the performance of two imbalanced datasets with the ratio of AMPs to non-AMPS equal to 1:3 (natural ratio of this dataset) and 1:2. From the empirical data, most classifiers do not learn the minority class well, often becoming more misclassified compared to the majority class. When the different ratio between classes is high, as the imbalance is more highly skewed, the effect of imbalanced training data will be more severe, as most learners will show more bias toward the majority class. However, in real life, the majority class is usually our class of interest, which is more focused. Therefore, developing well-balanced training data is an important step. There are two categories of methods that handle this class imbalance problem: Data-Level methods (e.g., data sampling), and Algorithm-Level methods (e.g., cost-sensitive and hybrid/ensemble) [56,57,58].
In this work, we have applied both the Data-Level and the Algorithm-Level methods to avoid the imbalance problem. Firstly, since AMP data are heterogeneous (various functional families, from various organisms), in order to ensure that the training sample can reflect all characteristics of the AMP sequences and remain generalized to all types of AMP data, proportionate stratified random sampling (Data-Level method) was applied. Therefore, the training data consisted of peptide sequences selected from both the AMP and non-AMP datasets to produce an equally balanced training dataset to reduce the likelihood of generating a predictive model biased toward the majority class. Then, balanced training data were used to train the predictive models. Secondly, our method is an ensemble or hybrid method (Algorithm-Level method), which is to say that it can also handle the imbalance problem (results in Section 3.3).
In this section, various single models were hyperparameter-optimized using a grid search, and the optimal selected parameters based on model selection are described in the Supplementary File S3: Parameter Optimization. There are many machine learning algorithms, and their performance is dependent on the characteristics of the data of interest. To detect the weaknesses and strengths of various algorithms on AMPs data, tenfold cross-validation was used to compare the performances of various single models, as shown in Table 3, consisting of eight single models, namely, MLP, SVM, KNN, RBF, LDA, NB, DT, and DL, and two existing state-of-the-art programs, namely, AMPScanner [16] (using the latest updated model in February 2020) and CAMP [11] (the results shown in Table 3 report only the highest performance of the four predictive models, RF, neural network (NN), SVM, and LDA).

Table 3.
Performance comparison of single machine learning models.
To evaluate the model, two testing datasets were used. Testing dataset 1 is the dataset, containing the latest and updated AMP and non-AMP sequences, compiled from public databases. It is available to download at [59]. Testing dataset 2 is a benchmark dataset S that has been used in testing several AMP predictive programs taken from [13]. As shown in Table 3. The SVM had the highest performance with an accuracy of 85.89% in the training data. The SVM uses few parameters that have been carefully selected using a grid search. However, the AUC of the SVM model is lower than that of the KNN, MLP, LDA and RBF models. The AUC depicts the tradeoff between the sensitivity and specificity values, which implies achieving a balance in predicting both true positive and true negative instances. Interestingly, the KNN model shows good performance in both accuracy and AUC (85.37% and 0.906, respectively). The advantage of the KNN model is that it is well suited for multimodal classes [60,61]. In fact, AMP-positive data can be considered multimodal class data composed of several types of AMPs, such as antibacterial, anticancer, antiviral, and antifungal AMPs. This may explain the KNN performance on the testing.
In addition, the two existing state-of-the-art programs, namely, AMPScanner (using the latest updated model in February 2020) and CAMP (reporting only the highest performance of the four predictive models, RF, NN, SVM, and LDA), were tested with an independent testing dataset, and their performance is reported in Table 3.
3.3. Ensemble Models have Better Performance than Single Models
Since the AMPs have diverse functions with heterogeneous data from various organisms, we hypothesize that the ensemble may be suitable for this type of data. Therefore, we investigated various types of ensemble models. The ensemble model has the ability to increase accuracy by combining the output of multiple diverse classifiers to reduce bias and variance. Moreover, an ensemble can improve efficiency by decomposing complex problems into multiple subproblems. A proper combination of diverse predictors through the ensemble method can efficiently exploit the strength of the single predictive models by considering multiple points of view to obtain a more accurate and robust (stable) prediction. Therefore, in this work, the ensemble models based on bagging, boosting, and voting techniques were also built and compared, as shown in Table 4. The five ensemble models include the RF, MaxProbVote (where the answer from model with a higher probability between the RF and KNN models is chosen), majority vote, XGBoost, and AdaBoost. Comparing the five ensemble models based on accuracy, the MaxProb voting of the RF and KNN has the highest overall accuracy of 87.41% with a high AUC of 0.925 using the training datasets. When comparing the performance based on testing dataset 1, the MaxProbVote model has the highest performance compared to other models in predicting the AMPs of testing dataset 1, but it has a lower performance in identifying AMP-positive sequences than the RF model in testing dataset 2. Table 4 also includes the performances of the five ensemble models integrated with the new hybrid feature (the new hybrid feature will be discussed in Section 3.4). When comparing the 10 ensemble models (with and without the hybrid feature), based on overall accuracy, we found that the majority vote model with the hybrid feature achieved the highest accuracy of 88.63% with an AUC of 0.927. However, MaxProbVote models provide better predictive tradeoffs between sensitivity and specificity than the majority vote model, with a higher AUC value of 0.946. This suggests that a tradeoff between the specificity and sensitivity of the MaxProbVote method is relatively more appropriate.

Table 4.
Performance comparison of ensemble machine learning models.
We found that the performances of ensembles based on bagging and voting methods are comparable (RF, MaxProbVote, and majority voting with and without the hybrid feature). However, for all of the predictive models, both single and ensemble models are included in the Ensemble-AMPPred program (therefore, users can choose and compare the results between these models, especially to use them as a decision support system for a situation in which conflicting predictions occur).
3.4. Hybrid Feature to Improve the Sensitivity of the Predictive Performance
To add some additional features for capturing the major and subtle patterns to differentiate actual positives from negatives, we include feature engineering and transformation experiments in this work. We propose a new feature by building a hybrid feature based on feature fusion using a logistic regression equation. The logistic regression model that had the highest sensitivity (Sn = 77.07%) among the others was selected for further use as a hybrid feature. For this new feature, APAAC1_5, CTDD66, AMPA, and Tango4 features were included in this logistic regression and applied as a hybrid feature. The hybrid feature was integrated into the ensemble to test whether this new proposed feature can further improve the sensitivity of the models. We also performed feature ranking based on various filtering feature selection techniques (including information gain, gain ratio, chi-square, consistency, and Pearson’s correlation feature ranking) and found that the newly added hybrid feature can be ranked in the top ranks (in the top 1–5 ranking of most feature selection methods). Moreover, the hybrid feature was the top ranked variable in the plots of variable importance during the process of building both the RF model and the XGBoost model (as shown in Supplementary Figures S2–S3), which indicates the highly significant contribution of the hybrid feature to the prediction performance.
To demonstrate the enhancement when adding the proposed new hybrid feature to the model, Table 4 shows that the overall ACC and AUC values obtained from the ensemble with the hybrid feature are 0.54–2.99% and 0.30–3.92% higher, respectively, than those of the model without the hybrid feature based on 10-fold cross-validation (CV) of the training datasets. Interestingly, we found that the hybrid feature can improve the sensitivity and overall accuracy of the ensemble model based on the bagging and voting methods (RF, MaxProbVote, and majority vote models). We highlight that our hybrid feature is more advantageous for use with bagging or voting ensemble strategies. In particular, including the hybrid feature showed significantly improved performance in the tradeoff between true positive and false positive prediction, as indicated by a better improvement in AUC values.
Actually, the MaxProbVote model with hybrid features (with an ACC of 88.13% and an AUC of 0.946) and the majority vote model with hybrid features (with an ACC of 88.63% and an AUC of 0.927) showed comparable performances. However, the MaxProbVote model with the hybrid feature was chosen as the default model in the Ensemble-AMPPred program because the AUC is higher (AUC is a measure of the value that shows that the model has a better tradeoff between both positive and negative predictions).
3.5. Comparison with Existing Prediction Methods
As shown in Table 3, we also reported the testing dataset 1 and 2 prediction results of the other two existing methods: CAMP and AMPScanner. (We initially planned to test more predictive programs; however, since our testing dataset is quite large, other webserver programs become unavailable or nonresponsive.) Based on the testing results using the two testing datasets, our ensemble models (in Table 4) can recognize both AMPs and non-AMPs based on accuracy in predicting positive and negative data compared with the currently available programs, AMPScanner (a DL model using a deep neural network (DNN) and long short-term memory (LSTM) deep learning) and CAMP (LDA, SVM, RF, and NN models), as shown in Table 3. Testing dataset 2, which contains benchmarked data, was not generated by our group. In contrast, testing dataset 1 is quite large, and the latest updated dataset (updated February 2020) was generated by our group. In addition to these two testing datasets, we added another smaller benchmark dataset that included all AMPs from the ADP3 database [62] (obtained in October 2020) and the smaller UniProt dataset as negative data to be compared with another two programs, i.e., iAMP-2 L and iAMPpred. The comparison result is in the Supplementary File S5. The results provide more details beyond the predictive performance summary. The information shows more details about the distribution of incorrect predictions of both false positive and false negative results of all five programs, including our Ensemble_AMPPred (MaxProbVote model), CAMP, AMPscanner, iAMP-2 L, and iAMPpred. This result also gives a better understanding of the different behaviors of different predictive models and identifies influential instances in the testing data, which is the weakness of individual predictive models that makes them perform poorly.
The contributions of this research are as follows: (1) To the best of our knowledge, we collected the largest AMP dataset from 15 public databases, in contrast to previous works that used one to two databases, such as the CAMP and/or APD database. As AMPs are highly heterogeneous, different subtypes of AMPs exist; therefore, we performed proportionate stratified random sampling by partitioning into homogeneous strata based on clustering. Representative sequences were selected from each cluster for using as training data. (2) We collected and employed as many features as possible. Sequence features are important for the antimicrobial activity and in vivo stability of AMPs. Such sequence features also contribute to AMP prediction. Different AMP predictors use different sets of features. For a state-of-the-art deep learning approach, the algorithm automatically extracts features (in the form of a matrix of the weight numbers from deep neural nets). Thus, the deep learning method does not possess a feature engineering step. In our method, feature extraction and selection were conducted. The features are relatively more explainable in terms of biological meanings. We attempted to capture some explainable relationship in the features, which may provide an advantage in AMP sequence design in the future. (3) We include the process of feature engineering, i.e., modeling, to describe or capture relationships between interpretable features to create the hybrid feature. Moreover, we expect that this type of information will be helpful in exploring or designing AMPs sequence in the future. Moreover, we plan to explore this type of feature in greater detail. (4) We investigated various types of machine learning technique for building predictors, including both single and ensemble techniques.
4. Conclusions
Ensemble-AMPPred is an AMP prediction and recognition program that contains various predictive models, including individual single models and ensemble models. The overall accuracy obtained by Ensemble-AMPPred is significantly higher than that obtained by the existing methods on the same benchmark dataset. We found that the ensemble model based on the voting technique, especially the MaxProbVote model, has a better tradeoff performance between sensitivity and specificity. Moreover, including the new hybrid feature into the ensemble-based models can improve the accuracy of these predictive models. All the predictive models based on single or ensemble machine learning algorithms are included in Ensemble-AMPPred and are available to download at [59]. Therefore, users can choose between models and can compare and distinguish the results between these models. Moreover, users can use these models as a decision support system for screening new AMPs prior to in vitro laboratory experiments or use them in a situation in which conflicting predictions occur.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/12/2/137/s1, Supplementary File S1: CD-HIT clustering result of AMP sequences (positive data), Supplementary File S2: Sequence similarities between training and testing sequences, Supplementary File S3: Ensemble AMP Prediction (Figure S1: The correlation between all features obtained by calculating the Pearson correlation coefficient, Figure S2: Variable importance plot. The importance of each of the features for predicting AMPs with a random forest. The most important feature that significantly contributed to the prediction performance was the hybrid feature, Figure S3: Variable importance plot. The importance of each of the features for predicting AMPs with XGBoost. The most important feature that significantly contributed to the prediction performance was the hybrid feature. Table S1: 517 feature descriptors, Table S2: A list of 92 features selected by CFS + Genetic Search), Supplementary File S4: Performance comparison of machine learning models when trained with imbalanced datasets, Supplementary File S5: Preliminary performance comparison of available AMP prediction tools.
Author Contributions
Conceptualization, S.L., A.H. and C.T.; methodology, S.L.; investigation, S.L.; writing—original draft preparation, S.L.; writing—review and editing, S.L., T.V., A.H. and C.T.; supervision, A.H. and C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Center for Genetic Engineering and Biotechnology (BIOTEC), CPM, the National Science and Technology Development Agency (NSTDA), Bangkok, Thailand. Grant number: P18-51620.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the supplementary files.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, Q.; Ke, H.; Li, D.; Wang, Q.; Fang, J.; Zhou, J. Recent progress in machine learning-based prediction of peptide activity for drug discovery. Curr. Top. Med. Chem. 2019, 19, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; Fuente-Nunez, C. Peptide design principles for antimicrobial applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef] [PubMed]
- Torrent, M.; Di Tommaso, P.; Pulido, D.; Nogués, M.V.; Notredame, C.; Boix, E.; Andreu, D. AMPA: An automated web server for prediction of protein antimicrobial regions. Bioinformatics 2011, 28, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.R.; Jhong, J.H.; Wang, Z.; Chen, S.; Wan, Y.; Horng, J.T.; Lee, T.Y. Characterization and identification of natural antimicrobial peptides on different organisms. Int. J. Mol. Sci. 2020, 21, 986. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238. [Google Scholar] [CrossRef]
- Maria-Neto, S.; Almeida, K.C.; Macedo, M.L.; Franco, O.L. Understanding bacterial resistance to antimicrobial peptides: From the surface to deep inside. Biochim. Biophys. Acta 2015, 1848, 3078–3088. [Google Scholar] [CrossRef]
- Cardoso, M.H.; Orozco, R.Q.; Rezende, S.B.; Rodrigues, G.; Oshiro, K.G.N.; Cândido, E.S.; Franco, O.L. Computer-aided design of antimicrobial peptides: Are we generating effective drug candidates? Front. Microbiol. 2020, 10, 3097. [Google Scholar] [CrossRef]
- Lata, S.; Sharma, B.K.; Raghava, G.P.S. Analysis and prediction of antibacterial peptides. BMC Bioinform. 2007, 8, 263–272. [Google Scholar] [CrossRef]
- Meher, P.; Sahu, T.; Saini, V.; Rao, A.R. Predicting antimicrobial peptides with improved accuracy by incorporating the compositional, physico-chemical and structural features into Chou’s general PseAAC. Sci. Rep. 2017, 7, 42362. [Google Scholar] [CrossRef]
- Fjell, C.; Jenssen, H.; Hilpert, K.; Cheung, W.A.; Pante, N.; Hancock, R.E.; Cherkasov, A. Identification of novel antibacterial peptides by chemoinformatics and machine learning. J. Med. Chem. 2006, 52, 2006–2015. [Google Scholar] [CrossRef]
- Waghu, F.; Barai, R.; Gurung, P.; Thomas, S. CAMPR3: A database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res. 2016, 44, D1094–D1097. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Mishra, N.K.; Raghava, G.P. AntiBP2: Improved version of antibacterial peptide prediction. BMC Bioinform. 2010, 11, S1–S19. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, P.; Lin, W.Z.; Jia, J.H.; Chou, K.C. iAMP-2L: A two- level multi-label classifier for identifying antimicrobial peptides and their functional types. Anal. Biochem. 2013, 436, 168–177. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Gabrielian, A.; Cruz, P.; Griggs, H.L.; Squires, R.B.; Hurt, D.E.; Grigolava, M.; Chubinidze, M.; Gogoladze, G.; Vishnepolsky, B.; et al. DBAASP v.2: An enhanced database of structure and antimicrobial/cytotoxic activity of natural and synthetic peptides. Nucleic Acids Res. 2016, 44, D1104–D1112. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Xu, D. Imbalanced multi-label learning for identifying antimicrobial peptides and their functional types. Bioinformatics 2016, 32, 3745–3752. [Google Scholar] [CrossRef]
- Veltri, D.; Kamath, U.; Shehu, A. Deep learning improves antimicrobial peptide recognition. Bioinformatics 2018, 34, 2740–2747. [Google Scholar] [CrossRef] [PubMed]
- Gabere, M.N.; Noble, W.S. Empirical comparison of web-based antimicrobial peptide prediction tools. Bioinformatics 2017, 33, 1921–1929. [Google Scholar] [CrossRef]
- Polikar, R. Ensemble based systems in decision making. IEEE Circuits Syst. Mag. 2006, 6, 21–45. [Google Scholar]
- Breiman, L. Bagging predictors. Mach. Learn. 1996, 26, 123–140. [Google Scholar] [CrossRef]
- Freund, Y. Boosting a weak learning algorithm by majority. Inf. Comput. 1995, 121, 256–285. [Google Scholar] [CrossRef]
- Dietterich, T.G. An experimental comparison of three methods for constructing ensembles of decision trees: Bagging, boosting, and randomization. Mach. Learn. 2000, 40, 139. [Google Scholar] [CrossRef]
- Wolpert, D.; Macready, W. No free lunch theorems for optimization. IEEE Trans. Evol. Comput. 1997, 1, 67–82. [Google Scholar] [CrossRef]
- Kuncheva, L. Combining Pattern Classifiers: Methods and Algorithms, 2nd ed.; Wiley: Hoboken, NJ, USA, 2014; Volume 8, pp. 263–272. [Google Scholar] [CrossRef]
- Li, W.; Godzik, A. Cd-hit: A fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 2006, 22, 1658–1659. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, V.; Subramaniyam, S.; Malik, A.; Lee, G.; Manavalan, B.; Yang, D. mACPpred: A support vector machine-based meta-Predictor for identification of anticancer peptides. Int. J. Mol. Sci. 2019, 20, 1964. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The universal protein knowledgebase. Nucleic Acids Res. 2017, 45, D158–D169. [Google Scholar] [CrossRef] [PubMed]
- Anekthanakul, K.; Hongsthong, A.; Senachak, J.; Ruengjitchatchawalya, M. SpirPep: An in-silico digestion-based platform to assist bioactive peptides discovery from a genome-wide database. BMC Bioinform. 2018, 19, 149. [Google Scholar] [CrossRef]
- Available online: http://www.jci-bioinfo.cn/iAMP/data.html (accessed on 17 February 2020).
- Available online: https://www.dveltri.com/ascan/v2/data/AMP_Scan2_Feb2020_Dataset.zip (accessed on 17 February 2020).
- Wang, G.; Li, X.; Wang, Z. APD2: The updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009, 37, D933–D937. [Google Scholar] [CrossRef] [PubMed]
- Hammami, R.; Zouhir, A.; Lay, C.; Hamida, J.; Fliss, I. BACTIBASE second release: A database and tool platform for bacteriocin characterization. BMC Microbiol. 2010, 10, 22. [Google Scholar] [CrossRef]
- Heel, A.; Jong, A.; Montalbán-López, M.; Kok, J.; Kuipers, O. BAGEL3: Automated identification of genes encoding bacteriocins and (non-)bactericidal posttranslationally modified peptides. Nucleic Acids Res. 2013, 41, W448–W453. [Google Scholar] [CrossRef]
- Thomas, S.; Karnik, S.; Barai, R.S.; Jayaraman, V.K.; Idicula-Thomas, S. CAMP: A useful resource for research on antimicrobial peptides. Nucleic Acids Res. 2010, 38, D774–D780. [Google Scholar] [CrossRef]
- Kang, X.; Dong, F.; Shi, C.; Liu, S.; Sun, J.; Chen, J.; Li, H.; Xu, H.; Lao, X.; Zheng, H. DRAMP 2.0, an updated data repository of antimicrobial peptides. Sci. Data 2019, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Seebah, S.; Suresh, A.; Zhuo, S.; Choong, Y.; Chua, H.; Chuon, D.; Beuerman, R.; Verma, C. Defensins knowledgebase: A manually curated database and information source focused on the defensins family of antimicrobial peptides. Nucleic Acids Res. 2007, 35, D265–D268. [Google Scholar] [CrossRef] [PubMed]
- Zamyatnin, A.A.; Borchikov, A.S.; Vladimirov, M.G.; Voronina, O.L. The EROP-Moscow oligopeptide database. Nucleic Acids Res. 2006, 34, D261–D266. [Google Scholar] [CrossRef] [PubMed]
- Gueguen, Y.; Garnier, J.; Robert, L.; Lefranc, M.P.; Mougenot, I.; de Lorgeril, J.; Janech, M.; Gross, P.S.; Warr, G.W.; Cuthbertson, B.; et al. Penbase, the shrimp antimicrobial peptide penaeidin database: Sequence-based classification and recommended nomenclature. Dev. Comp. Immunol. 2006, 30, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, H.; Lu, H.; Li, G.; Huang, Q. LAMP: A database linking antimicrobial peptides. PLoS ONE 2013, 8, e66557. [Google Scholar] [CrossRef] [PubMed]
- Hammami, R.; Hamida, J.; Vergoten, G.; Fliss, I. PhytAMP: A database dedicated to antimicrobial plant peptides. Nucleic Acids Res. 2009, 37, D963–D968. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z. RAPD: A database of recombinantly-produced antimicrobial peptides. FEMS Microbiol. Lett. 2008, 289, 126–129. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Tyagi, A.; Tuknait, A.; Anand, P.; Gupta, S.; Sharma, M.; Mathur, D.; Joshi, A.; Singh, S.; Gautam, A.; Raghava, G. CancerPPD: A database of anticancer peptides and proteins. Nucleic Acids Res. 2015, 43, D837–D843. [Google Scholar] [CrossRef]
- Mehta, D.; Anand, P.; Kumar, V.; Joshi, A.; Mathur, D.; Singh, S.; Tuknait, A.; Chaudhary, K.; Gautam, S.; Gautam, A.; et al. ParaPep: A web resource for experimentally validated antiparasitic peptide sequences and their structures. Database 2014, 2014. [Google Scholar] [CrossRef]
- Xiao, N.; Cao, D.S.; Zhu, M.F.; Xu, Q.S. protr/ProtrWeb: R package and web server for generating various numerical representation schemes of protein sequences. Bioinformatics 2015, 31, 1857–1859. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; ISBN 3-900051-07-0. [Google Scholar]
- Osorio, D.; Rondon-Villarreal, P.; Torres, R. Peptides: A package for data mining of antimicrobial peptides. R J. 2015, 7, 4–14. [Google Scholar] [CrossRef]
- Torrent, M.; Nogués, V.M.; Boix, E. A theoretical approach to spot active regions in antimicrobial proteins. BMC Bioinform. 2009, 10, 373. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Escamilla, A.M.; Rousseau, F.; Schymkowitz, J.; Serrano, L. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat. Biotech. 2004, 22, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, F.; Wang, X.; Chen, J.; Fang, L.; Chou, K. Pse-in-One: A web server for generating various modes of pseudo components of DNA, RNA, and protein sequences. Nucleic Acids Res. 2015, 43, W65–W71. [Google Scholar] [CrossRef]
- Chou, K.C. Using amphiphilic pseudo amino acid composition to predict enzyme subfamily classes. Bioinformatics 2005, 21, 10–19. [Google Scholar] [CrossRef]
- Chou, K.C. Some remarks on protein attribute prediction and pseudo amino acid composition (50th anniversary year review). J. Theor. Biol. 2011, 273, 236–247. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, L.; Zhang, T.; Zhao, Z.; Du, P. A brief review on software tools in generating Chou’s pseudo-factor representations for all types of biological sequences. Protein Pept. Lett. 2018, 25, 822–829. [Google Scholar] [CrossRef]
- Chang, C.; Lin, C. LIBSVM: A library for support vector machines. ACM Trans. Intell. Syst. Technol. 2011, 2, 21–27. [Google Scholar] [CrossRef]
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA data mining software: An update. SIGKDD Explor. Newsl. 2009, 11, 10–18. [Google Scholar] [CrossRef]
- Hall, M.A.; Holmes, G. Benchmarking attribute selection techniques for discrete class data mining. IEEE Trans. Knowl. Data Eng. 2003, 15, 1437–1447. [Google Scholar] [CrossRef]
- Leevy, J.; Khoshgoftaar, T.; Bauder, R.; Seliya, N. A survey on addressing high-class imbalance in big data. J. Big Data 2008, 5, 42. [Google Scholar] [CrossRef]
- Bauder, R.A.; Khoshgoftaar, T.M. The effects of varying class distribution on learner behavior for medicare fraud detection with imbalanced big data. Health Inf. Sci. Syst. 2018, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shamsuddin, S.M.; Ralescu, A.L. Classification with class imbalance problem: A review. Int. J. Adv. Soft Comput. Appl. 2015, 7, 176–204. [Google Scholar]
- Ensemble AMPPred. Available online: http://ncrna-pred.com/Hybrid_AMPPred.htm (accessed on 17 February 2020).
- Li, L.; Kuang, H.; Zhang, Y.; Zhou, Y.; Wang, K.; Wan, Y. Prediction of eukaryotic protein subcellular multi-localisation with a combined KNN-SVM ensemble classifier. J. Comput. Biol. Bioinform. Res. 2011, 3, 15–24. [Google Scholar]
- Wang, T.; Yang, J. Using the nonlinear dimensionality reduction method for the prediction of subcellular localization of gram-negative bacterial proteins. Mol. Divers. 2009, 13, 475–481. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).