Abstract
The evolution of antiretroviral therapies (ART) has tremendously improved the life expectancy of people living with human immunodeficiency virus (HIV) (PLWH), which is currently similar to the general population. However, as PLWH are now living longer, they exhibit various comorbidities such as a higher risk of cardiovascular disease (CVD) and non-acquired immunodeficiency syndrome (AIDS)-defined malignancies. Clonal hematopoiesis (CH) is the acquisition of somatic mutations by the hematopoietic stem cells, rendering them survival and growth benefit, thus leading to their clonal dominance in the bone marrow. Recent epidemiologic studies have highlighted that PLWH have a higher prevalence of CH, which in turn is associated with increased CVD risk. Thus, a link between HIV infection and a higher risk for CVD might be explained through the induction of inflammatory signaling in the monocytes carrying CH mutations. Among the PLWH, CH is associated with an overall poorer control of HIV infection; an association that requires further mechanistic evaluation. Finally, CH is linked to an increased risk of progression to myeloid neoplasms including myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML), which are associated with particularly poor outcomes among patients with HIV infection. These bidirectional associations require further molecular-level understanding, highlighting the need for more preclinical and prospective clinical studies. This review summarizes the current literature on the association between CH and HIV infection.
1. Introduction
Although the life expectancy of people living with HIV (PLWH) has improved steeply in the past decades with the introduction of safe and effective antiretroviral therapy (ART), HIV infection continues to be associated with various comorbidities [1,2], including but not limited to cardiovascular disease (CVD), accelerated aging [3,4] and non-acquired immunodeficiency syndrome (AIDS)-related neoplasms [5]. Given that ART suppresses the HIV viral load to an undetectable level, the majority of these comorbidities have been largely attributed to chronic low-level inflammation [6,7], altered T-cell biology [8], ART adverse effects [9,10] and the prothrombotic phenotype of PLWH [10,11]. The current research focuses on early risk assessment, identifying very high-risk individuals, and preventing these comorbidities. Preclinical and clinical research aims to understand the mechanisms mediating the association of HIV infection with these comorbidities.
Clonal hematopoiesis (CH) is the process of acquiring somatic mutations in the genes that affect the function of the hematopoietic stem cells (HSC) by providing a growth advantage to the mutated cells over the unmutated counterparts, resulting in the dominance of the malignant clone in the bone marrow [12]. CH without evidence of hematologic malignancy, dysplasia, or cytopenia with a variant allele frequency (VAF) of at least 2% is defined as clonal hematopoiesis of indeterminate potential (CHIP) [12,13]. In contrast, the CH associated with low counts in at least one cell lineage is defined as clonal cytopenia of unknown significance (CCUS) [13]. The prevalence of CH increases with aging and is estimated to be 15% among individuals older than 70 years [14]. CH has been associated with worse overall outcomes, such as a higher incidence of atherosclerosis and CVD, which is mediated by the induction of innate immune signaling and chronic low-level inflammation [15] and the increased risk of progression to myeloid neoplasms such as myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML) [16,17]. The most common CH-associated mutations are in the DNMT3A and TET2 genes, which encode the epigenetic regulators and cause altered transcriptional profiles in differentiated monocytes and macrophages, leading to the upregulation of the inflammatory cytokines such as IL-1β [18]. Less common mutations associated with CH are mutations in the genes such as ASXL1 and TP53, which provide more prominent survival and growth advantage to the mutated hematopoietic cells, introducing a higher risk of progression to myeloid neoplasms [12,14].
Patients with HIV previously presented with severe cytopenias in the setting of uncontrolled viremia and chronic infections, but the introduction of efficacious ART has dramatically decreased the incidence of these abnormalities. However, mild cytopenias, particularly anemias, continue to be commonly described in PLWH [19,20,21]. Recent studies highlight that CH is more common among PLWH compared to the general population [18,22,23] and is associated with altered viral control [24] and increased risk of CVD [25], providing a novel link between HIV infection and chronic inflammation in PLWH via CH. Lastly, it has been demonstrated that HIV infection alters the outcomes of patients with advanced myeloid stem cell neoplasms [26]. Thus, a bidirectional effect between HIV infection and abnormal hematopoiesis exists.
This review summarizes the current findings on the prevalence of CH among PLWH and the implication of CH in the control of HIV.
2. Clonal Hematopoiesis in Patients Living with HIV
Dharan et al. analyzed the prevalence and the characteristics of CH in 220 PLWH and 226 HIV-negative Australian adults over the age of 55 enrolled in the ARCHIVE study by performing targeted sequencing of genomic DNA extracted from the peripheral blood [18]. PLWH had a significantly higher prevalence of CH than the non-HIV controls (28.2% vs. 16.8%), with most of the mutations observed in the DNMT3A, TET2, and ASXL1 genes. The difference in the prevalence of the higher-risk ASXL1 mutations between the PLWH and the non-HIV controls was more prominent [18]. It was noted that the sub-analysis for VAF, gender and sexual orientation, ancestry, BMI, the extent of smoking exposure and alcohol use, recreational and injection drug use, annual household income, type of health insurance coverage, and HIV-specific characteristics showed no significant correlation [18]. These findings suggest that PLWH have a higher prevalence of CH and its associated mutations than non-HIV infected individuals. These observational conclusions are limited by the relatively small number of patients studied. Interestingly, the authors showed no correlation between the duration of HIV and the VAF or the presence of more than one mutation [18]. In this study population, the prevalence of cardiovascular comorbidities was similar between the PLWH and the controls (64.1 vs. 65.5%). The authors evaluated the prevalence of a mutation in the IL-6 receptor (IL6R p.Asp358Ala), suggested by a different study to play a role in CH-related cardiovascular risk, but found no difference between the two groups [18]. No other correlation or sub-analysis of CVD was made in this study. It is important to note that while the ARCHIVE study was designed and powered as a prospective cohort study with a 10-year follow-up of participants, these results were published in a single-time-point cross-sectional manner [18].
In another study, Bick et al. assessed the prevalence of CH in a multi-ethnic, randomly selected sample of 600 PLWH enrolled in the Swiss HIV Cohort Study (SHCS), aged between 21 and 83, and 8111 individuals with available exome sequences enrolled in the Atherosclerotic risk in the Community study (ARIC), aged between 45 and 84, as the population controls [22]. A significant association between HIV case status and CHIP was observed [22]. To account for the demographic imbalance of the cases and controls, the authors performed a 1:5 propensity matching strategy to select a subgroup of 230 cases and 1002 controls with similar baseline characteristics. This subset analysis detected CHIP in 7% of the exomes from the PLWH but only in 3% of the controls [22]. It is noted that the authors demonstrated a positive correlation between the prevalence of CH and ART duration but not with HIV infection duration [22]. It is possible that this correlation still underlies the importance of the chronicity of low-level inflammation in PLWH, even under ART, as a mechanism of chronic stress in the hematopoietic stem cells. This correlation may reflect that the most critical risk factor for the development of CH is advanced age.
Van der Heijden et al. performed a cross-sectional cohort study comparing the prevalence of CH in 217 individuals; primarily men, PLWH on stable ART from the 200 HIV cohort (between 24 and 74 years) with the prevalence of CH in a cohort of overweight individuals and a cohort of age- and sex-matched population controls [24]. The authors confirmed that the probability of CHIP was significantly higher in the PLWH compared to the HIV-uninfected overweight controls [24]. Regarding mutations in specific genes, the proportion of the CH mutations in genes other than DNMT3A, and specifically JAK2, STAT3, and TP53, was larger in the PLWH [24]. Furthermore, the authors performed a mutational signature analysis showing that the clock-like and the reactive oxygen species signatures contributed uniquely to CH in the PLWH. In contrast, DNA mismatch repair signatures contributed uniquely to CH in the HIV-uninfected controls [24]. C>A mutations were identified as contributing to CH in the PLWH with prior exposure to zidovudine (AZT) but not in the unexposed individuals [24]. These results highlighted that a different mutational process potentially drives CH in PLWH and that the underlying biology of CH pathogenesis is different between PLWH and non-infected individuals. Finally, this study highlighted that within the PLWH group, CH mutations carriers had elevated coagulation markers (D-dimer and von Willebrand Factor) compared to the PLWH without the CH mutation [24], which further suggests that CH in PLWH is associated with an increased risk of thrombosis and potentially worse cardiovascular outcomes.
In a more recent cross-sectional study, Wang et al. examined the differences in CHIP and coronary artery disease prevalence between PLWH and non-HIV-infected individuals [23]. The authors performed next-generation sequencing in genomic DNA extracted from the peripheral blood mononuclear cells of 118 men (86 PLWH and 32 non-HIV-infected individuals) aged between 42 and 70 from the Baltimore-Washington D.C. center of the Multicenter AIDS Cohort Study (MACS) cohort who had coronary computed tomography angiography (CTA) and a measurement of multiple serologic inflammatory biomarkers [23]. Using a VAF cut-off of 1% and excluding germline variants, the PLWH were almost two-fold more likely to have CH than the non-infected controls (64% vs. 38%) [23]. Using a VAF of less than 1% in the same population, the effect size was increased (23% vs. 6%) [23]. The use of the lower cut-off VAFs allowed for the detection of a significant number of mutations that would have been missed in the studies mentioned above. Thirty-five of 86 (40%) of the PLWH and 10 of 32 (31%) of the non-HIV-infected individuals carried mutations with VAF between 0.5 and 1%, which may explain the identification of mutations less commonly associated with CH in this study, such as somatic TP53 and ARID1A, which were only found in the PLWH [23]. Contrary to the studies by Dharan et al. and Bick et al., which identified ASXL1 as one of the most commonly mutated genes in PLWH participants, Wang et al., did not detect mutations in this gene despite the lower VAF cut-off [23]. Moreover, the authors demonstrated that moderate-to-severe coronary artery stenosis was significantly more common in PLWH with CH than those without CH (30% vs. 9%) even after adjustment for the ACC-AHA Pooled Cohort Equation [23]. This finding is essential as it links CH and CVD among PLWH, supporting the hypothesis that CH promotes atherosclerotic disease in these individuals, likely through the induction of inflammatory alterations in the monocytes and migrated macrophages. The robustness of this study is limited, though, by the relatively small sample size, especially for the uninfected controls, and the significant differences in baseline demographics such as race, BMI, and lack of female participants.
Overall, there is robust epidemiological evidence that clonal hematopoiesis is more prevalent in PLWH than in non-HIV-infected individuals. Chronic inflammation can potentially mediate these associations. Numerous studies have highlighted that various inflammatory markers such as IL-6, soluble CD14, and CD163 are elevated among the PLWH compared to the uninfected individuals [27,28]. It is well described that sub-acute or chronic inflammation drives CH, likely by the increased resistance of mutated clones to inflammatory signals [29,30,31]. Thus, it is possible that induced inflammation in PLWH, potentially in combination with patient-related factors such as age and comorbidities, could increase the risk of CH development, further promoting inflammation and negatively affecting innate immunity [32] (Figure 1). Finally, the combined effect of CH, persistent chronic inflammation, and patient-related factors increase the risk of CVD [33,34,35] (Figure 1).
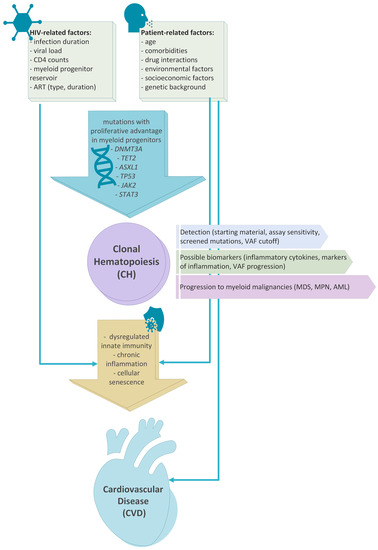
Figure 1.
Association between HIV infection, patients’ characteristics, development of clonal hematopoiesis and the risk of development cardiovascular disease and myeloid neoplasms. HIV infection and patient-related risk factors such as age, comorbidities and medications have been associated with de-regulated innate immunity and chronic inflammation. These biologic processes have also been linked to clonal hematopoiesis. Clonal hematopoiesis and chronic inflammation are both associated with high risk of cardiovascular disease development. Finally, patients with clonal hematopoiesis have a higher risk of progression to myeloid neoplasms such as MDS, MPN and AML.
However, the causality of these associations should be established with prospective studies adequately controlled for all possible confounders and adequately powered to detect a relationship between HIV infection duration, ART duration, viral load, or inflammatory markers. When designing such studies in the future, it is important to consider ways to decrease biases and maintain the generalizability of the results with broad participant eligibility criteria. Furthermore, technical details such as the sequencing starting material (whole blood vs. peripheral blood mononuclear cells), the sequencing ‘modalities’ sensitivity to detect low VAF, the variety of screened mutations, and the VAF cut-offs with the exclusion of germline mutations should be predetermined after weighing sensitivity with clinical meaningfulness of the findings. A large prospective study with long-term follow-up would be ideal to identify which aspect(s) of HIV infection drives this relationship with CH and the time course of the mutational landscape. Finally, this would allow the investigation of a possible impact of CH in developing CVD in PLWH as a mediator or inducer of inflammatory changes in the monocytes in these individuals, causing high-risk atherosclerotic lesions.
4. Effect of CH on the Course of HIV and Other Infections
While several recent studies have investigated the correlation between HIV infection and the development of CH [18,22,23,24], the associations of CH with the characteristics and the course of HIV infection and the risk for other infections in PLWH are less well known.
Van der Heijden explored the clinical correlates of PLWH with their CH mutation carrier status and found that the CH carriers were older with a longer duration of HIV infection and lower CD4 nadir [24]. On the contrary, no significant difference in CH prevalence was found concerning CD4/CD8 T-cell ratio and the most recent CD4 T-cell count [24]. Older age, lower CD4 nadir, and increased CD4/CD8 ratio were independently associated with the CH mutation prevalence, while HIV duration and the most recent CD4 T cell count were not [24]. The same study demonstrated that the PLWH with a CH mutation had at least once a detectable HIV viral load within a year before a visit supporting an existing HIV reservoir [24]. Similarly, the ratio of HIV cell-associated (CA)-RNA to CA-DNA, which represents the relative viral transcription level, was found to be increased in the CH-mutation carriers compared to the PLWH without the CH mutation [24]. Overall, these observations support that CH can negatively affect the course of HIV infection in PLWH.
Recent studies provided evidence that CH increases the risk of several other infections. Specifically, Bolton et al. found that CH increased the risk of Streptococcal and Clostridium difficile infections in patients with solid malignancies after adjusting for age, race, smoking, gender, cumulative exposure to cytotoxic therapy before the blood draw, cumulative exposure to cytotoxic therapy after the blood draw, and primary tumor site [48]. In the same study, the authors found that among COVID-19 positive patients, individuals with CH had a significantly higher risk for severe disease in a multivariable analysis showing a positive association between the VAF and the severity of illness [48]. The exact mechanisms underlying these associations remain unclear. Somatic alterations in the hematopoietic stem cells lead to altered inflammatory signaling in differentiated white blood cells, including the monocytes, migrated macrophages, and other cells participating in the innate immune reactions [49,50]. Thus, CH may affect the regulation of the innate immunity in the setting of infections leading to induced inflammatory responses with poor antimicrobial effect. Further preclinical studies and prospective clinical trials are required to shed light on the underlying biologic mechanisms linking CH to poor infection outcomes.
5. The Role of CH in the Development of Myeloid Neoplasms in PLWH and the Outcomes of PLWH Who Develop Myeloid Neoplasms
Identifying the subset of the PLWH who eventually develop a myeloid malignancy and analyzing their mutational status is an indirect way to examine the role of CH in the development of hematopoietic malignancy in these patients. While some of the molecular abnormalities associated with CH seem to be enriched in the PLWH who develop MDS compared with the HIV-negative counterparts (mainly ASXL1, DNMT3A and TP53 mutations, and higher risk cytogenetics) [26], progression of CH from >2% VAF and correlation with hematologic malignancy is not well defined [51]. The common mutations associated with CH (epigenetic factors DNMT3A, ASXL1, TET2, DNA damage repair genes such as PPM1D, TP53, signaling genes such as JAK2, and spliceosome components SF3B1, SRSF2) can act as drivers, more often in the PLWH who develop MDS than those who develop AML, where the most common alterations are adverse/intermediate karyotype or chromosome 7 abnormalities [52]. The role of other quantitative rather than qualitative factors, such as the role of the VAF percentage and the accumulation of more than one driver mutations, or the presence of CH without driver mutations in the PLWH who eventually develop a malignancy, should be taken into account when planning prospective follow-up studies of PLWH to understand the pathophysiology and need for surveillance of these patients.
The outcomes of PLWH who develop myeloid neoplasms such as MDS and AML are worse than the non-HIV patients [26,53]. It has been reported that patients with HIV and MDS (HIV+/MDS) exhibited more prominent cytopenias and a higher percentage of marrow blasts, a marker indicative of a higher risk for AML transformation, than the HIV-/MDS patients [26,53]. Similarly, despite being younger and potentially better candidates for chemotherapy and allogeneic bone marrow transplantation, the HIV+/AML patients have worse overall survival outcomes compared to the HIV-/AML patients [54,55]. Since these findings suggest that the development of myeloid neoplasms among PLWH is associated with particularly poor outcomes and CH in its turn predisposes to myeloid neoplasms, the need for better understanding of the biology of these associations and the early detection of PLWH with CH to facilitate close monitoring and potentially early treatment to prevent the progression to advanced myeloid neoplasms is warranted.
6. Conclusions
The optimization of ART has tremendously improved the outcomes of PLWH, who have now similar survival to the general population. However, PLWH have a higher prevalence of CVD and non-AIDS associated malignancies, with the underlying biology remaining unclear. The recent data support that PLWH have a higher prevalence of CH, with a potentially different pattern of acquisition of somatic mutations in their hematopoietic stem cells, which appears to be associated with a higher risk of CVD. The underlying biologic mechanism remains unclear, however HIV infection may increase the age-related replication stress to hematopoietic cells leading to a higher risk of acquisition of somatic mutations. Interestingly, CH among the PLWH is associated with lower CD4 nadir and worse HIV-related outcomes, potentially associated with worse infectious outcomes among individuals with CH. Finally, CH carries a significant risk of progression to myeloid neoplasms such as MDS and AML, which are strongly associated with worse outcomes among patients with HIV infection. Further basic science, translational studies, and prospective clinical trials are required to confirm these associations and elucidate the underlying biological mechanisms.
Author Contributions
Conceptualization, T.K. and S.K.; Literature Review S.C.V., I.C., T.K. and S.K.; S.C.V., I.C. and T.K; writing—original draft preparation, S.C.V., I.C, T.K. and S.K.; writing—review and editing, supervision, T.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marcus, J.L.; Leyden, W.A.; Alexeeff, S.E.; Anderson, A.N.; Hechter, R.C.; Hu, H.; Lam, J.O.; Towner, W.J.; Yuan, Q.; Horberg, M.A.; et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000–2016. JAMA Netw. Open 2020, 3, e207954. [Google Scholar] [CrossRef] [PubMed]
- Schouten, J.; Wit, F.W.; Stolte, I.G.; Kootstra, N.A.; van der Valk, M.; Geerlings, S.E.; Prins, M.; Reiss, P. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: The AGEhIV cohort study. Clin. Infect. Dis. 2014, 59, 1787–1797. [Google Scholar] [CrossRef]
- Hasse, B.; Ledergerber, B.; Furrer, H.; Battegay, M.; Hirschel, B.; Cavassini, M.; Bertisch, B.; Bernasconi, E.; Weber, R. Morbidity and aging in HIV-infected persons: The Swiss HIV cohort study. Clin. Infect. Dis. 2011, 53, 1130–1139. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Hubbard, D.; Monda, K.L.; Reading, S.R.; Chen, L.; Dluzniewski, P.J.; Burkholder, G.A.; Muntner, P.; Colantonio, L.D. Excess Risk for Atherosclerotic Cardiovascular Outcomes Among US Adults With HIV in the Current Era. J. Am. Heart Assoc. 2020, 9, e013744. [Google Scholar] [CrossRef]
- Shiels, M.S.; Engels, E.A. Evolving epidemiology of HIV-associated malignancies. Curr. Opin. HIV AIDS 2017, 12, 6–11. [Google Scholar] [CrossRef]
- Deeks, S.G. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009, 17, 118–123. [Google Scholar]
- Jalbert, E.; Crawford, T.Q.; D’Antoni, M.L.; Keating, S.M.; Norris, P.J.; Nakamoto, B.K.; Seto, T.; Parikh, N.I.; Shikuma, C.M.; Ndhlovu, L.C.; et al. IL-1Β enriched monocytes mount massive IL-6 responses to common inflammatory triggers among chronically HIV-1 infected adults on stable anti-retroviral therapy at risk for cardiovascular disease. PLoS ONE 2013, 8, e75500. [Google Scholar] [CrossRef]
- Feinstein, M.J.; Hsue, P.Y.; Benjamin, L.A.; Bloomfield, G.S.; Currier, J.S.; Freiberg, M.S.; Grinspoon, S.K.; Levin, J.; Longenecker, C.T.; Post, W.S. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e98–e124. [Google Scholar] [CrossRef]
- Zangerle, R.; Sarcletti, M. Antiretroviral drugs and the risk of myocardial infarction. N. Engl. J. Med. 2007, 357, 715–716, author reply 716–717. [Google Scholar]
- Friis-Møller, N.; Sabin, C.A.; Weber, R.; d’Arminio Monforte, A.; El-Sadr, W.M.; Reiss, P.; Thiébaut, R.; Morfeldt, L.; De Wit, S.; Pradier, C.; et al. Combination antiretroviral therapy and the risk of myocardial infarction. N. Engl. J. Med. 2003, 349, 1993–2003. [Google Scholar]
- Subbaraman, R.; Chaguturu, S.K.; Mayer, K.H.; Flanigan, T.P.; Kumarasamy, N. Adverse effects of highly active antiretroviral therapy in developing countries. Clin. Infect. Dis. 2007, 45, 1093–1101. [Google Scholar] [CrossRef]
- Gondek, L.P.; DeZern, A.E. Assessing clonal haematopoiesis: Clinical burdens and benefits of diagnosing myelodysplastic syndrome precursor states. Lancet Haematol. 2020, 7, e73–e81. [Google Scholar] [CrossRef]
- DeZern, A.E.; Malcovati, L.; Ebert, B.L. CHIP, CCUS, and Other Acronyms: Definition, Implications, and Impact on Practice. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 400–410. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef]
- Gibson, C.J.; Kim, H.T.; Zhao, L.; Murdock, H.M.; Hambley, B.; Ogata, A.; Madero-Marroquin, R.; Wang, S.; Green, L.; Fleharty, M.; et al. Donor Clonal Hematopoiesis and Recipient Outcomes After Transplantation. J. Clin. Oncol. 2022, 40, 189–201. [Google Scholar] [CrossRef]
- Young, A.L.; Tong, R.S.; Birmann, B.M.; Druley, T.E. Clonal hematopoiesis and risk of acute myeloid leukemia. Haematologica 2019, 104, 2410–2417. [Google Scholar] [CrossRef]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef]
- Dharan, N.J.; Yeh, P.; Bloch, M.; Yeung, M.M.; Baker, D.; Guinto, J.; Roth, N.; Ftouni, S.; Ognenovska, K.; Smith, D.; et al. HIV is associated with an increased risk of age-related clonal hematopoiesis among older adults. Nat. Med. 2021, 27, 1006–1011. [Google Scholar] [CrossRef]
- Ambler, K.L.; Vickars, L.M.; Leger, C.S.; Foltz, L.M.; Montaner, J.S.; Harris, M.; Dias Lima, V.; Leitch, H.A. Clinical Features, Treatment, and Outcome of HIV-Associated Immune Thrombocytopenia in the HAART Era. Adv. Hematol. 2012, 2012, 910954. [Google Scholar] [CrossRef]
- Sullivan, P.S.; Hanson, D.L.; Chu, S.Y.; Jones, J.L.; Ward, J.W. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: Results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood 1998, 91, 301–308. [Google Scholar] [CrossRef]
- Vannappagari, V.; Nkhoma, E.T.; Atashili, J.; Laurent, S.S.; Zhao, H. Prevalence, severity, and duration of thrombocytopenia among HIV patients in the era of highly active antiretroviral therapy. Platelets 2011, 22, 611–618. [Google Scholar] [CrossRef]
- Bick, A.G.; Popadin, K.; Thorball, C.W.; Uddin, M.M.; Zanni, M.V.; Yu, B.; Cavassini, M.; Rauch, A.; Tarr, P.; Schmid, P.; et al. Increased prevalence of clonal hematopoiesis of indeterminate potential amongst people living with HIV. Sci. Rep. 2022, 12, 577. [Google Scholar] [CrossRef]
- Wang, S.; Pasca, S.; Post, W.S.; Langan, S.; Pallavajjala, A.; Haley, L.; Gocke, C.D.; Budoff, M.; Haberlen, S.; Brown, T.T.; et al. Clonal hematopoiesis in men living with HIV and association with subclinical atherosclerosis. AIDS 2022, 36, 1521–1531. [Google Scholar] [CrossRef]
- van der Heijden, W.A.; van Deuren, R.C.; van de Wijer, L.; van den Munckhof, I.C.L.; Steehouwer, M.; Riksen, N.P.; Netea, M.G.; de Mast, Q.; Vandekerckhove, L.; de Voer, R.M.; et al. Clonal Hematopoiesis Is Associated With Low CD4 Nadir and Increased Residual HIV Transcriptional Activity in Virally Suppressed Individuals With HIV. J. Infect. Dis. 2022, 225, 1339–1347. [Google Scholar] [CrossRef]
- Wiley, B.; Parsons, T.M.; Burkart, S.; Young, A.L.; Erlandson, K.M.; Tassiopoulos, K.K.; Wu, K.; Gurnett, C.; Presti, R.M.; Bolton, K.L.; et al. Effect of Clonal Hematopoiesis on Cardiovascular Disease in People Living with HIV. Exp. Hematol. 2022, 114, 18–21. [Google Scholar] [CrossRef]
- Kaner, J.D.; Thibaud, S.; Jasra, S.; Wang, Y.; Janakiram, M.; Sharma, A.; Sridharan, A.; Elias, H.; Polineni, R.; Assal, A.; et al. HIV portends a poor prognosis in myelodysplastic syndromes. Leuk. Lymphoma 2019, 60, 3529–3535. [Google Scholar] [CrossRef]
- Neuhaus, J.; Jacobs, D.R., Jr.; Baker, J.V.; Calmy, A.; Duprez, D.; La Rosa, A.; Kuller, L.H.; Pett, S.L.; Ristola, M.; Ross, M.J.; et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J. Infect. Dis. 2010, 201, 1788–1795. [Google Scholar] [CrossRef]
- Kelesidis, T.; Kendall, M.A.; Yang, O.O.; Hodis, H.N.; Currier, J.S. Biomarkers of microbial translocation and macrophage activation: Association with progression of subclinical atherosclerosis in HIV-1 infection. J. Infect. Dis. 2012, 206, 1558–1567. [Google Scholar] [CrossRef]
- Avagyan, S.; Henninger, J.E.; Mannherz, W.P.; Mistry, M.; Yoon, J.; Yang, S.; Weber, M.C.; Moore, J.L.; Zon, L.I. Resistance to inflammation underlies enhanced fitness in clonal hematopoiesis. Science 2021, 374, 768–772. [Google Scholar] [CrossRef]
- Hormaechea-Agulla, D.; Matatall, K.A.; Le, D.T.; Kain, B.; Long, X.; Kus, P.; Jaksik, R.; Challen, G.A.; Kimmel, M.; King, K.Y. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNγ signaling. Cell Stem Cell 2021, 28, 1428–1442.e6. [Google Scholar] [CrossRef]
- Caiado, F.; Pietras, E.M.; Manz, M.G. Inflammation as a regulator of hematopoietic stem cell function in disease, aging, and clonal selection. J. Exp. Med. 2021, 218, e20201541. [Google Scholar] [CrossRef]
- Jaiswal, S.; Libby, P. Clonal haematopoiesis: Connecting ageing and inflammation in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 137–144. [Google Scholar] [CrossRef]
- Fidler, T.P.; Xue, C.; Yalcinkaya, M.; Hardaway, B.; Abramowicz, S.; Xiao, T.; Liu, W.; Thomas, D.G.; Hajebrahimi, M.A.; Pircher, J.; et al. The AIM2 inflammasome exacerbates atherosclerosis in clonal haematopoiesis. Nature 2021, 592, 296–301. [Google Scholar] [CrossRef]
- Fuster, J.J.; MacLauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef]
- Soares, C.; Kwok, M.; Boucher, K.A.; Haji, M.; Echouffo-Tcheugui, J.B.; Longenecker, C.T.; Bloomfield, G.S.; Ross, D.; Jutkowtiz, E.; Sullivan, J.L.; et al. Performance of Cardiovascular Risk Prediction Models Among People Living With HIV: A Systematic Review and Meta-analysis. JAMA Cardiol. 2022, 8, 139–149. [Google Scholar] [CrossRef]
- Fernandez-Montero, J.V.; Eugenia, E.; Barreiro, P.; Labarga, P.; Soriano, V. Antiretroviral drug-related toxicities—Clinical spectrum, prevention, and management. Expert Opin. Drug. Saf. 2013, 12, 697–707. [Google Scholar] [CrossRef]
- Moraes Filho, A.V.; Carvalho, C.J.; Carneiro, C.C.; Vale, C.R.; Lima, D.C.; Carvalho, W.F.; Vieira, T.B.; Silva, D.M.; Cunha, K.S.; Chen-Chen, L. Genotoxic and Cytotoxic Effects of Antiretroviral Combinations in Mice Bone Marrow. PLoS ONE 2016, 11, e0165706. [Google Scholar] [CrossRef]
- Shah, M.M.; Li, Y.; Christensen, R.D. Effects of perinatal zidovudine on hematopoiesis: A comparison of effects on progenitors from human fetuses versus mothers. AIDS 1996, 10, 1239–1247. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Valsalan, R.; Sheshadri, S.; Pandit, V.R.; Medep, V.; Agrawal, R.K. Zidovudine-induced reversible pure red cell aplasia. Indian J. Pharmacol. 2010, 42, 189–191. [Google Scholar]
- Lin, S.H.; Wang, Y.; Hartley, S.W.; Karyadi, D.M.; Lee, O.W.; Zhu, B.; Zhou, W.; Brown, D.W.; Beilstein-Wedel, E.; Hazra, R.; et al. In-utero exposure to zidovudine-containing antiretroviral therapy and clonal hematopoiesis in HIV-exposed uninfected newborns. AIDS 2021, 35, 1525–1535. [Google Scholar] [CrossRef]
- Sloand, E.M.; Maciejewski, J.; Kumar, P.; Kim, S.; Chaudhuri, A.; Young, N. Protease inhibitors stimulate hematopoiesis and decrease apoptosis and ICE expression in CD34(+) cells. Blood 2000, 96, 2735–2739. [Google Scholar] [CrossRef]
- Lewis, W.; Dalakas, M.C. Mitochondrial toxicity of antiviral drugs. Nat. Med. 1995, 1, 417–422. [Google Scholar] [CrossRef]
- Chen, C.H.; Cheng, Y.C. Delayed cytotoxicity and selective loss of mitochondrial DNA in cells treated with the anti-human immunodeficiency virus compound 2′,3′-dideoxycytidine. J. Biol. Chem. 1989, 264, 11934–11937. [Google Scholar] [CrossRef]
- Chen, C.H.; Vazquez-Padua, M.; Cheng, Y.C. Effect of anti-human immunodeficiency virus nucleoside analogs on mitochondrial DNA and its implication for delayed toxicity. Mol. Pharmacol. 1991, 39, 625–628. [Google Scholar]
- Brinkman, K.; Smeitink, J.A.; Romijn, J.A.; Reiss, P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet 1999, 354, 1112–1115. [Google Scholar] [CrossRef]
- Luo, H.; Mu, W.C.; Karki, R.; Chiang, H.H.; Mohrin, M.; Shin, J.J.; Ohkubo, R.; Ito, K.; Kanneganti, T.D.; Chen, D. Mitochondrial Stress-Initiated Aberrant Activation of the NLRP3 Inflammasome Regulates the Functional Deterioration of Hematopoietic Stem Cell Aging. Cell Rep. 2019, 26, 945–954.e4. [Google Scholar] [CrossRef]
- Morganti, C.; Ito, K. Mitochondrial Contributions to Hematopoietic Stem Cell Aging. Int. J. Mol. Sci. 2021, 22, 11117. [Google Scholar] [CrossRef]
- Bolton, K.L.; Koh, Y.; Foote, M.B.; Im, H.; Jee, J.; Sun, C.H.; Safonov, A.; Ptashkin, R.; Moon, J.H.; Lee, J.Y.; et al. Clonal hematopoiesis is associated with risk of severe COVID-19. Nat. Commun. 2021, 12, 5975. [Google Scholar] [CrossRef]
- Meisel, M.; Hinterleitner, R.; Pacis, A.; Chen, L.; Earley, Z.M.; Mayassi, T.; Pierre, J.F.; Ernest, J.D.; Galipeau, H.J.; Thuille, N.; et al. Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature 2018, 557, 580–584. [Google Scholar] [CrossRef]
- Cai, Z.; Kotzin, J.J.; Ramdas, B.; Chen, S.; Nelanuthala, S.; Palam, L.R.; Pandey, R.; Mali, R.S.; Liu, Y.; Kelley, M.R.; et al. Inhibition of Inflammatory Signaling in Tet2 Mutant Preleukemic Cells Mitigates Stress-Induced Abnormalities and Clonal Hematopoiesis. Cell Stem Cell 2018, 23, 833–849.e5. [Google Scholar] [CrossRef]
- Marnell, C.S.; Bick, A.; Natarajan, P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J. Mol. Cell Cardiol. 2021, 161, 98–105. [Google Scholar] [CrossRef]
- Forghieri, F.; Nasillo, V.; Bettelli, F.; Pioli, V.; Giusti, D.; Gilioli, A.; Mussini, C.; Tagliafico, E.; Trenti, T.; Cossarizza, A.; et al. Acute Myeloid Leukemia in Patients Living with HIV Infection: Several Questions, Fewer Answers. Int. J. Mol. Sci. 2020, 21, 1081. [Google Scholar] [CrossRef]
- Takahashi, K.; Yabe, M.; Shapira, I.; Pierce, S.; Garcia-Manero, G.; Varma, M. Clinical and cytogenetic characteristics of myelodysplastic syndrome in patients with HIV infection. Leuk. Res. 2012, 36, 1376–1379. [Google Scholar] [CrossRef]
- Sutton, L.; Guénel, P.; Tanguy, M.L.; Rio, B.; Dhedin, N.; Casassus, P.; Lortholary, O. Acute myeloid leukaemia in human immunodeficiency virus-infected adults: Epidemiology, treatment feasibility and outcome. Br. J. Haematol. 2001, 112, 900–908. [Google Scholar] [CrossRef]
- Aboulafia, D.M.; Meneses, M.; Ginsberg, S.; Siegel, M.S.; Howard, W.W.; Dezube, B.J. Acute myeloid leukemia in patients infected with HIV-1. AIDS 2002, 16, 865–876. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).