Abstract
Bidens pilosa L., a traditional Chinese medicinal herb, has been used in clinical practice for the treatment of inflammatory diseases and cancer. BPA, an extract derived from the whole herb of B. pilosa L., has been shown to possess potent immunomodulatory properties by regulating tumor-associated macrophages (TAMs) and regulatory T cells (Tregs) within the tumor microenvironment (TME) in a mouse syngeneic colorectal cancer (CRC) model. RT-PCR and flow cytometry analyses showed that BPA, together with its flavonoid and polyacetylene constituents, effectively suppressed the differentiation of M2-TAMs and Tregs by downregulating Arg-1 and CD25 expression. They had minimal effects on the expression of markers associated with M1-TAMs and promoted the proliferation of CD4+ T cells that were inhibited by M2-TAMs and Tregs. In mice, BPA markedly inhibited the growth of syngeneic CRC tumors, accompanied by decreased serum levels of the immunosuppressive cytokine IL-10 and reduced expression of the proliferative marker Ki67 in tumor tissues. Moreover, BPA downregulated the mRNA expression of markers associated with M2-TAMs and Tregs, while increasing markers associated with M1-TAMs. Western blot analyses of tumor tissues revealed that BPA reduced the expression of marker proteins associated with M2-TAMs and Tregs, while increasing the expression of the immune-stimulatory markers CD80, GITR and CD4. In addition, combined treatment with BPA and 5-fluorouracil (5-FU), a commonly used chemotherapeutic agent for CRC, notably enhanced the anti-tumor effect in mice. These findings indicate that BPA, an active extract of B. pilosa L., showed antitumor activity in mice by suppressing the differentiation of pro-tumorigenic TAMs and Tregs within the TME.
1. Introduction
Cancer is one of the most prevalent diseases worldwide and ranks as the second leading cause of death among humans [1]. Although traditional cancer treatments, such as surgery, chemotherapy, and radiotherapy, have made significant progress, they are still associated with notable adverse effects and risks of recurrence and metastasis. Consequently, the development of novel therapeutic strategies to combat cancer is imperative [2]. In recent years, immunotherapy has emerged as a promising anticancer strategy by mobilizing host immunity against malignancies [3], and the rapid development of tumor immunotherapy has not only provided cancer patients with new treatment options but has also highlighted the crucial role of the tumor microenvironment (TME) in the processes of tumor initiation and progression [4].
The TME refers to the microenvironment surrounding tumor cells, which includes the tumor cells themselves, adjacent non-tumor cells, extracellular matrix, blood vessels, immune cells, as well as various signaling molecules and cytokines. Within the TME, immunosuppressive cells such as TAMs, Tregs, and MDSCs activate protumor signaling cascades that promote tumor immune evasion and drive disease pathogenesis [4,5]. As the most plastic immune cells abundant in the TME, TAMs can differentiate into either M1 or M2 macrophages. M1 macrophages, activated by stimuli such as interferon-γ (IFN-γ), release pro-inflammatory cytokines (e.g., interleukin-1β, IL-1β) and chemokines (such as CXCL10). This enhances antigen presentation, promotes Th1 responses, and mediates anti-tumor effects [6]. In contrast, M2 macrophages, activated by IL-4 and IL-13, secrete anti-inflammatory cytokines (e.g., IL-10 and TGF-β) that facilitate tumor development by promoting growth, angiogenesis, and the differentiation of CD4+ T cells into Tregs [7,8,9]. Tregs, characterized by the expression of Foxp3, play a crucial role in establishing immune tolerance within the TME by suppressing the activity of CD4+ and CD8+ T cells. They achieve this through the secretion of cytokines such as IL-10 and TGF-β, as well as by decreasing the expression of CD80 or CD86 on antigen-presenting cells, thereby facilitating immune evasion [10]. Overall, TAMs and Tregs play pivotal roles in creating the immunosuppressive TME, exerting powerful effects on tumor progression and clinical outcomes [11]. Therapeutic interventions targeting immunosuppressive TAMs and Tregs within the TME show clinical promise. For instance, emactuzumab, a monoclonal antibody targeting CSF-1R, effectively depletes M2 macrophages in tenosynovial giant cell tumors (TGCT), thereby restoring T cell activity and reducing tumor burden [12]. Sunitinib, a chemotherapy agent and tyrosine kinase inhibitor (TKI), selectively reduces the abundance and function of Tregs in patients with renal cell carcinoma, thereby enhancing anti-tumor immunity and therapeutic efficacy [13].
Natural products have attracted considerable attention for their capacity to modulate immune cell functions within the TME and to provide a promising strategy for cancer immunotherapy. Traditional Chinese medicine has a long history of clinical use for therapeutic purposes. Bidens pilosa L., an annual herb from the Asteraceae family, is utilized as both a food source and a traditional medicine for humans and animals. Traditional records highlight its application in treating a variety of diseases, particularly inflammatory diseases and cancer [14,15]. A diverse array of bioactive compounds has been identified from B. pilosa L. by phytochemical investigations, including flavonoids, polyacetylene, phenolic acids, terpenoids, lipids, and alkaloids. These constituents confer a wide range of pharmacological effects, including anti-hyperglycemic, antihypertensive, anti-ulcer, antipyretic, analgesic, immunosuppressive, antibacterial, anti-inflammatory activities, as well as antioxidant and antitumor effects [16]. The anticancer activity of B. pilosa L. is well-documented; however, its specific immunomodulatory effects in the context of cancer are not yet fully understood.
In our previous work, we isolated compounds from B. pilosa L. and characterized their structures. Preliminary studies demonstrated that flavonoids and polyacetylenes isolated from B. pilosa L. notably suppressed cancer cell proliferation by inhibiting DNA topoisomerase I (Topo I) and disrupting mitotic progression [17,18]. However, the immunomodulatory potential of these compounds within the TME, particularly their effect on immunosuppressive cells, remains largely unexplored. Therefore, this study focuses on TAMs and Tregs to investigate how BPA, an extract derived from B. pilosa L., exerts antitumor effects by modulating these immune cells within the mouse TME.
2. Materials and Methods
2.1. Plant Origin
In June 2020, B. pilosa L. was collected from Liangwang Mountain in Kunming, China and subsequently identified by Jun Zhang of Kunming Plant Science and Biotechnology Co., Ltd. (Kunming, China). The voucher specimen (YMU-ZF20200624) has been stored in the Yunnan Key Laboratory of Chiral Functional Substances Research and Application, Yunnan Minzu University.
2.2. Preparation of B. pilosa L. Extract
Powdered whole plant of B. pilosa L. (20 kg) was cold-soaked in 95% methanol (MeOH) (60 L) for 24 h to obtain a filtrate, and the residue was then subjected to six additional extractions using 25 L of solvent each time. The crude extract weighing 2 kg was obtained by decompression concentration. Following the addition of 2 L of water, the crude extract was separated by petroleum ether (PE) and ethyl acetate (EtOAc). The EtOAc-solute fraction (210 g) was concentrated and fractionated on a silica gel column (60–100 mesh) (Qindao Marine Chemical Inc., Qingdao, China) followed by sequential elution using PE (35 g) and dichloromethane (DCM)-MeOH mixtures at volume ratios of (1:0 (11 g), 50:1 (28 g), 25:1 (9 g), 10:1 (81 g), 1:1 (17 g), and 0:1 (5 g)). The 10:1 DCM-MeOH fraction was concentrated and further purified by using MCI (Middle Chromatogram Isolated) gel (Mitsubishi Chemical, Japan) with 50% MeOH-50% H2O, yielding B. pilosa L. extract (BPA, 58.1 g) (Figure 1A). Compounds 1–8 were isolated from BPA and identified in HPLC trace of BPA. Additional details of the compounds are provided in Table S1.
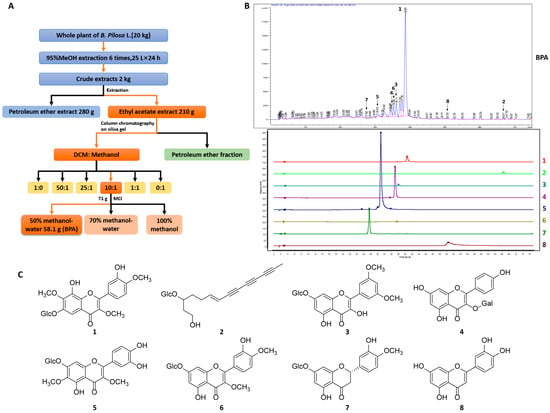
Figure 1.
Extraction, HPLC analysis, and structural characterization of BPA and its main constituents. (A) Extraction and isolation process of BPA. (B) HPLC trace of BPA. HPLC analysis of BPA was performed using an Agilent 1260 Infinity II HPLC system with an Agilent XDB-C18 chromatographic column (dimensions: 4.6 × 250 mm, particle size: 5 μm) under gradient elution conditions with methanol. The specific methanol gradient profile was: 0–5 min, 10–20%; 5–15 min, 20–30%; 15–20 min, 30–35%; 20–30 min, 35–40%; 30–60 min, 40–45%; 60–70 min, 45–50%; 70–75 min and 50–55%. The effluents were monitored at 254 nm with the column maintained at 30 °C and a flow rate of 1.0 mL/min. (C) Chemical structures of compounds 1–8, the major constituents of BPA.
2.3. Cell Culture
CT26. WT cells (colorectal cancer, murine) were obtained commercially (Guangzhou Cellcook Biotech Co., Ltd., Guangzhou, China) and cultured in Dulbecco’s Modified Eagle Medium (DMEM, Biological Industries, Cat. C3113-0500, Beit Shemesh, Israel) supplemented with 10% (v/v) FBS (VivaCell, Cat. C04001-500, Shanghai, China) and 1% glutamine (Biological Industries, Cat. E607004-0500) under standard conditions (37 °C, 5% CO2) in a humidified incubator (ThermoFisher, Waltham, MA, USA).
2.4. Animal Experiments
Male BALB/c or Kunming mice aged 6–8 weeks were purchased commercially (Henan Skobes Biotechnology Co., Ltd., Anyang, China) and maintained in specific pathogen-free (SPF) housing with controlled environmental parameters (temperature: 23 ± 2 °C; humidity: 55 ± 10%; 12 h light/dark). The animal Experiment Ethics Committee of Yunnan Minzu University approved all protocols (Issue No. YMU-2022-A027; YMU-AFEC-2023-A001; YMU-AFEC-2023-A009).
2.4.1. Isolation and Polarization of Peritoneal Macrophages
Peritoneal macrophages were isolated from Kunming mice by peritoneal lavage according to the method described by Zhao et al. [19], and then resuspended in RPMI 1640 medium (Biological Industries, Cat. C3010-0500) supplemented with 10% (v/v) FBS, 1% Penicillin-streptomycin (Sangon Biotech, Cat. E607011-0100, Shanghai, China). After 3 h for adherence, non-adherent cells were removed to yield peritoneal macrophages (M0). M0 macrophages were treated with 20 ng/mL of IL-4 (PeproTech, Cat. 214-14, Cranbury, NJ, USA) and 20 ng/mL of IL-13 (Sino Biological Inc., Cat. 50225-MNAH, Beijing, China) for 72 h to facilitate their transformation into M2 macrophages, or were treated with 1 μg/mL LPS (Sigma, Cat. 93572-42-0, St. Louis, MO, USA) for 24 h to promote their transformation into M1 macrophages.
2.4.2. Isolation and Differentiation of Induced Regulatory T Cells
Lymphocytes were isolated from the lymph nodes and spleens of Kunming mice as previously described [20]. The resulting cell suspension was passed through a 70-μm cell strainer (NEST, Cat. 258368, Wuxi, China), followed by centrifugation and resuspension in EasySep buffer to a final concentration of 1 × 108 cells/mL, then naïve CD4+ T cells were purified from this suspension using the EasySep mouse naïve CD4+ T cell isolation kit (Stemcell Technologies, Cat. 19765, Vancouver, BC, Canada) according to the manufacturer’s instructions. The purity of isolated CD4+ T cells was assessed by flow cytometry, and results are presented in Figure S2B. To obtain induced Tregs, 24-well plates were pre-coated with anti-CD3e (5 μg/mL, eBioscience, Cat. 16-0031-82, San Diego, CA, USA) overnight at 4 °C. Purified naïve CD4+ T cells were then added at 2 × 106 cells/well in Treg-polarizing medium supplemented with anti-CD28 (2 μg/mL, eBioscience, Cat. 16-0281-82), anti-IL-4 monoclonal antibody (5 μg/mL, eBioscience, Cat. 16-7041-81), anti-IFN-γ antibody (5 μg/mL, eBioscience, Cat. 16-7311-85), IL-2 (100 U/mL, Sino Biological Inc., Cat. 51061-MNAE, Beijing, China), TGF-β (4 ng/mL, Sino Biological Inc., Cat. 51061-MNAE), and rapamycin (100 ng/mL, Sigma, Cat. S115842). After that, cells were incubated at 37 °C for 96 h to induce into Tregs.
2.4.3. Syngeneic Tumor Model in Mice
CT26. WT cells in logarithmic growth phase were harvested, washed, and resuspended in DMEM at a concentration of 8 × 106 cells/mL and an aliquot of cell suspension (0.1 mL) was injected subcutaneously in the right flank of each BALB/c mouse to initiate tumor growth. Tumor length (a) and width (d) were measured with a vernier caliper, and tumor volume (V) was calculated as V = 0.5 × a × d2.
When subcutaneous tumors reached a volume of 50–100 mm3, mice were randomly assigned to five groups (n = 6 per group) using simple randomization. No significant differences in tumor volume or body weight were observed among the groups prior to treatment. The groups were defined as follows: The model group served as the negative control and received daily oral administration of 0.5% carboxymethylcellulose sodium (CMC-Na). The positive control group received 5-FU (MCE, Cat. HY-90006, Monmouth Junction, NJ, USA) at a dose of 30 mg/kg by intraperitoneal injection every three days. The BPA treatment groups were given daily oral BPA at 100 mg/kg or 50 mg/kg, each dissolved in 0.5% CMC-Na. The combination treatment group received 5-FU by intraperitoneal injections at 30 mg/kg every 3 days, together with daily BPA gavage at 50 mg/kg. Animals had free access to water and food throughout the study. Once tumor volumes reached approximately 1500 mm3, mice were euthanized, and serum and tissue specimens, including liver, heart, spleen, lungs, kidneys, stomach, and tumors, were collected for subsequent analyses. Normal BALB/c mice that did not undergo subcutaneous CT26. WT cell injections were used as sham controls. The investigators performing outcome assessments and the personnel administering treatments were blinded to group allocation.
2.5. MTT Assay
For M2-TAMs, M0 macrophages were seeded in 96-well plates at a density of 5 × 104 cells/well, followed by the addition of IL-4 and IL-13. After 24 h of stimulation, different concentration of BPA or other compounds were added and then incubated for 48 h. Cell viability was determined by MTT assay (Macklin, Cat. 298-93-1, Shanghai, China) and measured on a microplate reader (SpectraMax i3x, Molecular Devices, Sunnyvale, CA, USA). For Tregs, purified naïve CD4+ T cells were seeded at 5 × 105 cells/well in 96-well plates precoated with anti-CD3e. Corresponding stimulatory factors and test samples were then added. After 96 h of incubation, cells were stained with 0.04% trypan blue and viable cells were counted with a hemocytometer. IC50 values were calculated by the Reed&Muench method and are expressed as the mean ± SEM from at least three independent measurements, each performed in duplicate.
2.6. Flow Cytometry
M2 macrophages treated with or without samples were harvested and resuspended in PBS. To block Fc receptors, cells were incubated with anti-CD16/32 (Elabscience, Cat. E-AB-F0997A, Wuhan, China) for 10 min. After washing, cells were stained with PerCP/Cyanine 5.5 anti-mouse F4/80 (Elabscience, Cat. E-AB-F0995J), FITC anti-mouse CD206 antibody (Elabscience, Cat. E-AB-F1135C), and APC anti-mouse CD80 antibody (Elabscience, Cat. E-AB-F0992E) at 4 °C for 30 min. The population of CD206+ or CD80+ were analyzed in F4/80+ cells using a Beckman CytoFlex flow cytometry (Figure S2). Induced Tregs treated with or without samples were resuspended in PBS and stained with APC anti-mouse CD25 antibody (Elabscience, Cat. E-AB-F1102E) for 30 min at 4 °C. Cells were then fixed and permeabilized using the Transcription Factor Buffer Set kit (BD Biosciences, Cat. 562574, Franklin Lakes, NJ, USA) according to the manufacture’s instruction. After incubated with PE anti-mouse Foxp3 antibody (Elabscience, Cat. E-AB-F1238D) at 4 °C for 50 min, cells were analyzed by flow cytometry for CD25+Foxp3+ cell populations. Flow cytometry analysis results are based on at least three independent experiments.
2.7. Quantitative Real-Time PCR (qRT-PCR)
The total RNA of M1/M2 macrophages, Tregs and tumor tissues was extracted using a Total RNA Extractor (Trizol) Extraction Kit (TaKaRa, Cat. 9109, Kusatsu, Japan) and reverse-transcribed into cDNA with a reverse transcription kit (Vazyme, Cat. R223-01, Nanjing, China). The cDNA was used for PCR amplification in accordance with the method described in previous publication [21]. Relative gene expression levels were normalized to 18S rRNA and calculated via 2−ΔΔCt method. Quantitative RT-PCR results are from at least three independent experiments. Primer sequences used in this study are listed in Supplementary Table S2.
2.8. T Cell Proliferation Assay
Purified CD4+ T cells were labeled with 0.5 μM CFSE (MCE, Cat. HY-D0938) in PBS for 10 min at 37 °C, followed by washing and resuspension in complete RPMI-1640 medium. The cells were seeded in 96-well plates precoated with anti-CD3e and co-cultured with M2 macrophages treated with or without BPA at a 5:1 ratio (T cells: M2 macrophages), or with Tregs treated with or without BPA at a 2:1 ratio (T cells: Tregs), in the presence of anti-CD28 mAb. After 72 h incubation, cells were collected, resuspended in staining buffer, and the proliferation of CFSE-labeled CD4+ T cells was then assessed by flow cytometry. The analysis results are based on at least three independent experiments.
2.9. ELISA
Whole blood was collected from mice and allowed to clot at room temperature for 3 h. Serum was obtained by centrifugation. Serum IL-10 levels were detected using a mouse IL-10 ELISA Kit (Proteintech, Cat. KE10008, Wuhan, China) according to the manufacturer’s instructions.
2.10. Hematoxylin/Eosin Staining
Tissues from mice (tumor, heart, liver, spleen, lung, kidney, and stomach) were fixed in 10% neutral-buffered formalin for 48 h, dehydrated, embedded in paraffin, and sectioned. Sections were stained with hematoxylin and eosin (H&E) using commercial kits (Solarbio, Cat. G1140/G1100, Beijing, China) according to the manufacturer’s instructions. After mounting with neutral resin (Solarbio, Cat. G8590), images were captured with an inverted microscope (Leica DMi8, Wetzlar, Germany).
2.11. Immunohistochemical
Tumor sections were rehydrated and subjected to antigen retrieval in Tris-EDTA buffer (10 mM Tris, 1 mM EDTA, 0.05% Tween-20, pH 8.0) using an autoclave for 2 min. After washing, sections were blocked with 10% goat serum (Solarbio, Cat. SL038) in TBS containing 1% BSA (Coolaber, Cat. CA1381, Beijing, China) at 37 °C for 1 h, then incubated overnight at 4 °C with anti-Ki67 primary antibody (Proteintech, Cat. 28074-1-AP). A horseradish peroxidase-polymer-conjugated secondary antibody (ZSGB-BIO, Cat. PV-6001, Beijing, China) was applied at 37 °C for 30 min, followed by DAB detection (ZSGB-BIO, Cat. ZLI-9018) and hematoxylin counterstain. Slides were mounted and imaged using an inverted microscope.
2.12. Western Blot
Approximately 10 mg of tumor tissue was weighted and added to lysis buffer (2% SDS, 10% glycerol, 65 mM Tris-HCl, pH 6.8), then homogenized on ice using a tissue grinder. Lysate was sonicated and subsequently heated at 98 °C for 10 min. Further methodological details were performed as previously described [21]. Band densities were quantified by grayscale analysis in ImageJ software (version 1.53a) from at least three independent experiments. Antibody details used in this study is presented in Supplementary Table S3.
2.13. RNA Sequencing (RNA-Seq) and Bioinformatic Analysis
Total RNA was extracted from TAMs and Tregs treated with stimulatory factors and different concentrations of BPA using TRIzol reagent. RNA samples that met quality control criteria were used to construct sequencing libraries with the NEB library preparation protocol. Library insert size distributions were validated on an Agilent 2100 Bioanalyzer. Qualified libraries were quantified and sequenced on an Illumina platform (Novogene, Beijing, China).
For RNA-seq data analysis, sequencing quality was assessed from base-calling output generated by CASAVA. Sequenced reads were mapped to GRCm39 genome using hisat2 software (version 2.2.1), and gene-level read counts were generated with featureCounts. After normalization, differential gene expression analysis was carried out using the R package DESeq2 (version 4.5.1). Volcano plot analysis was performed with the R package ggplot2 (version 4.5.1) to identify differentially expressed genes (DEGs) with a p ≤ 0.05 and an absolute value of log2 fold change ≥ 1. Functional enrichment analysis of DEGs was conducted using the R package clusterProfiler (version 4.5.1) for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation.
2.14. Statistical Analysis
Quantitative data are expressed as mean ± SEM from at least three independent experiments. Statistical significance was determined using either one-way ANOVA or Student’s t-test in GraphPad Prism (version 8.0.2). A p value threshold of less than 0.05 was applied to define statistical significance.
3. Results
3.1. The Chemical Characterization of BPA
The chemical profile of BPA was characterized using HPLC through comparison with compounds isolated from B. pilosa L. Detailed extraction and compounds identification were described in our previous studies [18,19]. Seven flavonoids (1, 3–8) and one polyacetylene (2) were identified in the HPLC trace of BPA (Figure 1B,C), in which 1 and 3 are the main components. Both BPA and its chemical components (1–8) were subsequently used in vitro to assess their modulatory effects on TAMs and Tregs.
3.2. BPA and 1–8 Do Not Show Potent Cytotoxicity on M2 Macrophages and Tregs
M0 macrophages and naïve CD4+ T cells were differentiated into M2 macrophages and Tregs, respectively, and subsequently treated with the appropriate concentrations of BPA and compounds 1–8. Cytotoxicity assay revealed that, at 50 μM, most compounds had minimal inhibitory effects on M2 macrophage viability (Figure 2A). However, compounds 6 and 8 represented exceptions, reducing cell viability by approximately 30% and 40%, respectively. For Tregs (Figure 2B), most compounds reduced cell viability by approximately 10%, whereas 3 decreased it by roughly 30%. At the concentration of 50 μg/mL, BPA markedly suppressed the cell viability of Tregs by 55%. These results indicate that BPA and its monomeric compounds showed a weak inhibitory effect on both M2 macrophages and Tregs.
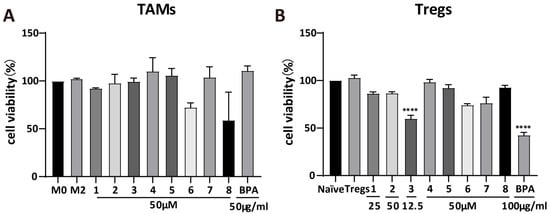
Figure 2.
Cytotoxicity of BPA and compounds 1–8. Cytotoxic effects of BPA and 1–8 on M2 macrophages (A) and Tregs (B). Statistical significance relative to the M2 macrophage or Tregs group is indicated as follows (n = 3): **** p < 0.0001.
3.3. BPA Inhibits the Differentiation of M0 Macrophages into M2 Macrophages and the Polarization of CD4+ T Cells into Tregs
TAMs and Tregs promote tumor progression through the establishment of immunosuppressive microenvironments. To assess the effects of BPA and its chemical constituents (1–8) on immune cell differentiation, specifically the conversion of M0 macrophages into either M1 or M2 macrophages, as well as the differentiation of T cells into Tregs, we performed qRT-PCR and flow cytometry analyses. Results from qRT-PCR revealed that LPS significantly enhanced the mRNA expression levels of iNOS (p = 0.026) and IL-1β (p = 0.0397), which are markers of M1 macrophages (Figure S1C,D). By contrast, BPA and compounds 1 and 3–8 did not induce significant changes in the expression of these transcripts relative to the M1 control (Figure S1C), indicating that they do not affect M1 differentiation. Results from flow cytometry confirmed this finding, showing no obvious changes in CD80+F4/80+ cell populations (M1 macrophages) after treatment (Figure S3E,F). However, almost all compounds and BPA notably decreased the mRNA expression of the M2 macrophage marker Arg-1 (Figure S1A) and the regulatory T cell marker CD25 (Figure S1B). Consistent with these findings, flow cytometric analysis demonstrated a pronounced reduction in immunosuppressive cell populations, including M2 macrophages (CD206+F4/80+) and Tregs (Foxp3+CD25+), upon treatment (Figure S3A–D). Furthermore, BPA and 1–8 significantly reduced the CD206+/CD80+ cell ratio in F4/80+ macrophages (p = 4.4 × 10−7, Figure S3G,H). Compounds 1 and 3, identified as the major constituents of BPA [18], were studied along with BPA in subsequent investigation. Flow cytometric analysis revealed that the population of M2 macrophages (CD206+F4/80+) showed dose-dependent reductions (Figure S4C,D) following treatment with BPA, 1, and 3. In contrast, the CD80+F4/80+ cell populations (M1 macrophages) remained unchanged (Figure S4A,B), suggesting a selective effect on M2 macrophages. Additionally, the CD206+/CD80+ cell ratio declined in a concentration-dependent manner with increasing concentrations of 1, 3, and BPA (Figure 3A,B). Notably, only compound 3 induced a dose-dependent suppression of Tregs. In contrast, 1 and BPA showed potent inhibitory effects on differentiation of Tregs, although not in a concentration-dependent manner (Figure 3C,D). This lack of concentration dependence may be due to the drug’s effects on Tregs cytotoxicity and other complex underlying mechanisms. Collectively, these data indicate that BPA exerts an in vitro immunomodulatory effect by inhibiting the proliferation of both M2-TAMs and Tregs.
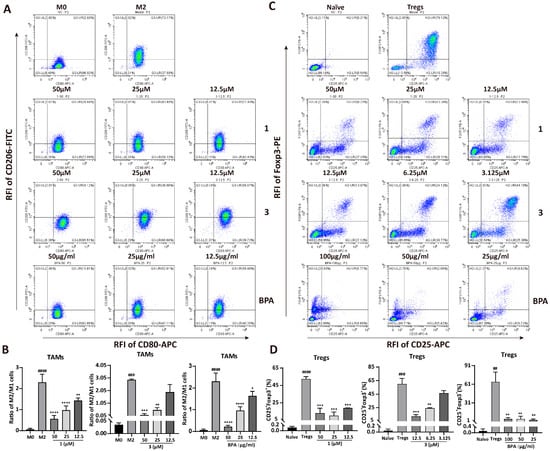
Figure 3.
Effect of BPA and compounds 1 and 3 on the differentiation of macrophages and CD4+ T cells. (A) Representative flow cytometry plots illustrating the effect of BPA, 1, and 3 on the differentiation of M2 macrophages. (B) Quantitative analysis of the ratios of M2 to M1 cells following treatment with various concentrations of BPA, 1 and 3. (C) Representative flow cytometry plots illustrating the effect of BPA, 1, and 3 on the differentiation of Tregs. (D) Histograms displaying the quantified results of the CD25+Foxp3+ double-positive cell population following treatment with different concentrations of BPA, 1 and 3. Statistical significance for all experiments is denoted as follows (n = 3): compared to the M0 cells or Naïve CD4+ T cells, ## p < 0.01, ### p < 0.001, #### p < 0.0001; compared to M2-TAMs or Tregs, * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001. RFI means Relative Fluorescence Intensity.
3.4. BPA Reverses M2 Macrophage- and Treg-Mediated Suppression of CD4+ T Cells
The anti-tumor immune response is compromised when M2 macrophages and Tregs suppress Th1 cytokine secretion, directly inhibiting the proliferation and activation of CD4+ T cells. This suppression subsequently promotes tumor progression. To determine whether 1, 3, and BPA can reverse this suppression, CD4+ T cell proliferation in co-culture was measured by CFSE staining and analyzed by flow cytometry. Results from Figure 4 exhibited that both M2 macrophages and Tregs significantly inhibited CD4+ T cell proliferation, with the proliferation rate decreased from 77.65% to 2.59% (p = 5.45 × 10−6) and 81.20% to 8.39% (p = 5.52 × 10−10), respectively. However, a concentration-dependent increase in CD4+ T cell proliferation was observed following pretreatment of M2 macrophages with 1, 3, or BPA (Figure 4A,B). In particular, compound 1 at the concentrations of 50, 25, and 12.5 μM markedly increased CD4+ T cells proliferation to 75.81%, 72.03%, and 65.21%, respectively (p = 0.000016, p = 0.000019, and p = 0.000026), compared to the M2 macrophage-suppressed CD4+ T cell group. As shown in Figure 4C,D, pretreatment of Tregs with compound 3 at 12.5, 6.25, and 3.125 μM significantly enhanced CD4+ T-cell proliferation from 8.39% to 79.79%, 82.65%, and 66.99%, respectively (p = 7.29 × 10−6, p = 4.97 × 10−6, and p = 0.000019). BPA showed a similar but less pronounced effect, whereas compound 1 did not cause statistically significant changes in regulatory T cell-mediated suppression. Therefore, BPA may attenuate the immunosuppressive activity of M2 macrophages and Tregs, leading to restoration of CD4+ T cell proliferation and a potential enhancement of antitumor immune response.
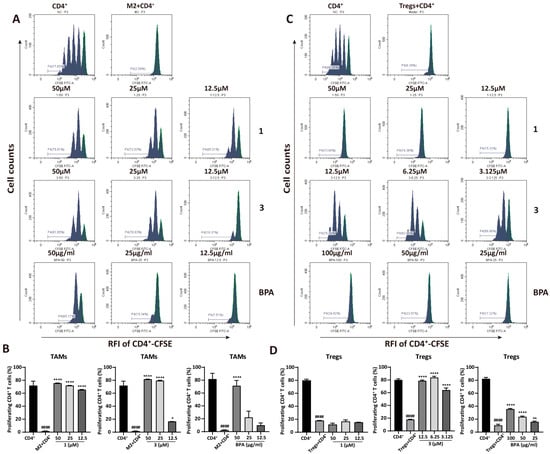
Figure 4.
BPA and compounds 1 and 3 enhance CD4+ T cell proliferation suppressed by M2 macrophages and Tregs. (A) Representative flow cytometry plots illustrating the effect of BPA, 1, and 3 on CD4+ T cell proliferation suppressed by M2 macrophages. (B) Quantification of CD4+ T cell proliferation following co-culture with M2 macrophages pretreated with BPA, 1 and 3. (C) Representative flow cytometry plots illustrating the effect of BPA, compounds 1, and 3 on CD4+ T cell proliferation inhibited by Tregs. (D) Quantification of CD4+ T cell proliferation following co-culture with Tregs pretreated with BPA, 1 and 3. Statistical significance is indicated as follows (n = 3): compared to the CD4+ group, #### p < 0.0001; Compared to the M2 or Tregs group, * p < 0.05, ** p < 0.01, **** p < 0.0001. RFI means Relative Fluorescence Intensity.
3.5. BPA Inhibits the Growth of Syngeneic Colorectal Cancer in Mice
Tumor growth rate and volume are key indicators of tumor progression. To validate the in vitro findings, a murine syngeneic tumor model was established using CT26. WT cells to assess the in vivo effects of BPA on tumor growth. Compared to model controls, BPA treatment suppressed tumor growth and reduced tumor weight (Figure 5A–C). Additionally, combined treatment with BPA and 5-FU exhibited stronger antitumor effect compared to 5-FU monotherapy, which served as the positive control. Notably, no significant body weight changes were observed across groups, suggesting that BPA has minimal systemic toxicity in mice (Figure S5A). H&E histopathological analysis also showed no obvious pathological changes in major mouse organs (heart, liver, spleen, lungs, kidneys, and stomach) compared to healthy controls (Figure S5B). These findings suggest that BPA inhibits colorectal cancer growth in mice without causing significant toxicity or organ damage.
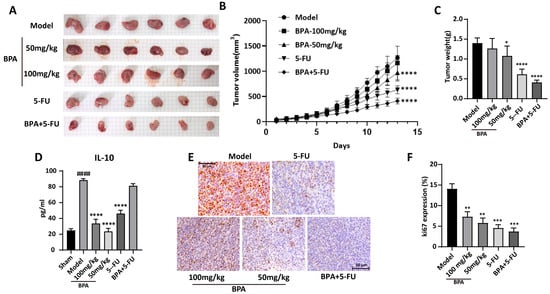
Figure 5.
The growth of CT26. WT syngeneic tumors in mice is inhibited by BPA. (A) Images of CT26. WT syngeneic tumors in mice from different treatment groups. Scale bar = 5 mm (each background grid square corresponds to 5 mm). (B) Tumor growth curves for syngeneic CT26.WT tumors in mice were generated from daily volume measurements during the treatment phase (n = 6). (C) Statistical analysis of tumor weight at the end of the study (n = 6). (D) Statistical analysis of serum IL-10 levels (n = 6). (E) Representative immunohistochemistry (IHC) images showing the expression of the Ki67 protein in tumor tissue sections from different treatment groups. Original magnification 400×. (F) Quantification of Ki-67 expression in tumor tissue. The proportion of Ki-67-positive area within sections was quantified using ImageJ software and is presented as a percentage of total tissue area. Statistical significance is denoted as follows: compared to the sham group, #### p < 0.0001; Compared to the model group, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
IL-10, a key immunosuppressive cytokine secreted by TAMs and Tregs, is essential for maintaining an immunosuppressive tumor microenvironment. ELISA quantification of Serum IL-10 revealed a significant increase in the model group (p = 6.71 × 10−10), which was substantially attenuated following BPA treatment (Figure 5D). Although the 5-FU and the combination treatments led to a reduction in IL-10 levels, these decreases were less pronounced compared to the effects observed with BPA alone. The anti-proliferative effect of BPA on tumor cells was investigated by immunohistochemical detection of Ki67, a key marker of cell proliferation. Notably, Ki67 expression was significantly decreased in the BPA-treated group compared to the model group (Figure 5E,F), consistent with the observed reduction in tumor volume and weight. Taken together, these results indicate the antitumor effect of BPA, either used alone or with conventional chemotherapy (e.g., 5-FU), for the treatment of colorectal cancer.
3.6. BPA Modulates the Expression of Genes and Proteins Associated with TAMs and Tregs in Mouse Tumor Tissues
The expression levels of genes in mouse tumor tissues were analyzed by qRT-PCR, and the results demonstrated that treatment with 100 mg/kg BPA significantly reduced the mRNA levels of the tumor-promoting factors, including Arg-1, YM-1, CCL2, CCL22, CD25, CCR4, and CCR10, secreted by TAMs and Tregs, compared to the model group, respectively (all p < 0.01). Whereas, the levels of TGF-β, CXCR3, and CCR8 showed only a slight reduction. This indicates that BPA might transform the TME from immunosuppressive to immune-supportive by modulating cytokine or chemokine levels within the tumor. Moreover, BPA also increased the mRNA levels of the tumor-inhibitory factors, including TNF-α, IL-1β, iNOS, CXCL10, and CCR7, compared to the model (Figure 6A). These findings indicates that BPA might promote a transition of the tumor microenvironment toward immune supportiveness by modulating cytokine and chemokine levels. The expression levels of proteins in tumors were analyzed by Western blot (Figure 6B,C), and the results exhibited that treatment with BPA led to a reduction in the expression levels of CD206 and PD-1. In contrast, there were no significant changes observed in the levels of CD25, CD80, and PD-L1. Notably, there was a significant increase in levels of GITR (100 mg/kg: p = 0.01; 50 mg/kg: p = 0.00069). Additionally, the ratio of CD206 to CD80 protein levels, which serves an indicator of the functional state of immune cells, particularly macrophages, was calculated and revealed a significant decrease in BPA-treated tumors compared to the model group (100 mg/kg: p = 0.062; 50 mg/kg: p = 0.0061). To investigate whether the immunoregulatory effect of BPA can enhance the anti-tumor effects of chemotherapy drugs, we established a combination treatment group of BPA and 5-FU, a clinically used drug for CRC chemotherapy. The results showed that the combination therapy significantly reduced the CD206/CD80 protein ratio (p = 0.0002) in tumor tissues (Figure 6B,C). These results indicate that BPA reduced the prevalence of M2 macrophages within the TME, either alone or in combination with 5-FU.
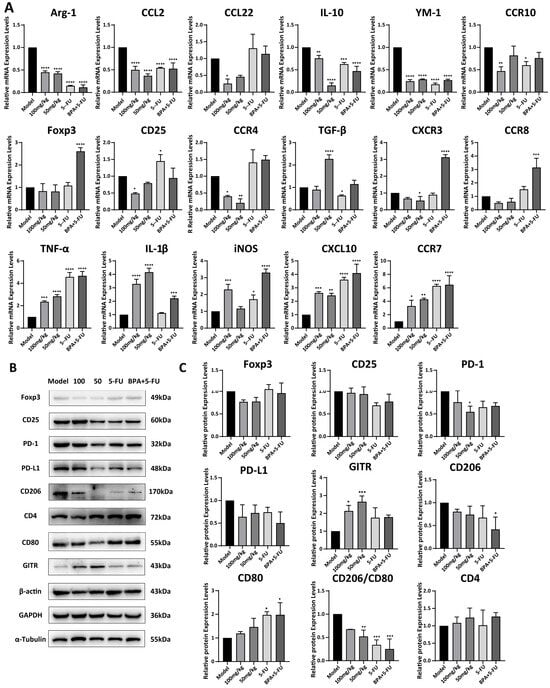
Figure 6.
Expression levels of mRNAs and proteins associated with TAMs and Tregs in BPA-treated mouse tumors. (A) Quantification of mRNA by qRT-PCR for TAM/Treg-associated tumor-promoting factors (Arg-1, YM-1, CCL2, CCL22, Foxp3, CD25, TGF-β, CXCR3, CCR4, CCR8, CCR10) and tumor-inhibitory factors (TNF-α, IL-1β, iNOS, CXCL10, CCR7). Data were normalized to 18 S rRNA and are shown relative to the model group (baseline control). (B) Representative immunoblots of tumor tissue for TAM- and Treg-associated proteins. Pro-tumor: CD206, Foxp3, CD25, PD-1, PD-L1; Anti-tumor: CD80, CD4, GITR. (C) Quantification of protein expression from Western blots. Values were normalized to α-tubulin, β-actin, or GAPDH as indicated. Statistical significance is denoted as follows (n = 6): compared to the model group, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
3.7. BPA Downregulates Pro-Tumor Pathways in TAMs and Tregs
Collectively, our results indicate that BPA inhibits the differentiation of TAMs toward the pro-tumor M2-like TAMs and of Naïve CD4+ T cells toward pro-tumor Tregs within the TME. However, the precise molecular pathways by which BPA modulates TAMs and Tregs remain unclear. To further elucidate the regulatory mechanisms, we induced M2 macrophages and Tregs in vitro in the presence of BPA and performed transcriptome sequencing, and the representative findings are presented in Figure 7. A total of 296 differentially expressed genes (DEGs) were identified between the BPA-treated and M2-TAMs groups (Figure 7A). Among these, genes encoding immunosuppressive factors (TGF-β2, CD28, Hgf) were significantly downregulated, whereas genes associated with inflammation and immune activation (Il-6, TNF, CXCL1, IL-1β) were markedly upregulated (Figure 7B). This coordinated dysregulation of these genes is likely to contribute to BPA-mediated inhibition of the differentiation of M0 macrophages into M2 macrophages. KEGG pathway enrichment analysis revealed significant alterations in the IL-17, NF-κB, and C-type lectin receptor signaling pathways (Figure 7C), all of which play important roles in immunomodulation. Compared with the Tregs group, BPA treatment identified 2935 DEGs, including marked alteration of cytokine- and chemokine-related genes (IL-17a, Cxcl-10, Cxcl3, IL12rb1, IL12rb2, etc.) and downregulation of genes involved in cell proliferation and survival (Ccna2, Cdk1, Aurkb, Mcm5, Pole, etc.). These transcriptional changes may underlie BPA-mediated inhibition of regulatory T cell differentiation and proliferation. KEGG pathway enrichment analysis revealed significant perturbations in the cell cycle, DNA replication and cytokine-cytokine receptor interaction (Figure 7D). The first two pathways, cell cycle and DNA replication, are involved in development as well as cellular survival and proliferation. These perturbations are consistent with BPA-induced in Tregs observed in Figure 2B.
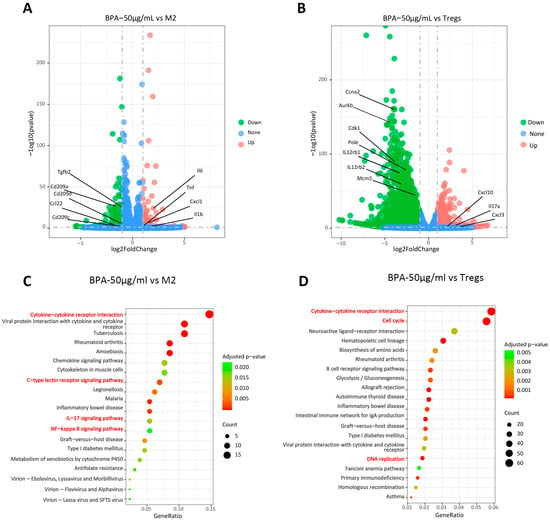
Figure 7.
BPA inhibits the expression of tumor-promoting related pathways in TAMs and Tregs. (A) Volcano plot of RNA-seq results for TAMs; (B) Volcano plot of RNA-seq results for Tregs, where red dots represent upregulated genes and blue dots represent downregulated genes. (C) Scatter plot of KEGG-enriched pathways from the biological process analysis of TAMs; (D) Scatter plot of KEGG-enriched pathways from the biological process analysis of Tregs. The signaling pathways highlighted in red are closely related to the effects of BPA.
4. Discussion
Flavonoids have been isolated from various plants and demonstrated diverse biological activities, including tumor suppression, immunomodulation, and antioxidant effects [22]. The Chinese medicinal herb B. pilosa L., traditionally used in cancer treatment, contains a large number of flavonoids (>100) that exhibit antioxidant, anticancer, and other bioactive properties [23]. In our investigation of the anti-tumor activity of plant-derived compounds, we focused on the chemical constituents of B. pilosa L. and identified a total of 80 compounds, including 19 flavonoids and 10 polyacetynes. Notably, 13 flavonoids and 5 polyacetynes were specifically isolated from BPA [18,19]. Cytotoxicity and DNA topoisomerase I (Topo I) inhibition assays revealed that several flavonoids and polyacetynes exhibited potent cytotoxicity on a panel of 6 cancer cell lines by inhibiting the activity of Topo I. However, most of the compounds isolated from BPA did not exhibit inhibition on tumor cell growth or on Topo I activity [18]. Consequently, we further explored the immunomodulatory effects of BPA and its chemical constituents in the context of tumor treatment, with a particular focus on the TME.
Given the pivotal role of the TME in tumorigenesis, progression, and metastatic dissemination, immunotherapy represents a promising strategy for reshaping the TME and enhancing antitumor immune response [24]. Although targeting TAMs and Tregs within the TME shows considerable therapeutic potential, current agents, including those in clinical investigation, lack robust in vitro validation [4]. This shortcoming has resulted in the absence of appropriate positive controls targeting TAMs and Tregs in our experimental designs. HPLC quantification confirmed that compounds 1–8 are the predominant constituents of BPA, with compound 1 exhibiting the highest abundance (Figure 1). Cytotoxicity results revealed that compared to TAMs, BPA and 1–8 exhibited weak cytotoxic activity against Tregs, whereas no significant cytotoxicity was observed in TAMs, except compound 8 (Figure 2A,B).
As the anti-tumor immune cells, M1-TAMs express iNOS and produce IL-1β, and display the surface marker CD86 and CD80; by contrast, as the tumor promoting immune cells, M2-TAMs express Arg-1 and YM-1, secrete CCL2, end display CD163 and CD206. Tregs expresses CD25 and Foxp3 [25,26]. Results from RT-PCR (Figure S1) and flow cytometry (Figure S3) revealed that BPA and 1–8 modulated the differentiation of M0 macrophages into M2-type macrophages and inhibited the conversion of Naïve CD4+ T cells into Tregs. The populations of CD80+CD206+ in F4/80+ cells were analyzed (Figure S3C–H), along with the population of CD25+Foxp3+ in CD4+ T cells (Figure S3A,B). The results indicated that BPA and 1–8 more effectively inhibited M2 polarization and suppressed regulatory T cell differentiation, while exhibiting no significant activity on M1 polarization (Figure S3).
During the purification process, compound 1 and 3 were obtained as a mixture through crystallization. Consequently, further investigation primarily focused on BPA and its main flavonoids constituents, 1 and 3. The results from flow cytometry analysis demonstrated that 1 and 3 inhibited the transformation of M0 macrophages and Naïve CD4+ T cells into their immunosuppressive forms in a concentration-dependent manner (Figure 3A–D). Specifically, BPA showed a concentration-dependent inhibition of the CD206+F4/80+ cell population (Figure S4C,D). Although BPA reduced the CD25+Foxp3+ cell population, this effect was not dose-dependent, likely due to its cytotoxic effects on Tregs (Figure 3C,D).
The results of qPCR and flow cytometry showed that the effects of BPA and 1 on Tregs were not concentration-dependent, which may be due to the dual regulatory role that flavonoid compounds exhibit in T-cell immunity. According to the reviewed literature, flavonoids exhibit an immune-enhancing effect at low doses, whereas at high doses, they may display an immunosuppressive effect [27]. Given that the components of BPA are primarily flavonoids, with compound 1 being the predominant one, we propose that this characteristic may explain why compound 1 and BPA did not produce concentration-dependent suppression of regulatory T cell differentiation. In addition, the presence of non-specific components in the extract may also contribute to this phenomenon. There may be trace amounts of undiscovered components in BPA that inhibit T-cell immune function, and their effects could become apparent as the concentration of the extract increases.
Tregs are a subset of CD4+ T cells characterized by their ineffective immune responses and immunosuppressive capabilities, which enable them to inhibit immune cell-mediated responses [28]. M2 macrophage-secreted cytokines actively suppress CD4+ T cell-mediated immune responses [29]. Additionally, IL-10 and TGF-β secreted by Tregs promote the differentiation of M0 macrophages into the M2 subtype, facilitating angiogenesis and establishing an immunosuppressive microenvironment [30]. Furthermore, chemokines secreted by TAMs, such as CCL22, recruit Tregs into the TME, further amplifying immunosuppression [31]. To assess the dynamics of CD4+ T cell proliferation, CFDA-SE-labeled CD4+ T cells were co-cultured with M2 macrophages or Tregs pretreated with BPA, 1 and 3. A concentration-dependent reversal of M2 macrophage-mediated suppression of CD4+ T cells was observed (Figure 4A,B). Additionally, compound 3 reversed CD4+ T cell proliferation that had been suppressed by Tregs (Figure 4C,D). In contrast, BPA and compound 1 did not significantly affect CD4+ T cells proliferation, possibly because they did not alleviate the IL-2 competition between Tregs and CD4+ T cells, a known mechanism by which Tregs suppress CD4+ T cell proliferation [32]. The above results indicate that BPA and its chemical constituents (1–8) exhibit immunomodulatory effects by suppressing tumor-promoting cells, M2 macrophages and Tregs, thereby potentially exerting antitumor activity by enhanced immune responses.
To validate these findings in a physiologically relevant context, we established a murine colorectal cancer syngeneic model to demonstrate the in vivo anti-tumor efficacy of BPA. Consistent with the in vitro studies, BPA treatment induced significant reductions in both tumor volume and weight compared to the model control (Figure 5A–C). Furthermore, BPA suppressed tumor cell proliferation, as shown by decreased Ki67 expression in IHC assays (Figure 5E,F). Importantly, BPA exhibited no apparent toxicity in mice, as reflected by unchanged body weights and normal histological findings in major organs (Figure S5). Interestingly, BPA exhibited stronger anti-tumor activity at 50 mg/kg than at 100 mg/kg. As an ethyl acetate extract of B. pilosa L., BPA is chemically complex; the higher dose may increase hepatic metabolic burden and allow accumulation of low-level toxic constituents that attenuates its intended effect. This aligns with reports that lower doses of plant-derived extracts can improve efficacy, possibly by allowing key bioactive components to act more effectively [33]. For BPA, optimized interactions of its constituents at lower concentrations may enhance target engagement while minimizing off-target effects. This underscores the importance of selecting an appropriate dosage for herbal extract treatments to achieve effective therapeutic outcomes, and highlights the need for comprehensive pharmacokinetic and toxicological studies to define safe and efficacious dosing regimens.
The anti-tumor mechanism of BPA was further explored in mice. Compared to the model control, BPA reduced the levels of IL-10 in mouse serum (Figure 5D), an immunosuppressive cytokine produced by both M2-TAMs and Tregs [34]. This suggests that BPA disrupts the cooperation between TAMs and Tregs that inhibits immunosuppression, as documented in the literature [35].
In addition, we also observed that the combination of BPA and 5-FU significantly reduce colorectal tumor growth and the effect is better than that of 5-FU alone. 5-FU is a cytotoxic chemotherapeutic agent used clinically for colorectal cancer; however, its effects on cancer are often accompanied by immunosuppression due to bone marrow toxicity. Although significant success has been achieved with tumor immunotherapy, only a minority of patients experience benefits, primarily because of the body’s limited immune response and the intricate, diverse immunosuppressive mechanisms at play [36]. Combining cytotoxic chemotherapy with immunotherapy can effectively decrease tumor burden and suppress production of immunosuppressive factors, thereby enhancing the efficacy of each treatment [37]. Based on this, the combination treatment of BPA and 5-FU in mice was established for comparison with 5-FU alone. 5-FU induces immunogenic cell death (ICD), leading to the release of tumor-associated antigens and danger-associated molecular patterns (DAMPs) that promote the recruitment of immune cells, including immunosuppressive subsets, into the TME [38]. In contrast, BPA targets the immune microenvironment by suppressing TAMs and Tregs and by promoting the expression of anti-tumor cytokines (Figure 6). This complementary mechanism suggests that BPA could counteract the immunosuppressive effects of 5-FU while enhancing its anti-tumor efficacy by restoring immune surveillance within the TME. These findings indicate that BPA may serve as an effective immunomodulatory agent, either alone or in combination with conventional chemotherapeutic such as 5-FU, to improve cancer treatment outcomes; however, additional validation is required.
CRC is a prevalent gastrointestinal malignancy characterized by complex pathogenesis influenced by a variety of factors, including environmental changes, genetic variations, and immunity [39]. Among these factors, immune imbalance and inflammatory responses play critical roles in the initiation and progression of CRC [40]. Within the TME, IFN-γ secreted by Th1 cells, CD8+ T cells, and NK cells effectively kills tumor cells [41]. However, Tregs and M2-TAMs promote tumorigenesis in CRC by inhibiting Th1 immune responses. Additionally, they limit T-cell trafficking to intestinal tumors via downregulation of endothelial CXCL10 and promote tumor progression through IL-6 expression. Through these and other immunosuppressive mechanisms, Tregs and TAMs, together with their secreted cytokines and chemokines, decrease anti-tumor immunity and are associated with reduced survival in CRC patients [42,43]. To investigate how BPA disrupts this network, we examined the mRNA levels of immunosuppressive factors in tumor tissues (Figure 6A). Compared to the model control, BPA treatment significantly upregulated the anti-tumor mediators including TNF-α, IL-1β, iNOS, CXCL10 and CCR7. When combined with 5-FU, BPA further enhanced the expression of these cytokines, with the exception of IL-1β, which correlated with a greater suppression of tumor growth. Conversely, BPA downregulated mRNA expression levels of the pro-tumor cytokines and chemokines such as Foxp3, CD25, CXCR3, CCR4, CCR8, CCR10, TGF-β, Arg-1, YM-1, CCL2, CCL22. Notably, certain immunosuppressive targets, such as CCL22, Foxp3, CCR4, CXCR3, CCR8, were significantly suppressed when combined BPA with 5-FU. Moreover, results from immunoblots further confirmed that BPA led to a reduction in the expression of immunosuppressive markers (Foxp3, CD25, PD-1, PD-L1, CD206) while increasing the levels of immune stimulatory proteins (CD80, CD4, GITR) in tumor tissues (Figure 6B,C). This divergence likely arises from mechanistic complementarity between BPA and 5-FU. BPA disrupts crosstalk between Tregs and TAMs by inhibiting IL-10 and CCL22 signaling, while 5-FU induces ICD, releasing DAMPs and antigens to destabilize immunosuppression. Consequently, the remodeling of the TME by 5-FU creates conditions that enhances BPA’s ability to suppress immunosuppressive pathways and enhance antitumor immune responses. Herein, experimental evidence (both animal and cellular studies) demonstrate that BPA modulates the activity of TAMs and Tregs within the TME, with its low-dose application showing greater therapeutic potential. Further research is warranted to explore these dose-dependent effects and optimize BPA formulations to balance efficacy and safety.
This article investigated the tumor immunomodulatory effects of BPA and its compounds from both in vivo and in vitro perspectives, and verified the research findings through transcriptome analysis. BPA can inhibit the formation of an immunosuppressive microenvironment by suppressing pathways such as IL-17 and NF-κB signaling pathways, thereby reducing the immunosuppressive effects of immune cells (Figure 7). In preliminary studies, we explored the immunomodulatory effects of flavonoids and polyacetylenes from B. pilosa L. through network pharmacology and found that they may regulate immune and inflammatory responses through multiple pathways, including the STAT3, AMPK, and NF-κB signaling [44], which is consistent with the findings of this study. Clinically, tumor immunotherapy agents are often used in conjunction with cytotoxic anticancer drugs to achieve synergistic effects through different mechanisms of action. However, the potential for synergistic enhancement between tumor immunotherapy agents that operate through different mechanisms is worthy of further exploration. As the key immunotherapeutic agents for cancer, inhibitors of the immune checkpoint PD-1/PD-L1 primarily restore T-cell immune function by blocking the PD-1/PD-L1 signaling pathway, thereby facilitating tumor cell destruction [45]. In our study, we observed that BPA treatment reduced the expression of PD-1 and PD-L1 in tumor tissues. These results prompt the hypothesis that BPA could be used in combination with PD-1 or PD-L1 inhibitors to augment their antitumor activity. Further preclinical investigations, particularly comprehensive pharmacokinetic analyses, rigorous toxicology assessments, and tumor re-challenge studies, are required to fully evaluate the efficacy and safety of these combination regimens.
5. Conclusions
In summary, BPA, an extract derived from B. pilosa L. and enriched in flavonoids, shows anti-tumor effects in mice by suppressing macrophages polarization toward M2-like TAMs and inhibiting the differentiation of Naïve CD4+ T cells into immunosuppressive Tregs within the TME. This promotes the activation and proliferation of effector CD4+ T cells. These findings provide a theoretical basis for the clinical anticancer use of the traditional medicinal herb B. pilosa L. and suggest that BPA suppresses pro-tumor immune cells, indicating antitumor efficacy in the treatment of CRC. Although BPA demonstrated immunotherapeutic effects, comprehensive preclinical studies to define optimal dosing, pharmacodynamics, and mechanisms of action are needed to validate its potential in tumor immunotherapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cells15020126/s1, Table S1: Chemical information of compounds 1–8; Table S2: Primer sequences used for qRT-PCR analysis; Table S3: Antibody information utilized in the Western blot experiment; Figure S1: The effects of BPA and 1–8 on M1/M2 macrophages and Tregs analyzed by qRT-PCR. (A) Arg-1 mRNA expression in M2 macrophages; (B) CD25 mRNA expression in Tregs; (C) iNOS mRNA expression in M1 macrophages; (D) IL-1β mRNA expression in M1 macrophages. Gene expression levels were quantified using qRT-PCR and normalized to 18SRNA as the reference gene. Statistical significance for all experiments is denoted as follows (n = 3): compared to the M0 or NaÏve group, # p < 0.05, ## p < 0.01 and #### p < 0.0001; compared to M2/M1/Tregs group, * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001; Figure S2: Flow cytometry plots for macrophage gating strategy and CD4+ T cell purity. (A) Gating strategy used for flow cytometric identification of macrophages. (B) Purity of CD4+ T cells. Left, CD4+ T cell purity among lymphocytes isolated from mouse lymph node and spleen. Right, CD4+ T cell purity after isolation using a CD4+ T cell isolation kit; Figure S3: Effects of BPA and 1–8 on M0 differentiation into M2 macrophages and CD4+ T cell differentiation into Tregs. (A) Representative flow cytometry plots of CD25+Foxp3+. (B) Quantification of CD25+Foxp3+ frequency. (C) Representative flow cytometry plots of F4/80+CD206+. (D) Quantification of F4/80+CD206+ frequency. (E) Representative flow cytometry plots of F4/80+CD80+. (F) Quantification of F4/80+CD80+ frequency. (G) Representative flow cytometry plots of CD206+/CD80+. (H) Quantification of CD206+/CD80+ frequency. Statistical significance for all experiments is denoted as follows (n = 3): compared to the M0/Naïve group, #### p < 0.0001; compared to M2 /Tregs group, * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001. RFI means Relative Fluorescence Intensity; Figure S4: Dose-dependent effects of BPA, 1, and 3 on M2 macrophages differentiation. (A) Flow cytometry analysis of F4/80+CD80+ under increasing concentrations of BPA (12.5, 25, 50 μg/mL) or 1 and 3 (12.5, 25, 50 μM). (B) The histograms for the quantified results of F4/80+CD80+ cells. (C) Analysis of F4/80+CD206+ cell population using flow cytometry. (D) The histograms for the quantified results of F4/80+CD206+ cells. Statistical significance for all experiments is denoted as follows (n = 3): compared to the M0 group, # p < 0.05, ### p < 0.001, and #### p < 0.0001; compared to M2 group, * p < 0.05, ** p < 0.01, and **** p < 0.0001. RFI means Relative Fluorescence Intensity; Figure S5: Body weight changes and representative H&E-stained images of mouse organs. (A) Statistical analysis of mouse body weight across all treatment groups. (B) Histopathological analysis of mouse visceral tissues (heart, liver, spleen, lungs, kidneys, and stomach) using H&E staining, indicating the absence of significant toxicity in the treatment groups. Magnified 200 times.
Author Contributions
Methodology, M.Z. and J.X.; Investigation, M.Z., J.X., W.S., Z.Y., Y.C., Y.T. and Y.-Q.Z.; Formal analysis Visualization, X.Y., Y.C. and Y.T.; Writing—original draft, M.Z.; Data curation, J.X.; Conceptualization, R.Z., B.F. and G.Z.; Funding acquisition, B.F. and G.Z.; Project administration, B.F. and G.Z.; Writing—review and editing, R.Z. and G.Z.; Supervision, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81960639) and the Project of Yunnan Provincial Department of Science and Technology (202205AC160084, 202402AN360010, 202401BC070018).
Institutional Review Board Statement
The animal study protocols were approved by the Ethics Committee for Experimental Animals of Yunnan Minzu University (Project code YMU-2022-A027, 2 September 2022; Project code YMU-AFEC-2023-A001, 3 February 2023; Project code YMU-AFEC-2023-A009, 4 April 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| TME | The tumor microenvironment |
| TAMs | Tumor-associated macrophages |
| Tregs | Regulatory T cells |
| MDSCs | Myeloid-derived suppressor cells |
| DCs | Dendritic cells |
| NK | Natural killer cells |
| IFN-γ | Interferon-γ |
| Arg-1 | Arginase-1 |
| iNOS | Inducible nitric oxide synthase |
| qRT-PCR | Quantitative real-time PCR |
| 5-FU | 5-Fluorouracil |
| IL-1β | Interleukin-1β |
| IHC | immunohistochemistry |
| CRC | Colorectal cancer |
| FCM | Flow cytometry |
References
- Fan, A.; Wang, B.; Wang, X.; Nie, Y.; Fan, D.; Zhao, X.; Lu, Y. Immunotherapy in colorectal cancer: Current achievements and future perspective. Int. J. Biol. Sci. 2021, 17, 3837–3849. [Google Scholar] [CrossRef]
- Johdi, N.A.; Sukor, N.F. Colorectal Cancer Immunotherapy: Options and Strategies. Front. Immunol. 2020, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Han, D.; Shen, C.; Lei, Q.; Zhang, Y. Mechanism and strategies of immunotherapy resistance in colorectal cancer. Front. Immunol. 2022, 13, 1016646. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Wang, J.; Lu, D.; Xu, X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Xu, Y.; Wu, J.; Wu, D.; Zhou, L.; Xia, X. TME-Related Biomimetic Strategies Against Cancer. Int. J. Nanomed. 2024, 19, 109–135. [Google Scholar] [CrossRef]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Kos, K.; Salvagno, C.; Wellenstein, M.D.; Aslam, M.A.; Meijer, D.A.; Hau, C.S.; Vrijland, K.; Kaldenbach, D.; Raeven, E.A.M.; Schmittnaegel, M.; et al. Tumor-associated macrophages promote intratumoral conversion of conventional CD4(+) T cells into regulatory T cells via PD-1 signalling. Oncoimmunology 2022, 11, 2063225. [Google Scholar] [CrossRef]
- Zhang, Q.; Sioud, M. Tumor-Associated Macrophage Subsets: Shaping Polarization and Targeting. Int. J. Mol. Sci. 2023, 24, 7493. [Google Scholar] [CrossRef]
- Cheng, K.; Cai, N.; Zhu, J.; Yang, X.; Liang, H.; Zhang, W. Tumor-associated macrophages in liver cancer: From mechanisms to therapy. Cancer Commun. 2022, 42, 1112–1140. [Google Scholar] [CrossRef]
- Tang, Y.; Cui, G.; Liu, H.; Han, Y.; Cai, C.; Feng, Z.; Shen, H.; Zeng, S. Converting “cold” to “hot”: Epigenetics strategies to improve immune therapy effect by regulating tumor-associated immune suppressive cells. Cancer Commun. 2024, 44, 601–636. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Gomez-Roca, C.A.; Italiano, A.; Le Tourneau, C.; Cassier, P.A.; Toulmonde, M.; D’Angelo, S.P.; Campone, M.; Weber, K.L.; Loirat, D.; Cannarile, M.A.; et al. Phase I study of emactuzumab single agent or in combination with paclitaxel in patients with advanced/metastatic solid tumors reveals depletion of immunosuppressive M2-like macrophages. Ann. Oncol. 2019, 30, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Shan, F.; Somasundaram, A.; Bruno, T.C.; Workman, C.J.; Vignali, D.A.A. Therapeutic targeting of regulatory T cells in cancer. Trends Cancer 2022, 8, 944–961. [Google Scholar] [CrossRef] [PubMed]
- Idris, O.A.; Kerebba, N.; Horn, S.; Maboeta, M.S.; Pieters, R. Phytochemical-Based Evidence of the Health Benefits of Bidens pilosa Extracts and Cytotoxicity. Chem. Afr. 2023, 6, 1767–1788. [Google Scholar] [CrossRef]
- Xuan, T.D.; Khanh, T.D. Chemistry and pharmacology of Bidens pilosa: An overview. J. Pharm. Investig. 2016, 46, 91–132. [Google Scholar] [CrossRef]
- Rodríguez-Mesa, X.M.; Contreras Bolaños, L.A.; Mejía, A.; Pombo, L.M.; Modesti Costa, G.; Santander González, S.P. Immunomodulatory Properties of Natural Extracts and Compounds Derived from Bidens pilosa L.: Literature Review. Pharmaceutics 2023, 15, 1491. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Ma, Q.T.; Wu, Y.; Duan, W.; Zeng, G.; Yin, J. Study on the chemical constituents of Bidens pilosa L. J. Yunnan Minzu Univ. (Nat. Sci. Ed.) 2022, 31, 627–632. [Google Scholar] [CrossRef]
- Zeng, G.; Wang, Y.; Zhu, M.; Yi, J.; Ma, J.; Yang, B.; Sun, W.; Dai, F.; Yin, J.; Zeng, G. Inhibition of DNA Topoisomerase Ι by Flavonoids and Polyacetylenes Isolated from Bidens pilosa L. Molecules 2024, 29, 3547. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Tian, P.X.; Han, F.; Zheng, J.; Xia, X.X.; Xue, W.J.; Ding, X.M.; Ding, C.G. Comparison of the characteristics of macrophages derived from murine spleen, peritoneal cavity, and bone marrow. J. Zhejiang Univ. Sci. B 2017, 18, 1055–1063. [Google Scholar] [CrossRef]
- Asadi, Z.; Ghazanfari, T.; Hatami, H. Anti-inflammatory Effects of Matricaria chamomilla Extracts on BALB/c Mice Macrophages and Lymphocytes. Iran. J. Allergy Asthma Immunol. 2020, 19, 63–73. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Z.; He, Z.; Yi, J.; Qiao, X.; Tan, C.; Xing, Y.; Zeng, Y.; Yang, D.; Yin, J.; et al. Cantharidin analogue alleviates dextran sulfate sodium-induced colitis in mice by inhibiting the activation of NF-κB signaling. Eur. J. Med. Chem. 2023, 260, 115731. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Shi, S.Y.; Cheng, F.; Wei, M.; Zou, K.; Yu, X.Q.; Chen, J.F. Polyacetylene Isomers Isolated from Bidens pilosa L. Suppress the Metastasis of Gastric Cancer Cells by Inhibiting Wnt/β-Catenin and Hippo/YAP Signaling Pathways. Molecules 2023, 28, 1837. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, T.; Pan, W.; Wang, T. B. pilosa L. Research progress on Chemical Components of Medicinal Plants. Chin. Med. Mater. 2017, 40, 748–753. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, L.; Xia, H.; Yan, Y.; Zhu, X.; Sun, F.; Sun, L.; Li, S.; Li, D.; Wang, J.; et al. Tumor microenvironment remodeling after neoadjuvant immunotherapy in non-small cell lung cancer revealed by single-cell RNA sequencing. Genome Med. 2023, 15, 14. [Google Scholar] [CrossRef]
- Ge, W.; Wu, W. Influencing Factors and Significance of Tumor-associated Macrophage Polarization in Tumor Microenvironment. Zhongguo Fei Ai Za Zhi 2023, 26, 228–237. [Google Scholar] [CrossRef]
- Qin, D.; Zhang, Y.; Shu, P.; Lei, Y.; Li, X.; Wang, Y. Targeting tumor-infiltrating tregs for improved antitumor responses. Front. Immunol. 2024, 15, 1325946. [Google Scholar] [CrossRef]
- Chen, X.M.; Ni, F. Research Progress on the Immunomodulatory Effects of Plant Flavonoids. J. Fujian Univ. Tradit. Chin. Med. 2010, 20, 69–70. [Google Scholar] [CrossRef]
- Dadey, R.E.; Workman, C.J.; Vignali, D.A.A. Regulatory T Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 105–134. [Google Scholar] [CrossRef]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Jiang, A.; Lin, A.; Liu, Z.; Cheng, X.; Wang, W.; Cheng, Q.; Zhang, J.; Wei, T.; et al. Potential anti-tumor effects of regulatory T cells in the tumor microenvironment: A review. J. Transl. Med. 2024, 22, 293. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Śledzińska, A.; Vila de Mucha, M.; Bergerhoff, K.; Hotblack, A.; Demane, D.F.; Ghorani, E.; Akarca, A.U.; Marzolini, M.A.V.; Solomon, I.; Vargas, F.A.; et al. Regulatory T Cells Restrain Interleukin-2- and Blimp-1-Dependent Acquisition of Cytotoxic Function by CD4(+) T Cells. Immunity 2020, 52, 151–166.e6. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Jia, Y.; Li, J.; Kuai, W.; Li, Y.; Guo, F.; Xu, X.; Zhao, Z.; Lv, J.; Li, Z. Gegen Qinlian decoction enhances the effect of PD-1 blockade in colorectal cancer with microsatellite stability by remodelling the gut microbiota and the tumour microenvironment. Cell Death Dis. 2019, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Guo, X.; Han, F.; He, Z.; Wang, Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm. Sin. B 2022, 12, 1163–1185. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, Y.; Shan, M.; Wang, S.; Chen, J.; Liu, Z.; Xu, Q. Targeting crosstalk of STAT3 between tumor-associated M2 macrophages and Tregs in colorectal cancer. Cancer Biol. Ther. 2023, 24, 2226418. [Google Scholar] [CrossRef]
- Cheng, R.; Li, S.; Ma, X.; Zhuang, W.; Lei, Y.; He, J.; Liang, C.; Nie, W.; Xie, H.Y. Intratumoral antigen-presenting cell activation by a nanovesicle for the concurrent tertiary lymphoid structure de novo neogenesis. Sci. Adv. 2025, 11, eadr1299. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C.X. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 156. [Google Scholar] [CrossRef]
- Taniura, T.; Iida, Y.; Kotani, H.; Ishitobi, K.; Tajima, Y.; Harada, M. Immunogenic chemotherapy in two mouse colon cancer models. Cancer Sci. 2020, 111, 3527–3539. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Mathai, M.; Zulli, A. A synopsis of modern—Day colorectal cancer: Where we stand. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188699. [Google Scholar] [CrossRef]
- Zhang, Z.; Bahaji Azami, N.L.; Liu, N.; Sun, M. Research Progress of Intestinal Microecology in the Pathogenesis of Colorectal Adenoma and Carcinogenesis. Technol. Cancer Res. Treat. 2023, 22, 15330338221135938. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Chu, X.; Tian, Y.; Lv, C. Decoding the spatiotemporal heterogeneity of tumor-associated macrophages. Mol. Cancer 2024, 23, 150. [Google Scholar] [CrossRef]
- Mirlekar, B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022, 10, 20503121211069012. [Google Scholar] [CrossRef]
- Ma, Q.T.; Zeng, G.Y.; Yi, J.M.; Zhu, M.H.; Yin, J.L.; Lai, Q.; Zeng, G.Z. Mechanisms of four polyacetylenes in Bidens pilosa L. onLPS-induced immune in flammation based on networkpharmacology. J. Qilu Univ. Technol. 2024, 38, 34–43. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, J.; Zhang, C.; Guan, Y.; Guo, Q. Progress on PD-1/PD-L1 Checkpoint Inhibitors in Lung Cancer. Zhongguo Fei Ai Za Zhi 2019, 22, 369–379. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.