Kinase-Independent Functions of MASTL in Cancer: A New Perspective on MASTL Targeting
Abstract
1. Introduction
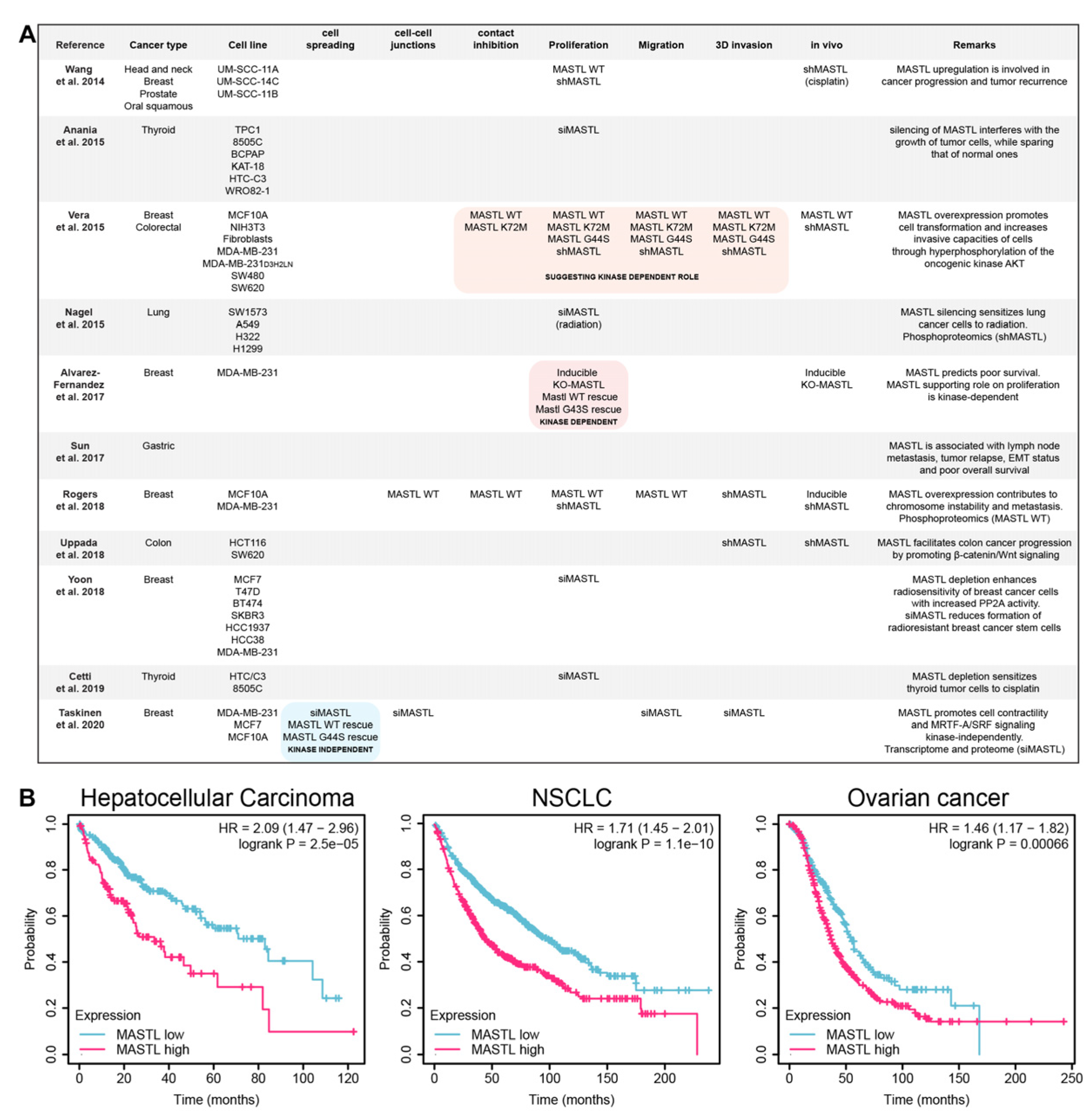
2. MASTL in Cancer
2.1. Possible Kinase-Dependent Functions of MASTL in Cancer
2.1.1. Glycogen Synthase Kinase 3 (GSK3), AKT, and Wnt Activity
2.1.2. Phosphorylation of Cytoskeletal- and Adhesion-Related Proteins
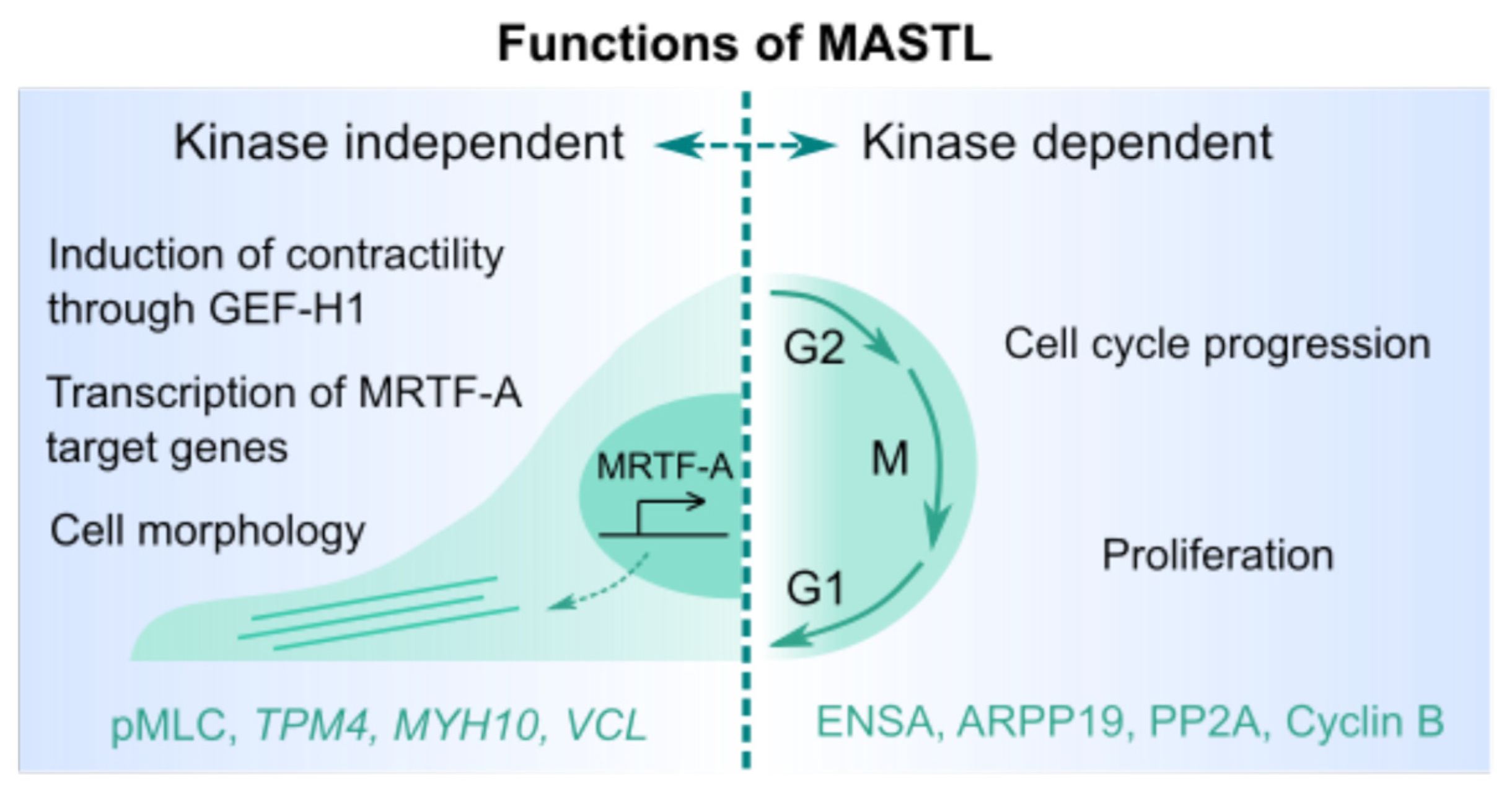
2.2. Possible Kinase-Independent Effects of MASTL on the Actomyosin Cytoskeleton
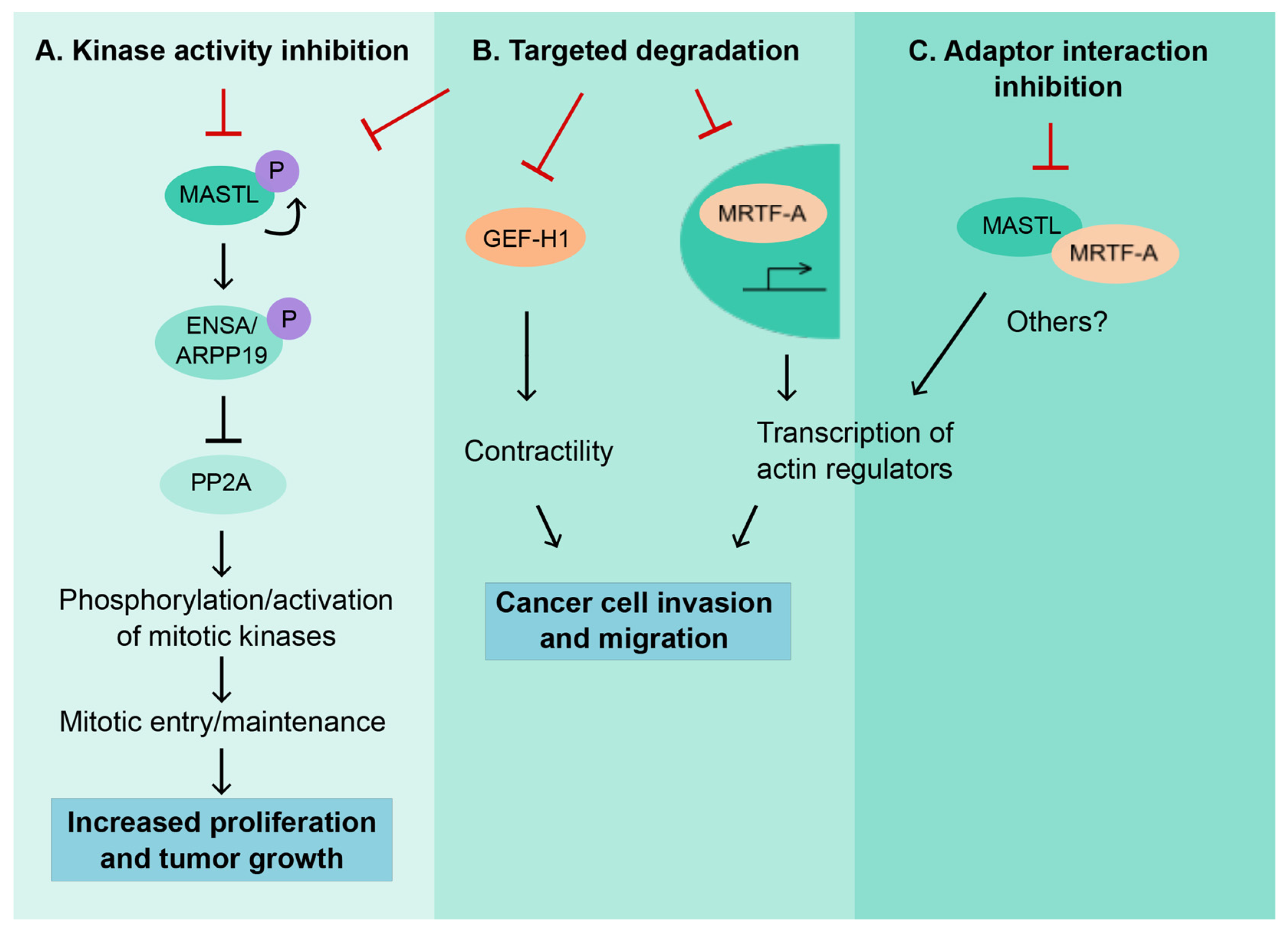
3. MASTL as a Therapeutic Target
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, J.; Fleming, S.L.; Williams, B.; Williams, E.V.; Li, Z.; Somma, P.; Rieder, C.L.; Goldberg, M.L. Greatwall kinase: A nuclear protein required for proper chromosome condensation and mitotic progression in Drosophila. J. Cell Biol. 2004, 164, 487–492. [Google Scholar] [CrossRef]
- Burgess, A.; Vigneron, S.; Brioudes, E.; Labbe, J.C.; Lorca, T.; Castro, A. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl. Acad. Sci. USA 2010, 107, 12564–12569. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, Y.; Li, Z.; Galas, S.; Goldberg, M.L. Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol. Cell 2006, 22, 83–91. [Google Scholar] [CrossRef]
- Alvarez-Fernandez, M.; Sanchez-Martinez, R.; Sanz-Castillo, B.; Gan, P.P.; Sanz-Flores, M.; Trakala, M.; Ruiz-Torres, M.; Lorca, T.; Castro, A.; Malumbres, M. Greatwall is essential to prevent mitotic collapse after nuclear envelope breakdown in mammals. Proc. Natl. Acad. Sci. USA 2013, 110, 17374–17379. [Google Scholar] [CrossRef]
- Hermida, D.; Mortuza, G.B.; Pedersen, A.K.; Pozdnyakova, I.; Nguyen, T.; Maroto, M.; Williamson, M.; Ebersole, T.; Cazzamali, G.; Rand, K.; et al. Molecular Basis of the Mechanisms Controlling MASTL. Mol. Cell Proteom. 2020, 19, 326–343. [Google Scholar] [CrossRef]
- Thul, P.J.; Lindskog, C. The human protein atlas: A spatial map of the human proteome. Protein Sci. 2018, 27, 233–244. [Google Scholar] [CrossRef]
- Rauch, J.; Volinsky, N.; Romano, D.; Kolch, W. The secret life of kinases: Functions beyond catalysis. Cell Commun. Signal. 2011, 9, 23. [Google Scholar] [CrossRef]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef]
- Marzec, K.; Burgess, A. The Oncogenic Functions of MASTL Kinase. Front. Cell Dev. Biol. 2018, 6, 162. [Google Scholar] [CrossRef]
- Wang, L.; Luong, V.Q.; Giannini, P.J.; Peng, A. Mastl kinase, a promising therapeutic target, promotes cancer recurrence. Oncotarget 2014, 5, 11479–11489. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.J.; Li, Y.L.; Wang, L.G.; Liu, L.Q.; Ma, H.; Hou, W.H.; Yu, J.M. Mastl overexpression is associated with epithelial to mesenchymal transition and predicts a poor clinical outcome in gastric cancer. Oncol. Lett. 2017, 14, 7283–7287. [Google Scholar] [CrossRef]
- Uppada, S.B.; Gowrikumar, S.; Ahmad, R.; Kumar, B.; Szeglin, B.; Chen, X.; Smith, J.J.; Batra, S.K.; Singh, A.B.; Dhawan, P. MASTL induces Colon Cancer progression and Chemoresistance by promoting Wnt/beta-catenin signaling. Mol. Cancer 2018, 17, 111. [Google Scholar] [CrossRef]
- Taskinen, M.E.; Narva, E.; Conway, J.R.W.; Hinojosa, L.S.; Lilla, S.; Mai, A.; De Franceschi, N.; Elo, L.L.; Grosse, R.; Zanivan, S.; et al. MASTL promotes cell contractility and motility through kinase-independent signaling. J. Cell Biol. 2020, 219. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.; McCloy, R.A.; Parker, B.L.; Gallego-Ortega, D.; Law, A.M.K.; Chin, V.T.; Conway, J.R.W.; Fey, D.; Millar, E.K.A.; O’Toole, S.; et al. MASTL overexpression promotes chromosome instability and metastasis in breast cancer. Oncogene 2018, 37, 4518–4533. [Google Scholar] [CrossRef] [PubMed]
- Vera, J.; Lartigue, L.; Vigneron, S.; Gadea, G.; Gire, V.; Del Rio, M.; Soubeyran, I.; Chibon, F.; Lorca, T.; Castro, A. Greatwall promotes cell transformation by hyperactivating AKT in human malignancies. Elife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Fernandez, M.; Sanz-Flores, M.; Sanz-Castillo, B.; Salazar-Roa, M.; Partida, D.; Zapatero-Solana, E.; Ali, H.R.; Manchado, E.; Lowe, S.; VanArsdale, T.; et al. Therapeutic relevance of the PP2A-B55 inhibitory kinase MASTL/Greatwall in breast cancer. Cell Death Differ. 2018, 25, 828–840. [Google Scholar] [CrossRef]
- Gyorffy, B.; Lanczky, A.; Szallasi, Z. Implementing an online tool for genome-wide validation of survival-associated biomarkers in ovarian-cancer using microarray data from 1287 patients. Endocr. Relat. Cancer 2012, 19, 197–208. [Google Scholar] [CrossRef]
- Gyorffy, B.; Surowiak, P.; Budczies, J.; Lanczky, A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS ONE 2013, 8, e82241. [Google Scholar] [CrossRef]
- Menyhart, O.; Nagy, A.; Gyorffy, B. Determining consistent prognostic biomarkers of overall survival and vascular invasion in hepatocellular carcinoma. R. Soc. Open Sci. 2018, 5, 181006. [Google Scholar] [CrossRef]
- Gharbi-Ayachi, A.; Labbe, J.C.; Burgess, A.; Vigneron, S.; Strub, J.M.; Brioudes, E.; Van-Dorsselaer, A.; Castro, A.; Lorca, T. The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 2010, 330, 1673–1677. [Google Scholar] [CrossRef]
- Hached, K.; Goguet, P.; Charrasse, S.; Vigneron, S.; Sacristan, M.P.; Lorca, T.; Castro, A. ENSA and ARPP19 differentially control cell cycle progression and development. J. Cell Biol. 2019, 218, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.; Lorca, T. Greatwall kinase at a glance. J. Cell Sci. 2018, 131, jcs222364. [Google Scholar] [CrossRef]
- Mochida, S.; Maslen, S.L.; Skehel, M.; Hunt, T. Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 2010, 330, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.C.; Filter, J.J.; Blake-Hodek, K.A.; Wadzinski, B.E.; Fuda, N.J.; Shalloway, D.; Goldberg, M.L. Greatwall-phosphorylated Endosulfine is both an inhibitor and a substrate of PP2A-B55 heterotrimers. Elife 2014, 3, e01695. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Hyun, S.Y.; Jang, Y.J. Dephosphorylation of Plk1 occurs through PP2A-B55/ENSA/Greatwall pathway during mitotic DNA damage recovery. Cell Cycle 2019, 18, 1154–1167. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Hemmings, B.A. PKB/Akt-dependent regulation of cell motility. J. Natl. Cancer Inst. 2013, 105, 393–404. [Google Scholar] [CrossRef]
- Wang, K.; Wang, X.; Zou, J.; Zhang, A.; Wan, Y.; Pu, P.; Song, Z.; Qian, C.; Chen, Y.; Yang, S.; et al. miR-92b controls glioma proliferation and invasion through regulating Wnt/beta-catenin signaling via Nemo-like kinase. Neuro Oncol. 2013, 15, 578–588. [Google Scholar] [CrossRef]
- Yang, C.M.; Ji, S.; Li, Y.; Fu, L.Y.; Jiang, T.; Meng, F.D. beta-Catenin promotes cell proliferation, migration, and invasion but induces apoptosis in renal cell carcinoma. Onco Targets Ther. 2017, 10, 711–724. [Google Scholar] [CrossRef]
- Hurtado, B.; Trakala, M.; Ximenez-Embun, P.; El Bakkali, A.; Partida, D.; Sanz-Castillo, B.; Alvarez-Fernandez, M.; Maroto, M.; Sanchez-Martinez, R.; Martinez, L.; et al. Thrombocytopenia-associated mutations in Ser/Thr kinase MASTL deregulate actin cytoskeletal dynamics in platelets. J. Clin. Invest. 2018, 128, 5351–5367. [Google Scholar] [CrossRef]
- Pearce, L.R.; Komander, D.; Alessi, D.R. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell Biol. 2010, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Blake-Hodek, K.A.; Williams, B.C.; Zhao, Y.; Castilho, P.V.; Chen, W.; Mao, Y.; Yamamoto, T.M.; Goldberg, M.L. Determinants for activation of the atypical AGC kinase Greatwall during M phase entry. Mol. Cell Biol. 2012, 32, 1337–1353. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.J.; Gandhi, M.J.; Shafizadeh, E.; Langer, N.B.; Pierce, E.L.; Paw, B.H.; Gilligan, D.M.; Drachman, J.G. In vivo inactivation of MASTL kinase results in thrombocytopenia. Exp. Hematol. 2009, 37, 901–908. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ly, D.L.; Waheed, F.; Lodyga, M.; Speight, P.; Masszi, A.; Nakano, H.; Hersom, M.; Pedersen, S.F.; Szaszi, K.; Kapus, A. Hyperosmotic stress regulates the distribution and stability of myocardin-related transcription factor, a key modulator of the cytoskeleton. Am. J. Physiol. Cell Physiol. 2013, 304, C115–C127. [Google Scholar] [CrossRef] [PubMed]
- Champion, L.; Linder, M.I.; Kutay, U. Cellular Reorganization during Mitotic Entry. Trends Cell Biol. 2017, 27, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Heng, Y.W.; Koh, C.G. Actin cytoskeleton dynamics and the cell division cycle. Int. J. Biochem. Cell Biol. 2010, 42, 1622–1633. [Google Scholar] [CrossRef]
- Kunda, P.; Baum, B. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 2009, 19, 174–179. [Google Scholar] [CrossRef]
- Bakal, C.J.; Finan, D.; LaRose, J.; Wells, C.D.; Gish, G.; Kulkarni, S.; DeSepulveda, P.; Wilde, A.; Rottapel, R. The Rho GTP exchange factor Lfc promotes spindle assembly in early mitosis. Proc. Natl. Acad. Sci. USA 2005, 102, 9529–9534. [Google Scholar] [CrossRef]
- Birkenfeld, J.; Nalbant, P.; Bohl, B.P.; Pertz, O.; Hahn, K.M.; Bokoch, G.M. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev. Cell 2007, 12, 699–712. [Google Scholar] [CrossRef]
- Birkenfeld, J.; Nalbant, P.; Yoon, S.H.; Bokoch, G.M. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: Is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol. 2008, 18, 210–219. [Google Scholar] [CrossRef]
- Chanet, S.; Sharan, R.; Khan, Z.; Martin, A.C. Myosin 2-Induced Mitotic Rounding Enables Columnar Epithelial Cells to Interpret Cortical Spindle Positioning Cues. Curr. Biol. CB 2017, 27, 3350–3358. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.P.; Helenius, J.; Stewart, M.P.; Cattin, C.J.; Hyman, A.A.; Muller, D.J. Cdk1-dependent mitotic enrichment of cortical myosin II promotes cell rounding against confinement. Nat. Cell Biol. 2015, 17, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Shaposhnikov, D.; Kuffer, C.; Storchova, Z.; Posern, G. Myocardin related transcription factors are required for coordinated cell cycle progression. Cell Cycle 2013, 12, 1762–1772. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshizaki, H.; Ohba, Y.; Parrini, M.C.; Dulyaninova, N.G.; Bresnick, A.R.; Mochizuki, N.; Matsuda, M. Cell type-specific regulation of RhoA activity during cytokinesis. J. Biol. Chem. 2004, 279, 44756–44762. [Google Scholar] [CrossRef]
- Voets, E.; Wolthuis, R.M. MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle 2010, 9, 3591–3601. [Google Scholar] [CrossRef]
- Dahlhaus, M.; Burkovski, A.; Hertwig, F.; Mussel, C.; Volland, R.; Fischer, M.; Debatin, K.M.; Kestler, H.A.; Beltinger, C. Boolean modeling identifies Greatwall/MASTL as an important regulator in the AURKA network of neuroblastoma. Cancer Lett. 2016, 371, 79–89. [Google Scholar] [CrossRef]
- Wang, L.; Fisher, L.A.; Wahl, J.K., 3rd; Peng, A. Monoclonal antibodies against Xenopus greatwall kinase. Hybridoma (Larchmt) 2011, 30, 469–474. [Google Scholar] [CrossRef]
- Ocasio, C.A.; Rajasekaran, M.B.; Walker, S.; Le Grand, D.; Spencer, J.; Pearl, F.M.; Ward, S.E.; Savic, V.; Pearl, L.H.; Hochegger, H.; et al. A first generation inhibitor of human Greatwall kinase, enabled by structural and functional characterisation of a minimal kinase domain construct. Oncotarget 2016, 7, 71182–71197. [Google Scholar] [CrossRef]
- Ammarah, U.; Kumar, A.; Pal, R.; Bal, N.C.; Misra, G. Identification of new inhibitors against human Great wall kinase using in silico approaches. Sci. Rep. 2018, 8, 4894. [Google Scholar] [CrossRef]
- Gorgulla, C.; Boeszoermenyi, A.; Wang, Z.F.; Fischer, P.D.; Coote, P.W.; Padmanabha Das, K.M.; Malets, Y.S.; Radchenko, D.S.; Moroz, Y.S.; Scott, D.A.; et al. An open-source drug discovery platform enables ultra-large virtual screens. Nature 2020, 580, 663–668. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conway, J.R.W.; Närvä, E.; Taskinen, M.E.; Ivaska, J. Kinase-Independent Functions of MASTL in Cancer: A New Perspective on MASTL Targeting. Cells 2020, 9, 1624. https://doi.org/10.3390/cells9071624
Conway JRW, Närvä E, Taskinen ME, Ivaska J. Kinase-Independent Functions of MASTL in Cancer: A New Perspective on MASTL Targeting. Cells. 2020; 9(7):1624. https://doi.org/10.3390/cells9071624
Chicago/Turabian StyleConway, James Ronald William, Elisa Närvä, Maria Emilia Taskinen, and Johanna Ivaska. 2020. "Kinase-Independent Functions of MASTL in Cancer: A New Perspective on MASTL Targeting" Cells 9, no. 7: 1624. https://doi.org/10.3390/cells9071624
APA StyleConway, J. R. W., Närvä, E., Taskinen, M. E., & Ivaska, J. (2020). Kinase-Independent Functions of MASTL in Cancer: A New Perspective on MASTL Targeting. Cells, 9(7), 1624. https://doi.org/10.3390/cells9071624




