Abstract
SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) transcription factors play critical roles in regulating diverse aspects of plant growth and development, including vegetative phase change, plant architecture, anthocyanin accumulation, lateral root growth, etc. In the present study, 15 SPL genes were identified based on the genome data of Codonopsis pilosula, a well-known medicinal plant. Phylogenetic analysis clustered CpSPLs into eight groups (G1-G8) along with SPLs from Arabidopsis thaliana, Solanum lycopersicum, Oryza sativa and Physcomitrella patens. CpSPLs in the same group share similar gene structure and conserved motif composition. Cis-acting elements responding to light, stress and phytohormone widely exist in their promoter regions. Our qRT-PCR results indicated that 15 CpSPLs were differentially expressed in different tissues (root, stem, leaf, flower and calyx), different developmental periods (1, 2 and 3 months after germination) and various conditions (NaCl, MeJA and ABA treatment). Compared with the control, overexpression of CpSPL2 or CpSPL10 significantly promoted not only the growth of hairy roots, but also the accumulation of total saponins and lobetyolin. Our results established a foundation for further investigation of CpSPLs and provided novel insights into their biological functions. As far as we know, this is the first experimental research on gene function in C. pilosula.
Keywords:
biomass; Codonopsis pilosula; expression patterns; hairy root; SPLs; secondary metabolites 1. Introduction
Transcription factors (TFs) function in various physiological and developmental processes via activating and/or repressing transcription of multiple target genes [1]. They have been usually divided into different families according to the sequence of DNA-binding domains and other conserved motifs [2]. SQUAMOSA-promoter binding protein-like (SPL or SBP) TFs are exclusive to plant and characterized by a highly conserved SBP domain and a nuclear localization signal (NLS) at the C-terminus. The SBP domain is approximately 76 amino acids and includes two zinc-binding sites (one zinc finger is C3H or C4, and the other is C2H4) essential for DNA binding, and the NLS partially overlaps with the second zinc finger [3,4,5,6]. AmSBP1 and AmSBP2 from Antirrhinum majus were the first discovered SBP-domain proteins in plants and were found to bind to the floral meristem identity gene SQUAMOSA promoter, so named them [3]. Since then, SPL genes have been identified in many plant species, including single-cell algae, mosses, gymnosperms and angiosperms [7]. With the rapid implication of high-throughput sequencing technology, more and more plant genome data have been released and genome-wide identification of the SPL gene family from model and non-model plants has been identified in Arabidopsis thaliana [8], Oryza sativa [9], Glycine max [10], Solanum lycopersicum [11], Malus domestica [12], Salvia miltiorrhiza [13], Vitis vinifera [14], Phyllostachys edulis [15], Capsicum annuum [16], Ricinus communis [17], etc.
The functions of SPL genes have been well characterized in the model plant Arabidopsis and they play important regulatory roles in diverse developmental progresses, including vegetative to reproductive phase transition, cotyledon- to vegetative-leaf transition, micro- and megasporogenesis, trichome formation, stamen filament elongation, axillary bud formation and lateral root growth [18,19,20,21,22,23]. Besides, they are involved in copper homeostasis, abiotic stress response, immune response and secondary metabolites production [24,25,26,27]. The functions of SPL genes from other species have also been identified. In rice, OsSPL14 has been found to promote panicle branching and grain productivity and OsSPL16 regulates grain yield and quality [28,29]. FvSPL10 from strawberry (Fragaria vesca) not only promotes early flowering, but also increases organ size, such as longer root, larger floral organ and seeds [30]. As a class of plant-specific gene family, some SPL genes are important candidates for improving plant agronomic traits by genetic engineering.
Codonopsis pilosula is a member of the Campanulaceae family. Its dried root, named “Dangshen” in Chinese, is one of the most widely used traditional Chinese medicine for replenishing qi (vital energy), strengthening body immunity, improving appetite, promoting gastrointestinal function, reducing blood pressure and curing gastric ulcers [31]. In addition, Dangshen is also a well-known health-care food in China and is listed in the “Food and Drug Homology Catalogue” approved by the National Health Commission of People’s Republic of China. Consequently, the demand for Dangshen is growing, and the yield and accumulation of bioactive metabolites is attracting more and more attention in the planting field [32,33]. Lobetyolin, alkaloids, polysaccharides and saponins are the major active ingredients in Dangshen, which are responsible for most of the pharmacological functions found in the medicine [34]. Lobetyolin, a general marker compound in Dangshen, has been well reported to exert multiple bioactivities, such as anti-cancer, anti-viral, anti-inflammatory, anti-oxidative, mucosal protective and xanthine oxidase inhibiting properties [35,36].
Although C. pilosula has received great attention on the chemical constituents and their pharmacological activities, relevant study of this species at the genetic level is lagging behind and only a few studies involved genes in C. pilosula [37,38,39,40]. Until now, the SPL gene family has never been reported in C. pilosula. Most recently, we have developed an efficient Agrobacterium rhizogenes-mediated transformation approach for transgenic hairy roots with this species [39], which lay a good foundation for genetic engineering of that species. Here, we identified 15 SPL genes based on the genome sequence of C. pilosula (data unpublished). Gene structure, conserved motif and cis-acting elements of 15 CpSPLs were systematically analyzed. Additionally, their spatiotemporal expression profiles in different tissues and expression patterns under various conditions (NaCl, MeJA and ABA treatment) were analyzed by qRT-PCR. Furthermore, we obtained CpSPL2 or CpSPL10 overexpressing transgenic hairy roots, and a significant increase was observed in the biomass and concentrations of total saponins and lobetyolin. As far as we know, this is the first experimental research on gene function in this species. These findings demonstrate that CpSPL2 and CpSPL10 positively regulate the growth of hairy roots and accumulation of active ingredients, which have great potential in improving the yield and quality of Dangshen.
2. Materials and Methods
2.1. Identification of SPL Genes in C. pilosula and Bioinformatic Analysis
The sequences of SBP domain (ID: PF03110), which were downloaded from Pfam database (http://pfam.xfam.org/, accessed on 20 July 2020), were used to search possible SPL genes in C. pilosula genome sequences (data unpublished) by HMMER (http://hmmer.org, accessed on 20 July 2020) with the e-value < 1 × 10−10. A total of 15 CpSPL genes containing a complete SBP domain were identified.
MEGA X software (https://mega.nz/, accessed on 25 July 2020) was used to construct the phylogenetic tree of 81 full-length SPL amino acid sequences, 15 from C. pilosula, 19 from O. sativa, 17 from S. lycopersicum, 14 from Physcomitrella patens and 16 from A. thaliana, with 1000 bootstraps in the Neighbor Joining (NJ) method. Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/, accessed on 25 July 2020) was used for gene structure analysis. The MEME program (http://meme-suite.org/, accessed on 25 July 2020) was used to for identification of the conserved motifs. The cis-acting elements of 15 CpSPL genes promoter regions (2000 bp upstream of the translation initiation codon “ATG”) were analyzed online (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 25 July 2020).
2.2. Plant Materials and Treatments
Seeds of C. pilosula were collected from Gansu Province, China. The botanical origin of the materials was identified by Professor ZheZhi Wang in Shaanxi Normal University. The specimens of the seeds were deposited in the herbarium of National Engineering Laboratory for Resource Development of Endangered Crude Drugs in Northwest of China, Shaanxi Normal University, Xi’an, China. The seeds of C. pilosula were germinated and incubated according to the method that we described previously [39].
For gene spatiotemporal expression analysis, the seeds were germinated and incubated in mixed soil of nutrient soil, perlite and vermiculite in a glass greenhouse with a temperature regime of 24 ± 2 °C, 40–50% relative humidity. The leaves, stems and roots were collected separately from one-month-old seedlings, two-month-old seedlings, and three-month-old plantlets, and the flower and calyx were collected from the plants at the flowering stage. To test CpSPLs responses to hormonal and stress treatments, two-week-old seedings were treated with 200 mmol NaCl, 200 µmol MeJA, and 100 µmol ABA, respectively, and samples were gathered after 6 h as we have described previously [40]. The control group was treated with the same amount of ddH2O and all the samples were collected 6 h after treatment.
2.3. Gene Expression Analysis
For qRT-PCR analysis, total RNA was extracted and then reverse transcribed into cDNA as we described previously [40]. All the primer sequences used for qRT-PCR are listed in Supplementary Material Table S1 and CpGAPDH was used as the internal control [40]. The relative expression levels of 15 CpSPLs were calculated according to the method described by Livak and Schmittgen [41]. All the experiments included three biological and three technical replicates.
2.4. Vector Construction and Hairy Root Transformation
The complete open reading frames of CpSPL2 and CpSPL10 were amplified through PCR using the specific primer pairs CpSPL2-F/R and CpSPL10-F/R (Supplementary Material Table S1), respectively, with the following PCR conditions: 95 °C, 3 min; 30 cycles of 95 °C, 10 s, 58 °C, 30 s, 72 °C, 45 s; 72 °C, 5 min. Then the products were digested with Pac I and Asc I and ligated into pMDC85 to generate overexpression (OE) vectors pMDC85-CpSPL2 and pMDC85-CpSPL10.
Transgenic hairy roots overexpressing CpSPL2 or CpSPL10 were obtained by Agrobacterium-mediated method according to the protocol established in our lab [39]. Briefly, the roots of four-week-old C. pilosula aseptic seedlings were cut into an average length of 0.5 cm. The surface of the explants was gently scratched with a scalpel back, inoculated on MS medium, and pre-cultured at 24 ± 2 °C for 2 days in dark. The pre-cultured explants were immersed in the infection solution for 5 min and inoculated onto 1/2 MS solid medium for co-cultivation for 2 days. After co-cultivation for 2 days, the explants were transferred to 1/2 MS solid medium supplemented with 200 mg/L cefotaxime sodium (Cef) and 2 mg/L hygromycin. In parallel, pMDC85 was introduced into C. pilosula as the empty vector control (EV). Every transgenic line was excised and sub-cultured separately as we described previously [39]. Five independent CpSPL2-OE lines, seven CpSPL10-OE lines and four EV lines were obtained, and then confirmed by genomic DNA PCR using primers hptII-F/R (Supplementary Material Table S1) for hygromycin phosphotransferase II gene (hptII), followed by expression analysis of CpSPL2 or CpSPL10 by qRT-PCR.
2.5. Determination of Lobetyolin and Total Saponins
Transgenic hairy roots sub-cultured for one month were used for determination of lobetyolin and total saponins.
To determine the concentration of lobetyolin, we ground 50 mg dried hairy roots into powder, followed by extracted three times with 10 mL methanol in an ultrasonic bath (Kunshan Instrument Co., Ltd., Kunshan, China) for 30, 20 and 15 min, respectively. The extracts were put together and the methanol solution was evaporated, followed by dissolving with methanol to 5 mL volumetric flask. After filtration with 0.22 μm microporous membrane, the solution was used for HPLC analysis on a Shimadzu LC-20A instrument (Shimadzu, Kyoto, Japan) equipped with an Agilent 5 TC-C18 column (250 × 4.6 mm, 5 μm). The mobile phase consisted of ultrapure water (A) and methanol (B) and the gradient condition was 0–5 min, 20–40% B; 5–10 min, 40–70% B; 10–12 min, 79–90% B; 12–25 min, 90% B. The separation was performed at 30 °C, with the flow rate of 1.0 mL/min and UV detector wavelength at 220 nm. The concentration of total saponins in transgenic hairy roots was determined as we described previously [39].
2.6. Statistical Analysis
All the experiments and data presented here involved at least three biological repeats. SPSS version 20.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical evaluation. The error bars indicate standard deviation. Significant difference of the mean values was set at p < 0.05.
3. Results
3.1. Genome-Wide Identification and Sequence Feature Analysis of CpSPLs
To identify possible SPL genes in C. pilosula genome sequences, we employed the SBP domain (PF03110) to search the databases by HMMER. A total of 15 SPLs containing complete SBP domain were identified based on the genome sequence of C. pilosula and their cDNA sequences were listed in Supplementary Material Table S2. Consulting the homologous AtSPLs in Arabidopsis, 15 CpSPLs were named from CpSPL1 to CpSPL15. The deduced CpSPLs exhibited great variations in terms of their molecular weight (MW), ranging from 17.98 (CpSPL5) to 119.80 KDa (CpSPL14). Similarly, the lengths of the CDS were found to be varied in the CpSPLs, from 480 (CpSPL5) to 3276 bp (CpSPL14). The detailed information, including the gene length, intron number, protein length, predicted MW and theoretical isoelectric point (pI), are listed in Table 1.

Table 1.
The information of 15 SPL genes in Codonopsis pilosula.
3.2. Phylogenetic Analysis of CpSPLs
We constructed a phylogenetic tree of 15 CpSPLs, 19 OsSPLs, 17 SlySPLs, 14 PpSPLs and 16 AtSPLs using MEGA X with NJ method. As shown in Figure 1, 81 SPLs from two species were classified into eight groups, named from G1 to G8, and each group consisted of at least one SPL from C. pilosula. There are only five members in G2 (CpSPL6, AtSPL6, Sly05g012040, Sly03g114850 and Sly12g038520) and six members in G3 (CpSPL7, AtSPL7, OsSPL9, Sly01g080670, Pp3c331330v3 and Pp3c331350v3). G6 is the largest group with three PpSPLs (Pp3c167480v3/Pp3c258630v3/Pp3c167490v3), five OsSPLs (OsSPL17/14/7/13/8), four SlySPLs (Sly10g078700/Sly02g77920/Sly10g009080/Sly07g062980), four CpSPLs (CpSPL3/4/9/15) and two AtSPLs (AtSPL9/15). In Arabidopsis, AtSPL2/10/11, three members closely related, regulate root regeneration by inhibiting auxin biosynthesis [42]. Phylogenetic tree clustered CpSPL2, CpSPL10, AtSPL2, AtSPL10 and AtSPL11 in G1, indicating CpSPL2 and CpSPL10 are probably involved in root growth.
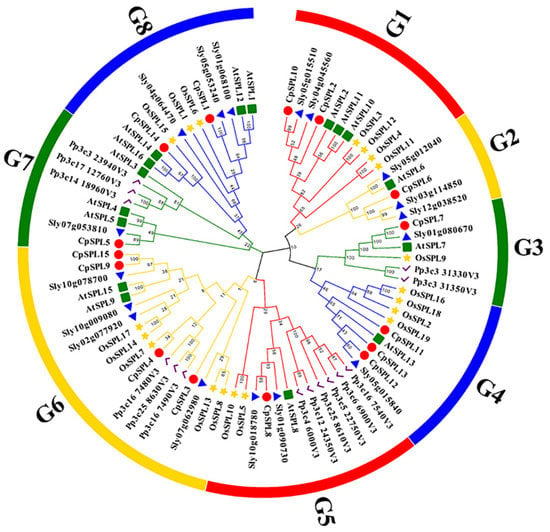
Figure 1.
An NJ phylogenetic tree of the SPLs from Codonopsis pilosula, Arabidopsis thaliana, Solanum lycopersicum, Oryza sativa and Physcomitrella patens.
3.3. Gene Structure and Conserved Motif Analysis
To clarify the structural diversities of 15 CpSPLs, we performed gene exon/intron structure analysis. The result displayed that the number of introns had a high variation and ranged from 1 to 10 (Figure 2). Interestingly, we found that most CpSPLs in the same group share a similar structure. For instance, CpSPL1 and CpSPL14, belonging to G8, have 10 introns, respectively (Figure 2).
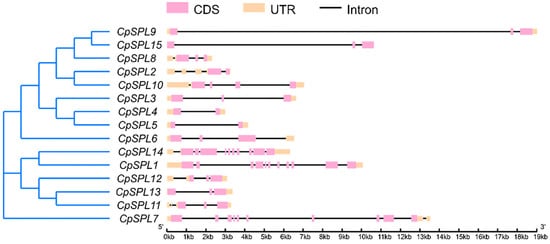
Figure 2.
Exon–intron organization structures of 15 SPL genes in Codonopsis pilosula. Exons are represented by pink rectangles, introns are represented by black lines, UTRs are represented by orange rectangles.
To explore the conserved motifs, 15 CpSPLs were subjected to analysis with MEME program. Among the 12 conserved motifs identified (Figure 3 and Supplementary Material Table S3), motif 1, motif 2 and motif 3 existed in all the 15 CpSPLs and formed the conserved SBP domain. Similar motif composition existed in the same group. For example, CpSPL2 and CpSPL10 in G1 all consisted of five conserved motifs (motif 1/2/3/10/11). The motif composition in CpSPL9 was completely consistent with that in CpSPL15, suggesting that CpSPL9 and CpSPL15 probably have similar and redundant functions in plant development.
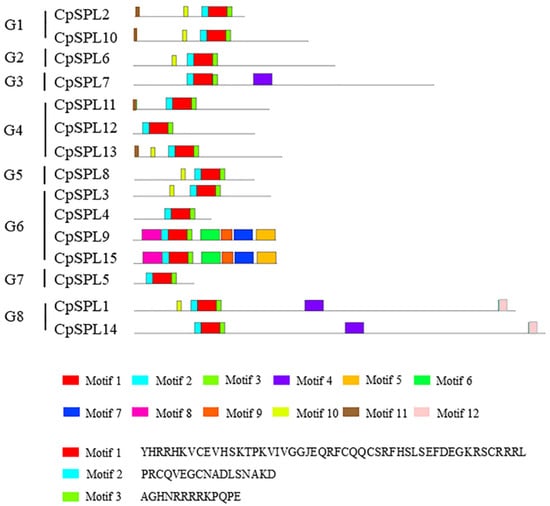
Figure 3.
The analysis of conserved motifs of SPLs in Codonopsis pilosula. Left panel: eight groups based on the NJ phylogenetic tree in Figure 1. Below panel: frames in different colors represent different protein motifs, and each motif has its own number.
3.4. Cis-Acting Elements Analysis of CpSPLs Promoter Regions
We analyzed the cis-acting elements of 15 CpSPLs promoter regions and light responsive elements (including G-box, GATA-motif, GTGGC-motif, AE-box, TCT-motif and chs-CMA2a), hormone responsive elements (such as gibberellin (GARE-motif), MeJA (CGTCA- and TGACG-motif), and abscisic acid (ABA) (ABRE)), stress responsive elements (such as drought (MBS), low-temperature (LTR), and anaerobic induction (ARE)), and CAT box related to meristem expression were found in their promoter regions (Supplementary Material Table S4). Among these cis-elements, MeJA-responsive elements existed in the promoter regions of almost all the CpSPLs except for CpSPL9 and CpSPL13, and ABA-responsive element (ABRE) existed in the promoter regions of 10 CpSPLs (including CpSPL1, CpSPL6-10, CpSPL12 and CpSPL14-15).
3.5. Spatiotemporal Expression Analysis of CpSPL Genes
We investigated the expression patterns of 15 CpSPLs in the leaves, stems and roots from one-month-old seedlings, two-month-old seedlings, and three-month-old plantlets, and the flower and calyx from the plants at the flowering stage by qRT-PCR assay. The results showed that most CpSPLs expressed in almost all the tissues (Figure 4). Compared with other genes, the expression level of CpSPL7 was more constant in all the tissues tested. CpSPL8 showed highest level in calyx. CpSPL5 was expressed at relatively higher levels in leaf and calyx. The expression levels of CpSPL3, CpSPL8, CpSPL10, CpSPL12 and CpSPL13 in the stems gradually decreased with the maturation of the seedlings. CpSPL1 and CpSPL14, two members in G1, showed similar expression patterns and their expression levels in the root increased gradually with the maturation of the seedlings. In addition, the expression patterns of CpSPL9 and CpSPL15 were highly similar, with higher levels in flowers and 3-month-old roots. In summary, spatiotemporal expression analysis results indicated that CpSPL genes exhibited various expression patterns, which provide preliminary information for understanding their potential functions in the development of C. pilosula.
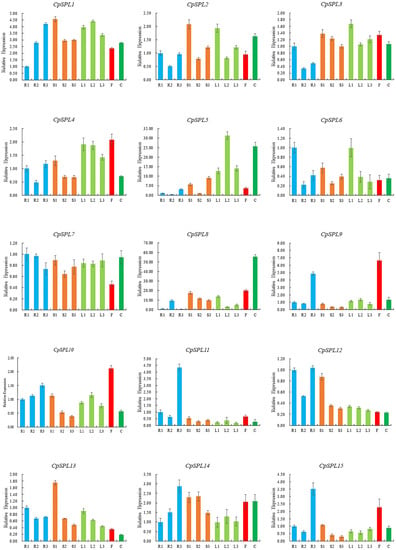
Figure 4.
Spatiotemporal expression analysis of 15 SPL genes in Codonopsis pilosula. R1, R2 and R3 represent roots from one-month-old seedlings, two-month-old seedlings and three-month-old plantlets, respectively; S1, S2 and S3 represent stems from one-month-old seedlings, two-month-old seedlings and three-month-old plantlets, respectively; L1, L2 and L3 represent leaves from one-month-old seedlings, two-month-old seedlings and three-month-old plantlets, respectively; F: flower; C: calyx.
3.6. Expression Profiles of CpSPLs under Various Conditions
To assess the expression profiles of 15 CpSPL genes under various treatments (NaCl, MeJA and ABA), a histogram was generated using the relative expression level (Figure 5). When treated with NaCl, the transcript levels of eight CpSPLs (CpSPL1, CpSPL2, CpSPL4, CpSPL6, CpSPL7, CpSPL11, CpSPL14 and CpSPL15) and four CpSPLs (CpSPL5, CpSPL8, CpSPL10 and CpSPL13) were significantly upregulated and downregulated, respectively. Among those, CpSPL2, CpSPL6 and CpSPL11 increased to 6.02, 5.66 and 7.94 times than the control, respectively, while CpSPL5 and CpSPL8 decreased to 20.00 and 12.50 times than the control, respectively (Figure 5A). For MeJA treatment, the transcript levels of CpSPL4, CpSPL6, CpSPL14 and CpSPL15 significantly increased, with the highest fold change in CpSPL15 (3.03-fold). The transcript levels of five CpSPL genes (CpSPL3, CpSPL5, CpSPL8, CpSPL10 and CpSPL11) significantly decreased, with 14.28- and 10.00-fold change in CpSPL5 and CpSPL8, respectively (Figure 5B). Under ABA treatment, eight CpSPLs (CpSPL1, CpSPL4, CpSPL6, CpSPL7, CpSPL9, CpSPL12, CpSPL14 and CpSPL15) responded positively to the treatment, while three genes (CpSPL3, CpSPL5 and CpSPL8) responded negatively to ABA treatment. Among those genes, CpSPL15 and CpSPL8 exhibited highest upregulation and downregulation, respectively (Figure 5C).
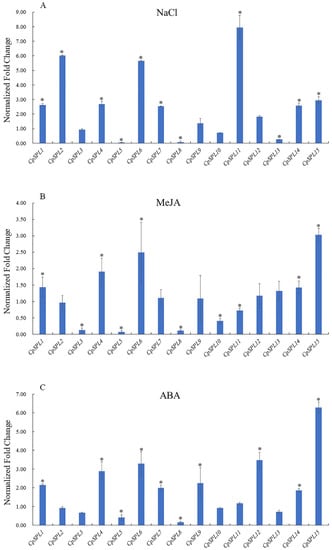
Figure 5.
Relative expression levels of 15 SPL genes in Codonopsis pilosula under various treatments. (A) Expression changes of 15 CpSPL genes when two-week-old seedings were treated with 200 mmol NaCl. (B) Expression changes of 15 CpSPL genes when two-week-old seedings were treated with 200 µmol methyl jasmonate (MeJA). (C) Expression changes of 15 CpSPL genes when two-week-old seedings were treated with 100 µmol abscisic acid (ABA). Change multiples are relative to the expression level of each gene treated with ddH2O (set to 1). One-way ANOVA (followed by Tukey’s comparisons) tested for significant differences among means (* indicates the value is significantly different from the control at p < 0.05).
3.7. Overexpression of CpSPL2 or CpSPL10 Promotes the Growth of C. pilosula Hairy Root
To investigate the function of CpSPL2 and CpSPL10 in root development, we generated CpSPL2-overexpressing or CpSPL10-overexpressing transgenic hairy roots. The expression level of CpSPL2 or CpSPL10 in the transgenics was examined by qRT-PCR (Figure 6A,B). Two independent CpSPL2-overexpressing lines (CpSPL2-OE3 and CpSPL2-OE5) and CpSPL10-overexpressing lines (CpSPL10-OE2 and CpSPL2-OE3) with dramatically elevated CpSPL2 or CpSPL10 expression were selected for further analysis. In comparison to the control, the hairy roots overexpressing CpSPL2 or CpSPL10 grew faster (Figure 6C,D). When the transgenic hairy roots with the length about 1.0 cm were cultured for one month, the biomass of CpSPL2-OE3, CpSPL2-OE5, CpSPL10-OE2 and CpSPL10-OE3 was 2.19, 1.98, 3.15 and 2.83 times that of the control (EV2), respectively (Figure 6E). Our results indicated that both CpSPL2 and CpSPL10 promote the growth of hairy roots.
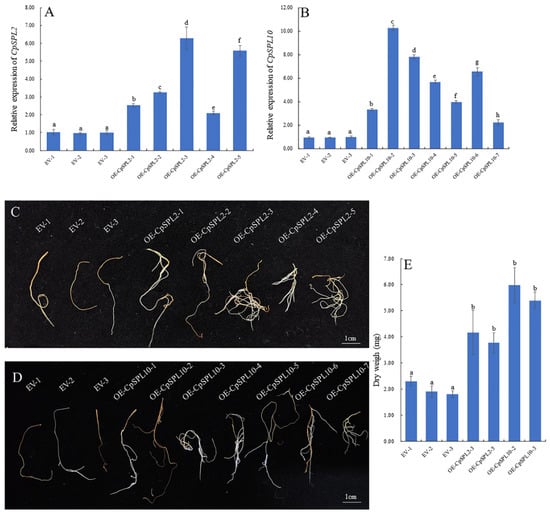
Figure 6.
Identification and growth phenotype of CpSPL2 or CpSPL10-overexpressing (OE) Codonopsis pilosula transgenic hairy roots. (A) Expression levels of CpSPL2 in CpSPL2-OE lines. (B) Expression levels of CpSPL10 in CpSPL10-OE lines. (C) Growth phenotype of empty vector (EV) and CpSPL2-OE lines when 1 cm hairy roots were cultured for one month. (D) Growth phenotype of EV and CpSPL10-OE lines when 1 cm hairy roots were cultured for one month. (E) The biomass of EV, CpSPL2-OE and CpSPL10-OE lines when 1 cm hairy roots were cultured for one month. One-way ANOVA (followed by Tukey’s comparisons) tested for significant differences among means (indicated by different letters at p < 0.05).
3.8. Overexpression of CpSPL2 or CpSPL10 Promotes Accumulation of Lobetyolin and Total Saponins in C. pilosula Hairy Root
To evaluate the impact of CpSPL2 or CpSPL10 on active ingredients, HPLC and UV spectrophotometer were used to determine the concentrations of lobetyolin and total saponins in those transgenic lines, respectively. It was surprising that the production of both lobetyolin and total saponins were greatly increased in CpSPL2-OE or CpSPL10-OE lines. The concentration of lobetyolin in CpSPL2-OE3, CpSPL2-OE5, CpSPL10-OE2 and CpSPL10-OE3 was 6.43, 6.25, 6.29 and 7.03 times that of the control (EV2), respectively (Figure 7A). The concentration of total saponins in CpSPL2-OE3, CpSPL2-OE5, CpSPL10-OE2 and CpSPL10-OE3 was 3.18, 2.72, 1.81 and 1.94 times that of the control (EV2), respectively (Figure 7B). In summary, CpSPL2 and CpSPL10 promote not only the growth of hairy roots but also accumulations of lobetyolin and total saponins.
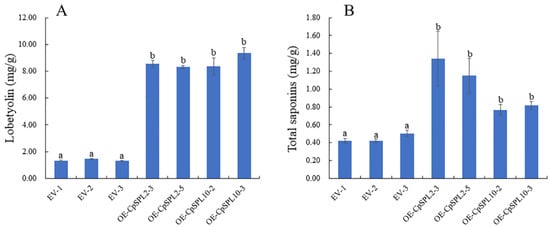
Figure 7.
Concentration of lobetyolin (A) and total saponins (B) contents in Codonopsis pilosula transgenic hairy roots. One-way ANOVA (followed by Tukey’s comparisons) tested for significant differences among means (indicated by different letters at p < 0.05).
4. Discussion
4.1. Identification of SPL Genes in C. pilosula
SPLs are plant-specific TFs and characterized by a highly conserved SBP domain [5,6]. They play critical roles in regulating diverse aspects of plant growth and development, including vegetative phase change, plant architecture, anthocyanin accumulation, lateral root growth, etc. [18,19,20,21,22,23,24]. Since its first discovery in A. majus [3], the SPL gene family from various plants has been isolated and identified. For instance, there are 16 SPL gene family members in Arabidopsis thaliana [8], 19 in Oryza sativa [9], 15 in Solanum lycopersicum [11], 15 in Salvia miltiorrhiza [13] and 15 in Ricinus communis [17]. However, little information is known about SPL gene family in C. pilosula, a famous species with important medical and edible values. Here, we identified 15 CpSPL genes in C. pilosula genome.
We constructed the phylogenetic tree of 15 from C. pilosula, 19 from O. sativa, 17 from S. lycopersicum, 14 from Physcomitrella patens and 16 from A. thaliana. In total, 81 SPL genes were divided into eight groups and each group had at least one CpSPL (Figure 1). CpSPL family members in the same group showed similar gene structure and motif composition (Figure 2 and Figure 3), which was consistent with previous report [17]. In Arabidopsis, members in the same group often have the same or similar function. For instance, AtSPL3, AtSPL4 and AtSPL5, clustered in G7, synergistically induce flowering under long-day photoperiod [43]. Most recently, AtSPL2, AtSPL10 and AtSPL11, members in G1, have been reported to inhibit root regeneration by dampening auxin biosynthesis [42]. We speculate that CpSPLs in the same group maybe have the same function, such as CpSPL2 and CpSPL10 in G1, CpSPL5 in G7 and so on.
4.2. CpSPL Genes’ Expression Patterns in C. pilosula
Gene expression patterns, to a large extent, will provide valuable information for its potential function [44]. In this study, the spatiotemporal expression patterns of 15 CpSPLs in the leaves, stems and roots from one-month-old seedlings, two-month-old seedlings, and three-month-old plantlets, and the flower and calyx from the plants at the flowering stage were detected by qRT-PCR (Figure 4). The results showed that CpSPL1 and CpSPL14 in G8 exhibited similar expression patterns, and the expression patterns of paralogous CpSPL9 and CpSPL15 in G6 showed high similarity. Our results were consistent with previous conclusion that paralogous SPL genes in the same group often showed similar expression profiles [45,46]. AtSPL9, AtSPL10 and AtSPL15 contribute to the vegetative to reproductive phase transition [8]. Here, CpSPL9, CpSPL10 and CpSPL15 expressed predominantly in the flower, suggesting they might function in the development of flower in C. pilosula.
Some SPL genes have been proved to be involved in abiotic stress. For example, in Arabidopsis, AtSPL1 and AtSPL12 function redundantly in thermotolerance and overexpression of AtSPL1 or AtSPL12 increased plant thermotolerance [47]. In alfalfa, silencing MsSPL13 enhanced tolerance to drought and heat stress (40 °C) [26,48], and downregulation of MsSPL8 led to enhanced salt and drought tolerance [49]. In the present study, we investigated the expression levels of 15 CpSPLs under various stress conditions, including NaCl, MeJA, or ABA treatment. We found that the expression levels of most CpSPL genes significantly changed under NaCl, MeJA and ABA treatment (Figure 5). Among those genes with significant change, CpSPL4, CpSPL6, CpSPL14 and CpSPL15 positively responded to all the treatments, while CpSPL5, CpSPL8 and CpSPL10 negatively responded to all the treatments. Compared with other genes, CpSPL5 and CpSPL8 showed higher fold change under different treatments. We speculate that those two genes are potential candidates involved in abiotic stress.
4.3. Functional Study of the CpSPL2 and CpSPL10 Genes
Since the medicinal and edible part of C. pilosula is the root, increasing root yield is one of the main goals of breeding for this species. In Arabidopsis, AtSPL2, AtSPL10 and AtSPL11, inhibit root regeneration by dampening auxin biosynthesis [42]. The miR156-targeted SPL10 is involved in regulating not only lateral root growth but also primary root growth [21,23]. Recently, it was reported that overexpression of FvSPL10, a SPL gene from Fragaria vesca, resulted in increased organs size, including longer root, larger floral organ and seeds [30]. We speculated that CpSPL2 and CpSPL10, two members clustered in the same group with AtSPL2/10/11 (Figure 1), were probably involved in the regulation of root development. To investigate the function of CpSPL2 and CpSPL10, we generated transgenic hairy roots overexpressing CpSPL2 or CpSPL10. Compared with the control, transgenic lines overexpressing CpSPL2 or CpSPL10 grew faster and the biomass of CpSPL2-OE3, CpSPL2-OE5, CpSPL10-OE2 and CpSPL10-OE3 was 2.19, 1.98, 3.15 and 2.83 times that of the control when the transgenic hairy roots with the length about 1.0 cm were cultured for one month (Figure 6). Our results indicated that overexpression of CpSPL2 or CpSPL10 significantly promote the growth of hairy root.
Furthermore, we determined the concentration of lobetyolin and total saponins in those transgenic lines. Unexpectedly, we found that overexpressing CpSPL2 or CpSPL10 dramatically promoted the accumulation of lobetyolin and total saponins in the hairy roots (Figure 7). Among 16 AtSPLs in Arabidopsis, AtSPL9 is the only one that has been reported to regulate biosynthesis of secondary metabolites [24,25]. AtSPL9 negatively regulates anthocyanin accumulation by preventing the formation of MBW complex [24], and it positively regulates the formation of (E)-β-caryophyllene by binding to the promoter of sesquiterpene synthase gene TPS21 and activates its expression [25]. Our results indicated that CpSPL2 and CpSPL10 are potential candidates for genetic improvement of C. pilosula because they can significantly promote not only the growth of hairy roots, but also accumulation of lobetyolin and total saponins. The molecular mechanism that CpSPL2 and CpSPL10 function in hairy roots needs to be addressed in the future.
5. Conclusions
In this study, we identified 15 CpSPL genes, which were supported by confirmation of the SBP domain, based on the genome data of C. pilosula. All CpSPLs were clustered into eight groups and members in the same group share similar gene structure and conserved motif composition. The spatiotemporal expression analysis of 15 CpSPLs showed that the CpSPL gene family had various expression patterns. The expression levels of most CpSPLs significantly changed under NaCl, MeJA, or ABA treatment, and CpSPL5 and CpSPL8 showed higher fold change under different treatments. Overexpression of CpSPL2 or CpSPL10 significantly promoted not only the growth of hairy roots, but also the accumulation of lobetyolin and total saponins. CpSPL2 and CpSPL10 are potential candidates for genetic improvement of C. pilosula. These results established a foundation for further investigation of CpSPLs and provided novel insights into their biological functions.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes12101588/s1, Table S1. Primer sequences used in the study, Table S2. cDNA sequences of 15 SPLs in Codonopsis pilosula, Table S3. The conserved motifs of SPL in Codonopsis pilosula, Table S4. Putative cis-acting elements present in SPL promoters of Codonopsis pilosula.
Author Contributions
J.Y. performed the experiments, analyzed the data and wrote the draft manuscript. Z.G. and W.W. prepared the materials and assisted in the experiments. X.Y. and X.C. conceived the study and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (grant number GK202107003).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Materials, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luscombe, N.M.; Austin, S.E.; Berman, H.M.; Thornton, J.M. An overview of the structures of protein-DNA complexes. Genome Biol. 2000, 1, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, D1040–D1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, J.; Saedler, H.; Huijser, P. A new family of DNA binding proteins includes putative transcriptional regulators of theAntirrhinum majus floral meristem identity geneSQUAMOSA. Mol. Genet. Genom. 1996, 250, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Kigawa, T.; Inoue, M.; Tateno, M.; Yamasaki, T.; Yabuki, T.; Aoki, M.; Seki, E.; Matsuda, T.; Nunokawa, E.; et al. A Novel Zinc-binding Motif Revealed by Solution Structures of DNA-binding Domains of Arabidopsis SBP-family Transcription Factors. J. Mol. Biol. 2004, 337, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Birkenbihl, R.P.; Jach, G.; Saedler, H.; Huijser, P. Functional Dissection of the Plant-specific SBP-Domain: Overlap of the DNA-binding and Nuclear Localization Domains. J. Mol. Biol. 2005, 352, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wang, X.; Gu, S.; Hu, Z.; Xu, H.; Xu, C. Comparative study of SBP-box gene family in Arabidopsis and rice. Gene 2008, 407, 1–11. [Google Scholar] [CrossRef]
- Preston, J.C.; Hileman, L.C. Functional Evolution in the Plant SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE (SPL) Gene Family. Front. Plant Sci. 2013, 4, 80. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Hu, T.; Zhao, J.; Park, M.-Y.; Earley, K.W.; Wu, G.; Yang, L.; Poethig, R.S. Developmental Functions of miR156-Regulated SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) Genes in Arabidopsis thaliana. PLoS Genet. 2016, 12, e1006263. [Google Scholar] [CrossRef] [Green Version]
- Xie, K.; Wu, C.; Xiong, L. Genomic Organization, Differential Expression, and Interaction of SQUAMOSA Promoter-Binding-Like Transcription Factors and microRNA156 in Rice. Plant Physiol. 2006, 142, 280–293. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, R.K.; Goel, R.; Kumari, S.; Dahuja, A. Genomic organization, phylogenetic comparison, and expression profiles of the SPL family genes and their regulation in soybean. Dev. Genes Evol. 2017, 227, 101–119. [Google Scholar] [CrossRef]
- Salinas, M.; Xing, S.; Höhmann, S.; Berndtgen, R.; Huijser, P. Genomic organization, phylogenetic comparison and differential expression of the SBP-box family of transcription factors in tomato. Planta 2011, 235, 1171–1184. [Google Scholar] [CrossRef]
- Li, J.; Hou, H.; Li, X.; Xiang, J.; Yin, X.; Gao, H.; Zheng, Y.; Bassett, C.L.; Wang, X. Genome-wide identification and analysis of the SBP-box family genes in apple (Malus × domestica Borkh.). Plant Physiol. Biochem. 2013, 70, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, B.; Zhao, D.; Li, C.; Shao, F.; Lu, S. Genome-wide analysis and molecular dissection of the SPL gene family in Salvia miltiorrhiza. J. Integr. Plant Biol. 2013, 56, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Li, J.; Gao, M.; Singer, S.D.; Wang, H.; Mao, L.; Fei, Z.; Wang, X. Genomic Organization, Phylogenetic Comparison and Differential Expression of the SBP-Box Family Genes in Grape. PLoS ONE 2013, 8, e59358. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Wang, Y.; Liu, H.; Wu, M.; Chu, W.; Chen, D.; Xiang, Y. Genome-wide identification and expression analysis of SBP-like transcription factor genes in Moso Bamboo (Phyllostachys edulis). BMC Genom. 2017, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-X.; Jin, J.-H.; He, Y.-M.; Lu, B.-Y.; Li, D.-W.; Chai, W.-G.; Khan, A.; Gong, Z.-H. Genome-Wide Identification and Analysis of the SBP-Box Family Genes under Phytophthora capsici Stress in Pepper (Capsicum annuum L.). Front. Plant Sci. 2016, 7, 504. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-D.; Ling, L.-Z. Genome-Wide Identification and Evolutionary Analysis of the SBP-Box Gene Family in Castor Bean. PLoS ONE 2014, 9, e86688. [Google Scholar] [CrossRef]
- Gandikota, M.; Birkenbihl, R.P.; Höhmann, S.; Cardon, G.H.; Saedler, H.; Huijser, P. The miRNA156/157 recognition element in the 3′ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J. 2010, 49, 683–693. [Google Scholar] [CrossRef] [Green Version]
- Lal, S.; Pacis, L.B.; Smith, H.M. Regulation of the SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE genes/microRNA156 Module by the Homeodomain Proteins PENNYWISE and POUND-FOOLISH in Arabidopsis. Mol. Plant 2011, 4, 1123–1132. [Google Scholar] [CrossRef] [Green Version]
- Hyun, Y.; Richter, R.; Vincent, C.; Martinez-Gallegos, R.; Porri, A.; Coupland, G. Multi-layered regulation of SPL15 and co-operation with SOC1 integrate endogenous flowering pathways at the Arabidopsis shoot meristem. Dev. Cell 2015, 37, 254–266. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Y.; Gruber, M.Y.; Hannoufa, A. miR156/SPL10 modulates lateral root development, branching and leaf mor-phology in Arabidopsis by silencing AGAMOUS-LIKE 79. Front. Plant Sci. 2017, 8, 2226. [Google Scholar] [CrossRef] [Green Version]
- Ye, B.; Zhang, K.; Wang, J. The role of miR156 in rejuvenation in Arabidopsis thaliana. J. Integr. Plant Biol. 2019, 62, 550–555. [Google Scholar] [CrossRef]
- Barrera-Rojas, C.H.; Rocha, G.H.B.; Polverari, L.; Brito, D.A.P.; Batista, D.S.; Notini, M.M.; Da Cruz, A.C.F.; Morea, E.G.O.; Sabatini, S.; Otoni, W.C.; et al. miR156-targeted SPL10 controls Arabidopsis root meristem activity and root-derived de novo shoot regeneration via cytokinin responses. J. Exp. Bot. 2019, 71, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.-Y.; de Felippes, F.F.; Liu, C.-J.; Weigel, D.; Wang, J.-W. Negative Regulation of Anthocyanin Biosynthesis in Arabidopsis by a miR156-Targeted SPL Transcription Factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.X.; Wang, L.J.; Zhao, B.; Shan, C.M.; Zhang, Y.H.; Chen, D.F.; Chen, X.Y. Progressive regulation of sesquiterpene bio-synthesis in Arabidopsis and Patchouli (Pogostemon cablin) by the miR156-targeted SPL transcription factors. Mol. Plant 2015, 8, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feyissa, B.A.; Arshad, M.; Gruber, M.Y.; Kohalmi, S.E.; Hannoufa, A. The interplay between miR156/SPL13 and DFR/WD40-1 regulate drought tolerance in alfalfa. BMC Plant Biol. 2019, 19, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mermod, M.; Takusagawa, M.; Kurata, T.; Kamiya, T.; Fujiwara, T.; Shikanai, T. SQUAMOSA promoter-binding protein-like 7 mediates copper deficiency response in the presence of high nitrogen in Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 835–846. [Google Scholar] [CrossRef]
- Miura, K.; Ikeda, M.; Matsubara, A.; Song, X.-J.; Ito, M.; Asano, K.; Matsuoka, M.; Kitano, H.; Ashikari, M. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat. Genet. 2010, 42, 545–549. [Google Scholar] [CrossRef]
- Wang, S.; Wu, K.; Yuan, Q.; Liu, X.; Liu, Z.; Lin, X.; Zeng, R.; Zhu, H.; Dong, G.; Qian, Q.; et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 2012, 44, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Bai, Y.; Ma, C.; Zhu, H.; Zheng, D.; Cheng, Z. Molecular Cloning and Characterization of SQUAMOSA-Promoter Binding Protein-Like Gene FvSPL10 from Woodland Strawberry (Fragaria vesca). Plants 2019, 8, 342. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.Z.; Dan, Y.; Liu, Y.Z.; Peng, Y. Committee for the Pharmacopoeia of the People’s Republic of China, Pharmacopoeia of the People’s Republic of China, Part I; China Medical Science Press: Beijing, China, 2020; pp. 293–294. [Google Scholar]
- Li, F.J.; Wang, Z.C.; Yang, K. Summary of recent research on Codonopsis pilosula. Technol. Inf. 2008, 35, 422–440. [Google Scholar]
- Li, D.; Li, Z.L. The research status of that Codonopsis pilosula polysaccharide is as an immune adjuvant. Guide of China Medicine 2013, 11, 56–57. [Google Scholar]
- He, J.Y.; Ma, N.; Zhu, S.; Komatsu, K.; Li, Z.Y.; Fu, W.M. The genus Codonopsis (Campanulaceae): A review of phytochemistry, bioactivity and quality control. J. Nat. Med. 2015, 69, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Yoon, I.-S.; Cho, S.-S. Effects of lobetyolin on xanthine oxidase activity in vitro and in vivo: Weak and mixed inhibition. Nat. Prod. Res. 2019, 35, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Tao, W.; Zhang, F.; Jie, Q.; He, Y.; Zhu, W.; Tan, J.; Shen, W.; Li, L.; Yang, Y.; et al. Lobetyolin induces apoptosis of colon cancer cells by inhibiting glutamine metabolism. J. Cell. Mol. Med. 2020, 24, 3359–3369. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.P.; Wang, N.; Cao, L.Y.; Sun, H.F. Transcriptome Sequencing of Codonopsis pilosula and Identification of Candidate Genes Involved in Polysaccharide Biosynthesis. PLoS ONE 2015, 10, e0117342. [Google Scholar] [CrossRef]
- Ji, J.-J.; Feng, Q.; Sun, H.-F.; Zhang, X.-J.; Li, X.-X.; Li, J.-K.; Gao, J.-P. Response of Bioactive Metabolite and Biosynthesis Related Genes to Methyl Jasmonate Elicitation in Codonopsis pilosula. Molecules 2019, 24, 533. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Yang, X.; Li, B.; Lu, X.; Kang, J.; Cao, X. Establishment of in vitro culture system for Codonopsis pilosula transgenic hairy roots. 3 Biotech 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Yang, J.; Yang, X.Z.; Kuang, Z.; Li, B.; Lu, X.Y.; Cao, X.Y.; Kang, J.F. Selection of suitable reference genes for qRT-PCR expression analysis of Codonopsis pilosula under different experimental conditions. Mol. Biol. Rep. 2020, 47, 4169–4181. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ye, B.-B.; Shang, G.-D.; Pan, Y.; Xu, Z.-G.; Zhou, C.-M.; Mao, Y.-B.; Bao, N.; Sun, L.; Xu, T.; Wang, J.-W. AP2/ERF Transcription Factors Integrate Age and Wound Signals for Root Regeneration. Plant Cell 2019, 32, 226–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, J.H.; Lee, H.J.; Ryu, J.Y.; Park, C.M. SPL3/4/5 integrate developmental aging and photoperiodic signals into the FT-FD module in Arabidopsis flowering. Mol. Plant 2016, 9, 1647–1659. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Zhang, S.; Chen, F.; Liu, B.; Wu, L.; Li, F.; Zhang, J.; Bao, M.; Liu, G. Genome-wide identification and characterization of the SBP-box gene family in Petunia. BMC Genom. 2018, 19, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, S. Molecular characterization of the SPL gene family in Populus trichocarpa. BMC Plant Biol. 2014, 14, 131. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.-F.; Zhou, J.-J.; Liu, S.-R.; Gan, Z.-M.; Zhang, J.-Z.; Hu, C.-G. Genome-Wide Identification and Characterization of SQUAMOSA—Promoter-Binding Protein (SBP) Genes Involved in the Flowering Development of Citrus Clementina. Biomolecules 2019, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Chao, L.; Liu, Y.-Q.; Chen, D.-Y.; Xue, X.-Y.; Mao, Y.-B.; Chen, X.-Y. Arabidopsis Transcription Factors SPL1 and SPL12 Confer Plant Thermotolerance at Reproductive Stage. Mol. Plant 2017, 10, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.; Arshad, M.; Hannoufa, A. Alfalfa response to heat stress is modulated by microRNA156. Physiol. Plant. 2018, 165, 830–842. [Google Scholar] [CrossRef]
- Gou, J.; Debnath, S.; Sun, L.; Flanagan, A.; Tang, Y.; Jiang, Q.; Wen, J.; Wang, Z.-Y. From model to crop: Functional characterization of SPL8 in M. truncatula led to genetic improvement of biomass yield and abiotic stress tolerance in alfalfa. Plant Biotechnol. J. 2017, 16, 951–962. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).