Eukaryotic Elongation Factor 3 Protects Saccharomyces cerevisiae Yeast from Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Growth Conditions
2.2. Oxidative Stress Assays
2.3. Generation of Expression Constructs
2.4. Electrophoresis and Western Blot Analysis
2.5. Translation Assay
2.6. RNA Isolation and Quantitative PCR
3. Results
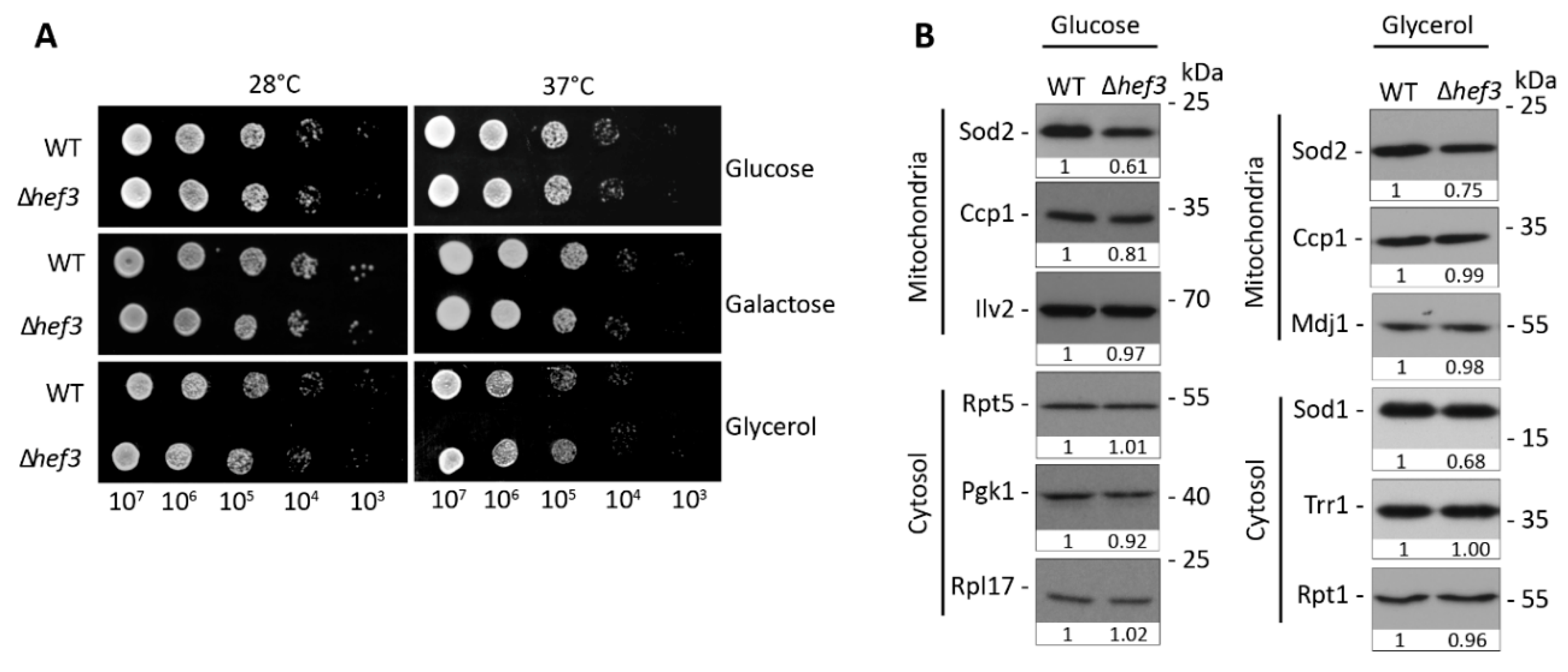
3.1. Hef3p Is Not Required under Normal Vegetative Growth Conditions
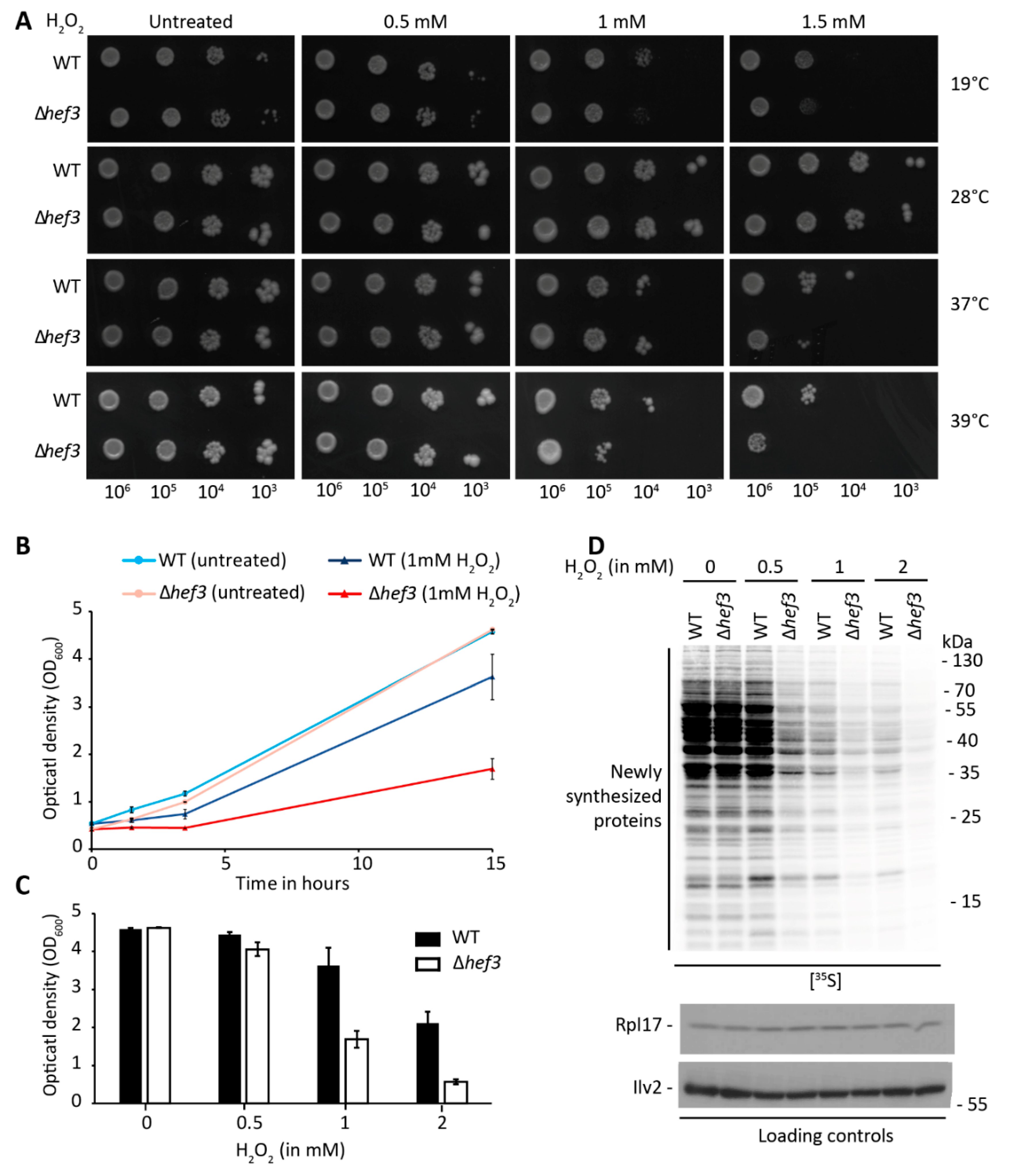
3.2. Deletion of HEF3 Results in Growth Defect upon Oxidative Stress
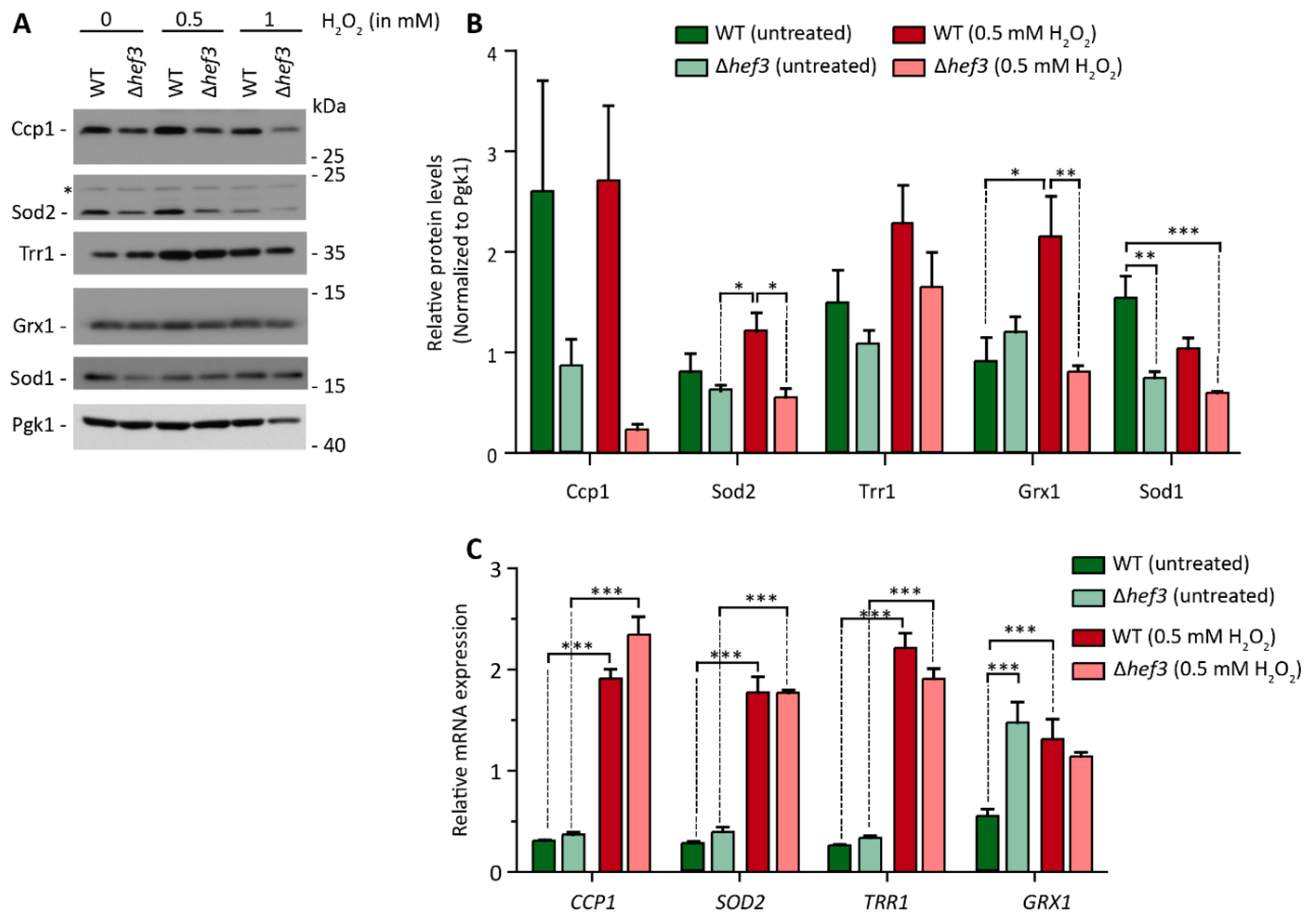
3.3. HEF3 Expression Is Regulated by Oxidative Stress Conditions
3.4. Hef3p Is Necessary for the Expression of Oxidative Stress Response Proteins at the Translational Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lindqvist, L.M.; Tandoc, K.; Topisirovic, I.; Furic, L. Cross-talk between protein synthesis, energy metabolism and autophagy in cancer. Curr. Opin. Genet. Dev. 2018, 48, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, A.N.; Perez, W.B.; Kinzy, T.G. The many roles of the eukaryotic elongation factor 1 complex. Wiley Interdiscip. Rev. RNA 2012, 3, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wei, T.; Abbott, C.M.; Harrich, D. The unexpected roles of eukaryotic translation elongation factors in RNA virus replication and pathogenesis. Microbiol. Mol. Biol. Rev. 2013, 77, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Negrutskii, B. Non-translational Connections of eEF1B in the Cytoplasm and Nucleus of Cancer Cells. Front. Mol. Biosci. 2020, 7, 56. [Google Scholar] [CrossRef]
- Rodnina, M.V.; Wintermeyer, W. Recent mechanistic insights into eukaryotic ribosomes. Curr. Opin. Cell Biol. 2009, 21, 435–443. [Google Scholar] [CrossRef]
- Shah, P.; Ding, Y.; Niemczyk, M.; Kudla, G.; Plotkin, J.B. Rate-limiting steps in yeast protein translation. Cell 2013, 153, 1589–1601. [Google Scholar] [CrossRef]
- Hershey, J.W.; Sonenberg, N.; Mathews, M.B. Principles of translational control: An overview. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Jackson, R.J.; Hellen, C.U.; Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef]
- Merrick, W.C.; Pavitt, G.D. Protein Synthesis Initiation in Eukaryotic Cells. Cold Spring Harb. Perspect. Biol. 2018, 10, a033092. [Google Scholar] [CrossRef]
- Pisareva, V.P.; Pisarev, A.V.; Hellen, C.U.; Rodnina, M.V.; Pestova, T.V. Kinetic analysis of interaction of eukaryotic release factor 3 with guanine nucleotides. J. Biol. Chem. 2006, 281, 40224–40235. [Google Scholar] [CrossRef]
- Pisarev, A.V.; Hellen, C.U.; Pestova, T.V. Recycling of eukaryotic posttermination ribosomal complexes. Cell 2007, 131, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.D.; Carvalho, J.F.; Merrick, W.C. Biological characterization of various forms of elongation factor 1 from rabbit reticulocytes. Arch. Biochem. Biophys. 1984, 234, 603–611. [Google Scholar] [CrossRef]
- Dever, T.E.; Dinman, J.D.; Green, R. Translation Elongation and Recoding in Eukaryotes. Cold Spring Harb. Perspect. Biol. 2018, 10, a032649. [Google Scholar] [CrossRef] [PubMed]
- Schuller, A.P.; Green, R. Roadblocks and resolutions in eukaryotic translation. Nat. Rev. Mol. Cell Biol. 2018, 19, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Skogerson, L.; Engelhardt, D. Dissimilarity in protein chain elongation factor requirements between yeast and rat liver ribosomes. J. Biol. Chem. 1977, 252, 1471–1475. [Google Scholar]
- Mateyak, M.K.; Pupek, J.K.; Garino, A.E.; Knapp, M.C.; Colmer, S.F.; Kinzy, T.G.; Dunaway, S. Demonstration of translation elongation factor 3 activity from a non-fungal species, Phytophthora infestans. PLoS ONE 2018, 13, e0190524. [Google Scholar] [CrossRef]
- Murina, V.; Kasari, M.; Takada, H.; Hinnu, M.; Saha, C.K.; Grimshaw, J.W.; Seki, T.; Reith, M.; Putrinš, M.; Tenson, T.; et al. ABCF ATPases Involved in Protein Synthesis, Ribosome Assembly and Antibiotic Resistance: Structural and Functional Diversification across the Tree of Life. J. Mol. Biol. 2019, 431, 3568–3590. [Google Scholar] [CrossRef]
- Uritani, M.; Miyazaki, M. Role of yeast peptide elongation factor 3 (EF-3) at the AA-tRNA binding step. J. Biochem 1988, 104, 118–126. [Google Scholar] [CrossRef]
- Andersen, C.B.; Becker, T.; Blau, M.; Anand, M.; Halic, M.; Balar, B.; Mielke, T.; Boesen, T.; Pedersen, J.S.; Spahn, C.M.; et al. Structure of eEF3 and the mechanism of transfer RNA release from the E-site. Nature 2006, 443, 663–668. [Google Scholar] [CrossRef]
- Triana-Alonso, F.J.; Chakraburtty, K.; Nierhaus, K.H. The elongation factor 3 unique in higher fungi and essential for protein biosynthesis is an E site factor. J. Biol. Chem. 1995, 270, 20473–20478. [Google Scholar] [CrossRef]
- Kovalchuke, O.; Kambampati, R.; Pladies, E.; Chakraburtty, K. Competition and cooperation amongst yeast elongation factors. Eur. J. Biochem. 1998, 258, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Kurata, S.; Nielsen, K.H.; Mitchell, S.F.; Lorsch, J.R.; Kaji, A.; Kaji, H. Ribosome recycling step in yeast cytoplasmic protein synthesis is catalyzed by eEF3 and ATP. Proc. Natl. Acad. Sci. USA 2010, 107, 10854–10859. [Google Scholar] [CrossRef] [PubMed]
- Young, D.J.; Guydosh, N.R.; Zhang, F.; Hinnebusch, A.G.; Green, R. Rli1/ABCE1 Recycles Terminating Ribosomes and Controls Translation Reinitiation in 3’UTRs in vivo. Cell 2015, 162, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Samra, N.; Atir-Lande, A.; Pnueli, L.; Arava, Y. The elongation factor eEF3 (Yef3) interacts with mRNA in a translation independent manner. BMC Mol. Biol. 2015, 16, 17. [Google Scholar] [CrossRef] [PubMed]
- Sandbaken, M.G.; Lupisella, J.A.; DiDomenico, B.; Chakraburtty, K. Protein synthesis in yeast. Structural and functional analysis of the gene encoding elongation factor 3. J. Biol. Chem. 1990, 265, 15838–15844. [Google Scholar]
- Wolfe, K.H. Origin of the Yeast Whole-Genome Duplication. PLoS Biol. 2015, 13, e1002221. [Google Scholar] [CrossRef]
- Wolfe, K.H.; Shields, D.C. Molecular evidence for an ancient duplication of the entire yeast genome. Nature 1997, 387, 708–713. [Google Scholar] [CrossRef]
- Sarthy, A.V.; McGonigal, T.; Capobianco, J.O.; Schmidt, M.; Green, S.R.; Moehle, C.M.; Goldman, R.C. Identification and kinetic analysis of a functional homolog of elongation factor 3, YEF3 in Saccharomyces cerevisiae. Yeast 1998, 14, 239–253. [Google Scholar] [CrossRef]
- Maurice, T.C.; Mazzucco, C.E.; Ramanathan, C.S.; Ryan, B.M.; Warr, G.A.; Puziss, J.W. A highly conserved intraspecies homolog of the Saccharomyces cerevisiae elongation factor-3 encoded by the HEF3 gene. Yeast 1998, 14, 1105–1113. [Google Scholar] [CrossRef]
- Yuan, D.S. Zinc-regulated genes in Saccharomyces cerevisiae revealed by transposon tagging. Genetics 2000, 156, 45–58. [Google Scholar]
- Komili, S.; Farny, N.G.; Roth, F.P.; Silver, P.A. Functional specificity among ribosomal proteins regulates gene expression. Cell 2007, 131, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Escalera-Fanjul, X.; Quezada, H.; Riego-Ruiz, L.; González, A. Whole-Genome Duplication and Yeast’s Fruitful Way of Life. Trends Genet. 2019, 35, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Soria, P.S.; McGary, K.L.; Rokas, A. Functional divergence for every paralog. Mol. Biol. Evol. 2014, 31, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, M.P.; Schatz, G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc. Natl. Acad. Sci. USA 1984, 81, 4819–4823. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.E.; Brown, T.A.; Trumpower, B.L. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990, 18, 3091–3092. [Google Scholar] [CrossRef]
- Jurkiewicz, A.; Leśniewska, E.; Cieśla, M.; Gorjão, N.; Kantidakis, T.; White, R.J.; Boguta, M.; Graczyk, D. Inhibition of tRNA Gene Transcription by the Immunosuppressant Mycophenolic Acid. Mol. Cell Biol. 2019, 40, e00294-19. [Google Scholar] [CrossRef]
- Morano, K.A.; Grant, C.M.; Moye-Rowley, W.S. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics 2012, 190, 1157–1195. [Google Scholar] [CrossRef]
- Topf, U.; Suppanz, I.; Samluk, L.; Wrobel, L.; Böser, A.; Sakowska, P.; Knapp, B.; Pietrzyk, M.K.; Chacinska, A.; Warscheid, B. Quantitative proteomics identifies redox switches for global translation modulation by mitochondrially produced reactive oxygen species. Nat. Commun. 2018, 9, 324. [Google Scholar] [CrossRef]
- Shenton, D.; Smirnova, J.B.; Selley, J.N.; Carroll, K.; Hubbard, S.J.; Pavitt, G.D.; Ashe, M.P.; Grant, C.M. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J. Biol. Chem. 2006, 281, 29011–29021. [Google Scholar] [CrossRef]
- Drummond, D.A.; Wilke, C.O. The evolutionary consequences of erroneous protein synthesis. Nat. Rev. Genet. 2009, 10, 715–724. [Google Scholar] [CrossRef]
- Scheper, G.C.; van der Knaap, M.S.; Proud, C.G. Translation matters: Protein synthesis defects in inherited disease. Nat. Rev. Genet. 2007, 8, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Himanen, S.V.; Sistonen, L. New insights into transcriptional reprogramming during cellular stress. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, G.W.; Fong, C.S.; Alic, N.; Higgins, V.J.; Dawes, I.W. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: Oxidative-stress-response genes. Proc. Natl. Acad. Sci. USA 2004, 101, 6564–6569. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Gerashchenko, M.V.; Lobanov, A.V.; Gladyshev, V.N. Genome-wide ribosome profiling reveals complex translational regulation in response to oxidative stress. Proc. Natl. Acad. Sci. USA 2012, 109, 17394–17399. [Google Scholar] [CrossRef] [PubMed]
- Ingolia, N.T.; Ghaemmaghami, S.; Newman, J.R.; Weissman, J.S. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 2009, 324, 218–223. [Google Scholar] [CrossRef]
- Simsek, D.; Tiu, G.C.; Flynn, R.A.; Byeon, G.W.; Leppek, K.; Xu, A.F.; Chang, H.Y.; Barna, M. The Mammalian Ribo-interactome Reveals Ribosome Functional Diversity and Heterogeneity. Cell 2017, 169, 1051–1065 e1018. [Google Scholar] [CrossRef]
- Gasch, A.P.; Spellman, P.T.; Kao, C.M.; Carmel-Harel, O.; Eisen, M.B.; Storz, G.; Botstein, D.; Brown, P.O. Genomic expression programs in the response of yeast cells to environmental changes. Mol. Biol. Cell 2000, 11, 4241–4257. [Google Scholar] [CrossRef]
- Kamath, A.; Chakraburtty, K. Role of yeast elongation factor 3 in the elongation cycle. J. Biol. Chem. 1989, 264, 15423–15428. [Google Scholar]
- Chan, C.T.; Pang, Y.L.; Deng, W.; Babu, I.R.; Dyavaiah, M.; Begley, T.J.; Dedon, P.C. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 2012, 3, 937. [Google Scholar] [CrossRef]
- Chan, C.T.; Dyavaiah, M.; DeMott, M.S.; Taghizadeh, K.; Dedon, P.C.; Begley, T.J. A quantitative systems approach reveals dynamic control of tRNA modifications during cellular stress. PLoS Genet. 2010, 6, e1001247. [Google Scholar] [CrossRef]
- Wang, Y.; Weisenhorn, E.; MacDiarmid, C.W.; Andreini, C.; Bucci, M.; Taggart, J.; Banci, L.; Russell, J.; Coon, J.J.; Eide, D.J. The cellular economy of the Saccharomyces cerevisiae zinc proteome. Metallomics 2018, 10, 1755–1776. [Google Scholar] [CrossRef] [PubMed]
- Venters, B.J.; Wachi, S.; Mavrich, T.N.; Andersen, B.E.; Jena, P.; Sinnamon, A.J.; Jain, P.; Rolleri, N.S.; Jiang, C.; Hemeryck-Walsh, C.; et al. A comprehensive genomic binding map of gene and chromatin regulatory proteins in Saccharomyces. Mol. Cell 2011, 41, 480–492. [Google Scholar] [CrossRef] [PubMed]
- El’skaya, A.V.; Ovcharenko, G.V.; Palchevskii, S.S.; Petrushenko, Z.M.; Triana-Alonso, F.J.; Nierhaus, K.H. Three tRNA binding sites in rabbit liver ribosomes and role of the intrinsic ATPase in 80S ribosomes from higher eukaryotes. Biochemistry 1997, 36, 10492–10497. [Google Scholar] [CrossRef] [PubMed]
- Yarunin, A.; Panse, V.G.; Petfalski, E.; Dez, C.; Tollervey, D.; Hurt, E.C. Functional link between ribosome formation and biogenesis of iron-sulfur proteins. EMBO J. 2005, 24, 580–588. [Google Scholar] [CrossRef]
- Pisarev, A.V.; Skabkin, M.A.; Pisareva, V.P.; Skabkina, O.V.; Rakotondrafara, A.M.; Hentze, M.W.; Hellen, C.U.; Pestova, T.V. The role of ABCE1 in eukaryotic posttermination ribosomal recycling. Mol. Cell 2010, 37, 196–210. [Google Scholar] [CrossRef]
- Kurata, S.; Shen, B.; Liu, J.O.; Takeuchi, N.; Kaji, A.; Kaji, H. Possible steps of complete disassembly of post-termination complex by yeast eEF3 deduced from inhibition by translocation inhibitors. Nucleic Acids Res. 2013, 41, 264–276. [Google Scholar] [CrossRef]
- Karcher, A.; Buttner, K.; Martens, B.; Jansen, R.P.; Hopfner, K.P. X-ray structure of RLI, an essential twin cassette ABC ATPase involved in ribosome biogenesis and HIV capsid assembly. Structure 2005, 13, 649–659. [Google Scholar] [CrossRef]
- Karcher, A.; Schele, A.; Hopfner, K.P. X-ray structure of the complete ABC enzyme ABCE1 from Pyrococcus abyssi. J. Biol. Chem. 2008, 283, 7962–7971. [Google Scholar] [CrossRef]
- Alhebshi, A.; Sideri, T.C.; Holland, S.L.; Avery, S.V. The essential iron-sulfur protein Rli1 is an important target accounting for inhibition of cell growth by reactive oxygen species. Mol. Biol Cell 2012, 23, 3582–3590. [Google Scholar] [CrossRef]


| Gene | Forward (5′–3′) | Reverse (5′–3′) | Reference |
|---|---|---|---|
| HEF3 | CGCTAAAGAACAGATTGCCT | TTCAGTTGCCTTTGTGATGG | Present study |
| YEF3 | GCTATCTCTGCTATGGTCGA | CAGCCTTGACTTCCTTCTTG | |
| CCP1 | ACAACGAACAGTGGGACTCT | ACTTGTCCTGGTCATTAGCGT | |
| TRR1 | CCGTCCCCATTTTCAGAAACA | GCACGCAAATGGTCTTTTCTG | |
| SOD2 | CTCTAGTTGCCATTGACGCC | TGCCAGCATCGAATCTTCTG | |
| GRX1 | CGTCGCATCCAAAACGTACT | GCGCCTTCCTTCATGTCATT | |
| ACT1 | CATGTTCCCAGGTATTGCCG | GTCAAAGAAGCCAAGATAGA | [36] |
| ALG9 | TCCATGATACAGGAGCAAGC | CTACCATCAGAACCGCATTC | |
| TDH1 | GGTATGGCTTTCAGAGTCCCA | AGACAACGGCATCTTCGGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gościńska, K.; Shahmoradi Ghahe, S.; Domogała, S.; Topf, U. Eukaryotic Elongation Factor 3 Protects Saccharomyces cerevisiae Yeast from Oxidative Stress. Genes 2020, 11, 1432. https://doi.org/10.3390/genes11121432
Gościńska K, Shahmoradi Ghahe S, Domogała S, Topf U. Eukaryotic Elongation Factor 3 Protects Saccharomyces cerevisiae Yeast from Oxidative Stress. Genes. 2020; 11(12):1432. https://doi.org/10.3390/genes11121432
Chicago/Turabian StyleGościńska, Karolina, Somayeh Shahmoradi Ghahe, Sara Domogała, and Ulrike Topf. 2020. "Eukaryotic Elongation Factor 3 Protects Saccharomyces cerevisiae Yeast from Oxidative Stress" Genes 11, no. 12: 1432. https://doi.org/10.3390/genes11121432
APA StyleGościńska, K., Shahmoradi Ghahe, S., Domogała, S., & Topf, U. (2020). Eukaryotic Elongation Factor 3 Protects Saccharomyces cerevisiae Yeast from Oxidative Stress. Genes, 11(12), 1432. https://doi.org/10.3390/genes11121432





