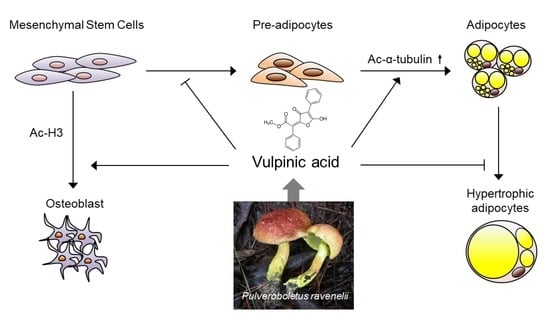
Vulpinic Acid Controls Stem Cell Fate toward Osteogenesis and Adipogenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Differentiation
2.2. Extraction and Isolation of Vulpinic Acid
2.3. Immunoblotting
2.4. Reverse Transcription and Quantitative Real-Time PCR (RT-qPCR)
2.5. Chromatin Immunoprecipitation and Real-Time PCR (ChIP-qPCR)
2.6. Oil-Red-O Staining
2.7. Statistical Analysis
3. Results
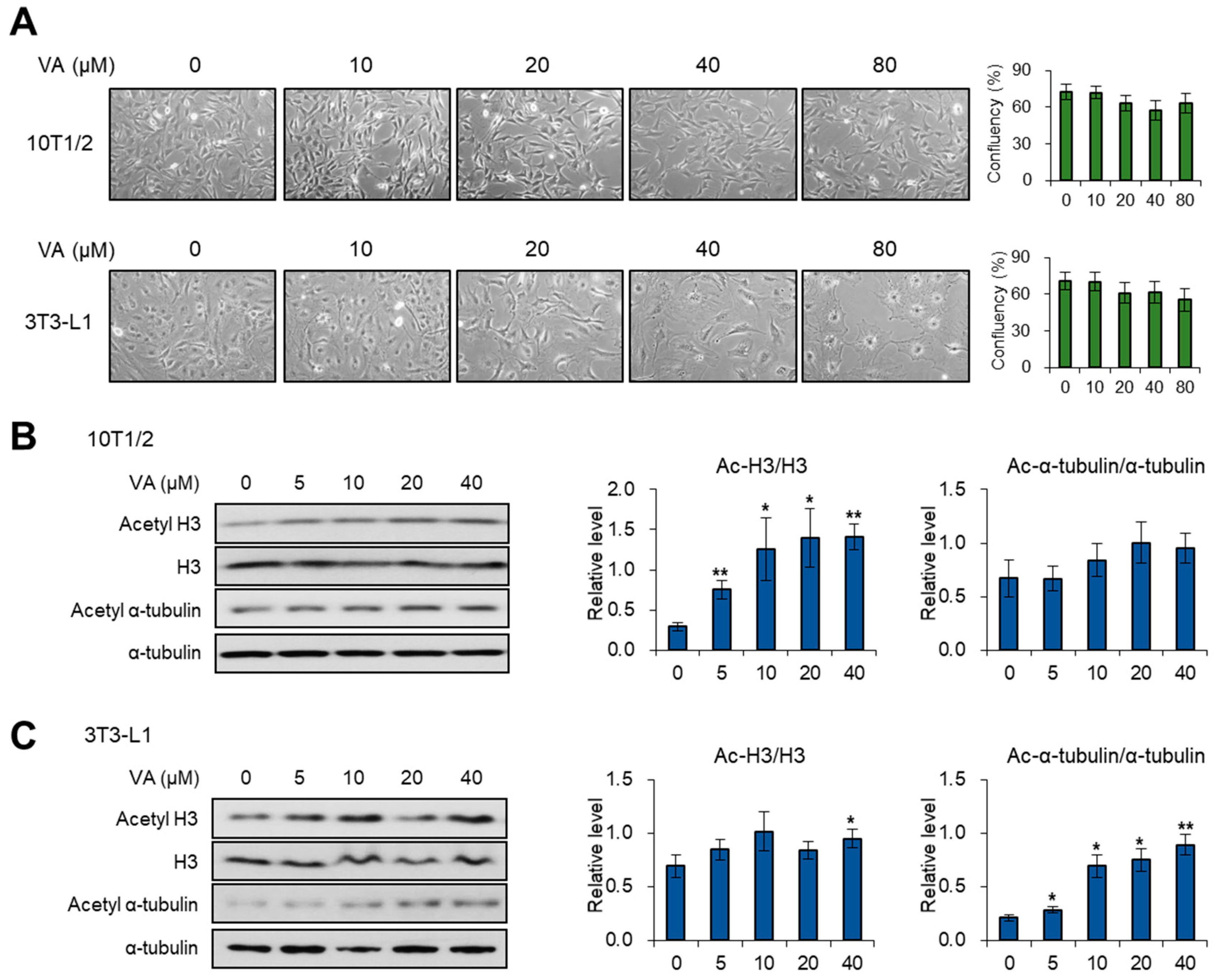
3.1. Vulpinic Acid Modulates Acetylation of Histone H3 and α Tubulin Depending on Cell Stage
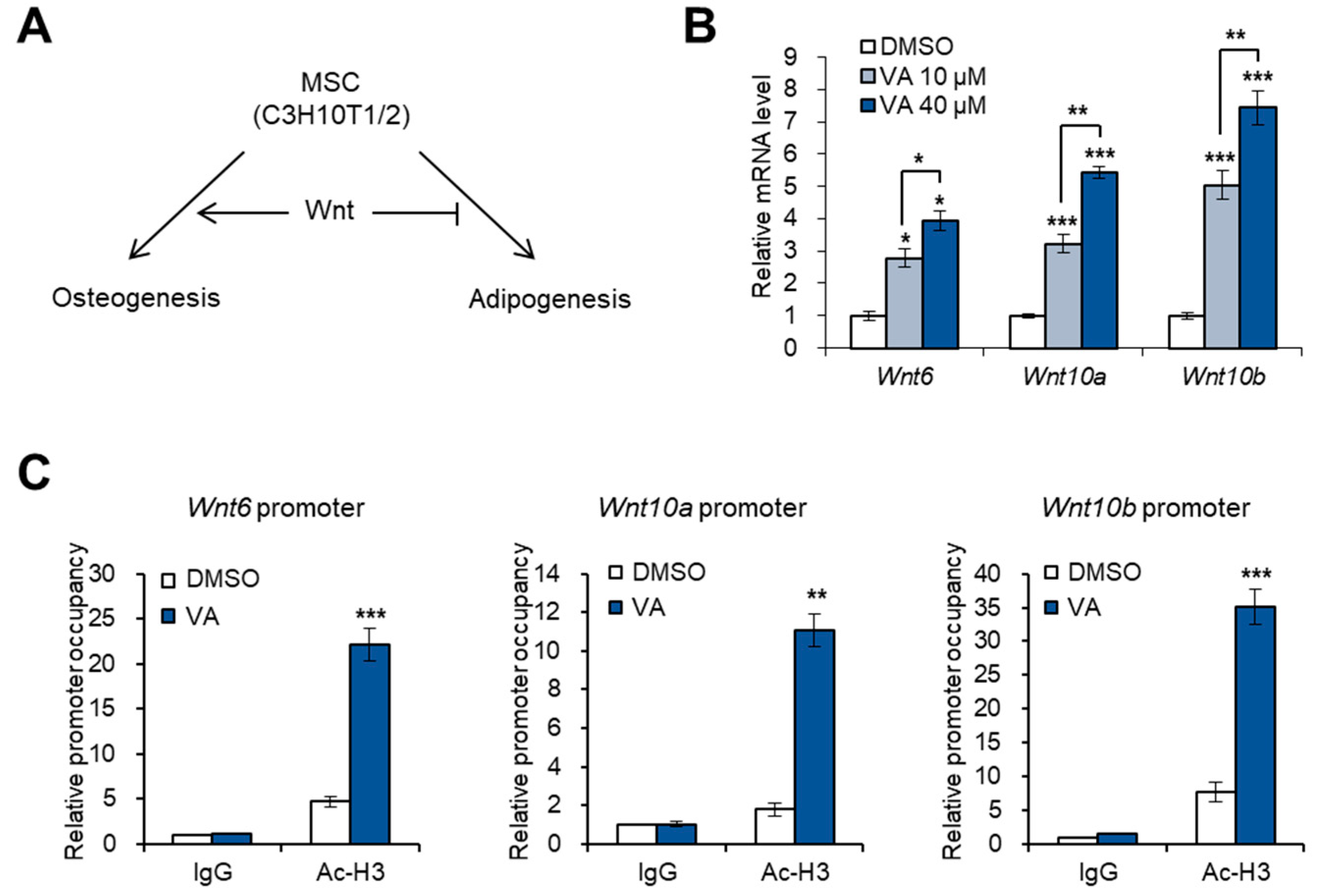
3.2. Vulpinic Acid Promotes the Expression of Wnt Genes via H3 Acetylation in 10T1/2 MSCs
3.3. Vulpinic Acid Induces Osteogenesis via H3 Acetylation in 10T1/2 MSCs
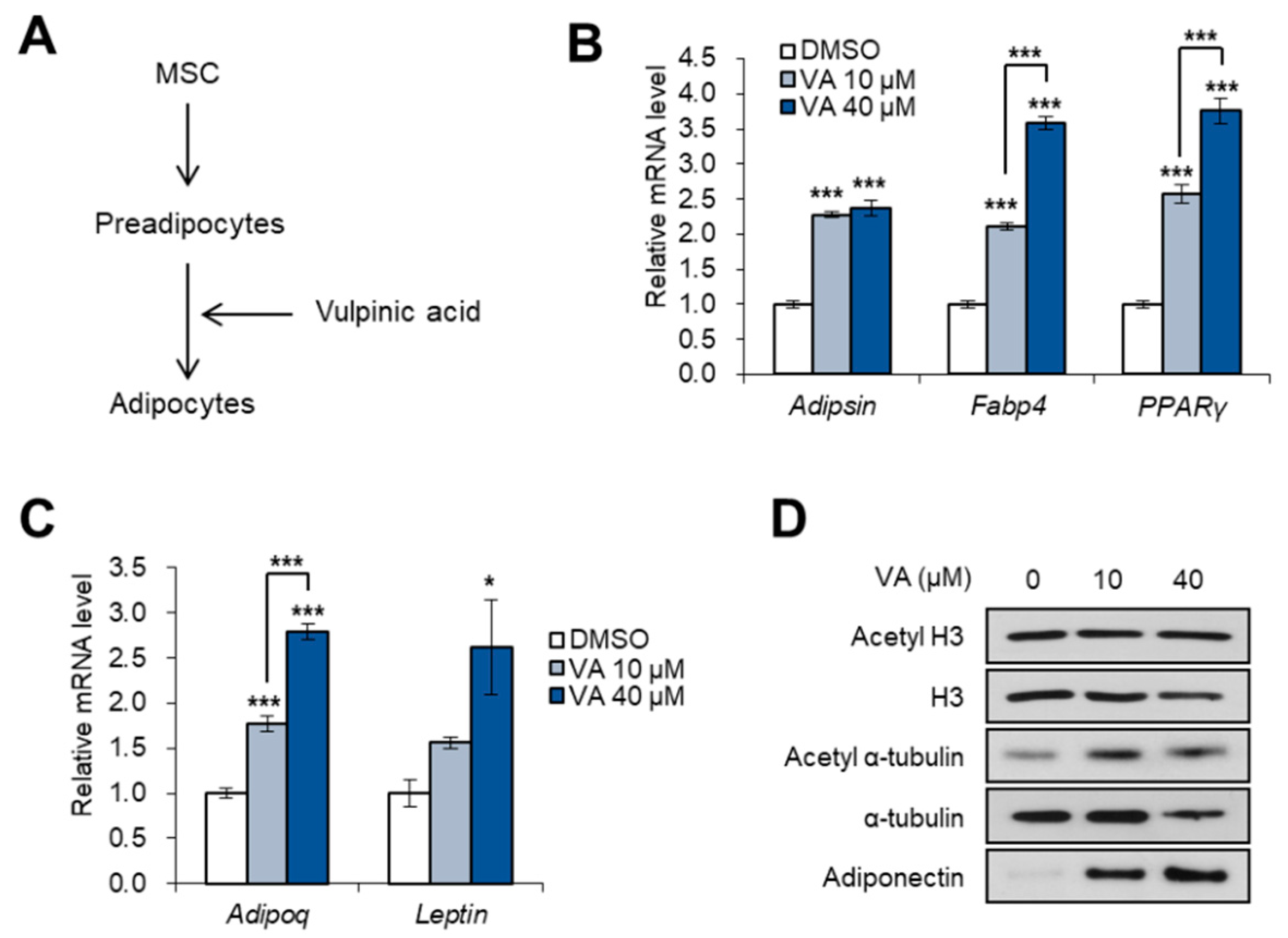
3.4. Vulpinic Acid Promotes Adipogenesis from 3T3-L1 Preadipocytes
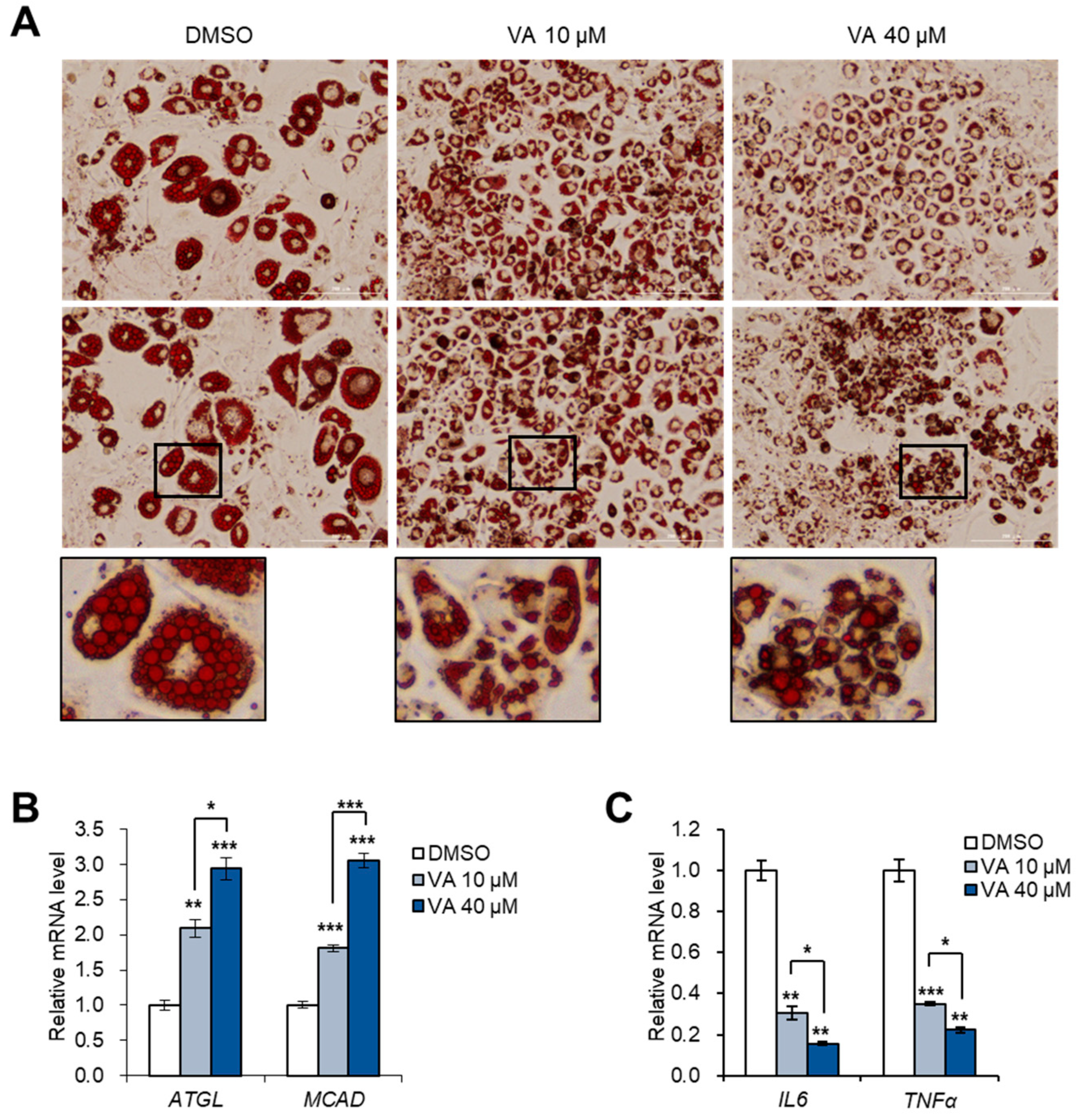
3.5. Vulpinic Acid Reduces the Characteristics of Hypertrophic Adipocytes
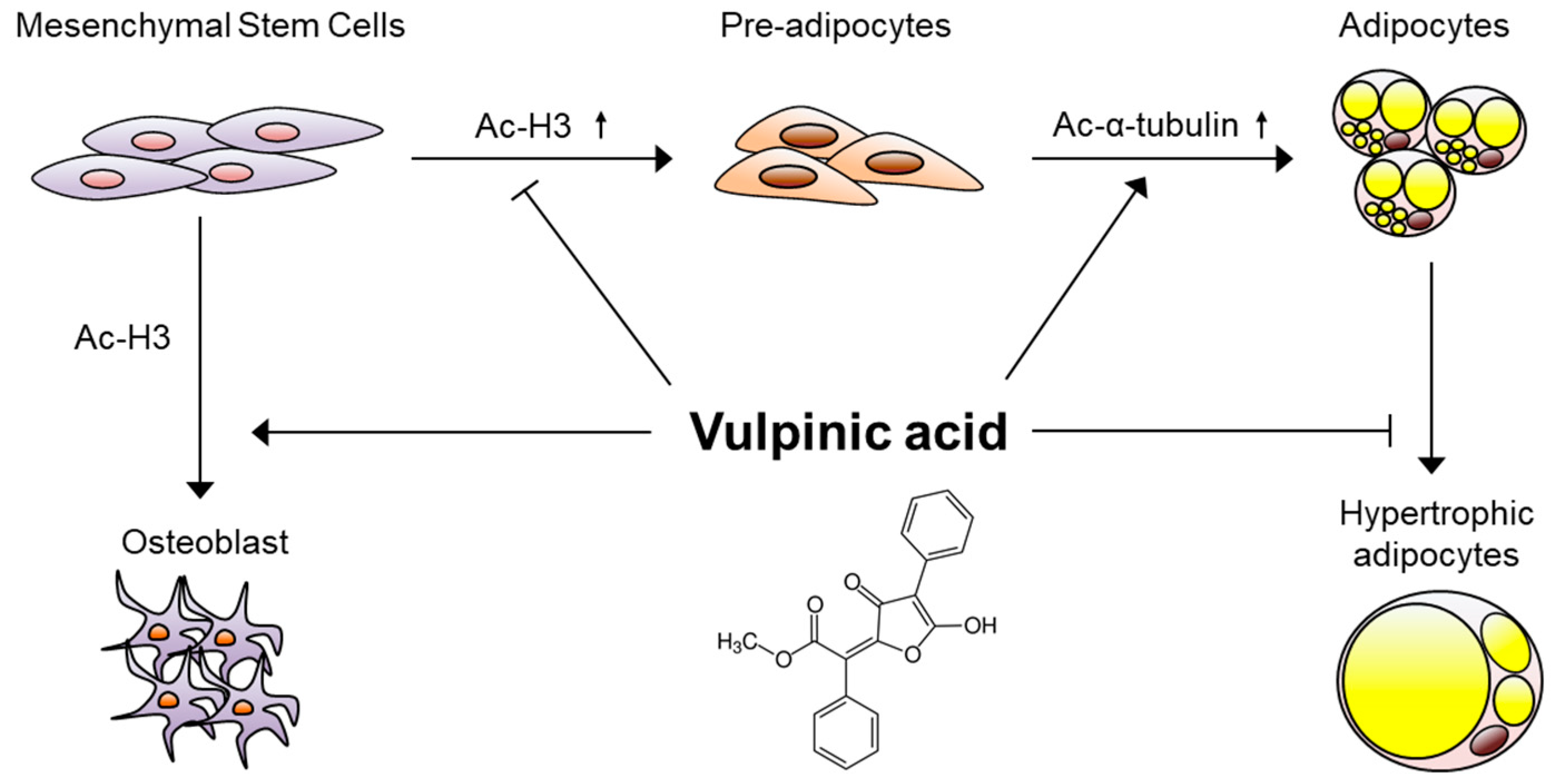
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baek, S.C.; Choi, E.; Eom, H.J.; Jo, M.S.; Kim, S.; So, H.M.; Kim, S.H.; Kang, K.S.; Kim, K.H. LC/MS-based analysis of bioactive compounds from the bark of Betula platyphylla var. japonica and their effects on regulation of adipocyte and osteoblast differentiation. Nat. Prod. Sci. 2018, 24, 235–240. [Google Scholar] [CrossRef]
- Augello, A.; De Bari, C. The regulation of differentiation in mesenchymal stem cells. Hum. Gene Ther. 2010, 21, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Shou, P.; Zheng, C.; Jiang, M.; Cao, G.; Yang, Q.; Cao, J.; Xie, N.; Velletri, T.; Zhang, X.; et al. Fate decision of mesenchymal stem cells: Adipocytes or osteoblasts? Cell Death Differ. 2016, 23, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, E.; Yang, S.M.; Ryoo, R.; Moon, E.; Kim, S.H.; Kim, K.H. Bioactive compounds from sclerotia extract of Poria cocos that control adipocyte and osteoblast differentiation. Bioorg. Chem. 2018, 81, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, Y.H.; Li, L.Y.; Lang, J.; Yeh, S.P.; Shi, B.; Yang, C.C.; Yang, J.Y.; Lin, C.Y.; Lai, C.C.; et al. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat. Cell Biol. 2011, 13, 87–94. [Google Scholar] [CrossRef]
- Hemming, S.; Cakouros, D.; Isenmann, S.; Cooper, L.; Menicanin, D.; Zannettino, A.; Gronthos, S. EZH2 and KDM6A act as an epigenetic switch to regulate mesenchymal stem cell lineage specification. Stem Cells 2014, 32, 802–815. [Google Scholar] [CrossRef]
- Gelman, L.; Zhou, G.; Fajas, L.; Raspé, E.; Fruchart, J.C.; Auwerx, J. p300 interacts with the N-and C-terminal part of PPARgamma2 in a ligand-independent and -dependent manner, respectively. J. Biol. Chem. 1999, 274, 7681–7688. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Wang, S.G.; Liu, S.X.; Li, J.P.; Zhang, Y.X.; Sun, Z.Y.; Fan, Q.M.; Tian, J.W. PPARγ forms a bridge between DNA methylation and histone acetylation at the C/EBPα gene promoter to regulate the balance between osteogenesis and adipogenesis of bone marrow stromal cells. FEBS J. 2013, 280, 5801–5814. [Google Scholar] [CrossRef]
- Huang, S.; Wang, S.; Bian, C.; Yang, Z.; Zhou, H.; Zeng, Y.; Li, H.; Han, Q.; Zhao, R.C. Upregulation of miR-22 promotes osteogenic differentiation and inhibits adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells by repressing HDAC6 protein expression. Stem Cells Dev. 2012, 21, 2531–2540. [Google Scholar] [CrossRef]
- Spiegelman, B.M.; Farmer, S.R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell 1982, 29, 53–60. [Google Scholar] [CrossRef]
- Yang, W.; Guo, X.; Thein, S.; Xu, F.; Sugii, S.; Baas, P.W.; Radda, G.K.; Han, W. Regulation of adipogenesis by cytoskeleton remodelling is facilitated by acetyltransferase MEC-17-dependent acetylation of α-tubulin. Biochem. J. 2013, 449, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, G.; Jiang, X.H. Fate determination in mesenchymal stem cells: A perspective from histone-modifying enzymes. Stem Cell Res. Ther. 2015, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, G.; Thompson, A.; Robison, R.; St Clair, L.L. Letharia vulpina, a vulpinic acid containing lichen, targets cell membrane and cell division processes in methicillin-resistant Staphylococcus aureus. Pharm. Biol. 2016, 54, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Cha, J.; Chiang, J.; Tran, G.; Giaever, G.; Nislow, C.; Hur, J.S.; Kwak, Y.S. A chemogenomic approach to understand the antifungal action of Lichen-derived vulpinic acid. J. Appl. Microbiol. 2016, 121, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Koparal, A.T. Anti-angiogenic and antiproliferative properties of the lichen substances (-)-usnic acid and vulpinic acid. Z. Naturforsch. C 2015, 70, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; So, H.M.; Roh, H.S.; Kim, J.; Yu, J.S.; Lee, S.; Seok, S.; Pang, C.; Baek, K.H.; Kim, K.H. Vulpinic acid contributes to the cytotoxicity of Pulveroboletus ravenelii to human cancer cells by inducing apoptosis. RSC Adv. 2017, 7, 35297–35304. [Google Scholar] [CrossRef]
- Kılıç, N.; Aras, S.; Cansaran-Duman, D. Determination of Vulpinic Acid Effect on Apoptosis and mRNA Expression Levels in Breast Cancer Cell Lines. Anticancer Agents Med. Chem. 2018, 18, 2032–2041. [Google Scholar] [CrossRef]
- Sahin, E.; Dabagoglu Psav, S.; Avan, I.; Candan, M.; Sahinturk, V.; Koparal, A.T. Vulpinic acid, a lichen metabolite, emerges as a potential drug candidate in the therapy of oxidative stress-related diseases, such as atherosclerosis. Hum. Exp. Toxicol. 2019, 38, 675–684. [Google Scholar] [CrossRef]
- Yi, S.A.; Um, S.H.; Lee, J.; Yoo, J.H.; Bang, S.Y.; Park, E.K.; Lee, M.G.; Nam, K.H.; Jeon, Y.J.; Park, J.W.; et al. S6K1 Phosphorylation of H2B Mediates EZH2 Trimethylation of H3: A Determinant of Early Adipogenesis. Mol. Cell 2016, 62, 443–452. [Google Scholar] [CrossRef]
- McCool, K.W.; Xu, X.; Singer, D.B.; Murdoch, F.E.; Fritsch, M.K. The role of histone acetylation in regulating early gene expression patterns during early embryonic stem cell differentiation. J. Biol. Chem. 2007, 282, 6696–6706. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Q.; Lee, J.E.; Su, I.H.; Ge, K. Histone H3K27 methyltransferase Ezh2 represses Wnt genes to facilitate adipogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 7317–7322. [Google Scholar] [CrossRef] [PubMed]
- Cawthorn, W.P.; Bree, A.J.; Yao, Y.; Du, B.; Hemati, N.; Martinez-Santibañez, G.; MacDougald, O.A. Wnt6, Wnt10a and Wnt10b inhibit adipogenesis and stimulate osteoblastogenesis through a β-catenin-dependent mechanism. Bone 2012, 50, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Su, X.; Gao, B.; Shuai, Y.; Chen, J.; Deng, Z.; Liao, L.; Jin, Y. Epigenetic inhibition of Wnt pathway suppresses osteogenic differentiation of BMSCs during osteoporosis. Cell Death Dis. 2018, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Luu, H.H.; Song, W.X.; Luo, X.; Manning, D.; Luo, J.; Deng, Z.L.; Sharff, K.A.; Montag, A.G.; Haydon, R.C.; He, T.C. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J. Orthop. Res. 2007, 25, 665–677. [Google Scholar] [CrossRef]
- Huang, Z.; Ren, P.G.; Ma, T.; Smith, R.L.; Goodman, S.B. Modulating osteogenesis of mesenchymal stem cells by modifying growth factor availability. Cytokine 2010, 51, 305–310. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 2004, 101, 9607–9611. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Sun, H.L.; Liang, H.; Li, K.; Fan, Q.M.; Zhao, Q.H. Dynamic and distinct histone modifications of osteogenic genes during osteogenic differentiation. J. Biochem. 2015, 158, 445–457. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Henninger, A.M.; Eliasson, B.; Jenndahl, L.E.; Hammarstedt, A. Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to type 2 diabetes. PLoS ONE 2014, 9, e105262. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Agarwal, S.; Gupta, K.K.; Ramteke, P.W.; Singh, M.P. Medicinal mushroom: Boon for therapeutic applications. 3 Biotech 2018, 8, 334. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-Obesity Effects of Medicinal and Edible Mushrooms. Molecules 2018, 23, 2880. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Zhao, C.; Zheng, S.; Mei, X.; Huang, K.; Wang, G.; He, X. Anti-obesity and hypolipidemic effect of water extract from Pleurotus citrinopileatus in C57BL/6J mice. Food Sci. Nutr. 2019, 7, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Na, H.G.; Park, Y.; Kim, M.A.; Lee, J.W.; So, G.; Kim, S.H.; Jang, K.H.; Kim, M.J.; Namkoong, S.; Koo, H.J.; et al. Secondary fermented extract of Chaga-Cheonggukjang attenuates the effects of obesity and suppresses inflammatory response in the liver and spleen of high-fat diet-induced obese mice. J. Microbiol. Biotechnol. 2019, 29, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.; Han, P.W. Cellularity of rat adipose tissue: Effects of growth, starvation, and obesity. J. Lipid Res. 1969, 10, 77–82. [Google Scholar] [PubMed]
- Björntorp, P.; Gustafson, A.; Persson, B. Adipose tissue fat cell size and number in relation to metabolism in endogenous hypertriglyceridemia. Acta Med. Scand. 1971, 190, 363–367. [Google Scholar] [CrossRef]
- Salans, L.B.; Knittle, J.L.; Hirsch, J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J. Clin. Investig. 1968, 47, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Foley, J.E.; Bogardus, C.; Tataranni, P.A.; Pratley, R.E. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia 2000, 43, 1498–1506. [Google Scholar] [CrossRef]
- Pasarica, M.; Xie, H.; Hymel, D.; Bray, G.; Greenway, F.; Ravussin, E.; Smith, S.R. Lower total adipocyte number but no evidence for small adipocyte depletion in patients with type 2 diabetes. Diabetes Care 2009, 32, 900–902. [Google Scholar] [CrossRef]
- Lönn, M.; Mehlig, K.; Bengtsson, C.; Lissner, L. Adipocyte size predicts incidence of type 2 diabetes in women. FASEB J. 2010, 24, 326–331. [Google Scholar] [CrossRef]
- Virtue, S.; Vidal-Puig, A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome—An allostatic perspective. Biochim. Biophys. Acta 2010, 1801, 338–349. [Google Scholar] [CrossRef]
- Gustafson, B.; Hedjazifar, S.; Gogg, S.; Hammarstedt, A.; Smith, U. Insulin resistance and impaired adipogenesis. Trends Endocrinol. Metab. 2015, 26, 193–200. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Forcioli-Conti, N.; Estève, D.; Bouloumié, A.; Dani, C.; Peraldi, P. The size of the primary cilium and acetylated tubulin are modulated during adipocyte differentiation: Analysis of HDAC6 functions in these processes. Biochimie 2016, 124, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Shi, S.; Wang, H.; Liao, K. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J. Cell Sci. 2009, 122, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Ren, A.; Qiu, Y.; Zhang, Y. Epigenetic Regulation of Osteogenic Differentiation of Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2016, 11, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, P.; Ge, J.; Cheng, J.; Dong, W.; Yuan, H.; Du, Y.; Yang, M.; Sun, R.; Jiang, H. Histone deacetylase 8 suppresses osteogenic differentiation of bone marrow stromal cells by inhibiting histone H3K9 acetylation and RUNX2 activity. Int. J. Biochem. Cell Biol. 2014, 54, 68–77. [Google Scholar] [CrossRef]
- Huynh, N.C.; Everts, V.; Pavasant, P.; Ampornaramveth, R.S. Inhibition of Histone Deacetylases Enhances the Osteogenic Differentiation of Human Periodontal Ligament Cells. J. Cell. Biochem. 2016, 117, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Kao, Y.H.; Chung, C.C.; Cheng, W.L.; Chen, Y.J. HDAC I inhibitor regulates RUNX2 transactivation through canonical and non-canonical Wnt signaling in aortic valvular interstitial cells. Am. J. Transl. Res. 2019, 11, 744–754. [Google Scholar] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, S.A.; Nam, K.H.; Kim, S.; So, H.M.; Ryoo, R.; Han, J.-W.; Kim, K.H.; Lee, J. Vulpinic Acid Controls Stem Cell Fate toward Osteogenesis and Adipogenesis. Genes 2020, 11, 18. https://doi.org/10.3390/genes11010018
Yi SA, Nam KH, Kim S, So HM, Ryoo R, Han J-W, Kim KH, Lee J. Vulpinic Acid Controls Stem Cell Fate toward Osteogenesis and Adipogenesis. Genes. 2020; 11(1):18. https://doi.org/10.3390/genes11010018
Chicago/Turabian StyleYi, Sang Ah, Ki Hong Nam, Sil Kim, Hae Min So, Rhim Ryoo, Jeung-Whan Han, Ki Hyun Kim, and Jaecheol Lee. 2020. "Vulpinic Acid Controls Stem Cell Fate toward Osteogenesis and Adipogenesis" Genes 11, no. 1: 18. https://doi.org/10.3390/genes11010018
APA StyleYi, S. A., Nam, K. H., Kim, S., So, H. M., Ryoo, R., Han, J.-W., Kim, K. H., & Lee, J. (2020). Vulpinic Acid Controls Stem Cell Fate toward Osteogenesis and Adipogenesis. Genes, 11(1), 18. https://doi.org/10.3390/genes11010018