In Vitro Modulation of Macrophage Inflammatory and Pro-Repair Properties Essential for Wound Healing by Calcium and Calcium-Alginate Dressings
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Conditioned Medium from Calcium-Alginate Dressings and a CaCl2 Solution
2.2. Human Monocyte Isolation and Purification
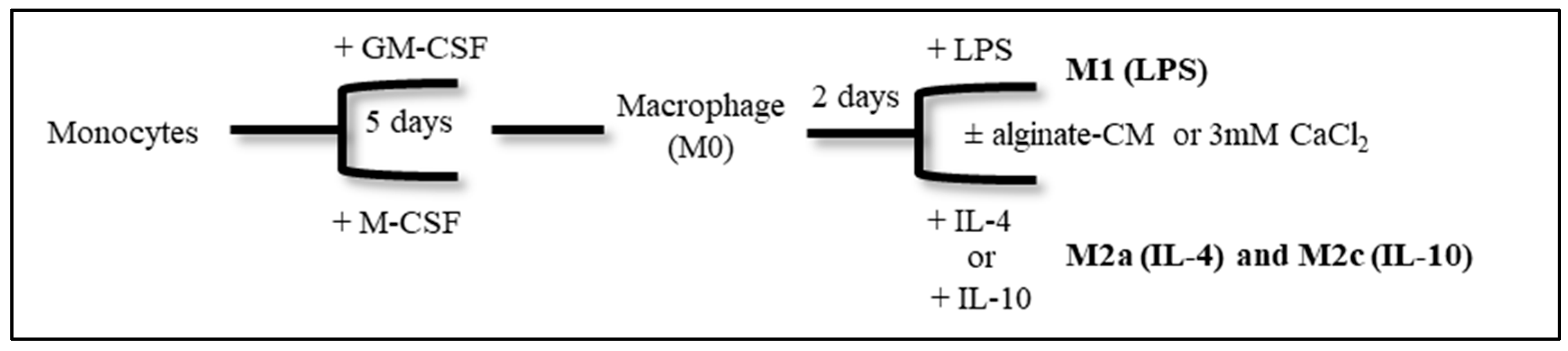
2.3. Monocyte Differentiation and Polarization In Vitro
2.4. MTT Assay
2.5. RNA Isolation and cDNA Synthesis
2.6. Gene Expression
2.6.1. Real-Time Quantitative PCR (RTq-PCR)
2.6.2. Microfluidic qPCR
2.7. Phenotypic Features Analysis by Flow Cytometry
2.8. Cytokine Production by ELISA
2.9. IncuCyte® Phagocytosis Assay
2.10. Human Dermal Fibroblast (HDF) Cell Culture and Stimulation with M0, M1, and M2 Macrophage Supernatants
2.11. Statistical Analysis
3. Results
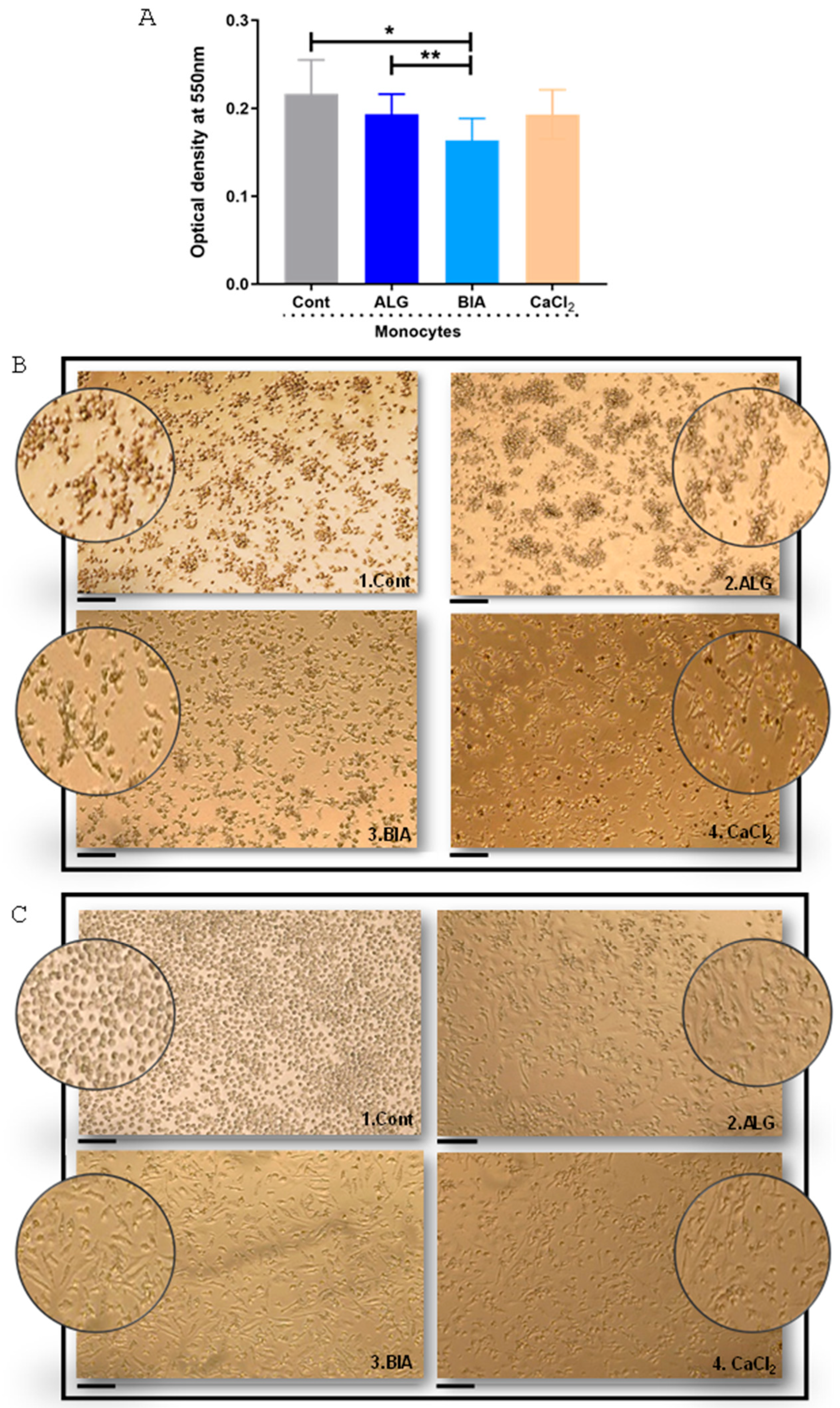
3.1. Monocyte Viability and Macrophage Morphology in the Presence of Alginate Dressings and CaCl2
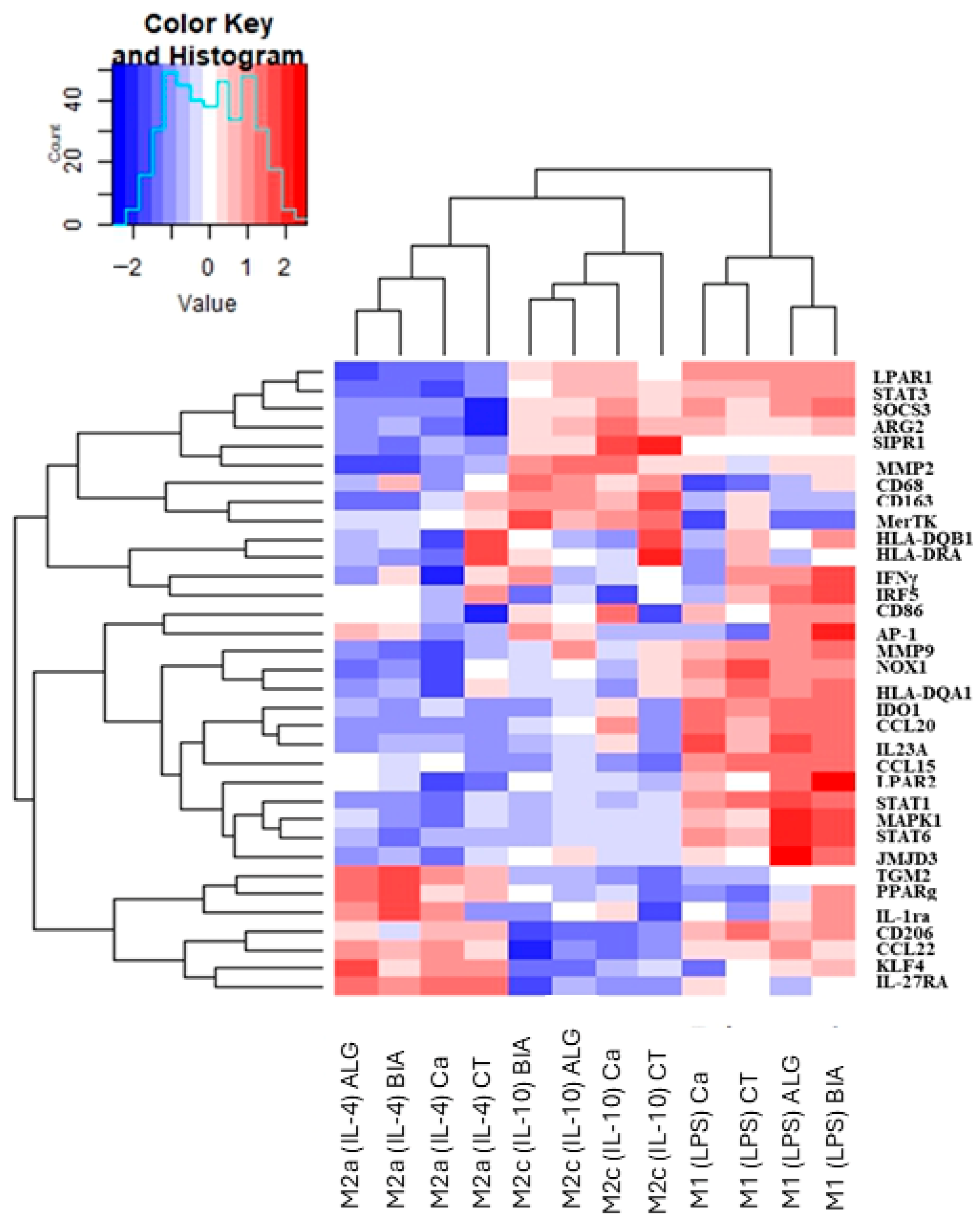
3.2. Clustering Analysis of M1, M2a, and M2c Macrophages Polarized in the Presence of Alginate Dressings or CaCl2
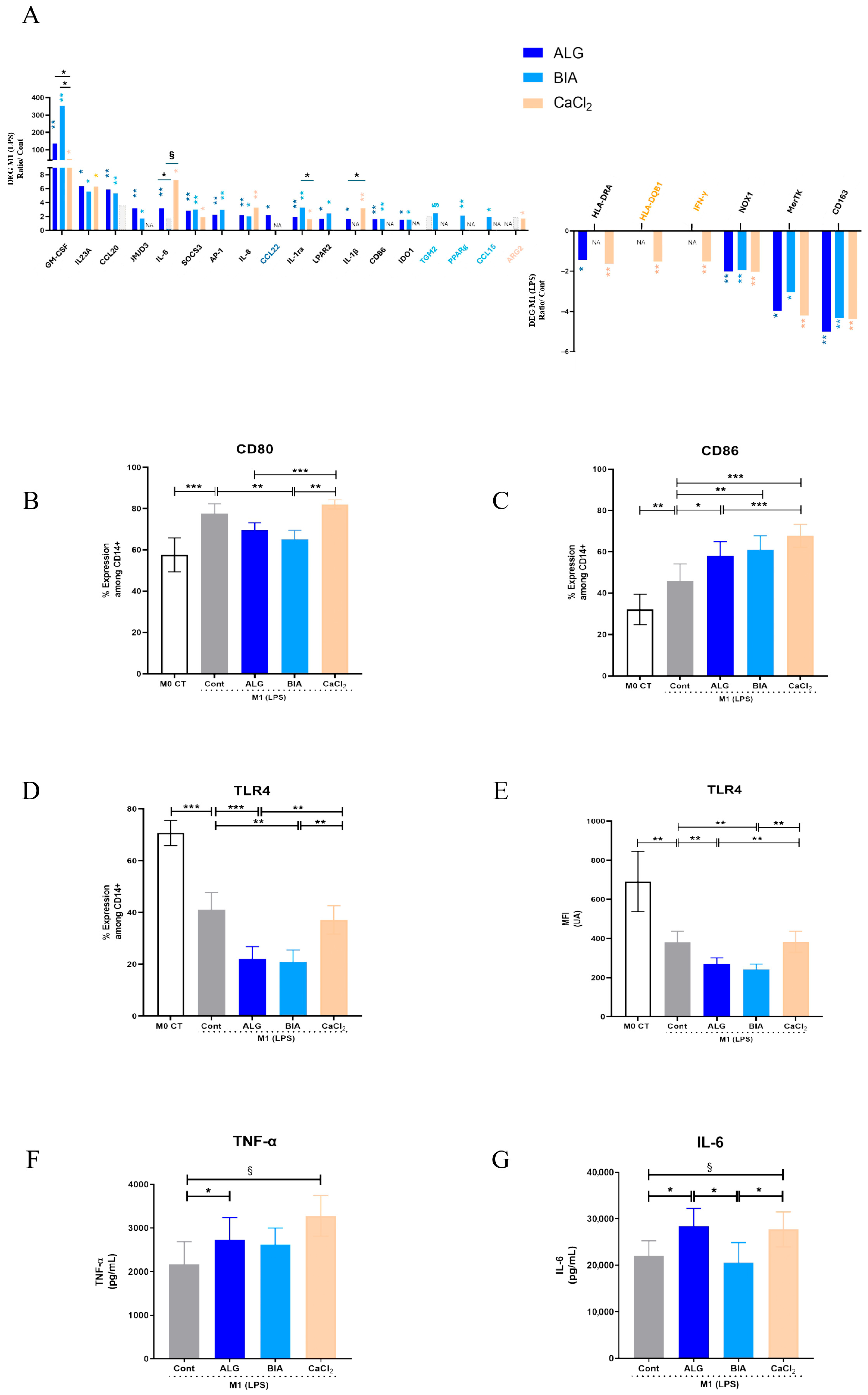
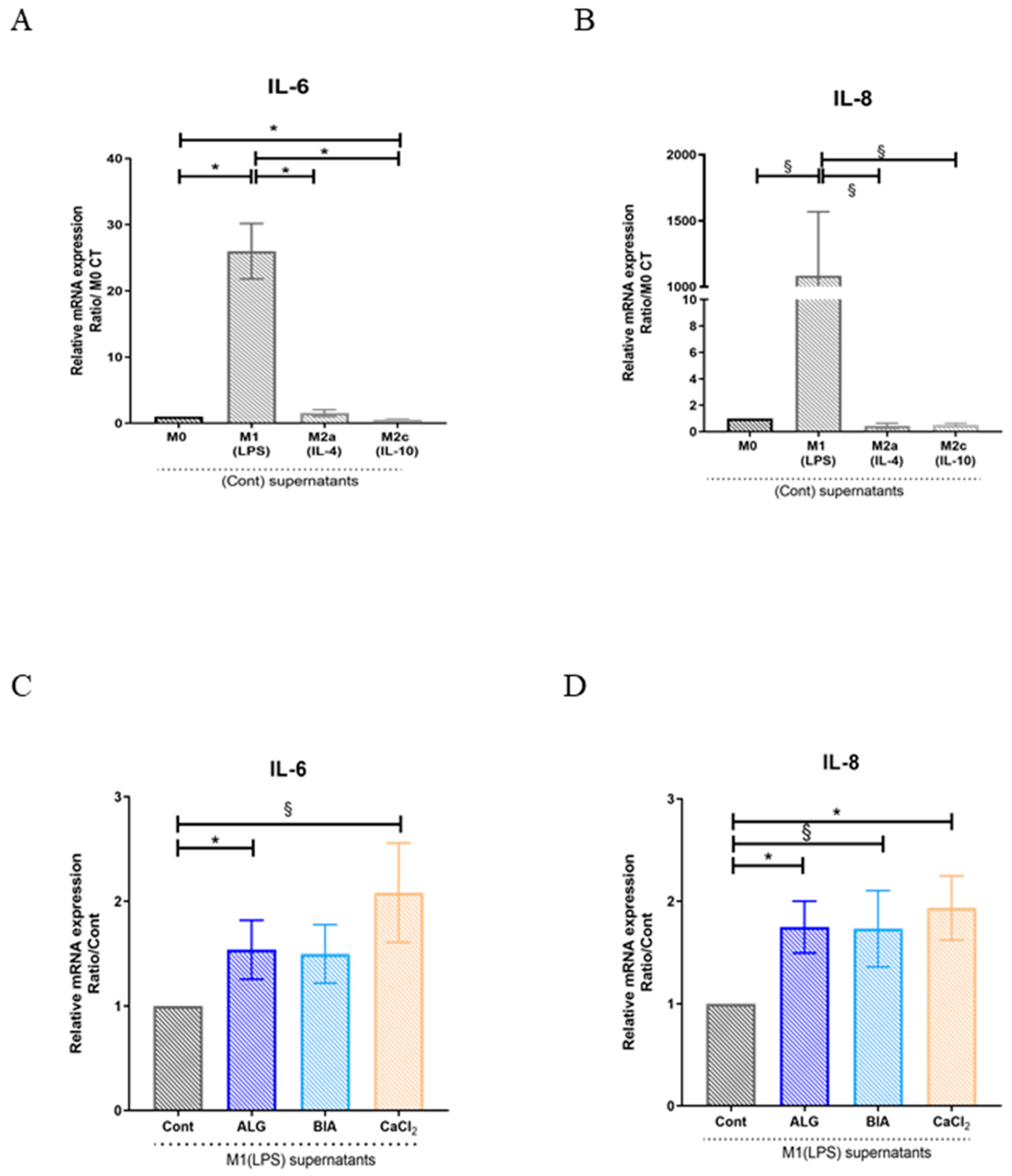
3.3. Focus on M1 Macrophages
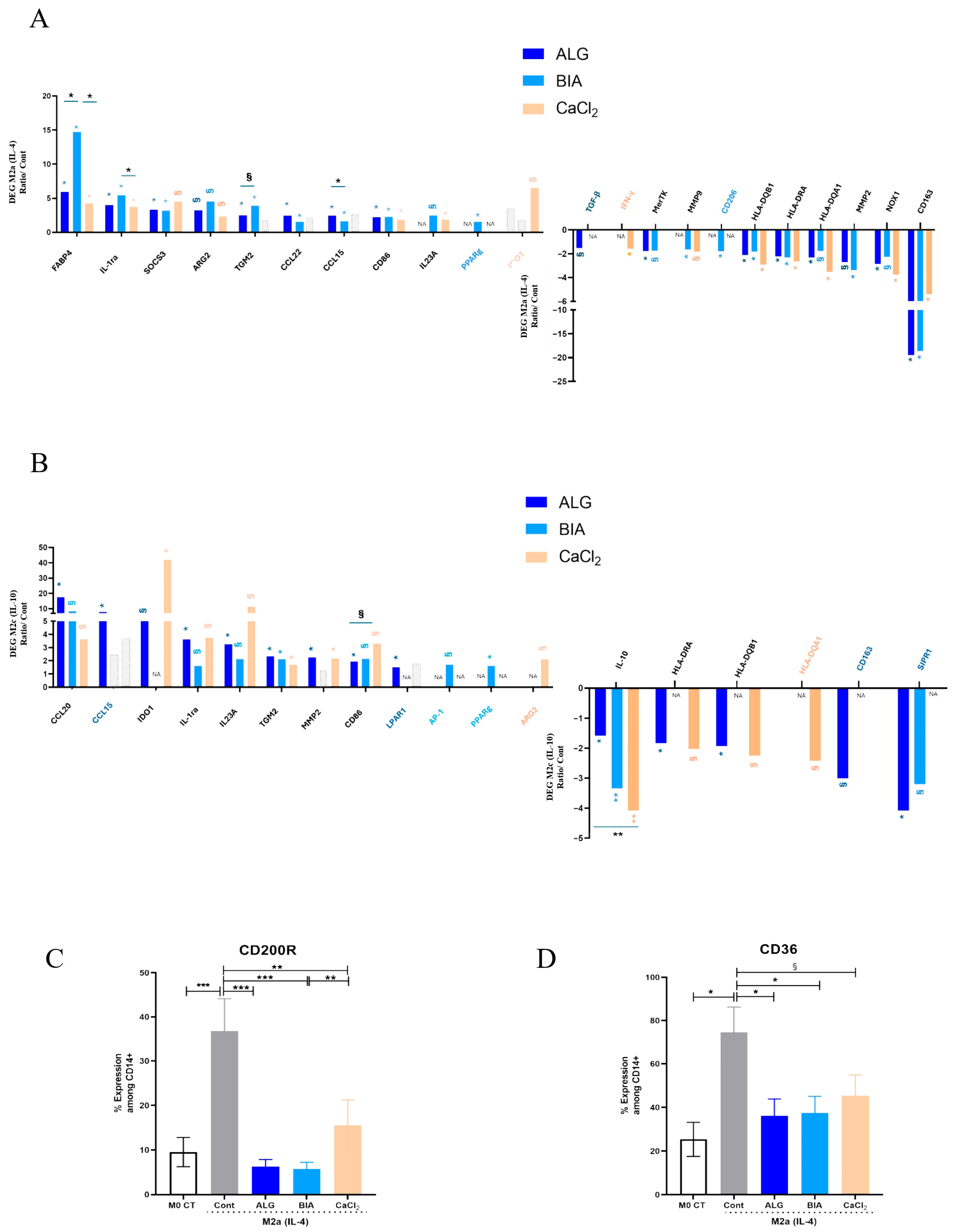
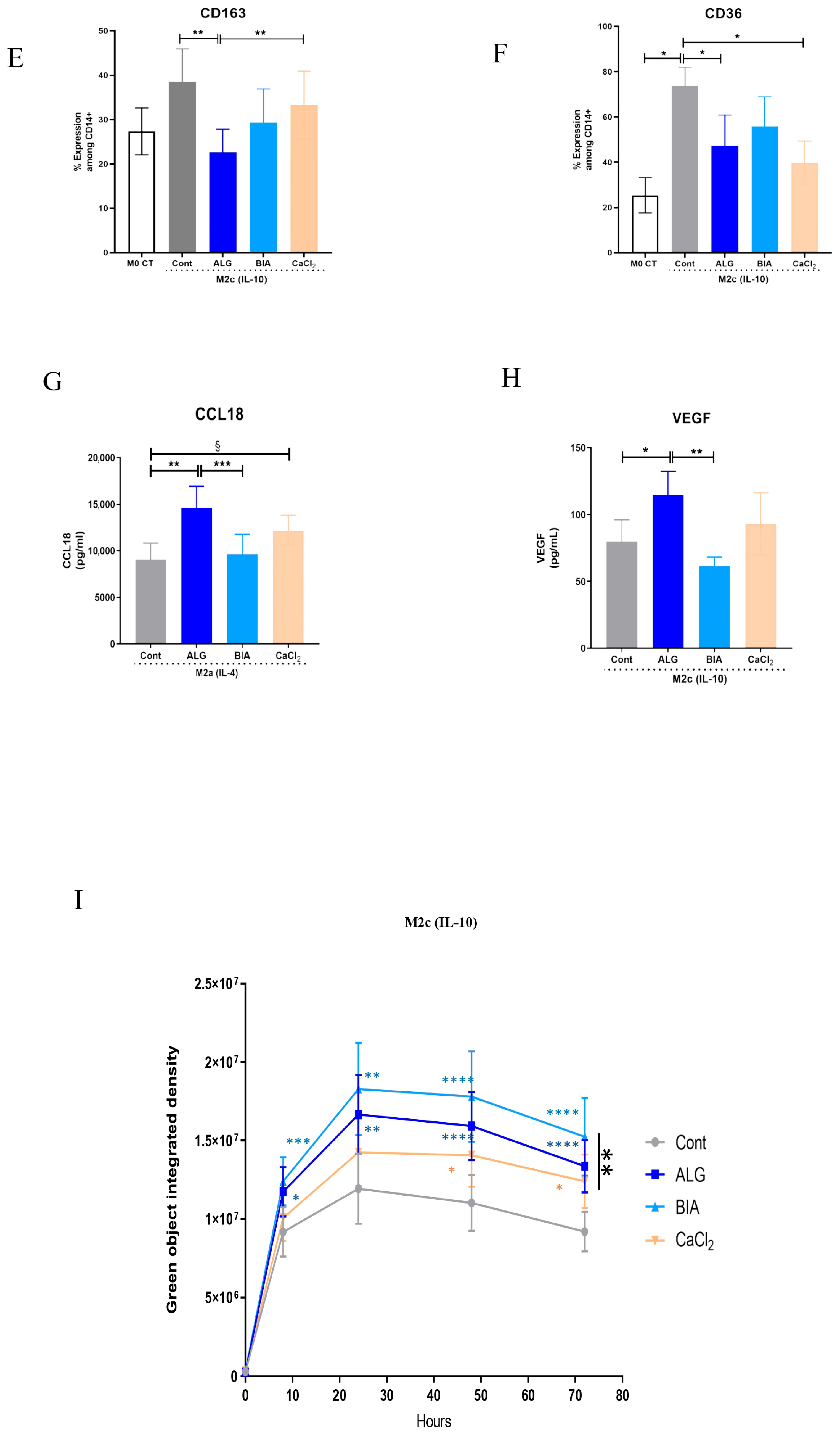
3.4. Focus on M2 Macrophages
3.5. Effects of Macrophage Paracrine Factors on Human Dermal Fibroblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghamangiz, S.; Jafari, A.; Maleki-Kakelar, H.; Azimi, H.; Mazloomi, E. Reprogram to Heal: Macrophage Phenotypes as Living Therapeutics. Life Sci. 2025, 371, 123601. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage Phenotypes Regulate Scar Formation and Chronic Wound Healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [PubMed]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, Functional, and Plasticity Features of Classical and Alternatively Activated Human Macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef]
- Sawaya, A.P.; Vecin, N.M.; Burgess, J.L.; Ojeh, N.; DiBartolomeo, G.; Stone, R.C.; Pastar, I.; Tomic-Canic, M. Calreticulin: A Multifunctional Protein with Potential Therapeutic Applications for Chronic Wounds. Front. Med. 2023, 10, 1207538. [Google Scholar] [CrossRef]
- Duong, L.; Radley, H.; Lee, B.; Dye, D.; Pixley, F.; Grounds, M.; Nelson, D.; Jackaman, C. Macrophage Function in the Elderly and Impact on Injury Repair and Cancer. Immun. Ageing 2021, 18, 4. [Google Scholar] [CrossRef]
- Linehan, E.; Fitzgerald, D. Ageing and the Immune System: Focus on Macrophages. Eur. J. Microbiol. Immunol. 2015, 5, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Castle, S.C. Clinical Relevance of Age-Related Immune Dysfunction. Clin. Infect. Dis. 2000, 31, 578–585. [Google Scholar] [CrossRef]
- Chen, V.Y.; Siegfried, L.G.; Tomic-Canic, M.; Stone, R.C.; Pastar, I. Cutaneous Changes in Diabetic Patients: Primed for Aberrant Healing? Wound Repair Regen. 2023, 31, 700–712. [Google Scholar] [CrossRef]
- Theocharidis, G.; Thomas, B.E.; Sarkar, D.; Mumme, H.L.; Pilcher, W.J.R.; Dwivedi, B.; Sandoval-Schaefer, T.; Sîrbulescu, R.F.; Kafanas, A.; Mezghani, I.; et al. Single Cell Transcriptomic Landscape of Diabetic Foot Ulcers. Nat. Commun. 2022, 13, 181. [Google Scholar] [CrossRef]
- Minsart, M.; Deroose, N.; Parmentier, L.; Van Vlierberghe, S.; Mignon, A.; Dubruel, P. Fine-Tuning the Endcap Chemistry of Acrylated Poly(Ethylene Glycol)-Based Hydrogels for Efficient Burn Wound Exudate Management. Macromol. Biosci. 2023, 23, 2200341. [Google Scholar] [CrossRef]
- Theocharidis, G.; Rahmani, S.; Lee, S.; Li, Z.; Lobao, A.; Kounas, K.; Katopodi, X.-L.; Wang, P.; Moon, S.; Vlachos, I.S.; et al. Murine Macrophages or Their Secretome Delivered in Alginate Dressings Enhance Impaired Wound Healing in Diabetic Mice. Biomaterials 2022, 288, 121692. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.-H.; Chang, L.; Ho, C.-Y.; Tsai, C.-H.; Liu, Y.-C.; Huang, W.-Y.; Wang, Y.-N.; Wang, W.-H.; Wang, T.-W. Immunomodulatory Hydrogel Orchestrates Pro-Regenerative Response of Macrophages and Angiogenesis for Chronic Wound Healing. Biomaterials 2025, 314, 122848. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The Versatility and Universality of Calcium Signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Qin, Y. The Characterization of Alginate Wound Dressings with Different Fiber and Textile Structures. J. Appl. Polym. Sci. 2006, 100, 2516–2520. [Google Scholar] [CrossRef]
- Qin, Y. The Gel Swelling Properties of Alginate Fibers and Their Applications in Wound Management. Adv. Technol. 2008, 19, 6–14. [Google Scholar] [CrossRef]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from Algae. In Biopolymers Online; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; ISBN 978-3-527-60003-8. [Google Scholar]
- Aderibigbe, B.A.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Duciel, L.; Proust, R.; Ponsen, A.; Ziarelli, F.; Coudreuse, A.; Jeanmichel, L.; Samardzic, M.; Uzan, G.; Des Courtils, C. Are All Alginate Dressings Equivalent? J. Biomed. Mater. Res. 2025, 113, e35557. [Google Scholar] [CrossRef]
- Adib, Y.; Boy, M.; Serror, K.; Dulphy, N.; des Courtils, C.; Duciel, L.; Boccara, D.; Mimoun, M.; Samardzic, M.; Bagot, M.; et al. Modulation of NK Cell Activation by Exogenous Calcium from Alginate Dressings in Vitro. Front. Immunol. 2023, 14, 1141047. [Google Scholar] [CrossRef]
- Peltier, S.; Adib, Y.; Nicosia, L.; Ly Ka So, S.; Da Silva, C.; Serror, K.; Duciel, L.; Proust, R.; Mimoun, M.; Bagot, M.; et al. In Vitro Effects of Wound-dressings on Key Wound Healing Properties of Dermal Fibroblasts. Exp. Dermatol. 2024, 33, e15098. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Induction of Multiple Cytokine Secretion from RAW264.7 Cells by Alginate Oligosaccharides. Biosci. Biotechnol. Biochem. 2007, 71, 238–241. [Google Scholar] [CrossRef]
- Iwamoto, M.; Kurachi, M.; Nakashima, T.; Kim, D.; Yamaguchi, K.; Oda, T.; Iwamoto, Y.; Muramatsu, T. Structure-Activity Relationship of Alginate Oligosaccharides in the Induction of Cytokine Production from RAW264.7 Cells. FEBS Lett. 2005, 579, 4423–4429. [Google Scholar] [CrossRef] [PubMed]
- Hirt, N.; Alkobtawi, M.; Manchon, E.; Proust, R.; Villain, E.; Lack, S.; Bourrat, E.; Courtils, C.d.; Bouaziz, J.-D.; Al-Daccak, R. A Novel Calcium Alginate Hydrogel Formulation to Enhance Monocyte/Macrophage Anti-Inflammatory Activity. Int. J. Biol. Macromol. 2025, 306, 141251. [Google Scholar] [CrossRef]
- Ge, F.; Zhu, L.; Yang, L.; Li, W.; Wei, S.; Du, G. The Soluble and Particulate Form of Alginates Positively Regulate Immune Response. Iran J. Immunol. 2018, 15, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Flo, T.H.; Ryan, L.; Latz, E.; Takeuchi, O.; Monks, B.G.; Lien, E.; Halaas, Ø.; Akira, S.; Skjåk-Bræk, G.; Golenbock, D.T.; et al. Involvement of Toll-like Receptor (TLR) 2 and TLR4 in Cell Activation by Mannuronic Acid Polymers. J. Biol. Chem. 2002, 277, 35489–35495. [Google Scholar] [CrossRef]
- Chauhan, A.; Sun, Y.; Sukumaran, P.; Quenum Zangbede, F.O.; Jondle, C.N.; Sharma, A.; Evans, D.L.; Chauhan, P.; Szlabick, R.E.; Aaland, M.O.; et al. M1 Macrophage Polarization Is Dependent on TRPC1-Mediated Calcium Entry. iScience 2018, 8, 85–102. [Google Scholar] [CrossRef]
- Nunes, P.; Demaurex, N. The Role of Calcium Signaling in Phagocytosis. J. Leukoc. Biol. 2010, 88, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Donaldson, K.; Borm, P.J.; Schins, R.P.; Dehnhardt, M.; Gilmour, P.; Jimenez, L.A.; Stone, V. Calcium and ROS-Mediated Activation of Transcription Factors and TNF-α Cytokine Gene Expression in Macrophages Exposed to Ultrafine Particles. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L344–L353. [Google Scholar] [CrossRef]
- Tang, S.; Chen, T.; Yang, M.; Wang, L.; Yu, Z.; Xie, B.; Qian, C.; Xu, S.; Li, N.; Cao, X.; et al. Extracellular Calcium Elicits Feedforward Regulation of the Toll-like Receptor-Triggered Innate Immune Response. Cell. Mol. Immunol. 2017, 14, 180–191. [Google Scholar] [CrossRef]
- ISO 10993-12; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- NF EN ISO 11885; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). Association Française de Normalisation: La Plaine Saint-Denis, France, 2008.
- Thomas, A. Alginates from Wound Dressings Activate Human Macrophages to Secrete Tumour Necrosis Factor-α. Biomaterials 2000, 21, 1797–1802. [Google Scholar] [CrossRef]
- Kapellos, T.S.; Taylor, L.; Lee, H.; Cowley, S.A.; James, W.S.; Iqbal, A.J.; Greaves, D.R. A Novel Real Time Imaging Platform to Quantify Macrophage Phagocytosis. Biochem. Pharmacol. 2016, 116, 107–119. [Google Scholar] [CrossRef]
- Zanoni, I.; Ostuni, R.; Marek, L.R.; Barresi, S.; Barbalat, R.; Barton, G.M.; Granucci, F.; Kagan, J.C. CD14 Controls the LPS-Induced Endocytosis of Toll-like Receptor. Cell 2011, 147, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.J.; Choi, S.-E.; Lee, N.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. CCL20 Induced by Visfatin in Macrophages via the NF-κB and MKK3/6-P38 Signaling Pathways Contributes to Hepatic Stellate Cell Activation. Mol. Biol. Rep. 2020, 47, 4285. [Google Scholar] [CrossRef]
- Lin, T.-J.; Maher, L.H.; Gomi, K.; McCurdy, J.D.; Garduno, R.; Marshall, J.S. Selective Early Production of CCL20, or Macrophage Inflammatory Protein 3α, by Human Mast Cells in Response to Pseudomonas Aeruginosa. Infect. Immun. 2003, 71, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, Q.; Hoover, D.M.; Staley, P.; Tucker, K.D.; Lubkowski, J.; Oppenheim, J.J. Many Chemokines Including CCL20/MIP-3alpha Display Antimicrobial Activity. J. Leukoc. Biol. 2003, 74, 448–455. [Google Scholar] [CrossRef]
- Fang, W.; Bi, D.; Zheng, R.; Cai, N.; Xu, H.; Zhou, R.; Lu, J.; Wan, M.; Xu, X. Identification and Activation of TLR4-Mediated Signalling Pathways by Alginate-Derived Guluronate Oligosaccharide in RAW264.7 Macrophages. Sci. Rep. 2017, 7, 1663. [Google Scholar] [CrossRef] [PubMed]
- Koorella, C.; Nair, J.R.; Murray, M.E.; Carlson, L.M.; Watkins, S.K.; Lee, K.P. Novel Regulation of CD80/CD86-Induced Phosphatidylinositol 3-Kinase Signaling by NOTCH1 Protein in Interleukin-6 and Indoleamine 2,3-Dioxygenase Production by Dendritic Cells. J. Biol. Chem. 2014, 289, 7747–7762. [Google Scholar] [CrossRef]
- Wang, Y.; Lawson, M.A.; Dantzer, R.; Kelley, K.W. LPS-Induced Indoleamine 2,3-Dioxygenase Is Regulated in an Interferon-c- 3 Independent Manner by a JNK Signaling Pathway in Primary Murine Microglia. Brain Behav. Immun. 2010, 24, 201. [Google Scholar] [CrossRef]
- Adema, G.J.; Hartgers, F.; Verstraten, R.; de Vries, E.; Marland, G.; Menon, S.; Foster, J.; Xu, Y.; Nooyen, P.; McClanahan, T.; et al. A Dendritic-Cell-Derived C–C Chemokine That Preferentially Attracts Naive T Cells. Nature 1997, 387, 713–717. [Google Scholar] [CrossRef]
- Chenivesse, C.; Chang, Y.; Azzaoui, I.; Ait Yahia, S.; Morales, O.; Plé, C.; Foussat, A.; Tonnel, A.-B.; Delhem, N.; Yssel, H.; et al. Pulmonary CCL18 Recruits Human Regulatory T Cells. J. Immunol. 2012, 189, 128–137. [Google Scholar] [CrossRef]
- Shimizu, Y.; Dobashi, K. CC-Chemokine CCL15 Expression and Possible Implications for the Pathogenesis of IgE-Related Severe Asthma. Med. Inflamm. 2012, 2012, e475253. [Google Scholar] [CrossRef]
- Kimura, S.; Tsuji, T. Mechanical and Immunological Regulation in Wound Healing and Skin Reconstruction. Int. J. Mol. Sci. 2021, 22, 5474. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, U.; Kuroda, E. Regulation of Macrophage-Derived Chemokine (MDC, CCL22) Production. Crit. Rev. Immunol. 2002, 22, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Toobian, D.; Ghosh, P.; Katkar, G.D. Parsing the Role of PPARs in Macrophage Processes. Front. Immunol. 2021, 12, 783780. [Google Scholar] [CrossRef]
- Rigamonti, E.; Chinetti-Gbaguidi, G.; Staels, B. Regulation of Macrophage Functions by PPAR-α, PPAR-γ, and LXRs in Mice and Men. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1050–1059. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively Activated Macrophages; a Double-Edged Sword in Allergic Asthma. J. Transl. Med. 2020, 18, 58. [Google Scholar] [CrossRef]
- Eligini, S.; Fiorelli, S.; Tremoli, E.; Colli, S. Inhibition of Transglutaminase 2 Reduces Efferocytosis in Human Macrophages: Role of CD14 and SR-AI Receptors. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 922–930. [Google Scholar] [CrossRef]
- Telci, D. Tissue Transglutaminase (TG2)—A Wound Response Enzyme. Front. Biosci. 2006, 11, 867. [Google Scholar] [CrossRef] [PubMed]
- Yiu, T.W.; Holman, S.R.; Kaidonis, X.; Graham, R.M.; Iismaa, S.E. Transglutaminase 2 Facilitates Murine Wound Healing in a Strain-Dependent Manner. Int. J. Mol. Sci. 2023, 24, 11475. [Google Scholar] [CrossRef]
- Silverstein, R.L.; Febbraio, M. CD36, a Scavenger Receptor Involved in Immunity, Metabolism, Angiogenesis, and Behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef]
- Pennathur, S.; Pasichnyk, K.; Bahrami, N.M.; Zeng, L.; Febbraio, M.; Yamaguchi, I.; Okamura, D.M. The Macrophage Phagocytic Receptor CD36 Promotes Fibrogenic Pathways on Removal of Apoptotic Cells during Chronic Kidney Injury. Am. J. Pathol. 2015, 185, 2232–2245. [Google Scholar] [CrossRef]
- Parks, B.W.; Black, L.L.; Zimmerman, K.A.; Metz, A.E.; Steele, C.; Murphy-Ullrich, J.E.; Kabarowski, J.H. CD36, but Not G2A, Modulates Efferocytosis, Inflammation, and Fibrosis Following Bleomycin-Induced Lung Injury. J. Lipid. Res. 2013, 54, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Luzina, I.G.; Tsymbalyuk, N.; Choi, J.; Hasday, J.D.; Atamas, S.P. CCL18-Stimulated Upregulation of Collagen Production in Lung Fibroblasts Requires Sp1 Signaling and Basal Smad3 Activity. J. Cell. Physiol. 2006, 206, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Won, K.-J.; Lee, R.; Cho, H.-S.; Hwang, S.-H.; Nah, S.-Y. Wound Healing Effect of Gintonin Involves Lysophosphatidic Acid Receptor/Vascular Endothelial Growth Factor Signaling Pathway in Keratinocytes. Int. J. Mol. Sci. 2021, 22, 10155. [Google Scholar] [CrossRef]
- Geraldo, L.H.M.; Spohr, T.C.L.d.S.; Amaral, R.F.d.; Fonseca, A.C.C.d.; Garcia, C.; Mendes, F.d.A.; Freitas, C.; dosSantos, M.F.; Lima, F.R.S. Role of Lysophosphatidic Acid and Its Receptors in Health and Disease: Novel Therapeutic Strategies. Signal Transduct. Target. Ther. 2021, 6, 45. [Google Scholar] [CrossRef] [PubMed]
- Rancoule, C.; Pradère, J.-P.; Gonzalez, J.; Klein, J.; Valet, P.; Bascands, J.-L.; Schanstra, J.P.; Saulnier-Blache, J.-S. Lysophosphatidic Acid-1-Receptor Targeting Agents for Fibrosis. Expert Opin. Investig. Drugs 2011, 20, 657–667. [Google Scholar] [CrossRef]
- Zhou, X.; Friedmann, K.S.; Lyrmann, H.; Zhou, Y.; Schoppmeyer, R.; Knörck, A.; Mang, S.; Hoxha, C.; Angenendt, A.; Backes, C.S.; et al. A Calcium Optimum for Cytotoxic T Lymphocyte and Natural Killer Cell Cytotoxicity: A Calcium Optimum for CTL and NK Cell Cytotoxicity. J. Physiol. 2018, 596, 2681–2698. [Google Scholar] [CrossRef]
- Fujisaki, H.; Futaki, S.; Yamada, M.; Sekiguchi, K.; Hayashi, T.; Ikejima, T.; Hattori, S. Respective Optimal Calcium Concentrations for Proliferation on Type I Collagen Fibrils in Two Keratinocyte Line Cells, HaCaT and FEPE1L-8. Regen. Ther. 2018, 8, 73–79. [Google Scholar] [CrossRef]
- Lalau, J.D.; Bresson, R.; Charpentier, P.; Coliche, V.; Erlher, S.; Ha Van, G.; Magalon, G.; Martini, J.; Moreau, Y.; Pradines, S.; et al. Efficacy and Tolerance of Calcium Alginate versus Vaseline Gauze Dressings in the Treatment of Diabetic Foot Lesions. Diabetes Metab. 2002, 28, 223–229. [Google Scholar]
- Sayag, J.; Meaume, S.; Bohbot, S. Healing Properties of Calcium Alginate Dressings. J. Wound Care 1996, 5, 357–362. [Google Scholar] [CrossRef]
| Gene Name | Forward Primer 5′-3′ | Reverse Primer 5′-3′ |
|---|---|---|
| Primers for RT-qPCR | ||
| α-SMA | GGCTGTTTTCCCATCCATTG | TGTCCCATTCCCACCATCA |
| β2M | TGCTGTCTCCATGTTTGATGTATCT | TCTCTGCTCCCCACCTCTAAGT |
| GM-CSF | AGGGCCCTTGACCATGA | GGGTTGCACAGGAAGTTTCC |
| COL1A | GGGCAAGACAGTGATTGAATACAA | ACGTCGAAGCCGAATTCCT |
| IL-1β | TACCTGTCCTGCGTGTTGAA | TCTTTGGGTAATTTTTGGGATCT |
| IL-8 | AGACAGCAGAGCACACAAGC | ATGGTTCCTTCCGGTGGT |
| IL-6 | TGGCTGAAAAAGATGGATGCT | GATGATTTTCACCAGGCAAGTCT |
| IL-10 | TGCCTTCAGCAGAGTGAAGA | GCAACCCAGGTAACCCTTAAA |
| TGF-β | GGGAAATTGAGGGCTTTC G | GAACCCGTTGATGTCCACTTG |
| FABP4 | ACGAGAGGATGATAAACTGGTGG | GCGAACTTCAGTCCAGGTCAAC |
| Primers for Microfluidic qPCR | ||
| (1) Transcription Factors | ||
| STAT1 | CAGCTTGACTCAAAATTCCTGGA | TGAAGATTACGCTTGCTTTTCCT |
| STAT3 | ATCACGCCTTCTACAGACTGC | CATCCTGGAGATTCTCTACCACT |
| STAT6 | CGAGTAGGGGAGATCCACCTT | GCAGGAGTTTCTATCAAGCTGTG |
| SOCS3 | AGCAGCGATGGAATTACCTGGAAC | TCCAGCCCAATACCTGACACAGAA |
| PPARγ | GAC-AGG-AAA-GAC-AAC-AGA-CAA-ATC | GGG-GTG-ATG-TGT-TTG-AAC-TTG |
| KLF4 | CCCACATGAAGCGACTTCCC | CAGGTCCAGGAGATCGTTGAA |
| MERTK | ACCTCTGTCGAATCAAAGCCC | CTGCACACTGGTTATGCTGAA |
| JMJD3 | CACCCCAGCAAACCATATTATGC | CACACAGCCATGCAGGGATT |
| AP-1 | TCCAAGTGCCGAAAAAGGAAG | CGAGTTCTGAGCTTTCAAGGT |
| IRF5 | GCTGTGCCCTTAACAAGAGC | TGCACCAAAAGAGTAATCCTCAG |
| MAPK1 | TACACCAACCTCTCGTACATCG | CATGTCTGAAGCGCAGTAAGATT |
| IDO1 | GCCAGCTTCGAGAAAGAGTTG | ATCCCAGAACTAGACGTGCAA |
| TGM2 | TGTGGCACCAAGTACCTGCTCA | GCACCTTGATGAGGTTGGACTC |
| (2) Receptors/Markers M1 or M2 | ||
| HLA-DQA1 | TCGCTCTGACCACCGTGAT | AGGGACCGTAAAACTGGTACAA |
| HLA-DQB1 | AGACTCTCCCGAGGATTTCGT | GAAGTAGCACATGCCCTTAAACT |
| HLA-DR A | AGTCCCTGTGCTAGGATTTTTCA | ACATAAACTCGCCTGATTGGTC |
| CD68 | CGAGCATCATTCTTTCACCAGCT | ATGAGAGGCAGCAAGATGGACC |
| CD86 | CTGCTCATCTATACACGGTTACC | GGAAACGTCGTACAGTTCTGTG |
| CD206 | GGGTTGCTATCACTCTCTATGC | TTTCTTGTCTGTTGCCGTAGTT |
| NOX1 | ACCGGGTTTATGATATTCCACCT | GATTTCGACAGACTGGCAAGA |
| (3) Wound-Healing-Associated Genes | ||
| ARG2 | CGCGAGTGCATTCCATCCT | TCCAAAGTCTTTTAGGTGGCAG |
| LPAR1 | CTTTGCTGGGTTGGCCTACTT | GCCATGTGCTAACAGTCAGTCT |
| LPAR2 | ACAGCCCGACTTTCACTTGAG | GCCCACAATGAGCATGACCA |
| SIPR1 | GCCTCTTCCTGCTAATCAGCG | GCAGTACAGAATGACGATGGAG |
| (4) Cytokines, Chemokines, and MMPs | ||
| CCL15 | TCCCAGGCCCAGTTCATAAAT | TGCTTTGTGAGATGTAGGAGGT |
| CCL20 | TGCTGTACCAAGAGTTTGCTC | CGCACACAGACAACTTTTTCTTT |
| CCL22 | ATTACGTCCGTTACCGTCTGC | TCCCTGAAGGTTAGCAACACC |
| IL-27RA | CCCCGTCTTCGTGAACCTAGA | ACATCTTCGGTAGTGGAACTGG |
| IL1-ra | CATTGAGCCTCATGCTCTGTT | CGCTGTCTGAGCGGATGAA |
| IL23A | GAGCCTTCTCTGCTCCCTGATA | GACTGAGGCTTGGAATCTGCTG |
| IFNG | TAATGCAGGTCATTCAGATGTAG | TGTCACTCTCCTCTTTCCAA |
| MMP2 | CTT-CCA-AGT-CTG-GAG-CGA-TGT | TAC-CGT-CAA-AGG-GGT-ATC-CAT |
| MMP9 | TGTACCGCTATGGTTACACTCG | GGCAGGGACAGTTGCTTCT |
| (5) Housekeeping Genes | ||
| GAPDH | CCAGGTGGTCTCCTCTGACTTC | GTGGTCGTTGAGGGCAATG |
| B-actin | TGC-CGA-CAG-GAT-GCA-GAA-G | CTC-AGG-AGG-AGC-AAT-GAT-CTT-GA |
| β2M | TGCTGTCTCCATGTTTGATGTATCT | TCTCTGCTCCCCACCTCTAAGT |
| M1 Phenotype | Common to All ALG, BIA, & Ca2+ 3 mM | Common to Alginates ALG & BIA | Common to ALG & Ca2+ 3 mM | Common to BIA & Ca2+ 3 mM | ALG Only | BIA Only | Ca2+ 3 mM Only |
|---|---|---|---|---|---|---|---|
| Upregulated genes | GM-CSF, IL-23A, SOCS3, IL-1ra, IL-8 | CCL20, JMJD3, AP-1, LPAR2, CD86, IDO1 | IL-1β, IL-6 | CCL22 | CCL15, PPARγ | ARG2 | |
| Downregulated genes | MerTK, NOX1, CD16 | HLA-DRA | HLA-DRQB1, IFN-γ |
| M2a Phenotype | Common to All ALG, BIA, & Ca2+ 3 mM | Common to Alginates ALG & BIA | Common to ALG & Ca2+ 3 mM | Common to BIA & Ca2+ 3 mM | ALG Only | BIA Only | Ca2+ 3 mM Only |
|---|---|---|---|---|---|---|---|
| Upregulated genes | FABP4, IL-1ra, SOCS3, CD86, ARG2 | TGM2, CCL22, CCL15 | IL-23A | PPARγ | IDO1 | ||
| Downregulated genes | NOX1, CD163, HLA-DRQA1, HLA-DRQB1, HLA-DRA | MerTK, MMP2 | MMP9 | TGF-β | CD206 | IFN-γ |
| M2c Phenotype | Common to All ALG, BIA, & Ca2+ 3 mM | Common to Alginates ALG & BIA | Common to ALG & Ca2+ 3 mM | Common to BIA & Ca2+ 3 mM | ALG Only | BIA Only | Ca2+ 3 mM Only |
|---|---|---|---|---|---|---|---|
| Upregulated genes | CCL20, IL-23A, CD86, TGM2, IL-1ra | IDO1, MMP2 | LPAR1, CCL15 | AP-1, PPARγ | ARG2 | ||
| Downregulated genes | IL-10 | HLA-DRQB1, HLA-DRA, SIPR1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adib, Y.; Serror, K.; Pinzon, J.A.; Duciel, L.; Delagrange, M.; Ducos, B.; Boccara, D.; Mimoun, M.; Chaouat, M.; Bensussan, A.; et al. In Vitro Modulation of Macrophage Inflammatory and Pro-Repair Properties Essential for Wound Healing by Calcium and Calcium-Alginate Dressings. Cells 2025, 14, 909. https://doi.org/10.3390/cells14120909
Adib Y, Serror K, Pinzon JA, Duciel L, Delagrange M, Ducos B, Boccara D, Mimoun M, Chaouat M, Bensussan A, et al. In Vitro Modulation of Macrophage Inflammatory and Pro-Repair Properties Essential for Wound Healing by Calcium and Calcium-Alginate Dressings. Cells. 2025; 14(12):909. https://doi.org/10.3390/cells14120909
Chicago/Turabian StyleAdib, Yara, Kevin Serror, Jose Amaya Pinzon, Laura Duciel, Marine Delagrange, Bertrand Ducos, David Boccara, Maurice Mimoun, Marc Chaouat, Armand Bensussan, and et al. 2025. "In Vitro Modulation of Macrophage Inflammatory and Pro-Repair Properties Essential for Wound Healing by Calcium and Calcium-Alginate Dressings" Cells 14, no. 12: 909. https://doi.org/10.3390/cells14120909
APA StyleAdib, Y., Serror, K., Pinzon, J. A., Duciel, L., Delagrange, M., Ducos, B., Boccara, D., Mimoun, M., Chaouat, M., Bensussan, A., Samardzic, M., Bagot, M., Des Courtils, C., & Michel, L. (2025). In Vitro Modulation of Macrophage Inflammatory and Pro-Repair Properties Essential for Wound Healing by Calcium and Calcium-Alginate Dressings. Cells, 14(12), 909. https://doi.org/10.3390/cells14120909









