Paradoxical Role of Matrix Metalloproteinases in Liver Injury and Regeneration after Sterile Acute Hepatic Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Acetaminophen-Induced Liver Injury Model
2.3. Alanine Aminotransferase Assay
2.4. Indocyanine Green Clearance Rate Assay
2.5. Histological Analysis
2.6. Confocal Intravital Imaging
2.7. Neutrophil Depletion and Pharmacological Interventions
2.8. Neutrophil Myeloperoxidase Activity Assay
2.9. Statistical Analysis
3. Results
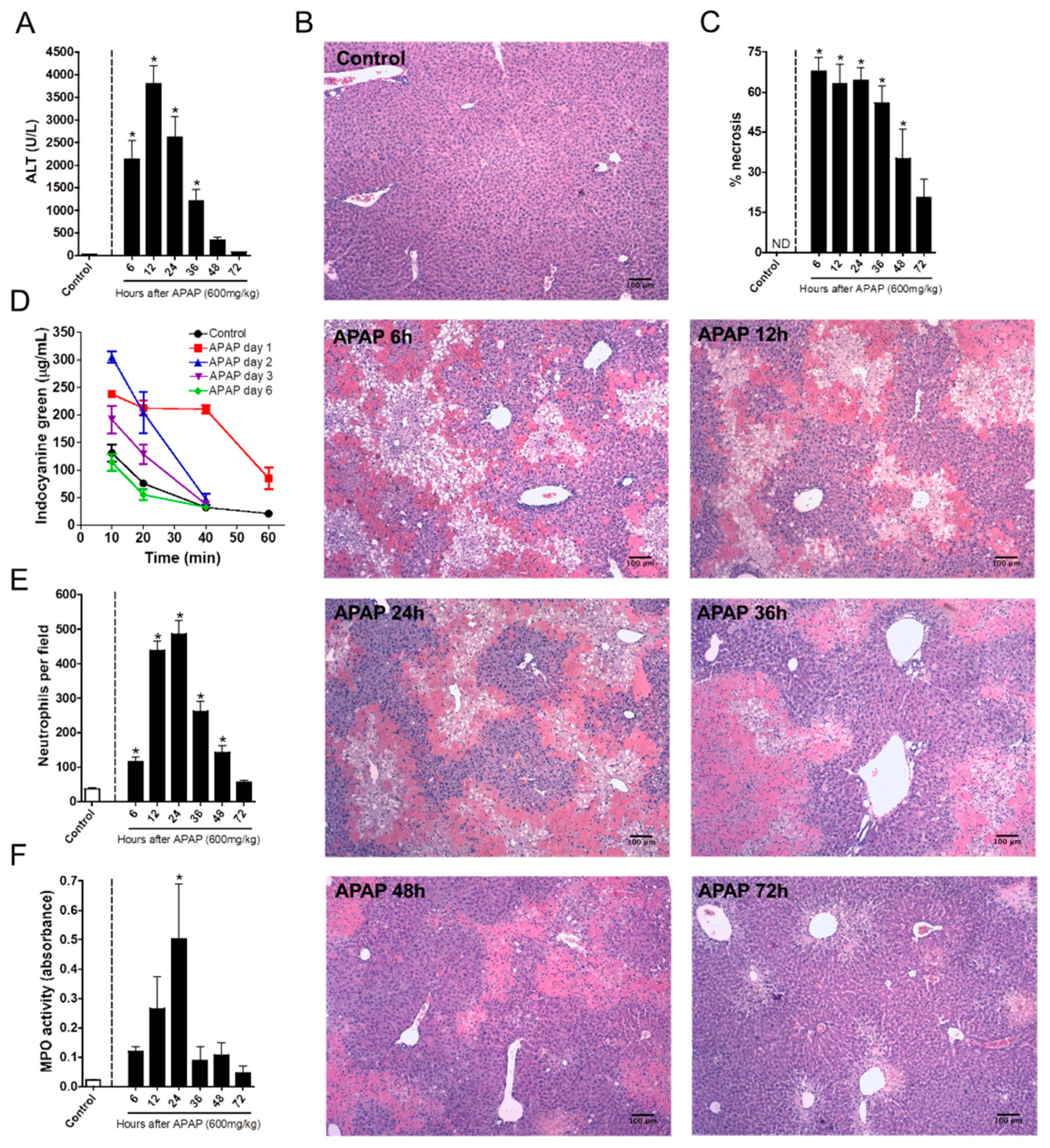
3.1. Neutrophil Recruitment Kinetics Correlate with Liver Injury
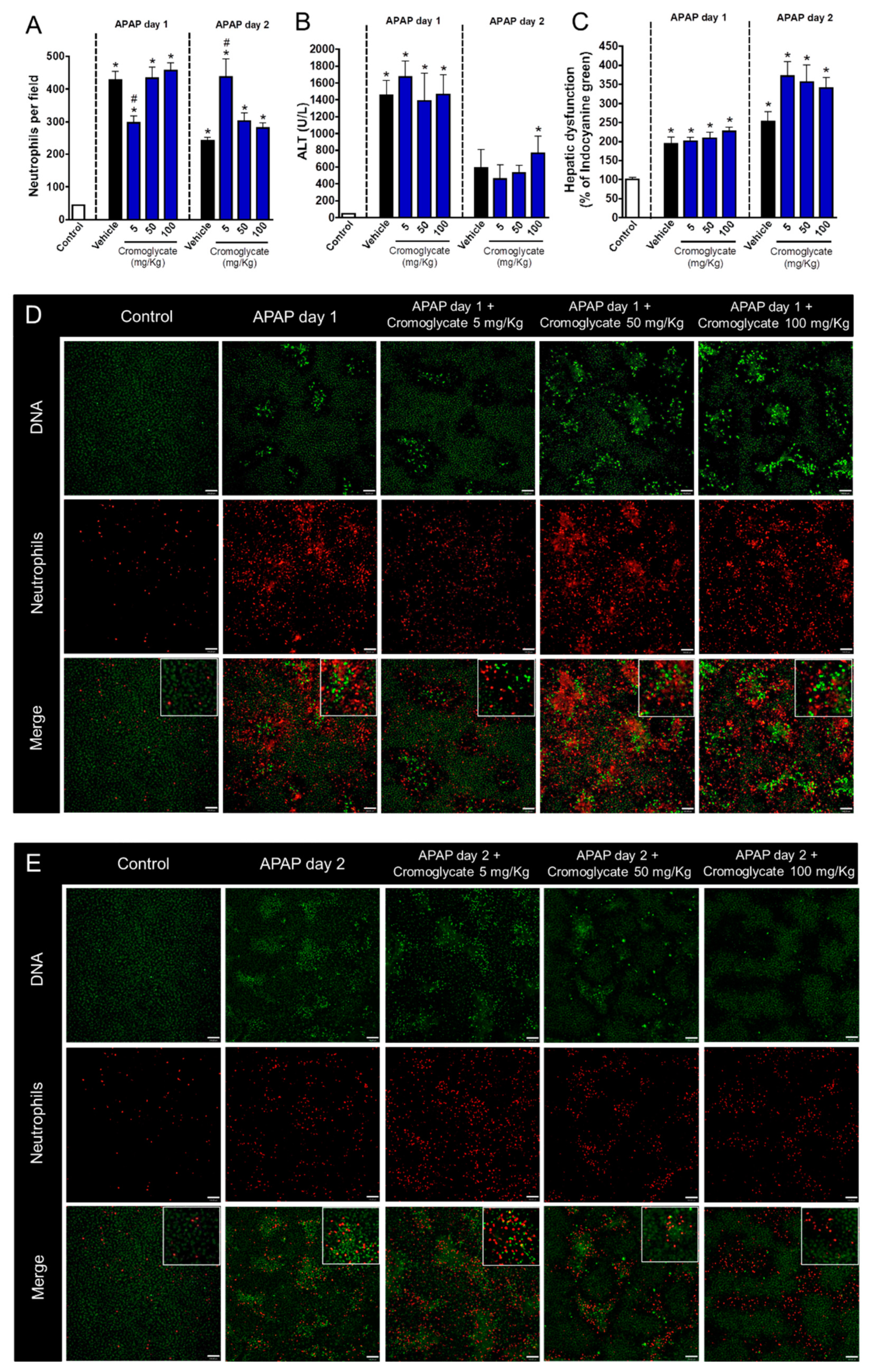
3.2. Neutrophil Degranulation Is Crucial to Tissue Repair after APAP-Induced Liver Injury
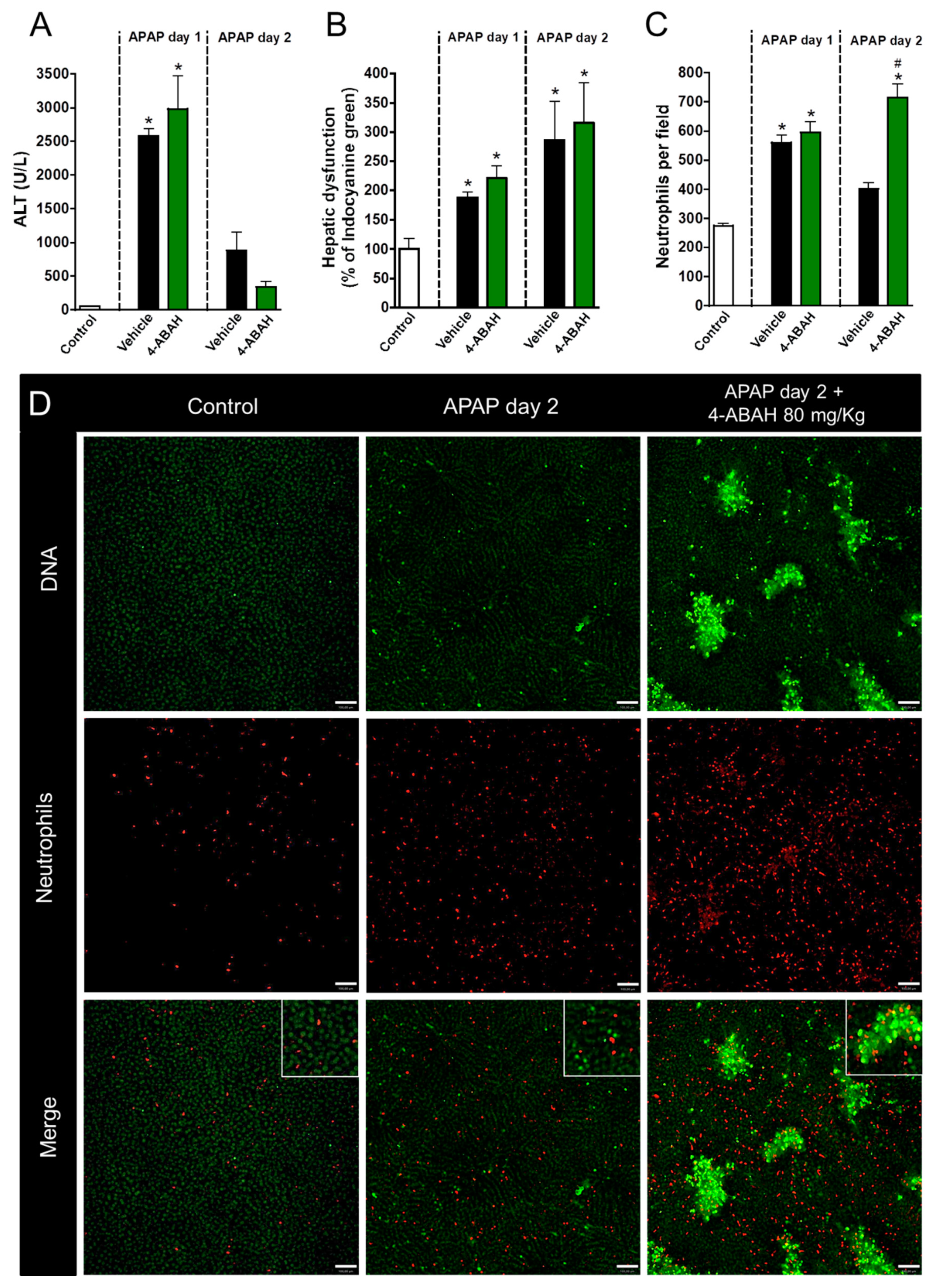
3.3. Neutrophil Proteases, but Not Myeloperoxidase, Participate in Liver Repair after APAP-Induced Injury
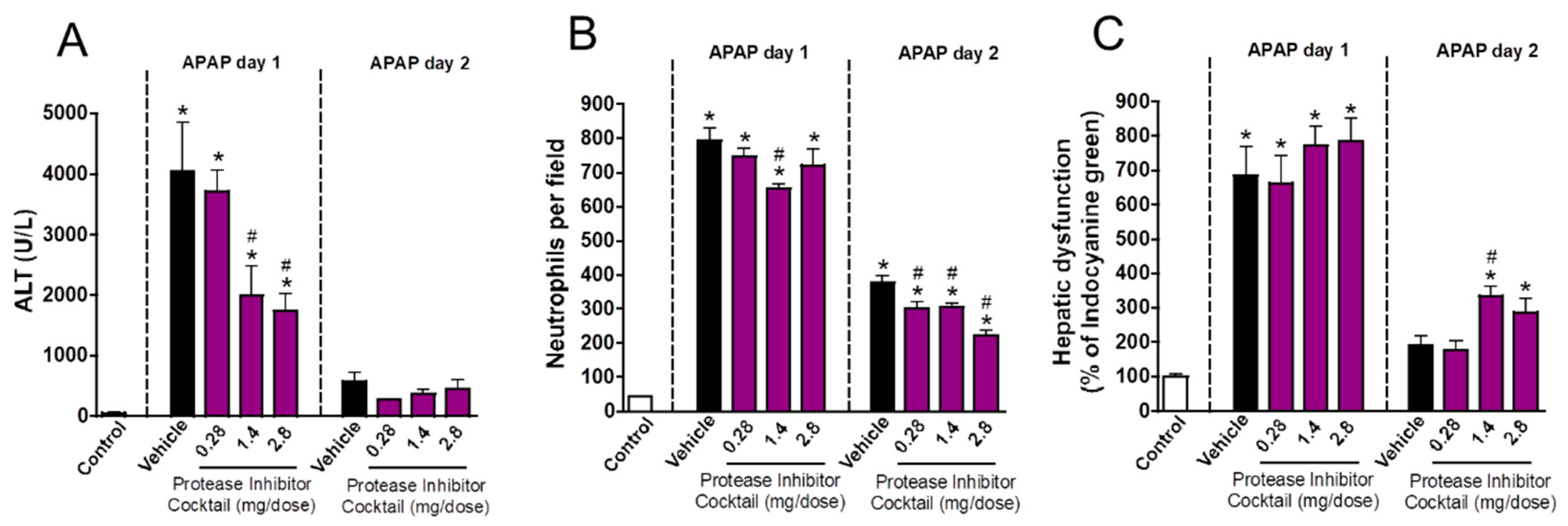
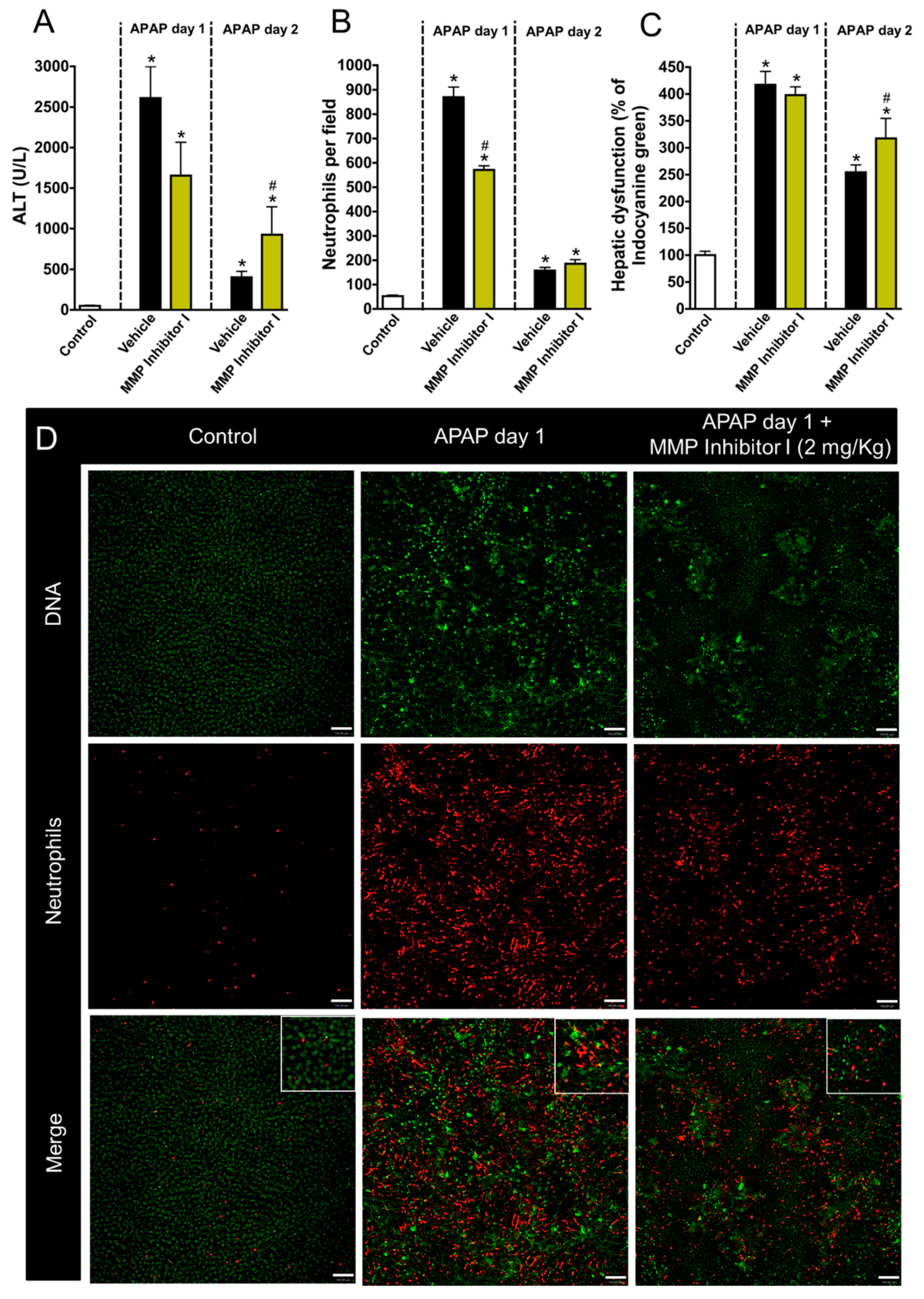
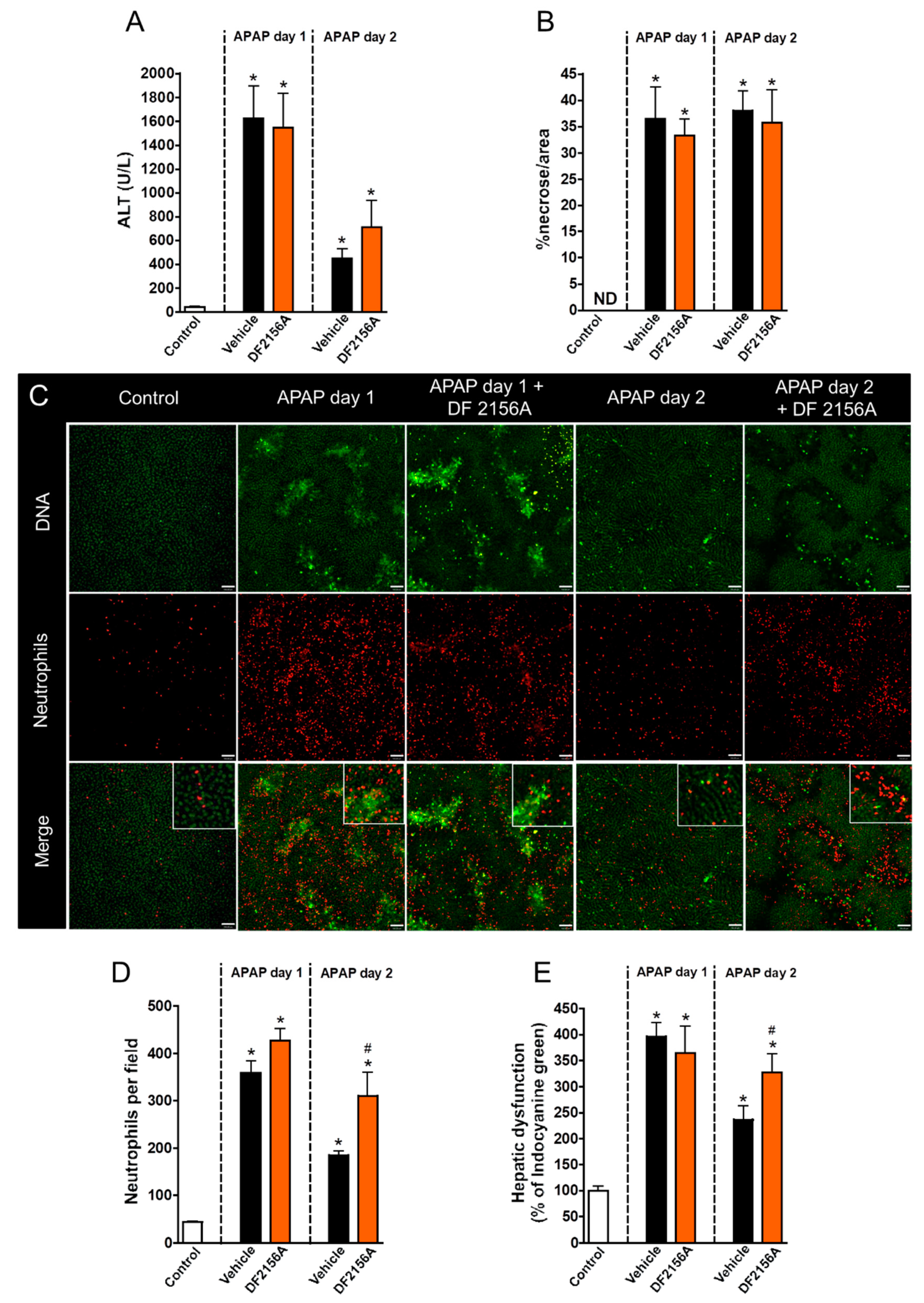
3.4. Neutrophil Matrix Metalloproteinases Mediate Liver Function Restoration
3.5. Inhibition of Neutrophil Migration Recapitulates the Delay in Liver Repair Observed during MMP Blockage
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- David, S.; Hamilton, J.P. Drug-induced liver injury. US Gastroenterol. Hepatol. Rev. 2010, 6, 73–80. [Google Scholar] [PubMed]
- Prescott, L.F.; Park, J.; Ballantyne, A.; Adriaenssens, P.; Proudfoot, A.T. Treatment of paracetamol (acetaminophen) poisoning with n-acetylcysteine. Lancet 1977, 2, 432–434. [Google Scholar] [CrossRef]
- Lee, W.M. Acetaminophen toxicity: Changing perceptions on a social/medical issue. Hepatology 2007, 46, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.; Babar, A.; Choudhary, M.; Kutner, M.; Pyrsopoulos, N. Acetaminophen-induced hepatotoxicity: A comprehensive update. J. Clin. Transl. Hepatol. 2016, 4, 131–142. [Google Scholar] [PubMed]
- Marques, P.E.; Amaral, S.S.; Pires, D.A.; Nogueira, L.L.; Soriani, F.M.; Lima, B.H.; Lopes, G.A.; Russo, R.C.; Avila, T.V.; Melgaco, J.G.; et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 2012, 56, 1971–1982. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Faurschou, M.; Borregaard, N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003, 5, 1317–1327. [Google Scholar] [CrossRef]
- Rosell, A.; Cuadrado, E.; Ortega-Aznar, A.; Hernandez-Guillamon, M.; Lo, E.H.; Montaner, J. Mmp-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type iv collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 2008, 39, 1121–1126. [Google Scholar] [CrossRef]
- Ohnishi, K.; Takagi, M.; Kurokawa, Y.; Satomi, S.; Konttinen, Y.T. Matrix metalloproteinase-mediated extracellular matrix protein degradation in human pulmonary emphysema. Lab. Invest. 1998, 78, 1077–1087. [Google Scholar]
- Suzuki, S.; Toledo-Pereyra, L.H.; Rodriguez, F.J.; Cejalvo, D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of fk506 and cyclosporine. Transplantation 1993, 55, 1265–1272. [Google Scholar]
- Sheron, N.; Bird, G.; Koskinas, J.; Portmann, B.; Ceska, M.; Lindley, I.; Williams, R. Circulating and tissue levels of the neutrophil chemotaxin interleukin-8 are elevated in severe acute alcoholic hepatitis, and tissue levels correlate with neutrophil infiltration. Hepatology 1993, 18, 41–46. [Google Scholar] [PubMed]
- Marques, P.E.; Oliveira, A.G.; Chang, L.; Paula-Neto, H.A.; Menezes, G.B. Understanding liver immunology using intravital microscopy. J. Hepatol. 2015, 63, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Sawant, K.V.; Poluri, K.M.; Dutta, A.K.; Sepuru, K.M.; Troshkina, A.; Garofalo, R.P.; Rajarathnam, K. Chemokine cxcl1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y. Neutrophil infiltration and chemokines. Crit. Rev. Immunol. 2006, 26, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.; Tauzin, S.; Hind, L.E.; Deng, Q.; Beebe, D.J.; Huttenlocher, A. Chemokine signaling and the regulation of bidirectional leukocyte migration in interstitial tissues. Cell Rep. 2017, 19, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.E.; Oliveira, A.G.; Pereira, R.V.; David, B.A.; Gomides, L.F.; Saraiva, A.M.; Pires, D.A.; Novaes, J.T.; Patricio, D.O.; Cisalpino, D.; et al. Hepatic DNA deposition drives drug-induced liver injury and inflammation in mice. Hepatology 2015, 61, 348–360. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Davis, D.C.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J. Pharmacol. Exp. Ther. 1973, 187, 185–194. [Google Scholar]
- Liu, Z.X.; Han, D.; Gunawan, B.; Kaplowitz, N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 2006, 43, 1220–1230. [Google Scholar] [CrossRef]
- Jaeschke, H.; Williams, C.D.; Ramachandran, A.; Bajt, M.L. Acetaminophen hepatotoxicity and repair: The role of sterile inflammation and innate immunity. Liver Int. 2012, 32, 8–20. [Google Scholar] [CrossRef]
- Daley, J.M.; Thomay, A.A.; Connolly, M.D.; Reichner, J.S.; Albina, J.E. Use of ly6g-specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 2008, 83, 64–70. [Google Scholar] [CrossRef]
- Jaeschke, H. How relevant are neutrophils for acetaminophen hepatotoxicity? Hepatology 2006, 43, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, M.; Kubes, P. The neutrophil in vascular inflammation. Nat. Med. 2011, 17, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Bourke, E.; Cassetti, A.; Villa, A.; Fadlon, E.; Colotta, F.; Mantovani, A. Il-1 beta scavenging by the type ii il-1 decoy receptor in human neutrophils. J. Immunol. 2003, 170, 5999–6005. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Palmer, G.; Vigne, S.; Lamacchia, C.; Rodriguez, E.; Talabot-Ayer, D.; Rose-John, S.; Chalaris, A.; Gabay, C. Mouse neutrophils express the decoy type 2 interleukin-1 receptor (il-1r2) constitutively and in acute inflammatory conditions. J. Leukoc. Biol. 2013, 94, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Norling, L.V.; Renshaw, D.; Cooper, D.; Leung, K.Y.; Perretti, M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood 2008, 112, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Mott, J.D.; Werb, Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004, 16, 558–564. [Google Scholar] [CrossRef]
- Overall, C.M.; McQuibban, G.A.; Clark-Lewis, I. Discovery of chemokine substrates for matrix metalloproteinases by exosite scanning: A new tool for degradomics. Biol. Chem. 2002, 383, 1059–1066. [Google Scholar] [CrossRef]
- McQuibban, G.A.; Butler, G.S.; Gong, J.H.; Bendall, L.; Power, C.; Clark-Lewis, I.; Overall, C.M. Matrix metalloproteinase activity inactivates the cxc chemokine stromal cell-derived factor-1. J. Biol. Chem. 2001, 276, 43503–43508. [Google Scholar] [CrossRef]
- Xie, Y.; McGill, M.R.; Dorko, K.; Kumer, S.C.; Schmitt, T.M.; Forster, J.; Jaeschke, H. Mechanisms of acetaminophen-induced cell death in primary human hepatocytes. Toxicol. Appl. Pharmacol. 2014, 279, 266–274. [Google Scholar] [CrossRef]
- Iorga, A.; Dara, L.; Kaplowitz, N. Drug-induced liver injury: Cascade of events leading to cell death, apoptosis or necrosis. Int. J. Mol. Sci. 2017, 18, 1018. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Abril, E.R.; Bethea, N.W.; McCuskey, R.S. Inhibition of matrix metalloproteinases minimizes hepatic microvascular injury in response to acetaminophen in mice. Toxicol. Sci. 2005, 83, 190–196. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarenga, D.M.; Mattos, M.S.; Lopes, M.E.; Marchesi, S.C.; Araújo, A.M.; Nakagaki, B.N.; Santos, M.M.; David, B.A.; De Souza, V.A.; Carvalho, É.; et al. Paradoxical Role of Matrix Metalloproteinases in Liver Injury and Regeneration after Sterile Acute Hepatic Failure. Cells 2018, 7, 247. https://doi.org/10.3390/cells7120247
Alvarenga DM, Mattos MS, Lopes ME, Marchesi SC, Araújo AM, Nakagaki BN, Santos MM, David BA, De Souza VA, Carvalho É, et al. Paradoxical Role of Matrix Metalloproteinases in Liver Injury and Regeneration after Sterile Acute Hepatic Failure. Cells. 2018; 7(12):247. https://doi.org/10.3390/cells7120247
Chicago/Turabian StyleAlvarenga, Débora Moreira, Matheus Silvério Mattos, Mateus Eustáquio Lopes, Sarah Cozzer Marchesi, Alan Moreira Araújo, Brenda Naemi Nakagaki, Mônica Morais Santos, Bruna Araújo David, Viviane Aparecida De Souza, Érika Carvalho, and et al. 2018. "Paradoxical Role of Matrix Metalloproteinases in Liver Injury and Regeneration after Sterile Acute Hepatic Failure" Cells 7, no. 12: 247. https://doi.org/10.3390/cells7120247
APA StyleAlvarenga, D. M., Mattos, M. S., Lopes, M. E., Marchesi, S. C., Araújo, A. M., Nakagaki, B. N., Santos, M. M., David, B. A., De Souza, V. A., Carvalho, É., Sousa Pereira, R. V., Marques, P. E., Mafra, K., De Castro Oliveira, H. M., De Miranda, C. D. M., Diniz, A. B., De Oliveira, T. H. C., Teixeira, M. M., Rezende, R. M., ... Menezes, G. B. (2018). Paradoxical Role of Matrix Metalloproteinases in Liver Injury and Regeneration after Sterile Acute Hepatic Failure. Cells, 7(12), 247. https://doi.org/10.3390/cells7120247





