Heparan Sulfates Regulate Axonal Excitability and Context Generalization through Ca2+/Calmodulin-Dependent Protein Kinase II
Abstract
1. Introduction
2. Materials and Methods
2.1. Immunoblot Analysis
2.2. Slice Preparation and In Vitro Electrophysiology
2.3. Immunocytochemistry in Hippocampal Cultured Neurons
2.4. In Vivo Intrahippocampal Injection
2.5. Fear Conditioning
2.6. Statistics
3. Results
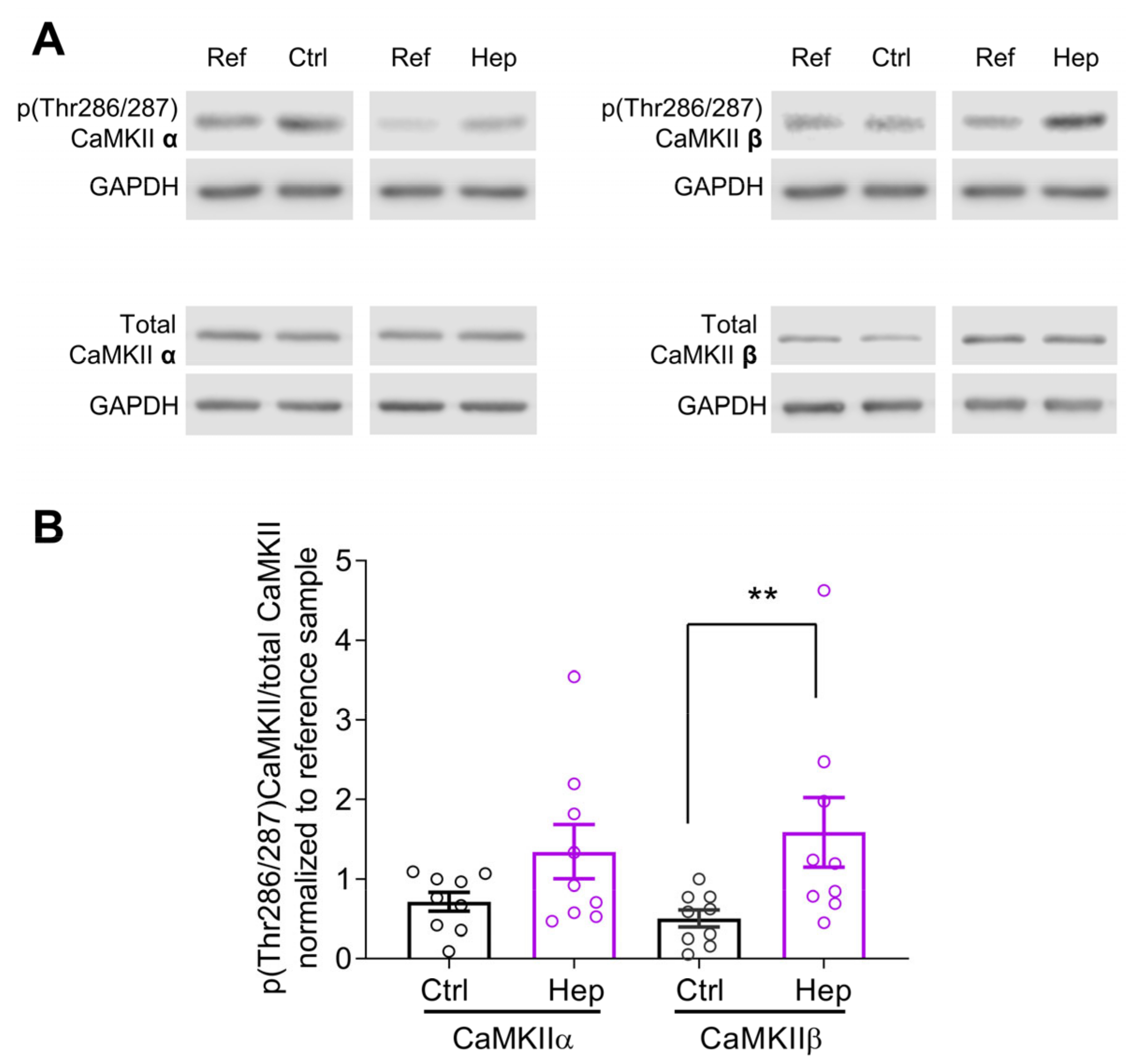
3.1. Heparinase Treatment Elevates CaMKIIβ Autophosphorylation in the Mouse Hippocampus
3.2. Enzymatic Digestion of HSHSs Does Not Change Synaptic Transmission to CA1 Pyramidal Neurons
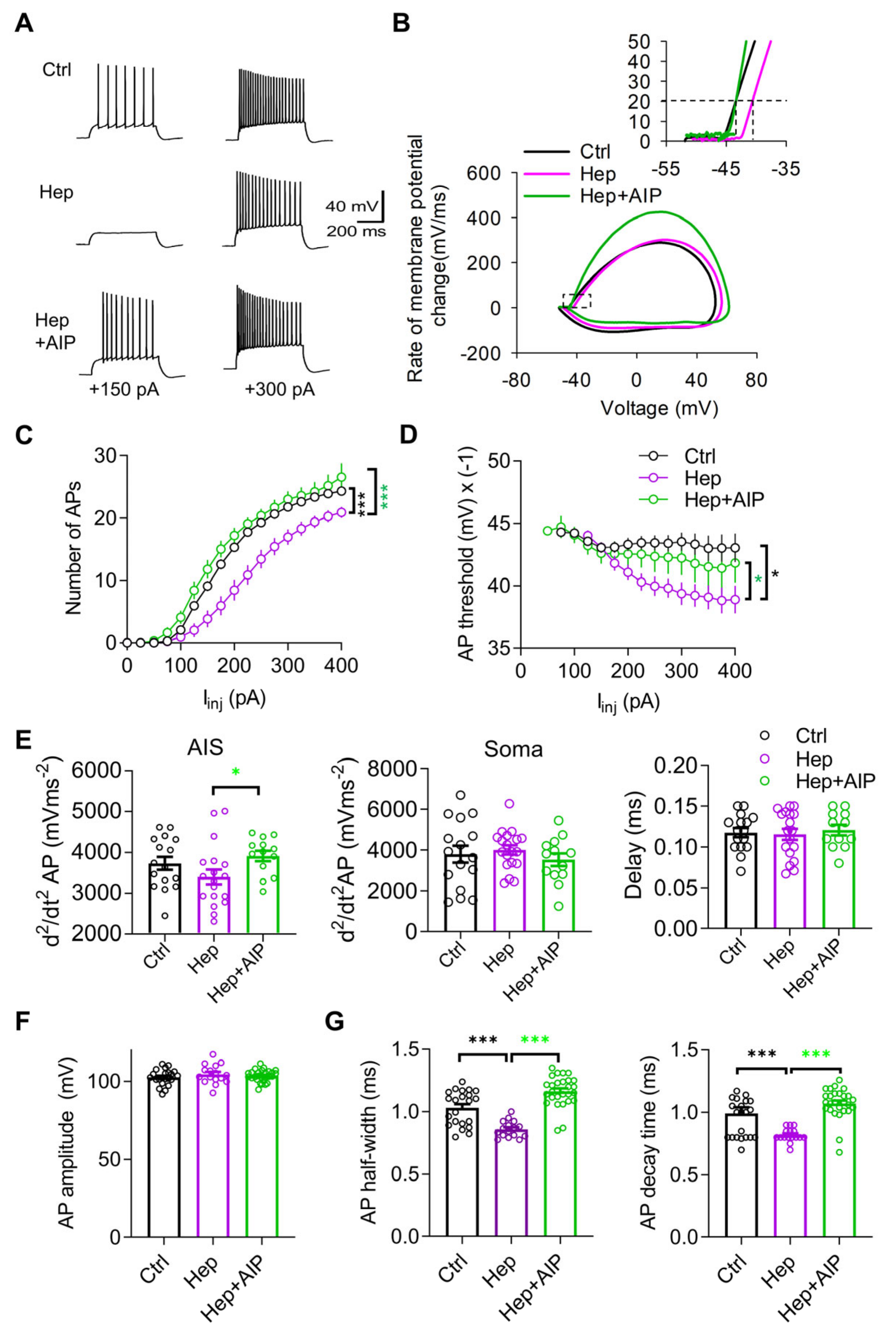
3.3. Impaired Neuronal Excitability after In Vivo Injection of Heparinase Is Rescued by CaMKII Inhibitor AIP
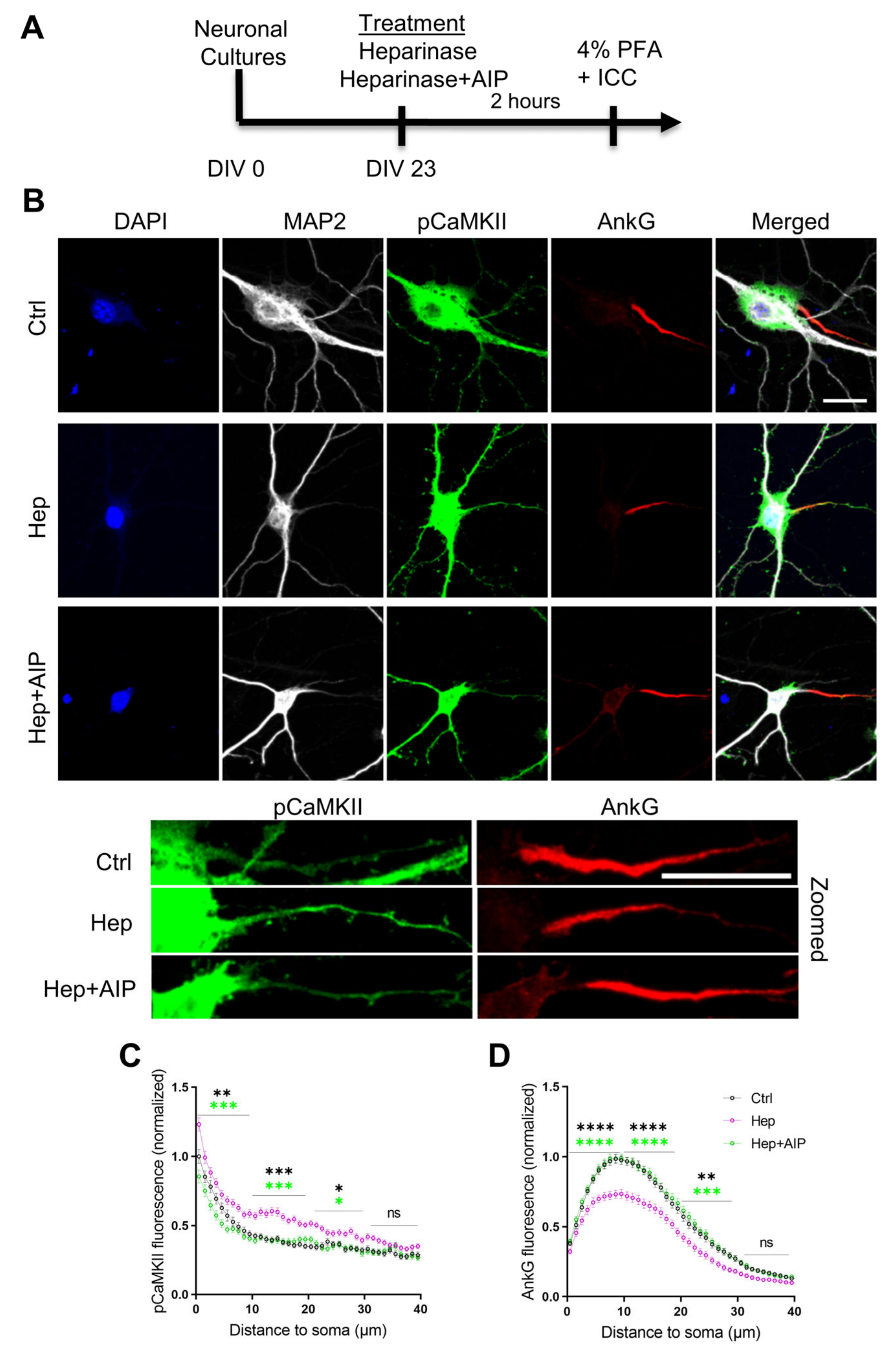
3.4. Increased Activity of CaMKII and Impaired Expression of AnkG at the Axon Initial Segment after Heparinase Treatment Are Abrogated by AIP
3.5. Impaired Recall of Contextual Memories after Heparinase Treatment Is Rescued by Co-Administration of AIP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ford-Perriss, M.; Turner, K.; Guimond, S.; Apedaile, A.; Haubeck, H.D.; Turnbull, J.; Murphy, M. Localisation of specific heparan sulfate proteoglycans during the proliferative phase of brain development. Dev. Dyn. 2003, 227, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Farhy-Tselnicker, I.; van Casteren, A.C.M.; Lee, A.; Chang, V.T.; Aricescu, A.R.; Allen, N.J. Astrocyte-Secreted Glypican 4 Regulates Release of Neuronal Pentraxin 1 from Axons to Induce Functional Synapse Formation. Neuron 2017, 96, 428–445.e413. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.P.; Sheng, M. Regulated expression and subcellular localization of syndecan heparan sulfate proteoglycans and the syndecan-binding protein CASK/LIN-2 during rat brain development. J. Neurosci. 1999, 19, 7415–7425. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.P.; Yang, F.C.; Kharazia, V.; Naisbitt, S.; Cohen, A.R.; Weinberg, R.J.; Sheng, M. Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J. Cell Biol. 1998, 142, 139–151. [Google Scholar] [CrossRef]
- Grootjans, J.J.; Zimmermann, P.; Reekmans, G.; Smets, A.; Degeest, G.; Durr, J.; David, G. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc. Natl. Acad. Sci. USA 1997, 94, 13683–13688. [Google Scholar] [CrossRef]
- Gao, Y.; Li, M.; Chen, W.; Simons, M. Synectin, syndecan-4 cytoplasmic domain binding PDZ protein, inhibits cell migration. J. Cell Physiol. 2000, 184, 373–379. [Google Scholar] [CrossRef]
- Ethell, I.M.; Hagihara, K.; Miura, Y.; Irie, F.; Yamaguchi, Y. Synbindin, A novel syndecan-2-binding protein in neuronal dendritic spines. J. Cell Biol. 2000, 151, 53–68. [Google Scholar] [CrossRef]
- Ethell, I.M.; Yamaguchi, Y. Cell surface heparan sulfate proteoglycan syndecan-2 induces the maturation of dendritic spines in rat hippocampal neurons. J. Cell Biol. 1999, 144, 575–586. [Google Scholar] [CrossRef]
- Zimmermann, P.; Zhang, Z.; Degeest, G.; Mortier, E.; Leenaerts, I.; Coomans, C.; Schulz, J.; N’Kuli, F.; Courtoy, P.J.; David, G. Syndecan recycling [corrected] is controlled by syntenin-PIP2 interaction and Arf6. Dev. Cell 2005, 9, 377–388. [Google Scholar] [CrossRef]
- Lauri, S.E.; Kaukinen, S.; Kinnunen, T.; Ylinen, A.; Imai, S.; Kaila, K.; Taira, T.; Rauvala, H. Regulatory role and molecular interactions of a cell-surface heparan sulfate proteoglycan (N-syndecan) in hippocampal long-term potentiation. J. Neurosci. 1999, 19, 1226–1235. [Google Scholar] [CrossRef]
- Kaksonen, M.; Pavlov, I.; Voikar, V.; Lauri, S.E.; Hienola, A.; Riekki, R.; Lakso, M.; Taira, T.; Rauvala, H. Syndecan-3-deficient mice exhibit enhanced LTP and impaired hippocampus-dependent memory. Mol. Cell. Neurosci. 2002, 21, 158–172. [Google Scholar] [CrossRef]
- McCroskery, S.; Bailey, A.; Lin, L.; Daniels, M.P. Transmembrane agrin regulates dendritic filopodia and synapse formation in mature hippocampal neuron cultures. Neuroscience 2009, 163, 168–179. [Google Scholar] [CrossRef]
- Coles, C.H.; Shen, Y.; Tenney, A.P.; Siebold, C.; Sutton, G.C.; Lu, W.; Gallagher, J.T.; Jones, E.Y.; Flanagan, J.G.; Aricescu, A.R. Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science 2011, 332, 484–488. [Google Scholar] [CrossRef]
- Dityatev, A.; Dityateva, G.; Sytnyk, V.; Delling, M.; Toni, N.; Nikonenko, I.; Muller, D.; Schachner, M. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J. Neurosci. 2004, 24, 9372–9382. [Google Scholar] [CrossRef]
- Irie, F.; Badie-Mahdavi, H.; Yamaguchi, Y. Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate. Proc. Natl. Acad. Sci. USA 2012, 109, 5052–5056. [Google Scholar] [CrossRef]
- Zhang, P.; Lu, H.; Peixoto, R.T.; Pines, M.K.; Ge, Y.; Oku, S.; Siddiqui, T.J.; Xie, Y.; Wu, W.; Archer-Hartmann, S.; et al. Heparan Sulfate Organizes Neuronal Synapses through Neurexin Partnerships. Cell 2018, 174, 1450–1464. [Google Scholar] [CrossRef]
- Korotchenko, S.; Cingolani, L.A.; Kuznetsova, T.; Bologna, L.L.; Chiappalone, M.; Dityatev, A. Modulation of network activity and induction of homeostatic synaptic plasticity by enzymatic removal of heparan sulfates. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2014, 369, 20140134. [Google Scholar] [CrossRef][Green Version]
- Garau, G.; Magotti, P.; Heine, M.; Korotchenko, S.; Lievens, P.M.; Berezin, V.; Dityatev, A. Heparin/heparan sulfates bind to and modulate neuronal L-type (Cav1.2) voltage-dependent Ca(2+) channels. Exp. Neurol. 2015, 274, 156–165. [Google Scholar] [CrossRef]
- Minge, D.; Senkov, O.; Kaushik, R.; Herde, M.K.; Tikhobrazova, O.; Wulff, A.B.; Mironov, A.; van Kuppevelt, T.H.; Oosterhof, A.; Kochlamazashvili, G.; et al. Heparan Sulfates Support Pyramidal Cell Excitability, Synaptic Plasticity, and Context Discrimination. Cereb. Cortex 2017, 27, 903–918. [Google Scholar] [CrossRef]
- Baucum, A.J., 2nd; Shonesy, B.C.; Rose, K.L.; Colbran, R.J. Quantitative proteomics analysis of CaMKII phosphorylation and the CaMKII interactome in the mouse forebrain. ACS Chem. Neurosci. 2015, 6, 615–631. [Google Scholar] [CrossRef]
- De Carvalho Myskiw, J.; Furini, C.R.; Benetti, F.; Izquierdo, I. Hippocampal molecular mechanisms involved in the enhancement of fear extinction caused by exposure to novelty. Proc. Natl. Acad. Sci. USA 2014, 111, 4572–4577. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.N.; Pattanayek, R.; Potet, F.; Rebbeck, R.T.; Blackwell, D.J.; Nikolaienko, R.; Sequeira, V.; Le Meur, R.; Radwanski, P.B.; Davis, J.P.; et al. The CaMKII inhibitor KN93-calmodulin interaction and implications for calmodulin tuning of NaV1.5 and RyR2 function. Cell Calcium 2019, 82, 102063. [Google Scholar] [CrossRef] [PubMed]
- Murakoshi, H.; Shin, M.E.; Parra-Bueno, P.; Szatmari, E.M.; Shibata, A.C.E.; Yasuda, R. Kinetics of Endogenous CaMKII Required for Synaptic Plasticity Revealed by Optogenetic Kinase Inhibitor. Neuron 2017, 94, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Meeks, J.P.; Mennerick, S. Action potential initiation and propagation in CA3 pyramidal axons. J. Neurophysiol. 2007, 97, 3460–3472. [Google Scholar] [CrossRef]
- Romberg, C.; Yang, S.; Melani, R.; Andrews, M.R.; Horner, A.E.; Spillantini, M.G.; Bussey, T.J.; Fawcett, J.W.; Pizzorusso, T.; Saksida, L.M. Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J. Neurosci. 2013, 33, 7057–7065. [Google Scholar] [CrossRef]
- Thiagarajan, T.C.; Piedras-Renteria, E.S.; Tsien, R.W. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron 2002, 36, 1103–1114. [Google Scholar] [CrossRef]
- Sametsky, E.A.; Disterhoft, J.F.; Ohno, M. Autophosphorylation of alphaCaMKII downregulates excitability of CA1 pyramidal neurons following synaptic stimulation. Neurobiol. Learn. Mem. 2009, 92, 120–123. [Google Scholar] [CrossRef]
- Giese, K.P.; Fedorov, N.B.; Filipkowski, R.K.; Silva, A.J. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science 1998, 279, 870–873. [Google Scholar] [CrossRef]
- Pellicena, P.; Schulman, H. CaMKII inhibitors: From research tools to therapeutic agents. Front. Pharmacol. 2014, 5, 21. [Google Scholar] [CrossRef]
- Ishida, A.; Kameshita, I.; Okuno, S.; Kitani, T.; Fujisawa, H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem. Biophys. Res. Commun. 1995, 212, 806–812. [Google Scholar] [CrossRef]
- Huang, C.Y.; Rasband, M.N. Axon initial segments: Structure, function, and disease. Ann. N. Y. Acad. Sci. 2018, 1420, 46–61. [Google Scholar] [CrossRef]
- Zhou, D.; Lambert, S.; Malen, P.L.; Carpenter, S.; Boland, L.M.; Bennett, V. AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J. Cell Biol. 1998, 143, 1295–1304. [Google Scholar] [CrossRef]
- Komada, M.; Soriano, P. [Beta]IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J. Cell Biol. 2002, 156, 337–348. [Google Scholar] [CrossRef]
- Hund, T.J.; Koval, O.M.; Li, J.; Wright, P.J.; Qian, L.; Snyder, J.S.; Gudmundsson, H.; Kline, C.F.; Davidson, N.P.; Cardona, N.; et al. A beta(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J. Clin. Investig. 2010, 120, 3508–3519. [Google Scholar] [CrossRef]
- Eshed, Y.; Feinberg, K.; Carey, D.J.; Peles, E. Secreted gliomedin is a perinodal matrix component of peripheral nerves. J. Cell Biol. 2007, 177, 551–562. [Google Scholar] [CrossRef]
- Hilgenberg, L.G.; Smith, M.A. Agrin signaling in cortical neurons is mediated by a tyrosine kinase-dependent increase in intracellular Ca2+ that engages both CaMKII and MAPK signal pathways. J. Neurobiol. 2004, 61, 289–300. [Google Scholar] [CrossRef]
- Ivins, J.K.; Litwack, E.D.; Kumbasar, A.; Stipp, C.S.; Lander, A.D. Cerebroglycan, a developmentally regulated cell-surface heparan sulfate proteoglycan, is expressed on developing axons and growth cones. Dev. Biol. 1997, 184, 320–332. [Google Scholar] [CrossRef]
- Litwack, E.D.; Ivins, J.K.; Kumbasar, A.; Paine-Saunders, S.; Stipp, C.S.; Lander, A.D. Expression of the heparan sulfate proteoglycan glypican-1 in the developing rodent. Dev. Dyn. 1998, 211, 72–87. [Google Scholar] [CrossRef]
- Condomitti, G.; Wierda, K.D.; Schroeder, A.; Rubio, S.E.; Vennekens, K.M.; Orlandi, C.; Martemyanov, K.A.; Gounko, N.V.; Savas, J.N.; de Wit, J. An Input-Specific Orphan Receptor GPR158-HSPG Interaction Organizes Hippocampal Mossy Fiber-CA3 Synapses. Neuron 2018, 100, 201–215. [Google Scholar] [CrossRef]
- Goutebroze, L.; Carnaud, M.; Denisenko, N.; Boutterin, M.C.; Girault, J.A. Syndecan-3 and syndecan-4 are enriched in Schwann cell perinodal processes. BMC Neurosci. 2003, 4, 29. [Google Scholar] [CrossRef]
- Luo, N.; Li, H.; Xiang, B.; Qiao, L.; He, J.; Ji, Y.; Liu, Y.; Li, S.; Lu, R.; Li, Y.; et al. Syndecan-4 modulates the proliferation of neural cells and the formation of CaP axons during zebrafish embryonic neurogenesis. Sci. Rep. 2016, 6, 25300. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.S.; Hodge, J.J.; Mehren, J.; Sun, X.X.; Griffith, L.C. Regulation of the Ca2+/CaM-responsive pool of CaMKII by scaffold-dependent autophosphorylation. Neuron 2003, 40, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Laabich, A.; Cooper, N.G. Neuroprotective effect of AIP on N-methyl-D-aspartate-induced cell death in retinal neurons. Brain Res. Mol. Brain Res. 2000, 85, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.J.; Wallace, R.S.; Nicholson, O.M.; Wilson, G.A.; McDonald, F.J.; Jones, P.P.; Baldi, J.C.; Lamberts, R.R.; Erickson, J.R. Inhibition of calcium/calmodulin-dependent kinase II restores contraction and relaxation in isolated cardiac muscle from type 2 diabetic rats. Cardiovasc. Diabetol. 2018, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Matsushima, S.; Okabe, K.; Ikeda, M.; Ishikita, A.; Tadokoro, T.; Enzan, N.; Yamamoto, T.; Sada, M.; Deguchi, H.; et al. Blockade of L-type Ca(2+) channel attenuates doxorubicin-induced cardiomyopathy via suppression of CaMKII-NF-kappaB pathway. Sci. Rep. 2019, 9, 9850. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, J.J.; Liu, X.D.; Yu, L.C. Inhibition of CaMKII activity in the nucleus accumbens shell blocks the reinstatement of morphine-seeking behavior in rats. Neurosci. Lett. 2012, 518, 167–171. [Google Scholar] [CrossRef]
- Duits, P.; Cath, D.C.; Lissek, S.; Hox, J.J.; Hamm, A.O.; Engelhard, I.M.; van den Hout, M.A.; Baas, J.M. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress. Anxiety 2015, 32, 239–253. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Linton, S.J. Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain 2012, 153, 1144–1147. [Google Scholar] [CrossRef]
- Dymond, S.; Dunsmoor, J.E.; Vervliet, B.; Roche, B.; Hermans, D. Fear Generalization in Humans: Systematic Review and Implications for Anxiety Disorder Research. Behav. Ther. 2015, 46, 561–582. [Google Scholar] [CrossRef]
- De Sousa, A.F.; Cowansage, K.K.; Zutshi, I.; Cardozo, L.M.; Yoo, E.J.; Leutgeb, S.; Mayford, M. Optogenetic reactivation of memory ensembles in the retrosplenial cortex induces systems consolidation. Proc. Natl. Acad. Sci. USA 2019, 116, 8576–8581. [Google Scholar] [CrossRef]
- Kazanskaya, G.M.; Tsidulko, A.Y.; Volkov, A.M.; Kiselev, R.S.; Suhovskih, A.V.; Kobozev, V.V.; Gaytan, A.S.; Aidagulova, S.V.; Krivoshapkin, A.L.; Grigorieva, E.V. Heparan sulfate accumulation and perlecan/HSPG2 up-regulation in tumour tissue predict low relapse-free survival for patients with glioblastoma. Histochem. Cell Biol. 2018, 149, 235–244. [Google Scholar] [CrossRef]
- Sears, J.C.; Broadie, K. Fragile X Mental Retardation Protein Regulates Activity-Dependent Membrane Trafficking and Trans-Synaptic Signaling Mediating Synaptic Remodeling. Front. Mol. Neurosci. 2017, 10, 440. [Google Scholar] [CrossRef]
- Bussini, S.; Meda, L.; Scarpini, E.; Clementi, E.; Conti, G.; Tiriticco, M.; Bresolin, N.; Baron, P. Heparan sulfate proteoglycan induces the production of NO and TNF-alpha by murine microglia. Immun. Ageing 2005, 2, 11. [Google Scholar] [CrossRef][Green Version]
- Chen, M.; Vincent, J.; Ezeanii, A.; Wakade, S.; Yerigenahally, S.; Mor, D.E. Heparan sulfate proteoglycans mediate prion-like alpha-synuclein toxicity in Parkinson’s in vivo models. Life Sci. Alliance 2022, 5, e202201366. [Google Scholar] [CrossRef]
- Liu, C.C.; Zhao, N.; Yamaguchi, Y.; Cirrito, J.R.; Kanekiyo, T.; Holtzman, D.M.; Bu, G. Neuronal heparan sulfates promote amyloid pathology by modulating brain amyloid-beta clearance and aggregation in Alzheimer’s disease. Sci. Transl. Med. 2016, 8, 332ra344. [Google Scholar] [CrossRef]
- Van Horssen, J.; Wesseling, P.; van den Heuvel, L.P.; de Waal, R.M.; Verbeek, M.M. Heparan sulphate proteoglycans in Alzheimer’s disease and amyloid-related disorders. Lancet Neurol. 2003, 2, 482–492. [Google Scholar] [CrossRef]
- Kazim, S.F.; Seo, J.H.; Bianchi, R.; Larson, C.S.; Sharma, A.; Wong, R.K.S.; Gorbachev, K.Y.; Pereira, A.C. Neuronal Network Excitability in Alzheimer’s Disease: The Puzzle of Similar versus Divergent Roles of Amyloid beta and Tau. eNeuro 2021, 8, ENEURO.0418-20.2020. [Google Scholar] [CrossRef]
- Lam, A.D.; Deck, G.; Goldman, A.; Eskandar, E.N.; Noebels, J.; Cole, A.J. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat. Med. 2017, 23, 678–680. [Google Scholar] [CrossRef]
- Zhao, D.; Watson, J.B.; Xie, C.W. Amyloid beta prevents activation of calcium/calmodulin-dependent protein kinase II and AMPA receptor phosphorylation during hippocampal long-term potentiation. J. Neurophysiol. 2004, 92, 2853–2858. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, I.; Kuznetsova, T.; Baidoe-Ansah, D.; Mirzapourdelavar, H.; Senkov, O.; Hayani, H.; Mironov, A.; Kaushik, R.; Druzin, M.; Johansson, S.; et al. Heparan Sulfates Regulate Axonal Excitability and Context Generalization through Ca2+/Calmodulin-Dependent Protein Kinase II. Cells 2023, 12, 744. https://doi.org/10.3390/cells12050744
Song I, Kuznetsova T, Baidoe-Ansah D, Mirzapourdelavar H, Senkov O, Hayani H, Mironov A, Kaushik R, Druzin M, Johansson S, et al. Heparan Sulfates Regulate Axonal Excitability and Context Generalization through Ca2+/Calmodulin-Dependent Protein Kinase II. Cells. 2023; 12(5):744. https://doi.org/10.3390/cells12050744
Chicago/Turabian StyleSong, Inseon, Tatiana Kuznetsova, David Baidoe-Ansah, Hadi Mirzapourdelavar, Oleg Senkov, Hussam Hayani, Andrey Mironov, Rahul Kaushik, Michael Druzin, Staffan Johansson, and et al. 2023. "Heparan Sulfates Regulate Axonal Excitability and Context Generalization through Ca2+/Calmodulin-Dependent Protein Kinase II" Cells 12, no. 5: 744. https://doi.org/10.3390/cells12050744
APA StyleSong, I., Kuznetsova, T., Baidoe-Ansah, D., Mirzapourdelavar, H., Senkov, O., Hayani, H., Mironov, A., Kaushik, R., Druzin, M., Johansson, S., & Dityatev, A. (2023). Heparan Sulfates Regulate Axonal Excitability and Context Generalization through Ca2+/Calmodulin-Dependent Protein Kinase II. Cells, 12(5), 744. https://doi.org/10.3390/cells12050744






