Highlights
- This study provides the first comprehensive analysis of long-term, region-specific changes in the entire glutamatergic system (iGluRs, mGluRs, EAATs) after neonatal febrile seizures.
- The most severe and persistent molecular alterations occur in the ventral hippocampus and medial prefrontal cortex, key nodes for emotional regulation and cognition.
- The behavioral outcome is characterized by a hyperanxious phenotype with locomotor and habituation deficits, but with working memory and sociability remaining unaffected.
- The findings reveal a precise neurobiological mechanism that could underlie the increased risk of anxiety-related disorders following early-life febrile seizures.
Abstract
Febrile seizures (FS) are a common childhood neurological event associated with an increased risk of long-term cognitive and emotional deficits, though the precise mechanisms remain elusive. Using a rat model, we investigated the long-term effects of FS induced on postnatal day 10, assessing outcomes in young adulthood (P45-55). We report region-specific neuronal loss in the hippocampus, more extensive in the ventral segment. Molecular analysis revealed a broad downregulation of genes encoding ionotropic and metabotropic glutamate receptors and excitatory amino acid transporters. These alterations were most severe and persistent in the ventral hippocampus and medial prefrontal cortex. Behaviorally, rats with neonatal FS exhibited a hyperanxious phenotype, characterized by reduced locomotor and exploratory activity and impaired habituation to a novel environment. In contrast, spatial working memory and social behavior remained intact. Our results provide the first comprehensive evidence that neonatal FS trigger long-term, region-specific disruptions of the glutamatergic system within hippocampal–prefrontal circuits. These findings identify vulnerable molecular targets and precise neurobiological mechanisms that may underlie the heightened risk of anxiety-related disorders following early-life FS, suggesting new avenues for therapeutic intervention.
1. Introduction
Febrile seizures (FS) are one of the most prevalent neurological disorders in children aged six months to five years, with a peak incidence in the second year of life [1]. These seizures typically occur at body temperatures above 38 °C in the absence of other triggering factors like CNS infection or trauma [1]. Although most FS episodes are benign, complex forms—such as those that are prolonged (>15 min), recurrent within 24 h, or progress to febrile status epilepticus—are associated with an elevated risk of cognitive impairments and mental disorders later in life, including schizophrenia [2], attention deficit hyperactivity disorder (ADHD) [3,4], and autism spectrum disorders [5]. Despite the established link, the specific disorders that arise after FS and which may subsequently cause these disorders remain unclear. Experimental models of FS also replicate cognitive and psychoemotional deficits [6,7,8,9,10,11,12], although the precise molecular mechanisms behind these impairments remain poorly understood.
The early postnatal period represents a critical window for the development of limbic and cortical circuits that support cognitive and emotional functions [13]. We hypothesize that FS during this vulnerable period disrupts the developmental trajectory of these circuits, thereby contributing to long-term impairments.
Clinical and electroencephalographic (EEG) studies implicate the hippocampus in FS in children [14,15] and animal models [16]. This structure, vital for learning, memory, and emotion [17,18], is highly vulnerable to hyperexcitability and a key focus in post-seizure cognitive research. Critically, the hippocampus is not uniform; it exhibits a functional dorsoventral gradient [17]. The dorsal hippocampus in rats is primarily involved in spatial memory, while the ventral part regulates emotional behaviors like anxiety and fear [18,19]. This regional specificity means negative impacts can be locally confined [20], a nuance often overlooked.
However, a singular focus on the hippocampus is insufficient to understand the long-term consequences of FS. Higher-order functions are mediated by distributed networks that include the medial prefrontal cortex (mPFC) and temporal cortex. The mPFC is crucial for executive functions and emotion regulation [21,22,23], and the temporal cortex for semantic memory and sensory processing [24,25]. These areas are densely interconnected with the hippocampus [21,22,26], and their dysfunction can significantly contribute to the cognitive and emotional deficits following FS. Consequently, a comprehensive investigation of impairments across all these interconnected brain regions is necessary to fully understand the long-term consequences of FS.
The pathogenesis of FS and its associated neuropsychiatric sequelae primarily involves genetic predispositions, hyperthermia-mediated mechanisms, and neuroinflammatory pathways [27]. Genetic factors prominently contribute through mutations in ion channel genes, including sodium channel subunits (SCN1A, SCN1B), GABAA receptor subunits (GABRG2, GABRD), and hyperpolarization-activated channels (HCN1, HCN2). These defects disrupt ionic homeostasis and reduce inhibition, predisposing to seizures [27,28].
Fever-induced hyperthermia directly triggers FS by modulating temperature-sensitive ion channels (TRP, TREK-1, HCN), causing membrane depolarization and increased excitability [27,29].
Simultaneously, inflammatory responses are central to FS. Proinflammatory cytokines, particularly IL-1β, enhance NMDA receptor function, impair glutamate clearance, and reduce GABAergic inhibition, promoting hyperexcitability [30,31]. IL-6 and TNF-α further destabilize excitation–inhibition balance [28,32]. The critical role of inflammation is confirmed by elevated seizure thresholds when IL-1 signaling is blocked [30,31].
Crucially, key FS mechanisms—notably neuroinflammation and hyperthermia—converge on the glutamatergic system [20,33]. We propose that this convergence underlies the prolonged neuronal hyperexcitability and behavioral disturbances seen following FS [34,35,36]. The impact of these pathological processes is likely most severe during early postnatal development, a critical period for the maturation of glutamate networks [37]. We therefore conducted a comprehensive investigation of the persistent, FS-induced alterations within the glutamatergic system.
Excessive release of glutamate and subsequent overactivation of its receptors represent the primary mechanism of excitotoxicity during seizures [38,39,40]. This cascade triggers dysregulated calcium influx, oxidative stress, and ultimately, neuronal death. Ionotropic glutamate receptors (iGluRs), specifically N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, are key players in this process. Furthermore, metabotropic glutamate receptors (mGluRs), which modulate synaptic transmission and plasticity, and excitatory amino acid transporters (EAATs), responsible for glutamate reuptake and homeostasis, critically shape the neuronal response to hyperexcitation.
Studies on immature neuron cultures and in a clonazepam-induced seizure model in newborn animals have demonstrated that early-life hyperexcitability induces short- and long-term alterations in the expression of glutamate receptor subunits [41,42]. Such alterations can cause an enduring imbalance between excitation and inhibition, thereby establishing a molecular basis for long-term functional impairments.
In contrast to the models described above, the possibility of persistent changes in the glutamatergic system following febrile seizures (FS) remains insufficiently explored. While previous studies have reported changes in a limited number of genes within this system [36,43,44], a comprehensive analysis of the long-term molecular consequences of FS is lacking. This is particularly true for a systematic assessment of the entire spectrum of glutamate receptors and transporters.
Therefore, we aimed to provide a comprehensive characterization of the delayed effects of FS, integrating the analysis of glutamatergic system gene expression with cognitive and emotional outcomes in young adult animals (P45–55). In rodents, this age corresponds to human adolescence [45]—an age when the first clinical manifestations of mental disorders are often observed, occurring, among other things, against the backdrop of puberty [46,47].
To investigate long-term, region-specific changes following FS, we used a rat model of seizures induced at P10 [48]. In young adult animals (P45-55), we assessed: (1) selective neuronal loss in dorsal vs. ventral hippocampal subfields CA1 and CA3; (2) gene expression of glutamatergic targets (NMDA, AMPA, mGluR subunits, EAATs) across limbic and cortical regions; and (3) a spectrum of behavioral outcomes including exploration, anxiety, and memory.
2. Materials and Methods
2.1. Animals
All experimental procedures utilized male Wistar rats obtained from the animal breeding facility of the Sechenov Institute of Evolutionary Physiology and Biochemistry, Russian Academy of Sciences (St. Petersburg, Russia). The animals were maintained in standard cages under a 12 h light/dark cycle with ad libitum access to food and water. For the study, both control and experimental cohorts were assembled from individuals of different litters to minimize litter-specific effects. All animal handling and experimental protocols were performed in strict adherence to the institutional guidelines of the Sechenov Institute, which are fully aligned with the European Union Directive 2010/63/EU on the protection of animals used for scientific purposes.
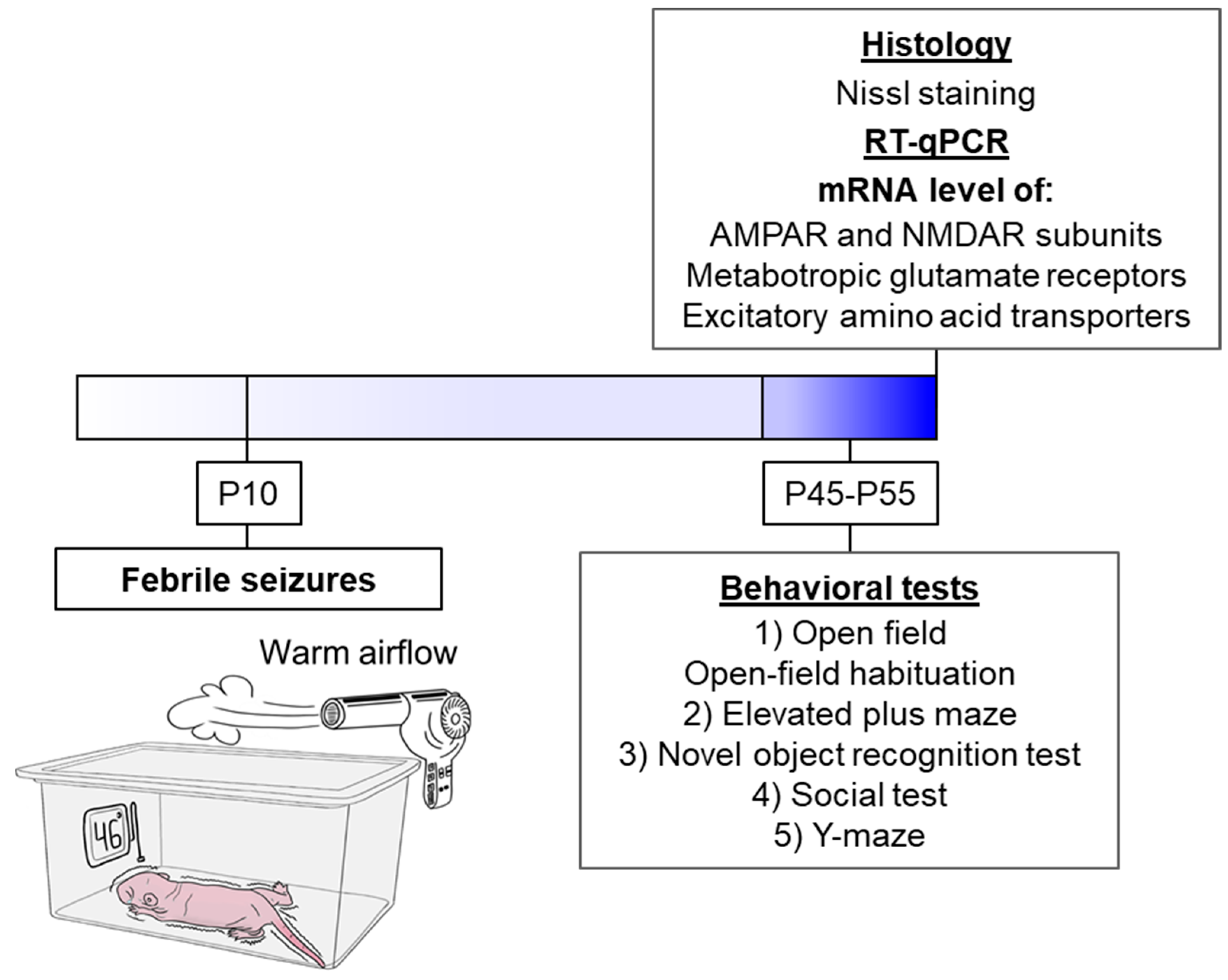
The schematic representation delineates the sequence of experimental procedures performed during the study (Figure 1).
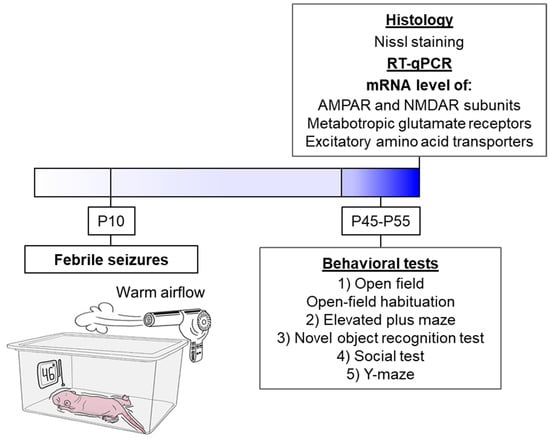
Figure 1.
The experimental design. The schematic diagram illustrates the experimental model of FS. The animal is placed in a chamber over which a flow of warm air is created so that a temperature of 46 °C at the bottom of the chamber. Under such conditions, the animal’s body temperature rises, and FS develops (N = 17). The littermates utilized as controls were removed from the nest for the same duration but were kept at room temperature (N = 23).
Sample size was determined a priori using GraphPad StatMate version 2.00 (GraphPad Software, San Diego, CA, USA) (α = 0.05, power = 0.8) based on effect sizes from prior work. Some values were removed after the experiment if they were identified as outliers. The total number of animals used for the study is shown in Table 1. For the analysis of mRNA expression, eight animals were randomly selected from each group after behavioral testing. Histological analysis was performed on a separate sample of animals.

Table 1.
Total number of animals in the study.
2.2. Febrile Seizure Model
Febrile seizures were provoked in P10 rat pups following a well-characterized hyperthermia paradigm [16]. This developmental stage in rats corresponds to the first year of human life in terms of CNS maturation, and it is at this point that rodents exhibit a seizure threshold and stereotypical seizure progression analogous to human FS [48]. Briefly, pups were individually placed in a glass chamber and subjected to a stream of heated air for 30 min, which maintained an ambient temperature of 46 °C (Figure 1). Core body temperature was monitored rectally at baseline (32–33 °C), at seizure onset (~39 °C), and at two-minute intervals thereafter. Most animals reached a core temperature of 39 °C within 10 min, displaying facial automatisms and unilateral body flexion, followed by myoclonic jerks of the hindlimbs that evolved into generalized clonic convulsions. These behavioral seizures have been electrophysiologically correlated with rhythmic discharges in limbic structures [16]. To prevent hyperthermia exceeding 40 °C, pups were briefly transferred to a cooled surface until their temperature returned to approximately 37 °C, after which they were placed back into the chamber. Only pups exhibiting clonic convulsions for a minimum of 15 min were included in the experimental group. Control littermates were separated from the dam for an equal duration but maintained at room temperature.
2.3. Behavioral Testing
A battery of behavioral tests was administered starting at postnatal day 45 (P45). The testing order was identical for all animal cohorts: (1) Open Field with a 3-day habituation protocol, (2) Elevated Plus Maze, (3) Novel Object Recognition, (4) Social Interaction Test, and (5) Y-Maze. Given that these paradigms are considered to induce mild stress, they were performed consecutively, with one test per day.
2.3.1. Open Field Test
Locomotor and anxiety-related behaviors were assessed in a circular open field arena (100 cm diameter, 30 cm high walls) under dim illumination (8 lux). Each rat was introduced to the center of the arena and allowed to explore freely for 3 min while being video-recorded. The resulting videos were analyzed offline with dedicated software (“Tracking” v3.2 and “Field 4” v4.0, Institute of Experimental Medicine, St. Petersburg, Russia). The total distance moved served as an index of general locomotor activity, while the duration of time spent in the central zone (defined as an area with a radius of 50 cm) was used to quantify anxiety-like behavior [49].
2.3.2. Open-Field Habituation
To evaluate habituation to a familiar environment, animals were exposed to the open field arena once daily for three consecutive days at a consistent time. The total distance traveled during each 3 min session was recorded and served as the primary metric for quantifying the reduction in exploratory activity over time.
2.3.3. Elevated Plus Maze
Anxiety-like behavior was further assessed using an elevated plus maze, comprising a central square (10 × 10 cm) from which two open arms and two enclosed arms (each 50 × 10 cm) extended. The apparatus was raised 40 cm above the floor. The closed arms featured 30 cm-high walls with opaque covers, creating a dimly lit environment (1 lux), while the open arms were more brightly illuminated (40 lux). At the start of a trial, a rat was placed in the central area facing an open arm and given 5 min to explore the maze freely. Its behavior was video-tracked, and the time spent in the open versus closed arms was used as the key indicator of anxiety [50].
2.3.4. Novel Object Recognition Test
Exploratory behavior and short-term recognition memory were evaluated using the novel object recognition test, based on established protocols [51,52]. Testing occurred in a Plexiglas chamber (60 × 30 × 40 cm) under video surveillance. Two distinct pairs of toys, selected to preclude inherent preferences and prevent climbing, served as objects. To minimize neophobia and baseline stress, rats were habituated to the empty testing chamber for 24 h prior to the experiment.
The test protocol included two 5 min trials separated by a 1 h inter-trial interval. In Trial 1 (acquisition), two identical objects were positioned in the chamber, and the rat’s exploratory behavior (sniffing, touching, or oral contact) towards them was recorded. The total exploration time in this trial provided a measure of initial exploratory drive.
In Trial 2 (retrieval), one of the familiar objects was replaced with a novel one. The time spent investigating the familiar and the novel object was recorded separately. A discrimination index (DI) was calculated as (Tnovel − Tfamiliar)/(Tnovel + Tfamiliar) to quantify recognition memory, where positive values indicate a preference for novelty.
2.3.5. Social Interaction Test
Social behavior was examined using a resident-intruder paradigm [53]. Resident rats were first habituated to the test cage (60 × 30 × 40 cm) for 24 h to reduce environmental novelty. Subsequently, an unfamiliar, age-matched male Wistar rat (the intruder) was introduced into the home cage for a 5 min session. Social interaction was quantified by measuring the duration of active social behaviors (e.g., sniffing the body or anogenital area of the intruder) displayed by the resident. Aggressive encounters were also timed. Furthermore, the duration of self-grooming by the resident rat was recorded as a potential measure of anxiety in a social context.
2.3.6. Y-Shaped Maze Spontaneous Alternation Test
Spatial working memory was evaluated in a Y-maze consisting of three identical arms (50 × 10 cm each) with 30 cm-high opaque walls. Each rat was placed in the central area and allowed to freely explore the maze for 8 min. An arm entry was recorded when all four paws crossed the threshold. Spontaneous alternation behavior, defined as sequential entry into three different arms (e.g., 1-2-3, 2-3-1), was considered a correct choice. Working memory performance was quantified using the alternation coefficient (CA), calculated as follows: CA = Nright/(Ntotal − 2), where Nright represents the number of correct entries into a new arm, and Ntotal is the total number of arm inputs, a measure of total locomotor activity.
2.4. mRNA Expression Analysis
Brain isolation for subsequent RT-qPCR was performed on day 55 of rat life. Based on the rat brain atlas [54], the following brain regions were dissected using an OTF5000 cryostat microtome (Bright Instrument, Luton, UK): the dorsal and ventral hippocampus, medial prefrontal cortex, and temporal cortex. Total RNA was extracted from these regions using ExtractRNA reagent (Evrogen, Moscow, Russia) according to the manufacturer’s protocol and stored in 75% ethanol at −20 °C.
RNA samples were treated with RQ1 DNase (Promega, Madison, WI, USA) to eliminate genomic DNA contamination. RNA concentration and purity were verified spectrophotometrically. First-strand cDNA was synthesized from 1 μg of total RNA using a mix of oligo-dT and random 9-mer primers (DNA-Synthesis, Moscow, Russia) with M-MLV reverse transcriptase (Evrogen).
Quantitative PCR was performed in 6 μL reactions containing diluted cDNA, TaqM polymerase (Alkor Bio, St. Petersburg, Russia), 3.5 mM MgCl2, and gene-specific primers/probes (Table 2; DNA-Synthesis). Amplification was performed using a CFX384 Real-Time System (Bio-Rad, Hercules, CA, USA) with four technical replicates per sample. The multiplexes used for housekeeping genes have been described previously [55]. The following multiplexes for the genes of interest were used in this study: Grin1 + Grin2a, Grin2b + Gria1 + Gria2, Grm1 + Grm3 + Grm5, Grm2 + Grm7 + Grm8, Slc1a1 + Slc1a3, except for Grm4 and Slc1a2, which were run in singleplex.
The relative expression of the genes was evaluated using the 2−ΔΔCt method [56]. The most stably expressed genes for each structure were selected using the RefFinder online tool (https://www.ciidirsinaloa.com.mx/RefFinder-master/ (accessed on 16 June 2025)). Normalization was performed using the following genes: Rpl13a, Pgk1, Ywhaz for dorsal hippocampus; Ppia, Gapdh for ventral hippocampus; Actb, Gapdh for temporal cortex; Pgk1, Ywhaz for medial prefrontal cortex. For the selection of reference genes, we relied on our previous findings from the same model [57]. The detailed results and stability rankings from the RefFinder analysis are provided in Table A1, Table A2, Table A3 and Table A4.

Table 2.
Primer and probe sequences for RT-qPCR.
Table 2.
Primer and probe sequences for RT-qPCR.
| Gene Symbol RefSeq Accession Number | Forward Primer, Reverse Primer, and Probe Sequences (5′→3′) | Final Concentrations, nM | References |
|---|---|---|---|
| Actb NM_031144 | TGTCACCAACTGGGACGATA GGGGTGTTGAAGGTCTCAAA FAM-CGTGTGGCCCCTGAGGAGCAC-BHQ1 | 200 200 | [58] (primers) [55] (probe) |
| Gapdh NM_017008 | TGCACCACCAACTGCTTAG GGATGCAGGGATGATGTTC R6G-ATCACGCCACAGCTTTCCAGAGGG-BHQ2 | 200 100 | [59] |
| B2m NM_012512 | TGCCATTCAGAAAACTCCCC GAGGAAGTTGGGCTTCCCATT ROX-ATTCAAGTGTACTCTCGCCATCCACCG-BHQ1 | 200 100 | [60] |
| Rpl13a NM_173340 | GGATCCCTCCACCCTATGACA CTGGTACTTCCACCCGACCTC FAM-CTGCCCTCAAGGTTGTGCGGCT-BHQ1 | 200 100 | [61] (primers) [55] (probe) |
| Sdha NM_130428 | AGACGTTTGACAGGGGAATG TCATCAATCCGCACCTTGTA R6G-ACCTGGTGGAGACGCTGGAGCT-BHQ2 | 200 100 | [62] (primers) [55] (probe) |
| Ppia NM_017101 | AGGATTCATGTGCCAGGGTG CTCAGTCTTGGCAGTGCAGA ROX-CACGCCATAATGGCACTGGTGGCA-BHQ1 | 200 100 | [63] |
| Hprt1 NM_012583 | TCCTCAGACCGCTTTTCCCGC TCATCATCACTAATCACGACGCTGG FAM-CCGACCGGTTCTGTCATGTCGACCCT-BHQ1 | 200 100 | [64] (primers) [55] (probe) |
| Pgk1 NM_053291 | ATGCAAAGACTGGCCAAGCTAC AGCCACAGCCTCAGCATATTTC R6G-TGCTGGCTGGATGGGCTTGGA-BHQ2 | 200 100 | [65] (primers) [55] (probe) |
| Ywhaz NM_013011 | GATGAAGCCATTGCTGAACTTG GTCTCCTTGGGTATCCGATGTC ROX-TGAAGAGTCGTACAAAGACAGCACGC-BHQ1 | 200 100 | [65] (primers) [55] (probe) |
| Grm1 NM_001114330.1 | GGGAATGCCAAGAAGAGGCAG CTGCAGTGTGGGGGTTTTCAA FAM-GCCCAGCAGCCAGTGTCCGTCGGC-BHQ1 | 400 200 | [66] |
| Grm2 NM_001105711.1 | TCCAGTGATTATCGGGTGCAG AACTTGGGTGCAAAGAGGCA FAM-TGCGTGTCCGTCAGCCTCAGTGGCT-BHQ1 | 200 100 | [66] |
| Grm3 NM_001105712.1 | CAGGAGTTGACGGTGCGAG GCCTGTCCTTCAGATAAGGGAG ROX-TCGGTGACGGGCTCTTTCAGCCCAA-BHQ2 | 200 200 | [66] |
| Grm4 NM_022666.1 | GGCAGTGCGAGCAGCTAAGG CCGGTCACTCCTACCAACCG FAM-CTCCCTGAGCTCCCCCGGAGCAGC-BHQ1 | 200 150 | [66] |
| Grm5 NM_017012.1 | ATGCATGTAGGAGACGGCAA TTTCCGTTGGAGCTTAGGGTTT HEX-CGTCCGCTGCCAGCAGATCCAGCA-BHQ2 | 400 200 | [67] (primers) [66] (probe) |
| Grm7 NM_031040.1 | CCAGACAACAAACACAACCAACC GCGTTCCCTTCTGTGTCTTCTTC HEX-TGCAGTGGGGCAAAGGAGTCCGAG-BHQ2 | 200 100 | [68] (primers) [66] (probe) |
| Grm8 NM_022202.1 | TCATCGGGCACTGGACAAAT CACGGTTTTCTTCCTCTCCCC ROX-TGTCTGCAGCCTGCCGTGCAAGCCC-BHQ2 | 300 100 | [66] |
| Grin1 NM_017010 | GTTCTTCCGCTCAGGCTTTG AGGGAAACGTTCTGCTTCCA FAM-CGGCATGCGCAAGGACAGCC-BHQ1 | 200 100 | [69] |
| Grin2a NM_012573 | GCTACACACCCTGCACCAATT CACCTGGTAACCTTCCTCAGTGA FAM-TGGTCAATGTGACTTGGGATGGCAA-BHQ1 | 200 100 | [70] |
| Grin2b NM_012574 | CCCAACATGCTCTCTCCCTTAA CAGCTAGTCGGCTCTCTTGGTT FAM-GACGCCAAACCTCTAGGCGGACAG-BHQ1 | 200 100 | [70] |
| Gria1 NM_031608 | TCAGAACGCCTCAACGCC TGTAGTGGTACCCGATGCCA ROX-TCCTGGGCCAGATCGTGAAGCTAGAAAA-BHQ1 | 200 100 | [71] |
| Gria2 NM_017261 | CAGTGCATTTCGGGTAGGGA TGCGAAACTGTTGGCTACCT FAM-TCGGAGTTCAGACTGACACCCCA-BHQ1 | 200 100 | [71] |
| Slc1a1 NM_013032.3 | CCTGCATCCCTCATCCCAC CTCCTACCACGATGCCCAGTA HEX-CCGCCGCGCTCCCCGATTCC-BHQ2 | 200 100 | [72] |
| Slc1a2 NM_001035233.1 | CCAGTGCTGGAACTTTGCCT TAAAGGGCTGTACCATCCAT FAM-AGCGTGTGACCAGATTCGTCCTCCCA-BHQ1 | 200 150 | [73] (primers) [74] (probe) |
| Slc1a3 NM_019225.2 | GCGCTGTCATTGTGGGTACA CAGAAGCTCCCCAGGAAAGG Cy5-CCTTGGATTTGCCCTCCGACCGT-BHQ3 | 200 100 | [75] |
2.5. Histology
2.5.1. Preparation of Brain Tissue for Histological Examination
For histological examination, P55 rats were deeply anesthetized and transcardially perfused with ice-cold PBS followed by 4% paraformaldehyde. Brains were post-fixed in 4% PFA for 48 h at 4 °C, cryoprotected in 30% sucrose, and rapidly frozen in chilled isopentane. Serial 20-μm coronal sections encompassing the dorsal and ventral hippocampus were cut on a cryostat. To control for potential lateralization, one hemisphere was randomly chosen per animal. Four sections from each hippocampal region, spaced 100 μm apart, were mounted on adhesive slides and stored at −20 °C until staining.
2.5.2. Staining of Sections Using the Nissl Method
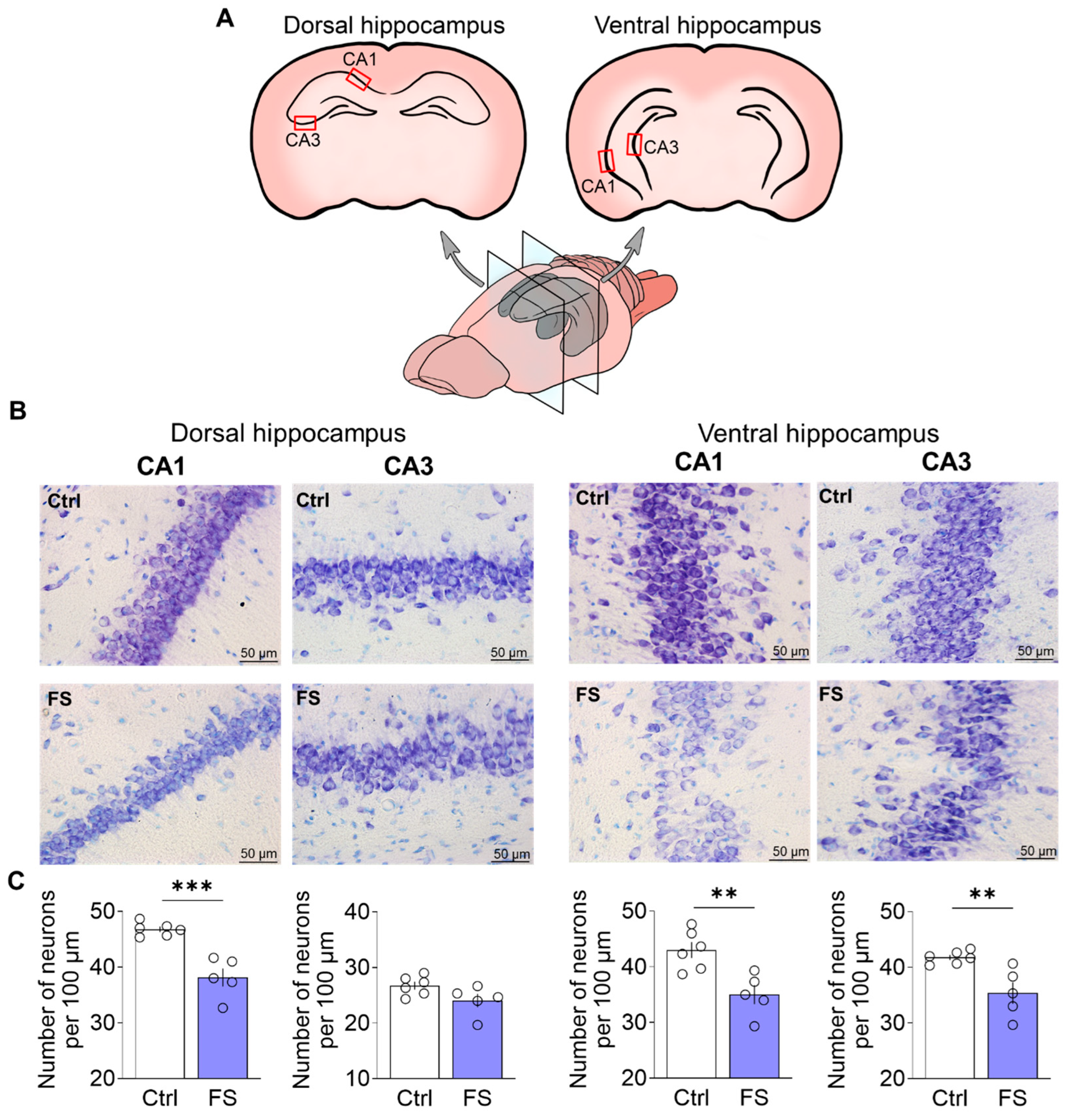
Nissl staining was performed as previously described in detail [76]. Images using a ×40 lens were obtained using a Leica AF7000 light microscope (Leica Microsystems, Wetlar, Germany). Quantitative analysis was performed visually in 100 µm of cell layer length in the CA1 and CA3 regions of the dorsal and ventral hippocampus in photographs using ImageJ 1.53k (U.S. National Institutes of Health, Bethesda, MD, USA). Cell counting was conducted by an experimenter blinded to the experimental groups. Sampling areas were consistently identified using standardized anatomical landmarks as illustrated in Figure 2A.
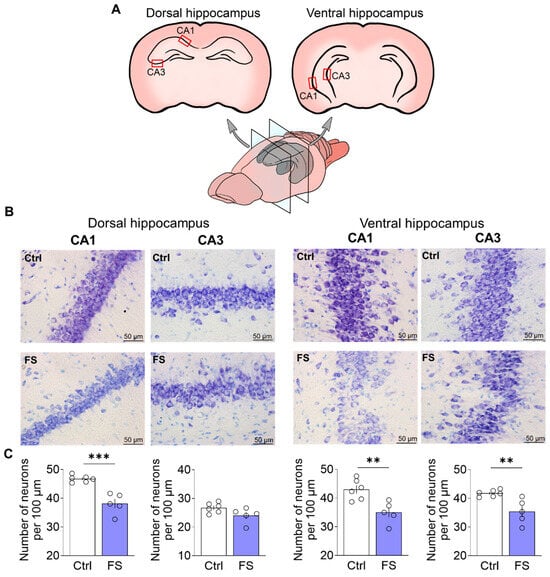
Figure 2.
(A) Schematic representation of dorsal and ventral hippocampal sections. The regions of the brain in which neurons were quantified are indicated. (B) CA1 and CA3 areas of the dorsal and ventral hippocampus of animals in the control (N = 6) and experimental groups (N = 5). (C) Diagrams illustrating the number of neurons per 100 µm of cell layer length in the CA1 and CA3 areas of the dorsal hippocampus and in the CA1 and CA3 areas of the ventral hippocampus. The dots represent the individual values for each rat. Student’s test: **—p < 0.01, ***—p < 0.001.
2.6. Statistical Analysis
Statistical analysis was conducted using the GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA). Potential outliers were identified with Dixon’s Q-test, and data distribution was assessed for normality using the Kolmogorov–Smirnov test. Based on distribution characteristics, we applied either parametric tests (Student’s t-test, mixed ANOVA with Sidak’s post hoc comparison) or non-parametric alternatives (Mann–Whitney U test). Statistical significance was defined as p < 0.05.
For qPCR data, results are displayed as box plots showing individual data points, quartiles, and median values. Multiple comparison correction was not applied because each gene was analyzed as an independent hypothesis. Behavioral data are expressed as means ± SEM for normally distributed parameters or medians with interquartile ranges for non-normally distributed measures.
Effect sizes were calculated as Cohen’s d for t-tests and η2 for ANOVA, with conventional thresholds for interpretation: 0.2 (small), 0.5 (medium), 0.8 (large), 1.5 (very large), and 2.5 (huge); for η2: 0.01 (small), 0.06 (medium), and 0.14 (large).
3. Results
3.1. Febrile Seizures Provoke Neuronal Loss in the Rat Hippocampus
To determine whether FS led to neuronal loss, we quantified neurons in the hippocampus of adult rats (P55). Given the functional differentiation along the dorsoventral axis [17], we separately analyzed neuronal counts in the CA1 and CA3 subfields of both the dorsal and ventral hippocampus. For this purpose, we used Nissl staining followed by quantitative analysis (Figure 2).
In the dorsal hippocampus, a significant difference in the number of neurons between control animals and animals after FS was observed in the CA1 region (CA1: control: 46.8 ± 0.5 neurons per 100 μm, 95% CI [45.5, 48.0], N = 6 rats; FS: 38.1 ± 1.6, 95% CI [33.7, 42.6], N = 5, t = 5.6, p < 0.001, d = 3.39). However, there were no differences between the groups in the CA3 region (CA3: control: 26.7 ± 0.7, 95% CI [24.9, 28.5], FS: 24.1 ± 1.2, 95% CI [20.8, 27.4], t = 2.01, p = 0.08).
In contrast to the dorsal hippocampus, FS induced significant neuronal loss in both the CA1 and CA3 regions of the ventral hippocampus (CA1: control: 43.0 ± 1.4, 95% CI [39.3, 46.7], FS: 35.0 ± 1.7, 95% CI [30.3, 39.7], t = 3.64, p = 0.005, d = 2.20; CA3: control: 41.8 ± 0.5, 95% CI [40.6, 43.0], FS: 35.4 ± 1.9, 95% CI [30.0, 40.8], t = 3.5, p = 0.007, d = 2.12).
3.2. Changes in Glutamate Receptors and Excitatory Amino Acid Transporters Gene Expression in Rat Brain After Febrile Seizures
We examined gene expression of NMDA and AMPA receptor subunits, mGluRs, as well as EAATs in the dorsal and ventral hippocampus, temporal and medial prefrontal cortex of rats that had undergone FS. The analysis was performed at P55.
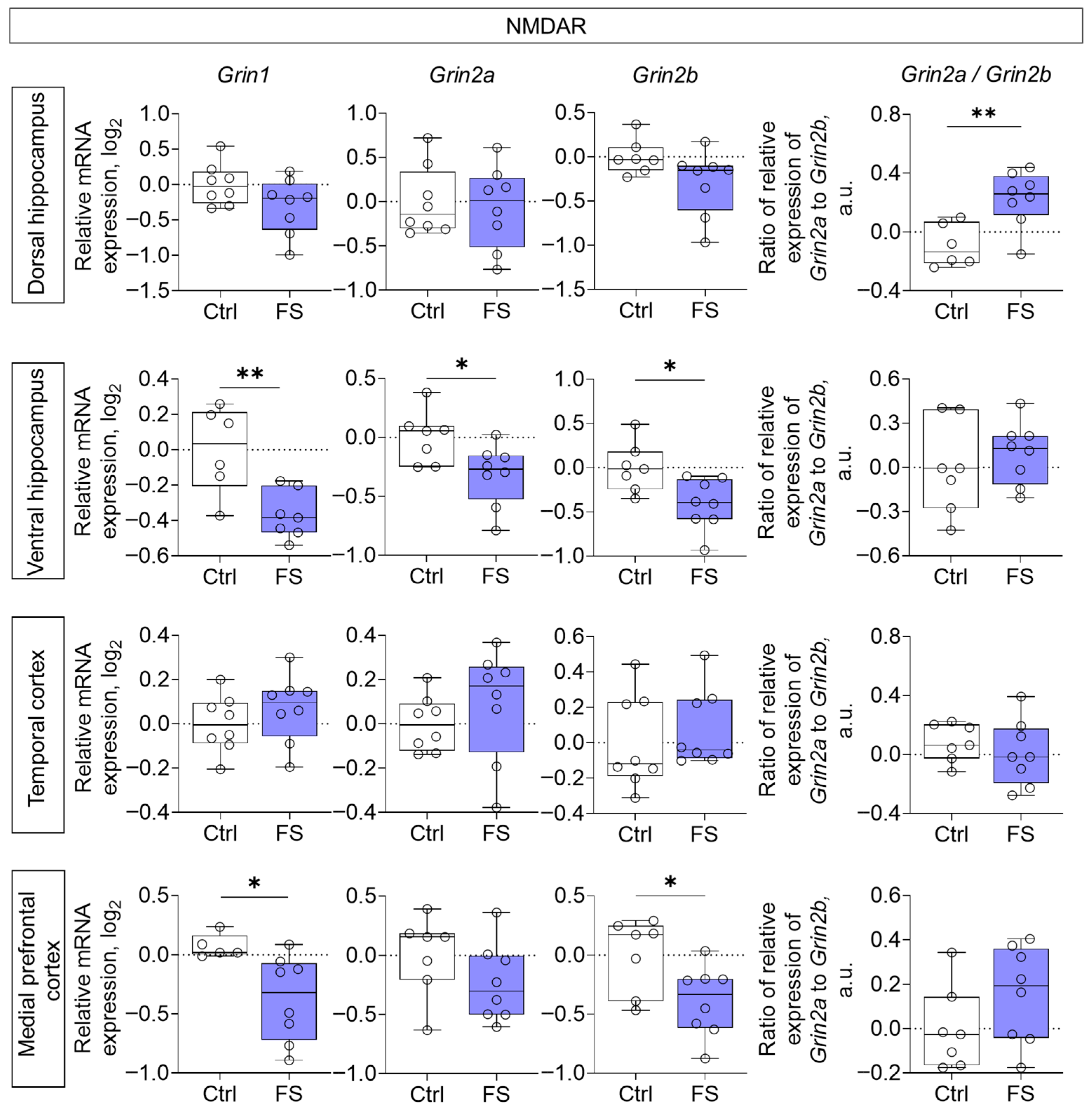
Decreased expression of all NMDA receptor subunits studied (Grin1, Grin2a, Grin2b) was noted in the ventral region of the hippocampus (Grin1: t = 3.4, p = 0.006, d = 1.91; Grin2a: t = 2.5, p = 0.026, d = 1.31; Grin2b: t = 2.8, p = 0.015, d = 1.46), while Grin1 and Grin2b gene expression was reduced in the medial prefrontal cortex (Grin1: t = 2.6, p = 0.023, d = 1.50; Grin2b: t = 2.5, p = 0.027, d = 1.23) of rats after FS (Figure 3). Additionally, we calculated the ratio of Grin2a/Grin2b mRNA production. This ratio reflects the balance between Grin2a and Grin2b mRNA, which affects the formation of receptor composition, and thus, the functional activity of the glutamatergic synapse and synaptic plasticity processes [77,78]. We found that it was increased in the dorsal hippocampus (t = 3.5, p = 0.005, d= 1.86).
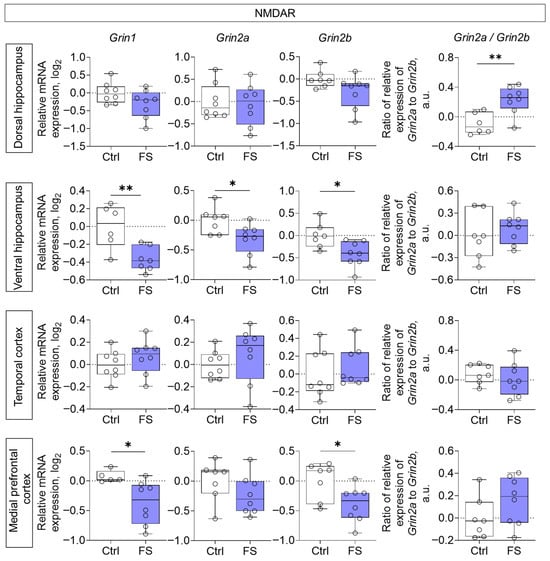
Figure 3.
Relative gene expression of NMDA receptor subunits (Grin1, Grin2a, Grin2b) and ratio of Grin2a/Grin2b mRNA production in the dorsal and ventral hippocampus, temporal and medial prefrontal cortex of rats in a model of FS at P55. Ctrl–animals weaned for the time of modeling seizures from the nest, but kept in normothermic conditions (N = 6–8), FS–animals after modeling FS at P10 (N = 7–8). Data are presented as individual values with minimum and maximum values (error whiskers), sample median (horizontal line), and first and third quartiles. Student’s test: *—p < 0.05, **—p < 0.01.
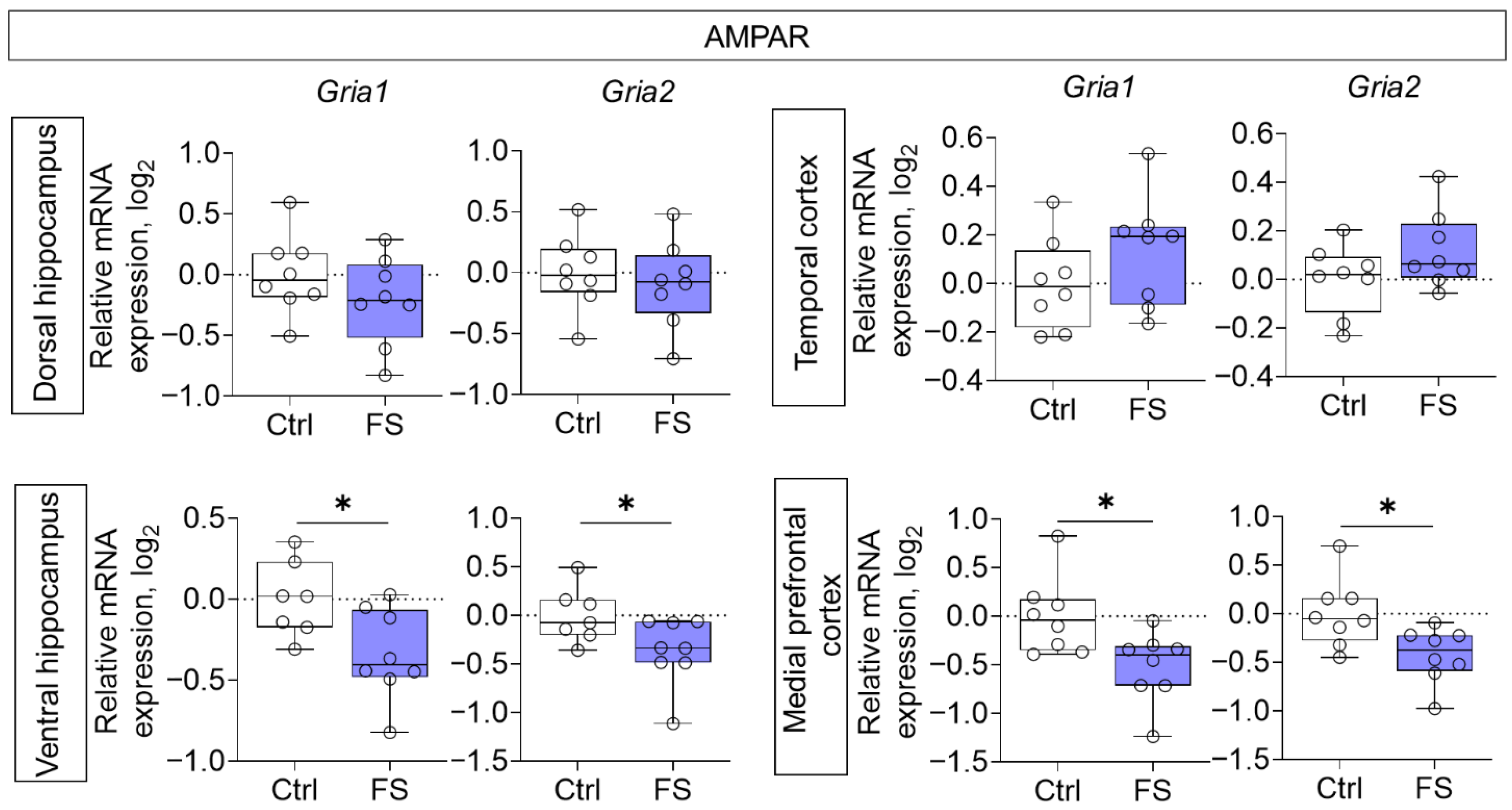
Decreased mRNA production of genes of both investigated AMPA receptor subunits (Gria1, Gria2) was detected in the ventral region of the hippocampus (Gria1: t = 2.5, p = 0.025, d = 1.31; Gria2: t = 2.2, p = 0.044, d = 1.15) and in the medial prefrontal cortex (Gria1: t = 2.7, p = 0.017, d = 1.35; Gria2: t = 2.7, p = 0.019, d = 1.33) (Figure 4).
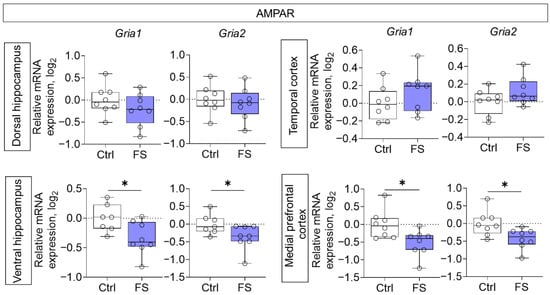
Figure 4.
Relative gene expression of AMPA receptor subunits (Gria1, Gria2) in the dorsal and ventral hippocampus, temporal and medial prefrontal cortex of rats in a model of FS at P55. Ctrl–animals weaned for the time of modeling seizures from the nest, but kept in normothermic conditions (N = 7–8), FS–animals after modeling FS at P10 (N = 7–8). Data are presented as individual values with minimum and maximum values (error whiskers), sample median (horizontal line), and first and third quartiles. Student’s test: *—p < 0.05.
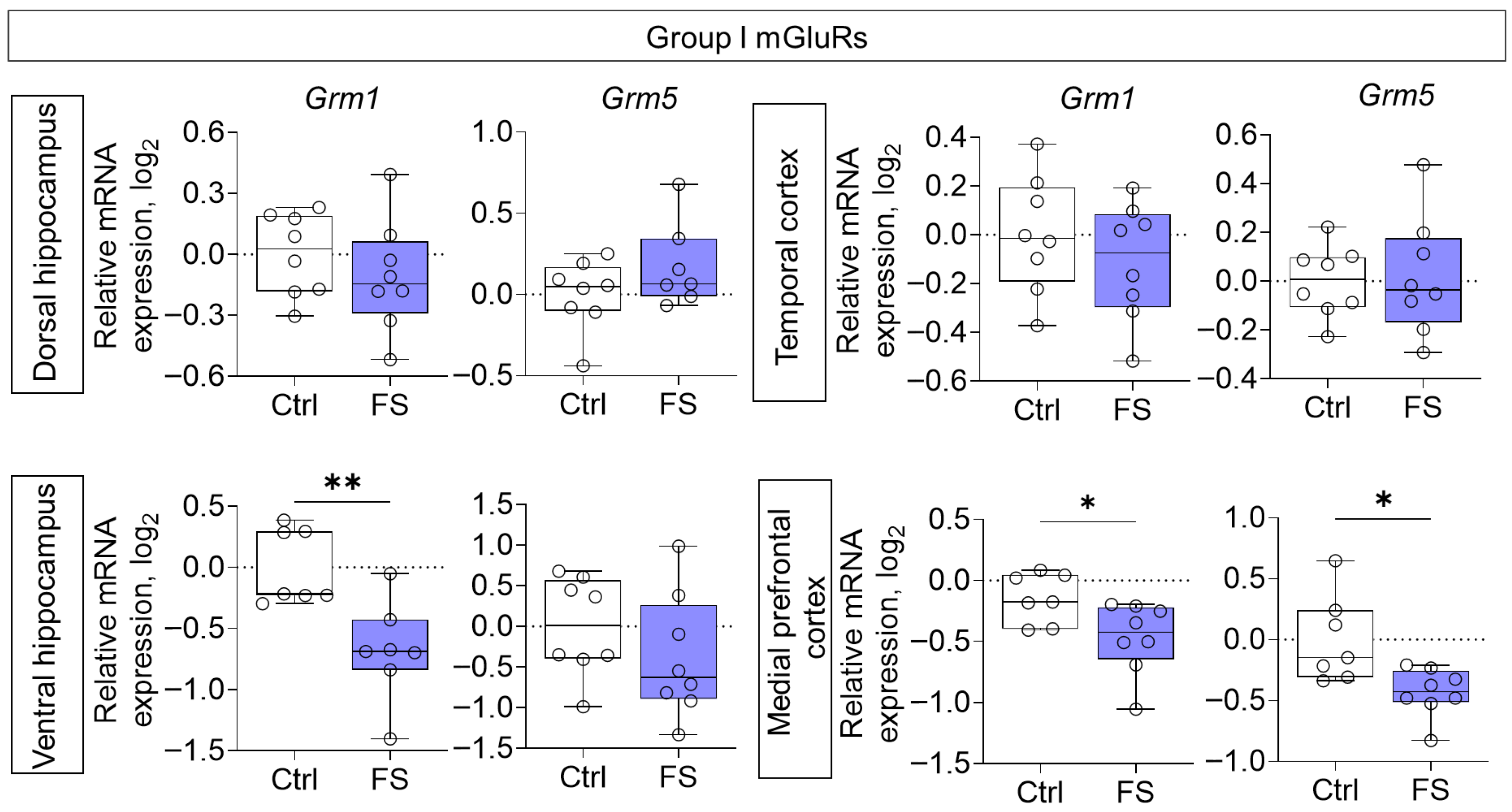
Reduced gene expression of members of group I mGluRs was also observed in the ventral hippocampal region (Grm1: Mann–Whitney test U7,7 = 4, p = 0.007) and medial prefrontal cortex (Grm1: t = 2.5, p = 0.029, d = 1.27; Grm5: t = 2.9, p = 0.012, d = 1.52) of rats after FS (Figure 5).
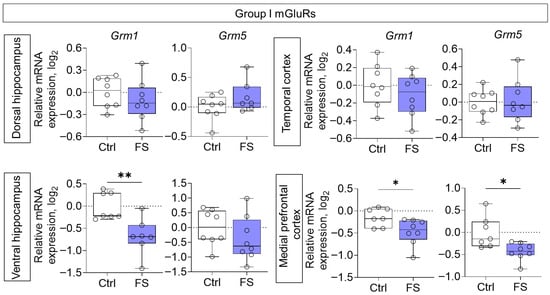
Figure 5.
Relative gene expression of the group I mGluRs (Grm1, Grm5) in the dorsal and ventral hippocampus, temporal and medial prefrontal cortex of rats in a model of FS at P55. Ctrl–animals weaned for the time of modeling seizures from the nest, but kept in normothermic conditions (N = 7–8), FS–animals after modeling FS at P10 (N = 7–8). Data are presented as individual values with minimum and maximum values (error whiskers), sample median (horizontal line), and first and third quartiles. Student’s test: *—p < 0.05; Mann–Whitney test: **—p < 0.01.
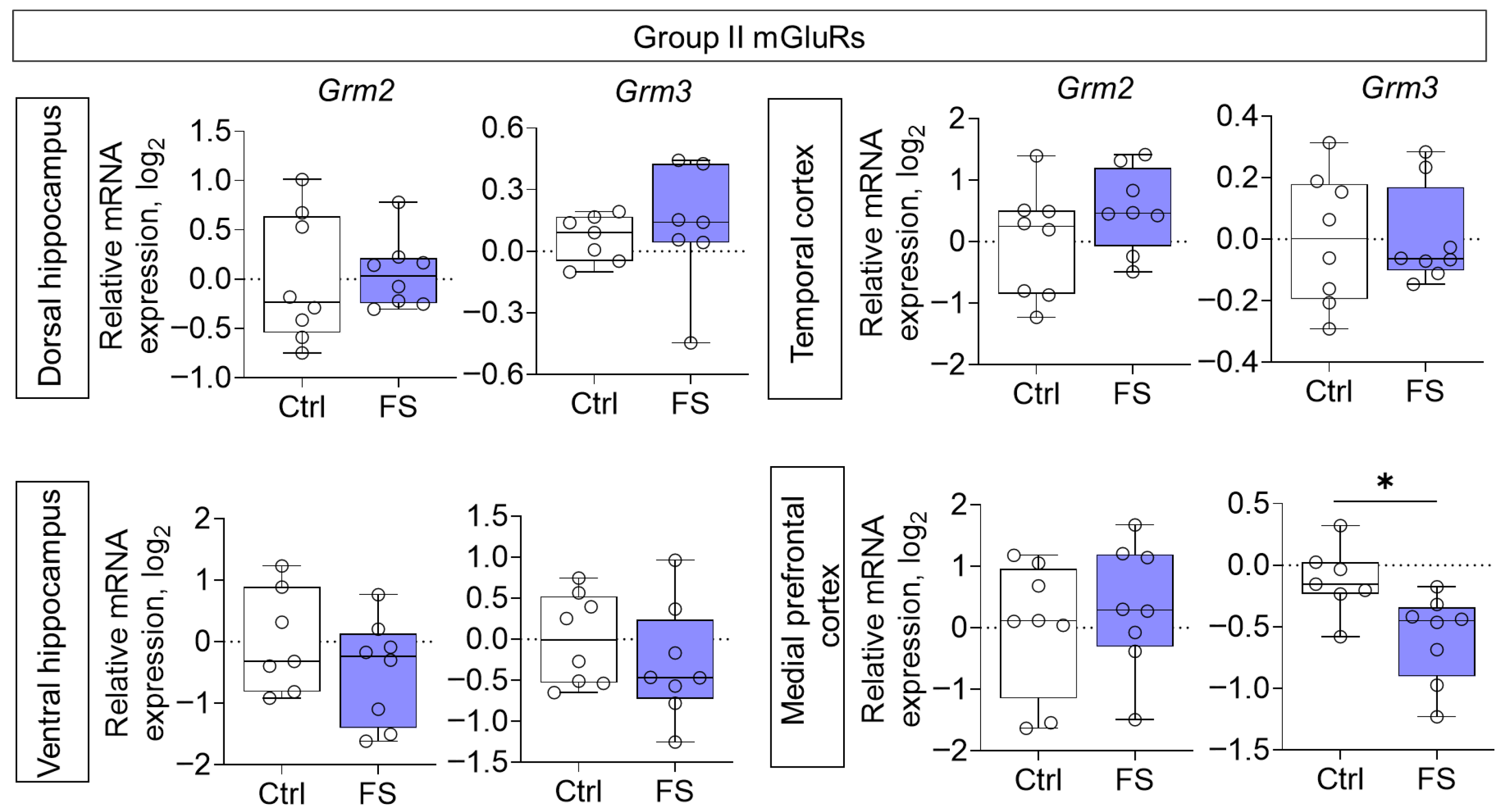
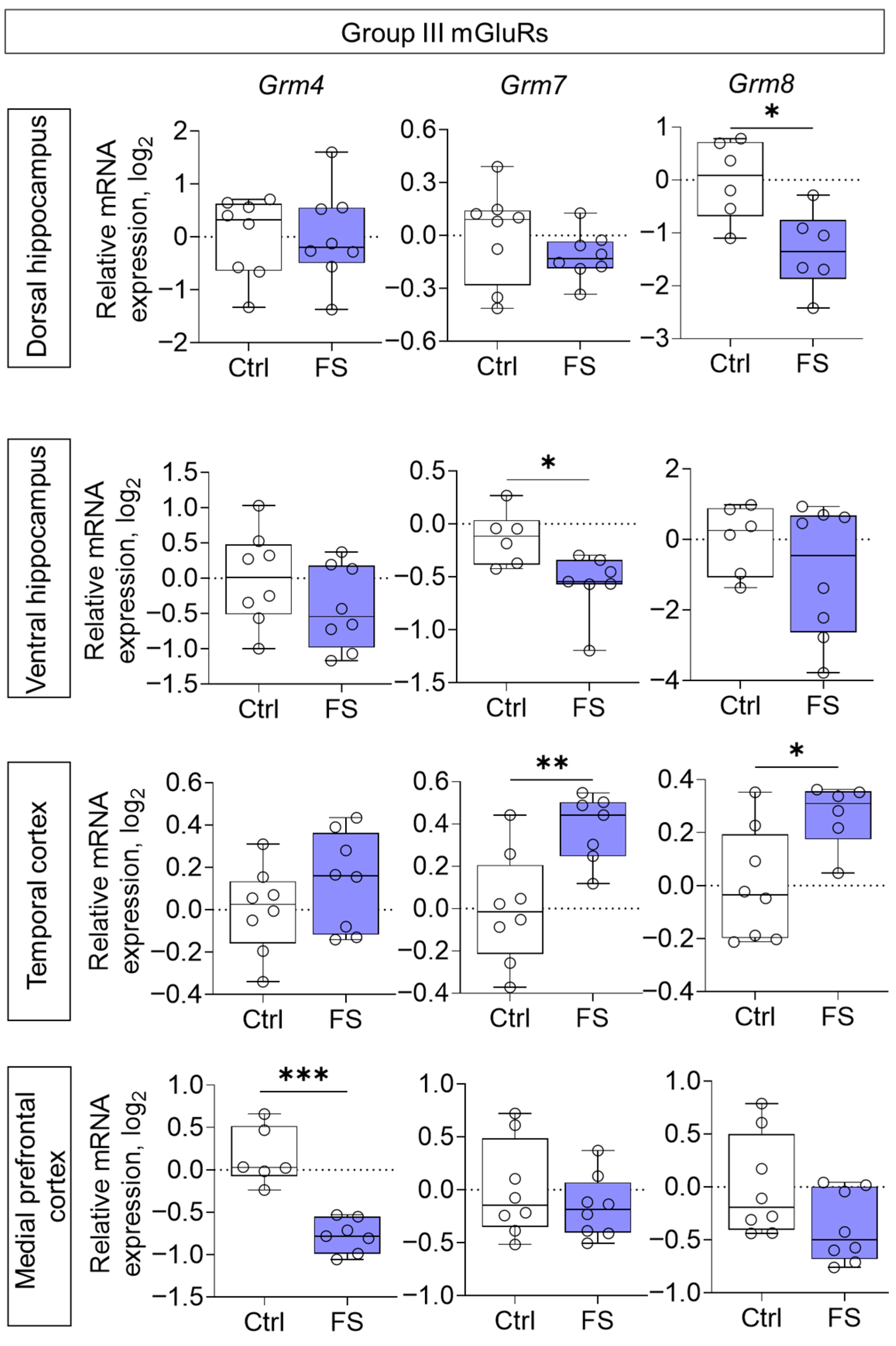
The changes in mRNA production of group II mGluRs were less pronounced: we found a decrease in Grm3 gene expression only in the medial prefrontal cortex (t = 2.8, p = 0.015, d = 1.45) (Figure 6). By contrast, the expression of group III metabotropic glutamate receptor genes was altered in all of the rat brains examined after they had suffered FS at an early age (Figure 7). In particular, the production of Grm4 mRNA was reduced in the medial prefrontal cortex (t = 6.2, p < 0.001, d = 3.44). Meanwhile, Grm7 gene expression changed in different directions: it decreased in the ventral hippocampus (Mann–Whitney test U6,7 = 4, p = 0.014) and increased in the temporal cortex (t = 3.3, p = 0.006, d = 1.71). Similar changes in the temporal cortex were also seen with Grm8 gene expression (t = 2.8, p = 0.017, d = 1.50). However, Grm8 mRNA production decreased in the dorsal region of the hippocampus (t = 3.1, p = 0.011, d = 1.80).
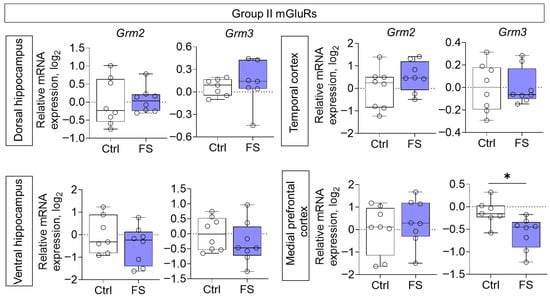
Figure 6.
Relative gene expression of the group II mGluRs (Grm2, Grm3) in the dorsal and ventral hippocampus, temporal and medial prefrontal cortex of rats in a model of FS at P55. Ctrl–animals weaned for the time of modeling seizures from the nest, but kept in normothermic conditions (N = 6–8), FS–animals after modeling FS at P10 (N = 7–8). Data are presented as individual values with minimum and maximum values (error whiskers), sample median (horizontal line), and first and third quartiles. Student’s test: *—p < 0.05.
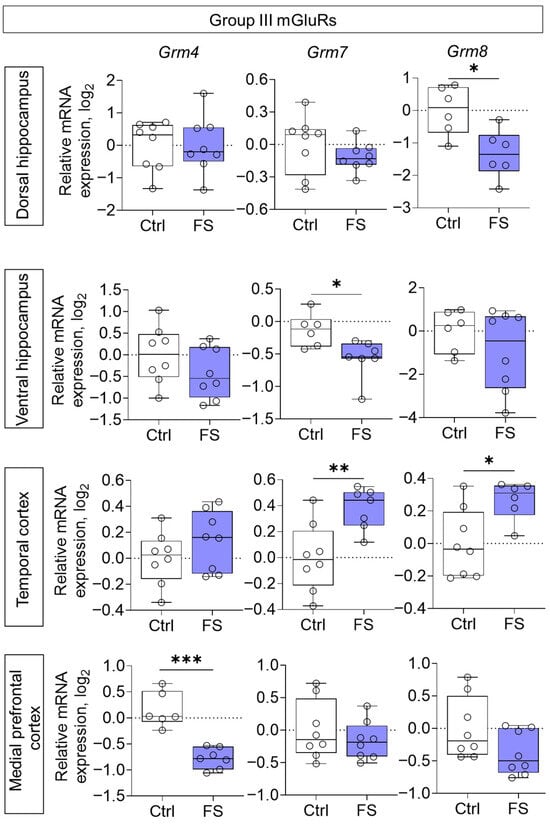
Figure 7.
Relative gene expression of the group III mGluRs (Grm4, Grm7, Grm8) in the dorsal and ventral hippocampus, temporal and medial prefrontal cortex of rats in a model of FS at P55. Ctrl–animals weaned for the time of modeling seizures from the nest, but kept in normothermic conditions (N = 6–8), FS–animals after modeling FS at P10 (N = 6–8). Data are presented as individual values with minimum and maximum values (error whiskers), sample median (horizontal line), and first and third quartiles. Mann–Whitney test: *—p < 0.05 (Grm7 gene expression in the ventral hippocampus); the Student’s test: *—p < 0.05, **—p < 0.01, ***—p < 0.001.
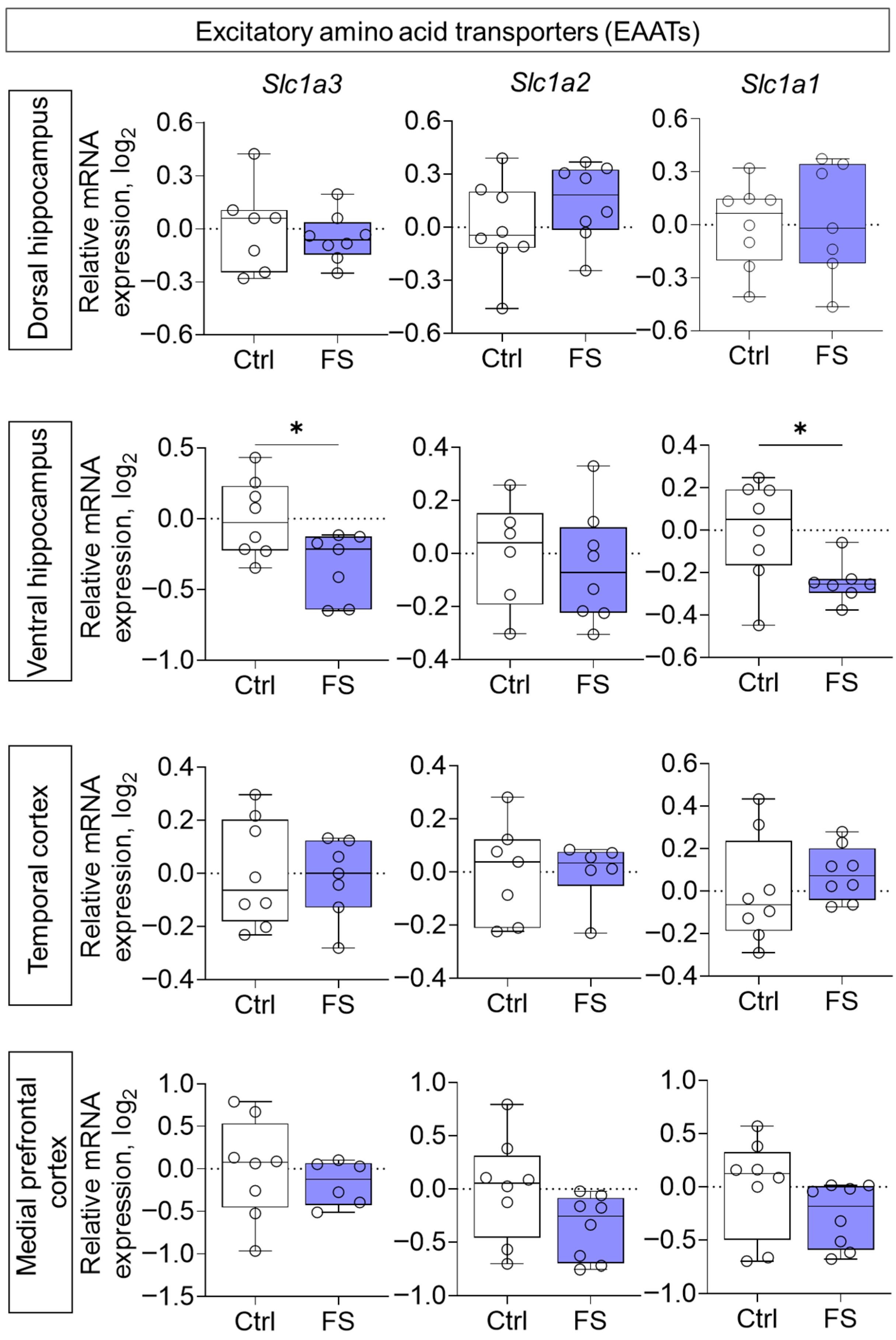
Furthermore, the expression of genes encoding EAATs was studied. A decline in expression was observed following FS, exclusively in the ventral hippocampus (Figure 8, Slc1a3 (encoding EAAT1): t = 2.5, p = 0.026, d = 1.31; Slc1a1 (encoding EAAT3): t = 2.6, p = 0.024, d = 1.33).
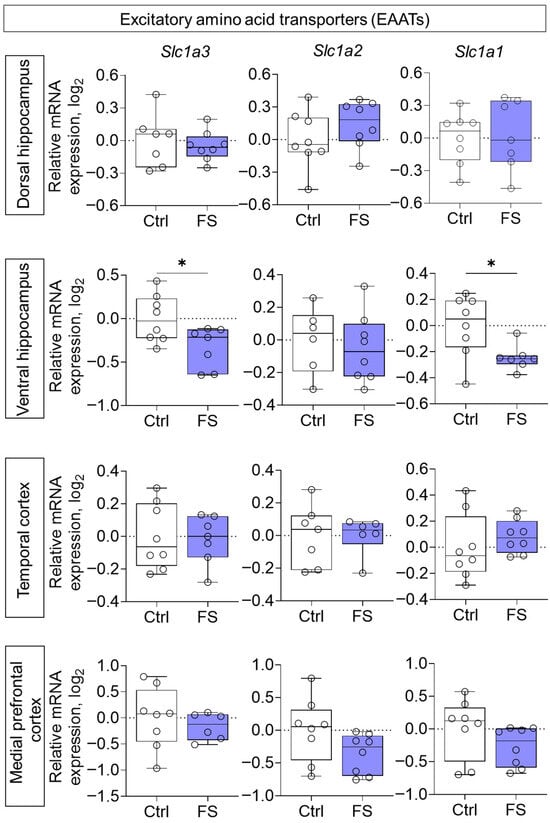
Figure 8.
Relative gene expression of the excitatory amino acid transporters (Slc1a3, Slc1a2, Slc1a1) in the dorsal and ventral hippocampus, temporal and medial prefrontal cortex of rats in a model of FS at P55. Ctrl–animals weaned for the time of modeling seizures from the nest, but kept in normothermic conditions (N = 7–8), FS–animals after modeling FS at P10 (N = 6–8). Data are presented as individual values with minimum and maximum values (error whiskers), sample median (horizontal line), and first and third quartiles. Student’s test: *—p < 0.05.
Thus, long-term changes in the expression iGluRs, mGluRs and EAATs are observed in the rat brain following neonatal FS. The most pronounced alterations were observed in the medial prefrontal cortex and ventral hippocampus. Such region-specific changes in the expression of genes encoding components of the glutamatergic system may underlie a variety of behavioral disorders. So, in this study, we evaluated the behavior of adult rats that had undergone FS.
3.3. Behavioral Alterations
3.3.1. Decline in Locomotor Activity and Exploratory Behavior
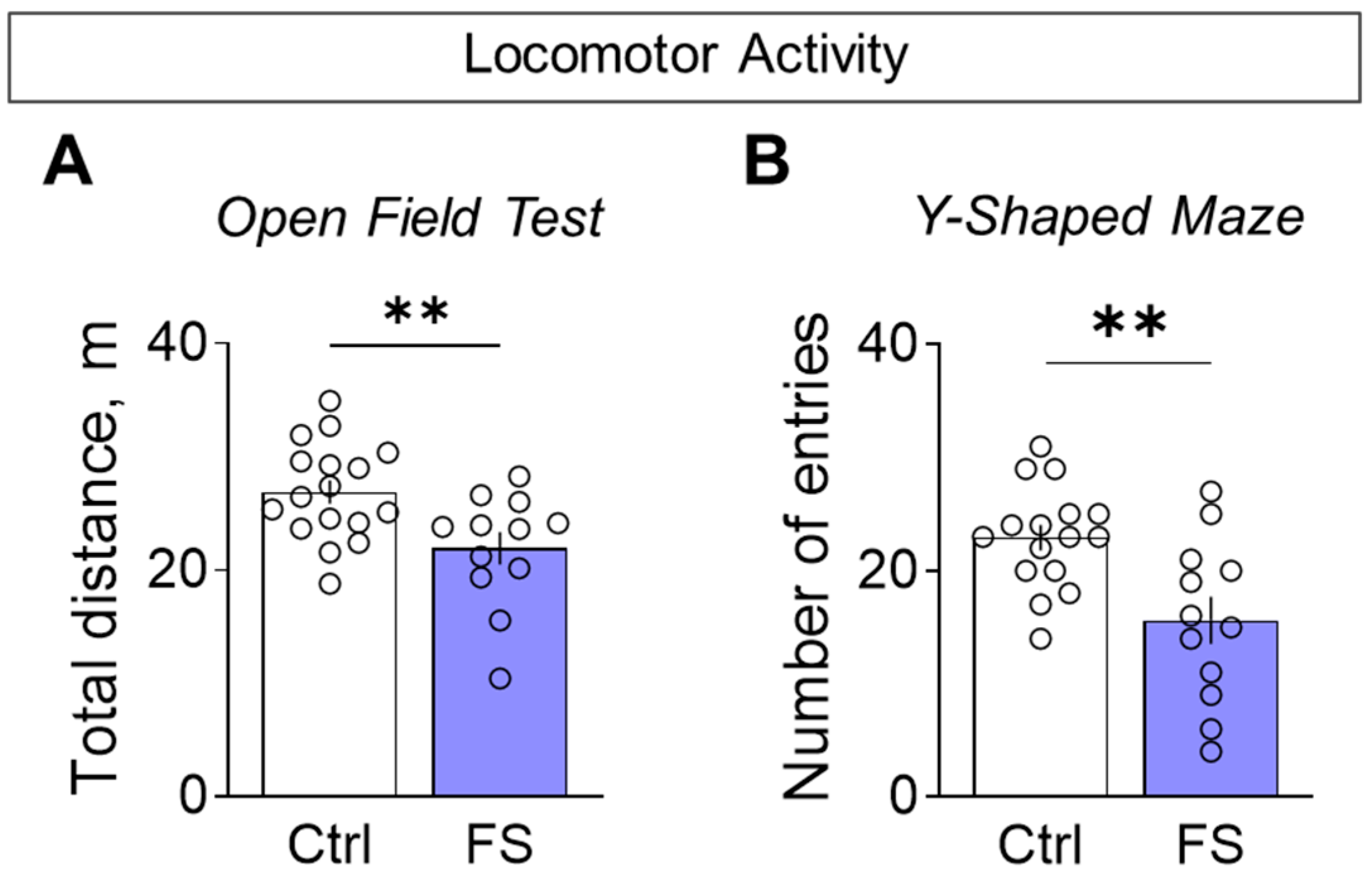
Rats with a history of FS were less active when exploring novel environments. This hypolocomotion was consistent across tests: they traveled a shorter distance in the open field (Figure 9A, control: 26.9 ± 1.1 m, 95% CI [24.7, 29.1], N = 17; FS: 21.9 ± 1.5 m, 95% CI [18.7, 25.1], N = 12; t = 2.9, p = 0.008, d = 1.01) and entered fewer arms in the Y-maze (Figure 9B, control: 22.9 ± 1.1 entries, 95% CI [20.5, 25.4], N = 16; FS: 15.6 ± 2.1 entries, 95% CI [11.0, 20.2], N = 12; t = 3.3, p = 0.003, d = 1.26) compared to control.
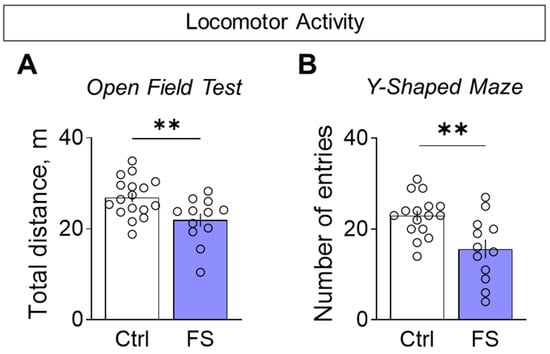
Figure 9.
Locomotor activity assessment. (A) Total distance traveled in the open field test. (B) The number of visited arms in the Y-shaped maze. Ctrl–animals weaned for the time of modeling seizures from the nest, but kept in normothermic conditions (N = 16–17), FS–animals after modeling FS at P10 (N = 12). Data are presented as means and standard errors of the mean. The circles show the individual values for each rat. Student’s test: **—p < 0.01.
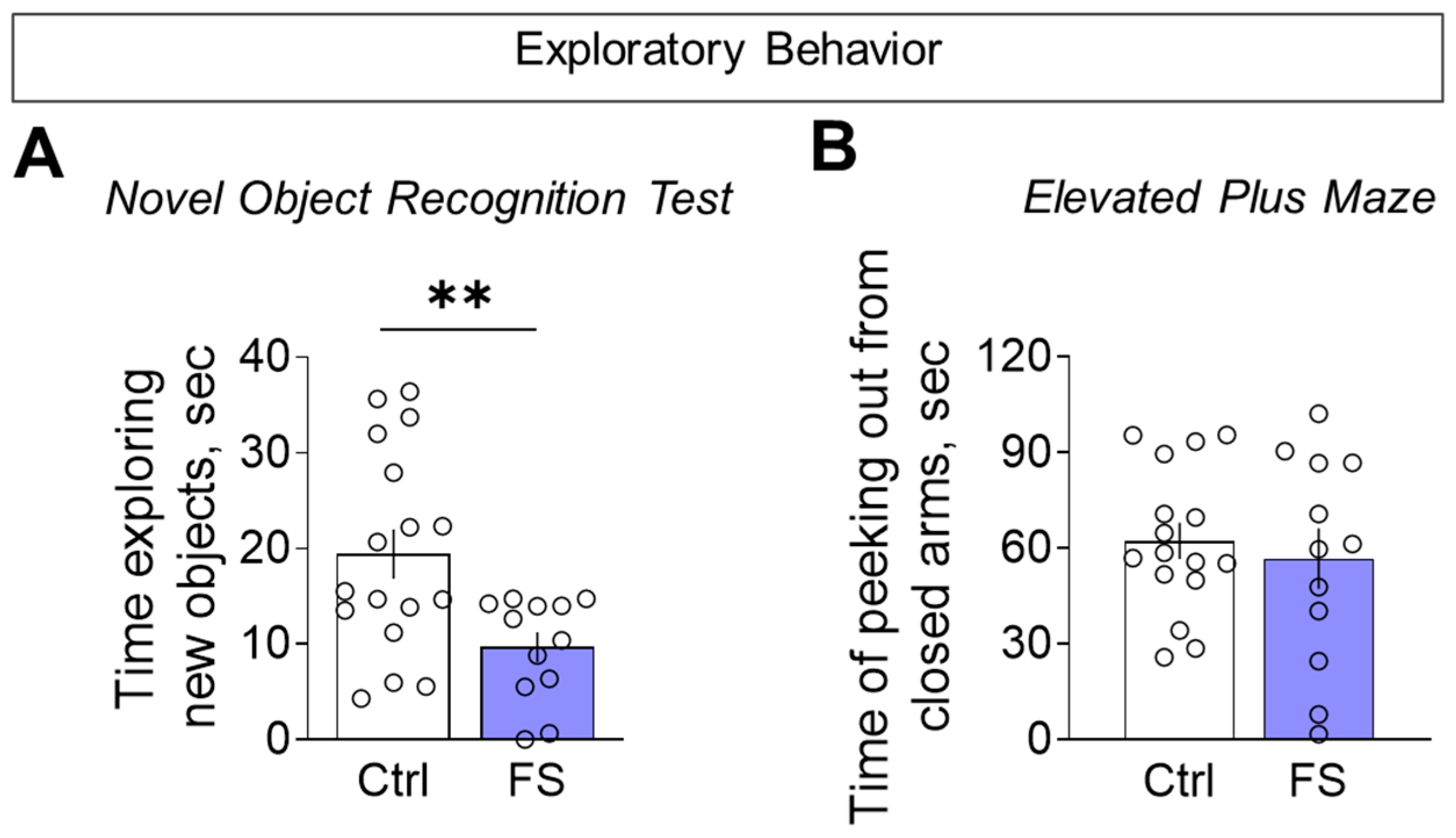
To determine whether reduced motor activity reflected a broader decline in exploration, we directly assessed exploratory behavior. In the novel object recognition test, FS rats investigated the unfamiliar objects for less time than controls during the initial presentation (Figure 10A, control: 19.4 ± 2.6 s, 95% CI [14.0, 24.9], N = 17; FS: 9.7 ± 1.6 s, 95% CI [6.2, 13.1], N = 12; t = 2.9, p = 0.007, d= 1.10). We found no difference between the groups in the elevated plus maze for the time spent head-dipping from the closed arms, a behavior used to assess exploratory activity (Figure 10B, control: 62.3 ± 5.7 s, 95% CI [50.2, 74.3], N = 16; FS: 56.7 ± 9.5 s, 95% CI [35.7, 77.7], N = 12; t = 0.5, p = 0.6). This confirmed a specific deficit in exploratory drive, which was not solely attributable to general hypolocomotion.
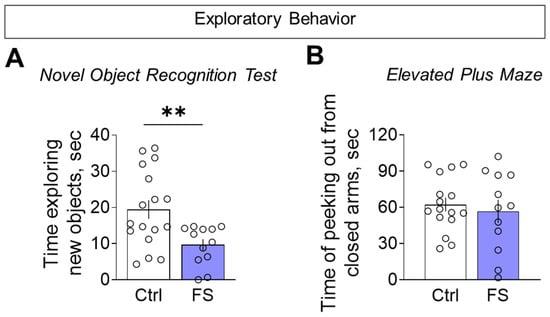
Figure 10.
Exploratory behavior assessment. (A) Time exploring unfamiliar identical objects when they were first presented in the novel object recognition test. (B) Time of peeking out from closed arms in the elevated plus maze. Ctrl–animals weaned for the time of modeling seizures from the nest, but kept in normothermic conditions (N = 16–17), FS–animals after modeling FS at P10 (N = 12). Data are presented as means and standard errors of the mean. The circles show the individual values for each rat. Student’s test: **—p < 0.01.
3.3.2. Cognitive Functions
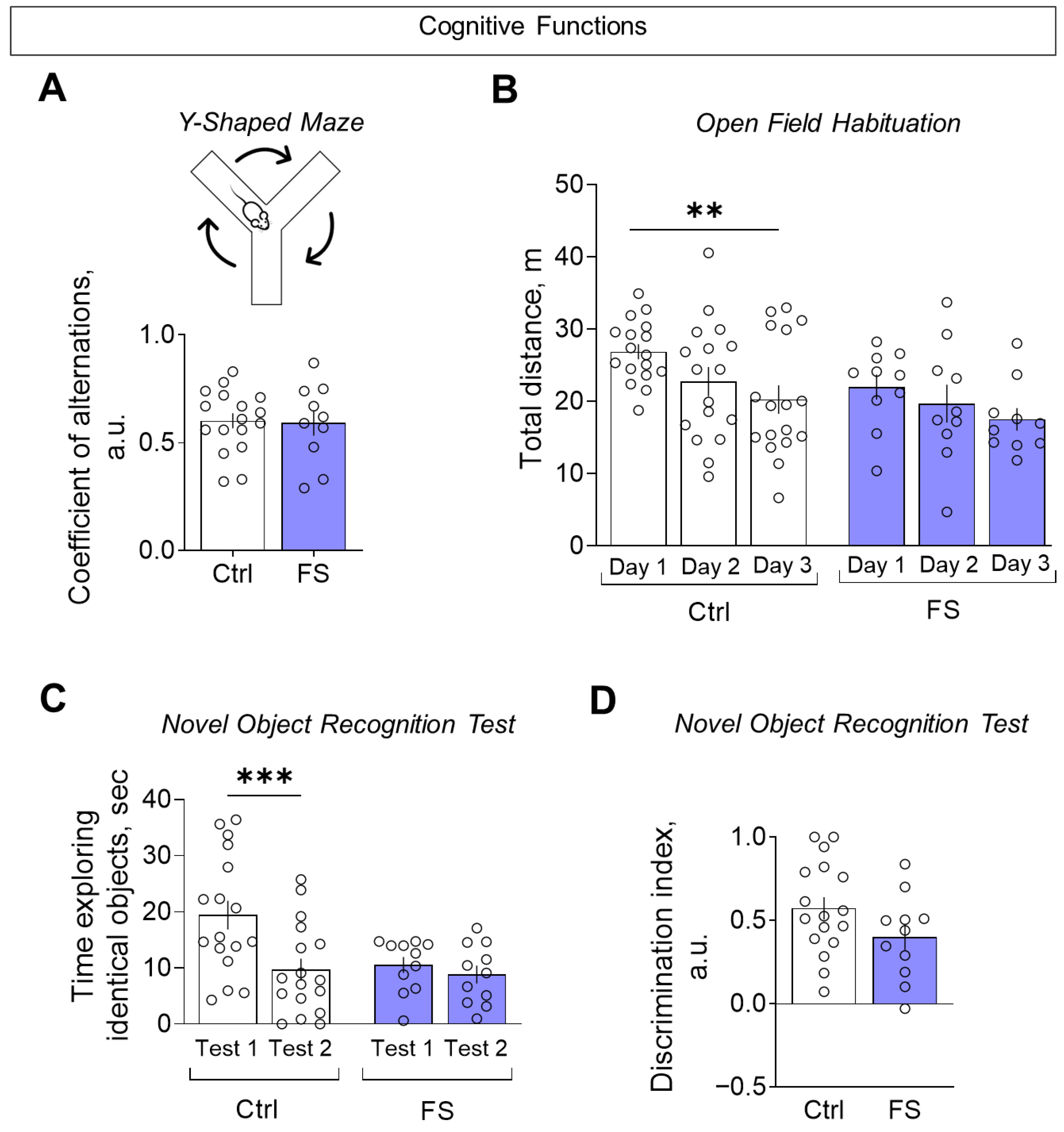
The assessment of cognitive functions was conducted using three distinct tests: the Y-maze, the open field habituation and the novel object recognition test. In the Y-shaped maze, we did not observe any changes in the coefficient of alternation (Figure 11A, control: 0.60 ± 0.04, 95% CI [0.53, 0.68], N = 17; FS: 0.59 ± 0.06, 95% CI [0.46, 0.72], N = 10; t = 0.16, p = 0.87), suggesting no impairment of spatial working memory.
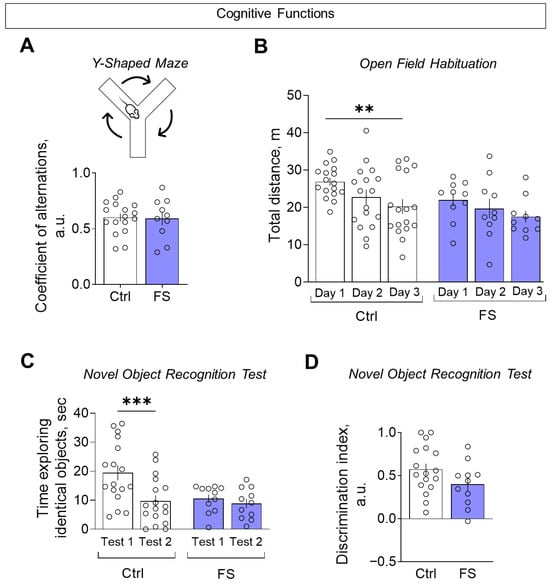
Figure 11.
Assessment of cognitive functions. (A) Coefficient of alternations in the Y-shaped maze. (B) Distance traveled in the open field test over 3 consecutive days. (C) The time taken to examine the same object when first presented (test 1) and one hour later (test 2) in the novel object recognition test. (D) Discrimination index for assessing preference for a new object compared to a familiar object in the novel object recognition test. Ctrl–animals weaned for the time of modeling seizures from the nest, but kept in normothermic conditions (N = 17), FS–animals after modeling FS at P10 (N = 10–12). Data are presented as means and standard errors of the mean. The circles show the individual values for each rat. Sidak’s post hoc test: **—p < 0.01, ***—p < 0.001.
In the open field habituation test, FS rats failed to reduce their exploratory activity over three consecutive days, unlike controls, indicating impaired habituation (Figure 11B, mixed ANOVA F(FS)2,50 = 6.55, p = 0.003, η2 = 0.21). A similar deficit in habituation was seen in the novel object recognition test: FS rats spent similar time exploring a familiar object upon re-presentation, while controls showed a significant decrease (Figure 11C, mixed ANOVA F(FS × time)1,26 = 5.28, p = 0.03, η2 = 0.29). Finally, when assessed by the discrimination index (where +1 indicates a total preference for the novel object), there was no significant difference between the groups (Figure 11D, control: 0.57 ± 0.07, 95% CI [0.30, 0.72], N = 17; FS: 0.40 ± 0.08, 95% CI [0.23, 0.57], N = 11; t = 1.67, p = 0.11), indicating that the ability to discriminate novelty per se was intact.
3.3.3. Social Interaction
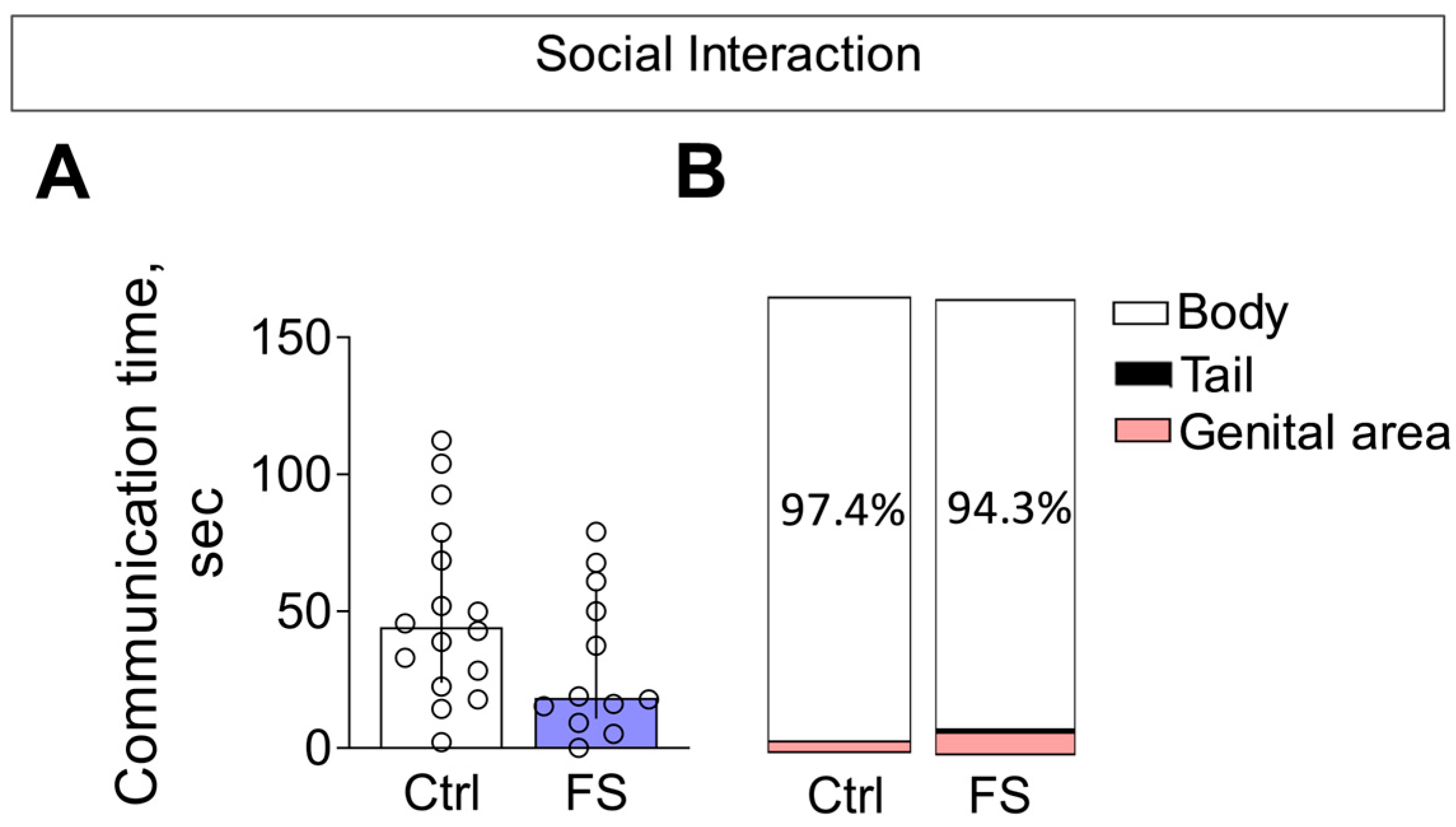
In the social interaction test, we observed no alterations in the total time spent in social interaction between rats with a history of FS and control animals (Figure 12A, Mann–Whitney test U16,12 = 63, p = 0.13).
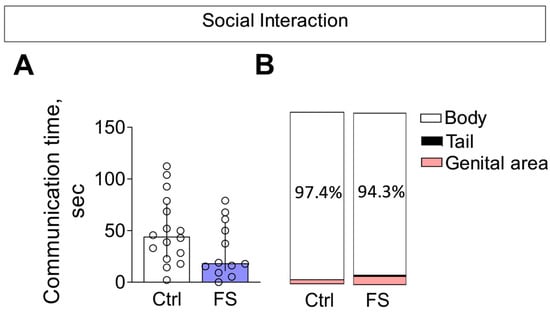
Figure 12.
Social interaction. (A) Time of communication. Ctrl–animals weaned for the time of modeling seizures from the nest, but kept in normothermic conditions (N = 16), FS–animals after modeling FS at P10 (N = 12). Data are presented as median and interquartile range. The circles show the individual values for each rat. (B) Structure of communicative behavior.
The composition of communicative behaviors was also similar between groups (Figure 12B). In both control and FS rats, the majority of social investigation was directed toward the body of the unfamiliar conspecific. Notably, the time spent sniffing the genital area—a behavior associated with higher confidence—was low (<5%) and did not differ between groups. Furthermore, we detected no differences in the occurrence of aggressive behaviors (Mann–Whitney test U16,12 = 79, p = 0.4).
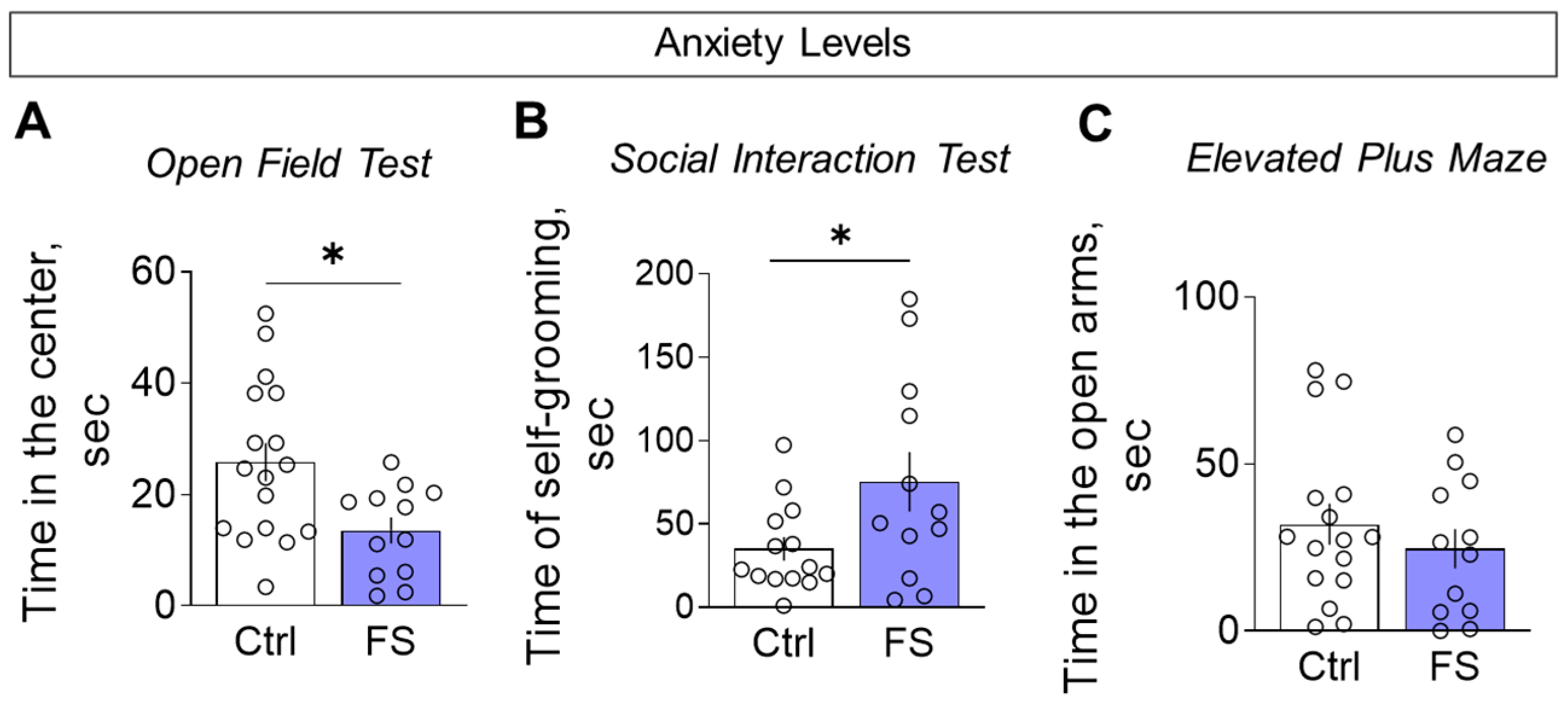
3.3.4. Anxiety Levels
We assessed anxiety-like behavior using multiple parameters. Compared to controls, FS rats spent less time in the center of the open field (Figure 13A, control: 25.8 ± 3.4 s, 95% CI [18.5, 33.1], N = 17; FS: 13.5 ± 2.4 s, 95% CI [8.4, 18.7], N = 12; t = 2.6, p = 0.012, d = 1.02) and more time engaged in self-grooming during the social interaction test (Figure 13B, control: 35.0 ± 7.0 s, 95% CI [19.8, 50.2], N = 14; FS: 75.3 ± 17.8 s, 95% CI [36.0, 114.5], N = 12; t = 2.2, p = 0.036, d = 0.87), collectively indicating a heightened anxiety-like state.
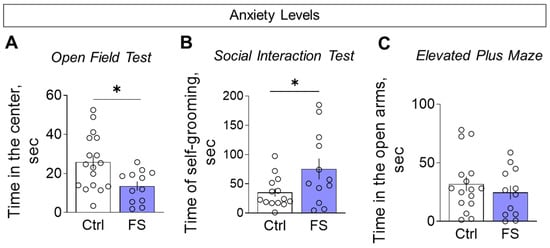
Figure 13.
Assessment of anxiety levels. (A) Time in the central zone in the open field test. (B) Time spent on grooming in the social interaction test. (C) Time in open arms in the elevated plus maze. Ctrl–animals weaned for the time of modeling seizures from the nest, but kept in normothermic conditions (N = 14–17), FS–animals after modeling FS at P10 (N = 12). Data are presented as M ± SEM. The circles show the individual values for each rat. Student’s test: *—p < 0.05.
However, no significant differences were observed between the groups in time spent in the open arms (Figure 13C, control: 32.0 ± 6.1 s, 95% CI [18.9, 45.0], N = 16; FS: 24.7 ± 5.9 s, 95% CI [11.7, 37.7], N = 12; t = 0.8, p = 0.41).
Consequently, mature animals that have experienced prolonged FS at an early age exhibit a specific behavioral phenotype characterized by increased anxiety, decreased motor activity and exploratory behavior, and impaired habituation to familiar environments, without impairing working memory, recognition of new objects and social behavior.
4. Discussion
Febrile seizures (FS) are a common neurological disorder in early childhood, yet considerable debate persists regarding the long-term histopathological, neurochemical, and behavioral consequences of complex FS forms [79,80].
Both clinical and experimental evidence indicates that the profound brain hyperactivation during severe neonatal FS can result in cognitive deficits [34]. A leading hypothesis posits that this hyperactivation triggers excitotoxic and neuroinflammatory cascades, which disrupt normal developmental trajectories and produce long-term alterations, frequently linked to hippocampal dysfunction [76,81,82,83]. However, these studies did not account for the structural-functional heterogeneity of the hippocampus—particularly the distinct roles of its dorsal and ventral segments in spatial memory and emotional regulation, respectively [84]. Furthermore, the neurochemical mechanisms underlying FS-associated neuropsychiatric disorders remain poorly understood.
This study provides a comprehensive analysis of the long-term consequences in young adult rats (P45-55) following experimental FS induced at a developmentally critical period (P10). We report an uneven pattern of neuronal loss, alongside significant and regionally distinct alterations in the expression of genes encoding ionotropic (NMDA, AMPA) and mGluRs, as well as EAATs in the dorsal and ventral hippocampus, temporal cortex, and medial prefrontal cortex. These molecular changes were accompanied by a spectrum of behavioral abnormalities, including impaired cognitive function (exploratory behavior and memory), diminished motor activity in a novel environment, and heightened anxiety.
4.1. The Histopathological Changes
Our data confirm earlier reports of hippocampal neuronal loss following prolonged FS [85] and extend these findings by revealing a specific topographical pattern of vulnerability. Excitotoxicity and neuroinflammation represent two principal mechanisms likely mediating seizure-induced neuronal death. Fever directly enhances neuronal excitability [27] and hyperthermia-induced seizures in immature rats involve amplified glutamate-triggered intracellular Ca2+ release—a process whose pharmacological inhibition attenuates FS severity [86]. Inflammatory processes also play a critical role in modulating glutamate dynamics during FS. Fever-associated elevations in pro-inflammatory cytokines, particularly IL-1β, have been shown to promote glutamate release from both neurons and glial cells. These effects lead to accumulation of extracellular glutamate and increased NMDA receptor-mediated calcium influx, thereby contributing to neuronal hyperexcitability and potentially inducing excitotoxicity and neuronal loss [27,31,87]. However, further studies are needed to determine the exact mechanism of neuronal death after FS.
In this study, we performed the first differential analysis of neuronal loss in the dorsal and ventral hippocampus in an FS model. This regional assessment revealed an important nuance: while neuronal loss in the dorsal hippocampus was confined to the CA1 subfield, more extensive damage was observed in the ventral hippocampus, with cell loss evident in both CA1 and CA3 regions. This finding is significant as it suggests that the ventral hippocampus, which is more strongly implicated in emotional regulation, may be particularly vulnerable to functional impairment early in life.
4.2. The Molecular Changes
Our findings demonstrate that neonatal FS induce long-term, region-specific alterations in the expression of iGluR and mGluR, as well as EAATs, with the most pronounced changes observed in the ventral hippocampus and medial prefrontal cortex. Earlier research provided fragmented insights, describing alterations in specific components like mGluR5, EAAT2, and GluA1 in the cortex of young animals [36,43] or reporting no changes in hippocampal NMDA subunits at similar time points [44]. Our systematic approach, in contrast, uncovers a broader and persistent dysregulation across multiple glutamatergic genes, revealing a complex, region-specific molecular footprint of early-life FS that underpins the diverse functional impairments.
Notably, in the ventral hippocampus and medial prefrontal cortex, FS led to reduced expression of Grin2a (ventral hippocampus only) and Grin2b genes, with a parallel downregulation of the obligatory Grin1 subunit [88]. This coordinated downregulation points toward a general reduction in NMDA receptor density in these regions.
Discrepancies with some earlier reports, which found no changes in NMDA subunit gene expression [44,89], may be explained by differences in experimental design or the timing of assessment. Notably, those studies that did detect alterations often found them at the post-translational level, such as impaired GluN2A phosphorylation after repetitive FS [44] or sustained increases in GluN2B phosphorylation following prolonged seizures [89], highlighting that functional deficits can occur independently of transcriptional changes.
In the dorsal hippocampus of experimental rats, we found no changes in the ex-pression of the studied NMDA receptor subunits. Grin2b gene expression tended to decrease, but the differences did not reach statistical significance. However, we observed an elevated Grin2a/Grin2b gene expression ratio in the dorsal hippocampus of experimental rats. Assessing this ratio is critical for understanding NMDA receptor functionality, as the balance between the GluN2A and GluN2B subunits directly influences receptor kinetics, synaptic plasticity, and descending signaling [90]. The normal neurodevelopmental transition from predominant Grin2b expression prenatally to elevated Grin2a expression postnatally is required for the maturation of hippocampal synaptic networks. It has been suggested that decreased Grin2a/Grin2b ratio may lower the seizure threshold by enhancing NMDA receptor-mediated excitability [91]. Therefore, the increase in the Grin2a/Grin2b ratio observed in our model may represent a compensatory mechanism aimed at reducing the hyperexcitability of hippocampal neurons.
We found reduced mRNA levels of the AMPA receptor subunits Gria1 and Gria2 specifically in the ventral hippocampus and medial prefrontal cortex. This contrasts with earlier reports of increased GluA1 protein at acute time points [43], suggesting a dynamic temporal regulation of AMPA receptor expression post-FS. Our findings align with a delayed decrease in GluA2 protein reported in a different FS model [92]. The downregulation of Gria1, a key subunit for LTP induction [93], likely contributes to the synaptic plasticity and memory deficits we and others have observed [7]. Moreover, the reduction in Gria2 implies an increased formation of Ca2+-permeable AMPA receptors, which can exacerbate neuronal Ca2+ loading and promote excitotoxic damage [94,95].
Beyond iGluRs, we extended our analysis to mGluRs, providing the first comprehensive profile of their transcriptional changes in this model. Transcript levels of group I mGluRs (Grm1 and Grm5) were diminished in the ventral hippocampus and medial prefrontal cortex. These postsynaptic receptors normally enhance intracellular Ca2+ signaling and tune synaptic strength [96,97]. Their downregulation therefore likely exacerbates the impairment of glutamatergic synaptic function. The apparent discrepancy with a prior study showing increased Grm5 expression probably reflects distinct temporal dynamics, as that analysis was performed at a much earlier post-FS interval.
We also identified significant alterations in the less-explored groups II and III mGluRs. Specifically, expression of Grm3 and Grm4 (mPFC), Grm7 (ventral hippocampus), and Grm8 (dorsal hippocampus) was reduced. These presynaptic receptors function as autoreceptors, suppressing glutamate release upon activation [96,98]. Their downregulation could thus impair this auto-inhibitory feedback, potentially leading to enhanced synaptic glutamate levels. Conversely, the elevated expression of Grm7 and Grm8 in the temporal cortex might reflect a region-specific, homeostatic adaptation to counter neuronal hyperexcitability.
Our analysis of glutamate transporters revealed a specific reduction in the expression of Slc1a3 (EAAT1) and Slc1a1 (EAAT3) within the ventral hippocampus, while Slc1a2 (EAAT2) remained unchanged. Although EAAT2 handles the bulk of hippocampal glutamate clearance, the contribution of EAAT3 at specific synapses can be substantial (~20%) [99,100,101]. Critically, both glial EAAT1 and neuronal EAAT3 play non-redundant roles in fine-tuning extracellular glutamate dynamics [101,102,103]. Thus, their coordinated downregulation likely disrupts precise glutamate homeostasis in the ventral hippocampus, creating a permissive environment for excitotoxicity and network dysfunction, as demonstrated in knockdown models [101].
Thus, the induction of FS during a critical period of glutamatergic system development leads to long-term alterations in the expression of various glutamatergic system components. These alterations, in turn, may underlie the impairments in synaptic plasticity and behavior.
4.3. Linking Molecular Disruptions to Behavioral Deficits
Cognitive deficits represent one of the most consistently reported long-term consequences of early-life FS in animal models. A substantial body of evidence, using paradigms ranging from the Morris water maze and active avoidance to fear conditioning, has documented persistent memory impairments in both young [10] and adult animals [6,7,8,9] with a history of FS. In the present study, we employed a complementary set of behavioral assays—open field habituation, novel object recognition, and Y-maze spontaneous alternation—to probe different aspects of learning and memory that are particularly dependent on hippocampal and prefrontal function.
The observed habituation deficit in the open field aligns closely with our molecular and histopathological findings. This form of non-associative memory is known to require intact NMDA receptor signaling (involving Grin1 and Grin2a [104] and is highly sensitive to ventral hippocampal dysfunction [105]—precisely the circuits where we documented the most severe neuronal loss and glutamatergic downregulation.
However, it cannot be ruled out that the poorer performance of experimental rats in the Open Field habituation test may be associated not with memory impairment per se, but with a deficit in exploratory behavior. We demonstrated that experimental rats were less active when exploring a novel environment (exhibiting reduced motor activity in the Open Field test). This lower baseline level of exploratory behavior in the experimental group may have influenced the results of the memory test [106].
Similar deficits in exploratory behavior were observed during the learning stage of the Novel Object Recognition test, when two identical objects are initially presented. Rats with a history of FS spent less time exploring the objects compared to control rats.
The reduced level of exploratory activity in rats that experienced FS in the neonatal period may be linked to the decreased expression of NMDA receptor subunit genes in the ventral hippocampus that we identified, since it has previously been shown that NMDA receptor blockade in the ventral hippocampus reduces exploratory behavior in the Open Field [107].
The prefrontal cortex, along with the hippocampus, plays a key role in the implementation of cognitive functions. Having extensive connections with various brain structures, including direct projections to the ventral hippocampus, it integrates emotional and cognitive components of behavior, which is necessary for working memory processes [21,22,23]. The prefrontal cortex-hippocampus circuit also is crucial for recognition memory in rodents [108].
There is compelling evidence from rodent studies that both NMDA and AMPA receptors in the frontal cortex are critically involved in working and recognition memory mechanisms [109,110,111]. In particular, multiple pharmacological investigations have demonstrated that when NMDA receptors (particularly GluN2B-containing) and AMPA receptors are blocked in the medial prefrontal cortex, rodents exhibit significant impairments in working memory performance [110].
A notable finding of our study was the absence of significant deficits in novel object recognition or Y-maze working memory, despite the substantial downregulation of key AMPA and NMDA receptor genes in the medial prefrontal cortex and hippocampus. This result aligns with the report by Mesquita et al., who also found working memory to be intact in adult rats after neonatal FS [112].
This apparent dissociation between molecular and behavioral findings can be interpreted in several ways. First, the medial prefrontal cortex is functionally heterogeneous. The observed molecular deficits might be more pronounced in subregions like the infralimbic and prelimbic cortices [113,114,115], which are critically involved in emotional regulation and extinction learning—functions that were impaired in our rats—rather than in the spatial and recognition memory circuits probed by our tests. Second, compensatory mechanisms involving other neurotransmitter systems (e.g., dopamine, acetylcholine) not assessed in this study might have preserved performance in simpler memory tasks. Finally, the Y-maze and standard novel object recognition test may lack the sensitivity to detect subtle deficits, requiring more challenging cognitive paradigms to unmask functional impairments.
That molecular changes do lead to functional behavioral consequences is clearly demonstrated in the field of anxiety regulation. Our data reveal a consistent hyperanxious phenotype, evidenced by reduced center time in the open field and increased self-grooming in the social test. This behavioral profile co-occurs with the most severe histopathological and molecular deficits in the ventral hippocampus, a key node in anxiety circuits [116]. Interestingly, this effect was test-specific, as anxiety-like behavior in the elevated plus maze was unaltered.
Differences in anxiety-related behavior observed across tests may be due to differences in the stress response of experimental and control animals. Rats typically experience higher levels of stress in the Open Field and Social Interaction tests compared to the Elevated Plus Maze, where the closed arms provide a safe haven. It is well known that various adverse environmental factors, including those that trigger febrile seizures, can have long-term effects on the development of stress-response systems [117]. However, further research is needed to fully understand this issue.
Reports on anxiety-like behaviors following neonatal FS in rodents remain inconsistent. While some studies, like ours, describe an increase in anxiety-like behavior [11,112], others have reported decreased anxiety [8,118]. Some authors have also reported the development of depressive-like changes in rats that experienced FS at an early age [11,12], but we have not studied these forms of behavior.
We also found no impairment of social behavior in rats that had undergone FS at an early age. These results support clinical studies that found no impairment of social functioning in adolescents with a history of FS [119].
In summary, the behavioral profile observed—characterized by heightened anxiety, reduced exploration, and impaired habituation, but spared working memory and social function—exhibits a compelling regional alignment with the most pronounced molecular and cellular deficits in the ventral hippocampus and medial prefrontal cortex. This selectivity underscores the vulnerability of specific limbic circuits to early-life FS and provides a neurobiological framework for understanding the ensuing neuropsychiatric sequelae.
4.4. Conclusion and Future Directions
Our study demonstrates that neonatal FS lead to long-term, region-specific alterations characterized by neuronal loss in the hippocampus and a persistent downregulation of glutamatergic signaling components—including ionotropic, metabotropic receptors, and excitatory amino acid transporters—primarily within the ventral hippocampus and medial prefrontal cortex. These molecular and cellular deficits are accompanied by a distinct behavioral phenotype of increased anxiety, reduced exploratory drive, and impaired habituation, while working memory and social interaction remain intact.
While our study delineates robust correlations, establishing causality requires further investigation. Our previous electrophysiological work in the same model demonstrated impaired hippocampal LTP that was rescued by D-serine, an NMDA receptor co-agonist [7]. The NMDA receptor hypofunction suggested by our current transcriptional data, combined with this pharmacological evidence, positions D-serine supplementation as a compelling therapeutic strategy worthy of future behavioral testing in this model, consistent with its promise in other neuropsychiatric conditions [120,121,122,123].
Furthermore, the distinct alterations in mGluR gene expression provide a strong rationale for exploring their specific contributions to the FS phenotype. Given the established role of mGluRs in modulating synaptic transmission and plasticity, and the availability of selective pharmacological tools [124,125,126], future studies should employ these ligands to directly test whether correcting mGluR dysfunction can ameliorate the behavioral deficits we observed.
However, this study has several limitations that highlight the need for further research. First, the analysis was conducted at a single time point (P45-55). Tracking the age-dependent dynamics of these changes—from the acute post-seizure period through adolescence into adulthood—would reveal the developmental trajectory of the observed deficits and identify potential critical windows for intervention. Second, the reported mRNA levels should be interpreted as a proxy for transcriptional activity and may not directly correlate with the final protein abundance or functional activity of the receptors due to potential post-transcriptional and post-translational regulation. Confirming these findings at the protein level and using electrophysiological techniques is essential to determine whether the observed transcriptional changes translate into functional impairments in synaptic transmission and plasticity.
Furthermore, the present study was conducted exclusively in male rats. Given known sex differences in neural development and seizure susceptibility [8,127], a direct comparison of the long-term consequences of FS in males and females is a crucial next step to fully understand the neurobiological impact of early-life seizures.
It is important to note the limitations of our rat model when generalizing the conclusions to human anxiety risk. The model does not account for the full spectrum of human genetic vulnerability and environmental influences that shape clinical anxiety. The anxiety-like behaviors we measured, while translationally relevant, are not identical to the subjective experience and diagnostic criteria of human anxiety disorders. These limitations, however, highlight key directions for future research, such as validating these molecular findings in human post-mortem studies or using more complex genetic models to better mimic the human condition.
Furthermore, the hyperthermia model cannot fully replicate febrile seizures associated with infectious fever, as it lacks exposure to bacterial agents, which significantly increase the risk of severe neuroinflammation.
Collectively, our findings delineate a robust link between early-life FS, selective disruption of the glutamatergic system in limbic circuits, and specific long-term behavioral impairments. This precise identification of vulnerable circuits and molecular targets provides a foundation for developing targeted therapeutic strategies aimed at preventing or mitigating the neuropsychiatric consequences of FS.
Author Contributions
Conceptualization, A.V.Z. and O.E.Z.; methodology, O.E.Z., A.A.K., M.V.Z. and A.V.G.; validation, A.V.Z. and O.E.Z.; formal analysis, O.E.Z., A.A.K., M.V.Z. and A.V.G.; investigation, O.E.Z., A.A.K., M.V.Z. and A.V.G.; writing—original draft preparation, A.V.G., A.A.K. and O.E.Z.; writing—review and editing, A.V.Z. and O.E.Z.; visualization, A.V.G.; supervision, A.V.Z. and O.E.Z.; project administration, A.V.Z.; funding acquisition, A.V.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the state assignment of Ministry of Science and Higher Education of the Russian Federation (assignment 075-00263-25-00).
Institutional Review Board Statement
The study was conducted according to the EU Directive. 2010/63/EU for animal experiments and approved by the Ethics Committee of the Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences (ethical permit number 1-17, 26 January 2023).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| ANOVA | Analysis of Variance |
| CA | Cornu Ammonis (followed by number: CA1, CA3) |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| cDNA | complementary DNA |
| Ctrl | Control |
| DI | Discrimination Index |
| DNA | Deoxyribonucleic acid |
| EAAT | Excitatory amino acid transporter (followed by number: EAAT1, EAAT2, EAAT3) |
| EEG | Electroencephalography |
| FS | Febrile Seizures |
| Gria1/2 | Gene names for AMPA receptor subunits (GluA1/2 proteins) |
| Grin1 | Gene name for obligatory NMDA receptor subunit (GluN1 protein) |
| Grin2a/2b | Gene names for NMDA receptor subunits (GluN2A/2B proteins) |
| Grm1-8 | Gene names for metabotropic glutamate receptors (mGluR1-8 proteins) |
| iGluR | Ionotropic glutamate receptor |
| IL-1β | Interleukin-1 beta |
| LTP | Long-Term Potentiation |
| mGluR | Metabotropic glutamate receptor (Groups: I, II, III) |
| mPFC | medial Prefrontal Cortex |
| mRNA | Messenger RNA |
| NMDA | N-methyl-D-aspartate |
| p | Postnatal day (followed by number: P10, P50) |
| PBS | Phosphate-Buffered Saline |
| PFA | Paraformaldehyde |
| RT-qPCR | Reverse Transcription quantitative Polymerase Chain Reaction |
| Slc1a1 | Gene name for EAAT3 (Solute carrier family 1 member 1) |
| Slc1a2 | Gene name for EAAT2 (Solute carrier family 1 member 2) |
| Slc1a3 | Gene name for EAAT1 (Solute carrier family 1 member 3) |
Appendix A

Table A1.
Reference gene stability values within the dorsal hippocampus of rats obtained by four algorithms with the RefFinder online tool.
Table A1.
Reference gene stability values within the dorsal hippocampus of rats obtained by four algorithms with the RefFinder online tool.
| Dorsal Hippocampus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delta CT | BestKeeper | NormFinder | GeNorm | Comprehensive Ranking | ||||||
| Rank | Gene | Average of STDV | Gene | Std Dev | Gene | Stability Value | Gene | Stability Value | Gene | Geomean of Ranking Values |
| 1 | Pgk1 | 0.23 | Pgk1 | 0.09 | Rpl13a | 0.131 | Pgk1 | 0.090 | Pgk1 | 1.19 |
| 2 | Rpl13a | 0.23 | Ywhaz | 0.14 | Pgk1 | 0.134 | Ywhaz | 0.090 | Ywhaz | 2.06 |
| 3 | Ywhaz | 0.24 | Rpl13a | 0.16 | Ywhaz | 0.160 | Ppia | 0.198 | Rpl13a | 2.21 |
| 4 | Ppia | 0.26 | Ppia | 0.17 | Ppia | 0.193 | Rpl13a | 0.210 | Ppia | 3.94 |
| 5 | Actb | 0.29 | Actb | 0.17 | Actb | 0.224 | Actb | 0.235 | Actb | 4.73 |
| 6 | Hprt1 | 0.31 | Hprt1 | 0.25 | Hprt1 | 0.256 | Hprt1 | 0.260 | Hprt1 | 6.00 |

Table A2.
Reference gene stability values within the ventral hippocampus of rats obtained by four algorithms with the RefFinder online tool.
Table A2.
Reference gene stability values within the ventral hippocampus of rats obtained by four algorithms with the RefFinder online tool.
| Ventral Hippocampus | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delta CT | BestKeeper | NormFinder | GeNorm | Comprehensive Ranking | ||||||
| Rank | Gene | Average of STDV | Gene | Std Dev | Gene | Stability Value | Gene | Stability Value | Gene | Geomean of Ranking Values |
| 1 | Ppia | 0.29 | Rpl13a | 0.22 | Ppia | 0.085 | Actb | 0.093 | Ppia | 1.68 |
| 2 | Gapdh | 0.30 | Ppia | 0.36 | Gapdh | 0.182 | Gapdh | 0.093 | Gapdh | 1.86 |
| 3 | Actb | 0.30 | Gapdh | 0.38 | Actb | 0.186 | Pgk1 | 0.208 | Actb | 2.59 |
| 4 | Pgk1 | 0.33 | Pgk1 | 0.41 | Pgk1 | 0.227 | Ppia | 0.231 | Pgk1 | 3.72 |
| 5 | B2m | 0.41 | Actb | 0.43 | B2m | 0.334 | B2m | 0.294 | Rpl13a | 3.83 |
| 6 | Rpl13a | 0.45 | B2m | 0.44 | Rpl13a | 0.400 | Rpl13a | 0.347 | B2m | 5.23 |

Table A3.
Reference gene stability values within the temporal cortex of rats obtained by four algorithms with the RefFinder online tool.
Table A3.
Reference gene stability values within the temporal cortex of rats obtained by four algorithms with the RefFinder online tool.
| Temporal Cortex | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delta CT | BestKeeper | NormFinder | GeNorm | Comprehensive Ranking | ||||||
| Rank | Gene | Average of STDV | Gene | Std Dev | Gene | Stability Value | Gene | Stability Value | Gene | Geomean of Ranking Values |
| 1 | Gapdh | 0.19 | Actb | 0.14 | Gapdh | 0.102 | Actb | 0.069 | Gapdh | 1.19 |
| 2 | Actb | 0.20 | Gapdh | 0.18 | Actb | 0.124 | Gapdh | 0.069 | Actb | 1.41 |
| 3 | Hprt1 | 0.22 | Rpl13a | 0.20 | Hprt1 | 0.152 | Rpl13a | 0.158 | Rpl13a | 3.46 |
| 4 | Rpl13a | 0.23 | Pgk1 | 0.23 | Rpl13a | 0.169 | Hprt1 | 0.193 | Hprt1 | 3.66 |
| 5 | Ppia | 0.24 | Hprt1 | 0.23 | Ppia | 0.188 | Ppia | 0.206 | Ppia | 5.23 |
| 6 | Pgk1 | 0.25 | Ppia | 0.26 | Pgk1 | 0.206 | Pgk1 | 0.221 | Pgk1 | 5.42 |

Table A4.
Reference gene stability values within the medial prefrontal cortex of rats obtained by four algorithms with the RefFinder online tool.
Table A4.
Reference gene stability values within the medial prefrontal cortex of rats obtained by four algorithms with the RefFinder online tool.
| Medial Prefrontal Cortex | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Delta CT | BestKeeper | NormFinder | GeNorm | Comprehensive Ranking | ||||||
| Rank | Gene | Average of STDV | Gene | Std Dev | Gene | Stability Value | Gene | Stability Value | Gene | Geomean of Ranking Values |
| 1 | Pgk1 | 0.30 | Rpl13a | 0.20 | Pgk1 | 0.070 | Pgk1 | 0.155 | Pgk1 | 1.32 |
| 2 | Ywhaz | 0.32 | Ppia | 0.33 | Ywhaz | 0.167 | Ywhaz | 0.155 | Ywhaz | 2.00 |
| 3 | Ppia | 0.35 | Pgk1 | 0.34 | Ppia | 0.218 | Hprt1 | 0.187 | Ppia | 2.91 |
| 4 | Hprt1 | 0.36 | Ywhaz | 0.40 | Hprt1 | 0.258 | Ppia | 0.238 | Rpl13a | 3.34 |
| 5 | Rpl13a | 0.42 | Hprt1 | 0.46 | Rpl13a | 0.320 | Rpl13a | 0.298 | Hprt1 | 3.94 |
| 6 | B2m | 0.55 | B2m | 0.46 | B2m | 0.501 | B2m | 0.381 | B2m | 6.00 |
References
- Leung, A.K.C.; Hon, K.L.; Leung, T.N.H. Febrile Seizures: An Overview. Drugs Context 2018, 7, 212536. [Google Scholar] [CrossRef]
- Vestergaard, M.; Pedersen, C.B.; Christensen, J.; Madsen, K.M.; Olsen, J.; Mortensen, P.B. Febrile Seizures and Risk of Schizophrenia. Schizophr. Res. 2005, 73, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, E.N.; Larsen, J.T.; Petersen, L.; Christensen, J.; Dalsgaard, S. Childhood Epilepsy, Febrile Seizures, and Subsequent Risk of ADHD. Pediatrics 2016, 138, e20154654. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Yousefichaijan, P.; Safi Arian, S.; Ebrahimi, S.; Naziri, M. Comparison of Relation between Attention Deficit Hyperactivity Disorder in Children with and without Simple Febrile Seizure Admitted in Arak Central Iran. Iran. J. Child Neurol. 2016, 10, 56–61. [Google Scholar]
- Gillberg, C.; Lundström, S.; Fernell, E.; Nilsson, G.; Neville, B. Febrile Seizures and Epilepsy: Association with Autism and Other Neurodevelopmental Disorders in the Child and Adolescent Twin Study in Sweden. Pediatr. Neurol. 2017, 74, 80–86.e2. [Google Scholar] [CrossRef]
- Dubé, C.M.; Zhou, J.-L.; Hamamura, M.; Zhao, Q.; Ring, A.; Abrahams, J.; McIntyre, K.; Nalcioglu, O.; Shatskih, T.; Baram, T.Z.; et al. Cognitive Dysfunction after Experimental Febrile Seizures. Exp. Neurol. 2009, 215, 167–177. [Google Scholar] [CrossRef]
- Griflyuk, A.V.; Postnikova, T.Y.; Zaitsev, A.V. Prolonged Febrile Seizures Impair Synaptic Plasticity and Alter Developmental Pattern of Glial Fibrillary Acidic Protein (GFAP)-Immunoreactive Astrocytes in the Hippocampus of Young Rats. Int. J. Mol. Sci. 2022, 23, 12224. [Google Scholar] [CrossRef]
- Kloc, M.L.; Marchand, D.H.; Holmes, G.L.; Pressman, R.D.; Jeremy, M. Cognitive Impairment Following Experimental Febrile Seizures Is Determined by Sex and Seizure Duration. Epilepsy Behav. 2023, 126, 108430. [Google Scholar] [CrossRef]
- Dai, Y.-J.; Wu, D.-C.; Feng, B.; Chen, B.; Tang, Y.-S.; Jin, M.-M.; Zhao, H.-W.; Dai, H.-B.; Wang, Y.; Chen, Z. Prolonged Febrile Seizures Induce Inheritable Memory Deficits in Rats through DNA Methylation. CNS Neurosci. Ther. 2019, 25, 601–611. [Google Scholar] [CrossRef]
- Yagoubi, N.; Jomni, Y.; Sakly, M. Hyperthermia-Induced Febrile Seizures Have Moderate and Transient Effects on Spatial Learning in Immature Rats. Behav. Neurol. 2015, 2015, 924303. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.H.; Kim, S.-W.; Im, H.; Song, Y.; Kim, S.J.; Lee, Y.R.; Kim, G.W.; Hwang, C.; Park, D.-K.; Kim, D.-S. Febrile Seizures Cause Depression and Anxiogenic Behaviors in Rats. Cells 2022, 11, 3228. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Early-Life Hyperthermic Seizures Upregulate Adenosine A(2A) Receptors in the Cortex and Promote Depressive-like Behavior in Adult Rats. Epilepsy Behav. 2018, 86, 173–178. [Google Scholar] [CrossRef]
- Rice, D.; Barone, S., Jr. Critical Periods of Vulnerability for the Developing Nervous System: Evidence from Humans and Animal Models. Environ. Health Perspect. 2000, 108, 511–533. [Google Scholar]
- Hamelin, S.; Vercueil, L. A Simple Febrile Seizure with Focal Onset. Epileptic Disord. 2014, 16, 112–115. [Google Scholar] [CrossRef]
- Neville, B.; Gindner, D. Febrile Seizures Are a Syndrome of Secondarily Generalized Hippocampal Epilepsy. Dev. Med. Child Neurol. 2010, 52, 1151–1153. [Google Scholar] [CrossRef]
- Baram, T.Z.; Gerth, A.; Schultz, L. Febrile Seizures: An Appropriate-Aged Model Suitable for Long-Term Studies. Dev. Brain Res. 1997, 98, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.B.; Moser, E.I. Functional Differentiation in the Hippocampus. Hippocampus 1998, 8, 608–619. [Google Scholar] [CrossRef]
- Barkus, C.; McHugh, S.B.; Sprengel, R.; Seeburg, P.H.; Rawlins, J.N.P.; Bannerman, D.M. Hippocampal NMDA Receptors and Anxiety: At the Interface between Cognition and Emotion. Eur. J. Pharmacol. 2010, 626, 49–56. [Google Scholar] [CrossRef]
- Grigoryan, G.; Segal, M. Lasting Differential Effects on Plasticity Induced by Prenatal Stress in Dorsal and Ventral Hippocampus. Neural Plast. 2016, 2016, 2540462. [Google Scholar] [CrossRef]
- Zubareva, O.E.; Postnikova, T.Y.; Grifluk, A.V.; Schwarz, A.P.; Smolensky, I.V.; Karepanov, A.A.; Vasilev, D.S.; Veniaminova, E.A.; Rotov, A.Y.; Kalemenev, S.V.; et al. Exposure to Bacterial Lipopolysaccharide in Early Life Affects the Expression of Ionotropic Glutamate Receptor Genes and Is Accompanied by Disturbances in Long-Term Potentiation and Cognitive Functions in Young Rats. Brain Behav. Immun. 2020, 90, 3–15. [Google Scholar] [CrossRef]
- Vertes, R.P. Interactions among the Medial Prefrontal Cortex, Hippocampus and Midline Thalamus in Emotional and Cognitive Processing in the Rat. Neuroscience 2006, 142, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Bai, W.; Xia, M.; Tian, X. Directional Hippocampal-Prefrontal Interactions during Working Memory. Behav. Brain Res. 2018, 338, 1–8. [Google Scholar] [CrossRef]
- Jin, J.; Maren, S. Prefrontal-Hippocampal Interactions in Memory and Emotion. Front. Syst. Neurosci. 2015, 9, 170. [Google Scholar] [CrossRef]
- Furtak, S.C.; Wei, S.-M.; Agster, K.L.; Burwell, R.D. Functional Neuroanatomy of the Parahippocampal Region in the Rat: The Perirhinal and Postrhinal Cortices. Hippocampus 2007, 17, 709–722. [Google Scholar] [CrossRef]
- Squire, L.R.; Stark, C.E.L.; Clark, R.E. The Medial Temporal Lobe. Annu. Rev. Neurosci. 2004, 27, 279–306. [Google Scholar] [CrossRef]
- Agster, K.L.; Burwell, R.D. Hippocampal and Subicular Efferents and Afferents of the Perirhinal, Postrhinal, and Entorhinal Cortices of the Rat. Behav. Brain Res. 2013, 254, 50–64. [Google Scholar] [CrossRef]
- Dubé, C.M.; Brewster, A.L.; Richichi, C.; Zha, Q.; Baram, T.Z. Fever, Febrile Seizures and Epilepsy. Trends Neurosci. 2007, 30, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Sawires, R.; Buttery, J.; Fahey, M. A Review of Febrile Seizures: Recent Advances in Understanding of Febrile Seizure Pathophysiology and Commonly Implicated Viral Triggers. Front. Pediatr. 2021, 9, 801321. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Vezzani, A.; Bartfai, T. Chapter 12—Mechanisms of Fever and Febrile Seizures: Putative Role of the Interleukin-1 System. In Febrile Seizures; Baram, T.Z., Shinnar, S., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 169–188. ISBN 978-0-12-078141-6. [Google Scholar]
- Dubé, C.M.; Brewster, A.L.; Baram, T.Z. Febrile Seizures: Mechanisms and Relationship to Epilepsy. Brain Dev. 2009, 31, 366–371. [Google Scholar] [CrossRef]
- Reid, A.Y.; Galic, M.A.; Teskey, G.C.; Pittman, Q.J. Febrile Seizures: Current Views and Investigations. Can. J. Neurol. Sci. 2009, 36, 679–686. [Google Scholar] [CrossRef]
- Millichap, J.G.J.; Millichap, J.G.J. Role of Viral Infections in the Etiology of Febrile Seizures. Pediatr. Neurol. 2006, 35, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D. Early-Life Infection Is a Vulnerability Factor for Aging-Related Glial Alterations and Cognitive Decline. Neurobiol. Learn. Mem. 2010, 94, 57–64. [Google Scholar] [CrossRef]
- Yi, Y.; Zhong, C.; Hu, W. The Long-Term Neurodevelopmental Outcomes of Febrile Seizures and Underlying Mechanisms. Front. Cell Dev. Biol. 2023, 11, 1186050. [Google Scholar] [CrossRef]
- Griflyuk, A.V.; Postnikova, T.Y.; Malkin, S.L.; Zaitsev, A.V. Alterations in Rat Hippocampal Glutamatergic System Properties after Prolonged Febrile Seizures. Int. J. Mol. Sci. 2023, 24, 16875. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Glutamatergic System Is Affected in Brain from an Hyperthermia-Induced Seizures Rat Model. Cell. Mol. Neurobiol. 2021, 42, 1501–1512. [Google Scholar] [CrossRef]
- Bellone, C.; Lüscher, C. Drug-Evoked Plasticity: Do Addictive Drugs Reopen a Critical Period of Postnatal Synaptic Development? Front. Mol. Neurosci. 2012, 5, 75. [Google Scholar] [CrossRef]
- Barker-Haliski, M.; White, H.S. Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022863. [Google Scholar] [CrossRef]
- Green, J.L.; Dos Santos, W.F.; Fontana, A.C.K. Role of Glutamate Excitotoxicity and Glutamate Transporter EAAT2 in Epilepsy: Opportunities for Novel Therapeutics Development. Biochem. Pharmacol. 2021, 193, 114786. [Google Scholar] [CrossRef]
- Wasterlain, C.G.; Shirasaka, Y. Seizures, Brain Damage and Brain Development. Brain Dev. 1994, 16, 279–295. [Google Scholar] [CrossRef]
- Swann, J.W.; Le, J.T.; Lam, T.T.; Owens, J.; Mayer, A.T. The Impact of Chronic Network Hyperexcitability on Developing Glutamatergic Synapses. Eur. J. Neurosci. 2007, 26, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Kubová, H.; Bendová, Z.; Moravcová, S.; Pačesová, D.; Rocha, L.L.; Mareš, P. Neonatal Clonazepam Administration Induces Long-Lasting Changes in Glutamate Receptors. Front. Mol. Neurosci. 2018, 11, 382. [Google Scholar] [CrossRef]
- Crespo, M.; León-Navarro, D.A.; Martín, M. Na(+)/K(+)- and Mg(2+)-ATPases and Their Interaction with AMPA, NMDA and D(2) Dopamine Receptors in an Animal Model of Febrile Seizures. Int. J. Mol. Sci. 2022, 23, 14638. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Kuo, Y.; Huang, A.; Huang, C. Repetitive Febrile Seizures in Rat Pups Cause Long-Lasting Deficits in Synaptic Plasticity and NR2A Tyrosine Phosphorylation. Neurobiol. Dis. 2005, 18, 466–475. [Google Scholar] [CrossRef]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain Development in Rodents and Humans: Identifying Benchmarks of Maturation and Vulnerability to Injury across Species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Marceau, K.; Ruttle, P.L.; Shirtcliff, E.A.; Essex, M.J.; Susman, E.J. Developmental and Contextual Considerations for Adrenal and Gonadal Hormone Functioning during Adolescence: Implications for Adolescent Mental Health. Dev. Psychobiol. 2015, 57, 742–768. [Google Scholar] [CrossRef] [PubMed]
- Trotman, H.D.; Holtzman, C.W.; Ryan, A.T.; Shapiro, D.I.; MacDonald, A.N.; Goulding, S.M.; Brasfield, J.L.; Walker, E.F. The Development of Psychotic Disorders in Adolescence: A Potential Role for Hormones. Horm. Behav. 2013, 64, 411–419. [Google Scholar] [CrossRef]
- Griflyuk, A.V.; Postnikova, T.Y.; Zaitsev, A.V. Animal Models of Febrile Seizures: Limitations and Recent Advances in the Field. Cells 2024, 13, 1895. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The Open-Field Test: A Critical Review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of Open:Closed Arm Entries in an Elevated plus-Maze as a Measure of Anxiety in the Rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Herrman, L.; Palmatier, M.I.; Bevins, R.A. Rats’ Novel Object Interaction as a Measure of Environmental Familiarity. Learn. Motiv. 2006, 37, 131–148. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The Novel Object Recognition Memory: Neurobiology, Test Procedure, and Its Modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- File, S.E.; Hyde, J.R. Can Social Interaction Be Used to Measure Anxiety? Br. J. Pharmacol. 1978, 62, 19–24. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006; ISBN 9780080475134. [Google Scholar]
- Schwarz, A.P.; Malygina, D.A.; Kovalenko, A.A.; Trofimov, A.N.; Zaitsev, A.V. Multiplex QPCR Assay for Assessment of Reference Gene Expression Stability in Rat Tissues/Samples. Mol. Cell. Probes 2020, 53, 101611. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kovalenko, A.A.; Zakharova, M.V.; Schwarz, A.P.; Zubareva, O.E.; Zaitsev, A.V. Identification of Reliable Reference Genes for Use in Gene Expression Studies in Rat Febrile Seizure Model. Int. J. Mol. Sci. 2024, 25, 11125. [Google Scholar] [CrossRef]
- Bonefeld, B.E.; Elfving, B.; Wegener, G. Reference Genes for Normalization: A Study of Rat Brain Tissue. Synapse 2008, 62, 302–309. [Google Scholar] [CrossRef]
- Lin, W.; Burks, C.A.; Hansen, D.R.; Kinnamon, S.C.; Gilbertson, T.A. Taste Receptor Cells Express PH-Sensitive Leak K + Channels. J. Neurophysiol. 2004, 92, 2909–2919. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yamauchi, A.; Nishimura, M.; Ueda, N.; Naito, S. Soybean Oil Fat Emulsion Prevents Cytochrome P450 MRNA Down-Regulation Induced by Fat-Free Overdose Total Parenteral Nutrition in Infant Rats. Biol. Pharm. Bull. 2005, 28, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Swijsen, A.; Nelissen, K.; Janssen, D.; Rigo, J.-M.; Hoogland, G. Validation of Reference Genes for Quantitative Real-Time PCR Studies in the Dentate Gyrus after Experimental Febrile Seizures. BMC Res. Notes 2012, 5, 685. [Google Scholar] [CrossRef] [PubMed]
- Pohjanvirta, R.; Niittynen, M.; Lindén, J.; Boutros, P.C.; Moffat, I.D.; Okey, A.B. Evaluation of Various Housekeeping Genes for Their Applicability for Normalization of MRNA Expression in Dioxin-Treated Rats. Chem. Biol. Interact. 2006, 160, 134–149. [Google Scholar] [CrossRef]
- Malkin, S.L.; Amakhin, D.V.; Veniaminova, E.A.; Kim, K.K.; Zubareva, O.E.; Magazanik, L.G.; Zaitsev, A.V. Changes of AMPA Receptor Properties in the Neocortex and Hippocampus Following Pilocarpine-Induced Status Epilepticus in Rats. Neuroscience 2016, 327, 146–155. [Google Scholar] [CrossRef]
- Cook, N.L.; Vink, R.; Donkin, J.J.; van den Heuvel, C. Validation of Reference Genes for Normalization of Real-Time Quantitative RT-PCR Data in Traumatic Brain Injury. J. Neurosci. Res. 2009, 87, 34–41. [Google Scholar] [CrossRef]
- Langnaese, K.; John, R.; Schweizer, H.; Ebmeyer, U.; Keilhoff, G. Selection of Reference Genes for Quantitative Real-Time PCR in a Rat Asphyxial Cardiac Arrest Model. BMC Mol. Biol. 2008, 9, 53. [Google Scholar] [CrossRef]
- Kovalenko, A.A.; Zakharova, M.V.; Schwarz, A.P.; Dyomina, A.V.; Zubareva, O.E.; Zaitsev, A.V. Changes in Metabotropic Glutamate Receptor Gene Expression in Rat Brain in a Lithium-Pilocarpine Model of Temporal Lobe Epilepsy. Int. J. Mol. Sci. 2022, 23, 2725. [Google Scholar] [CrossRef] [PubMed]
- Dulman, R.S.; Auta, J.; Teppen, T.; Pandey, S.C. Acute Ethanol Produces Ataxia and Induces Fmr1 Expression via Histone Modifications in the Rat Cerebellum. Alcohol. Clin. Exp. Res. 2019, 43, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yan, C.-H.; Wu, S.-H.; Yu, X.-D.; Yu, X.-G.; Shen, X.-M. Developmental Lead Exposure Alters Gene Expression of Metabotropic Glutamate Receptors in Rat Hippocampal Neurons. Neurosci. Lett. 2007, 413, 222–226. [Google Scholar] [CrossRef]
- Giza, C.C.; Maria, N.S.S.; Hovda, D.A. N-Methyl-D-Aspartate Receptor Subunit Changes after Traumatic Injury to the Developing Brain. J. Neurotrauma 2006, 23, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Floyd, D.W.; Jung, K.-Y.; McCool, B.A. Chronic Ethanol Ingestion Facilitates N-Methyl-D-Aspartate Receptor Function and Expression in Rat Lateral/Basolateral Amygdala Neurons. J. Pharmacol. Exp. Ther. 2003, 307, 1020–1029. [Google Scholar] [CrossRef]
- Proudnikov, D.; Yuferov, V.; Zhou, Y.; LaForge, K.S.; Ho, A.; Kreek, M.J. Optimizing Primer—Probe Design for Fluorescent PCR. J. Neurosci. Methods 2003, 123, 31–45. [Google Scholar] [CrossRef]
- Zakharova, M.V.; Kovalenko, A.A.; Zubareva, O.E.; Schwarz, A.P.; Postnikova, T.Y.; Trofimova, A.M.; Zaitsev, A.V. Pentylenetetrazole-Induced Seizures Cause Short-Term Changes in the Phenotype of Microglial and Astroglial Cells in the Hippocampus and Temporal Cortex of Young Male Wistar Rats. J. Neurosci. Res. 2024, 102, e25385. [Google Scholar] [CrossRef]
- O’Donovan, S.M.; Hasselfeld, K.; Bauer, D.; Simmons, M.; Roussos, P.; Haroutunian, V.; Meador-Woodruff, J.H.; McCullumsmith, R.E. Glutamate Transporter Splice Variant Expression in an Enriched Pyramidal Cell Population in Schizophrenia. Transl. Psychiatry 2015, 5, e579. [Google Scholar] [CrossRef]
- Dyomina, A.V.; Zubareva, O.E.; Smolensky, I.V.; Vasilev, D.S.; Zakharova, M.V.; Kovalenko, A.A.; Schwarz, A.P.; Ischenko, A.M.; Zaitsev, A.V. Anakinra Reduces Epileptogenesis, Provides Neuroprotection, and Attenuates Behavioral Impairments in Rats in the Lithium–Pilocarpine Model of Epilepsy. Pharmaceuticals 2020, 13, 340. [Google Scholar] [CrossRef]
- Zakharova, M.V.; Dyomina, A.V.; Kovalenko, A.A.; Zubareva, O.E.; Ischenko, A.M.; Zaitsev, A.V. Anakinra Promotes M2 Microglia Activation during the Latent Phase of the Lithium-Pilocarpine Model of Temporal Lobe Epilepsy. J. Evol. Biochem. Physiol. 2024, 60, 672–689. [Google Scholar] [CrossRef]
- Postnikova, T.Y.; Griflyuk, A.V.; Amakhin, D.V.; Kovalenko, A.A.; Soboleva, E.B.; Zubareva, O.E.; Zaitsev, A.V. Early Life Febrile Seizures Impair Hippocampal Synaptic Plasticity in Young Rats. Int. J. Mol. Sci. 2021, 22, 8218. [Google Scholar] [CrossRef] [PubMed]
- Karantysh, G.V.; Fomenko, M.P.; Menzheritskii, A.M.; Prokof’ev, V.N.; Ryzhak, G.A.; Butenko, E.V. Effect of Pinealon on Learning and Expression of NMDA Receptor Subunit Genes in the Hippocampus of Rats with Experimental Diabetes. Neurochem. J. 2020, 14, 314–320. [Google Scholar] [CrossRef]
- Bar-Shira, O.; Maor, R.; Chechik, G. Gene Expression Switching of Receptor Subunits in Human Brain Development. PLoS Comput. Biol. 2015, 11, e1004559. [Google Scholar] [CrossRef]
- Mewasingh, L.D.; Chin, R.F.M.; Scott, R.C. Current Understanding of Febrile Seizures and Their Long-Term Outcomes. Dev. Med. Child Neurol. 2020, 62, 1245–1249. [Google Scholar] [CrossRef]
- Gould, L.; Delavale, V.; Plovnick, C.; Wisniewski, T.; Devinsky, O. Are Brief Febrile Seizures Benign? A Systematic Review and Narrative Synthesis. Epilepsia 2023, 64, 2539–2549. [Google Scholar] [CrossRef]
- Scott, R.C. What Are the Effects of Prolonged Seizures in the Brain? Epileptic Disord. 2014, 16, S6–S11. [Google Scholar] [CrossRef]
- Cendes, F. Febrile Seizures and Mesial Temporal Sclerosis. Curr. Opin. Neurol. 2004, 17, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Sokol, D.K.; Demyer, W.E.; Edwards-Brown, M.; Sanders, S.; Garg, B. From Swelling to Sclerosis: Acute Change in Mesial Hippocampus after Prolonged Febrile Seizure. Seizure 2003, 12, 237–240. [Google Scholar] [CrossRef]
- Gulyaeva, N.V. Functional Neurochemistry of the Ventral and Dorsal Hippocampus: Stress, Depression, Dementia and Remote Hippocampal Damage. Neurochem. Res. 2019, 44, 1306–1322. [Google Scholar] [CrossRef]
- Nazem, A.; Jafarian, A.H.; Sadraie, S.H.; Gorji, A.; Kheradmand, H.; Radmard, M.; Haghir, H. Neuronal Injury and Cytogenesis after Simple Febrile Seizures in the Hippocampal Dentate Gyrus of Juvenile Rat. Child’s Nerv. Syst. 2012, 28, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Hino, H.; Takahashi, H.; Suzuki, Y.; Tanaka, J.; Ishii, E.; Fukuda, M. Anticonvulsive Effect of Paeoniflorin on Experimental Febrile Seizures in Immature Rats: Possible Application for Febrile Seizures in Children. PLoS ONE 2012, 7, e42920. [Google Scholar] [CrossRef]
- Mosili, P.; Maikoo, S.; Mabandla, M.V.; Qulu, L. The Pathogenesis of Fever-Induced Febrile Seizures and Its Current State. Neurosci. Insights 2020, 15, 2633105520956973. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Furukawa, H.; Wollmuth, L.P.; Gibb, A.J.; Traynelis, S.F. Structure, Function, and Allosteric Modulation of NMDA Receptors. J. Gen. Physiol. 2018, 150, 1081–1105. [Google Scholar] [CrossRef]
- Chen, B.; Feng, B.; Tang, Y.; You, Y.; Wang, Y.; Hou, W.; Hu, W.; Chen, Z. Blocking GluN2B Subunits Reverses the Enhanced Seizure Susceptibility after Prolonged Febrile Seizures with a Wide Therapeutic Time-Window. Exp. Neurol. 2016, 283, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA Receptor Subunit Diversity: Impact on Receptor Properties, Synaptic Plasticity and Disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Acutain, M.F.; Baez, M.V. Reduced Expression of GluN2A Induces a Delay in Neuron Maturation. J. Neurochem. 2024, 168, 4001–4013. [Google Scholar] [CrossRef]
- Reid, A.Y.; Riazi, K.; Campbell Teskey, G.; Pittman, Q.J. Increased Excitability and Molecular Changes in Adult Rats after a Febrile Seizure. Epilepsia 2013, 54, e45–e48. [Google Scholar] [CrossRef]
- Herring, B.E.; Nicoll, R.A. Long-Term Potentiation: From CaMKII to AMPA Receptor Trafficking. Annu. Rev. Physiol. 2016, 78, 351–365. [Google Scholar] [CrossRef]
- Jaskova, K.; Pavlovicova, M.; Jurkovicova, D. Calcium Transporters and Their Role in the Development of Neuronal Disease and Neuronal Damage. Gen. Physiol. Biophys. 2012, 31, 375–382. [Google Scholar] [CrossRef]
- Tang, X.-J.; Xing, F. Calcium-Permeable AMPA Receptors in Neonatal Hypoxic-Ischemic Encephalopathy (Review). Biomed. Rep. 2013, 1, 828–832. [Google Scholar] [CrossRef]
- Niswender, C.M.; Conn, P.J. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 295–322. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.-M.; Bodepudi, A.; Chu, X.-P.; Wang, J.Q. Group I Metabotropic Glutamate Receptors and Interacting Partners: An Update. Int. J. Mol. Sci. 2022, 23, 840. [Google Scholar] [CrossRef]
- Nicoletti, F.; Bockaert, J.; Collingridge, G.L.; Conn, P.J.; Ferraguti, F.; Schoepp, D.D.; Wroblewski, J.T.; Pin, J.P. Metabotropic Glutamate Receptors: From the Workbench to the Bedside. Neuropharmacology 2011, 60, 1017–1041. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, A.V.; Smolensky, I.V.; Jorratt, P.; Ovsepian, S.V. Neurobiology, Functions, and Relevance of Excitatory Amino Acid Transporters (EAATs) to Treatment of Refractory Epilepsy. CNS Drugs 2020, 34, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Holmseth, S.; Dehnes, Y.; Huang, Y.H.; Follin-Arbelet, V.V.; Grutle, N.J.; Mylonakou, M.N.; Plachez, C.; Zhou, Y.; Furness, D.N.; Bergles, D.E.; et al. The Density of EAAC1 (EAAT3) Glutamate Transporters Expressed by Neurons in the Mammalian CNS. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 6000–6013. [Google Scholar] [CrossRef]
- Rothstein, J.D.; Dykes-Hoberg, M.; Pardo, C.A.; Bristol, L.A.; Jin, L.; Kuncl, R.W.; Kanai, Y.; Hediger, M.A.; Wang, Y.; Schielke, J.P.; et al. Knockout of Glutamate Transporters Reveals a Major Role for Astroglial Transport in Excitotoxicity and Clearance of Glutamate. Neuron 1996, 16, 675–686. [Google Scholar] [CrossRef]
- Bjørn-Yoshimoto, W.E.; Underhill, S.M. The Importance of the Excitatory Amino Acid Transporter 3 (EAAT3). Neurochem. Int. 2016, 98, 4–18. [Google Scholar] [CrossRef]
- Tzingounis, A.V.; Wadiche, J.I. Glutamate Transporters: Confining Runaway Excitation by Shaping Synaptic Transmission. Nat. Rev. Neurosci. 2007, 8, 935–947. [Google Scholar] [CrossRef]
- Cercato, M.C.; Vázquez, C.A.; Kornisiuk, E.; Aguirre, A.I.; Colettis, N.; Snitcofsky, M.; Jerusalinsky, D.A.; Baez, M.V. GluN1 and GluN2A NMDA Receptor Subunits Increase in the Hippocampus during Memory Consolidation in the Rat. Front. Behav. Neurosci. 2016, 10, 242. [Google Scholar] [CrossRef]
- Daenen, E.W.; Van der Heyden, J.A.; Kruse, C.G.; Wolterink, G.; Van Ree, J.M. Adaptation and Habituation to an Open Field and Responses to Various Stressful Events in Animals with Neonatal Lesions in the Amygdala or Ventral Hippocampus. Brain Res. 2001, 918, 153–165. [Google Scholar] [CrossRef]
- Leussis, M.P.; Bolivar, V.J. Habituation in Rodents: A Review of Behavior, Neurobiology, and Genetics. Neurosci. Biobehav. Rev. 2006, 30, 1045–1064. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Wang, T.; Chen, L. Dopamine D1 Receptor in the Medial Prefrontal Cortex Mediates Anxiety-like Behaviors Induced by Blocking Glutamatergic Activity of the Ventral Hippocampus in Rats. Brain Res. 2019, 1704, 59–67. [Google Scholar] [CrossRef]
- Chao, O.Y.; de Souza Silva, M.A.; Yang, Y.-M.; Huston, J.P. The Medial Prefrontal Cortex-Hippocampus Circuit That Integrates Information of Object, Place and Time to Construct Episodic Memory in Rodents: Behavioral, Anatomical and Neurochemical Properties. Neurosci. Biobehav. Rev. 2020, 113, 373–407. [Google Scholar] [CrossRef] [PubMed]
- van Vugt, B.; van Kerkoerle, T.; Vartak, D.; Roelfsema, P.R. The Contribution of AMPA and NMDA Receptors to Persistent Firing in the Dorsolateral Prefrontal Cortex in Working Memory. J. Neurosci. 2020, 40, 2458–2470. [Google Scholar] [CrossRef]
- Davies, D.A.; Greba, Q.; Howland, J.G. GluN2B-Containing NMDA Receptors and AMPA Receptors in Medial Prefrontal Cortex Are Necessary for Odor Span in Rats. Front. Behav. Neurosci. 2013, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Morici, J.F.; Bekinschtein, P.; Weisstaub, N.V. Medial Prefrontal Cortex Role in Recognition Memory in Rodents. Behav. Brain Res. 2015, 292, 241–251. [Google Scholar] [CrossRef]
- Mesquita, A.R.; Tavares, H.B.; Silva, R.; Sousa, N. Febrile Convulsions in Developing Rats Induce a Hyperanxious Phenotype Later in Life. Epilepsy Behav. 2006, 9, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L.; Wood, C.M.; Roberts, A.C. The Ventromedial Prefrontal Cortex and Emotion Regulation: Lost in Translation? J. Physiol. 2023, 601, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Mercado, D.; Padilla-Coreano, N.; Quirk, G.J. Dissociable Roles of Prelimbic and Infralimbic Cortices, Ventral Hippocampus, and Basolateral Amygdala in the Expression and Extinction of Conditioned Fear. Neuropsychopharmacology 2011, 36, 529–538. [Google Scholar] [CrossRef]
- Suzuki, S.; Saitoh, A.; Ohashi, M.; Yamada, M.; Oka, J.-I.; Yamada, M. The Infralimbic and Prelimbic Medial Prefrontal Cortices Have Differential Functions in the Expression of Anxiety-like Behaviors in Mice. Behav. Brain Res. 2016, 304, 120–124. [Google Scholar] [CrossRef]
- Parfitt, G.M.; Nguyen, R.; Bang, J.Y.; Aqrabawi, A.J.; Tran, M.M.; Seo, D.K.; Richards, B.A.; Kim, J.C. Bidirectional Control of Anxiety-Related Behaviors in Mice: Role of Inputs Arising from the Ventral Hippocampus to the Lateral Septum and Medial Prefrontal Cortex. Neuropsychopharmacology 2017, 42, 1715–1728. [Google Scholar] [CrossRef]
- Majidi, J.; Kosari-Nasab, M.; Salari, A.-A. Developmental Minocycline Treatment Reverses the Effects of Neonatal Immune Activation on Anxiety- and Depression-like Behaviors, Hippocampal Inflammation, and HPA Axis Activity in Adult Mice. Brain Res. Bull. 2016, 120, 1–13. [Google Scholar] [CrossRef]
- Remonde, C.G.; Gonzales, E.L.; Adil, K.J.; Jeon, S.J.; Shin, C.Y. Augmented Impulsive Behavior in Febrile Seizure-Induced Mice. Toxicol. Res. 2023, 39, 37–51. [Google Scholar] [CrossRef]
- Sillanpää, M.; Suominen, S.; Rautava, P.; Aromaa, M. Academic and Social Success in Adolescents with Previous Febrile Seizures. Seizure 2011, 20, 326–330. [Google Scholar] [CrossRef][Green Version]
- Klatte, K.; Kirschstein, T.; Otte, D.; Pothmann, L.; Müller, L.; Tokay, T.; Kober, M.; Uebachs, M.; Zimmer, A.; Beck, H. Impaired D-Serine-Mediated Cotransmission Mediates Cognitive Dysfunction in Epilepsy. J. Neurosci. 2013, 33, 13066–13080. [Google Scholar] [CrossRef]
- Nagai, T.; Yu, J.; Kitahara, Y.; Nabeshima, T.; Yamada, K. D-Serine Ameliorates Neonatal PolyI:C Treatment-Induced Emotional and Cognitive Impairments in Adult Mice. J. Pharmacol. Sci. 2012, 120, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Guercio, G.D.; Bevictori, L.; Vargas-Lopes, C.; Madeira, C.; Oliveira, A.; Carvalho, V.F.; d’Avila, J.C.; Panizzutti, R. D-Serine Prevents Cognitive Deficits Induced by Acute Stress. Neuropharmacology 2014, 86, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Otte, D.-M.; Barcena de Arellano, M.L.; Bilkei-Gorzo, A.; Albayram, O.; Imbeault, S.; Jeung, H.; Alferink, J.; Zimmer, A. Effects of Chronic D-Serine Elevation on Animal Models of Depression and Anxiety-Related Behavior. PLoS ONE 2013, 8, e67131. [Google Scholar] [CrossRef]
- Pitsikas, N. The Metabotropic Glutamate Receptors: Potential Drug Targets for the Treatment of Anxiety Disorders? Eur. J. Pharmacol. 2014, 723, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Hovelsø, N.; Sotty, F.; Montezinho, L.P.; Pinheiro, P.S.; Herrik, K.F.; Mørk, A. Therapeutic Potential of Metabotropic Glutamate Receptor Modulators. Curr. Neuropharmacol. 2012, 10, 12–48. [Google Scholar] [CrossRef] [PubMed]
- Henter, I.D.; Park, L.T.; Zarate, C.A. Novel Glutamatergic Modulators for the Treatment of Mood Disorders: Current Status. CNS Drugs 2021, 35, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Nunez, J.L.; McCarthy, M.M. Evidence for an Extended Duration of GABA-Mediated Excitation in the Developing Male Versus Female Hippocampus. Dev. Neurobiol. 2007, 14, 1879–1890. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).