Unraveling the Significance of DGCR8 and miRNAs in Thyroid Carcinoma
Abstract
1. Introduction
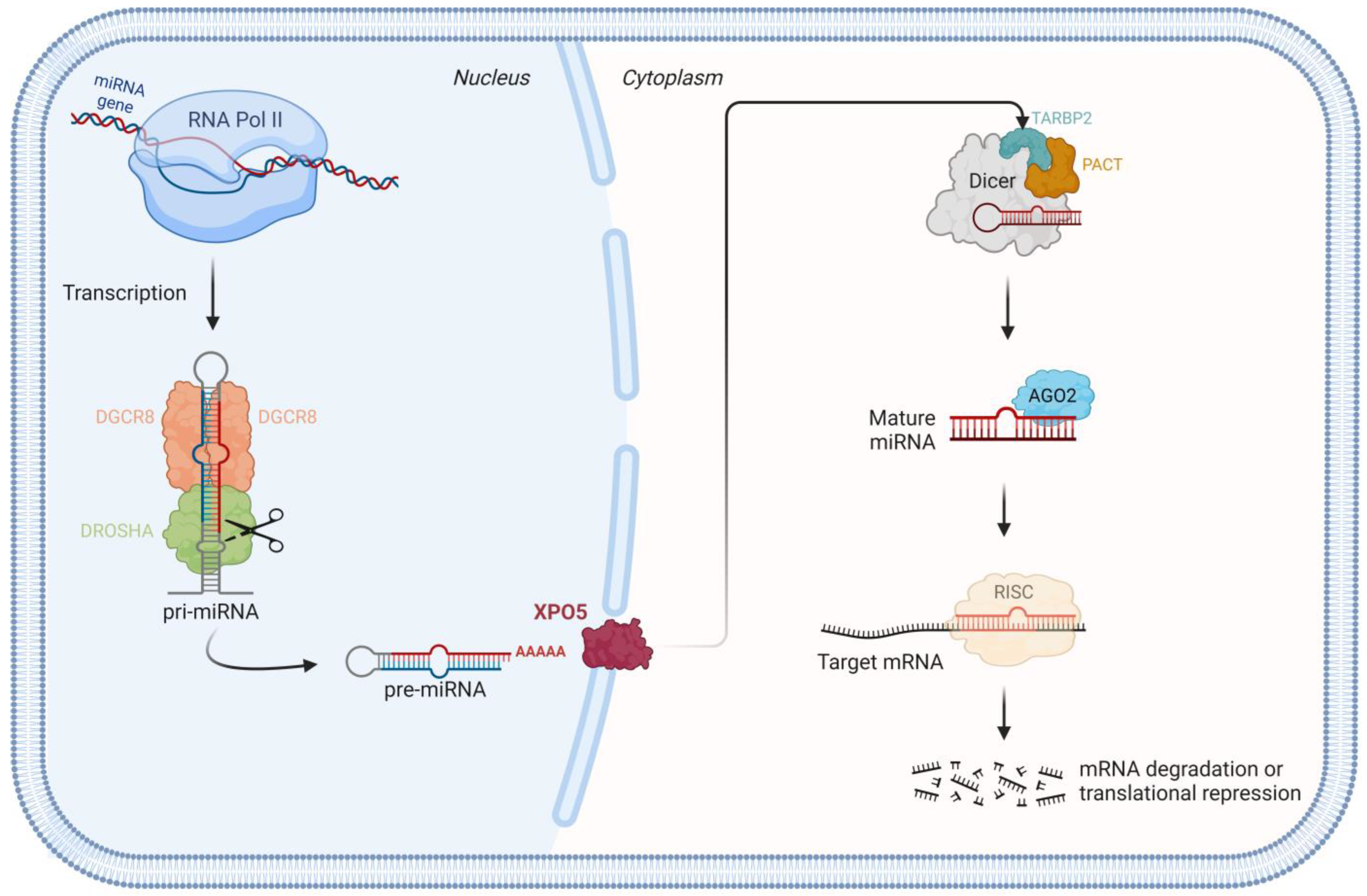
1.1. miRNA Biogenesis Pathway
1.2. miRNAs as Powerful Biomarkers in Cancer
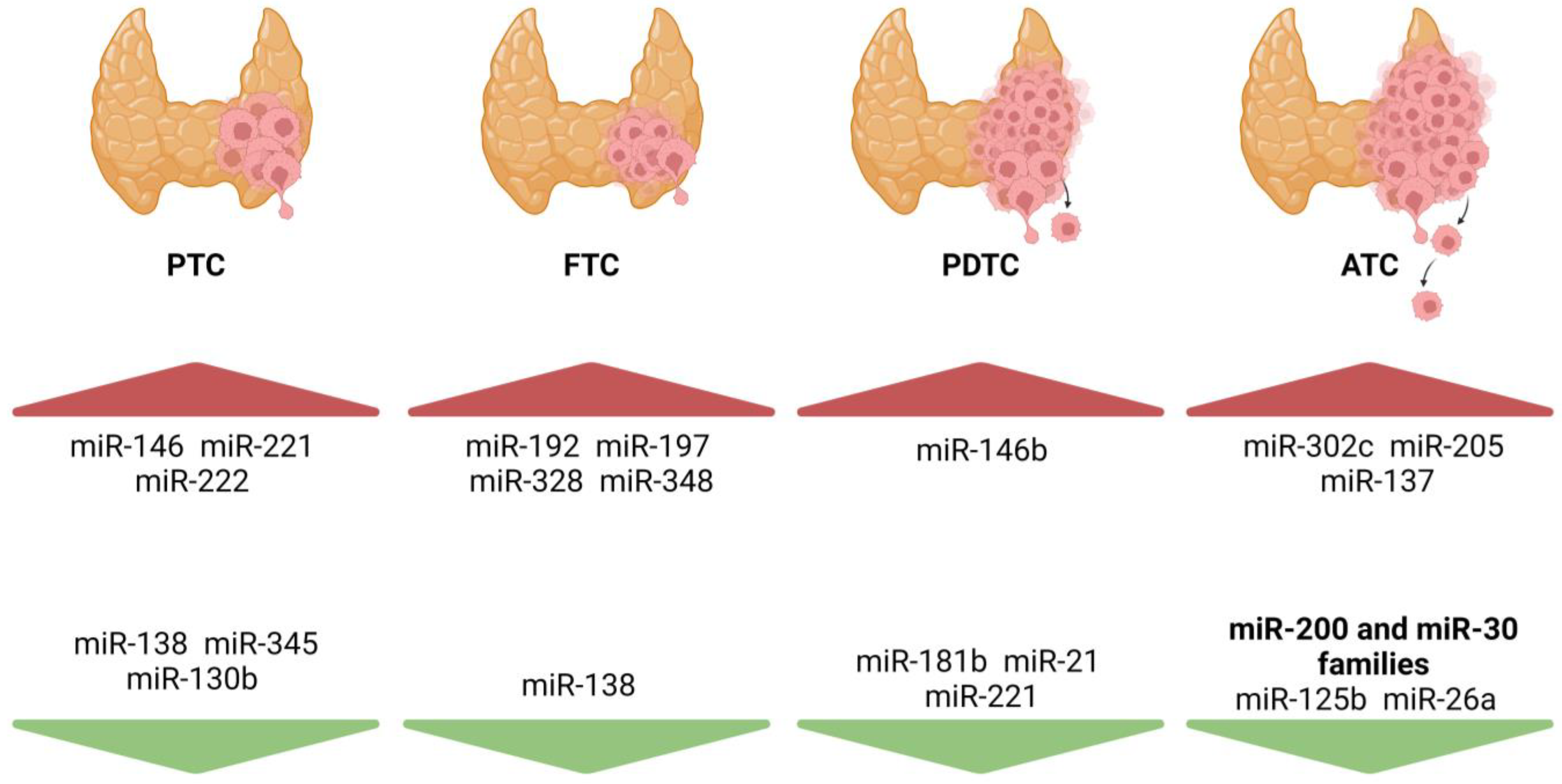
1.3. miRNAs in Thyroid Tumorigenesis
1.4. DGCR8, a miRNA Biogenesis Component, Is Dysregulated in Thyroid Tumors
1.5. DGCR8 E518K Hotspot Mutation
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Wen, J.; Lv, Z.; Ding, H.; Fang, X.; Sun, M. Association of miRNA biosynthesis genes DROSHA and DGCR8 polymorphisms with cancer susceptibility: A systematic review and meta-analysis. Biosci. Rep. 2018, 38, BSR20180072. [Google Scholar] [CrossRef]
- Ouellet, D.L.; Perron, M.P.; Gobeil, L.A.; Plante, P.; Provost, P. MicroRNAs in gene regulation: When the smallest governs it all. J. Biomed. Biotechnol. 2006, 2006, 69616. [Google Scholar] [CrossRef]
- Marini, F.; Luzi, E.; Brandi, M.L. MicroRNA Role in Thyroid Cancer Development. J. Thyroid. Res. 2011, 2011, 407123. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Jo, M.H.; Choi, Y.G.; Park, J.; Kwon, S.C.; Hohng, S.; Kim, V.N.; Woo, J.S. Functional Anatomy of the Human Microprocessor. Cell 2015, 161, 1374–1387. [Google Scholar] [CrossRef]
- Macias, S.; Cordiner, R.A.; Caceres, J.F. Cellular functions of the microprocessor. Biochem. Soc. Trans. 2013, 41, 838–843. [Google Scholar] [CrossRef]
- Liao, G.R.; Tseng, Y.Y.; Tseng, C.Y.; Lo, C.Y.; Hsu, W.L. The orf virus (ORFV) protein OV20.0 interacts with the microprocessor complex subunit DGCR8 to regulate miRNA biogenesis and ORFV infection. FEBS Lett. 2021, 595, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Shiohama, A.; Sasaki, T.; Noda, S.; Minoshima, S.; Shimizu, N. Nucleolar localization of DGCR8 and identification of eleven DGCR8-associated proteins. Exp. Cell Res. 2007, 313, 4196–4207. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.C.; Jorcyk, C.L.; Oxford, J.T. DICER1 Syndrome: DICER1 Mutations in Rare Cancers. Cancers 2018, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Rivera, B.; Nadaf, J.; Fahiminiya, S.; Apellaniz-Ruiz, M.; Saskin, A.; Chong, A.S.; Sharma, S.; Wagener, R.; Revil, T.; Condello, V.; et al. DGCR8 microprocessor defect characterizes familial multinodular goiter with schwannomatosis. J. Clin. Investig. 2020, 130, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Boufraqech, M.; Klubo-Gwiezdzinska, J.; Kebebew, E. MicroRNAs in the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 603–619. [Google Scholar] [CrossRef]
- Guo, W.T.; Wang, Y. Dgcr8 knockout approaches to understand microRNA functions in vitro and in vivo. Cell Mol. Life Sci. 2019, 76, 1697–1711. [Google Scholar] [CrossRef] [PubMed]
- Slade, I.; Bacchelli, C.; Davies, H.; Murray, A.; Abbaszadeh, F.; Hanks, S.; Barfoot, R.; Burke, A.; Chisholm, J.; Hewitt, M.; et al. DICER1 syndrome: Clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J. Med. Genet. 2011, 48, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Fuziwara, C.S.; Kimura, E.T. MicroRNAs in thyroid development, function and tumorigenesis. Mol. Cell. Endocrinol. 2017, 456, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Triboulet, R.; Thornton, J.E.; Gregory, R.I. A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28-let-7 pathway. Nature 2013, 497, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Heo, I.; Joo, C.; Cho, J.; Ha, M.; Han, J.; Kim, V.N. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol. Cell 2008, 32, 276–284. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhao, Z.; Cai, Q.; Zhang, Y.; Zhang, P.; Shi, S.; Xie, H.; Peng, X.; Yin, W.; Tao, Y.; et al. miRNA-based biomarkers, therapies, and resistance in Cancer. Int. J. Biol. Sci. 2020, 16, 2628–2647. [Google Scholar] [CrossRef]
- Pong, S.K.; Gullerova, M. Noncanonical functions of microRNA pathway enzymes—Drosha, DGCR8, Dicer and Ago proteins. FEBS Lett. 2018, 592, 2973–2986. [Google Scholar] [CrossRef]
- Park, J.L.; Kim, S.K.; Jeon, S.; Jung, C.K.; Kim, Y.S. MicroRNA Profile for Diagnostic and Prognostic Biomarkers in Thyroid Cancer. Cancers 2021, 13, 632. [Google Scholar] [CrossRef]
- Markopoulos, G.S.; Roupakia, E.; Tokamani, M.; Chavdoula, E.; Hatziapostolou, M.; Polytarchou, C.; Marcu, K.B.; Papavassiliou, A.G.; Sandaltzopoulos, R.; Kolettas, E. A step-by-step microRNA guide to cancer development and metastasis. Cell. Oncol. 2017, 40, 303–339. [Google Scholar] [CrossRef]
- Baumann, V.; Winkler, J. miRNA-based therapies: Strategies and delivery platforms for oligonucleotide and non-oligonucleotide agents. Future Med. Chem. 2014, 6, 1967–1984. [Google Scholar] [CrossRef]
- Leonardi, G.C.; Candido, S.; Carbone, M.; Colaianni, V.; Garozzo, S.F.; Cina, D.; Libra, M. microRNAs and thyroid cancer: Biological and clinical significance (Review). Int. J. Mol. Med. 2012, 30, 991–999. [Google Scholar] [CrossRef]
- Santiago, K.; Chen Wongworawat, Y.; Khan, S. Differential MicroRNA-Signatures in Thyroid Cancer Subtypes. J. Oncol. 2020, 2020, 2052396. [Google Scholar] [CrossRef]
- Forte, S.; La Rose, C.; Pecce, V.; Rosignolo, F.; Memeo, L. The Role of MicroRNAs in Thyroid Carcinomas. Anticancer Res. 2015, 35, 2037–2047. [Google Scholar]
- Ghafouri-Fard, S.; Shirvani-Farsani, Z.; Taheri, M. The role of microRNAs in the pathogenesis of thyroid cancer. Noncoding RNA Res. 2020, 5, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Chiosea, S.I.; Nikiforov, Y.E. MicroRNA expression profiles in thyroid tumors. Endocr. Pathol. 2009, 20, 85–91. [Google Scholar] [CrossRef]
- Perdas, E.; Stawski, R.; Nowak, D.; Zubrzycka, M. The Role of miRNA in Papillary Thyroid Cancer in the Context of miRNA Let-7 Family. Int. J. Mol. Sci. 2016, 17, 909. [Google Scholar] [CrossRef]
- Raue, R.; Frank, A.C.; Syed, S.N.; Brune, B. Therapeutic Targeting of MicroRNAs in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 2210. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Abdel-Mageed, A.B.; Mondal, D.; Kandil, E. MicroRNA expression profiles in differentiated thyroid cancer, a review. Int. J. Clin. Exp. Med. 2013, 6, 74–80. [Google Scholar] [PubMed]
- Ramirez-Moya, J.; Wert-Lamas, L.; Riesco-Eizaguirre, G.; Santisteban, P. Impaired microRNA processing by DICER1 downregulation endows thyroid cancer with increased aggressiveness. Oncogene 2019, 38, 5486–5499. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Kim, J.; Park, W.J.; Jeong, K.J.; Kang, S.H.; Kwon, S.Y.; Kim, S.; Park, J.W. Racial Differences in Expression Levels of miRNA Machinery-Related Genes, Dicer, Drosha, DGCR8, and AGO2, in Asian Korean Papillary Thyroid Carcinoma and Comparative Validation Using the Cancer Genome Atlas. Int. J. Genom. 2017, 2017, 5789769. [Google Scholar] [CrossRef]
- Rodrigues, L.; Canberk, S.; Macedo, S.; Soares, P.; Vinagre, J. DGCR8 Microprocessor Subunit Mutation and Expression Deregulation in Thyroid Lesions. Int. J. Mol. Sci. 2022, 23, 14812. [Google Scholar] [CrossRef]
- Vardapour, R.; Kehl, T.; Kneitz, S.; Ludwig, N.; Meese, E.; Lenhof, H.P.; Gessler, M. The DGCR8 E518K mutation found in Wilms tumors leads to a partial miRNA processing defect that alters gene expression patterns and biological processes. Carcinogenesis 2022, 43, 82–93. [Google Scholar] [CrossRef]
- Pelletier, D.; Rivera, B.; Fabian, M.R.; Foulkes, W.D. miRNA biogenesis and inherited disorders: Clinico-molecular insights. Trends Genet. 2023, 39, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Tseng, G.C.; Steward, D.; Diorio, D.; Nikiforov, Y.E. MicroRNA expression profiling of thyroid tumors: Biological significance and diagnostic utility. J. Clin. Endocrinol. Metab. 2008, 93, 1600–1608. [Google Scholar] [CrossRef] [PubMed]
- Christofer Juhlin, C.; Mete, O.; Baloch, Z.W. The 2022 WHO classification of thyroid tumors: Novel concepts in nomenclature and grading. Endocr. Relat. Cancer 2023, 30, e220293. [Google Scholar] [CrossRef]
- Jung, C.K.; Bychkov, A.; Kakudo, K. Update from the 2022 World Health Organization Classification of Thyroid Tumors: A Standardized Diagnostic Approach. Endocrinol. Metab. 2022, 37, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Romei, C.; Tacito, A.; Molinaro, E.; Piaggi, P.; Cappagli, V.; Pieruzzi, L.; Matrone, A.; Viola, D.; Agate, L.; Torregrossa, L.; et al. Clinical, pathological and genetic features of anaplastic and poorly differentiated thyroid cancer: A single institute experience. Oncol. Lett. 2018, 15, 9174–9182. [Google Scholar] [CrossRef] [PubMed]
- Lodewijk, L.; Prins, A.M.; Kist, J.W.; Valk, G.D.; Kranenburg, O.; Rinkes, I.H.; Vriens, M.R. The value of miRNA in diagnosing thyroid cancer: A systematic review. Cancer Biomark. 2012, 11, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Vriens, M.R.; Weng, J.; Suh, I.; Huynh, N.; Guerrero, M.A.; Shen, W.T.; Duh, Q.Y.; Clark, O.H.; Kebebew, E. MicroRNA expression profiling is a potential diagnostic tool for thyroid cancer. Cancer 2012, 118, 3426–3432. [Google Scholar] [CrossRef]
- Celano, M.; Rosignolo, F.; Maggisano, V.; Pecce, V.; Iannone, M.; Russo, D.; Bulotta, S. MicroRNAs as Biomarkers in Thyroid Carcinoma. Int. J. Genom. 2017, 2017, 6496570. [Google Scholar] [CrossRef]
- Visone, R.; Pallante, P.; Vecchione, A.; Cirombella, R.; Ferracin, M.; Ferraro, A.; Volinia, S.; Coluzzi, S.; Leone, V.; Borbone, E.; et al. Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. Oncogene 2007, 26, 7590–7595. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Nikiforova, M. Update on Molecular Testing for Cytologically Indeterminate Thyroid Nodules. Arch. Pathol. Lab. Med. 2018, 142, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Y.; Chen, L.; Zhao, J.; Guo, M.; Zhao, X.; Wen, Z.; He, Z.; Chen, C.; Xu, L. MiRNA-Based Therapies for Lung Cancer: Opportunities and Challenges? Biomolecules 2023, 13, 877. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, H.R.; Kim, H.; Yang, S.C.; Park, M.; Yoon, J.A.; Lim, H.J.; Hong, S.H.; DeMayo, F.J.; Lydon, J.P.; et al. Deficiency in DGCR8-dependent canonical microRNAs causes infertility due to multiple abnormalities during uterine development in mice. Sci. Rep. 2016, 6, 20242. [Google Scholar] [CrossRef] [PubMed]
- Jeker, L.T.; Zhou, X.; Blelloch, R.; Bluestone, J.A. DGCR8-mediated production of canonical microRNAs is critical for regulatory T cell function and stability. PLoS ONE 2013, 8, e66282. [Google Scholar] [CrossRef]
- Hang, Q.; Zeng, L.; Wang, L.; Nie, L.; Yao, F.; Teng, H.; Deng, Y.; Yap, S.; Sun, Y.; Frank, S.J.; et al. Non-canonical function of DGCR8 in DNA double-strand break repair signaling and tumor radioresistance. Nat. Commun. 2021, 12, 4033. [Google Scholar] [CrossRef] [PubMed]
- Bezman, N.A.; Cedars, E.; Steiner, D.F.; Blelloch, R.; Hesslein, D.G.; Lanier, L.L. Distinct requirements of microRNAs in NK cell activation, survival, and function. J. Immunol. 2010, 185, 3835–3846. [Google Scholar] [CrossRef]
- Daum, P.; Ottmann, S.R.; Meinzinger, J.; Schulz, S.R.; Corte-Real, J.; Hauke, M.; Roth, E.; Schuh, W.; Mielenz, D.; Jack, H.M.; et al. The microRNA processing subunit DGCR8 is required for a T cell-dependent germinal center response. Front. Immunol. 2022, 13, 991347. [Google Scholar] [CrossRef]
- Zhu, C.; Chen, C.; Huang, J.; Zhang, H.; Zhao, X.; Deng, R.; Dou, J.; Jin, H.; Chen, R.; Xu, M.; et al. SUMOylation at K707 of DGCR8 controls direct function of primary microRNA. Nucleic Acids Res. 2015, 43, 7945–7960. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.Y.; Pan, Q.W.; Wang, M.H.; Ai, X.; Yan, Y.Z.; Tian, Y.; Jin, Y.T.; Tang, P.; Jiang, J.; Ren, Z.X. DGCR8 promotes the metastasis in triple-negative breast cancer by epigenetically regulating TGF-beta. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
- Triboulet, R.; Chang, H.M.; Lapierre, R.J.; Gregory, R.I. Post-transcriptional control of DGCR8 expression by the Microprocessor. RNA 2009, 15, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Pedersen, J.S.; Kwon, S.C.; Belair, C.D.; Kim, Y.K.; Yeom, K.H.; Yang, W.Y.; Haussler, D.; Blelloch, R.; Kim, V.N. Posttranscriptional crossregulation between Drosha and DGCR8. Cell 2009, 136, 75–84. [Google Scholar] [CrossRef]
- Gomez-Cabello, D.; Callejas, S.; Benguria, A.; Moreno, A.; Alonso, J.; Palmero, I. Regulation of the microRNA processor DGCR8 by the tumor suppressor ING1. Cancer Res. 2010, 70, 1866–1874. [Google Scholar] [CrossRef]
- Zhou, X.H.; Lin, W.; Ren, Y.M.; Liu, S.; Fan, B.Y.; Wei, Z.J.; Shi, G.D.; Cheng, X.; Hao, Y.; Feng, S.Q. Comparison of DNA Methylation in Schwann Cells before and after Peripheral Nerve Injury in Rats. BioMed Res. Int. 2017, 2017, 5393268. [Google Scholar] [CrossRef]
- Han, J.; Wang, J.Z.; Yang, X.; Yu, H.; Zhou, R.; Lu, H.C.; Yuan, W.B.; Lu, J.C.; Zhou, Z.J.; Lu, Q.; et al. METTL3 promote tumor proliferation of bladder cancer by accelerating pri-miR221/222 maturation in m6A-dependent manner. Mol. Cancer 2019, 18, 110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Medvid, R.; Melton, C.; Jaenisch, R.; Blelloch, R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat. Genet. 2007, 39, 380–385. [Google Scholar] [CrossRef]
- Puppin, C.; Durante, C.; Sponziello, M.; Verrienti, A.; Pecce, V.; Lavarone, E.; Baldan, F.; Campese, A.F.; Boichard, A.; Lacroix, L.; et al. Overexpression of genes involved in miRNA biogenesis in medullary thyroid carcinomas with RET mutation. Endocrine 2014, 47, 528–536. [Google Scholar] [CrossRef]
- Nicolson, N.G.; Murtha, T.D.; Dong, W.; Paulsson, J.O.; Choi, J.; Barbieri, A.L.; Brown, T.C.; Kunstman, J.W.; Larsson, C.; Prasad, M.L.; et al. Comprehensive Genetic Analysis of Follicular Thyroid Carcinoma Predicts Prognosis Independent of Histology. J. Clin. Endocrinol. Metab. 2018, 103, 2640–2650. [Google Scholar] [CrossRef]
- Kumar, M.S.; Lu, J.; Mercer, K.L.; Golub, T.R.; Jacks, T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat. Genet. 2007, 39, 673–677. [Google Scholar] [CrossRef]
- Paulsson, J.O.; Rafati, N.; DiLorenzo, S.; Chen, Y.; Haglund, F.; Zedenius, J.; Juhlin, C.C. Whole-genome Sequencing of Follicular Thyroid Carcinomas Reveal Recurrent Mutations in MicroRNA Processing Subunit DGCR8. J. Clin. Endocrinol. Metab. 2021, 106, 3265–3282. [Google Scholar] [CrossRef]
- Condello, V.; Poma, A.M.; Macerola, E.; Vignali, P.; Paulsson, J.O.; Zedenius, J.; Basolo, F.; Juhlin, C.C. Prevalence, Molecular Landscape and Clinical Impact of DICER1 and DGCR8 Mutated Follicular-Patterned Thyroid Nodules. J. Clin. Endocrinol. Metab. 2024; corrected proof. [Google Scholar] [CrossRef]
- Hata, A.; Kashima, R. Dysregulation of microRNA biogenesis machinery in cancer. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 121–134. [Google Scholar] [CrossRef]
- Castro, P.; Eknaes, M.; Teixeira, M.R.; Danielsen, H.E.; Soares, P.; Lothe, R.A.; Sobrinho-Simoes, M. Adenomas and follicular carcinomas of the thyroid display two major patterns of chromosomal changes. J. Pathol. 2005, 206, 305–311. [Google Scholar] [CrossRef] [PubMed]
- de Kock, L.; Rivera, B.; Foulkes, W.D. Pineoblastoma is uniquely tolerant of mutually exclusive loss of DICER1, DROSHA or DGCR8. Acta Neuropathol. 2020, 139, 1115–1118. [Google Scholar] [CrossRef]
- Wu, M.K.; Sabbaghian, N.; Xu, B.; Addidou-Kalucki, S.; Bernard, C.; Zou, D.; Reeve, A.E.; Eccles, M.R.; Cole, C.; Choong, C.S.; et al. Biallelic DICER1 mutations occur in Wilms tumours. J. Pathol. 2013, 230, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Nogue, C.; Chong, A.S.; Grau, E.; Han, H.; Dorca, E.; Roca, C.; Mosquera, J.L.; Lazaro, C.; Foulkes, W.D.; Brunet, J.; et al. DGCR8 and the six hit, three-step model of schwannomatosis. Acta Neuropathol. 2022, 143, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Schultz, K.A.P.; Rednam, S.P.; Kamihara, J.; Doros, L.; Achatz, M.I.; Wasserman, J.D.; Diller, L.R.; Brugieres, L.; Druker, H.; Schneider, K.A.; et al. PTEN, DICER1, FH, and Their Associated Tumor Susceptibility Syndromes: Clinical Features, Genetics, and Surveillance Recommendations in Childhood. Clin. Cancer Res. 2017, 23, e76–e82. [Google Scholar] [CrossRef] [PubMed]
- Canberk, S.; Ferreira, J.C.; Pereira, L.; Batista, R.; Vieira, A.F.; Soares, P.; Sobrinho Simoes, M.; Maximo, V. Analyzing the Role of DICER1 Germline Variations in Papillary Thyroid Carcinoma. Eur. Thyroid. J. 2021, 9, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Astuti, D.; Morris, M.R.; Cooper, W.N.; Staals, R.H.; Wake, N.C.; Fews, G.A.; Gill, H.; Gentle, D.; Shuib, S.; Ricketts, C.J.; et al. Germline mutations in DIS3L2 cause the Perlman syndrome of overgrowth and Wilms tumor susceptibility. Nat. Genet. 2012, 44, 277–284. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.; Da Cruz Paula, A.; Soares, P.; Vinagre, J. Unraveling the Significance of DGCR8 and miRNAs in Thyroid Carcinoma. Cells 2024, 13, 561. https://doi.org/10.3390/cells13070561
Rodrigues L, Da Cruz Paula A, Soares P, Vinagre J. Unraveling the Significance of DGCR8 and miRNAs in Thyroid Carcinoma. Cells. 2024; 13(7):561. https://doi.org/10.3390/cells13070561
Chicago/Turabian StyleRodrigues, Lia, Arnaud Da Cruz Paula, Paula Soares, and João Vinagre. 2024. "Unraveling the Significance of DGCR8 and miRNAs in Thyroid Carcinoma" Cells 13, no. 7: 561. https://doi.org/10.3390/cells13070561
APA StyleRodrigues, L., Da Cruz Paula, A., Soares, P., & Vinagre, J. (2024). Unraveling the Significance of DGCR8 and miRNAs in Thyroid Carcinoma. Cells, 13(7), 561. https://doi.org/10.3390/cells13070561






