Abstract
Crohn’s disease (CD), also known as terminal ileitis, has been the focus of gastroenterological diagnostics and therapy for decades. Although significant therapeutic progress has been made in recent years, largely due to an improved understanding of the pathophysiology and evolving treatment strategies for Crohn’s disease, many new antibody-based therapies demonstrate clinical response rates of only 30–50%. Predictive biomarkers for differential therapeutic responses may therefore be critical for personalized treatment selection, but such markers have not yet been clinically validated for the majority of patients treated with prednisone or monoclonal antibodies targeting integrin pathways, TNF-α, or IL-23. In this review, the diagnostic potential of microRNA (miRNA) dysregulation in patients with Crohn’s disease is explored, emphasizing the potential utility of specific miRNA expression profiles in guiding targeted therapy. Notably, reduced expression of miR-29 is associated with planned treatment using ustekinumab (an IL-23 signaling inhibitor), elevated miR-23a levels in inflamed tissue may inform the use of TNF-α inhibitors, increased miR-155 expression is relevant for patients considered for JAK inhibitor therapy, and altered levels of miR-126 and miR-486 may support the selection of vedolizumab. Assessment of these dysregulated miRNAs—such as through comparative profiling in inflamed versus non-inflamed tissue from the same patients—could serve as a predictive biomarker panel to optimize individualized immunosuppressive treatment strategies in Crohn’s disease. We also examine the role of microRNAs in regulating TRP channels and their involvement in the mechanisms of action of selected complementary medicines.
1. Introduction
Crohn’s disease (CD), also known as terminal ileitis, is a chronic, relapsing inflammatory disorder affecting any part of the gastrointestinal tract, typified by discontinuous, transmural inflammation. It often leads to complications such as strictures, fistulas, and extra-intestinal manifestations. Accurate diagnosis is essential to optimize management and mitigate complications. Today, no single gold-standard test exists for diagnosing CD or guiding treatment decisions. Instead, CD is diagnosed using a multimodal approach that combines clinical evaluation with laboratory tests (including inflammatory markers and fecal calprotectin), endoscopic procedures with biopsies, and cross-sectional imaging such as MRI enterography or intestinal ultrasound, to confirm chronic intestinal inflammation and exclude alternative causes. Routine genetic or serological testing is not recommended for standard diagnostic work-up so far.
Until recently, first-line treatment for Crohn’s disease primarily involved broad T-cell-targeting drugs such as corticosteroids or azathioprine, both associated with considerable side effects, especially with long-term use. Systemic or topical steroids are still primarily employed in the acute phase. Approximately 70% of patients respond rapidly, but recurrence remains a major clinical challenge. For instance, an epidemiological study reported a relapse rate exceeding 50% within 12 months under combined therapy with glucocorticoids and 5-aminosalicylates (5-ASA) [1]. This high recurrence rate raises the question of whether traditional therapeutic goals in Crohn’s disease—including mucosal healing, stenosis prevention, and avoidance of surgery—can be sustainably achieved with “classical” therapies. The answer is likely no, and experienced clinicians are increasingly adopting novel therapeutic strategies that are both effective and durable.
In contrast, rheumatoid arthritis (RA) treatment with targeted antibody therapies is well established, resulting in symptom resolution, improved quality of life, and inhibition of joint destruction. As part of the “hit hard and early” strategy, potent and targeted immunosuppressants, including TNF-α blockers or interleukin-23 (IL-23) antagonists, have been introduced early in treatment for over 15 years. However, in countries like Germany, these therapies are largely restricted to outpatient settings due to costs and the lack of predictive biomarkers for therapeutic response, with success rates averaging 30–50% [1].
The development of neutralizing antibodies against TNF-α or IL-23 has introduced new treatment options for Crohn’s disease, similar to those in RA. Yet in practice, many patients fail to achieve durable responses, often due to economic limitations or treatment resistance. Thus, novel biomarkers are required prior to therapy initiation, since modern therapies are extremely cost-intensive, and predictive markers could potentially prevent ineffective treatments in up to 30–40% of patients. This review will present and discuss emerging tools based on specific therapy regimens.
2. The Role of microRNAs in Chronic Inflammatory Disorders and Cancer Diseases
Approximately 20 years ago, miRNAs were initially shown to play a key role in colorectal cancer (CRC). These small, non-coding RNA molecules, composed of 22–25 nucleotides, regulate mRNA translation by acting at the RNA-induced silencing complex (RISC). Humans express over 1000 known miRNAs, with many more presumed to exist. Recent studies have shown that miRNAs can influence programmed cell death by interacting with promoter regions of cell cycle regulators. Currently, around 1400 distinct miRNAs have been identified that bind to specific mRNA sequences, thereby inhibiting protein translation [2].
An increasing body of evidence suggests that microRNA (miRNA) dysregulation plays a causal or indicative role in many chronic inflammatory diseases of the gastrointestinal tract [3,4,5]. Differences in circulating miRNA levels—either in blood or in tissue samples—can serve as biomarkers for diagnosis, treatment response, or prognosis. Tissue-specific deregulation of miRNA expression has been reported in diseases such as multiple sclerosis and Crohn’s disease [6,7,8,9,10].
In recent years, circulating and fecal microRNAs (miRNAs) have gained attention as promising novel biomarkers for predicting therapeutic responses in patients with inflammatory bowel disease (IBD). Batra et al. reported significant alterations in the expression of seven miRNAs following treatment, distinguishing responders from non-responders in a small cohort of pediatric IBD patients receiving anti-TNF therapy [11]. However, a separate study investigating miRNA polymorphisms and their association with anti-TNF treatment response in Crohn’s disease (CD) found no significant correlations between the dysregulation of specific miRNAs (miR-146, miR-196a, miR-221, miR-224) and clinical outcomes in patients treated with anti-TNF monoclonal antibodies [12]. These conflicting findings underscore the need for further large-scale, well-controlled studies to validate the predictive value of miRNAs for treatment stratification in IBD.
3. MicroRNAs Dysregulation in Inactive (Quiescent) Crohn’s Disease
In a pioneering 2010 study, Fasseu et al. identified 14 and 23 miRNAs differentially expressed (0.001 < p < 0.05) in patients with inactive ulcerative colitis (UC) and inactive Crohn’s disease (CD), respectively [13]. Of these, eight miRNAs were commonly dysregulated in both diseases (miR-26a-5p, miR-29a-3p, miR-29b-5p, miR-30c-5p, miR-126-3p, miR-127-3p, miR-196a-5p, and miR-324-3p). Further analyses revealed that miR-26a-5p, miR-29b-5p, miR-126-3p, miR-127-3p, and miR-324-3p exhibited coordinated differential regulation in non-inflamed and inflamed colonic mucosa of patients with inflammatory bowel diseases. Other miRNAs, including miR-196b-5p, miR-199a-3p, miR-199b-5p, miR-320a-5p, miR-150-5p, and miR-223-3p, were differentially expressed between non-inflamed UC and CD tissue samples. Based on these findings, it has been proposed that miRNA dysregulation plays an important role in inflammation onset and relapse in IBD patients with quiescent mucosa [13].
4. MicroRNA Dysregulation in Active Crohn’s Disease
Initial evidence of altered miRNA expression in active IBD was reported by Wu et al., who found that patients with active UC exhibited elevated levels of eight miRNAs (miR-16, -21, -23a, -24, -29a, -126, -195, and Let-7f) and reduced levels of three miRNAs (miR-192, -375, and -422b) in intestinal mucosa compared to healthy individuals [14]. More recent work by de Sibia et al. identified ~15 upregulated and 6 downregulated miRNAs in active CD, including miR-16, -21, -23b, -31, -101, -106, -146, -191, -199a-5p, -206, -223, -340, -362–3p, and miR-375 [15]. An overview of the role of these microRNAs is given in Table 1 and Figure 1. Notably, miR-486 and miR-320a are particularly involved in traditional treatment responses with steroids (see Figure 1), as prednisone downregulates both [2]. Morilla et al. identified specific miRNAs associated with responses to corticosteroids, infliximab, and cyclosporine [16]. Cordes et al. reported that miR-320a levels were significantly increased in active IBD and strongly correlated with endoscopic disease activity, supporting its use as a non-invasive biomarker [17].

Table 1.
General biomarkers of activity in patients with CD, serving as possible diagnostic markers.
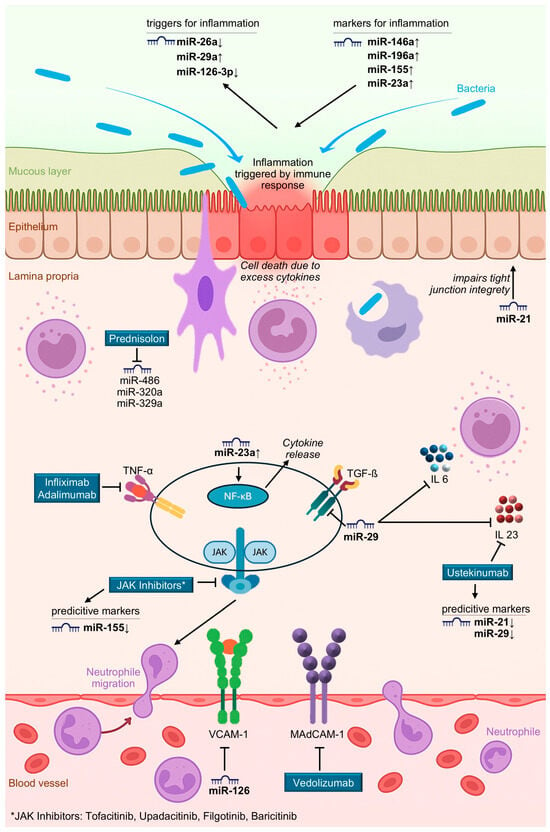
Figure 1.
Illustration of selected MicroRNA dysregulation and Drug Targets in inflammatory bowel disease.
5. TRP Channels in Crohn’s Disease
Transient Receptor Potential (TRP) channels are a diverse family of ion channels located on cell membranes, where they regulate calcium, sodium, and other ion influx in response to stimuli such as temperature, mechanical stress, pH, and chemical signals. They are central to sensory processes, pain signaling, inflammation, and barrier integrity, making them relevant modulators to Crohn’s disease. TRP channels can be regulated by microRNAs (miRNAs), linking epigenetic mechanisms to channel function.
For example, the transient receptor potential vanilloid 1 (TRPV1) and ankyrin 1 (TRPA1) channels, predominantly expressed on capsaicin-sensitive sensory neurons, contribute to hyperalgesia and neurogenic inflammation [23,24]. In Crohn’s patients, TRPA1 promoter methylation is dysregulated, correlating with reduced pressure pain thresholds and suggesting epigenetic control of its expression [25]. While direct evidence in inflammatory bowel disease is limited, studies in irritable bowel syndrome show that miR-199 upregulates TRPV1, thereby enhancing visceral pain—an effect that is plausibly relevant to IBD [26].
TRPV4 is highly expressed in the intestinal epithelium and is frequently upregulated in IBD tissue, where it promotes barrier dysfunction, cytokine release, and altered motility [27,28,29]. Pharmacological blockade of TRPV4 alleviates experimental colitis [30]. Furthermore, TRPV4 is a validated target of miR-203; overexpression of this miRNA reduces TRPV4 levels and downstream signaling. Given TRPV4’s pro-inflammatory role in the gut, therapeutic delivery of miR-203 mimics represents a promising strategy to attenuate TRPV4-mediated pathology in Crohn’s disease [31].
6. Treatment with TNF-α Antibodies and the Role of Specific microRNAs
In Crohn’s disease, chronic inflammation is characterized by an accumulation of activated monocytes/macrophages that secrete TNF-α. Clinical studies have demonstrated the key role of TNF-α, and large international multicenter trials have assessed the efficacy of infliximab, a chimeric monoclonal antibody against TNF-α. Despite its effectiveness, infliximab induces remission in only ~30% of patients. In the ACCENT I trial, long-term response rates ranged between 17 and 22%, compared to 9% in the placebo group [32]. No predictive biomarkers for infliximab response have been validated.
Felwick et al. reported that miR-23a levels were significantly increased in Crohn’s epithelium relative to healthy tissue. miR-23a targets TNF-α inhibitor protein 3, leading to NF-κB activation, increased epithelial permeability, and elevated cytokine release, mimicking Crohn’s disease pathology [33].
7. Treatment with Vedolizumab and microRNA Dysregulation
Vedolizumab, a humanized integrin antagonist used in therapy of CD and ulcerative colitis (UC), shows a risk ratio (RR) of 2.01 (95% CI: 1.5–2.71) in three RCTs [34,35,36], achieving a significant therapy improvement in patients with CD. However, a meta-analysis showed no significant difference between Ustekinumab and Vedolizumab in patients refractory to TNF-α therapy [37]. miRNAs relevant to vedolizumab signaling include miR-126 (downregulated), affecting β-integrin activation. miR-126 also modulates VCAM-1, and its suppression mimics vedolizumab’s inhibition of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) [38]. Harris et al. and Pathak et al. have demonstrated similar effects [39,40].
8. Treatment with Ustekinumab and microRNA Dysregulation
Ustekinumab targets IL-12/23 and demonstrates high efficacy in psoriasis and Crohn’s disease, particularly in TNF-refractory cases [41,42]. Using this new antibody, the UNITI-1 and UNITI-2 studies and meta-analyses report excellent efficacy in over 50% of CD patients, with RR values of 1.76 (95% CI: 1.4–2.22) and treatment success can be sustained for almost three years. However, dose escalation was required in over 80% of cases [43]. Ustekinumab shows the best long-term mucosal healing of all agents evaluated.
In this context, miRNAs such as miR-21 and miR-29 have been shown to regulate IL-17 and IL-23 expression, suggesting potential as predictive markers for ustekinumab response. miR-29 downregulates TGF-β, IL-6, and IL-23, mimicking ustekinumab’s effect [44,45]. miR-21 and miR-223 impair tight junction integrity, while miR-200 and miR-93 support it. miR-149 promotes a pathogenic microbiota shift.
Taken together, evaluation of these dysregulated miRNAs in inflamed versus healthy tissue could serve as a predictive biomarker panel to optimize individualized immunosuppressive treatment strategies in Crohn’s disease. In detail, maybe as a panel of investigated microRNA expression, reduced expression of miR-29 can favor a planned treatment using ustekinumab (an IL-23 signaling inhibitor), while elevated miR-23a levels in inflamed tissue may suggest the use of TNF-α inhibitors. Increased miR-155 expression could be relevant for patients considered for JAK inhibitor therapy, and altered levels of miR-126 and miR-486 may support the selection of vedolizumab. An overview summarizing the targets of mode of actions is provided in Figure 1 and Table 2, outlining targets and molecular pathways involved in the microRNA used as predictors.

Table 2.
Quintessence–Selective microRNA-biomarkers with a potential of predicting therapeutic response to antibody-based therapy in patients with Crohn’s Disease, based on elevated microRNA expression in inflamed tissue.
9. Treatment with Complementary Medicine and microRNA Dysregulation
In recent years, traditional medicine-derived approaches have attracted growing interest as part of complementary and alternative medicine, with numerous studies investigating their potential role in Crohn’s disease. Among these, probiotics and microbiota modulation are the most extensively explored. Evidence suggests that the gut microbiota can reduce mucosal inflammation and improve epithelial barrier function. Strains such as Escherichia coli Nissle 1917 [46], as well as specific Lactobacillus and Bifidobacterium mixes [47,48], have been evaluated as potential therapeutic candidates. However, their clinical benefit has been demonstrated more consistently in ulcerative colitis than in Crohn’s. Importantly, microbiota can modulate host microRNA profiles in ways consistent with reduced inflammation and improved barrier integrity in experimental models, although human results remain variable and strain-specific [49]. For example, several probiotic strains reduce miR-155 expression in experimental colitis [50], an miRNA typically associated with pro-inflammatory pathways. In addition, some probiotics regulate miR-223 [49], which is linked to neutrophil activity and inflammasome regulation.
Another complementary approach is curcumin, the active compound in turmeric. Although clinical evidence is stronger for ulcerative colitis, mechanistic studies support its potential role in Crohn’s. Curcumin primarily acts by suppressing inflammatory pathways, particularly NF-κB signaling, thereby modulating immune responses [51]. Recent findings suggest that curcumin promotes intestinal epithelial renewal in part through downregulation of miR-195-3p, which otherwise inhibits epithelial proliferation and wound repair [52]. In cancer and inflammatory models, curcumin has also been shown to alter the expression of several key miRNAs, including miR-21, miR-34a, and miR-155, further highlighting its broad impact on inflammatory signaling pathways relevant to IBD [53].
Omega-3 polyunsaturated fatty acids (EPA and DHA) represent another widely studied intervention. These lipids influence cell membrane composition and eicosanoid balance. While mechanistic studies demonstrate modulation of inflammatory gene expression and epigenetic regulators, large high-quality clinical trials have shown that omega-3 supplementation is probably ineffective for maintaining remission in Crohn’s [54]. Nonetheless, omega-3 fatty acids appear to be safe, though they may cause diarrhea or upper gastrointestinal discomfort. In vitro and nutrimiromics studies show that EPA and DHA regulate several miRNAs involved in inflammatory responses of macrophages and epithelial cells, including miR-21, miR-146a, and miR-155, which are central players in NF-κB signaling and cytokine regulation [55].
Vitamin D has also been linked to IBD pathophysiology. Low vitamin D levels are associated with worse outcomes, while supplementation has been shown in some studies to reduce relapse risk and improve disease course [56]. Vitamin D signaling influences autophagy, barrier function, and innate immunity [56]. Correlative studies further suggest that vitamin D status is associated with distinct circulating miRNA profiles, and supplementation may alter levels of miRNAs involved in inflammatory and autophagy pathways, such as miR-142-3p [57].
Finally, several herbal compounds, such as Boswellia serrata, possess anti-inflammatory and anti-fibrotic properties demonstrated in animal colitis models and small human trials, often in combination with curcumin [58,59]. Evidence is emerging that these extracts can modulate miRNA expression in models of intestinal inflammation, generally reducing pro-inflammatory miRNA signatures and enhancing barrier markers. While specific effects are compound- and model-dependent, studies in other diseases have shown that Boswellia regulates miR-155 and to some extent other inflammation-related miRNAs [60,61]. To date, however, miRNA regulation by Boswellia has not been investigated in Crohn’s disease.
In summary, there is strong mechanistic plausibility that complementary interventions such as probiotics, curcumin, omega-3 fatty acids, vitamin D, and Boswellia may exert part of their therapeutic effects through modulation of host miRNA expression. This, in turn, could influence cytokine signaling, autophagy, tight junction integrity, and host-microbiota interactions. Nevertheless, most current evidence comes from cell culture systems and animal colitis models, with relatively few and often small human studies. Further well-designed clinical investigations are required before definitive conclusions can be drawn about the role of miRNA regulation in the efficacy of these complementary therapies in Crohn’s disease.
10. Strength of Evidence and Practical Takeaways
Although microRNAs are increasingly investigated as potential biomarkers for CD, their clinical application faces major challenges. One key limitation is their inherent instability, as they are rapidly degraded by RNases present in blood, stool, and other biological samples. This instability raises concerns about the reproducibility and reliability of circulating or fecal miRNA measurements as predictive biomarkers. To overcome these obstacles, approaches such as sample stabilization, encapsulation of miRNAs in extracellular vesicles, or normalization against stable reference molecules are being explored. Therefore, robust methodological advances are required before they can be translated into routine clinical practice. Firstly, one could analyze miRNA-associated proteins (such as Argonaute-bound complexes) [62]. Argonaute proteins bind the guide strand of the miRNA and mediate recognition of complementary sequences in target mRNAs. When miRNAs are bound to Argonaute proteins they are protected from RNase-mediated degradation, which enhances stability and makes them more reliable for measurement in blood or stool samples. Secondly, one could investigate exosomal miRNA. Exosomal microRNAs are packaged inside exosomes and secreted by cells into the extracellular environment. Finally downstream Cytokines could be a target of interest. Molecules like TNF-α, IL-6, IL-23, and IL-12 are already well-established in the inflammatory cascade of CD and can be quantified with standardized immunoassays (e.g., ELISA). In practice, a combined biomarker panel—integrating stable exosomal miRNAs, miRNA-protein complexes, and key cytokines—may be superior to any single biomarker type. Such a multimodal approach could improve both specificity and sensitivity in predicting therapeutic responses in CD, however so far no panel has been established yet.
Regardless of the method of measurement, several of the microRNAs discussed in this article appear mechanistically plausible and are supported by associative human data. Many have been reproducibly shown to be dysregulated in inflammatory bowel disease. However, variability in pre-analytical handling and platform-specific differences continue to limit analytical validity, which to date remains only moderate [63]. Similarly, the clinical validity of candidate panels remains uncertain. Existing studies have largely been conducted in small cohorts, leaving the current body of evidence insufficient to support routine clinical application.
As a result, no multi-miRNA panel has yet been prospectively validated to guide therapeutic decision-making in CD, and none are currently incorporated into clinical guidelines [1]. A comparable situation is observed in other gastrointestinal disorders. Panels have been proposed for early detection of colorectal cancer [64,65,66] and cholangiocarcinoma [67], but all remain at an early stage of validation. The investigated cohorts are typically modest in size, underscoring the need for large-scale, multicenter studies to establish both robustness and generalizability.
To move toward clinical implementation of miRNA panels in Crohn’s disease, three sequential steps are essential. First, assay protocols and cutoff values must be standardized, with harmonized approaches to sample collection, processing, and quantification, along with reproducible detection methods and clinically meaningful thresholds. Second, prospective multicenter validation is required. Candidate panels must be evaluated in large, geographically diverse cohorts of patients starting defined therapies, with baseline profiles correlated against standardized outcomes such as remission, mucosal healing, and biochemical response, and replicated across independent populations. Finally, clinical utility must be tested in impact studies. Randomized trials comparing panel-guided therapy with standard care should assess not only remission and mucosal healing but also safety, cost-effectiveness, and the avoidance of ineffective treatments.
Beyond their role as biomarkers, miRNAs also hold therapeutic potential in Crohn’s disease. By modulating the expression of key inflammatory mediators, they can suppress pathogenic pathways or enhance protective responses. For example, delivery of miRNA mimics may restore downregulated anti-inflammatory miRNAs such as miR-29 or miR-126, thereby reducing pro-inflammatory cytokine release and strengthening epithelial barrier function. Conversely, inhibition of upregulated pro-inflammatory miRNAs, such as miR-155 or miR-23a, using antagomirs or locked nucleic acid inhibitors, may attenuate NF-κB signaling and immune cell activation. Therapeutic approaches under investigation include systemic administration, tissue-targeted nanoparticles, and exosome-based delivery systems, offering a precision strategy to modulate disease-specific pathways. While preclinical studies in murine colitis models are encouraging [68], successful translation into clinical practice will require rigorous evaluation of safety, specificity, and long-term efficacy.
11. Conclusions
Over the past two decades, advances in our understanding of the molecular mechanisms underlying Crohn’s disease have paved the way for more targeted and effective therapeutic approaches. Despite these achievements, response rates to antibody-based therapies remain suboptimal, underscoring the urgent need for predictive biomarkers that can guide treatment decisions and minimize unnecessary exposure to ineffective and costly therapies.
Mounting evidence highlights the role of microRNAs as central regulators of immune signaling pathways and epithelial barrier function in Crohn’s disease. Specific dysregulation patterns—such as reduced miR-29 in patients likely to benefit from ustekinumab, elevated miR-23a in those eligible for TNF-α inhibitors, or altered expression of miR-126, miR-486, and miR-155 in the context of vedolizumab, corticosteroid, and JAK inhibitor therapy—suggest that miRNA profiling holds considerable promise as a tool for personalized treatment stratification.
While the preliminary findings are compelling, current data are largely derived from small cohorts or exploratory studies, and no miRNA-based biomarker panel has yet achieved clinical validation. Future research must therefore focus on large-scale, multicenter, and longitudinal studies to establish standardized assays, validate predictive accuracy, and assess the reproducibility of these findings across diverse patient populations. Integration of miRNA profiling with established clinical parameters may ultimately enable a precision medicine framework for Crohn’s disease, improving patient outcomes while optimizing healthcare resources.
Author Contributions
C.P. and L.F. both wrote the initial version of the manuscript and contributed equally. Both author created the figure. C.P. was the responsible project leader. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available throughout the manuscript.
Conflicts of Interest
The authors declare that they have no competing interests for this article.
References
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Wei, T.; Janson, P.C.; Saaf, A.; Lundeberg, L.; Tengvall-Linder, M.; Norstedt, G.; Alenius, H.; Homey, B.; Scheynius, A.; et al. MicroRNAs: Novel regulators involved in the pathogenesis of psoriasis? PLoS ONE 2007, 2, e610. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Mahidhara, G.; Kanwar, R.K. MicroRNA in human cancer and chronic inflammatory diseases. Front. Biosci. 2010, 2, 1113–1126. [Google Scholar] [CrossRef]
- Yang, S.C.; Alalaiwe, A.; Lin, Z.C.; Lin, Y.C.; Aljuffali, I.A.; Fang, J.Y. Anti-Inflammatory microRNAs for Treating Inflammatory Skin Diseases. Biomolecules 2022, 12, 1072. [Google Scholar] [CrossRef]
- Keller, A.; Leidinger, P.; Bauer, A.; Elsharawy, A.; Haas, J.; Backes, C.; Wendschlag, A.; Giese, N.; Tjaden, C.; Ott, K.; et al. Toward the blood-borne miRNome of human diseases. Nat. Methods 2011, 8, 841–843. [Google Scholar] [CrossRef]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat. Rev. Genet. 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Bond, C.E.; Nancarrow, D.J.; Wockner, L.F.; Wallace, L.; Montgomery, G.W.; Leggett, B.A.; Whitehall, V.L. Microsatellite stable colorectal cancers stratified by the BRAF V600E mutation show distinct patterns of chromosomal instability. PLoS ONE 2014, 9, e91739. [Google Scholar] [CrossRef]
- Jung, H.; Kim, J.S.; Lee, K.H.; Tizaoui, K.; Terrazzino, S.; Cargnin, S.; Smith, L.; Koyanagi, A.; Jacob, L.; Li, H.; et al. Roles of microRNAs in inflammatory bowel disease. Int. J. Biol. Sci. 2021, 17, 2112–2123. [Google Scholar] [CrossRef]
- James, J.P.; Riis, L.B.; Malham, M.; Hogdall, E.; Langholz, E.; Nielsen, B.S. MicroRNA Biomarkers in IBD-Differential Diagnosis and Prediction of Colitis-Associated Cancer. Int. J. Mol. Sci. 2020, 21, 7893. [Google Scholar] [CrossRef]
- Batra, S.K.; Heier, C.R.; Diaz-Calderon, L.; Tully, C.B.; Fiorillo, A.A.; van den Anker, J.; Conklin, L.S. Serum miRNAs Are Pharmacodynamic Biomarkers Associated with Therapeutic Response in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Papaconstantinou, I.; Kapizioni, C.; Legaki, E.; Xourgia, E.; Karamanolis, G.; Gklavas, A.; Gazouli, M. Association of miR-146 rs2910164, miR-196a rs11614913, miR-221 rs113054794 and miR-224 rs188519172 polymorphisms with anti-TNF treatment response in a Greek population with Crohn’s disease. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Fasseu, M.; Treton, X.; Guichard, C.; Pedruzzi, E.; Cazals-Hatem, D.; Richard, C.; Aparicio, T.; Daniel, F.; Soule, J.C.; Moreau, R.; et al. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS ONE 2010, 5, e13160. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zikusoka, M.; Trindade, A.; Dassopoulos, T.; Harris, M.L.; Bayless, T.M.; Brant, S.R.; Chakravarti, S.; and Kwon, J.H. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology 2008, 135, 1624–1635.e24. [Google Scholar] [CrossRef]
- Sibia, C.F.; Quaglio, A.E.V.; Oliveira, E.C.S.; Pereira, J.N.; Ariede, J.R.; Lapa, R.M.L.; Severino, F.E.; Reis, P.P.; Sassaki, L.Y.; Saad-Hossne, R. microRNA-mRNA Networks Linked to Inflammation and Immune System Regulation in Inflammatory Bowel Disease. Biomedicines 2024, 12, 422. [Google Scholar] [CrossRef]
- Morilla, I.; Uzzan, M.; Laharie, D.; Cazals-Hatem, D.; Denost, Q.; Daniel, F.; Belleannee, G.; Bouhnik, Y.; Wainrib, G.; Panis, Y.; et al. Colonic MicroRNA Profiles, Identified by a Deep Learning Algorithm, That Predict Responses to Therapy of Patients with Acute Severe Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2019, 17, 905–913. [Google Scholar] [CrossRef]
- Cordes, F.; Demmig, C.; Bokemeyer, A.; Bruckner, M.; Lenze, F.; Lenz, P.; Nowacki, T.; Tepasse, P.; Schmidt, H.H.; Schmidt, M.A.; et al. MicroRNA-320a Monitors Intestinal Disease Activity in Patients with Inflammatory Bowel Disease. Clin. Transl. Gastroenterol. 2020, 11, e00134. [Google Scholar] [CrossRef]
- Yu, T.; Wang, P.; Wu, Y.; Zhong, J.; Chen, Q.; Wang, D.; Chen, H.; Hu, S.; Wu, Q. MiR-26a Reduces Inflammatory Responses via Inhibition of PGE2 Production by Targeting COX-2. Inflammation 2022, 45, 1484–1495. [Google Scholar] [CrossRef]
- Yao, X.C.; Wu, J.J.; Yuan, S.T.; Yuan, F.L. Recent insights and perspectives into the role of the miRNA-29 family in innate immunity (Review). Int. J. Mol. Med. 2025, 55, 53. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Gu, J.; Zhuang, T.; Zhang, J.; Fan, C.; Li, Y.; Zhao, M.; Chen, R.; Wang, R.; Kong, Y.; et al. MicroRNA-126: From biology to therapeutics. Biomed. Pharmacother. 2025, 185, 117953. [Google Scholar] [CrossRef]
- Ghorab, R.A.; Fouad, S.H.; Sherief, A.F.; El-Sehsah, E.M.; Shamloul, S.; Taha, S.I. MiR-146a (rs2910164) Gene Polymorphism and Its Impact on Circulating MiR-146a Levels in Patients with Inflammatory Bowel Diseases. Inflammation 2024, 48, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Raisch, J.; Darfeuille-Michaud, A.; Nguyen, H.T. Role of microRNAs in the immune system, inflammation and cancer. World J. Gastroenterol. 2013, 19, 2985–2996. [Google Scholar] [CrossRef] [PubMed]
- Cseko, K.; Beckers, B.; Keszthelyi, D.; Helyes, Z. Role of TRPV1 and TRPA1 Ion Channels in Inflammatory Bowel Diseases: Potential Therapeutic Targets? Pharmaceuticals 2019, 12, 48. [Google Scholar] [CrossRef]
- Akbar, A.; Yiangou, Y.; Facer, P.; Brydon, W.G.; Walters, J.R.; Anand, P.; Ghosh, S. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut 2010, 59, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Gombert, S.; Rhein, M.; Winterpacht, A.; Munster, T.; Hillemacher, T.; Leffler, A.; Frieling, H. Transient receptor potential ankyrin 1 promoter methylation and peripheral pain sensitivity in Crohn’s disease. Clin. Epigenet. 2019, 12, 1. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, L.; Larson, S.; Basra, S.; Merwat, S.; Tan, A.; Croce, C.; Verne, G.N. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut 2016, 65, 797–805. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, J.; Zhu, M.; Mukherjee, A.; Zhang, H. Transient Receptor Potential Channels and Inflammatory Bowel Disease. Front. Immunol. 2020, 11, 180. [Google Scholar] [CrossRef]
- D’Aldebert, E.; Cenac, N.; Rousset, P.; Martin, L.; Rolland, C.; Chapman, K.; Selves, J.; Alric, L.; Vinel, J.P.; Vergnolle, N. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology 2011, 140, 275–285. [Google Scholar] [CrossRef]
- Zielinska, M.; Jarmuz, A.; Wasilewski, A.; Salaga, M.; Fichna, J. Role of transient receptor potential channels in intestinal inflammation and visceral pain: Novel targets in inflammatory bowel diseases. Inflamm. Bowel Dis. 2015, 21, 419–427. [Google Scholar] [CrossRef]
- Fichna, J.; Mokrowiecka, A.; Cygankiewicz, A.I.; Zakrzewski, P.K.; Malecka-Panas, E.; Janecka, A.; Krajewska, W.M.; Storr, M.A. Transient receptor potential vanilloid 4 blockade protects against experimental colitis in mice: A new strategy for inflammatory bowel diseases treatment? Neurogastroenterol. Motil. 2012, 24, e557–e560. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Zhu, W.; Wang, L. MicroRNA-203 up-regulates nitric oxide expression in temporomandibular joint chondrocytes via targeting TRPV4. Arch. Oral Biol. 2013, 58, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Felwick, R.K.; Dingley, G.J.R.; Martinez-Nunez, R.; Sanchez-Elsner, T.; Cummings, J.R.F.; Collins, J.E. MicroRNA23a Overexpression in Crohn’s Disease Targets Tumour Necrosis Factor Alpha Inhibitor Protein 3, Increasing Sensitivity to TNF and Modifying the Epithelial Barrier. J. Crohn’s Colitis 2020, 14, 381–392. [Google Scholar] [CrossRef]
- MacDonald, J.K.; Nguyen, T.M.; Khanna, R.; Timmer, A. Anti-IL-12/23p40 antibodies for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2016, 11, CD007572. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Fedorak, R.N.; Scherl, E.; Fleisher, M.R.; Katz, S.; Johanns, J.; Blank, M.; Rutgeerts, P.; Ustekinumab Crohn’s Disease Study, G. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology 2008, 135, 1130–1141. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Gasink, C.; Gao, L.L.; Blank, M.A.; Johanns, J.; Guzzo, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Rutgeerts, P.; et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N. Engl. J. Med. 2012, 367, 1519–1528. [Google Scholar] [CrossRef]
- Sands, B.E.; Feagan, B.G.; Rutgeerts, P.; Colombel, J.F.; Sandborn, W.J.; Sy, R.; D’Haens, G.; Ben-Horin, S.; Xu, J.; Rosario, M.; et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology 2014, 147, 618–627.e3. [Google Scholar] [CrossRef]
- de Oliveira, E.C.S.; Quaglio, A.E.V.; Grillo, T.G.; Di Stasi, L.C.; Sassaki, L.Y. MicroRNAs in inflammatory bowel disease: What do we know and what can we expect? World J. Gastroenterol. 2024, 30, 2184–2190. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.; Grillo, A.R.; Scarpa, M.; Brun, P.; D’Inca, R.; Nai, L.; Banerjee, A.; Cavallo, D.; Barzon, L.; Palu, G.; et al. MiR-155 modulates the inflammatory phenotype of intestinal myofibroblasts by targeting SOCS1 in ulcerative colitis. Exp. Mol. Med. 2015, 47, e164. [Google Scholar] [CrossRef]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Gasink, C.; Chan, D.; Lang, Y.; Pollack, P.; Colombel, J.F.; Wolf, D.C.; Jacobstein, D.; Johanns, J.; Szapary, P.; et al. Efficacy of Ustekinumab for Inducing Endoscopic Healing in Patients with Crohn’s Disease. Gastroenterology 2018, 155, 1045–1058. [Google Scholar] [CrossRef]
- Barkai, L.J.; Gonczi, L.; Balogh, F.; Angyal, D.; Farkas, K.; Farkas, B.; Molnar, T.; Szamosi, T.; Schafer, E.; Golovics, P.A.; et al. Efficacy, drug sustainability, and safety of ustekinumab treatment in Crohn’s disease patients over three years. Sci. Rep. 2024, 14, 14909. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.J.; Noh, J.H.; Kim, J.K.; Eun, J.W.; Jung, K.H.; Kim, M.G.; Chang, Y.G.; Shen, Q.; Kim, S.J.; Park, W.S.; et al. MicroRNA-29c functions as a tumor suppressor by direct targeting oncogenic SIRT1 in hepatocellular carcinoma. Oncogene 2014, 33, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.G.; Pekow, J. The emerging role of miRNAs in inflammatory bowel disease: A review. Ther. Adv. Gastroenterol. 2015, 8, 4–22. [Google Scholar] [CrossRef]
- Malchow, H.A. Crohn’s disease and Escherichia coli—A new approach in therapy to maintain remission of colonic Crohn’s disease? J. Clin. Gastroenterol. 1997, 25, 653–658. [Google Scholar] [CrossRef]
- Fedorak, R.N.; Feagan, B.G.; Hotte, N.; Leddin, D.; Dieleman, L.A.; Petrunia, D.M.; Enns, R.; Bitton, A.; Chiba, N.; Pare, P.; et al. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn’s disease. Clin. Gastroenterol. Hepatol. 2015, 13, 928–935.e2. [Google Scholar]
- Estevinho, M.M.; Yuan, Y.; Rodriguez-Lago, I.; Sousa-Pimenta, M.; Dias, C.C.; Barreiro-de Acosta, M.; Barreiro-de Acosta, M.; Jairath, V.; Magro, F. Efficacy and safety of probiotics in IBD: An overview of systematic reviews and updated meta-analysis of randomized controlled trials. United Eur. Gastroenterol. J. 2024, 12, 960–981. [Google Scholar] [CrossRef]
- Barcenas-Preciado, V.; Mata-Haro, V. Probiotics in miRNA-Mediated Regulation of Intestinal Immune Homeostasis in Pigs: A Physiological Narrative. Microorganisms 2024, 12, 1606. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Marzban, H.; Goleij, P.; Sahebkar, A.; Morshedi, K.; Rezaei, S.; Mahjoubin-Tehran, M.; Tarrahimofrad, H.; Hamblin, M.R.; Mirzaei, H. Effects of therapeutic probiotics on modulation of microRNAs. Cell Commun. Signal. 2021, 19, 4. [Google Scholar] [CrossRef]
- Vecchi Brumatti, L.; Marcuzzi, A.; Tricarico, P.M.; Zanin, V.; Girardelli, M.; Bianco, A.M. Curcumin and inflammatory bowel disease: Potential and limits of innovative treatments. Molecules 2014, 19, 21127–21153. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, D.; Zhang, X.; Qing, M.; Li, X.; Chou, Y.; Chou, Y.; Chen, G.; Li, N. Curcumin promotes renewal of intestinal epithelium by miR-195–3p. J. Ethnopharmacol. 2024, 320, 117413. [Google Scholar] [CrossRef]
- Li, J.; Chai, R.; Chen, Y.; Zhao, S.; Bian, Y.; Wang, X. Curcumin Targeting Non-Coding RNAs in Colorectal Cancer: Therapeutic and Biomarker Implications. Biomolecules 2022, 12, 1339. [Google Scholar] [CrossRef]
- Lev-Tzion, R.; Griffiths, A.M.; Leder, O.; Turner, D. Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2014, 2014, CD006320. [Google Scholar] [CrossRef] [PubMed]
- Quintanilha, B.J.; Reis, B.Z.; Duarte, G.B.S.; Cozzolino, S.M.F.; Rogero, M.M. Nutrimiromics: Role of microRNAs and Nutrition in Modulating Inflammation and Chronic Diseases. Nutrients 2017, 9, 1168. [Google Scholar] [CrossRef]
- Reich, K.M.; Fedorak, R.N.; Madsen, K.; Kroeker, K.I. Vitamin D improves inflammatory bowel disease outcomes: Basic science and clinical review. World J. Gastroenterol. 2014, 20, 4934–4947. [Google Scholar] [CrossRef] [PubMed]
- McGillis, L.; Bronte-Tinkew, D.M.; Dang, F.; Capurro, M.; Prashar, A.; Ricciuto, A.; Greenfield, L.; Lozano-Ruf, A.; Siddiqui, I.; Hsieh, A.; et al. Vitamin D deficiency enhances expression of autophagy-regulating miR-142–3p in mouse and “involved” IBD patient intestinal tissues. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G171–G184. [Google Scholar] [CrossRef]
- Laudadio, I.; Leter, B.; Palone, F.; Cucchiara, S.; Carissimi, C.; Scafa, N.; Secci, D.; Vitali, R.; Stronati, L. Inhibition of intestinal inflammation and fibrosis by Scutellaria Baicalensis georgi and Boswellia serrata in human epithelial cells and fibroblasts. Immun. Inflamm. Dis. 2024, 12, e70036. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, D.; Rancan, S.; Orso, G.; Dall’Acqua, S.; Brun, P.; Giron, M.C.; Carrara, M.; Castagliuolo, I.; Ragazzi, E.; Caparrotta, L.; et al. Boswellia serrata Preserves Intestinal Epithelial Barrier from Oxidative and Inflammatory Damage. PLoS ONE 2015, 10, e0125375. [Google Scholar] [CrossRef]
- Sayed, A.S.; Gomaa, I.E.O.; Bader, M.; El Sayed, N. Role of 3-Acetyl-11-Keto-Beta-Boswellic Acid in Counteracting LPS-Induced Neuroinflammation via Modulation of miRNA-155. Mol. Neurobiol. 2018, 55, 5798–5808. [Google Scholar] [CrossRef]
- Salama, R.M.; Abbas, S.S.; Darwish, S.F.; Sallam, A.A.; Elmongy, N.F.; El Wakeel, S.A. Regulation of NOX/p38 MAPK/PPARalpha pathways and miR-155 expression by boswellic acids reduces hepatic injury in experimentally-induced alcoholic liver disease mouse model: Novel mechanistic insight. Arch. Pharm. Res. 2023, 46, 323–338. [Google Scholar] [CrossRef]
- Matsuyama, H.; Suzuki, H.I. Systems and Synthetic microRNA Biology: From Biogenesis to Disease Pathogenesis. Int. J. Mol. Sci. 2019, 21, 132. [Google Scholar] [CrossRef]
- Masi, L.; Capobianco, I.; Magri, C.; Marafini, I.; Petito, V.; Scaldaferri, F. MicroRNAs as Innovative Biomarkers for Inflammatory Bowel Disease and Prediction of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 7991. [Google Scholar] [CrossRef]
- Kanaan, Z.; Roberts, H.; Eichenberger, M.R.; Billeter, A.; Ocheretner, G.; Pan, J.; Rai, S.N.; Jorden, J.; Williford, A.; Galandiuk, S. A plasma microRNA panel for detection of colorectal adenomas: A step toward more precise screening for colorectal cancer. Ann. Surg. 2013, 258, 400–408. [Google Scholar] [CrossRef]
- Zheng, G.; Du, L.; Yang, X.; Zhang, X.; Wang, L.; Yang, Y.; Li, J.; Wang, C. Serum microRNA panel as biomarkers for early diagnosis of colorectal adenocarcinoma. Br. J. Cancer 2014, 111, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.; Sin, R.W.; Cheung, D.H.; Wong, C.K.; Lam, C.L.; Leung, W.K.; Law, W.L.; Foo, D.C. Identification and evaluation of a serum microRNA panel to diagnose colorectal cancer patients. Int. J. Cancer 2025, 156, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.L.; Puik, J.R.; Le Large, T.Y.S.; Heger, M.; Dijk, F.; Funel, N.; Wurdinger, T.; Garajova, I.; van Grieken, N.C.T.; van de Wiel, M.A.; et al. Unravelling the Diagnostic Dilemma: A MicroRNA Panel of Circulating MiR-16 and MiR-877 as A Diagnostic Classifier for Distal Bile Duct Tumors. Cancers 2019, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, Y.N.; Kamel, A.M.; Medhat, M.A.; Hetta, H.F. MicroRNA signatures in the pathogenesis and therapy of inflammatory bowel disease. Clin. Exp. Med. 2024, 24, 217. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).