Abstract
KV channel-interacting proteins (KChIP1-4) belong to a family of Ca2+-binding EF-hand proteins that are able to bind to the N-terminus of the KV4 channel α-subunits. KChIPs are predominantly expressed in the brain and heart, where they contribute to the maintenance of the excitability of neurons and cardiomyocytes by modulating the fast inactivating-KV4 currents. As the auxiliary subunit, KChIPs are critically involved in regulating the surface protein expression and gating properties of KV4 channels. Mechanistically, KChIP1, KChIP2, and KChIP3 promote the translocation of KV4 channels to the cell membrane, accelerate voltage-dependent activation, and slow the recovery rate of inactivation, which increases KV4 currents. By contrast, KChIP4 suppresses KV4 trafficking and eliminates the fast inactivation of KV4 currents. In the heart, IKs, ICa,L, and INa can also be regulated by KChIPs. ICa,L and INa are positively regulated by KChIP2, whereas IKs is negatively regulated by KChIP2. Interestingly, KChIP3 is also known as downstream regulatory element antagonist modulator (DREAM) because it can bind directly to the downstream regulatory element (DRE) on the promoters of target genes that are implicated in the regulation of pain, memory, endocrine, immune, and inflammatory reactions. In addition, all the KChIPs can act as transcription factors to repress the expression of genes involved in circadian regulation. Altered expression of KChIPs has been implicated in the pathogenesis of several neurological and cardiovascular diseases. For example, KChIP2 is decreased in failing hearts, while loss of KChIP2 leads to increased susceptibility to arrhythmias. KChIP3 is increased in Alzheimer’s disease and amyotrophic lateral sclerosis, but decreased in epilepsy and Huntington’s disease. In the present review, we summarize the progress of recent studies regarding the structural properties, physiological functions, and pathological roles of KChIPs in both health and disease. We also summarize the small-molecule compounds that regulate the function of KChIPs. This review will provide an overview and update of the regulatory mechanism of the KChIP family and the progress of targeted drug research as a reference for researchers in related fields.
1. Introduction
KV channel-interacting proteins (KChIPs) are a family of Ca2+-binding EF-hand proteins consisting of four members: KChIP1, KChIP2, KChIP3 (DREAM/calsenilin), and KChIP4 (CALP), encoded by KCNIP1-4, respectively. KChIPs have high sequence similarity and share the same conserved C-terminal core domains. However, the N-termini of KChIPs are variable in sequence and length, which defines the distinctions among them. KChIP1, KChIP3, and KChIP4 are highly expressed in the brain, whereas KChIP2 is predominantly expressed in the heart [1]. Other tissues, such as lung [2], kidney [3], and gastrointestinal tract [4,5,6], also express low levels of KChIPs (Table 1).
The biological functions of KChIPs are diverse. In 1998, KChIP3, the first reported member of the KChIP family, was identified as a presenilin (PS)-binding protein and was initially named “calsenilin” [7]. A year later, the transcriptional regulatory activity of KChIP3 was reported by Carrion et al., as it can bind to the downstream regulatory element (DRE) on the promoter of the human prodynorphin (PDYN) gene, repressing PDYN transcription. KChIP3 is therefore coined the acronym DREAM (downstream regulatory element antagonist modulator) [8]. In 2000, An et al. used the yeast two-hybrid system to identify three proteins of 216-, 252-, and 256-amino acid length that interacted with KV4 channels, and named them KChIP1, KChIP2, and KChIP3. Interestingly, they found that KChIP3 was translated from the same DNA sequence as the previously reported calsenilin and DREAM [9]. These three landmark papers marked the beginning of research into the functions of KChIPs. Academic research has uncovered the functions of KChIPs, which revolve around these three aspects. First, KChIPs can interact directly with intracellular proteins to regulate various cellular functions through non-transcriptional mechanisms. For example, in neurons, KChIPs interact with PS to regulate the activity of γ-secretase [7,10]. In platelets, KChIP3 exerts a procoagulant effect by directly binding to and activating phosphatidylinositol 3-kinase-Iβ [11]. Second, KChIPs can also regulate physiological and pathological responses at the transcriptional level. In addition to KChIP3, other KChIPs also have transcriptional activity to modulate the transcription of genes regarding circadian rhythm, pain, memory, inflammatory response, immune response, and hormone secretion. Last but not least, KChIPs regulate the subcellular localization and gating properties of fast-inactivating KV4 channels mainly in neurons and cardiomyocytes. In addition, KChIPs are involved in the regulation of other important ion channels in the heart, including KV1.5, CaV1.2, and NaV1.5. Therefore, KChIPs play a pivotal role in controlling the electrophysiological function of cardiomyocytes. Among these, the functions of KChIPs as transcriptional regulators and auxiliary subunits of ion channels have been studied the most, which is the focus of this review (Figure 1).
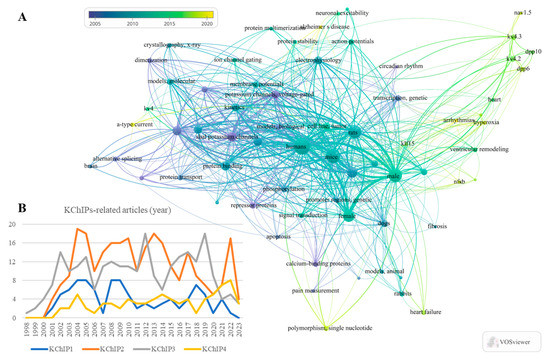
Figure 1.
Statistics of the KChIPs-related publications and the network visualization of key words. (A) Bibliometrics information acquired by VOSviewer shows the key words that occurs frequently in these KChIPs-related publications. (B) The statistics of the KChIPs-related publications since the year 1998.
KChIPs have been implicated in the pathogenesis of several diseases, including cardiac arrhythmias, cardiac hypertrophy, neurodegenerative diseases, and epilepsy. As research deepens and the role of KChIPs in physiological and pathological conditions become better understood through the use of some transgenic or gene knockout animal models, the evidence for the association of KChIPs with these diseases is increasing. Small molecules acting on KChIPs have been shown to enhance or inhibit the activity of KChIPs [12], which is instructive for the development of drugs targeting KChIPs to treat related diseases. However, a growing number of recent studies have shown the diversity of functions of KChIPs. For example, KChIP3 knockout rats and mice appear to have different pain hypersensitivity responses to drug stimulation. The small molecule NS5806 has different effects in different species, even in different parts of the same species. The emergence of these questions indicates the complexity of the function of KChIPs, and there are still many questions in this field that researchers urgently need to answer. In this review, we summarized the function of KChIPs and their multiple roles in disease progression. Meanwhile, we updated the small-molecule drugs targeting KChIPs to provide new guidance for future basic and translational research in this field.

Table 1.
The expression and post-translational modification of KChIPs.
Table 1.
The expression and post-translational modification of KChIPs.
| KChIP1 | KChIP2 | KChIP3 | KChIP4 | |
|---|---|---|---|---|
| Aliases | NA | NA | DREAM [8]; Calsenilin [7] | CALP [10] |
| Tissue distribution | Brain [1]; Heart [13]; Intestine [4,5]; Stomach [6]; Pancreas [14] | Brain [15]; Heart [1]; Lung [2]; Immune System [16] | Brain [1]; Heart [13,17]; Lung [2]; Immune System [16]; Nasal Mucosa [18] | Brain [1]; Kidney [3] |
| Post-translational modification | Myristoylation [19] | Palmitoylation [20] | Palmitoylation [20] Phosphorylation [21] Sumoylation [22] | Palmitoylation [20] |
| Interacted KV subtypes | KV4.1 [23,24] KV4.2 [9,24,25,26] KV4.3 [9,23,25,26] | KV4.1 [27] KV4.2 [9,27] KV4.3 [27] | KV4.1 [28] KV4.2 [9,29,30,31] KV4.3 [32,33,34] | KV4.2 [30] KV4.3 [25,35] |
NA, not available.
2. The Molecular Properties of KChIPs
2.1. The Structure of KChIPs
KChIPs are bipolar proteins of approximately 217–270 amino acids, with a C-terminal core domain and a variable N-terminal domain containing multiple post-translational modification sites (Figure 2). The C-terminal domain is highly conserved and contains a region that interacts with potassium voltage-gated channel subfamily D member (KV4) and four EF-hand motifs [36,37]. Of the four EF-hands, EF-3 and EF-4 can bind Ca2+, EF-2 can bind Mg2+, and EF-1 is degenerate [36,38]. KChIPs undergo conformational modifications when bound to divalent cations [39]. There are several post-translational modification sites at the N-terminus of KChIPs. For example, KChIP1 has an N-terminal myristoylation motif [19]. KChIP2 can undergo dynamic palmitoylation (Cys45 and Cys46) [20,40], namely palmitoylation and depalmitoylation. KChIP3 can undergo sumoylation (K26 and K90) [22], palmitoylation (Cys45 and Cys46) [20], and phosphorylation (Ser63 and Ser95) [21,41]. These diverse post-translational modification sites and patterns in the N-terminus determine the subcellular localization of KChIPs. Myristoylation of KChIP1, palmitoylation of KChIP2, and palmitoylation and phosphorylation (Ser95) of KChIP3 all enhance KChIPs’ localization to the cell membrane. In contrast, depalmitoylation of KChIP2 and sumoylation of KChIP3 increase their nuclear distribution. Furthermore, phosphorylation of KChIP3 at Ser63 inhibits its cleavage by caspase-3 [41].
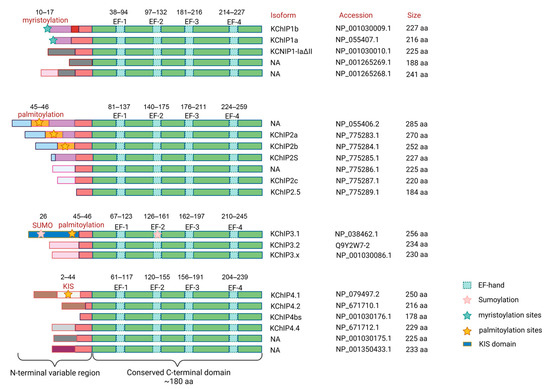
Figure 2.
Diagram of the protein structure of the KChIP family. KChIPs are bipolar proteins of approximately 178–285 amino acids in size. Each KChIP contains an N-terminal domain and a C-terminal domain. The N-terminal domain differs between KChIPs and has multiple post-translational modification sites, whereas the C-terminal domain contains four conserved EF-hand Ca2+-binding motifs. Human KCNIP1 has five transcript variants encoding five different isoforms (KChIP1a, KChIP1b, KChIP1-1adetall, and another two unnamed isoforms). KCNIP2 has seven transcript variants and encodes seven different isoforms (KChIP2a, KChIP2b, KChIP2s, KChIP2c, KChIP2.5, and another two unnamed isoforms). KCNIP3 has three transcript variants encoding three different isoforms (KChIP3.1, KChIP3.2, and KChIP3.x). KCNIP4 has seven transcript variants encoding six different isoforms (KChIP4.1, KChIP4.2, KChIP4bs, KChIP4.4, and another two unnamed isoforms), of which variant 3 (NM_147182.4) and variant 6 (NM_001035004.2) encode the same isoform 3 (NP_001030176.1). Functional domains are indicated by boxes with different colors. The post-translational modification sites are marked with pentagrams of different colors. The corresponding protein names and accession numbers refer to NCBI or Uniprot database. NA, not available.
KChIP4.4 (KChIP4a) [25] and KChIP3x (KChIP3b) [30], which possess the functional KV4 channel inhibitory domain (KID), an N-terminal membrane-spanning segment, are able to exert the inhibitory effect on KV4 channels. The KID contains an ER retention motif consisting of six hydrophobic and aliphatic residues 12–17, which interferes with KV4 surface expression. Residues 19–21 (VKL motif), adjacent to the ER retention motif, enhance KV4 inactivation and keep it in the closed state, thereby inhibiting channel current [35,37,42].
2.2. Regulation of KChIPs Expression
2.2.1. Transcriptional Level
Some physiological functions or pathological conditions are underpinned by changes in the expression levels of KChIPs. Unfortunately, mechanistic studies of the expression regulation of KChIPs are limited and have mainly focused on KChIP2. A number of signaling pathways have been identified that affect the transcription of KChIP2, including NF-κB [43], CaMKII [44], NFAT [45], MAPK [46,47,48], and Notch [49]. Our previous work showed that NF-κB can directly bind to the promoter region of the Kcnip2 gene and repress its transcription [43]. Several factors that downregulate KChIP2 expression, including ligands of TGF-β receptors [50], α-adrenergic receptors [51], β-adrenergic receptors [52], and C-reactive protein [53], are partially dependent on the activation of the NF-κB pathway. Additionally, Ca2+ reduces the mRNA expression of KChIP2 through the Ca2+/calmodulin-dependent protein kinase II (CaMKII) [44] and the calcineurin/NFAT signaling pathway [45]. Under physiological conditions, the inhibition of MEK1 increases, whereas the activation of MEK1 decreases KChIP2 mRNA level. In phenylephrine-induced hypertrophic cardiomyocytes, inhibition of JNK1 rescues the downregulation of KChIP2. These findings suggest that mitogen-activated protein kinase (MAPK) pathways are also involved in the regulation of KChIP2 expression [30]. Notch signaling inhibits the expression of KChIP2 expression and thereby contributes to the electrophysiological differences between neonatal and adult cardiomyocytes [49]. Several transcription factors have also been reported to bind directly to the promoter region of KCNIP2 to regulate its expression. For example, cyclic AMP response element binding protein (CREB) [54], Krüppel-like factor-4 [55], and Krüppel-like factor-5 [56] are able to promote KCNIP2 transcription (Figure 3).
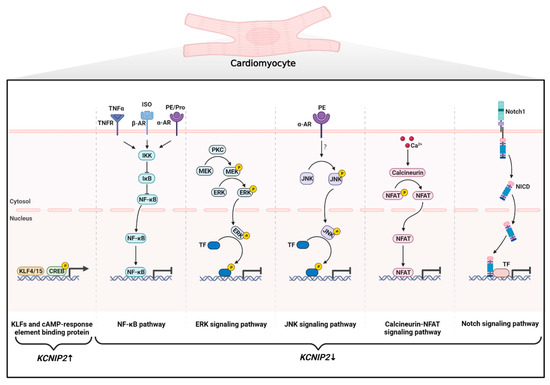
Figure 3.
Regulation of KChIP2 expression at the transcriptional level in cardiomyocytes. Positive regulation of KCNIP2 transcription includes: (1) members of the Krüppel-like factor family (KLF4 and KLF15) and (2) CREB directly binding to the promoter regions of the KCNIP2 gene. Negative regulation of KCNIP2 transcription includes: (1) Activation of TNFR, α-AR, and β-AR increases NF-κB activity and thus exerts an inhibitory effect on the KCNIP2 promoter. (2) Two branches of the MAPK signaling pathway are involved in the downregulation of KChIP2 expression: Activation of PKC phosphorylates and activates MEK, which in turn activates ERK. JNK can be phosphorylated and activated by phenylephrine (PE). Both ERK and JNK can reduce KCNIP2 mRNA levels by activating associated transcription factors. (3) The Ca2+-activated phosphatase calcineurin dephosphorylates NFAT, which then translocates to the nucleus to repress KCNIP2 transcription. (4) The Notch1 receptor is cleaved by γ-secretase to generate NICD following receptor–ligand interaction. NICD then translocates to the nucleus and interacts with transcriptional regulators to inhibit KCNIP2 expression. CREB: cAMP response element-binding protein; TNFR: tumor necrosis factor receptor; α-AR: α-adrenergic receptor; β-AR: β-adrenergic receptor; Pro: propranolol; NF-κB: nuclear factor κB; IκB: NF-κB inhibitor; IKK: IκB kinase; PKC: protein kinase C; MEK: mitogen-activated protein kinase kinase; ERK: extracellular regulated protein kinase; JNK: c-Jun N-terminal kinase; TF: Transcription factor; NFAT: nuclear factor of activated T cells; NICD: intracellular domain of the Notch receptor.
2.2.2. Protein Level
It is worth noting that as an integral component of the KV4 channel complex, the protein level of KChIPs is tightly coupled to KV4 and dipeptidyl-peptidase-related proteins (DPPs). On the one hand, deletion of KV4.2 significantly reduces KChIP1, KChIP2, and KChIP3 expression in the mouse brain. In particular, deletion of KV4.2 results in reduced expression of KChIP2 and KChIP3 in the hippocampus, KChIP2 in the striatum, and KChIP1 and KChIP3 in the cerebellum [57]. Furthermore, KChIP2, KChIP3, and KChIP4 protein expression levels in cortical pyramidal neurons were extremely low in mice with a targeted deletion of either KV4.2 or KV4.3 [15]. Nerbonne et al. showed that loss of KV4.2 in cortical pyramidal neurons resulted in targeted degradation of KChIP3 protein [58]. On the other hand, DPPs also affect the expression of KChIPs. Downregulation of DPP6 reduces KV4.2 and KChIPs in CA1 hippocampal neurons [59]. Similarly, knockdown of DPP10 in the dorsal root ganglion (DRG) neurons resulted in downregulation of KChIP1 and KChIP2 [60].
In addition, members of the KChIP subfamily can have an effect on the expression of other KChIPs. For example, when KChIP3 was deficient in the cortex, the expression of other KChIPs were increased to compensate for KChIP3 deficiency [15,61]. Interestingly, there is evidence that KChIP3 can be a negative regulator of its own expression [61].
3. Biological Function of KChIPs
3.1. KChIPs Are Auxiliary Subunits of KV4 Channels
3.1.1. The Interaction of KChIPs with KV4 Channels
KV4 channels are members of the KV channel superfamily. In mammals, the KV4-family consists of four members: KV4.1, KV4.2, and two splice variants of KV4.3. All KV4 channels share a functional core that is assembled as a tetramer of pore-forming α-subunits around a central pore [12]. The KV4 α-subunit contains an N-terminal cytoplasmic domain with an N-terminal hydrophobic segment, the KV channel assembly domain (T1 domain) under the tetrameric channel pore domains, a transmembrane domain with six transmembrane helices S1–S6, and the C-terminal cytoplasmic domain [62]. The KV4 channels are highly expressed in the brain, heart, and smooth muscle cells. The neuronal KV4 channels underlie the transient A-type current (IA), sustaining the homeostatic excitability of neurons [32]. In cardiomyocytes, KV4 channels control the early repolarization phase of the action potential by mediating the transient outward current (Ito) [63]. In gastrointestinal smooth muscle cells, KV4 currents are involved in shaping the slow-wave activity and mechanical responses [4].
However, KV4 channels can not carry out normal physiological processes on their own. Two auxiliary subunits, KChIPs and DPPs, are essential for the physiological function of KV4 channels. Mechanistically, KChIP1-3 can bind to the cytoplasmic domains of KV4 α-subunits, thereby increasing total KV4 current, slowing channel inactivation, and accelerating recovery from inactivation. An et al. also found that KChIP4a, which contains the KID, can eliminate the fast inactivation of the KV4 current. KChIPs are anchored laterally to the T1 domains of KV4 and clamp two adjacent KV4 N-terminals in a 4:4 ratio [64,65]. Recently, Kise et al. reported the cryo-electron microscopy structures of KV4.2–KChIP1 octamer complex. They suggested that the structure of the KV4.2–KChIP1 octamer complex has dimensions of around 105 Å × 105 Å × 100 Å [62]. The X-ray crystal structures of the KChIP1-KV4 complex have revealed some of the interaction sites that mediate the regulatory effects of KChIPs. Wang et al. reported two key sites for KChIP-KV4 interaction by resolving the crystal structures of the KChIP1-KV4 complex formed by the N-terminus of human KV4.3 (residues 6–145) and human KChIP1 (residues 38–217). At the first site, the hydrophobic pocket formed by the H10 helix of KChIP1 interacts with the N-terminal hydrophobic segment of KV4.3, which represents the main binding mode of KChIP1 with KV4. The N-terminal hydrophobic peptide of KV4.3 is sequestered in an elongated hydrophobic groove on the surface of KChIP1 and replaces the H10 helix of KChIP1. In addition, KChIP1 can interact with the neighboring KV4.3 N-terminals through hydrophobic interactions and hydrogen bonds. The second site is mainly formed by the interaction between the KChIP1 H2 helix and the KChIP-specific docking loop of the neighboring T1 domain through the hydrophobic interactions and salt bridges, stabilizing the KV4.3 tetramerization. In the second site, the conserved Phe73 of KV4 fits tightly into a hydrophobic cavity that is formed by the residues Leu39, Leu42, Leu53, Tyr57, and Phe108 in KChIP1. Meanwhile, residues Lys50, Arg51, and Lys61 of KChIP1 form salt bridges with Glu77, Asp78, and Glu70 of KV4.3, respectively [65]. The first site is responsible for KV4 inactivation and the second site for stabilizing the tetramer of KV4. Based on crystallographic studies by Wang et al., Cattee et al. defined the third site of KV4.3-KChIP1 interaction using all-atom molecular dynamics simulations. Residues R51, R58, and E63 on the KChIP1 H2 helix, which is also involved in the formation of the second site, interact with residues D39 and R60 on the KV4.3 T1N linker to form the third site, providing further stability to the KV4.3 tetrameric intracellular domain [65,66]. In addition, KChIP1 is able to capture the C-terminal cytoplasmic S6 helix on KV4.2 through the hydrophobic interactions [62].
3.1.2. KChIPs Modulate the Gating Properties of KV4 Channels
KV4 are the rapidly inactivating (A-type) KV potassium channels that generate currents at subthreshold membrane potentials. They are characterized by fast activation, fast inactivation, and fast recovery from inactivation. The inactivation of KV4 channels is classified into two types: open-state inactivation and closed-state inactivation. Closed-state inactivation is the main type, indicating that KV4 channels can be inactivated directly from the closed state [67]. Binding of KChIPs to the N-terminus of KV4 modulates the gating properties of KV4 channels. Specifically, KChIP1-3 augment KV4 currents through the following electrophysiological effects: shifting the activation midpoint of voltage activation to more negative potentials, slower inactivation, and acceleration of recovery from inactivation [9]. When co-expressed with KChIPs, the activation time of KV4 was slightly prolonged compared to KV4 alone, while the midpoint for KV4 of voltage activation significantly shifted to more hyperpolarized potentials [9]. In contrast, the modulation of KV4 gating by KChIPs is mainly manifested in the inactivation kinetics. Heterologous co-expression of KV4 and KChIPs significantly prolongs the inactivation time of KV4 channels. To be specific, KChIPs eliminate open-state inactivation and accelerate closed-state inactivation of KV4 channels [9,27,62]. However, it is still unclear about the molecular mechanism by which KChIPs control KV4 inactivation. The EF-hands were reportedly involved in the regulation of KV4.3 inactivation by sensing intracellular Ca2+ levels [68]. Recently, breakthroughs have been made in the structural basis of KChIPs that regulate the inactivation kinetics of KV4 channels. Kise et al. reported that KChIP1 is able to capture and sequester both the N-terminal hydrophobic segment and the C-terminus of KV4.2 channels. KChIP1, on the one hand, binds the C-terminal intracellular S6 helix to stabilize the S6 conformation. It also binds the N-terminal hydrophobic segment and two T1 domains from the neighboring subunit of KV4.2. Together, these KChIP1-mediated structural features prevent open-state inactivation and accelerate closed-state inactivation of KV4.2 [62]. By truncating the N-terminal or C-terminal helix of KV4.2, respectively, Ye et al. demonstrated that the interactions of KChIP2 with the KV4.2 N-terminal helix play a more prominent role in modulating channel inactivation [69]. Moreover, KChIPs accelerate the rate of recovery of KV4 channels from inactivation in a Ca2+-independent manner [68].
It is interesting to note that a specific KChIP isoform, KChIP4a, has been reported to delay KV4.3 channel activation, abolish rapid inactivation, and impede channel closure after opening. Therefore, co-expression of KChIP4a with KV4 α-subunits converts the A-type KV4 current to a slowly inactivating delayed rectifier-type potassium current [25]. The similar suppressive effect on KV4 currents was also found for KChIP3x (KChIP3b) in subsequent research [30].
3.1.3. KChIPs Modulate the Trafficking of KV4 Channels
KV4 trafficking to the cell surface are vital for the maintenance of current density [9,27]. The efficient trafficking of KV4 to the cell surface depends on KChIP binding to its N-terminal domain [27]. Shibata et al. postulated that in the absence of KChIP binding, the hydrophobic domains of multiple KV4.2 subunits will oligomerize, resulting in aggregation, misfolding, and consequently retention in the ER for degradation. Interaction of KChIPs with KV4.2 leads to changes in the overall molecular properties of KV4.2 by concealing the N-terminal hydrophobic domain. These changes include an increased protein stability, phosphorylation, detergent solubility, and cell surface localization [70]. Further research has shown that in the absence of KChIP1, the KV4 protein can be released from the ER but is then trapped in the Golgi complex. When KV4 channels and KChIP1 are co-expressed in heterologous expression systems, their translocation to the plasma membrane is facilitated [27,28]. In addition, KChIP2 and KChIP3 have also been shown to increase KV4.2 trafficking from the ER and Golgi complex to the plasma membrane [71]. In detail, KV4.2 is targeted by KChIP2 to cholesterol-rich lipid rafts in the cell membrane [72]. By contrast, KChIP4a, which contains the KID motif, does not have these effects on KV4.2. Meanwhile, KChIP4a suppresses KV4 trafficking by forming a ternary plasma membrane complex with KV4.2 and other KChIPs [70]. Therefore, the role of KChIPs in KV4.2 trafficking may also contribute to its regulation on KV4 current (Figure 4).
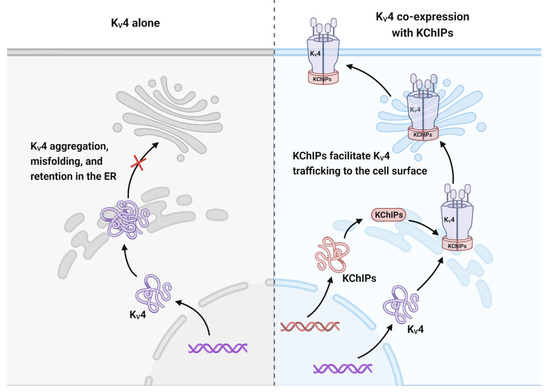
Figure 4.
KChIPs allow KV4 trafficking to the cell surface. KV4 channels cannot be trafficked to the cell membrane on their own because they are aggregated, misfolded, and retained in the ER for degradation. Co-expression of KChIP1-3 releases KV4 channels from ER retention and redistributes them to the cell surface.
3.1.4. KChIP Ligands Affect KChIP Regulation on KV4
Since KChIPs are essential for the control of electrophysiological processes in cells, inhibiting or enhancing their effect on KV4 channels is a promising avenue for pharmacological research in the treatment of KV4-mediated channelopathies. Several small molecules that bind to KChIPs have been shown to modulate KV4 currents (Table 2). For example, the binding of arachidonic acid to the hydrophobic C-terminus of KChIP1 accelerates KV4 inactivation and decreases current amplitude [26]. CL-888, a diaryl–urea compound, can bind to KChIP1 [73] and KChIP3 [74] to counteract their regulatory effects on KV4, reducing peak current amplitude and accelerating inactivation kinetics. IQM-PC330 and IQM-PC332, the derivatives of CL-888 modified by Lopez-Hurtado et al., have a more refined blocking effect on KV4 currents. IQM-PC330 and IQM-PC332 inhibit KV4.3 channels not only by reducing peak current amplitude and accelerating their inactivation but also by delaying their recovery from inactivation. Mechanistically, they act as KChIP3 ligands to reverse the regulatory function of KChIP3 on KV4.3 channel gating properties. It is interesting to note that the hydrophobic combination of IQM-PC332 and KChIP3 accelerates the activation kinetics of KV4.3 current at low concentrations (0.01 to 0.1 M) and reverses its effect on channel gating at higher concentrations. However, inactivation recovery kinetics were significantly reduced at all concentrations [75]. They also identified IQM-266 as a novel KChIP3 ligand with similar inhibitory properties to IQM-PC330 and IQM-PC332 [76]. Recently, IQM-266 was reported to bind KChIP2 and increase KV4.3/KChIP2 currents [77].
The sulfonylurea compound NS5806, a ligand of KChIP3, has been demonstrated to activate KV4.3 channels in neurons. NS5806 delays the inactivation of IA and slightly reduces the maximum peak current [78]. This effect is based on the Ca2+-dependent binding of NS5806 to the hydrophobic site at the C-terminus of KChIP3, which facilitated the binding affinity between KChIP3 and KV4.3 as well as reducing their dissociation rate [34]. In cardiomyocytes, however, the control of KV4 currents by NS5806 is controversial. In canine cardiomyocytes, NS5806 functions as an Ito activator to increase the amplitude of the KV4.3/KChIP2 peak current and significantly slow the current decay [79], while in mouse ventricular myocytes NS5806 has the opposite effect, as shown by a decrease in the amplitude of native Ito and significant acceleration of current inactivation [80]. Furthermore, in rabbit ventricular myocytes, NS5806 dramatically raised Ito amplitude, while in atrial myocytes, the Ito amplitude was repressed [81]. These conflicting results suggest that more than one site may be involved in the interaction between NS5806 and KV4 channel complexes, and that NS5806 may act as both an agonist and an antagonist of Ito on the same channel complexes. The exact mechanism by which NS5806 elicits different responses in different species and cell types remains unclear. Indeed, studies comparing the effects of reported small molecule drugs targeting KChIPs in different species are still lacking. It is therefore necessary to test and compare these small molecules in more animal models. More interestingly, repaglinide, a commonly used antidiabetic drug, can bind directly to KChIP3 and exert an inhibitory effect on KV4 channels [74]. In fact, accumulated research has shown that repaglinide and glibenclamide can competitively bind to the SUR subunit of cardiovascular and neurological KATP (ATP-sensitive potassium) channels to inhibit IKATP [82,83]. However, the magnitude and kinetics of KV4 currents were not affected by glibenclamide [74]. Mechanistically, repaglinide can bind to the Ca2+-dependent EF-hand protein of the neuronal calcium sensor family to antagonize its biological function [84]. It is regrettable that the effects of repaglinide against other KChIPs in other organs have not been reported. Given that repaglinide is already a mature drug that can improve type 2 diabetes and cardiovascular events, further investigation of the effect of repaglinide on KV4 currents in different tissue will be a potential way to improve KChIPs-related neurological and cardiovascular diseases. Altogether, the discovery of these KChIP ligands has improved our knowledge of the interaction between KV4 and KChIPs and has paved the way for future pharmaceutical development for the treatment of neurodegenerative and cardiovascular diseases involving KV4/KChIPs.

Table 2.
Small-molecule compounds that interact with KChIPs and their role in KV4 currents.
Table 2.
Small-molecule compounds that interact with KChIPs and their role in KV4 currents.
| Compounds | KChIP1 | KChIP2 | KChIP3 | KChIP4 |
|---|---|---|---|---|
| Arachidonic acid | Accelerate KV4 inactivation; Reduce current amplitude [26] | NA | NA | NA |
| CL-888 | Reduce peak current amplitude; Accelerate inactivation kinetics [73] | NA | Reduce peak current amplitude; Accelerate inactivation kinetics [74] | NA |
| IQM-PC330 IQM-PC332 | NA | NA | Reduce peak current amplitude; Accelerate inactivation kinetics; Delay recovery from inactivation [75] | NA |
| IQM-266 | NA | Increase current [77] | Reduce peak current amplitude; Accelerate inactivation kinetics; Delay recovery from inactivation [76] | NA |
| NS5806 | NA | Play opposite roles in different species, even in different tissues from one species [79,80,81] | Activate neural KV4.3; Delay inactivation kinetics; Reduce the maximum peak current slightly [78] | NA |
| Repaglinide | NA | NA | Inhibit KV4 channels [74] | NA |
NA, not available.
3.2. Role of KChIPs in Regulating Other Ion Channels
3.2.1. KV1.5
KV1.5 is another vital potassium channel characterized by rapid activation and rapid inactivation that is highly expressed in both the brain and heart. In the human heart, KV1.5 is expressed abundantly in atrial myocytes and mediates the ultra-rapid delayed rectifier current (IKr). In the adult mouse heart, KV1.5, which encodes the slow delayed rectifier K+ current (IKs), is involved in the repolarization of ventricular myocytes [85]. Several studies have demonstrated the contribution of KChIPs in the modulation of KV1.5 channels. In contrast to their augmenting effect on KV4 currents, KChIPs negatively regulate KV1.5-encoded K+ currents. In transiently transfected HEK293 cells, KChIP1 and KChIP2 attenuate KV1.5 currents by inhibiting the trafficking of KV1.5 channels to the cell surface [86]. Moreover, in the ventricles of Kcnip2−/− mice, KV1.5 mRNA levels were significantly increased and IKs were upregulated [87]. However, the structural basis of KChIP1 and KChIP2 negative regulation of cardiomyocyte KV1.5 channels in vivo remains unclear and needs further investigation.
3.2.2. CaV1.2
The high-voltage-activated L-type Ca2+ channel, CaV1.2, located on the t-tubule sarcolemma, is the major calcium channel type in cardiomyocytes. CaV1.2 channels are macromolecular complexes that are composed of α1, α2δ, and β subunits [88]. The cardiac L-type Ca2+ current (ICa,L) mediated by CaV1.2 is essential for cardiomyocyte depolarization and contraction. KChIPs have a dual effect on the regulation of the cardiac L-type Ca2+ current (ICa,L). On one hand, KChIP2 regulates the ICa,L through direct interaction with the intracellular N-terminus of the CaV1.2 α1C subunit. Without raising CaV1.2 protein expression or trafficking to the plasma membrane, KChIP2 increases ICa,L current density by impeding the N-terminal inhibitory module [89]. On the other hand, KChIP2 and KChIP3 bind and repress the transcription of the Cacnb2 [90] and Cacna1c [17] genes, respectively, which encode the corresponding β2-subunit and α1C-subunit of CaV1.2 channels.
3.2.3. NaV1.5
NaV1.5, which is encoded by SCN5A, is highly expressed in the heart. By mediating the rapid influx of Na+, NaV1.5 dominates the rapid depolarization phase of action potential. Previous studies suggest that KV4.3 and NaV1.5 work coordinately and can regulate each other [91]. Deschênes et al. found that co-expression of KChIP2 with NaV1.5 increased NaV1.5 current density but had no effect on Na+ current gating properties, whereas silencing of Kcnip2 resulted in a significant decrease in Scn5a mRNA level and complete inhibition of voltage-dependent Na+ currents [92]. However, this evidence is based on heterologous expression systems. The electrophysiological significance of the cross-regulation of KChIP2, NaV1.5, and KV4.3 in cardiomyocytes requires further study.
3.3. KChIPs Are Ca2+-Dependent Transcriptional Factors
KChIP3 is the first KChIP identified to have transcriptional activity, as it can bind to the downstream regulatory element (DRE) downstream of the TATA box in the human PDYN gene promoter [8]. Subsequent studies have demonstrated that all the KChIPs have Ca2+-dependent DRE binding affinities that block gene transcription in the form of homotetramer or heterotetramer. The binding of KchIP3 to DRE sequences is regulated by divalent cations [8]. Tetramer formation is required for KchIP3 in binding to DRE. Mg2+ and Ca2+ are the most important factors affecting the ability of KchIP3 to bind to DNA. The combination of Mg2+ and KchIP3 stabilizes the tertiary structure of the protein and promotes DNA binding [93]. In contrast, as intracellular Ca2+ levels increase, the Ca2+-bound KchIP3 switches to a denser dimer structure [94], preventing KchIP3-DRE interaction. Furthermore, direct interaction of nucleoprotein C-terminal binding protein [95] and αCREM [96] with KChIP3 enhances and inhibits their transcriptional repressive functions, respectively. In the nucleus, in addition to direct binding to DRE in target genes, KChIP3 also forms transcriptional regulatory complexes with nuclear proteins such as cAMP response element-binding protein [97], thyroid transcription factor 1 [98], and nuclear receptors [99]. Genes regulated by KChIP3 include but are not limited to the following: Pdyn, Fos [100], Hrk [101], Bdnf [102], Ifng, Il2 and Il4 [103], Klf9 [16], Npas4, Nr4a1, Mef2c, Junb [61], Tnfaip3 [104], Tg [98], Ttf2/Foxe1, Pax8 [105], Aanat, Fra-2, Crem [106], GnRH [107], Gfap [108], Ncx3 [109], Cacna1c [17], Mid1 [110], Cant1 [111], PAX6, NRG1 [112], and GCM1 [113] (Figure 5).
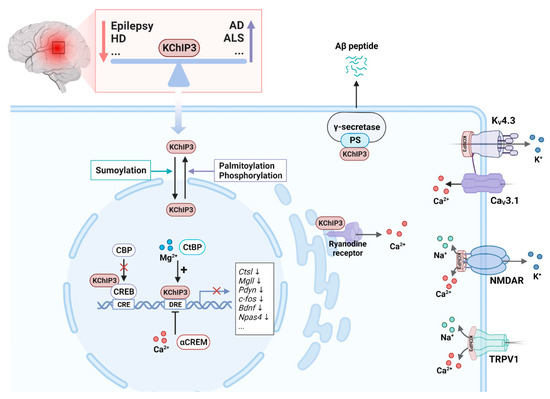
Figure 5.
Function of KChIP3 in neurons. Altered KChIP3 expression is associated with several nervous system disorders. For example, KChIP3 is downregulated in epilepsy and Huntington’s disease (HD) and upregulated in Alzheimer’s disease (AD) and amyotrophic lateral sclerosis (ALS). KChIP3 has physiological functions in both the cytoplasm and the nucleus. Sumoylated KChIP3 translocates to the nucleus to repress the transcription of associated genes by interacting with the DRE sequences. This interaction can be enhanced by Mg2+ and C-terminal binding protein (CtBP) and inhibited by Ca2+ and cyclic AMP-responsive element modulator α (αCREM). KChIP3 can also repress target gene transcription by interacting with the transcription factor CREB to interfere with CREB phosphorylation and CREB-binding protein (CBP) recruitment. Palmitoylation and phosphorylation of KChIP3 increase its location in the cytoplasm, where it interacts with presenilin (PS) to regulate the enzymatic activity of the γ-secretase. In the cytoplasm, KChIP3 regulates intracellular K+, Ca2+, and Na+ currents by interacting with KV4, N-methyl-D-aspartate receptor (NMDAR), transient receptor potential vanilloid 1 (TRPV1), and the ryanodine receptor.
Through the transcriptional regulation of the above-mentioned genes, KChIP3 has been shown to be multifunctional and is critically involved in the regulation of physiological and pathological functions of neurons, endocrine cells, immune cells, endothelial cells, and hematopoietic cells. For example, in the pineal gland, KChIP1-4 are involved in the regulation of rhythmically expressed genes engaged in circadian rhythms. By binding to the DRE sites of arylalkylamine N-acetyltransferase (Aanat), inducible cAMP early repressor (Crem), and Fos-related antigen-2 (Fra-2), KChIP1-4 are able to repress the basal and induced transcription of these circadian-rhythm-related genes [106]. In the pancreas, KChIP3 has been detected in islet α- and β-cells. KChIP3 represses the transcription of the Pdyn gene in a Ca2+-dependent manner and thereby affects glucagon release [114]. In the thyroid, KChIP3 inhibits the expression of thyroglobulin by binding directly to the DRE of the thyroglobulin gene and blocking thyroid-specific transcription factors such as TTF-1, TTF-2, and Pax8 [98,105]. KChIP3, as well as KChIP2, are expressed in T- and B cells to regulate immunological responses. In T lymphocytes, KChIP2 and KChIP3 act as transcriptional repressors to inhibit the expression of IFN-γ, IL-2, and IL-4 [103]. By inhibiting the transcription of the proliferation-related gene Klf9 and the protein-translation-related gene Eif4g3 in B cells, KChIP2 and KChIP3 govern B-cell proliferation and IgM and IgG protein synthesis [16]. In lung endothelial cells, neutrophils, and macrophages, KChIP3 can promote the NF-κB-pathway-mediated inflammatory response by suppressing the expression of the TNFAIP3 gene, which encodes the anti-inflammatory deubiquitinase A20 [104,115].
4. KChIPs and Diseases
4.1. Neurological Diseases
4.1.1. Epilepsy
The International League Against Epilepsy (ILAE) classification of epilepsy types includes focal epilepsy, generalized epilepsy, combined generalized and focal epilepsy, and unknown epilepsy [116]. All of these seizure types share the pathophysiological characteristic of increased neuronal excitability and synchronicity. Among them, temporal lobe epilepsy is the most common focal epilepsy that can further be subdivided into mesial temporal lobe epilepsy and lateral or neocortical temporal lobe epilepsy [117]. Adults with intractable epilepsy are most commonly affected by epilepsy of the mesial temporal lobe, which is the chronic and pharmacoresistant form of epilepsy. In this type of epilepsy, seizures originate from the hippocampus, entorhinal cortex, amygdala, and parahippocampal gyrus [118]. Abundantly expressed in the hippocampus, KChIPs control the frequency of slow repetitive spike firing and attenuate action potential backpropagation by modulating the properties of KV4 channels. Thus, KChIPs play a key role in maintaining neuronal excitability [119]. To date, several studies have implicated the downregulation of KChIPs in temporal lobe epilepsy. For example, the hippocampus of patients with medically refractory temporal lobe epilepsy had significantly reduced KChIP3 immunoreactivity compared to normal brains [120]. Consistently, downregulation of KChIP expression in specific hippocampal subfields (CA1 and CA3, but not CA2) was observed in rodent models of temporal lobe epilepsy that progressed to status epilepticus, i.e., very prolonged seizures. For example, in the hippocampus of the pilocarpine-induced rat epilepsy models, loss of KChIP1 immunoreactivity in interneurons and reduction of KChIP2 in the stratum radiatum of the CA1 region were observed [121]. In the kainic-acid-induced mouse epilepsy model, KChIP3 expression was reduced in the cortical area and CA3 region of the hippocampus in status epilepticus [120]. The use of KChIP-deficient transgenic mice provided further evidence for the role of KChIPs in the pathogenesis of epilepsy. In Kcnip2−/− mice, the excitability of hippocampal neurons was enhanced and the susceptibility to epilepsy induced by kindling was increased. In the hippocampal pyramidal neurons from Kcnip2−/− mice, IA showed a reduced amplitude and shift in V½ for steady-state inactivation to hyperpolarized potentials [122]. Nevertheless, the role of KChIPs in the pathogenesis of epilepsy remains to be further elucidated using neuron-specific Kcnip overexpression or knockout animal models.
4.1.2. Pain
A key factor in the development and maintenance of neuropathic pain is neuronal excitability. The physiological pain circuit can be briefly summarized as follows: physicochemical signals from noxious stimuli transduces through ion channels and purinergic channels to evoke action potentials. These action potentials are amplified by Na+ channels to produce pain. The electrical signals are carried by unmyelinated C-fibres and thinly myelinated Aδ-fibres to the DRG in the body and the trigeminal ganglion (TG) in the face, where their cell bodies are located and project to the dorsal horn of the spinal cord and the medulla oblongata, respectively. After integration and processing, the pain input is transmitted via several ascending tracts to various projection sites in the brain. To process the sensory and discriminative aspects of pain, the lateral spinothalamic tract projects to the lateral thalamus. Medial projections of the spinothalamic and parabrachial tracts to the medial thalamus and limbic structures mediate the emotional and aversive components of pain. In pathological conditions such as inflammation, neuropathy, and diabetes, physiological pain is converted into pathological pain, which manifests as increased sensitivity to painful stimuli (hyperalgesia) [123].
As proposed by Costigan et al.: “No DREAM, no pain” [124], indicating that KChIP3 is critically involved in the regulation of pain. KChIP3 is expressed in the neurons of the ventral and dorsal horns of the spinal cord and the TG of mice [22,100]. Using Kcnip3−/− mice, Cheng et al. proposed that loss of KChIP3 attenuates pain responses. Kcnip3−/− mice showed significantly reduced pain behaviors in models of visceral pain induced by MgSO4 and acetic acid, chemical pain induced by formalin, inflammatory pain induced by capsaicin and carrageenan, and neuropathic pain induced by cuff implantation around the sciatic nerve [100]. This result was further substantiated by Rivera et al. using daDREAM (dominant active DREAM)-transgenic mice. daDREAM is a Ca2+-insensitive double mutant (EF-hand, leucine-charged residue-rich domains) KChIP3 that actively represses KChIP3 target genes and prevents the Ca2+-dependent derepression function of KChIP3. In contrast to Kcnip3−/− mice, which showed a basal state of analgesia, daDREAM transgenic mice displayed a state of basal hyperalgesia. However, the daDREAM transgenic mice showed impaired response to inflammatory pain induced by Complete Freund’s adjuvant (CFA) [102]. In the above studies, the modulation of the basal threshold of pain by KChIP3 was attributed to the inhibition of the expression of PDYN, which can be cleaved into dynorphin in the spinal cord and acts as an endogenous ligand of κ-opioid receptors to exert analgesic effects [125]. Meanwhile, the regulation of spinal sensitization by KChIP3 was considered to be dependent on BDNF which is a well-established regulator of synaptic plasticity and plays a modulatory role in spinal nociceptive processing. BDNF in the physiological dose range facilitates nociception, whereas high doses produce analgesia or hypoalgesia [126]. Both Pdyn and Bdnf promoter regions contain the DRE sequence [8,61], the transcription of which could be inhibited by KChIP3. Notably, in daDREAM transgenic mice, orofacial injection of formalin (acute trigeminal nerve stimulation) induced hyperalgesia with a concomitant reduction in BDNF [127]. This finding was contrary to that reported by Rivera above, which may be because BDNF plays different and specific roles in the control of nociception in the trigeminal ganglion and in the spinal cord. Furthermore, in trigeminal neurons, KChIP3 specifically downregulates Mgll (encodes monoglyceride lipase) and Ctsl (encodes cathepsin L), which are associated with nociception [127].
Most of the conclusions drawn from the mouse model have been confirmed in the rat model. For example, upregulation of KChIP3 in the nuclear compartment was observed in a formalin-injection-induced acute pain model in rats [128]. Minocycline administration attenuated tactile allodynia and chemical hyperalgesia in diabetic rats, accompanied by downregulation of KChIP3 protein in the spinal cord [129]. However, the “no DREAM, no pain” hypothesis does not seem to apply to Kcnip3−/− rats. Guo et al. recently found that global knockout of KChIP3 using CRISPR/Cas9 technology increased pain sensitivity in rats to acute and chronic inflammatory pain induced by formalin and CFA [130,131], which is in contrast to what was found in studies of Kcnip3−/− mice by Cheng et al. [100]. This is a big wake-up call that KChIP3, in addition to repressing the expression of genes involved in pain processing, may have other functions in pain transmission and processing in rats. But the specificity of KChIP3 in nociception and pain processing at different levels could not be demonstrated in transgenic animals with global KChIP3 knockout. Indeed, KChIP3 is highly expressed in rat DRG neurons [132], whereas previously reported KChIP3 protein in mouse DRG could not be detected by Western blot [100]. Some reports suggest that the mechanism of KChIP3 in pain modulation in rats involves its interaction with several receptors and ion channels involved in pain-sensing and transmission in DRG neurons. These include the N-methyl-D-aspartate receptor (NMDAR) [133], transient receptor potential vanilloid 1 (TRPV1) channel [131], and KV4 [132]. Activation of NMDAR in the central nervous system is required for central sensitization. KChIP3 significantly inhibited the surface expression of NMDARs and NMDAR-mediated currents by directly interacting with NMDAR through its N-terminal residues 21-40 [131]. TRPV1, which is also known as the thermosensitive protein, can sense noxious heat and chemical stimuli simultaneously. In primary sensory neurons, TRPV1 acts as an integrator of the painful afferent sensation and as a key initiator of the efferent neurogenic inflammation [134]. In the rat inflammatory pain model induced by CFA, TRPV1 in nociceptive sensory neurons undergoes functional sensitization, resulting in Ca2+ influx. Meanwhile, upregulation of KChIP3 protein expression resulted in enhanced binding of KChIP3 to the TRPV1, inhibiting TRPV1 cell surface localization and thereby exerting an analgesic effect [131]. Furthermore, by regulating KV4 in DRG neurons that meditate somatodendritic ISA, KChIP3 is involved in the transmission of pain signals [132]. Notably, CaV3 channels and KV4 channels, the important contributors to pain control, were found to form a signaling complex in rat cerebellar, hippocampal, and neocortical regions [135]. Recently, a novel KChIP3 ligand, IQM-PC332, was reported to reduce mechanical hypersensitivity in rats subjected to chronic constriction injury of the sciatic nerve. Mechanistically, IQM-PC332 binds to KChIP3 and reduces ionic currents mediated by TRPV1 channels, KV4.3 channels, low-voltage-activated T-type Ca2+ channels, and high-voltage-activated Ca2+ channels in DRG neurons [136]. This is a potential chemical tool and the elucidation of the structural basis of the analgesic effect of IQM-PC332 will be one of the possible steps towards a better understanding of how KChIP3 controls pain. In conclusion, KChIP3 may act at multiple levels to modulate pain. It will be necessary to use tissue-specific KChIP3 knockout models or site-specific inhibition of KChIP3 to further elucidate its complex mechanism in pain modulation (Figure 6).
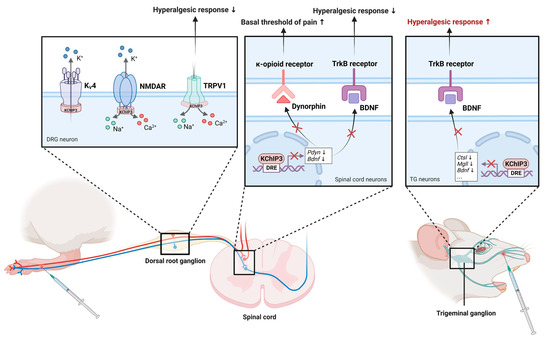
Figure 6.
The role of KChIP3 in the modulation of pain. KChIP3 is involved in pain control in both the spinal cord/DRG (left) and the trigeminal ganglion (right). In DRG neurons, KChIP3 modulates pain transmission by directly interacting with KV4 channels, TRPV1 channels, and NMDAR. On the one hand, KChIP3 contributes to the central transmission of pain signals by increasing KV4 currents. On the other hand, KChIP3 exerts analgesic effects by inhibiting the surface expression of TRPV1 and NMDAR. In the spinal cord, KChIP3 directly binds the genes encoding dynorphin and BDNF and represses their expression. In trigeminal ganglion neurons, KChIP3 binds and inhibits Bdnf, Ctsl, and Mgll to regulate trigeminal noxious perception.
4.1.3. Memory Dysfunction
Learning is the process by which new information about the world is acquired, and memory is the process by which knowledge is stored. The cellular basis of learning and memory is thought to be the process by which synapses undergo bidirectional changes in synaptic strength, known as synaptic plasticity. Among the different types of synaptic plasticity, two opposite forms of synaptic plasticity, long-term potentiation (LTP) and long-term depression (LTD), have been the most studied [137]. LTP is the strengthening of synapses following repeated synaptic activity. Inhibition of LTP results in impaired learning or failure to retain memories, while LTD is the synaptic framework of weakening synaptic strengths, contributing to learning by engaging in a functional interplay with LTP [138].
Several brain regions are involved in memory, including the hippocampus, neocortex, amygdala, basal ganglia, and cerebellum. Of these, the hippocampus is essential for the spatial representation of the environment and the ability to recall specific events, or ‘episodic memory’ [139]. KChIP3 is highly expressed in the hippocampus and is also involved in the regulation of learning and memory. Alexander et al. reported that KChIP3 knockout mice exhibited enhanced memory in a contextual fear-conditioning paradigm. They also found that translocation of KChIP3 from the membrane to the nucleus was increased in mouse hippocampal neurons following the fear conditioning training paradigm [33]. In the cell nucleus, KChIP3 functions as a transcriptional repressor to regulate the expression of genes involved in memory formation, such as Pdyn, Fos, Bdnf, and Npas4 [33,61]. In addition, Fontán et al. reported that loss of KChIP3 not only enhances LTP and improves learning and memory in young mice, but also improves cognition and slows age-related brain degeneration in old age [140]. Mechanistically, KChIP3 interacts with CREB, a key transcription factor involved in memory, and negatively regulates CREB-dependent transcription. This raises the threshold for CREB activation in learning and memory. Therefore, in the Kcnip3−/− mice, the threshold for CREB phosphorylation and CREB-CBP interaction in learning and memory is lowered, and the timing of CREB phosphorylation and CBP recruitment to CREB is shortened, thereby promoting CREB-dependent transcription during learning. Consistent results were also confirmed in daDREAM-transgenic mice. Transgenic mice expressing the daDREAM showed significant impairments in learning and memory [61].
The mechanism by which KChIP3 is involved in memory regulation also involves its protein–protein interactions. NMDAR is the major ionotropic glutamate receptor in the central nervous system and plays an important role in both synaptic transmission and plasticity [141]. NMDARs are required for LTP and LTD of synaptic transmission [142]. When Ca2+ binds to KChIP3, the interaction between KChIP3 and PSD95 is released, allowing NMDA to function as a normal receptor. The EF-hands mutant KChIP3, which lacks Ca2+ binding ability, inhibits NMDAR function in mouse hippocampal CA1, impairs LTD, and thereby reduces consolidation of hippocampus-dependent contextual fear-conditioned reflexive memory [143]. In addition, KV4.2 channels play an important role in both synaptic plasticity and cognition, which in part contributes to the regulatory effect of KChIP3 on learning and memory [144].
4.1.4. Alzheimer’s Disease
Amyloid beta-protein (Aβ) deposition is the main feature of AD, which has a toxic effect on neurons [145]. PS is the catalytic core of the high-molecular-weight enzyme complex γ-secretase, which can mediate γ-cleavage of β-amyloid precursor protein to produce Aβ [146]. KChIP3 was initially discovered as being capable of binding to the C-terminal fragment of presenilin 1 (PS1) and PS2 via the C-terminal 103 amino acids encoded by ALG3 [7]. The binding of KChIP3 to PS is dependent on the presence of Ca2+ [147]. Existing studies have shown that mutations of PS1 and PS2 lead to increased Aβ formation and apoptosis, thus causing early familial Alzheimer’s disease (AD) [148]. In the brains of patients with AD, the expression of KChIP3 was increased in neurons and reactive astrocytes, which is consistent with the regions with pathological changes of AD [149]. This suggests that KChIP3 may be involved in the pathogenesis of AD. Zaidi et al. subsequently showed that KChIP3 stabilizes the structure of the PS in the cerebellum and hippocampus [150]. Jo et al. reported KChIP3 as a pro-apoptotic protein that facilitates cell death induced by Aβ production [151]. Overexpression of KChIP3 increased the enzymatic activity of the presenilin-γ-secretase complex in HEK293 cells [152]. Aβ increased KChIP3 expression in cultured neurons while blocking its expression protected neuronal cells from Aβ toxicity [149]. Repaglinide has been shown to inhibit the KChIP3-PS2 interaction, suggesting a novel avenue for future AD treatment [153].
KChIP3 also regulates the intracellular Ca2+ signaling, the dysregulation of which has been implicated in the development of AD [154]. In AD models, increased Ca2+ release from ryanodine-sensitive Ca2+ stores have been observed in neurons [155]. Lilliehook et al. showed that overexpression of KChIP3 can enhance apoptosis by increasing ER Ca2+ release in glioma cells [156]. Fedrizzi et al. reported that transient co-expression of KChIP3 with PS facilitated Ca2+ release from the ER in neurons [157]. Mechanistically, KChIP3 binds to the DRE sequence of the isoform 3 of the Na+/Ca2+ exchanger (Ncx3) gene to repress its transcription, thereby regulating intracellular Ca2+ homeostasis [109]. In mouse hippocampal and cortical neurons, KChIP3 regulates intracellular calcium-induced Ca2+ release through direct protein–protein interaction with the ryanodine receptor [158]. Furthermore, KChIP3 appears to be involved in age-related brain degeneration [140], which is a well-known clinical symptom of AD. Although the current molecular understanding of the relationship between KChIP3 and AD pathogenesis is limited and insufficient, these findings clearly support KChIP3 as an appropriate target for further research into AD. Therefore, unravelling the diversity and complexity of KChIP3’s functions would pave the way for developing more effective treatments for AD.
4.1.5. Other Neurodegenerative Disorders
In addition to AD, KChIP3 has been implicated in the pathogenesis of other neurodegenerative disorders. Recent evidence suggests that KChIP3 is closely linked to Huntington’s disease (HD) [159] and amyotrophic lateral sclerosis (ASL) [160]. Firstly, the levels of KChIP3 protein are significantly reduced in the hippocampus samples of HD patients. To be specific, KChIP3 interacts with activating transcription factor 6 (ATF6) to suppress the pro-survival unfolded protein response, a common feature of neurodegenerative diseases. In the mouse model of HD, inhibition of the KChIP3-ATF6 interaction delays the onset of cognitive deficits. In addition, treatment with repaglinide has been shown to delay the onset and progression of motor and cognitive decline and extend lifespan by blocking the interaction of KChIP3 and ATF6 [74,159], suggesting that KChIP3 may be a novel target for the treatment of related diseases. Second, upregulation of KChIP3 has been observed in motor neurons and astrocytes in the spinal cord and frontal cortex of ALS patients [160]. The main feature of ALS is the relentless loss of motor neurons and increased reactive astrogliosis [161]. Cebolla et al. showed that KChIP3 is able to promote astrocyte differentiation of cortical precursors via cAMP-dependent calcium signaling. The neonatal cortex of Kcnip3−/− mice has a reduced number of astrocytes and an increased number of neurons [108]. Nevertheless, studies linking KChIP3 to these diseases have been limited. More evidence is needed to elucidate the mechanisms by which KChIP3 is involved in these neurodegenerative diseases.
4.2. Cardiovascular Diseases
4.2.1. Arrhythmias
Cardiac arrhythmias are classified mechanistically into two categories: focal activity due to enhanced or abnormal pulse generation and reentry due to conduction disturbances [162]. Early afterdepolarizations preceding full repolarization and delayed afterdepolarizations occurring after full repolarization are the most common causes of focal arrhythmias. Prolongation of action potentials caused by INa,L (late Na+ current), the ICa,L, or INCX, or decreases in the repolarizing potassium currents (IKr, IKs, IK1) can lead to early afterdepolarizations. As mentioned above, KChIPs have been shown to regulate a wide variety of ion channels in cardiomyocytes, including those that control the depolarization and repolarization of cardiac action potentials. Thus, KChIPs are critically involved in the pathogenesis of arrhythmias, as demonstrated by some of the available evidence, either directly or indirectly. For example, KChIP2 was upregulated in aging porcine atria [163] and was significantly reduced in TLR4-activated inflammatory responses [164] and chronic NMDAR activation [165]. Atrial fibrillation could be induced in zebrafish hearts overexpressing KCNIP1 [13]. Consistently, Kcnip2−/− mice have an increased susceptibility to arrhythmias, manifested by a prolonged elevation in the ST segment on the electrocardiogram [166]. In fact, the ST segment coincides in time with the action potential plateau, and increasing outward current (mainly Ito and IK-ATP) or decreasing inward current (mainly INa and ICa) can promote the occurrence of ST segment elevation [167]. Therefore, Kcnip2−/− mice may have compensatory remodeling against the loss of Ito. Mechanistically, KChIP2 on the one hand directly regulates the subcellular localization and gating properties of several ion channels as an auxiliary subunit in the cardiomyocyte. On the other hand, KChIP2 also controls the expression of ion channel subunits at the transcriptional level. In ventricular myocytes from Kcnip2−/− mice, Ito,f and INa are abolished, ICa,L is downregulated, and IKs and Ito,s (the slow transient outward K+ current) are upregulated [87,92,168]. Recently, KChIP2 was reported to act as a transcriptional repressor by binding directly to the promoters of miR-34b/c, a miRNA that directly affects SCN5A (NaV1.5), SCN1B (NaVβ1), and KCND3 (KV4.3) gene expression. Inhibition of miR-34b/c can block the induction of arrhythmia [169]. Whether there are other potential ion channel proteins, or whether the genes encoding these ion channels are regulated by KChIP2, is a topic that deserves further investigation. Due to the multiple roles of KChIP2 in regulating ion channels in the heart, a thorough understanding of the detailed molecular mechanisms by which abnormal KChIP2 levels increase arrhythmias susceptibility is key to the development of novel therapies for the prevention and treatment of cardiac arrhythmias.
4.2.2. Cardiac Remodeling
Cardiac remodeling is defined as persistent changes in cardiac structure and function in response to physiological or pathological stimuli. Pathophysiological events that cause a decrease in contractility and/or an increase in wall stress, such as ischemia/reperfusion, myocardial infarction, pressure and volume overload, hypertension, and neuroendocrine stimulation, often result in adverse cardiac remodeling [170]. KChIP2 expression is altered in many cardiovascular events, including ischemic cardiomyopathy [171], myocarditis [172], mitral valve disease [173], inflammatory cytokine-induced myocardial injury [50,174], myocardial infarction [175], and type 2 diabetes mellitus [176]. Previous studies have shown that KChIP2 expression is significantly downregulated in hypertrophic myocardium, which is associated with reduced Ito [166,177]. Some of these mechanisms have been validated in vitro models of hypertrophy. For example, overactivation of the JNK pathway was found to cause downregulation of KChIP2 in neonatal ventricular myocytes treated with phenylephrine, a robust inducer of hypertrophy [46]. In addition, our previous study demonstrated that the KChIP2 expression was decreased in phenylephrine-induced hypertrophic cardiomyocytes. Mechanistically, phenylephrine-activated NF-κB binds to the promoter region of the Kcnip2 gene and directly represses its transcription. Overexpression of muscle-specific mitsugumin 53 upregulates KChIP2 through inhibition of NF-κB and thereby reversed phenylephrine-induced cardiomyocyte hypertrophy [43]. In addition, Jin et al. found that adenoviral overexpression of KChIP2 in vivo significantly attenuated the development of left ventricular hypertrophy in aortic-banded rats. This protective effect of KChIP2 was achieved by inhibiting MAPK signaling activity and reducing calcineurin/NFAT expression [178].
Cardiac memory is a specific form of cardiac remodeling that is manifested by the persistence of inverted T waves after the restoration of sinus rhythm. The T wave “remembers” the QRS complex from the paced or arrhythmia phase following a short alteration in the sequence of ventricular depolarization caused by pacing or arrhythmia [179]. An important electrophysiological mechanism responsible for cardiac memory is the reduction in Ito density and its significantly prolonged recovery from inactivation [180]. The decreased expression of KChIP2 during this phase is essential for the occurrence of cardiac memory. According to Ozgen et al., left ventricular pacing induces the degradation of the transcription factor CREB by initiating the production of myocardial angiotensin II and the synthesis of reactive oxygen species, which results in the downregulation of KChIP2 expression [54].
4.2.3. Heart Failure
There is a growing number of evidence that KChIP2 also plays an important role in the pathogenesis of heart failure. The mRNA and protein expression of KChIP2 was significantly downregulated in the failing heart [181]. Meanwhile, autoantibodies against KChIP2 have been detected in patients with dilated cardiomyopathy. In vitro incubation of anti-KChIP2 antibody facilitates necrotic cell death in rat cardiomyocytes, suggesting that KChIP2 autoantibodies may be involved in the pathogenesis of dilated cardiomyopathy [182]. Candesartan, the angiotensin Ⅱ receptor blocker, attenuates KChIP2 downregulation in dilated cardiomyopathy and is involved in preventing severe electrical remodeling in inherited dilated cardiomyopathy [183]. Nassal et al. found that reduction of KChIP2 in guinea pig cardiomyocytes significantly increased ICa,L and prolonged action potentials by increasing CaV1.2 protein expression [184]. They also found that KChIP2, like the neuronal KChIP isoforms, can regulate ryanodine receptor activity by interacting with PS. Loss of KChIP2 resulted in reduced ryanodine receptor activity due to a decrease in its binding affinity to PS, which disrupted calcium-induced calcium release events. This further leads to impaired contractility of cardiomyocytes and promotes the onset of heart failure [185]. Interestingly, Speerschneider et al. showed that although KChIP2 is downregulated in heart failure, the reduction of Ito,f does not promote the development of heart failure. On the contrary, reduction of Ito exerts antiarrhythmic effects in mouse heart [186]. Likewise, Grubb et al. showed that while the downregulation of repolarization currents in heart failure was exacerbated in Kcnip2−/− mice, there was less prolongation of action potentials associated with heart failure due to compensation by upregulation of IKs and Ito,s [187]. In summary, the role of KChIP2 in heart failure remains controversial. Further research is needed to determine whether targeting KChIP2 has therapeutic implications in heart failure.
5. Concluding Remarks
KChIPs are collections of multifunctional proteins involved in a wide range of physiological processes and pathological diseases. Thanks to the contributions of many outstanding researchers in this field over the last 25 years, the understanding of their structure, regulation, biological function in health and diseases, and small-molecule drugs targeting KChIPs has been achieved. KChIPs are auxiliary subunits of KV4 channels and are critical for the trafficking and gating properties of KV4 channels. Meanwhile, KChIPs are also transcriptional regulators that repress the transcription of target genes in various systems throughout the body to maintain normal physiological functions, including the nervous, cardiovascular, respiratory, immune, and endocrine systems. To date, an increasing number of reports have partially linked dysregulation of KChIPs expression to disease. These include AD, HD, epilepsy, and memory dysfunction in the nervous system, arrhythmia, remodeling, and heart failure in the cardiovascular system. Some small-molecule drugs that regulate them have been shown to alleviate symptoms or provide therapeutic benefits in these diseases, most notably repaglinide, which has the most significant therapeutic effect in HD. In addition, many small molecule ligands targeting KChIPs have emerged in recent years, and basic research has shown good effects in activating or inhibiting KChIPs. However, our understanding of KChIPs, a class of proteins with similar functions but different characteristics, is still only the tip of the iceberg. There are still many questions that researchers need to answer. For example, the structural basis for the regulation of KV4 channels by KChIPs as auxiliary subunits remains elusive. The regulatory function of KChIP2 on KV1.5 and NaV1.5 found in the heterologous expression system has not yet been verified in vivo. Altered expression of KChIPs has been found in many clinical disease samples, but the elucidation of their causal relationship to these diseases and the mechanisms involved is far from complete. In addition, some conflicting results that have been reported in this field further confirm the functional complexity of KChIPs. For example, the role of KChIP3 in pain appears to differ between rats and mice. Small-molecule compounds such as NS5806, a ligand for KChIP2, has different effects in different species or even different parts of the same species, which will significantly hamper the development of targeted therapies. Therefore, despite the development of several small molecules targeting KChIPs, further efforts are needed to demonstrate their translational value in treating disease.
Author Contributions
Conceptualization, J.L.; original draft preparation, L.-Y.W. and Y.-J.S.; writing—review and editing, C.-L.Z. and J.L.; visualization, L.-Y.W. and Y.-J.S.; supervision, C.-L.Z. and J.L.; funding acquisition, C.-L.Z. and J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Natural Science Foundation of China (81970250, 32271151, 82000056), National Science Foundation of Guangdong Province Grants (2022A1515012310), Shenzhen Key Laboratory of Metabolism and Cardiovascular Homeostasis (grant no.: ZDSYS20190902092903237), and Basic Research Foundation of Shenzhen Grants (JCYJ20210324094006018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rosati, B.; Pan, Z.; Lypen, S.; Wang, H.S.; Cohen, I.; Dixon, J.E.; McKinnon, D. Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. J. Physiol. 2001, 533, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Maniak, P.J.; Ingbar, D.H.; O’Grady, S.M. Adult alveolar epithelial cells express multiple subtypes of voltage-gated K+ channels that are located in apical membrane. Am. J. Physiol. Cell Physiol. 2003, 284, C1614–C1624. [Google Scholar] [CrossRef] [PubMed]
- Bonne, A.; Vreede, L.; Kuiper, R.P.; Bodmer, D.; Jansen, C.; Eleveld, M.; van Erp, F.; Arkesteijn, G.; Hoogerbrugge, N.; van Ravenswaaij, C.; et al. Mapping of constitutional translocation breakpoints in renal cell cancer patients: Identification of KCNIP4 as a candidate gene. Cancer Genet. Cytogenet. 2007, 179, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Amberg, G.C.; Koh, S.D.; Hatton, W.J.; Murray, K.J.; Monaghan, K.; Horowitz, B.; Sanders, K.M. Contribution of Kv4 channels toward the A-type potassium current in murine colonic myocytes. J. Physiol. 2002, 544, 403–415. [Google Scholar] [CrossRef]
- Beckett, E.A.; McCloskey, C.; O’Kane, N.; Sanders, K.M.; Koh, S.D. Effects of female steroid hormones on A-type K+ currents in murine colon. J. Physiol. 2006, 573, 453–468. [Google Scholar] [CrossRef]
- Zhang, Y.; MacLean, J.N.; An, W.F.; Lanning, C.C.; Harris-Warrick, R.M. KChIP1 and frequenin modify shal-evoked potassium currents in pyloric neurons in the lobster stomatogastric ganglion. J. Neurophysiol. 2003, 89, 1902–1909. [Google Scholar] [CrossRef]
- Buxbaum, J.D.; Choi, E.K.; Luo, Y.; Lilliehook, C.; Crowley, A.C.; Merriam, D.E.; Wasco, W. Calsenilin: A calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment. Nat. Med. 1998, 4, 1177–1181. [Google Scholar] [CrossRef]
- Carrión, A.M.; Link, W.A.; Ledo, F.; Mellström, B.; Naranjo, J.R. DREAM is a Ca2+-regulated transcriptional repressor. Nature 1999, 398, 80–84. [Google Scholar] [CrossRef]
- An, W.F.; Bowlby, M.R.; Betty, M.; Cao, J.; Ling, H.P.; Mendoza, G.; Hinson, J.W.; Mattsson, K.I.; Strassle, B.W.; Trimmer, J.S.; et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature 2000, 403, 553–556. [Google Scholar] [CrossRef]
- Morohashi, Y.; Hatano, N.; Ohya, S.; Takikawa, R.; Watabiki, T.; Takasugi, N.; Imaizumi, Y.; Tomita, T.; Iwatsubo, T. Molecular cloning and characterization of CALP/KChIP4, a novel EF-hand protein interacting with presenilin 2 and voltage-gated potassium channel subunit Kv4. J. Biol. Chem. 2002, 277, 14965–14975. [Google Scholar] [CrossRef]
- Cho, J. Downstream Regulatory Element Antagonist Modulator (DREAM), a target for anti-thrombotic agents. Pharmacol. Res. 2017, 117, 283–287. [Google Scholar] [CrossRef]
- Cercós, P.; Peraza, D.A.; Benito-Bueno, A.; Socuéllamos, P.G.; Aziz-Nignan, A.; Arrechaga-Estévez, D.; Beato, E.; Peña-Acevedo, E.; Albert, A.; González-Vera, J.A.; et al. Pharmacological Approaches for the Modulation of the Potassium Channel K(V)4.x and KChIPs. Int. J. Mol. Sci. 2021, 22, 1419. [Google Scholar] [CrossRef]
- Tsai, C.T.; Hsieh, C.S.; Chang, S.N.; Chuang, E.Y.; Ueng, K.C.; Tsai, C.F.; Lin, T.H.; Wu, C.K.; Lee, J.K.; Lin, L.Y.; et al. Genome-wide screening identifies a KCNIP1 copy number variant as a genetic predictor for atrial fibrillation. Nat. Commun. 2016, 7, 10190. [Google Scholar] [CrossRef]
- Martinez-Pinna, J.; Marroqui, L.; Hmadcha, A.; Lopez-Beas, J.; Soriano, S.; Villar-Pazos, S.; Alonso-Magdalena, P.; Dos Santos, R.S.; Quesada, I.; Martin, F.; et al. Oestrogen receptor β mediates the actions of bisphenol-A on ion channel expression in mouse pancreatic beta cells. Diabetologia 2019, 62, 1667–1680. [Google Scholar] [CrossRef]
- Norris, A.J.; Foeger, N.C.; Nerbonne, J.M. Interdependent roles for accessory KChIP2, KChIP3, and KChIP4 subunits in the generation of Kv4-encoded IA channels in cortical pyramidal neurons. J. Neurosci. 2010, 30, 13644–13655. [Google Scholar] [CrossRef]
- Savignac, M.; Mellström, B.; Bébin, A.G.; Oliveros, J.C.; Delpy, L.; Pinaud, E.; Naranjo, J.R. Increased B cell proliferation and reduced Ig production in DREAM transgenic mice. J. Immunol. 2010, 185, 7527–7536. [Google Scholar] [CrossRef]
- Ronkainen, J.J.; Hänninen, S.L.; Korhonen, T.; Koivumäki, J.T.; Skoumal, R.; Rautio, S.; Ronkainen, V.P.; Tavi, P. Ca2+-calmodulin-dependent protein kinase II represses cardiac transcription of the L-type calcium channel alpha(1C)-subunit gene (Cacna1c) by DREAM translocation. J. Physiol. 2011, 589, 2669–2686. [Google Scholar] [CrossRef]
- Lee, J.Y.; Byun, J.Y.; Lee, S.H. Proteomic analysis of normal human nasal mucosa: Establishment of a two-dimensional electrophoresis reference map. Clin. Biochem. 2009, 42, 692–700. [Google Scholar] [CrossRef]
- O’Callaghan, D.W.; Hasdemir, B.; Leighton, M.; Burgoyne, R.D. Residues within the myristoylation motif determine intracellular targeting of the neuronal Ca2+ sensor protein KChIP1 to post-ER transport vesicles and traffic of Kv4 K+ channels. J. Cell Sci. 2003, 116, 4833–4845. [Google Scholar] [CrossRef]
- Takimoto, K.; Yang, E.K.; Conforti, L. Palmitoylation of KChIP splicing variants is required for efficient cell surface expression of Kv4.3 channels. J. Biol. Chem. 2002, 277, 26904–26911. [Google Scholar] [CrossRef]
- Ruiz-Gomez, A.; Mellström, B.; Tornero, D.; Morato, E.; Savignac, M.; Holguín, H.; Aurrekoetxea, K.; González, P.; González-García, C.; Ceña, V.; et al. G protein-coupled receptor kinase 2-mediated phosphorylation of downstream regulatory element antagonist modulator regulates membrane trafficking of Kv4.2 potassium channel. J. Biol. Chem. 2007, 282, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, M.; Casafont, I.; Ghimire, K.; Rojas, A.M.; Valencia, A.; Lafarga, M.; Mellström, B.; Naranjo, J.R. Sumoylation regulates nuclear localization of repressor DREAM. Biochim. Biophys. Acta 2011, 1813, 1050–1058. [Google Scholar] [CrossRef]
- Beck, E.J.; Bowlby, M.; An, W.F.; Rhodes, K.J.; Covarrubias, M. Remodelling inactivation gating of Kv4 channels by KChIP1, a small-molecular-weight calcium-binding protein. J. Physiol. 2002, 538, 691–706. [Google Scholar] [CrossRef]
- Nakamura, T.Y.; Nandi, S.; Pountney, D.J.; Artman, M.; Rudy, B.; Coetzee, W.A. Different effects of the Ca(2+)-binding protein, KChIP1, on two Kv4 subfamily members, Kv4.1 and Kv4.2. FEBS Lett. 2001, 499, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Holmqvist, M.H.; Cao, J.; Hernandez-Pineda, R.; Jacobson, M.D.; Carroll, K.I.; Sung, M.A.; Betty, M.; Ge, P.; Gilbride, K.J.; Brown, M.E.; et al. Elimination of fast inactivation in Kv4 A-type potassium channels by an auxiliary subunit domain. Proc. Natl. Acad. Sci. USA 2002, 99, 1035–1040. [Google Scholar] [CrossRef]
- Holmqvist, M.H.; Cao, J.; Knoppers, M.H.; Jurman, M.E.; Distefano, P.S.; Rhodes, K.J.; Xie, Y.; An, W.F. Kinetic modulation of Kv4-mediated A-current by arachidonic acid is dependent on potassium channel interacting proteins. J. Neurosci. 2001, 21, 4154–4161. [Google Scholar] [CrossRef]
- Bähring, R.; Dannenberg, J.; Peters, H.C.; Leicher, T.; Pongs, O.; Isbrandt, D. Conserved Kv4 N-terminal domain critical for effects of Kv channel-interacting protein 2.2 on channel expression and gating. J. Biol. Chem. 2001, 276, 23888–23894. [Google Scholar] [CrossRef]
- Matsuyoshi, H.; Takimoto, K.; Yunoki, T.; Erickson, V.L.; Tyagi, P.; Hirao, Y.; Wanaka, A.; Yoshimura, N. Distinct cellular distributions of Kv4 pore-forming and auxiliary subunits in rat dorsal root ganglion neurons. Life Sci. 2012, 91, 258–263. [Google Scholar] [CrossRef]
- Jerng, H.H.; Kunjilwar, K.; Pfaffinger, P.J. Multiprotein assembly of Kv4.2, KChIP3 and DPP10 produces ternary channel complexes with ISA-like properties. J. Physiol. 2005, 568, 767–788. [Google Scholar] [CrossRef] [PubMed]
- Jerng, H.H.; Pfaffinger, P.J. Multiple Kv channel-interacting proteins contain an N-terminal transmembrane domain that regulates Kv4 channel trafficking and gating. J. Biol. Chem. 2008, 283, 36046–36059. [Google Scholar] [CrossRef]
- Kunjilwar, K.; Strang, C.; DeRubeis, D.; Pfaffinger, P.J. KChIP3 rescues the functional expression of Shal channel tetramerization mutants. J. Biol. Chem. 2004, 279, 54542–54551. [Google Scholar] [CrossRef]
- Jerng, H.H.; Pfaffinger, P.J.; Covarrubias, M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol. Cell. Neurosci. 2004, 27, 343–369. [Google Scholar] [CrossRef]
- Alexander, J.C.; McDermott, C.M.; Tunur, T.; Rands, V.; Stelly, C.; Karhson, D.; Bowlby, M.R.; An, W.F.; Sweatt, J.D.; Schrader, L.A. The role of calsenilin/DREAM/KChIP3 in contextual fear conditioning. Learn. Mem. 2009, 16, 167–177. [Google Scholar] [CrossRef]
- Gonzalez, W.G.; Pham, K.; Miksovska, J. Modulation of the voltage-gated potassium channel (Kv4.3) and the auxiliary protein (KChIP3) interactions by the current activator NS5806. J. Biol. Chem. 2014, 289, 32201–32213. [Google Scholar] [CrossRef]
- Schwenk, J.; Zolles, G.; Kandias, N.G.; Neubauer, I.; Kalbacher, H.; Covarrubias, M.; Fakler, B.; Bentrop, D. NMR analysis of KChIP4a reveals structural basis for control of surface expression of Kv4 channel complexes. J. Biol. Chem. 2008, 283, 18937–18946. [Google Scholar] [CrossRef]
- Néant, I.; Haiech, J.; Kilhoffer, M.C.; Aulestia, F.J.; Moreau, M.; Leclerc, C. Ca(2+)-Dependent Transcriptional Repressors KCNIP and Regulation of Prognosis Genes in Glioblastoma. Front. Mol. Neurosci. 2018, 11, 472. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Liang, P.; Zhou, J.; Lu, Y.; Lei, L.; Bian, X.; Wang, K. Auxiliary KChIP4a suppresses A-type K+ current through endoplasmic reticulum (ER) retention and promoting closed-state inactivation of Kv4 channels. J. Biol. Chem. 2013, 288, 14727–14741. [Google Scholar] [CrossRef]
- Jerng, H.H.; Pfaffinger, P.J. Modulatory mechanisms and multiple functions of somatodendritic A-type K(+) channel auxiliary subunits. Front. Cell. Neurosci. 2014, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Bähring, R. Kv channel-interacting proteins as neuronal and non-neuronal calcium sensors. Channels 2018, 12, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.; Workman, S.W.; Jiang, M.; Hu, J.; Sifa, I.; Bernas, T.; Tang, W.; Deschenes, I.; Tseng, G.N. Dynamic palmitoylation regulates trafficking of K channel interacting protein 2 (KChIP2) across multiple subcellular compartments in cardiac myocytes. J. Mol. Cell. Cardiol. 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Choi, E.K.; Miller, J.S.; Zaidi, N.F.; Salih, E.; Buxbaum, J.D.; Wasco, W. Phosphorylation of calsenilin at Ser63 regulates its cleavage by caspase-3. Mol. Cell. Neurosci. 2003, 23, 495–506. [Google Scholar] [CrossRef]
- Liang, P.; Wang, H.; Chen, H.; Cui, Y.; Gu, L.; Chai, J.; Wang, K. Structural Insights into KChIP4a Modulation of Kv4.3 Inactivation. J. Biol. Chem. 2009, 284, 4960–4967. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, G.; Zhang, C.; Ding, W.; Cheng, W.; Luo, Y.; Wei, C.; Liu, J. MG53, A Novel Regulator of KChIP2 and I(to,f), Plays a Critical Role in Electrophysiological Remodeling in Cardiac Hypertrophy. Circulation 2019, 139, 2142–2156. [Google Scholar] [CrossRef]
- Wagner, S.; Hacker, E.; Grandi, E.; Weber, S.L.; Dybkova, N.; Sossalla, S.; Sowa, T.; Fabritz, L.; Kirchhof, P.; Bers, D.M.; et al. Ca/calmodulin kinase II differentially modulates potassium currents. Circ. Arrhythmia Electrophysiol. 2009, 2, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Rossow, C.F.; Dilly, K.W.; Santana, L.F. Differential calcineurin/NFATc3 activity contributes to the Ito transmural gradient in the mouse heart. Circ. Res. 2006, 98, 1306–1313. [Google Scholar] [CrossRef]
- Jia, Y.; Takimoto, K. Mitogen-activated protein kinases control cardiac KChIP2 gene expression. Circ. Res. 2006, 98, 386–393. [Google Scholar] [CrossRef]
- Huang, H.; Tang, Y.; Wu, G.; Mei, Y.; Liu, W.; Liu, X.; Wan, N.; Liu, Y.; Huang, C. ALK7 protects against pathological cardiac hypertrophy in mice. Cardiovasc. Res. 2015, 108, 50–61. [Google Scholar] [CrossRef]
- Ying, S.; Cao, H.; Hu, H.; Wang, X.; Tang, Y.; Huang, C. Alk7 Depleted Mice Exhibit Prolonged Cardiac Repolarization and Are Predisposed to Ventricular Arrhythmia. PLoS ONE 2016, 11, e0149205. [Google Scholar] [CrossRef]
- Borghetti, G.; Eisenberg, C.A.; Signore, S.; Sorrentino, A.; Kaur, K.; Andrade-Vicenty, A.; Edwards, J.G.; Nerkar, M.; Qanud, K.; Sun, D.; et al. Notch signaling modulates the electrical behavior of cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H68–H81. [Google Scholar] [CrossRef]
- Kaur, K.; Zarzoso, M.; Ponce-Balbuena, D.; Guerrero-Serna, G.; Hou, L.; Musa, H.; Jalife, J. TGF-β1, released by myofibroblasts, differentially regulates transcription and function of sodium and potassium channels in adult rat ventricular myocytes. PLoS ONE 2013, 8, e55391. [Google Scholar] [CrossRef] [PubMed]
- Panama, B.K.; Latour-Villamil, D.; Farman, G.P.; Zhao, D.; Bolz, S.S.; Kirshenbaum, L.A.; Backx, P.H. Nuclear factor kappaB downregulates the transient outward potassium current I(to,f) through control of KChIP2 expression. Circ. Res. 2011, 108, 537–543. [Google Scholar] [CrossRef]
- Panama, B.K.; Korogyi, A.S.; Aschar-Sobbi, R.; Oh, Y.; Gray, C.B.; Gang, H.; Brown, J.H.; Kirshenbaum, L.A.; Backx, P.H. Reductions in the Cardiac Transient Outward K+ Current Ito Caused by Chronic β-Adrenergic Receptor Stimulation Are Partly Rescued by Inhibition of Nuclear Factor κB. J. Biol. Chem. 2016, 291, 4156–4165. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mai, J.T.; Wang, F.; Lin, Y.Q.; Yuan, W.L.; Luo, N.S.; Fang, M.C.; Wang, J.F.; Chen, Y.X. Effects of C-reactive protein on K(+) channel interaction protein 2 in cardiomyocytes. Am. J. Transl. Res. 2015, 7, 922–931. [Google Scholar]
- Ozgen, N.; Lau, D.H.; Shlapakova, I.N.; Sherman, W.; Feinmark, S.J.; Danilo, P., Jr.; Rosen, M.R. Determinants of CREB degradation and KChIP2 gene transcription in cardiac memory. Heart Rhythm 2010, 7, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.K.; Liu, W.; Zi, M.; Li, Y.; Wang, S.; Tsui, H.; Prehar, S.; Castro, S.; Zhang, H.; Ji, Y.; et al. Stress-Activated Kinase Mitogen-Activated Kinase Kinase-7 Governs Epigenetics of Cardiac Repolarization for Arrhythmia Prevention. Circulation 2017, 135, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, D.; Haldar, S.M.; Wan, X.; McCauley, M.D.; Ripperger, J.A.; Hu, K.; Lu, Y.; Eapen, B.L.; Sharma, N.; Ficker, E.; et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 2012, 483, 96–99. [Google Scholar] [CrossRef]
- Menegola, M.; Trimmer, J.S. Unanticipated region- and cell-specific downregulation of individual KChIP auxiliary subunit isotypes in Kv4.2 knock-out mouse brain. J. Neurosci. 2006, 26, 12137–12142. [Google Scholar] [CrossRef]
- Nerbonne, J.M.; Gerber, B.R.; Norris, A.; Burkhalter, A. Electrical remodelling maintains firing properties in cortical pyramidal neurons lacking KCND2-encoded A-type K+ currents. J. Physiol. 2008, 586, 1565–1579. [Google Scholar] [CrossRef]
- Sun, W.; Maffie, J.K.; Lin, L.; Petralia, R.S.; Rudy, B.; Hoffman, D.A. DPP6 establishes the A-type K(+) current gradient critical for the regulation of dendritic excitability in CA1 hippocampal neurons. Neuron 2011, 71, 1102–1115. [Google Scholar] [CrossRef]
- Kuo, Y.L.; Cheng, J.K.; Hou, W.H.; Chang, Y.C.; Du, P.H.; Jian, J.J.; Rau, R.H.; Yang, J.H.; Lien, C.C.; Tsaur, M.L. K(+) Channel Modulatory Subunits KChIP and DPP Participate in Kv4-Mediated Mechanical Pain Control. J. Neurosci. 2017, 37, 4391–4404. [Google Scholar] [CrossRef]
- Mellström, B.; Sahún, I.; Ruiz-Nuño, A.; Murtra, P.; Gomez-Villafuertes, R.; Savignac, M.; Oliveros, J.C.; Gonzalez, P.; Kastanauskaite, A.; Knafo, S.; et al. DREAM controls the on/off switch of specific activity-dependent transcription pathways. Mol. Cell. Biol. 2014, 34, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Kise, Y.; Kasuya, G.; Okamoto, H.H.; Yamanouchi, D.; Kobayashi, K.; Kusakizako, T.; Nishizawa, T.; Nakajo, K.; Nureki, O. Structural basis of gating modulation of Kv4 channel complexes. Nature 2021, 599, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.P.; Campbell, D.L. Transient outward potassium current, ‘Ito’, phenotypes in the mammalian left ventricle: Underlying molecular, cellular and biophysical mechanisms. J. Physiol. 2005, 569, 7–39. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.A.; Furst, J.; Gutierrez, D.; Butler, M.H.; Xu, S.; Goldstein, S.A.; Grigorieff, N. Three-dimensional structure of I(to); Kv4.2-KChIP2 ion channels by electron microscopy at 21 Angstrom resolution. Neuron 2004, 41, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, Y.; Liu, Q.; Huang, Y.; Shen, Y.; Chen, L.; Chen, Y.; Yang, Q.; Hao, Q.; Wang, K.; et al. Structural basis for modulation of Kv4 K+ channels by auxiliary KChIP subunits. Nat. Neurosci. 2007, 10, 32–39. [Google Scholar] [CrossRef]
- Catte, A.; Ferbel, L.; Bhattacharjee, N.; Jan Akhunzada, M.; D’Agostino, T.; Brancato, G. In silico investigation of the interaction between the voltage-gated potassium channel Kv4.3 and its auxiliary protein KChIP1. Phys. Chem. Chem. Phys. 2019, 21, 25290–25301. [Google Scholar] [CrossRef]
- Fineberg, J.D.; Szanto, T.G.; Panyi, G.; Covarrubias, M. Closed-state inactivation involving an internal gate in Kv4.1 channels modulates pore blockade by intracellular quaternary ammonium ions. Sci. Rep. 2016, 6, 31131. [Google Scholar] [CrossRef]
- Patel, S.P.; Campbell, D.L.; Strauss, H.C. Elucidating KChIP effects on Kv4.3 inactivation and recovery kinetics with a minimal KChIP2 isoform. J. Physiol. 2002, 545, 5–11. [Google Scholar] [CrossRef]
- Ye, W.; Zhao, H.; Dai, Y.; Wang, Y.; Lo, Y.H.; Jan, L.Y.; Lee, C.H. Activation and closed-state inactivation mechanisms of the human voltage-gated K(V)4 channel complexes. Mol. Cell 2022, 82, 2427–2442.e2424. [Google Scholar] [CrossRef]
- Shibata, R.; Misonou, H.; Campomanes, C.R.; Anderson, A.E.; Schrader, L.A.; Doliveira, L.C.; Carroll, K.I.; Sweatt, J.D.; Rhodes, K.J.; Trimmer, J.S. A fundamental role for KChIPs in determining the molecular properties and trafficking of Kv4.2 potassium channels. J. Biol. Chem. 2003, 278, 36445–36454. [Google Scholar] [CrossRef]
- Foeger, N.C.; Marionneau, C.; Nerbonne, J.M. Co-assembly of Kv4 {alpha} subunits with K+ channel-interacting protein 2 stabilizes protein expression and promotes surface retention of channel complexes. J. Biol. Chem. 2010, 285, 33413–33422. [Google Scholar] [CrossRef]
- Li, Y.; Duan, H.; Yi, J.; Wang, G.; Cheng, W.; Feng, L.; Liu, J. Kv4.2 phosphorylation by PKA drives Kv4.2-KChIP2 dissociation, leading to Kv4.2 out of lipid rafts and internalization. Am. J. Physiol. Cell Physiol. 2022, 323, C190–C201. [Google Scholar] [CrossRef]
- Bowlby, M.R.; Chanda, P.; Edris, W.; Hinson, J.; Jow, F.; Katz, A.H.; Kennedy, J.; Krishnamurthy, G.; Pitts, K.; Ryan, K.; et al. Identification and characterization of small molecule modulators of KChIP/Kv4 function. Bioorg. Med. Chem. 2005, 13, 6112–6119. [Google Scholar] [CrossRef]
- Naranjo, J.R.; Zhang, H.; Villar, D.; González, P.; Dopazo, X.M.; Morón-Oset, J.; Higueras, E.; Oliveros, J.C.; Arrabal, M.D.; Prieto, A.; et al. Activating transcription factor 6 derepression mediates neuroprotection in Huntington disease. J. Clin. Investig. 2016, 126, 627–638. [Google Scholar] [CrossRef]
- Lopez-Hurtado, A.; Peraza, D.A.; Cercos, P.; Lagartera, L.; Gonzalez, P.; Dopazo, X.M.; Herranz, R.; Gonzalez, T.; Martin-Martinez, M.; Mellström, B.; et al. Targeting the neuronal calcium sensor DREAM with small-molecules for Huntington’s disease treatment. Sci. Rep. 2019, 9, 7260. [Google Scholar] [CrossRef]
- Peraza, D.A.; Cercós, P.; Miaja, P.; Merinero, Y.G.; Lagartera, L.; Socuéllamos, P.G.; Izquierdo García, C.; Sánchez, S.A.; López-Hurtado, A.; Martín-Martínez, M.; et al. Identification of IQM-266, a Novel DREAM Ligand That Modulates K(V)4 Currents. Front. Mol. Neurosci. 2019, 12, 11. [Google Scholar] [CrossRef]
- de Benito-Bueno, A.; Socuellamos, P.G.; Merinero, Y.G.; Cercos, P.; Izquierdo, C.; Daniel-Mozo, M.; Marín-Olivero, I.; Perez-Lara, A.; Gonzalez-Vera, J.A.; Orte, A.; et al. Modulation of K(V)4.3-KChIP2 Channels by IQM-266: Role of DPP6 and KCNE2. Int. J. Mol. Sci. 2022, 23, 9170. [Google Scholar] [CrossRef]
- Witzel, K.; Fischer, P.; Bähring, R. Hippocampal A-type current and Kv4.2 channel modulation by the sulfonylurea compound NS5806. Neuropharmacology 2012, 63, 1389–1403. [Google Scholar] [CrossRef]
- Calloe, K.; Soltysinska, E.; Jespersen, T.; Lundby, A.; Antzelevitch, C.; Olesen, S.P.; Cordeiro, J.M. Differential effects of the transient outward K(+) current activator NS5806 in the canine left ventricle. J. Mol. Cell. Cardiol. 2010, 48, 191–200. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Wang, C.; Wang, Y.; Zou, R.; Shi, C.; Guan, B.; Gamper, N.; Xu, Y. Auxiliary subunits control biophysical properties and response to compound NS5806 of the Kv4 potassium channel complex. FASEB J. 2020, 34, 807–821. [Google Scholar] [CrossRef]
- Cheng, H.; Cannell, M.B.; Hancox, J.C. Differential responses of rabbit ventricular and atrial transient outward current (I(to)) to the I(to) modulator NS5806. Physiol. Rep. 2017, 5, e13172. [Google Scholar] [CrossRef] [PubMed]
- Maqoud, F.; Scala, R.; Tragni, V.; Pierri, C.L.; Perrone, M.G.; Scilimati, A.; Tricarico, D. Zoledronic Acid as a Novel Dual Blocker of KIR6.1/2-SUR2 Subunits of ATP-Sensitive K(+) Channels: Role in the Adverse Drug Reactions. Pharmaceutics 2021, 13, 1350. [Google Scholar] [CrossRef] [PubMed]
- Maqoud, F.; Scala, R.; Hoxha, M.; Zappacosta, B.; Tricarico, D. ATP-sensitive Potassium Channel Subunits in Neuroinflammation: Novel Drug Targets in Neurodegenerative Disorders. CNS Neurol. Disord. Drug Targets 2022, 21, 130–149. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, M.; Wahl, P.; Holmes, W.E.; Ashcroft, F.M. Effect of repaglinide on cloned beta cell, cardiac and smooth muscle types of ATP-sensitive potassium channels. Diabetologia 2001, 44, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Ravens, U.; Wettwer, E. Ultra-rapid delayed rectifier channels: Molecular basis and therapeutic implications. Cardiovasc. Res. 2011, 89, 776–785. [Google Scholar] [CrossRef]
- Li, H.; Guo, W.; Mellor, R.L.; Nerbonne, J.M. KChIP2 modulates the cell surface expression of Kv 1.5-encoded K(+) channels. J. Mol. Cell. Cardiol. 2005, 39, 121–132. [Google Scholar] [CrossRef]
- Thomsen, M.B.; Sosunov, E.A.; Anyukhovsky, E.P.; Ozgen, N.; Boyden, P.A.; Rosen, M.R. Deleting the accessory subunit KChIP2 results in loss of I(to,f) and increased I(K,slow) that maintains normal action potential configuration. Heart Rhythm 2009, 6, 370–377. [Google Scholar] [CrossRef]
- Hofmann, F.; Flockerzi, V.; Kahl, S.; Wegener, J.W. L-type CaV1.2 calcium channels: From in vitro findings to in vivo function. Physiol. Rev. 2014, 94, 303–326. [Google Scholar] [CrossRef]
- Thomsen, M.B.; Wang, C.; Ozgen, N.; Wang, H.G.; Rosen, M.R.; Pitt, G.S. Accessory subunit KChIP2 modulates the cardiac L-type calcium current. Circ. Res. 2009, 104, 1382–1389. [Google Scholar] [CrossRef]
- Thomsen, M.B.; Foster, E.; Nguyen, K.H.; Sosunov, E.A. Transcriptional and electrophysiological consequences of KChIP2-mediated regulation of CaV1.2. Channels 2009, 3, 308–310. [Google Scholar] [CrossRef]
- Clatot, J.; Neyroud, N.; Cox, R.; Souil, C.; Huang, J.; Guicheney, P.; Antzelevitch, C. Inter-Regulation of K(v)4.3 and Voltage-Gated Sodium Channels Underlies Predisposition to Cardiac and Neuronal Channelopathies. Int. J. Mol. Sci. 2020, 21, 5057. [Google Scholar] [CrossRef]
- Deschênes, I.; Armoundas, A.A.; Jones, S.P.; Tomaselli, G.F. Post-transcriptional gene silencing of KChIP2 and Navbeta1 in neonatal rat cardiac myocytes reveals a functional association between Na and Ito currents. J. Mol. Cell. Cardiol. 2008, 45, 336–346. [Google Scholar] [CrossRef]
- Osawa, M.; Dace, A.; Tong, K.I.; Valiveti, A.; Ikura, M.; Ames, J.B. Mg2+ and Ca2+ differentially regulate DNA binding and dimerization of DREAM. J. Biol. Chem. 2005, 280, 18008–18014. [Google Scholar] [CrossRef]
- Lusin, J.D.; Vanarotti, M.; Li, C.; Valiveti, A.; Ames, J.B. NMR structure of DREAM: Implications for Ca(2+)-dependent DNA binding and protein dimerization. Biochemistry 2008, 47, 2252–2264. [Google Scholar] [CrossRef]
- Zaidi, N.F.; Kuplast, K.G.; Washicosky, K.J.; Kajiwara, Y.; Buxbaum, J.D.; Wasco, W. Calsenilin interacts with transcriptional co-repressor C-terminal binding protein(s). J. Neurochem. 2006, 98, 1290–1301. [Google Scholar] [CrossRef]
- Ledo, F.; Carrión, A.M.; Link, W.A.; Mellström, B.; Naranjo, J.R. DREAM-alphaCREM interaction via leucine-charged domains derepresses downstream regulatory element-dependent transcription. Mol. Cell. Biol. 2000, 20, 9120–9126. [Google Scholar] [CrossRef]
- Ledo, F.; Kremer, L.; Mellström, B.; Naranjo, J.R. Ca2+-dependent block of CREB-CBP transcription by repressor DREAM. EMBO J. 2002, 21, 4583–4592. [Google Scholar] [CrossRef]
- Rivas, M.; Mellström, B.; Naranjo, J.R.; Santisteban, P. Transcriptional repressor DREAM interacts with thyroid transcription factor-1 and regulates thyroglobulin gene expression. J. Biol. Chem. 2004, 279, 33114–33122. [Google Scholar] [CrossRef]
- Scsucova, S.; Palacios, D.; Savignac, M.; Mellström, B.; Naranjo, J.R.; Aranda, A. The repressor DREAM acts as a transcriptional activator on Vitamin D and retinoic acid response elements. Nucleic Acids Res. 2005, 33, 2269–2279. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Pitcher, G.M.; Laviolette, S.R.; Whishaw, I.Q.; Tong, K.I.; Kockeritz, L.K.; Wada, T.; Joza, N.A.; Crackower, M.; Goncalves, J.; et al. DREAM is a critical transcriptional repressor for pain modulation. Cell 2002, 108, 31–43. [Google Scholar] [CrossRef]
- Sanz, C.; Mellstrom, B.; Link, W.A.; Naranjo, J.R.; Fernandez-Luna, J.L. Interleukin 3-dependent activation of DREAM is involved in transcriptional silencing of the apoptotic Hrk gene in hematopoietic progenitor cells. EMBO J. 2001, 20, 2286–2292. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Arconada, I.; Benedet, T.; Roza, C.; Torres, B.; Barrio, J.; Krzyzanowska, A.; Avendaño, C.; Mellström, B.; Lopez-Garcia, J.A.; Naranjo, J.R. DREAM regulates BDNF-dependent spinal sensitization. Mol. Pain 2010, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Savignac, M.; Pintado, B.; Gutierrez-Adan, A.; Palczewska, M.; Mellström, B.; Naranjo, J.R. Transcriptional repressor DREAM regulates T-lymphocyte proliferation and cytokine gene expression. EMBO J. 2005, 24, 3555–3564. [Google Scholar] [CrossRef] [PubMed]
- Tiruppathi, C.; Soni, D.; Wang, D.M.; Xue, J.; Singh, V.; Thippegowda, P.B.; Cheppudira, B.P.; Mishra, R.K.; Debroy, A.; Qian, Z.; et al. The transcription factor DREAM represses the deubiquitinase A20 and mediates inflammation. Nat. Immunol. 2014, 15, 239–247. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, B.; Di Palma, T.; Mascia, A.; Motti, M.L.; Viglietto, G.; Nitsch, L.; Zannini, M. The transcriptional repressor DREAM is involved in thyroid gene expression. Exp. Cell Res. 2005, 305, 166–178. [Google Scholar] [CrossRef]
- Link, W.A.; Ledo, F.; Torres, B.; Palczewska, M.; Madsen, T.M.; Savignac, M.; Albar, J.P.; Mellström, B.; Naranjo, J.R. Day-night changes in downstream regulatory element antagonist modulator/potassium channel interacting protein activity contribute to circadian gene expression in pineal gland. J. Neurosci. 2004, 24, 5346–5355. [Google Scholar] [CrossRef]
- Leclerc, G.M.; Boockfor, F.R. Calcium influx and DREAM protein are required for GnRH gene expression pulse activity. Mol. Cell. Endocrinol. 2007, 267, 70–79. [Google Scholar] [CrossRef]
- Cebolla, B.; Fernández-Pérez, A.; Perea, G.; Araque, A.; Vallejo, M. DREAM mediates cAMP-dependent, Ca2+-induced stimulation of GFAP gene expression and regulates cortical astrogliogenesis. J. Neurosci. 2008, 28, 6703–6713. [Google Scholar] [CrossRef]
- Gomez-Villafuertes, R.; Torres, B.; Barrio, J.; Savignac, M.; Gabellini, N.; Rizzato, F.; Pintado, B.; Gutierrez-Adan, A.; Mellström, B.; Carafoli, E.; et al. Downstream regulatory element antagonist modulator regulates Ca2+ homeostasis and viability in cerebellar neurons. J. Neurosci. 2005, 25, 10822–10830. [Google Scholar] [CrossRef]
- Dierssen, M.; Fedrizzi, L.; Gomez-Villafuertes, R.; de Lagran, M.M.; Gutierrez-Adan, A.; Sahún, I.; Pintado, B.; Oliveros, J.C.; Dopazo, X.M.; Gonzalez, P.; et al. Reduced Mid1 Expression and Delayed Neuromotor Development in daDREAM Transgenic Mice. Front. Mol. Neurosci. 2012, 5, 58. [Google Scholar] [CrossRef]
- Calì, T.; Fedrizzi, L.; Ottolini, D.; Gomez-Villafuertes, R.; Mellström, B.; Naranjo, J.R.; Carafoli, E.; Brini, M. Ca2+-activated nucleotidase 1, a novel target gene for the transcriptional repressor DREAM (downstream regulatory element antagonist modulator), is involved in protein folding and degradation. J. Biol. Chem. 2012, 287, 18478–18491. [Google Scholar] [CrossRef] [PubMed]
- Fontán-Lozano, A.; Capilla-Gonzalez, V.; Aguilera, Y.; Mellado, N.; Carrión, A.M.; Soria, B.; Hmadcha, A. Impact of transient down-regulation of DREAM in human embryonic stem cell pluripotency: The role of DREAM in the maintenance of hESCs. Stem Cell Res. 2016, 16, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Baczyk, D.; Kibschull, M.; Mellstrom, B.; Levytska, K.; Rivas, M.; Drewlo, S.; Lye, S.J.; Naranjo, J.R.; Kingdom, J.C. DREAM mediated regulation of GCM1 in the human placental trophoblast. PLoS ONE 2013, 8, e51837. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, D.A.; Cho, J.; Landa, L.R., Jr.; Tamarina, N.A.; Roe, M.W.; Buxbaum, J.D.; Philipson, L.H. Downstream regulatory element antagonistic modulator regulates islet prodynorphin expression. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E587–E595. [Google Scholar] [CrossRef]
- Li, J.; Kumari, T.; Barazia, A.; Jha, V.; Jeong, S.Y.; Olson, A.; Kim, M.; Lee, B.K.; Manickam, V.; Song, Z.; et al. Neutrophil DREAM promotes neutrophil recruitment in vascular inflammation. J. Exp. Med. 2022, 219, e20211083. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshé, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef]
- Maizuliana, H.; Usui, N.; Terada, K.; Kondo, A.; Inoue, Y. Clinical, semiological, electroencephalographic, and neuropsychological features of "pure" neocortical temporal lobe epilepsy. Epileptic Disord. 2020, 22, 55–65. [Google Scholar] [CrossRef]
- Maillard, L.; Vignal, J.P.; Gavaret, M.; Guye, M.; Biraben, A.; McGonigal, A.; Chauvel, P.; Bartolomei, F. Semiologic and electrophysiologic correlations in temporal lobe seizure subtypes. Epilepsia 2004, 45, 1590–1599. [Google Scholar] [CrossRef]
- Villa, C.; Combi, R. Potassium Channels and Human Epileptic Phenotypes: An Updated Overview. Front. Cell. Neurosci. 2016, 10, 81. [Google Scholar] [CrossRef]
- Hong, Y.M.; Jo, D.G.; Lee, M.C.; Kim, S.Y.; Jung, Y.K. Reduced expression of calsenilin/DREAM/KChIP3 in the brains of kainic acid-induced seizure and epilepsy patients. Neurosci. Lett. 2003, 340, 33–36. [Google Scholar] [CrossRef]
- Monaghan, M.M.; Menegola, M.; Vacher, H.; Rhodes, K.J.; Trimmer, J.S. Altered expression and localization of hippocampal A-type potassium channel subunits in the pilocarpine-induced model of temporal lobe epilepsy. Neuroscience 2008, 156, 550–562. [Google Scholar] [CrossRef]
- Wang, H.G.; He, X.P.; Li, Q.; Madison, R.D.; Moore, S.D.; McNamara, J.O.; Pitt, G.S. The auxiliary subunit KChIP2 is an essential regulator of homeostatic excitability. J. Biol. Chem. 2013, 288, 13258–13268. [Google Scholar] [CrossRef]
- Kuner, R. Central mechanisms of pathological pain. Nat. Med. 2010, 16, 1258–1266. [Google Scholar] [CrossRef]
- Costigan, M.; Woolf, C.J. No DREAM, No pain. Closing the spinal gate. Cell 2002, 108, 297–300. [Google Scholar] [CrossRef]
- Tseng, P.Y.; Hoon, M.A. Molecular Genetics of Kappa Opioids in Pain and Itch Sensations. Handb. Exp. Pharmacol. 2022, 271, 255–274. [Google Scholar] [CrossRef]
- Ren, K.; Dubner, R. Pain facilitation and activity-dependent plasticity in pain modulatory circuitry: Role of BDNF-TrkB signaling and NMDA receptors. Mol. Neurobiol. 2007, 35, 224–235. [Google Scholar] [CrossRef]
- Benedet, T.; Gonzalez, P.; Oliveros, J.C.; Dopazo, J.M.; Ghimire, K.; Palczewska, M.; Mellstrom, B.; Naranjo, J.R. Transcriptional repressor DREAM regulates trigeminal noxious perception. J. Neurochem. 2017, 141, 544–552. [Google Scholar] [CrossRef]
- Long, I.; Suppian, R.; Ismail, Z. Increases in mRNA and DREAM protein expression in the rat spinal cord after formalin induced pain. Neurochem. Res. 2011, 36, 533–539. [Google Scholar] [CrossRef]
- Ismail, C.A.N.; Suppian, R.; Aziz, C.B.A.; Long, I. Minocycline attenuates the development of diabetic neuropathy by modulating DREAM and BDNF protein expression in rat spinal cord. J. Diabetes Metab. Disord. 2019, 18, 181–190. [Google Scholar] [CrossRef]
- Guo, Y.P.; Zhi, Y.R.; Liu, T.T.; Wang, Y.; Zhang, Y. Global Gene Knockout of Kcnip3 Enhances Pain Sensitivity and Exacerbates Negative Emotions in Rats. Front. Mol. Neurosci. 2019, 12, 5. [Google Scholar] [CrossRef]
- Tian, N.X.; Xu, Y.; Yang, J.Y.; Li, L.; Sun, X.H.; Wang, Y.; Zhang, Y. KChIP3 N-Terminal 31-50 Fragment Mediates Its Association with TRPV1 and Alleviates Inflammatory Hyperalgesia in Rats. J. Neurosci. 2018, 38, 1756–1773. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.F.; Wang, W.C.; Huang, C.Y.; Du, P.H.; Yang, J.H.; Tsaur, M.L. Coexpression of auxiliary subunits KChIP and DPPL in potassium channel Kv4-positive nociceptors and pain-modulating spinal interneurons. J. Comp. Neurol. 2016, 524, 846–873. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, P.; Liang, P.; Liu, T.; Liu, X.; Liu, X.Y.; Zhang, B.; Han, T.; Zhu, Y.B.; Yin, D.M.; et al. The DREAM protein negatively regulates the NMDA receptor through interaction with the NR1 subunit. J. Neurosci. 2010, 30, 7575–7586. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.; Mehaffey, W.H.; Iftinca, M.; Rehak, R.; Engbers, J.D.; Hameed, S.; Zamponi, G.W.; Turner, R.W. Regulation of neuronal activity by Cav3-Kv4 channel signaling complexes. Nat. Neurosci. 2010, 13, 333–337. [Google Scholar] [CrossRef]
- Socuéllamos, P.G.; Olivos-Oré, L.A.; Barahona, M.V.; Cercós, P.; Pérez Pascual, M.; Arribas-Blázquez, M.; Naranjo, J.R.; Valenzuela, C.; Gutiérrez-Rodríguez, M.; Artalejo, A.R. IQM-PC332, a Novel DREAM Ligand with Antinociceptive Effect on Peripheral Nerve Injury-Induced Pain. Int. J. Mol. Sci. 2022, 23, 2142. [Google Scholar] [CrossRef]
- Ma, S.; Zuo, Y. Synaptic modifications in learning and memory—A dendritic spine story. Semin. Cell Dev. Biol. 2022, 125, 84–90. [Google Scholar] [CrossRef]
- Bin Ibrahim, M.Z.; Benoy, A.; Sajikumar, S. Long-term plasticity in the hippocampus: Maintaining within and ‘tagging’ between synapses. FEBS J. 2022, 289, 2176–2201. [Google Scholar] [CrossRef]
- Lisman, J.; Buzsáki, G.; Eichenbaum, H.; Nadel, L.; Ranganath, C.; Redish, A.D. Viewpoints: How the hippocampus contributes to memory, navigation and cognition. Nat. Neurosci. 2017, 20, 1434–1447. [Google Scholar] [CrossRef]
- Fontán-Lozano, A.; Romero-Granados, R.; del-Pozo-Martín, Y.; Suárez-Pereira, I.; Delgado-García, J.M.; Penninger, J.M.; Carrión, A.M. Lack of DREAM protein enhances learning and memory and slows brain aging. Curr. Biol. 2009, 19, 54–60. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Li, X.H.; Zhuo, M. NMDA receptors and synaptic plasticity in the anterior cingulate cortex. Neuropharmacology 2021, 197, 108749. [Google Scholar] [CrossRef]
- Dore, K.; Malinow, R. Elevated PSD-95 Blocks Ion-flux Independent LTD: A Potential New Role for PSD-95 in Synaptic Plasticity. Neuroscience 2021, 456, 43–49. [Google Scholar] [CrossRef]
- Wu, L.J.; Mellström, B.; Wang, H.; Ren, M.; Domingo, S.; Kim, S.S.; Li, X.Y.; Chen, T.; Naranjo, J.R.; Zhuo, M. DREAM (downstream regulatory element antagonist modulator) contributes to synaptic depression and contextual fear memory. Mol. Brain 2010, 3, 3. [Google Scholar] [CrossRef]
- Lugo, J.N.; Brewster, A.L.; Spencer, C.M.; Anderson, A.E. Kv4.2 knockout mice have hippocampal-dependent learning and memory deficits. Learn. Mem. 2012, 19, 182–189. [Google Scholar] [CrossRef]
- Soria Lopez, J.A.; González, H.M.; Léger, G.C. Alzheimer’s disease. Handb. Clin. Neurol. 2019, 167, 231–255. [Google Scholar] [CrossRef]
- Robakis, N.K. Cell signaling abnormalities may drive neurodegeneration in familial Alzheimer disease. Neurochem. Res. 2014, 39, 570–575. [Google Scholar] [CrossRef]
- Pham, K.; Miksovska, J. Molecular insight of DREAM and presenilin 1 C-terminal fragment interactions. FEBS Lett. 2016, 590, 1114–1122. [Google Scholar] [CrossRef]
- Levy-Lahad, E.; Wasco, W.; Poorkaj, P.; Romano, D.M.; Oshima, J.; Pettingell, W.H.; Yu, C.E.; Jondro, P.D.; Schmidt, S.D.; Wang, K.; et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 1995, 269, 973–977. [Google Scholar] [CrossRef]
- Jo, D.G.; Lee, J.Y.; Hong, Y.M.; Song, S.; Mook-Jung, I.; Koh, J.Y.; Jung, Y.K. Induction of pro-apoptotic calsenilin/DREAM/KChIP3 in Alzheimer’s disease and cultured neurons after amyloid-beta exposure. J. Neurochem. 2004, 88, 604–611. [Google Scholar] [CrossRef]
- Zaidi, N.F.; Berezovska, O.; Choi, E.K.; Miller, J.S.; Chan, H.; Lilliehook, C.; Hyman, B.T.; Buxbaum, J.D.; Wasco, W. Biochemical and immunocytochemical characterization of calsenilin in mouse brain. Neuroscience 2002, 114, 247–263. [Google Scholar] [CrossRef]
- Jo, D.G.; Chang, J.W.; Hong, H.S.; Mook-Jung, I.; Jung, Y.K. Contribution of presenilin/gamma-secretase to calsenilin-mediated apoptosis. Biochem. Biophys. Res. Commun. 2003, 305, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.G.; Jang, J.; Kim, B.J.; Lundkvist, J.; Jung, Y.K. Overexpression of calsenilin enhances gamma-secretase activity. Neurosci. Lett. 2005, 378, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, R.; González, P.; Lopez-Hurtado, A.; Dopazo, X.M.; Mellström, B.; Naranjo, J.R. Inhibition of the Neuronal Calcium Sensor DREAM Modulates Presenilin-2 Endoproteolysis. Front. Mol. Neurosci. 2018, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Cascella, R.; Cecchi, C. Calcium Dyshomeostasis in Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4914. [Google Scholar] [CrossRef] [PubMed]
- Ferreiro, E.; Resende, R.; Costa, R.; Oliveira, C.R.; Pereira, C.M. An endoplasmic-reticulum-specific apoptotic pathway is involved in prion and amyloid-beta peptides neurotoxicity. Neurobiol. Dis. 2006, 23, 669–678. [Google Scholar] [CrossRef]
- Lilliehook, C.; Chan, S.; Choi, E.K.; Zaidi, N.F.; Wasco, W.; Mattson, M.P.; Buxbaum, J.D. Calsenilin enhances apoptosis by altering endoplasmic reticulum calcium signaling. Mol. Cell. Neurosci. 2002, 19, 552–559. [Google Scholar] [CrossRef]
- Fedrizzi, L.; Lim, D.; Carafoli, E.; Brini, M. Interplay of the Ca2+-binding protein DREAM with presenilin in neuronal Ca2+ signaling. J. Biol. Chem. 2008, 283, 27494–27503. [Google Scholar] [CrossRef]
- Grillo, M.A.; Grillo, S.L.; Gerdes, B.C.; Kraus, J.G.; Koulen, P. Control of Neuronal Ryanodine Receptor-Mediated Calcium Signaling by Calsenilin. Mol. Neurobiol. 2019, 56, 525–534. [Google Scholar] [CrossRef]
- López-Hurtado, A.; Burgos, D.F.; González, P.; Dopazo, X.M.; González, V.; Rábano, A.; Mellström, B.; Naranjo, J.R. Inhibition of DREAM-ATF6 interaction delays onset of cognition deficit in a mouse model of Huntington’s disease. Mol. Brain 2018, 11, 13. [Google Scholar] [CrossRef]
- Larrodé, P.; Calvo, A.C.; Moreno-Martínez, L.; de la Torre, M.; Moreno-García, L.; Molina, N.; Castiella, T.; Iñiguez, C.; Pascual, L.F.; Mena, F.J.M.; et al. DREAM-Dependent Activation of Astrocytes in Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2018, 55, 1–12. [Google Scholar] [CrossRef]
- Singh, D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J. Neuroinflam. 2022, 19, 206. [Google Scholar] [CrossRef]
- Tse, G. Mechanisms of cardiac arrhythmias. J. Arrhythmia 2016, 32, 75–81. [Google Scholar] [CrossRef]
- Kant, R.; Hu, Z.; Malhotra, J.K.; Krogh-Madsen, T.; Christini, D.J.; Heerdt, P.M.; Abbott, G.W. NHE isoform switching and KChIP2 upregulation in aging porcine atria. PLoS ONE 2013, 8, e82951. [Google Scholar] [CrossRef]
- Monnerat-Cahli, G.; Alonso, H.; Gallego, M.; Alarcón, M.L.; Bassani, R.A.; Casis, O.; Medei, E. Toll-like receptor 4 activation promotes cardiac arrhythmias by decreasing the transient outward potassium current (Ito) through an IRF3-dependent and MyD88-independent pathway. J. Mol. Cell. Cardiol. 2014, 76, 116–125. [Google Scholar] [CrossRef]
- Shi, S.; Liu, T.; Li, Y.; Qin, M.; Tang, Y.; Shen, J.Y.; Liang, J.; Yang, B.; Huang, C. Chronic N-methyl-D-aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. Pacing Clin. Electrophysiol. 2014, 37, 1367–1377. [Google Scholar] [CrossRef]
- Kuo, H.C.; Cheng, C.F.; Clark, R.B.; Lin, J.J.; Lin, J.L.; Hoshijima, M.; Nguyêñ-Trân, V.T.; Gu, Y.; Ikeda, Y.; Chu, P.H.; et al. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell 2001, 107, 801–813. [Google Scholar] [CrossRef]
- Hlaing, T.; DiMino, T.; Kowey, P.R.; Yan, G.X. ECG repolarization waves: Their genesis and clinical implications. Ann. Noninvasive Electrocardiol. 2005, 10, 211–223. [Google Scholar] [CrossRef]
- Foeger, N.C.; Wang, W.; Mellor, R.L.; Nerbonne, J.M. Stabilization of Kv4 protein by the accessory K(+) channel interacting protein 2 (KChIP2) subunit is required for the generation of native myocardial fast transient outward K(+) currents. J. Physiol. 2013, 591, 4149–4166. [Google Scholar] [CrossRef]
- Nassal, D.M.; Wan, X.; Liu, H.; Maleski, D.; Ramirez-Navarro, A.; Moravec, C.S.; Ficker, E.; Laurita, K.R.; Deschênes, I. KChIP2 is a core transcriptional regulator of cardiac excitability. eLife 2017, 6, e17304. [Google Scholar] [CrossRef]
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling--concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef]
- Liu, X.S.; Jiang, M.; Zhang, M.; Tang, D.; Clemo, H.F.; Higgins, R.S.; Tseng, G.N. Electrical remodeling in a canine model of ischemic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H560–H571. [Google Scholar] [CrossRef] [PubMed]
- Wakisaka, Y.; Niwano, S.; Niwano, H.; Saito, J.; Yoshida, T.; Hirasawa, S.; Kawada, H.; Izumi, T. Structural and electrical ventricular remodeling in rat acute myocarditis and subsequent heart failure. Cardiovasc. Res. 2004, 63, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Kim, K.B.; Ahn, H.; Cho, H.J.; Choi, Y.S. Remodeling of ion channel expression in patients with chronic atrial fibrillation and mitral valvular heart disease. Korean J. Intern. Med. 2010, 25, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Kawada, H.; Niwano, S.; Niwano, H.; Yumoto, Y.; Wakisaka, Y.; Yuge, M.; Kawahara, K.; Izumi, T. Tumor necrosis factor-alpha downregulates the voltage gated outward K+ current in cultured neonatal rat cardiomyocytes: A possible cause of electrical remodeling in diseased hearts. Circ. J. 2006, 70, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.Z.; Jiang, H.; Li, L.; Xie, P.; Li, J.Y.; Lu, Z.B.; He, B. Semaphorin 3A attenuates electrical remodeling at infarct border zones in rats after myocardial infarction. Tohoku J. Exp. Med. 2011, 225, 51–57. [Google Scholar] [CrossRef]
- Sato, T.; Kobayashi, T.; Kuno, A.; Miki, T.; Tanno, M.; Kouzu, H.; Itoh, T.; Ishikawa, S.; Kojima, T.; Miura, T.; et al. Type 2 diabetes induces subendocardium predominant reduction in transient outward K+ current with downregulation of Kv4.2 and KChIP2. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1054–H1065. [Google Scholar] [CrossRef]
- Näbauer, M.; Kääb, S. Potassium channel down-regulation in heart failure. Cardiovasc. Res. 1998, 37, 324–334. [Google Scholar] [CrossRef]
- Jin, H.; Hadri, L.; Palomeque, J.; Morel, C.; Karakikes, I.; Kaprielian, R.; Hajjar, R.; Lebeche, D. KChIP2 attenuates cardiac hypertrophy through regulation of Ito and intracellular calcium signaling. J. Mol. Cell. Cardiol. 2010, 48, 1169–1179. [Google Scholar] [CrossRef]
- Chiale, P.A.; Etcheverry, D.; Pastori, J.D.; Fernandez, P.A.; Garro, H.A.; González, M.D.; Elizari, M.V. The multiple electrocardiographic manifestations of ventricular repolarization memory. Curr. Cardiol. Rev. 2014, 10, 190–201. [Google Scholar] [CrossRef]
- Yu, H.; McKinnon, D.; Dixon, J.E.; Gao, J.; Wymore, R.; Cohen, I.S.; Danilo, P., Jr.; Shvilkin, A.; Anyukhovsky, E.P.; Sosunov, E.A.; et al. Transient outward current, Ito1, is altered in cardiac memory. Circulation 1999, 99, 1898–1905. [Google Scholar] [CrossRef]
- Radicke, S.; Cotella, D.; Graf, E.M.; Banse, U.; Jost, N.; Varró, A.; Tseng, G.N.; Ravens, U.; Wettwer, E. Functional modulation of the transient outward current Ito by KCNE beta-subunits and regional distribution in human non-failing and failing hearts. Cardiovasc. Res. 2006, 71, 695–703. [Google Scholar] [CrossRef]
- Choudhury, S.; Schnell, M.; Bühler, T.; Reinke, Y.; Lüdemann, J.; Nießner, F.; Brinkmeier, H.; Herda, L.R.; Staudt, A.; Kroemer, H.K.; et al. Antibodies against potassium channel interacting protein 2 induce necrosis in isolated rat cardiomyocytes. J. Cell. Biochem. 2014, 115, 678–689. [Google Scholar] [CrossRef]
- Odagiri, F.; Inoue, H.; Sugihara, M.; Suzuki, T.; Murayama, T.; Shioya, T.; Konishi, M.; Nakazato, Y.; Daida, H.; Sakurai, T.; et al. Effects of candesartan on electrical remodeling in the hearts of inherited dilated cardiomyopathy model mice. PLoS ONE 2014, 9, e101838. [Google Scholar] [CrossRef]
- Nassal, D.M.; Wan, X.; Liu, H.; Deschênes, I. Myocardial KChIP2 Expression in Guinea Pig Resolves an Expanded Electrophysiologic Role. PLoS ONE 2016, 11, e0146561. [Google Scholar] [CrossRef]
- Nassal, D.M.; Wan, X.; Liu, H.; Laurita, K.R.; Deschênes, I. KChIP2 regulates the cardiac Ca2+ transient and myocyte contractility by targeting ryanodine receptor activity. PLoS ONE 2017, 12, e0175221. [Google Scholar] [CrossRef]
- Speerschneider, T.; Grubb, S.; Metoska, A.; Olesen, S.P.; Calloe, K.; Thomsen, M.B. Development of heart failure is independent of K+ channel-interacting protein 2 expression. J. Physiol. 2013, 591, 5923–5937. [Google Scholar] [CrossRef]
- Grubb, S.; Speerschneider, T.; Occhipinti, D.; Fiset, C.; Olesen, S.P.; Thomsen, M.B.; Calloe, K. Loss of K+ currents in heart failure is accentuated in KChIP2 deficient mice. J. Cardiovasc. Electrophysiol. 2014, 25, 896–904. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).