Highlights
What are the main findings?
- Skeletal muscle and adipose tissue are primarily mediated by cytokines and exosomes.
- This intricate interplay is pivotal in the pathogenesis of numerous metabolic disorders, encompassing obesity and muscle atrophy, as well as influencing meat quality in animal production.
What are the implications of the main findings?
- Adipokines and myokines have bidirectional roles in key biological processes—such as muscle satellite cell differentiation, mitochondrial thermogenesis, insulin sensitivity, and lipid metabolism.
- By synthesizing these findings, we aim to provide novel insights into the treatment of metabolic diseases and the improvement of animal production.
Abstract
Adipose tissue and skeletal muscle are the foremost energy depots and locomotor organs; they orchestrate metabolic homeostasis through the secretion of cytokines via autocrine, paracrine, and endocrine pathways. This intricate interplay is pivotal in the pathogenesis of numerous metabolic disorders, encompassing obesity and muscle atrophy, as well as influencing meat quality in animal production. Despite its significance, unraveling the molecular mechanisms underlying muscle–adipose crosstalk remains a major challenge. Recent advancements in multi-omics technologies have facilitated the identification of a multitude of cytokines derived from adipose tissue and muscle, including adipokines, lipokines, myokines, and myogenic exosomes and adipose-derived exosomes containing various biomolecules. The functional roles of these cytokines have been elucidated through meticulous studies employing trans-well cultures and recombinant proteins. In this comprehensive review, we summarize the bidirectional roles of adipokines and myokines in key biological processes—such as muscle satellite cell differentiation, mitochondrial thermogenesis, insulin sensitivity, and lipid metabolism. By synthesizing these findings, we aim to provide novel insights into the treatment of metabolic diseases and the improvement of animal production.
Keywords:
skeletal muscle; adipose tissue; crosstalk; cytokines; adipokines; myokines; lipokines; exosomes 1. Introduction
Adipose tissue was initially identified as an energy storage organ, with excessive accumulation linked to metabolic-related diseases like type 2 diabetes mellitus (T2DM), hypertension, hyperlipidemia, cardiovascular disease, and chronic kidney disease in humans [1,2,3]. In livestock, adipose tissue deposition varies anatomically and holds different economic values and intramuscular fat deposition; in particular, their marbling score and economic worth can be enhanced by improving meat quality attributes like tenderness, flavor, and juiciness [4,5]. Over the past decades, adipose tissue has emerged as an endocrine organ, regulating metabolism through adipokines, such as leptin and adiponectin; they are predominantly secreted by adipocytes and play key roles in regulating appetite, insulin sensitivity, and inflammation [6]. For example, leptin regulates energy expenditure and food intake, while adiponectin enhances insulin sensitivity and fatty acid oxidation [7]. In terms of livestock and poultry growth, leptin concentration is positively correlated with weight gain [8,9]. In addition, leptin genotypes are closely related to the reproductive and productive traits of animals [10,11]. Dysregulation of adipokine secretion is closely associated with metabolic diseases and can also affect muscle metabolism and animal production [12,13]. Lipokines are lipid-derived signaling molecules, such as palmitoleate and 12,13-diHOME, which regulate systemic metabolic homeostasis independently of classical adipokine pathways [14]. Unlike peptide-based adipokines, lipokines are lipid metabolites that can modulate insulin sensitivity, lipid oxidation, and inflammation in distant tissues, including skeletal muscle [15]. Beyond maintaining homeostasis in energy regulation, metabolism, and cardiovascular function, adipokines exert long-term endocrine effects on metabolic status and reproduction [16]. The diverse signaling landscape of adipose tissue is further highlighted by the unique mechanisms of lipokines, which expand our understanding of their systemic influence.
Skeletal muscles account for about 40% of body weight and are crucial for motor function, respiration, and energy balance [16,17]. Similarly, muscle tissue releases various cytokines, known as “myokines”, that foster communication between muscle and other organs (such as adipose tissue or liver) [18,19]. Muscle integrity is closely related to age and metabolic health. Aging triggers coordinated dysregulation and muscle decline, manifested as intramuscular fat accumulation, insulin resistance, and mitochondrial dysfunction. Simultaneously, adipose tissue causes inflammation and ectopic fat redistribution [20]. This interaction is not only a major driver of sarcopenia, obesity, and type 2 diabetes [21,22], but also directly determines the quantity and quality of animal production [23,24]. Myokines, including myostatin, interleukin-6 (IL-6), and irisin, are produced and released by skeletal muscle, especially during contraction. These molecules influence muscle growth, adipose tissue browning, and systemic glucose homeostasis [25]. Myostatin, for example, negatively regulates muscle mass, and its inhibition is associated with improved lean meat yield in livestock [26]. Irisin promotes the browning of white adipose tissue and enhances energy expenditure, offering potential therapeutic avenues for obesity and metabolic disease [27,28]. In addition, irisin is closely related to animal reproduction, but further research is needed to clarify its function [29,30]. Its action highlights the diversity of adipose-derived signals.
Exosomes are small extracellular vesicles (30–150 nanometers) that can be actively secreted by almost all types of cells, including adipocytes and immune cells in adipose tissue, as well as skeletal muscle cells [31]. These tissue-specific exosomes form a sophisticated inter-organ communication network and influence processes such as satellite cell differentiation, mitochondrial thermogenesis, and lipid metabolism through their complex bioactive cargo (including proteins, lipids, various nucleic acids, and RNAs) [32]. The application of multi-omics and co-culture systems has greatly expanded the catalog of secreted factors involved in muscle–fat communication. Understanding this cytokine-mediated crosstalk—especially in the context of satellite cell differentiation, mitochondrial thermogenesis, insulin sensitivity, and lipid metabolism—will provide valuable insights for treating human adipose and muscle-related diseases, as well as for improving animal production.
2. The Origin of Adipocytes and Skeletal Muscle Cells
Adipocytes are usually classified into white, beige, and brown types based on their function. Anatomically, they are distributed in specific areas of the body. For example, brown adipocytes are found in the interscapular fat depot of rodents and the supraclavicular region in humans, while other major white fat depots include subcutaneous, visceral, intramuscular, and perirenal areas [33,34]. White adipocytes store excess energy in the form of triglycerides, which are utilized after fasting stimulation [35]. In fact, primary white adipocytes cultured in vitro also have a multilocular appearance. In contrast, brown adipocytes also exhibit a multilocular phenotype and express thermogenic gene profiles that uncouple mitochondrial fatty acid oxidation and ATP synthesis, enabling them to use lipids in a thermogenic way [36]. Although beige adipocytes are located within white adipose tissue, they function similarly to brown adipocytes [37]. They promote thermogenesis by upregulating uncoupling protein 1 (UCP-1), thereby helping to regulate body temperature homeostasis [38]. In the context of animal production, intramuscular fat cells, also known as marbling fat, are highly desirable for enhancing the flavor and palatability of meat [39]. Although all these fat cells originate from mesenchymal stem cells (MSCs) in the mesoderm, they undergo differentiation from distinct progenitors and possess specific transcriptional regulation (Figure 1).
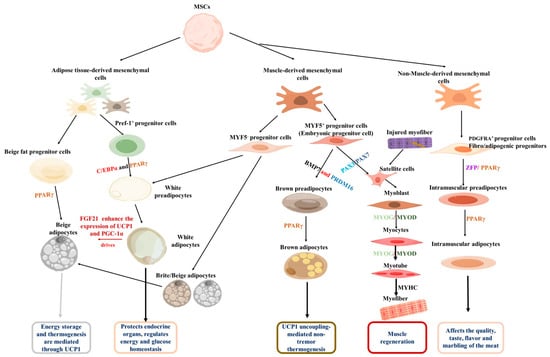
Figure 1.
Origins of adipose tissue and skeletal muscles. Both originate from MSCs, and under the regulation of adipogenic transcription factors such as PPARγ, they are guided to differentiate into the adipocyte lineage, ultimately generating white, brown, and beige adipocytes, and eventually forming mature adipose tissue. Under the regulation of myogenic regulatory factors such as Myf5 and MyoD, they are driven to differentiate into myogenic cells, which ultimately fuse to form multinucleated myotubes and muscle fibers. Additionally, intramuscular fat is generated under the regulation of PDGFRA.
The adipogenic process begins with the commitment of MSCs to a fat progenitor cell fate [40]. These progenitor cells then differentiate into beige or white preadipocytes under the influence of specific adipogenic factors. It is reported that MYF5- progenitor cells and early adipose progenitor cells are the source of white preadipocytes [41,42]. Furthermore, MYF5- progenitor cells also have the ability to differentiate into beige adipocytes [43]. Upon exposure to cold environments or after exercise, fibroblast growth factor 21 (FGF21) can enhance the expression of UCP1 and PGC-1α, thereby promoting the conversion of white adipocytes into beige adipocytes [43,44]. Conversely, MYF5+ progenitor cells produce both brown adipocytes and myoblasts; bone morphogenetic protein 7 (BMP7) and PR domain-containing 16 (PRDM16) drive the differentiation of MYF5+ progenitor cells into brown preadipocytes, which eventually develop into mature brown adipocytes [45,46]. Regarding intramuscular adipocytes, non-muscle-derived mesenchymal stem cells have been identified as their primary source [47]. These cells are marked by platelet-derived growth factor receptor α (PDGFRA, also known as CD140a) and possess biopotency, enabling them to differentiate into either lipid-rich adipocytes or collagen I-expressing fibroblasts; they are, therefore, defined as fibro/adipogenic progenitors (FAPs) [37]. Zinc finger protein (Zfp) plays a key role in FAP’s commitment to the adipogenic lineage. It induces PPARγ expression, promoting the transition of FAPs to preadipocytes and their subsequent conversion into mature adipocytes [48]. Elucidating the molecular regulation of distinct adipocyte progenitors is crucial, as it will enable the targeted modulation of fat development and function.
During muscle cell formation, a normal stage of embryonic development, PAX3 and PAX7 act synergistically as myogenic factors to stimulate the differentiation of MYF5+ progenitor cells into myoblasts [48,49]. Myogenic differentiation antigen 1 (MyoD) is a master regulator of myogenesis. It is expressed in the early stages of myogenic differentiation and induces the expression of other myogenesis-related genes. Another key regulator, myogenin (MyoG), promotes myoblast differentiation into myocytes [49]. Subsequently, myocytes differentiate and fuse to form myotubes [50], which ultimately mature into myofibers [50]. In the context of muscle injury, damaged muscle fibers stimulate the proliferation of muscle satellite cells, which, in turn, carry out subsequent muscle development and promote muscle regeneration [49]. By delving deeper into these comprehensive molecular mechanisms and regulatory networks, researchers will be able to develop intervention strategies to promote muscle regeneration, improve muscle function, and find treatments for related muscle diseases.
3. Effect of Adipose-Derived Cytokines on Skeletal Muscles
Adipose tissue releases cytokines, including adipokines, lipokines, and exosomes, maintaining individual energy homeostasis and participating in the pathogenesis of many metabolic diseases [51]. These molecules can affect adipose tissue itself, and other cells or organs (such as the heart and muscles) [52]. For skeletal muscle, these cytokines directly affect the proliferation, differentiation, and apoptosis of muscle satellite cells. In addition, adipose-derived cytokines are involved in fat ectopic deposition and insulin sensitivity of skeletal muscle, subsequently regulating its metabolism and function, and ultimately affecting human health and animal production (Figure 2).
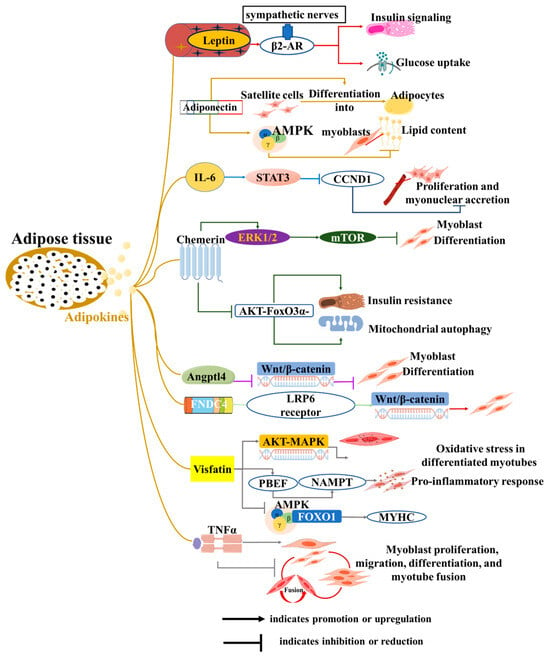
Figure 2.
Roles of adipokines from adipose tissue to skeletal muscle development and function. The diagram illustrates how adipocytes influence various biological functions of muscle cells, including proliferation, differentiation, myotube formation and fusion, insulin resistance, and mitochondrial function, through the secretion of adipokines.
3.1. Adipokines
Adipokines, a series of biologically active proteins and peptides secreted by adipocytes, are identified through various techniques, including transcriptomic and proteomic analyses. Leptin is the first adipokine to be uncovered [1] and plays an important role in communication among adipose tissue, the central nervous system (CNS), and skeletal muscle to maintain energy homeostasis [2]. In skeletal muscle, leptin increases glucose uptake and enhances insulin signaling via the activation of sympathetic nerves and β2-adrenergic receptors (β2-AR) [53]. Adiponectin, another well-known adipokine, not only improves insulin sensitivity in skeletal muscle but also exerts anti-atherosclerotic effects [54]. Interestingly, its effects appear to be context-dependent. In vitro, adiponectin induces the differentiation of goat skeletal muscle satellite cells into adipocytes [55]. Conversely, adiponectin reduces lipid content in chicken myoblasts by activating the AMPK pathway [56]. Adipocytes also secrete IL-6, which is known to induce insulin resistance and muscle mass loss in skeletal muscle [57]. Additionally, IL-6 inhibits satellite cell proliferation and myonuclear accretion in myofibers by regulating the expression of the signal transducer and activator of transcription 3 (STAT3) and its target gene, cyclin D1 (CCND1) [58]. Studies using co-culture systems have demonstrated that adipocytes inhibit the differentiation of C2C12 myoblasts and induce the expression of IL-6 in muscle cells [59]. This finding highlights the direct crosstalk between adipocytes and muscle cells. However, the critical role of IL-6 in this specific process requires further confirmation.
Recently, adipokines were identified, such as Chemerin, angiopoietin-like 4 (Angptl4), and fibronectin type III domain-containing 4 (FNDC4). The exogenous addition of Chemerin promotes the proliferation of both myoblasts and smooth muscle cells, while also inhibiting myoblast differentiation through the ERK1/2 and downstream mTOR signaling pathways [60,61]. Additionally, Chemerin induces insulin resistance, mitochondrial autophagy, and dysfunction in skeletal muscle via an AKT-FoxO3a-dependent signaling pathway [62]. White adipose tissue secretes Angptl4 in response to the systemic administration of dexamethasone (DEX), which directly stimulates cAMP-dependent PKA signaling and lipolysis [63]. In myoblasts, Angptl4 inhibits C2C12 myogenic differentiation by suppressing the Wnt/β-catenin pathway [64]. Conversely, FNDC4 has been identified as a novel adipokine that not only induces browning of human visceral adipose tissue but also promotes myoblast differentiation by interacting with the LRP6 receptor to activate the Wnt/β-catenin pathway [65,66]. In bovines, FNDC4 also promotes the migration and differentiation of skeletal muscle-derived satellite cells via focal adhesion kinase [67]. In addition, visfatin and tumor necrosis factor α (TNFα) derived from adipose tissue also regulate myogenesis, myocyte fusion, and muscle regeneration [68,69,70,71,72]. In summary, although these novel adipose-derived factors play crucial regulatory roles in myoblast differentiation and skeletal muscle function, direct evidence confirming that they are secreted by adipocytes to exert these specific effects is still lacking. Elucidating this direct endocrine axis represents an important direction for future research.
3.2. Lipokines
Bioactive lipids, referred to as “lipid factors” or “lipokines” that are produced by adipose tissue, represent a significant area of research in understanding the complex interactions between adipose and distant tissues, including muscle and liver [51]. They affect various physiological processes beyond metabolism, including immune responses and muscle function [73] (Figure 3). Lysophosphatidic acid (LPA) promotes the differentiation of myoblasts into myotubes and modulates muscle cell proliferation and survival [74]. Sphingosine-1-phosphate (S1P) induces myoblast differentiation through upregulation of gap junctional protein connexin (Cx43) protein expression [75]. Palmitoleic acid was previously shown to improve glucose homeostasis by reducing hepatic glucose production and enhancing insulin-stimulated glucose uptake in skeletal muscle [76], but it has also been reported to inhibit myogenic differentiation of C2C12 myoblasts through phosphorylation-dependent MyoD inactivation [77]. Branched fatty acid esters of hydroxy fatty acids (FAHFAs) were recently uncovered in white adipose tissues and exhibited antidiabetic and anti-inflammatory effects [78]. Melha Benlebna et al. [79] reported that eleven FAHFAs belonging to different families inhibited C2C12 myoblast proliferation, and two of the most active lipids (9-PAHPA and 9-OAHPA) induced a switch toward a more oxidative contractile phenotype of skeletal muscle in mice.
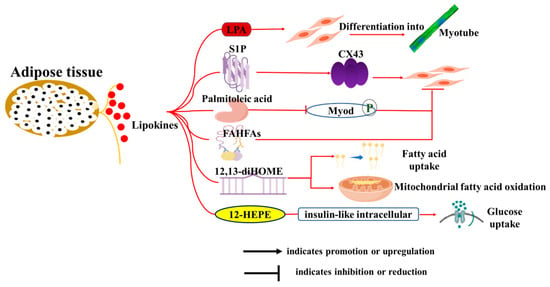
Figure 3.
Roles of lipokines from adipose tissue on skeletal muscle development and function. The diagram illustrates how adipocytes influence various biological functions of muscle cells, including proliferation, differentiation, myotube formation and fusion, fatty acid uptake, mitochondrial function, and glucose uptake through the secretion of adipokines and lipokines.
Recently, considerable attention has been given to BAT-secreted oxylipins, which form a structurally diverse family of oxidized polyunsaturated fatty acid derivatives, such as 12,13-dihydroxy-9Z-octadecenoic acid (12,13-diHOME) and 12-HEPE. Evidence showed that 12,13-diHOME treatment increased fatty acid uptake and mitochondrial fatty acid oxidation in mice skeletal muscle: a correlation that has also been observed in human vastus lateralis muscle fibers [80,81]. Similarly, 12-HEPE was demonstrated to promote glucose uptake into skeletal muscle through activation of the PI3K-mTOR-Akt-GLUT pathway [82]. Taken together, these newly discovered lipokines play a crucial role in regulating muscle function and metabolism, suggesting their potential as targets for effective interventions to improve muscle mass and treat skeletal muscle-related diseases.
3.3. Adipocyte-Derived Exosomes
Exosomes, small extracellular vesicles (30–150 nm) secreted by cells such as adipocytes, play a vital role in intercellular communication by transferring proteins, lipids, and RNAs [83]. Adipose-derived exosomes act as key mediators in the crosstalk between adipose tissue and skeletal muscle, influencing metabolic homeostasis and energy balance. Beyond their well-documented microRNA components, exosomes carry various protein cytokines and signaling molecules that contribute to muscle biology and systemic metabolism [84]. For instance, adipose-derived exosomes transport cytokines such as Laminins, Reelin, and PEDF, enhancing the growth of skeletal muscle [85]. Similarly, exosome-mediated delivery of HSPs and SOD2 promotes skeletal muscle generation [86].
Current research on exosome function primarily focuses on their microRNA (miRNA) components. Notably, miR-27a is secreted by both adipose tissue and muscle, with adipose-derived miR-27a specifically inhibiting insulin signaling in C2C12 cells and myocytes by downregulating PPARγ expression [87]. Both miR-155 and miR-155-5p also originate from adipose-derived exosomes [88], and miR-155 mimics treatment inhibits expression of the myogenic enhancer factor 2A (MEF2A) to repress myoblast differentiation [89]. Recently, Maki Itokazu and colleagues reported novel exosomes delivering miRNA eLet-7d-3p, which targets the transcription factor HMGA2 to inhibit the proliferation of muscle stem cells [90]. Furthermore, gonadal white adipose tissue-derived exosomal miR-222 has been implicated in downregulating IRS1 and p-AKT levels in skeletal muscle, contributing to impaired insulin sensitivity and glucose intolerance [91]. Collectively, adipose-derived exosomes represent a crucial mechanism underlying the crosstalk between adipose tissue and skeletal muscle. Although miRNAs are prominent components involved in this process, the full spectrum of adipose-derived exosome components that influence muscle biology and metabolic health remains largely unexplored, warranting further investigation (Table 1).

Table 1.
Roles of exosomes from adipose tissue on skeletal muscle.
4. Muscle-Derived Cytokine Regulation of Adipocytes
Skeletal muscle is another endocrine organ with significant secretory capabilities, producing and releasing myokines or exosomes during physical activity or in response to diverse stimuli from skeletal muscle cells (myocytes) [93,94]. These secretory molecules play pivotal roles in regulating both local and systemic metabolic processes, inflammation, muscle growth, repair, as well as interactions with adipose tissue, liver, bone, brain, and other organs [95,96]. Notably, over 100 myokines have been identified to date [97], and the secretome suggests that the skeletal muscle encompasses more than 300 secreted proteins [98]. In recent years, a surge of research has illuminated the crucial regulatory function of myokines in the crosstalk between skeletal muscle and adipose tissue. This section of the literature review will summarize the intricate interactions and regulatory mechanisms between muscle-derived secretory factors and adipose tissue, offering fresh perspectives that could revolutionize the treatment of metabolism-related diseases and enhance animal production (Figure 4).
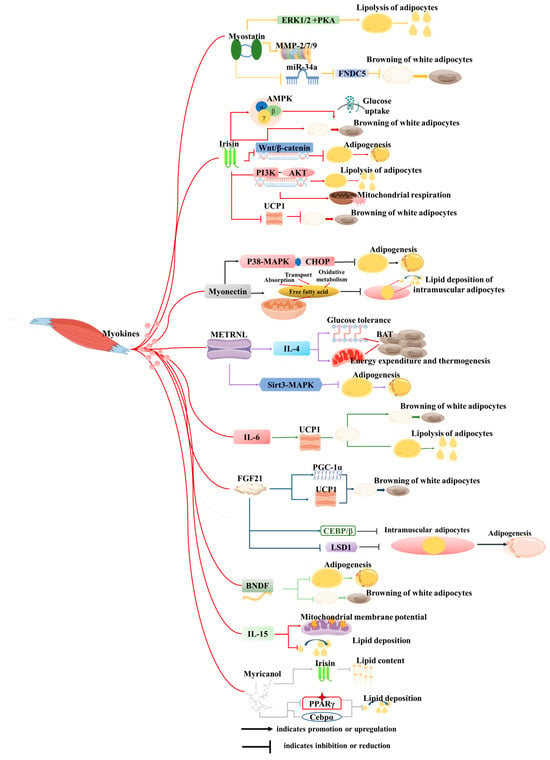
Figure 4.
Roles of cytokines derived from skeletal muscle on adipose tissue. This figure illustrates how myokines secreted by muscles influence biological processes of adipocytes through signaling mechanisms, including proliferation, differentiation, adipogenesis, lipolysis, browning, mitochondrial function, and oxidative stress, among others.
4.1. Myokines
Over the past few decades, many myokines have been identified, including irisin, myostatin (MSTN), meteorin-like (METRNL), β-aminoisobutyric acid (BAIBA), IL-6, fibroblast growth factor 21 (FGF21), brain-derived neurotrophic factor (BDNF), interleukin-15 (IL-15), and others (Figure 3). These myokines are synthesized within muscle tissues and play pivotal roles in regulating biological processes such as lipolysis, insulin sensitivity, and glucose uptake [99,100]. Furthermore, many of them may serve as therapeutic targets for the treatment of metabolic-related diseases.
- (1)
- Myostatin
Myostatin (MSTN) has garnered extensive research attention across diverse species [101]. MSTN-knockout mice exhibit a remarkable phenotype characterized by increased muscle mass, decreased adipose tissue deposition, and a heightened resistance to obesity induced by high-fat diets or genetic mutations [102]. Further investigation has elucidated that these phenotypic changes are primarily mediated through the inhibition of MSTN signaling within muscle tissue, but not adipose tissue [102]. Specifically, knockdown of MSTN downregulates the expression of Jmjd3 via the SMAD2/SMAD3 complex, effectively inhibiting the trans-differentiation of myocytes into adipocytes [90]. Consistent with these findings, MSTN-knockout pigs and goats display similar phenotypic traits to those observed in mice [103,104]. Additionally, MSTN knockdown hinders the differentiation of adipocyte precursors into mature adipocytes by suppressing the expression of MMP-2/7/9 [101]. In vitro studies have demonstrated that direct treatment of 3T3-L1 cells with MSTN recombinant protein promotes its proliferation while inhibiting adipogenesis [105]. The reduction in fat mass following inhibition of MSTN is well documented, but its underlying mechanisms and specific effects on different fat depots are complex and context-dependent. For example, in MSTN knockout mice, fat reduction is not only associated with an increase in overall metabolic rate driven by enlarged skeletal muscle mass but also linked to direct effects on adipose tissue metabolism. Studies have shown that myostatin deficiency can promote the browning of white adipose tissue (WAT), enhancing thermogenesis and energy expenditure [106]. Although previous studies have suggested that myostatin inhibition can reduce fat, its heterogeneous effects on fat storage and metabolism warrant further investigation, particularly in the context of obesity and metabolic syndrome.
- (2)
- Irisin
Irisin, first identified by Pontus Boström and colleagues, emerges as a muscle-derived factor induced by exercise, possessing the capacity to induce browning of white adipocytes [27]. Treatment of preadipocytes with recombinant irisin or overexpression of FNDC5 results in the inhibition of adipogenesis, accompanied by the downregulation of crucial adipogenic markers and activation of the Wnt pathway [107]. Irisin treatment of adipocytes enhances mitochondrial respiration and lipolysis through the PI3K-AKT signaling pathway in a time-dependent manner [108]. In human adipocytes and adipose tissue, irisin treatment upregulates the expression of thermogenesis-related genes and elevates phosphorylation levels of p38, ERK, and STAT3 [109]. Furthermore, in vivo studies have demonstrated that sustained muscle-specific ROCK1 activation downregulates the secretion of irisin from skeletal muscle, resulting in decreased circulating irisin levels, reduced UCP1 expression in both brown and white adipose tissue, and impaired glucose tolerance [110]. Conversely, irisin overexpression has been shown to counteract high-fat diet-induced obesity by promoting the browning of white adipose tissue and enhancing UCP1 expression in vivo [27]. As a dynamic myokine, the secretion of irisin is influenced by various physiological conditions. However, the question of how these fluctuations in irisin levels correlate with alterations in fat metabolism requires further exploration.
- (3)
- Myonectin
Myonectin (CTRP15), a novel member of the C1q/TNF-related protein (CTRP) family classified as a myokine, exhibits distinct expression patterns under fasting and refeeding conditions. Specifically, its levels are suppressed during fasting but undergo a significant increase following refeeding [111]. This myokine plays a pivotal role in facilitating fatty acid uptake in both adipocytes and hepatocytes [111]. Interestingly, myonectin inhibits adipogenesis in 3T3-L1 preadipocytes by modulating the p38 MAPK pathway and CHOP [112]. Furthermore, in porcine models, myonectin has been shown to inhibit intramuscular adipocyte lipid deposition by enhancing fatty acid uptake and promoting oxidative metabolism within mitochondria [113]. In vivo, myonectin deletion in the whole body promotes adipose fat storage and reduces liver steatosis [114]. Nevertheless, further investigation is required to ascertain whether these observed phenotypic changes are indeed attributable to myonectin originating from skeletal muscle.
- (4)
- Metrnl
Meteorin-like (Metrnl) is a circulating factor induced in muscle after exercise and in adipose tissue upon exposure to the cold [115]. It stimulates energy expenditure, beige fat thermogenesis, and improves glucose tolerance by increasing IL-4 expression and promoting the alternative activation of adipose tissue macrophages [115]. In addition, administration of recombinant Metrnl alleviates lipid accumulation and inhibits kidney failure [116]. In humans, Metrnl inhibits human adipocyte differentiation by decreasing the expression of PPARγ and CEBPα [117]. Given the observed beneficial effects of Metrnl in experimental animals, correlation studies have been conducted to investigate the relationship between serum Metrnl levels and indicators such as body weight and blood lipids in both humans and animals [118,119,120]. Notably, this study revealed that Metrnl levels are significantly lower in obese or overweight individuals, hinting at its potential as a therapeutic target for the treatment of lipid metabolism-related diseases.
- (5)
- BAIBA
β-aminoisobutyric acid (BAIBA) is a small-molecule myokine [115] that induces the browning of white adipose tissue and fatty acid β-oxidation in hepatocytes both in vitro and in vivo through a PPARα-mediated mechanism [121]. Simultaneously, BAIBA reduces LPS-induced and high-fat diet-induced inflammation and insulin resistance in an AMPK-PPARα-dependent manner [122,123]. Clinical studies in humans have found that the serum level of BAIBA is inversely related to the fat mass index [124,125]: a correlation that is similar to observations in mice or rats [126]. This collective evidence indicates that BAIBA may be a promising therapeutic target for excessive fat accumulation.
- (6)
- Other cytokines
Notably, IL-6 stimulates lipolysis and browning of white adipose tissue by enhancing the expression of UCP1 [127]. In contrast, FGF-21 is predominantly secreted by adipose tissue and skeletal muscle, with skeletal muscle contributing to lower levels of FGF-21 expression under normal circumstances. However, muscle FGF-21 release is elevated under conditions like fasting, exercise, and mitochondrial myopathy [128]. FGF21 promotes WAT browning by upregulating UCP1 and PGC-1α expression [129]. Furthermore, BDNF has been shown to inhibit the differentiation of 3T3-L1 adipocytes and the browning process in differentiated adipocytes [130]. IL-15 was previously identified as secreted by muscle, but its myogenic nature has not been reported, and its function is usually studied through the process of exogenous addition. IL-15 enhances mitochondrial membrane potential and decreases lipid deposition in adipose tissue of mice [131], and its overexpression in skeletal muscle of mice results in reduced trunk fat mass [132]. In zebrafish fed a high-fat diet, Myricanol inhibited lipid accumulation by suppressing adipogenic factors, including PPARγ and C/EBPα [133]. Furthermore, Myricanol stimulated irisin production and secretion from myotubes to reduce lipid content in 3T3-L1 adipocytes [134].
Among the muscle-secreted factors discussed above, myostatin and irisin stand out as the two most extensively examined across different species for their regulatory roles in adipose tissue. In contrast, research on other myokines in domestic animals remains limited, particularly regarding their impact on animal production, highlighting a critical area for future studies.
4.2. Muscle-Derived Exosomes
Muscle-derived exosomes, small extracellular vesicles (M-EVs), serve as equally crucial intercellular messengers. They transfer bioactive cargo, including myocyte-specific proteins, lipids, and nucleic acids, to recipient cells, thereby participating in the regulation of diverse physiological and pathological processes. Acting as key vehicles in the crosstalk between skeletal muscle and other tissues—particularly adipose tissue—muscle-derived exosomes play an indispensable role in modulating systemic metabolic homeostasis, inflammatory responses, and energy balance [135,136]. For instance, skeletal muscle-secreted exosomes transport myokines—muscle-specific cytokines—such as IL-15 and myostatin. The exosomal packaging of these factors protects them from degradation and ensures their targeted delivery to adipose tissue. Upon uptake by adipocytes, these myokines exert distinct regulatory effects on lipid metabolism, inflammatory status, and insulin sensitivity. Studies have shown that IL-15 can stimulate the proliferation of FAPs and prevent the adipogenesis of FAPs. The growth of FAPs caused by IL-15 was mediated through the JAK-STAT pathway [137]. Conversely, exosomal myostatin conveys a contrasting signal to adipose tissue. Studies have confirmed that myostatin delivered via exosomes suppresses adipocyte differentiation [138]. This interference contributes to adipose tissue dysfunction and is mechanistically linked to the development of obesity and associated metabolic disorders [139].
Muscle-derived exosomes carry various myokines and other signaling molecules, which can mutually influence the metabolism and function of adipose tissue [138]. Current research on M-EVs mainly focuses on miRNAs. Differential expression of miRNAs between M-EVs and adipose-derived EVs has been reported, with miR-146a-5p and miR-221-5p highly enriched in M-EVs. Further KEGG analysis revealed that these miRNAs are primarily involved in lipid-related metabolic processes, potentially through multiple signaling pathways [140]. Specifically, miR-146a-5p negatively regulates the PPARγ signaling pathway by targeting the GDF5 gene, thereby modulating adipogenesis and fatty acid absorption [141]. During muscle regeneration, muscle stem cells (MuSCs) and their derived myoblasts/myotubes secrete extracellular vesicles enriched in miR-206-3p and miR-27a/b-3p, which repress adipogenesis in FAPs, enabling complete muscle regeneration [142]. Furthermore, the role of exosomes released by different muscle fibers in muscle–fat interactions remains to be elucidated (Table 2). An additional question is, do these exosomes also regulate lipid balance in various adipose tissue types? Therefore, a systematic investigation of the heterogeneity of M-EVs and their regulatory mechanisms on adipose tissue development is crucial for understanding the intricate balance between muscle and adipose tissue interactions.

Table 2.
Roles of exosomes from muscle on adipose tissue.
5. Concluding Remarks and Future Perspectives
This review systematically delineates the intricate bidirectional communication between skeletal muscle and adipose tissue, primarily mediated by cytokines and exosomes. We consolidate evidence that adipose-derived signaling molecules, particularly those packaged within exosomes, are critical regulators of skeletal muscle metabolism, insulin sensitivity, and mitochondrial function. Conversely, we detail how muscle-derived myokines and exosomal cargo, such as miRNAs, govern adipose tissue biology by modulating lipolysis, lipogenesis, and browning. The central argument of this review is that fat–muscle interactions regulate distinct physiological processes through the secretion of various factors via different molecular mechanisms. These mechanisms may be related to human diseases (such as sarcopenia and diabetes) and the production performance of animals (such as lean meat percentage and feed conversion efficiency). The intricate interaction between muscle and adipose tissue is largely mediated by a complex network of signaling molecules, with exosomes serving as a crucial delivery system for these factors. However, several pressing challenges warrant further investigation: (1) The role of exosomes as specialized carriers in transporting cytokines and other bioactive molecules between tissues under different physiological and metabolic conditions remains to be fully elucidated. (2) Beyond proteins and miRNAs, the potential roles of other exosomal cargoes, such as lipids, circRNAs, and lncRNAs, in mediating muscle–adipose crosstalk require systematic exploration. (3) The in vivo functions and underlying molecular mechanisms of many identified muscle- and adipose-derived factors need further validation to establish their physiological relevance. (4) Critically, translating these fundamental discoveries into agricultural applications is essential. A deeper understanding of this tissue crosstalk is expected to provide novel strategies for improving livestock growth efficiency, body composition, and ultimately, animal production, thereby promoting sustainable animal agriculture.
Author Contributions
Conceptualization, A.L., B.Y. and Y.X.; Methodology, Y.L., B.Y. and H.H.; Software, B.Y. and Z.Z.; Validation, D.L., Z.Z. and P.S.; Formal Analysis, D.L., Z.Z. and P.S.; Investigation, P.S.; Resources, J.L.; Data Curation, H.H. and D.L.; Writing—Original Draft Preparation, A.L. and J.L.; Writing—Review and Editing, A.L. and Y.X.; Visualization, Y.L. and J.L.; Supervision, Y.L. and H.H.; Project Administration, Y.X. and A.L.; Funding Acquisition, Y.X. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities, Southwest Minzu University (ZYN2024186), the National Natural Sciences Foundation of China (31902154), and the Natural Science Foundation of Sichuan Province (26ZRMS1726).
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
We thank Figdraw (www.figdraw.com, accessed on 4 November 2025) for their assistance in creating the charts.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
T2DM, type 2, diabetes mellitus; IL-6, interleukin-6; UCP-1, uncoupling protein 1; MSCs, mesenchymal stem cells; Pref-1, preadipocyte factor-1; C/EBPα, CCAAT enhancer binding protein alpha; PPARγ, peroxisome proliferator-activated receptor γ; MYF5,myogenic factor 5; BMP7, bone morphogenetic protein 7; PRDM16, PR domain-containing 16; PDGFRA, platelet-derived growth factor receptor α; FAPs, fibro/adipogenic progenitors; Zfp, zinc finger protein; MyoD, myogenic differentiation antigen 1; MYOG, myogenin; CNS, central nervous system; β2-AR, β2-adrenergic receptors; STAT3, signal transducer and activator of transcription 3; CCND1, cyclin D1; Angptl4, angiopoietin-like 4; FNDC4, fibronectin type III domain-containing 4; ERK1/2, extracellular signal-regulated kinase 1 and 2; MTOR, mechanistic target of rapamycin; AKT, protein kinase B; FoxO3a, -protein kinase B forkhead box O3a; DEX, dexamethasone; LRP6, low-density lipoprotein receptor-related protein 6; TNFα, tumor necrosis factor α; LPA, lysophosphatidic acid; S1P, sphingosine-1-phosphate; Cx43, connexin 43; FAHFAs, fatty acid esters of hydroxy fatty acids; 12,13-diHOME, 12,13- dihydroxy-9Z-octadecenoic acid; 12-HEPE, 12-hydroxyeicosapentaenoic acid; miR-27a, microRNA 27a; miR-155, microRNA155; MEF2A, myogenic enhancer factor 2 A; HMGA2, high mobility group AT-Hook 2; miR-222, microRNA222; IRS1, insulin receptor substrate 1; PEDF, pigment epithelium-derived factor; HSPs, heat shock proteins; SOD2, superoxide dismutase 2; TSC1, tuberous sclerosis complex 1; MSTN, myostatin; METRNL, meteorin-like; BIBIA, β-aminoisobutyric acid; FGF21, fibroblast growth factor 21; BDNF, brain-derived neurotrophic factor; IL-15, interleukin-15; SMAD2/SMAD3, mothers against decapentaplegic homolog 2/mothers against decapentaplegic homolog 3; WAT, white adipose tissue; FNDC5, fibronectin type III domain-containing 5; PI3K, phosphatidylinositide 3-kinases; AKT, phosphatidylinositide 3-kinases protein kinase B; ROCK1, rho-associated coiled-coil containing protein kinase 1; CTRP15, tumor necrosis factor-related protein15 or myonectin; CTRP, Cancer Therapeutics Response Portal; MAPK, mitogen-activated protein kinase; IL, 4-interleukin 4; LPS, lipopolysaccharide; AMPK, adenosine monophosphate-activated protein kinase; BNDF, brain-derived neurotrophic factor; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; JAK-STAT, janus kinase-signal transducer and activator of transcription; M-EVs, muscle-derived exosomes; MuSCs, muscle stem cells; GDF5, growth differentiation factor 5.
References
- Gavaldà-Navarro, A.; Villarroya, J.; Cereijo, R.; Giralt, M.; Villarroya, F. The endocrine role of brown adipose tissue: An update on actors and actions. Rev. Endocr. Metab. Disord. 2022, 23, 31–41. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Hou, T.; Song, Y. A review of the relationship between epicardial adipose tissue and cerebral white matter microstructural damage in type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2025, 227, 112384. [Google Scholar] [PubMed]
- Jia, Z.; Wang, Z.; Pan, H.; Zhang, J.; Wang, Q.; Zhou, C.; Liu, J. Crosstalk between fat tissue and muscle, brain, liver, and heart in obesity: Cellular and molecular perspectives. Eur. J. Med. Res. 2024, 29, 637. [Google Scholar]
- Han, Y.; Akhtar, M.F.; Chen, W.; Liu, X.; Zhao, M.; Shi, L.; Khan, M.Z.; Wang, C. Potential candidate genes influencing meat production phenotypic traits in sheep: A review. Front. Vet. Sci. 2025, 12, 1616533. [Google Scholar] [CrossRef]
- Pei, X.; Xie, Y.; Liu, Y.; Cai, X.; Hong, L.; Yang, X.; Zhang, L.; Zhang, M.; Zheng, X.; Ning, K.; et al. Imaging-based adipose biomarkers for predicting clinical outcomes of cancer patients treated with immune checkpoint inhibitors: A systematic review. Front. Oncol. 2023, 13, 1198723. [Google Scholar] [CrossRef]
- Ahima, R.S.; Lazar, M.A. Adipokines and the peripheral and neural control of energy balance. Mol. Endocrinol. 2008, 22, 1023–1031. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Montelli, N.L.L.L.; Alvarenga, T.I.R.C.; Almeida, A.K.; Alvarenga, F.A.P.; Furusho-Garcia, I.F.; Greenwood, P.L.; Pereira, I.G. Associations of feed efficiency with circulating IGF-1 and leptin, carcass traits and meat quality of lambs. Meat Sci. 2021, 173, 108379. [Google Scholar] [CrossRef]
- Luo, G.; Wang, L.; Hu, S.; Du, K.; Wang, J.; Lai, S. Association of leptin mRNA expression with meat quality trait in Tianfu black rabbits. Anim. Biotechnol. 2022, 33, 480–486. [Google Scholar] [CrossRef]
- Yadav, T.; Magotra, A.; Kumar, R.; Bangar, Y.C.; Garg, A.R.; Kumar, S.; Jeet, V.; Malik, B.S. Evaluation of candidate genotype of leptin gene associated with fertility and production traits in Hardhenu (Bos taurus × Bos indicus) cattle. Reprod. Domest. Anim. 2020, 55, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Angel, S.P.; Bagath, M.; Sejian, V.; Krishnan, G.; Bhatta, R. Expression patterns of candidate genes reflecting the growth performance of goats subjected to heat stress. Mol. Biol. Rep. 2018, 45, 2847–2856. [Google Scholar] [CrossRef]
- Hausman, G.J.; Basu, U.; Du, M.; Fernyhough-Culver, M.; Dodson, M.V. Intermuscular and intramuscular adipose tissues: Bad vs. good adipose tissues. Adipocyte 2014, 3, 242–255. [Google Scholar] [CrossRef]
- Komolka, K.; Albrecht, E.; Wimmers, K.; Michal, J.J.; Maak, S. Molecular heterogeneities of adipose depots—Potential effects on adipose-muscle cross-talk in humans, mice and farm animals. J. Genom. 2014, 2, 31–44. [Google Scholar] [CrossRef]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008, 134, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.D.; Tseng, Y.H. Deciphering adipose tissue heterogeneity. Ann. N. Y. Acad. Sci. 2018, 1411, 5–20. [Google Scholar] [PubMed]
- Li, X.; Fu, X.; Yang, G.; Du, M. Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals. Animal 2020, 14, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.Y.; Choi, K.M. Impact of Adipose Tissue and Lipids on Skeletal Muscle in Sarcopenia. J. Cachexia Sarcopenia Muscle 2025, 16, e70000. [Google Scholar] [CrossRef]
- Gallagher, E.J.; Leroith, D.; Karnieli, E. Insulin resistance in obesity as the underlying cause for the metabolic syndrome. Mt. Sinai J. Med. 2010, 77, 511–523. [Google Scholar] [CrossRef]
- Yi, J.; Chen, J.; Yao, X.; Zhao, Z.; Niu, X.; Li, X.; Sun, J.; Ji, Y.; Shang, T.; Gong, L.; et al. Myokine-mediated muscle-organ interactions: Molecular mechanisms and clinical significance. Biochem. Pharmacol. 2025, 242 Pt 2, 117326. [Google Scholar] [CrossRef]
- Guo, L.; Quan, M.; Pang, W.; Yin, Y.; Li, F. Cytokines and exosomal miRNAs in skeletal muscle-adipose crosstalk. Trends Endocrinol. Metab. TEM 2023, 34, 666–681. [Google Scholar] [CrossRef]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef]
- Giordani, L.; He, G.J.; Negroni, E.; Sakai, H.; Law, J.Y.C.; Siu, M.M.; Wan, R.; Corneau, A.; Tajbakhsh, S.; Cheung, T.H.; et al. High-Dimensional Single-Cell Cartography Reveals Novel Skeletal Muscle-Resident Cell Populations. Mol. Cell 2019, 74, 609–621.e6. [Google Scholar]
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Edward, F. Adolph distinguished lecture: Muscle as an endocrine organ: IL-6 and other myokines. J. Appl. Physiol. (1985) 2009, 107, 1006–1014. [Google Scholar] [PubMed]
- McPherron, A.C.; Lee, S.J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 1997, 94, 12457–12461. [Google Scholar] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar]
- Stanford, K.I.; Goodyear, L.J. Muscle-Adipose Tissue Cross Talk. Cold Spring Harb. Perspect. Med. 2018, 8, a029801. [Google Scholar] [CrossRef]
- Shokrollahi, B.; Shang, J.H.; Saadati, N.; Ahmad, H.I.; Yang, C.Y. Reproductive roles of novel adipokines apelin, visfatin, and irisin in farm animals. Theriogenology 2021, 172, 178–186. [Google Scholar] [CrossRef]
- Shokrollahi, B.; Jang, S.S.; Lee, H.J.; Ahmad, H.I.; Sesay, A.R.; Ghazikhani Shad, A.; Morammazi, S.; Abdelnour, S.A. Exploring the potential roles of apelin, visfatin, and irisin in energy regulation in farm animals: An overview. Front. Vet. Sci. 2024, 11, 1435788. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Guescini, M.; Canonico, B.; Lucertini, F.; Maggio, S.; Annibalini, G.; Barbieri, E.; Luchetti, F.; Papa, S.; Stocchi, V. Muscle Releases Alpha-Sarcoglycan Positive Extracellular Vesicles Carrying miRNAs in the Bloodstream. PLoS ONE 2015, 10, e0125094. [Google Scholar] [CrossRef]
- Dobre, M.Z.; Virgolici, B.; Timnea, O. Key Roles of Brown, Subcutaneous, and Visceral Adipose Tissues in Obesity and Insulin Resistance. Curr. Issues Mol. Biol. 2025, 47, 343. [Google Scholar] [CrossRef]
- Park, A.; Kim, W.K.; Bae, K.H. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J. Stem Cells 2014, 6, 33–42. [Google Scholar] [CrossRef]
- Cho, C.H.; Patel, S.; Rajbhandari, P. Adipose tissue lipid metabolism: Lipolysis. Curr. Opin. Genet. Dev. 2023, 83, 102114. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Boychenko, S.; Egorova, V.S.; Brovin, A.; Egorov, A.D. White-to-Beige and Back: Adipocyte Conversion and Transcriptional Reprogramming. Pharmaceuticals 2024, 17, 790. [Google Scholar] [CrossRef]
- Cousin, B.; Cinti, S.; Morroni, M.; Raimbault, S.; Ricquier, D.; Pénicaud, L.; Casteilla, L. Occurrence of brown adipocytes in rat white adipose tissue: Molecular and morphological characterization. J. Cell Sci. 1992, 103 Pt 4, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, R.G.J.; Hubalek, S.; Melke, J.; Messmer, T.; Cantoni, F.; Mei, A.; Hueber, R.; Mitic, R.; Remmers, D.; Moutsatsou, P.; et al. Muscle-derived fibro-adipogenic progenitor cells for production of cultured bovine adipose tissue. npj Sci. Food 2022, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Choo, K.B.; Chen, C.F. The MicroRNA-signaling-peroxisome proliferator-activated receptor gamma connection in the modulation of adipogenesis: Bioinformatics projection on chicken. Poult. Sci. 2022, 101, 101950. [Google Scholar] [PubMed]
- Jing, K.; Heo, J.Y.; Song, K.S.; Seo, K.S.; Park, J.H.; Kim, J.S.; Jung, Y.J.; Jo, D.Y.; Kweon, G.R.; Yoon, W.H.; et al. Expression regulation and function of Pref-1 during adipogenesis of human mesenchymal stem cells (MSCs). Biochim. Biophys. Acta 2009, 1791, 816–826. [Google Scholar]
- Sanchez-Gurmaches, J.; Guertin, D.A. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun. 2014, 5, 4099. [Google Scholar] [CrossRef]
- Sidossis, L.; Kajimura, S. Brown and beige fat in humans: Thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Investig. 2015, 125, 478–486. [Google Scholar] [CrossRef]
- Shan, T.; Liang, X.; Bi, P.; Zhang, P.; Liu, W.; Kuang, S. Distinct populations of adipogenic and myogenic Myf5-lineage progenitors in white adipose tissues. J. Lipid Res. 2013, 54, 2214–2224. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Xue, R.; Wan, Y.; Zhang, S.; Zhang, Q.; Ye, H.; Li, Y. Role of bone morphogenetic protein 4 in the differentiation of brown fat-like adipocytes. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E363–E372. [Google Scholar] [CrossRef]
- Seale, P.; Bjork, B.; Yang, W.; Kajimura, S.; Chin, S.; Kuang, S.; Scimè, A.; Devarakonda, S.; Conroe, H.M.; Erdjument-Bromage, H.; et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454, 961–967. [Google Scholar] [CrossRef]
- Uezumi, A.; Fukada, S.; Yamamoto, N.; Takeda, S.; Tsuchida, K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 2010, 12, 143–152. [Google Scholar] [CrossRef]
- Lepper, C.; Fan, C.M. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 2010, 48, 424–436. [Google Scholar] [CrossRef]
- Chen, B.; You, W.; Wang, Y.; Shan, T. The regulatory role of Myomaker and Myomixer-Myomerger-Minion in muscle development and regeneration. Cell Mol. Life Sci. 2020, 77, 1551–1569. [Google Scholar] [CrossRef]
- Isesele, P.O.; Mazurak, V.C. Regulation of Skeletal Muscle Satellite Cell Differentiation by Omega-3 Polyunsaturated Fatty Acids: A Critical Review. Front. Physiol. 2021, 12, 682091. [Google Scholar] [CrossRef] [PubMed]
- Le Lay, S.; Scherer, P.E. Exploring adipose tissue-derived extracellular vesicles in inter-organ crosstalk: Implications for metabolic regulation and adipose tissue function. Cell Rep. 2025, 44, 115732. [Google Scholar] [CrossRef]
- Kojta, I.; Chacińska, M.; Błachnio-Zabielska, A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients 2020, 12, 1305. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Huenchullan, S.F.; Tam, C.S.; Ban, L.A.; Ehrenfeld-Slater, P.; Mclennan, S.V.; Twigg, S.M. Skeletal muscle adiponectin induction in obesity and exercise. Metabolism 2020, 102, 154008. [Google Scholar] [CrossRef]
- Wang, L.; Xue, K.; Wang, Y.; Niu, L.; Li, L.; Zhong, T.; Guo, J.; Feng, J.; Song, T.; Zhang, H. Molecular and functional characterization of the adiponectin (AdipoQ) gene in goat skeletal muscle satellite cells. Asian-Australas. J. Anim. Sci. 2018, 31, 1088–1097. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, D.; Lin, H.; Li, H.; Zhao, J.; Jiao, H.C.; Wang, X. Adiponectin Reduces Lipid Content in Chicken Myoblasts by Activating AMPK Signaling Pathway. Biosci. Rep. 2022, 42, BSR20212549. [Google Scholar] [CrossRef]
- Plomgaard, P.; Penkowa, M.; Pedersen, B.K. Fiber type specific expression of TNF-alpha, IL-6 and IL-18 in human skeletal muscles. Exerc. Immunol. Rev. 2005, 11, 53–63. [Google Scholar]
- Kurosaka, M.; Machida, S. Interleukin-6-induced satellite cell proliferation is regulated by induction of the JAK2/STAT3 signalling pathway through cyclin D1 targeting. Cell Prolif. 2013, 46, 365–373. [Google Scholar] [CrossRef]
- Seo, K.; Suzuki, T.; Kobayashi, K.; Nishimura, T. Adipocytes suppress differentiation of muscle cells in a co-culture system. Anim. Sci. J. 2019, 90, 423–434. [Google Scholar] [CrossRef]
- Peng, L.; Chen, Y.; Li, Y.; Feng, P.; Zheng, Y.; Dong, Y.; Yang, Y.; Wang, R.; Li, A.; Yan, J.; et al. Chemerin Regulates the Proliferation and Migration of Pulmonary Arterial Smooth Muscle Cells via the ERK1/2 Signaling Pathway. Front. Pharmacol. 2022, 13, 767705. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, F.; Kong, X.; Yuan, X.; Wang, W.; Huang, R.; Li, T.; Geng, M.; Wu, G.; Yin, Y. Chemerin regulates proliferation and differentiation of myoblast cells via ERK1/2 and mTOR signaling pathways. Cytokine 2012, 60, 646–652. [Google Scholar] [CrossRef]
- Xie, Q.; Deng, Y.; Huang, C.; Liu, P.; Yang, Y.; Shen, W.; Gao, P. Chemerin-induced mitochondrial dysfunction in skeletal muscle. J. Cell Mol. Med. 2015, 19, 986–995. [Google Scholar] [CrossRef]
- Kersten, S. Role and mechanism of the action of angiopoietin-like protein ANGPTL4 in plasma lipid metabolism. J. Lipid Res. 2021, 62, 100150. [Google Scholar] [CrossRef]
- Son, Y.; Lorenz, W.W.; Paton, C.M. Linoleic acid-induced ANGPTL4 inhibits C2C12 skeletal muscle differentiation by suppressing Wnt/β-catenin. J. Nutr. Biochem. 2023, 116, 109324. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Fernández-Quintana, B.; Paniagua, M.; Hernández-Pardos, A.W.; Valentí, V.; Moncada, R.; Catalán, V.; Becerril, S.; Gómez-Ambrosi, J.; Portincasa, P.; et al. FNDC4, a novel adipokine that reduces lipogenesis and promotes fat browning in human visceral adipocytes. Metabolism 2020, 108, 154261. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Wang, Y.; Yan, Y.; Tong, H.; Li, S. Fibronectin type III domain containing four promotes differentiation of C2C12 through the Wnt/β-catenin signaling pathway. FASEB J. 2020, 34, 7759–7772. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Z.; Pang, Y.; Tong, H.; Yan, Y.; Li, S.; Li, S. Fibronectin type III domain-containing 4 promotes the migration and differentiation of bovine skeletal muscle-derived satellite cells via focal adhesion kinase. Cell Adh. Migr. 2020, 14, 153–164. [Google Scholar] [CrossRef]
- Oita, R.C.; Ferdinando, D.; Wilson, S.; Bunce, C.; Mazzatti, D.J. Visfatin induces oxidative stress in differentiated C2C12 myotubes in an Akt- and MAPK-independent, NFkB-dependent manner. Pflug. Arch. 2010, 459, 619–630. [Google Scholar] [CrossRef]
- Romacho, T.; Azcutia, V.; Vázquez-Bella, M.; Matesanz, N.; Cercas, E.; Nevado, J.; Carraro, R.; Rodríguez-Mañas, L.; Sánchez-Ferrer, C.F.; Peiró, C. Extracellular PBEF/NAMPT/visfatin activates pro-inflammatory signalling in human vascular smooth muscle cells through nicotinamide phosphoribosyltransferase activity. Diabetologia 2009, 52, 2455–2463. [Google Scholar] [CrossRef]
- Zhou, L.N.; Lin, Y.N.; Gu, C.J.; Zhou, J.P.; Sun, X.W.; Cai, X.T.; Du, J.; Li, Q.Y. AMPK/FOXO1 signaling pathway is indispensable in visfatin-regulated myosin heavy chain expression in C2C12 myotubes. Life Sci. 2019, 224, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Wu, W.T.; Chen, Y.H.; Chen, L.R.; Hsu, W.H.; Lin, Y.L.; Han, D.S. Enhanced serum levels of tumor necrosis factor-α, interleukin-1β, and -6 in sarcopenia: Alleviation through exercise and nutrition intervention. Aging 2023, 15, 13471–13485. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Nie, J.R.; Shang, P.; Zhang, B.; Yan, D.W.; Hao, X.; Zhang, H. Tumor necrosis factor α deficiency promotes myogenesis and muscle regeneration. Zool. Res. 2024, 45, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Lynes, M.D.; Kodani, S.D.; Tseng, Y.H. Lipokines and Thermogenesis. Endocrinology 2019, 160, 2314–2325. [Google Scholar] [CrossRef]
- Ray, R.; Sinha, S.; Aidinis, V.; Rai, V. Atx regulates skeletal muscle regeneration via LPAR1 and promotes hypertrophy. Cell Rep. 2021, 34, 108809. [Google Scholar] [CrossRef]
- Squecco, R.; Sassoli, C.; Nuti, F.; Martinesi, M.; Chellini, F.; Nosi, D.; Zecchi-Orlandini, S.; Francini, F.; Formigli, L.; Meacci, E. Sphingosine 1-phosphate induces myoblast differentiation through Cx43 protein expression: A role for a gap junction-dependent and -independent function. Mol. Biol. Cell 2006, 17, 4896–4910. [Google Scholar] [CrossRef]
- Bolsoni-Lopes, A.; Festuccia, W.T.; Chimin, P.; Farias, T.S.; Torres-Leal, F.L.; Cruz, M.M.; Andrade, P.B.; Hirabara, S.M.; Lima, F.B.; Alonso-Vale, M.I. Palmitoleic acid (n-7) increases white adipocytes GLUT4 content and glucose uptake in association with AMPK activation. Lipids Health Dis. 2014, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Matsuba, I.; Fujita, R.; Iida, K. Palmitic Acid Inhibits Myogenic Activity and Expression of Myosin Heavy Chain MHC IIb in Muscle Cells through Phosphorylation-Dependent MyoD Inactivation. Int. J. Mol. Sci. 2023, 24, 5847. [Google Scholar] [CrossRef]
- Lee, J.; Moraes-Vieira, P.M.; Castoldi, A.; Aryal, P.; Yee, E.U.; Vickers, C.; Parnas, O.; Donaldson, C.J.; Saghatelian, A.; Kahn, B.B. Branched Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs) Protect against Colitis by Regulating Gut Innate and Adaptive Immune Responses. J. Biol. Chem. 2016, 291, 22207–22217. [Google Scholar] [CrossRef]
- Benlebna, M.; Balas, L.; Pessemesse, L.; Bonafos, B.; Fouret, G.; Pavlin, L.; Goustard, B.; Gaillet, S.; Durand, T.; Coudray, C.; et al. FAHFAs Regulate the Proliferation of C2C12 Myoblasts and Induce a Shift toward a More Oxidative Phenotype in Mouse Skeletal Muscle. Int. J. Mol. Sci. 2020, 21, 9046. [Google Scholar] [CrossRef]
- Stanford, K.I.; Lyne, M.D.; Takahashi, H.; Baer, L.A.; Arts, P.J.; May, F.J.; Lehnig, A.C.; Middelbeek, R.J.W.; Richard, J.J.; So, K.; et al. 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell Metab. 2018, 27, 1111–1120.e3. [Google Scholar] [CrossRef]
- Nicholson, T.; Church, C.; Baker, D.J.; Jones, S.W. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J. Inflamm. 2018, 15, 9. [Google Scholar] [CrossRef]
- Leiria, L.O.; Wang, C.H.; Lynes, M.D.; Yang, K.; Shamsi, F.; Sato, M.; Sugimoto, S.; Chen, E.Y.; Bussberg, V.; Narain, N.R.; et al. 12-Lipoxygenase Regulates Cold Adaptation and Glucose Metabolism by Producing the Omega-3 Lipid 12-HEPE from Brown Fat. Cell Metab. 2019, 30, 768–783.e7. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, opportunity, and perspective on exosome isolation—Efforts for efficient exosome-based theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef] [PubMed]
- Weiliang, Z.; Lili, G. Research Advances in the Application of Adipose-Derived Stem Cells Derived Exosomes in Cutaneous Wound Healing. Ann. Dermatol. 2021, 33, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Li, H.; Zhou, Y.; Gu, B.; Xu, Y.; Fu, Q.; Peng, X.; Cao, N.; Fu, Q.; Jin, M.; et al. Therapeutic Potential of Human Adipose-Derived Stem Cell Exosomes in Stress Urinary Incontinence—An in Vitro and in Vivo Study. Cell Physiol. Biochem. 2018, 48, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Mellows, B.; Sheard, J.; Antonioli, M.; Kretz, O.; Chambers, D.; Zeuner, M.T.; Tomkins, J.E.; Denecke, B.; Musante, L.; et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res. Ther. 2019, 10, 116. [Google Scholar] [CrossRef]
- Yu, Y.; Du, H.; Wei, S.; Feng, L.; Li, J.; Yao, F.; Zhang, M.; Hatch, G.M.; Chen, L. Adipocyte-Derived Exosomal MiR-27a Induces Insulin Resistance in Skeletal Muscle Through Repression of PPARγ. Theranostics 2018, 8, 2171–2188. [Google Scholar]
- Chen, D.; Jiang, X.; Zou, H. hASCs-derived exosomal miR-155-5p targeting TGFβR2 promotes autophagy and reduces pyroptosis to alleviate intervertebral disc degeneration. J. Orthop. Translat. 2023, 39, 163–176. [Google Scholar] [CrossRef]
- Seok, H.Y.; Tatsuguchi, M.; Callis, T.E.; He, A.; Pu, W.T.; Wang, D.Z. miR-155 inhibits expression of the MEF2A protein to repress skeletal muscle differentiation. J. Biol. Chem. 2011, 286, 35339–35346. [Google Scholar] [CrossRef]
- Itokazu, M.; Onodera, Y.; Mori, T.; Inoue, S.; Yamagishi, K.; Moritake, A.; Iwawaki, N.; Shigi, K.; Takehara, T.; Higashimoto, Y.; et al. Adipose-derived exosomes block muscular stem cell proliferation in aged mouse by delivering miRNA Let-7d-3p that targets transcription factor HMGA2. J. Biol. Chem. 2022, 298, 102098. [Google Scholar]
- Li, D.; Song, H.; Shuo, L.; Wang, L.; Xie, P.; Li, W.; Liu, J.; Tong, Y.; Zhang, C.Y.; Jiang, X.; et al. Gonadal white adipose tissue-derived exosomal MiR-222 promotes obesity-associated insulin resistance. Aging 2020, 12, 22719–22743. [Google Scholar] [CrossRef]
- Fang, X.; Stroud, M.J.; Ouyang, K.; Fang, L.; Zhang, J.; Dalton, N.D.; Gu, Y.; Wu, T.; Peterson, K.L.; Huang, H.D.; et al. Adipocyte-specific loss of PPARγ attenuates cardiac hypertrophy. JCI Insight 2016, 1, e89908. [Google Scholar] [CrossRef]
- Hoffmann, C.; Weigert, C. Skeletal Muscle as an Endocrine Organ: The Role of Myokines in Exercise Adaptations. Cold Spring Harb. Perspect. Med. 2017, 7, a029793. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Akerström, T.C.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. (1985) 2007, 103, 1093–1098. [Google Scholar] [CrossRef]
- Bortoluzz, S.; Scannapieco, P.; Cestaro, A.; Danieli, G.A.; Schiaffino, S. Computational reconstruction of the human skeletal muscle secretome. Proteins 2006, 62, 776–792. [Google Scholar] [CrossRef]
- Malicka, A.; Ali, A.; MacCannell, A.D.V.; Roberts, L.D. Brown and beige adipose tissue-derived metabokine and lipokine inter-organ signalling in health and disease. Exp. Physiol. 2025, 110, 918–935. [Google Scholar] [CrossRef]
- Huh, J.Y. The role of exercise-induced myokines in regulating metabolism. Arch. Pharm. Res. 2018, 41, 14–29. [Google Scholar] [CrossRef]
- Eckardt, K.; Görgens, S.W.; Raschke, S.; Eckel, J. Myokines in insulin resistance and type 2 diabetes. Diabetologia 2014, 57, 1087–1099. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Steensberg, A.; Schjerling, P. Muscle-derived interleukin-6: Possible biological effects. J. Physiol. 2001, 536 Pt 2, 329–337. [Google Scholar] [CrossRef]
- Dumas, S.N.; Guo, C.A.; Kim, J.K.; Friedline, R.H.; Ntambi, J.M. Interleukin-6 derived from cutaneous deficiency of stearoyl-CoA desaturase- 1 may mediate metabolic organ crosstalk among skin, adipose tissue and liver. Biochem. Biophys. Res. Commun. 2019, 508, 87–91. [Google Scholar] [CrossRef]
- Gao, L.; Yang, M.; Wang, X.; Yang, L.; Bai, C.; Li, G. Mstn knockdown decreases the trans-differentiation from myocytes to adipocytes by reducing Jmjd3 expression via the SMAD2/SMAD3 complex. Biosci. Biotechnol. Biochem. 2019, 83, 2090–2096. [Google Scholar] [CrossRef]
- Guo, T.; Jou, W.; Chanturiya, T.; Portas, J.; Gavrilova, O.; McPherron, A.C. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS ONE 2009, 4, e4937. [Google Scholar] [CrossRef]
- Zhu, Z.; Ali, A.; Wang, J.; Qi, S.; Hua, Z.; Ren, H.; Zhang, L.; Gu, H.; Molenaar, A.; Babar, M.E.; et al. Myostatin increases the expression of matrix metalloproteinase genes to promote preadipocytes differentiation in pigs. Adipocyte 2022, 11, 266–275. [Google Scholar] [CrossRef]
- He, Z.; Zhang, T.; Jiang, L.; Zhou, M.; Wu, D.; Mei, J.; Cheng, Y. Use of CRISPR/Cas9 technology efficiently targetted goat myostatin through zygotes microinjection resulting in double-muscled phenotype in goats. Biosci. Rep. 2018, 38, BSR20180742. [Google Scholar] [CrossRef]
- Zhu, H.J.; Pan, H.; Zhang, X.Z.; Li, N.S.; Wang, L.J.; Yang, H.B.; Gong, F.Y. The effect of myostatin on proliferation and lipid accumulation in 3T3-L1 preadipocytes. J. Mol. Endocrinol. 2015, 54, 217–226. [Google Scholar] [CrossRef]
- Ge, X.; Sathiakumar, D.; Lua, B.J.; Kukreti, H.; Lee, M.; McFarlane, C. Myostatin signals through miR-34a to regulate Fndc5 expression and browning of white adipocytes. Int. J. Obes. 2017, 41, 137–148. [Google Scholar] [CrossRef]
- Ma, E.B.; Sahar, N.E.; Jeong, M.; Huh, J.Y. Irisin Exerts Inhibitory Effect on Adipogenesis Through Regulation of Wnt Signaling. Front. Physiol. 2019, 10, 1085. [Google Scholar] [CrossRef]
- Vliora, M.; Grillo, E.; Corsini, M.; Ravelli, C.; Nintou, E.; Karligiotou, E.; Flouris, A.D.; Mitola, S. Irisin regulates thermogenesis and lipolysis in 3T3-L1 adipocytes. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130085. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, C.; Wang, H.; Foss, R.M.; Clare, M.; George, E.V.; Li, S.; Katz, A.; Cheng, H.; Ding, Y.; et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E530–E541. [Google Scholar] [CrossRef]
- Zhou, X.; Li, R.; Liu, X.; Wang, L.; Hui, P.; Chan, L.; Saha, P.K.; Hu, Z. ROCK1 reduces mitochondrial content and irisin production in muscle suppressing adipocyte browning and impairing insulin sensitivity. Sci. Rep. 2016, 6, 29669. [Google Scholar] [CrossRef]
- Seldin, M.M.; Peterson, J.M.; Byerly, M.S.; Wei, Z.; Wong, G.W. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J. Biol. Chem. 2012, 287, 11968–11980. [Google Scholar] [CrossRef]
- Park, T.J.; Park, A.; Kim, J.; Kim, J.Y.; Han, B.S.; Oh, K.J.; Lee, E.W.; Lee, S.C.; Bae, K.H.; Kim, W.K. Myonectin inhibits adipogenesis in 3T3-L1 preadipocytes by regulating p38 MAPK pathway. BMB Rep. 2021, 54, 124–129. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Z.; Xi, J.; Liu, Y.; Zheng, Z.; Li, N.; Li, Z.; Liang, S.; Li, Q.; Zhang, H.; et al. Effects of myonectin on porcine intramuscular adipocyte differentiation and exogenous free fatty acid utilization. Anim. Biotechnol. 2023, 34, 3757–3764. [Google Scholar] [CrossRef] [PubMed]
- Little, H.C.; Rodriguez, S.; Lei, X.; Tan, S.Y.; Stewart, A.N.; Sahagun, A.; Sarver, D.C.; Wong, G.W. Myonectin deletion promotes adipose fat storage and reduces liver steatosis. FASEB J. 2019, 33, 8666–8687. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Jin, B.; Wu, Y.; Xu, L.; Chang, X.; Hu, L.; Wang, G.; Huang, Y.; Song, L.; et al. Metrnl Alleviates Lipid Accumulation by Modulating Mitochondrial Homeostasis in Diabetic Nephropathy. Diabetes 2023, 72, 611–626. [Google Scholar] [CrossRef]
- Löffler, D.; Landgraf, K.; Rockstroh, D.; Schwartze, J.T.; Dunzendorfer, H.; Kiess, W.; Körner, A. METRNL decreases during adipogenesis and inhibits adipocyte differentiation leading to adipocyte hypertrophy in humans. Int. J. Obes. 2017, 41, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Chang, X.; Wang, J.; Bian, N.; An, Y.; Wang, G.; Liu, J. Serum Metrnl levels are decreased in subjects with overweight or obesity and are independently associated with adverse lipid profile. Front. Endocrinol. 2022, 13, 938341. [Google Scholar] [CrossRef]
- Alizadeh, H. Myokine-mediated exercise effects: The role of myokine meteorin-like hormone (Metrnl). Growth Factors 2021, 39, 71–78. [Google Scholar] [CrossRef]
- Qi, Q.; Hu, W.J.; Zheng, S.L.; Zhang, S.L.; Le, Y.Y.; Li, Z.Y.; Miao, C.Y. Metrnl deficiency decreases blood HDL cholesterol and increases blood triglyceride. Acta Pharmacol. Sin. 2020, 41, 1568–1575. [Google Scholar] [CrossRef]
- Roberts, L.D.; Boström, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef]
- Jung, T.W.; Park, H.S.; Choi, G.H.; Kim, D.; Lee, T. β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway. J. Biomed. Sci. 2018, 25, 27. [Google Scholar] [CrossRef]
- Jung, T.W.; Hwang, H.J.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Choi, K.M. BAIBA attenuates insulin resistance and inflammation induced by palmitate or a high fat diet via an AMPK-PPARδ-dependent pathway in mice. Diabetologia 2015, 58, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Katano, S.; Yano, T.; Kouzu, H.; Nagaoka, R.; Numazawa, R.; Yamano, K.; Fujisawa, Y.; Ohori, K.; Nagano, N.; Fujito, T.; et al. Circulating level of β-aminoisobutyric acid (BAIBA), a novel myokine-like molecule, is inversely associated with fat mass in patients with heart failure. Heart Vessel. 2024, 39, 35–47. [Google Scholar] [CrossRef]
- Lyssikatos, C.; Wang, Z.; Liu, Z.; Warden, S.J.; Brotto, M.; Bonewald, L. L-β-aminoisobutyric acid, L-BAIBA, a marker of bone mineral density and body mass index, and D-BAIBA of physical performance and age. Sci. Rep. 2023, 13, 17212. [Google Scholar] [CrossRef]
- Tanianskii, D.A.; Jarzebska, N.; Birkenfeld, A.L.; O’Sullivan, J.F.; Rodionov, R.N. Beta-Aminoisobutyric Acid as a Novel Regulator of Carbohydrate and Lipid Metabolism. Nutrients 2019, 11, 524. [Google Scholar] [CrossRef]
- Han, J.; Meng, Q.; Shen, L.; Wu, G. Interleukin-6 induces fat loss in cancer cachexia by promoting white adipose tissue lipolysis and browning. Lipids Health Dis. 2018, 17, 14. [Google Scholar] [CrossRef]
- Keipert, S.; Ost, M.; Johann, K.; Imber, F.; Jastroch, M.; van Schothorst, E.M.; Keijer, J.; Klaus, S. Skeletal muscle mitochondrial uncoupling drives endocrine cross-talk through the induction of FGF21 as a myokine. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E469–E482. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, D.H.; Moon, S.; Gu, B.; Mantik, K.E.K.; Kwak, H.B.; Ryu, J.K.; Kang, J.H. Ketogenic diet with aerobic exercise can induce fat browning: Potential roles of β-hydroxybutyrate. Front. Nutr. 2024, 11, 1443483. [Google Scholar] [CrossRef]
- Jo, D.; Son, Y.; Yoon, G.; Song, J.; Kim, O.Y. Role of Adiponectin and Brain Derived Neurotrophic Factor in Metabolic Regulation Involved in Adiposity and Body Fat Browning. J. Clin. Med. 2020, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Barra, N.G.; Palanivel, R.; Denou, E.; Chew, M.V.; Gillgrass, A.; Walker, T.D.; Kong, J.; Richards, C.D.; Jordana, M.; Collins, S.M.; et al. Interleukin-15 modulates adipose tissue by altering mitochondrial mass and activity. PLoS ONE 2014, 9, e114799. [Google Scholar] [CrossRef]
- Nielsen, A.R.; Hojman, P.; Erikstrup, C.; Fischer, C.P.; Plomgaard, P.; Mounier, R.; Mortensen, O.H.; Broholm, C.; Taudorf, S.; Krogh-Madsen, R.; et al. Association between interleukin-15 and obesity: Interleukin-15 as a potential regulator of fat mass. J. Clin. Endocrinol. Metab. 2008, 93, 4486–4493. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Feng, Y.; Liu, J.; Pan, R.; Lee, S.M.; Lin, L. Myricanol mitigates lipid accumulation in 3T3-L1 adipocytes and high fat diet-fed zebrafish via activating AMP-activated protein kinase. Food Chem. 2019, 270, 305–314. [Google Scholar] [CrossRef]
- Shen, S.; Liao, Q.; Zhang, T.; Pan, R.; Lin, L. Myricanol modulates skeletal muscle-adipose tissue crosstalk to alleviate high-fat diet-induced obesity and insulin resistance. Br. J. Pharmacol. 2019, 176, 3983–4001. [Google Scholar] [CrossRef] [PubMed]
- Castaño, C.; Mirasierra, M.; Vallejo, M.; Novials, A.; Párrizas, M. Delivery of muscle-derived exosomal miRNAs induced by HIIT improves insulin sensitivity through down-regulation of hepatic FoxO1 in mice. Proc. Natl. Acad. Sci. USA 2020, 117, 30335–30343. [Google Scholar] [CrossRef]
- Huang, M.; Cheng, S.; Li, Z.; Chen, J.; Wang, C.; Li, J.; Zheng, H. Preconditioning Exercise Inhibits Neuron Ferroptosis and Ameliorates Brain Ischemia Damage by Skeletal Muscle-Derived Exosomes via Regulating miR-484/ACSL4 Axis. Antioxid. Redox Signal. 2024, 41, 769–792. [Google Scholar] [CrossRef]
- Kang, X.; Yang, M.Y.; Shi, Y.X.; Xie, M.M.; Zhu, M.; Zheng, X.L.; Zhang, C.K.; Ge, Z.L.; Bian, X.T.; Lv, J.T.; et al. Interleukin-15 facilitates muscle regeneration through modulation of fibro/adipogenic progenitors. Cell Commun. Signal. 2018, 16, 42. [Google Scholar] [CrossRef]
- Luo, J.; Pu, Q.; Wu, X. Recent Advances of Exosomes Derived from Skeletal Muscle and Crosstalk with Other Tissues. Int. J. Mol. Sci. 2024, 25, 10877. [Google Scholar] [CrossRef]
- Feldman, B.J.; Streeper, R.S.; Farese, R.V., Jr.; Yamamoto, K.R. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc. Natl. Acad. Sci. USA 2006, 103, 15675–15680. [Google Scholar] [CrossRef]
- Li, W.; Wen, S.; Wu, J.; Zeng, B.; Chen, T.; Luo, J.; Shu, G.; Wang, S.B.; Zhang, Y.; Xi, Q. Comparative Analysis of MicroRNA Expression Profiles Between Skeletal Muscle- and Adipose-Derived Exosomes in Pig. Front. Genet. 2021, 12, 631230. [Google Scholar] [CrossRef]
- Qin, M.; Xing, L.; Wu, J.; Wen, S.; Luo, J.; Chen, T.; Fan, Y.; Zhu, J.; Yang, L.; Liu, J.; et al. Skeletal Muscle-Derived Exosomal miR-146a-5p Inhibits Adipogenesis by Mediating Muscle-Fat Axis and Targeting GDF5-PPARγ Signaling. Int. J. Mol. Sci. 2023, 24, 4561. [Google Scholar] [CrossRef]
- Yu, Y.; Su, Y.; Wang, G.; Lan, M.; Liu, J.; Garcia Martin, R.; Brandao, B.B.; Lino, M.; Li, L.; Liu, C.; et al. Reciprocal communication between FAPs and muscle cells via distinct extracellular vesicle miRNAs in muscle regeneration. Proc. Natl. Acad. Sci. USA 2024, 121, e2316544121. [Google Scholar] [CrossRef]
- Trajkovski, M.; Ahmed, K.; Esau, C.C.; Stoffel, M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat. Cell Biol. 2012, 14, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Shintani-Ishida, K.; Tsurumi, R.; Ikegaya, H. Decrease in the expression of muscle-specific miRNAs, miR-133a and miR-1, in myoblasts with replicative senescence. PLoS ONE 2023, 18, e0280527. [Google Scholar] [CrossRef]
- Liu, W.; Bi, P.; Shan, T.; Yang, X.; Yin, H.; Wang, Y.X.; Liu, N.; Rudnicki, M.A.; Kuang, S. miR-133a regulates adipocyte browning in vivo. PLoS Genet. 2013, 9, e1003626. [Google Scholar] [CrossRef]
- Vienberg, S.; Geiger, J.; Madsen, S.; Dalgaard, L.T. MicroRNAs in metabolism. Acta Physiol. 2017, 219, 346–361. [Google Scholar] [CrossRef]
- Díez-Sainz, E.; Milagro, F.I.; Aranaz, P.; Riezu-Boj, J.I.; Batrow, P.L.; Contu, L.; Gautier, N.; Amri, E.Z.; Mothe-Satney, I.; Lorente-Cebrián, S. Human miR-1 Stimulates Metabolic and Thermogenic-Related Genes in Adipocytes. Int. J. Mol. Sci. 2024, 26, 276. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).