Abstract
Spinal cord injury (SCI) affects millions of individuals worldwide. Currently, there is no cure, and treatment options to promote neural recovery are limited. An innovative approach to improve outcomes following SCI involves the recruitment of endogenous populations of neural stem cells (NSCs). NSCs can be isolated from the neuroaxis of the central nervous system (CNS), with brain and spinal cord populations sharing common characteristics (as well as regionally distinct phenotypes). Within the spinal cord, a number of NSC sub-populations have been identified which display unique protein expression profiles and proliferation kinetics. Collectively, the potential for NSCs to impact regenerative medicine strategies hinges on their cardinal properties, including self-renewal and multipotency (the ability to generate de novo neurons, astrocytes, and oligodendrocytes). Accordingly, endogenous NSCs could be harnessed to replace lost cells and promote structural repair following SCI. While studies exploring the efficacy of this approach continue to suggest its potential, many questions remain including those related to heterogeneity within the NSC pool, the interaction of NSCs with their environment, and the identification of factors that can enhance their response. We discuss the current state of knowledge regarding populations of endogenous spinal cord NSCs, their niche, and the factors that regulate their behavior. In an attempt to move towards the goal of enhancing neural repair, we highlight approaches that promote NSC activation following injury including the modulation of the microenvironment and parenchymal cells, pharmaceuticals, and applied electrical stimulation.
1. Spinal Cord Injury
Spinal cord injury (SCI) in mammals is a debilitating condition which leads to a spectrum of sensory and motor deficits. To date there is no cure for this condition. Deficits that occur due to SCI include those caused by the initial mechanical damage which results in damage to axons, breakdown of the blood-spinal cord barrier, as well as a cascade of secondary events that worsen the extent of injury [1,2,3,4]. Secondary events following SCI include vascular damage, inflammation, excitotoxicity, cell death, and the activation of astrocytes [2,5,6,7]. Combined, these events result in the infiltration of circulatory factors and cellular contents into the spinal cord [8,9]. Blood-borne macrophages enter the spinal cord and resident microglia react to the injury by releasing cytokines and chemokines (such as IL-1β, IL-6, and TNF-α) resulting in a rapid inflammatory response in the first several weeks following SCI [6,10,11]. Additionally, myelin associated factors such as myelin oligodendrocyte glycoprotein (MOG), myelin-associated glycoprotein (MAG), and myelin basic protein (MBP) are released into the cellular milieu [12,13]. Concurrently, neurotoxicity results from the release of excitatory neurotransmitters exacerbating neuronal and oligodendrocyte cell death through necrosis, and later apoptosis [5]. Oligodendrocyte precursor cells (OPCs), which comprise 5–10% of the cells in the spinal cord parenchyma, are activated and undergo morphological changes and increased proliferation at early times post-SCI [14,15].
The secondary injury following SCI leads to edema, chronic demyelination, and the formation of a glial scar which is composed of multiple cell populations including astrocytes, OPCs and fibroblast-like cells [16,17,18,19]. Within the glial scar, resident astrocytes are activated and proliferate, hypertrophy, and migrate to the site of injury [20,21,22,23]. The glial scar serves both positive and negative functions following SCI. While it limits the spread of injury, it also restricts structural repair of the spinal cord [16,17,19] Following injury, reactive astrocytes upregulate several genes including Nes, Axin2, and matrix metalloproteinase genes (Mmp2, Mmp13), relative to naïve astrocytes. Subsequently, reactive astrocytes become scar-forming astrocytes within the glial scar and express a different set of genes including Cdh2, Sox9, and Slit2, among others. In line with the appearance of distinct subsets of astrocytes following injury, recent work suggests that sub-populations of astrocytes may also be responsible for enhancing neurite outgrowth from parenchymal neurons following SCI, highlighting the dual role of the glial scar [19,24,25,26,27]. Schwann cells, the myelinating cells in the peripheral nervous system (PNS), have been shown to migrate into the CNS from the PNS in response to injury where they contribute to the glial scar and may play a role in remyelination [28]. Finally, in response to SCI it has been shown that NSCs and their progeny (together termed neural precursor cells, NPCs) in the periventricular zone (PVZ) surrounding the central canal within the spinal cord proliferate, migrate to the site of injury, and differentiate into mature neural cells [29,30]. Hence, while NSCs in the mammalian spinal cord are responsive to injury, their activation is insufficient for structural repair or functional recovery following SCI [30,31,32].
Notably, in regenerative competent species, a similar sequence of cellular events results in strikingly different outcomes, with structural repair and functional recovery observed across multiple groups including teleost fish, urodeles and some lizards [33,34,35]. Interestingly, the hallmark feature that is best correlated with spinal cord repair and fully recovered function, is the activation of endogenous spinal cord NSCs. In regeneration-competent species, NSCs are rapidly recruited to the site of injury where they give rise to differentiated cells that integrate into the newly formed tissue, underscoring the potential of stem cell-mediated regeneration in the CNS [34,35,36,37]. In the hunt for strategies aimed at harnessing endogenous mammalian NSCs for repair, clues can be found by (1) focusing future studies on understanding the mechanisms of spinal cord development, with the goal of recapitulation following injury [38,39,40] and/or (2) studying the properties of regenerative competent species to understand the cell and microenvironmental factors that facilitate NSC activation and genuine CNS regeneration [33,39,41,42]. Towards the goal of facilitating neural repair in mammals endogenous NSCs represent a promising target due to their potential to replace lost or damaged cells and re-establish the neural networks in the spinal cord. Here, we will examine distinct NSC populations found in the adult spinal cord, explore their lineage dynamics and kinetics following injury and their response to external cues [43].
2. Endogenous Neural Stem Cells
NSCs are rare, slowly dividing cells found along the entire neuraxis of the developing and mature CNS [44,45,46,47,48] and demonstrate two key stem cell properties: self-renewal and multipotentiality. These properties are demonstrated using the in vitro “neurosphere” assay [45,49,50]. The neurosphere assay is a robust and powerful approach to isolate and study the properties of NSCs and their progeny. In vivo, NSCs derived from the PVZ in the spinal cord do not display multipotency but instead, their progeny are aneurogenic under homeostatic conditions [51,52]. This aneurogenic phenotype is related to the microenvironment within the spinal cord. This was eloquently shown with cell transplant experiments where spinal cord derived NPCs gave rise to neurons following injection into the hippocampus, a neurogenic region of the adult forebrain [53]. These findings reveal the multipotency of spinal cord NSCs that is masked in the spinal cord milieu [54,55]. The aneurogenic phenotype of spinal cord NSCs is also seen following SCI, where activated NSCs exclusively generate gliogenic progeny, producing mostly astrocytes, which are found at the site of injury [30,56].
3. Adult Neural Stem Cells: A Heterogeneous Population of Cells
NSCs within the mammalian spinal cord can be sub-divided into several functionally and molecularly distinct populations. A number of NSC populations can be isolated from the PVZ surrounding the central canal (Figure 1). Common to all NSCs is their ability to generate clonally derived, multipotent colonies of cells in vitro [48]. Similar to NSCs found in the brain, the majority of NSCs in the spinal cord are definitive NSCs [57], while a rare population of primitive NSCs have also been identified [48]. Most recently, single-cell sequencing (scRNA-seq) and proteomics, have facilitated a comprehensive profile of PVZ heterogeneity and identified a novel, MSX1+ population of NSCs [58,59]. Finally, recent work has demonstrated that NSC properties are elicited from a population of spinal cord niche cells known as cerebrospinal fluid contacting neurons (CSFcNs) in vitro. These cells have a neuronal phenotype in vivo yet in vitro, CSFcNs generate multipotent, self-renewing colonies, shedding light on a potential new source of NSCs [60].
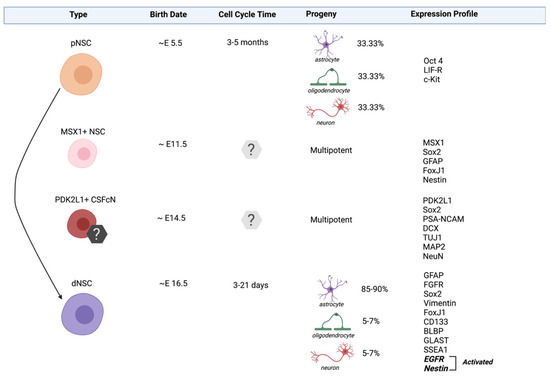
Figure 1.
Neural stem cell populations in the spinal cord. Spinal cord derived cells that exhibit stem cell properties include primitive neural stem cells (pNSCs), MSX1+ NSCs, PDK2L1+ CSFcNs and definitive neural stem cells (dNSCs). The lineage relationship (solid arrow) is established between pNSC and dNSC populations. The lineage relationship of MSX1+ NSCs and PDK2L1+ CSFcNs is unknown. The expression profiles listed include genes known to be specific to the identities of a given population (e.g., Oct4, MSX1, and PDK2L1) and those related to their stemness.
Heterogeneity within the NSC pool is also defined on the basis of the stem cell “state” (i.e., quiescent versus activated). Most NSCs in the mammalian CNS exist in quiescence, a mitotically dormant state, without undergoing proliferation or differentiation [61]. Several physiological elements such as growth factors and physical exercise (for example) can cause quiescent NSCs to become activated to proliferate and generate NPCs [62,63]. Conversely, stress and old age are examples of factors that reduce proliferation and enhance NSC quiescence [62]. The ability of stem cells to switch between quiescence and proliferation has important implications for enhancing endogenous repair.
Increased resolution pertaining to the identification and potential of distinct NSC populations is fundamental to exploiting these populations for neural repair in mammals [59]. Here, we provide a current and more detailed description of the spinal cord NSC pools (Figure 1).
3.1. Definitive Neural Stem Cells (dNSCs)
Definitive neural stem cells (dNSCs) are the most abundant NSCs within the adult spinal cord. They are first identified on ~ embryonic (E)16.5 along the developing neuraxis and they persist into adulthood in the PVZ. dNSCs primarily exist in a quiescent state [64,65,66,67] and are identified based on their expression of markers including the intermediate filament proteins Nestin, Vimentin and Glial Fibrillary Acidic Protein (GFAP) [68], as well as transcription factor, SRY-Box transcription factor 2 (Sox2) [69] and cilia marker, forkhead box J1 (FoxJ1) [70]. In vitro, dNSCs are responsive to fibroblast growth factor (FGF) and epidermal growth factor (EGF), and form clonally-derived, multipotent, and self-renewing neurospheres in these conditions [71]. Following injury, dNSCs are activated [72] to proliferate and generate progeny and migrate to the site of injury where they primarily give rise to glial cells [48,73]. Lineage tracking studies have revealed that ~95% of the progeny differentiate into astrocytes which contribute to the glial scar and ~2–5% differentiate into oligodendrocytes [29,32,74]. Considering their abundance, responsiveness to injury and their ability to migrate to the site of damage, dNSCS are important targets for neural repair.
3.2. Primitive Neural Stem Cells (pNSCs)
Primitive NSC (pNSCs) are first isolated from the developing nervous system at E5.5 [75] and they persist throughout embryonic development and into adulthood in the PVZ of the adult mammalian forebrain [76,77,78,79] and spinal cord [76,77]. In the CNS, it is estimated that pNSCs are 1000-fold less frequent than dNSCs [76,77,78,79]. pNSCs are identified based on their expression of low levels of the pluripotency marker organic cation/carnitine transporter 4 (Oct4) as well as leukemia inhibitory factor (LIF) receptor and tyrosine-protein kinase Kit receptor (cKit) [77,79]. In vitro, pNSCs can be cultured using the neurosphere assay in the presence of LIF to form multipotent, self-renewing colonies. Similar to dNSCs, pNSCs can be activated following SCI leading to an increase in the size of the pNSC pool at early times following injury [48]. Different from dNSCs, pNSCs do not migrate from the PVZ as LIF responsive neurospheres have not been isolated from the lesion site following SCI [48,76]. To date, lineage tracking of these rare pNSCs has been performed in the brain [74], but remains elusive in the spinal cord.
While pNSCs are significantly less abundant than dNSCs, this population has properties that could potentially augment endogenous neural repair and regeneration following SCI. Unlike the more prominent dNSCs that primarily give rise to astrocyte progeny following differentiation in vitro and in vivo, Oct4+ pNSCs from along the neuraxis generate equal numbers of neurons, oligodendrocytes, and astrocytes in vitro [48], potentially conferring a benefit for the replacement of lost cells following SCI. Also relevant is that fact that pNSCs are lineally related to the abundant dNSCs (Figure 1). Studies using transgenic mice and antimitotic agents to specifically deplete dNSCs have shown that pNSCs can be recruited to replace the lost dNSCs, as well as contribute to neurogenesis in the adult forebrain [77,78]. Whether neurons are derived directly from the pNSCs or whether progeny must first pass through a dNSC intermediate stage, is still unknown. What is clear, however, is that pNSCs are upstream of dNSCs and are responsive to injury through enhanced proliferation. Together, these findings support the conclusion that pNSC activation could serve as a novel approach to increase the numbers of endogenous NSCs available for neural repair.
3.3. MSX1+ NSCs
Msh Homeobox 1 (MSX1) expressing NSCs are novel, radial glia-like stem cells that were identified in the spinal cord using RNA profiling, immunohistochemistry, and transgenic mouse models [58]. A conditional MSX1-CreERT2 mouse crossed to a tdTomato reporter (MSX1-Cre:tdTom) was injected with tamoxifen on E11.5 and tdTomato+ cells were identified in vivo when tissue was examined at E13. MSX1+ NSCs are restricted to the dorsal region of the PVZ and share several molecular markers with dNSCs including Nestin, GFAP, Sox2 and FoxJ1 [58,59]. Further, MSX1+ cells express cRET (GDNF and NTN growth factor receptors) which are found on hematopoetic stem cell populations and Id4 which is highly enriched in quiescent NSCs from the forebrain [80]. Similar to pNSCs and dNSCs, MSX1+ NSCS are a largely quiescent population in vivo [58]. MSX1+ cells isolated from tamoxifen labeled MSX1-Cre:tdTom mice can proliferate to form td-Tom+ neurospheres that are multipotent upon differentiation, thereby eliciting a cardinal property of stem cells. Intriguingly, MSX1+ radial-like NSCs have been previously identified in regeneration-competent species, where they have been shown as crucial for the success of tail regeneration [81]. While the response of MSX1+ NSCs following SCI has not yet been explored in mammals, the observed properties of the MSX1+ NSCs support the possibility that they represent an intermediate population between pNSCs and dNSCs in the NSC lineage, making them candidates for activation and recruitment following injury.
3.4. Quiescent vs. Activated NSC States
In the forebrain, the baseline activation state of resident NSCs has been studied in detail [67,80,82,83] and dNSCs (the best characterized) are shown to exist in two states, namely quiescent (qNSCs) and activated (aNSCs). qNSCs have a radial glia-like morphology and a slow cell cycle time of ~3–21 days [67]. qNSCs play a fundamental role in maintaining the stem cell pool into adulthood via asymmetric divisions [84]. Adult forebrain qNSCs are maintained in their slow cycling state using bone morphogenetic protein (BMP) and Notch signaling [85,86,87]. Conversely, aNSCs are non-radial, have short processes and are rapidly dividing with a short cell cycle time of <24 h in the adult mouse [88,89]. aNSCs express EGFR, proliferation markers such as Ki67 and proliferating cell nuclear antigen (PCNA) [67,89,90] whereas qNSCs do not express EGFR but do express the Helix-Loop-Helix transcriptional regulator Id2 and the transcription factor Sox9 [89]. scRNA-seq experiments have also revealed that alongside qNSCs and aNSCs, a primed-quiescent transitionary stage exists between the two [80,91]. These “primed” NSCs showed decreased Notch and BMP signaling and an increase in lineage-specific transcription factors and protein synthesis (i.e., EGFR, cyclin-dependent kinase 2 (CDK2)) [80]. With regard to neural repair, modulating the activation state of forebrain NSCs by pulling them out of quiescence has been shown to result from injury alone, as well as following the administration of growth factors, cytokines and drugs [61,92,93,94,95,96] Indeed, brain repair and improved functional outcomes have been correlated with the activation of endogenous NSCs [91,97,98,99].
NSCs in the spinal cord have been less well characterized in terms of their quiescent and activated states. However, it is clear that the proliferative profile of endogenous NSCs in the spinal cord is significantly less than the forebrain under homeostatic conditions. Proliferation markers such as Ki67 only identify rare mitotically active cells in the spinal cord PVZ suggesting that the vast majority of resident NSCs are in a quiescent state. In the case of MSX1+/ID4+ NSCs, their already quiescent phenotype can be further enhanced (decreased cell proliferation) in the presence of BMP6 in vitro [58]. Hence, with a sound knowledge of the factors that regulate the activation state of NSCs, the spinal cord NSC subpopulations could be recruited to contribute to SCI repair as has been demonstrated in the brain.
4. The NSC Niche
Understanding stem cell-intrinsic differences and lineage dynamics are fundamental to enhancing the potential of endogenous NSC populations following SCI, combined with the knowledge that NSC behavior is tightly linked to the environment, or “niche”, where they reside [55,100,101,102]. The stem cell niche plays a critical role in stem cell activation, proliferation kinetics and cell fate through cell-cell interactions including the release of secreted factors and cell-contact mediated interactions with neighboring cells [103], as well as cell adhesion [104]. In the CNS, factors such as age and sex also play important roles in NSC behavior [105]. For example, the aged niche is less capable of supporting stem survival and/or activation, which has important implications for stem cell-based therapies to treat the aged population. Hence, the microenvironment surrounding endogenous NSCs may be a key limiting factor in the realization of stem cell mediated neural repair [106,107].
The PVZ surrounding the central canal, including immediately adjacent parenchyma cells, comprises the spinal cord niche (Figure 2). The PVZ in the spinal cord is synonymous with the well-characterized subventricular zone (SVZ) of the brain in terms of developmental origin. However, the cellular architecture and molecular signaling within the spinal cord is less well characterized [29,58,100,108]. Notably, many morphologically and functionally distinct cells within the spinal cord niche share similar marker expression. As such, it is important to look beyond marker expression and examine cell behavior when identifying stem cell populations, including the fundamental properties of self-renewal and multipotency. Important recent work has begun to unravel the distinct characteristics in the mammalian (including human) spinal cord niche [58,59].
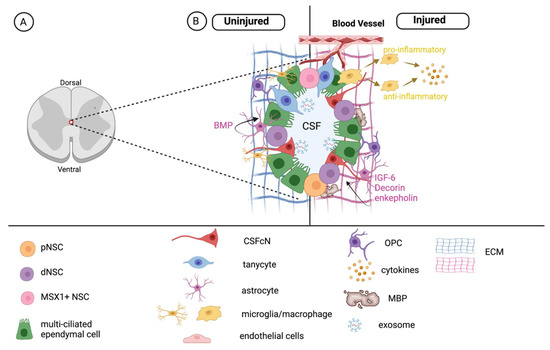
Figure 2.
The NSC niche in the spinal cord. (A) Location of the central canal and PVZ. (B) The PVZ is composed of stem cells, multi-ciliated ependymal cells, cerebrospinal fluid contacting cells (CSFcNs), and tanycytes and is influenced by niche-associated astrocytes, oligodendrocyte progenitor cells (OPCs), microglia/macrophages, matrix components and exosomes. Each of these factors regulate NSCs in the uninjured (left) spinal cord. Following injury (right), the microenvironment is modified and leads to changes in the NSC pool.
4.1. Ependymal Cells: A Population of NSCs?
Ciliated ependymal cells lining the central canal, identified by their expression of CD133 (Prominin1), FoxJ1 and Sox2 [109,110,111], play an important role in the circulation of cerebrospinal fluid [112]. Ciliated gene expression is affected by SCI (including expression of FOXJ1) and culture conditions, including the neurosphere assay [59]. Interestingly, ependymal cells have been ascribed as a source of multipotent NSCs that are activated following injury or following in vitro isolation [29,30,113]. In this regard, lineage tracking studies have reported varying results in terms of the potency and “stemness” of ependymal cells [29,30,31] Using transgenic mouse models that utilize the human promoter element to label FOXJ1 expressing cells, ependymal-derived cells were observed proliferating, migrating to the site of injury and differentiating into mature astrocytes and oligodendrocytes in models of SCI [29,30,32,73] Conversely, utilizing a different mouse model to label FOXJ1 ependymal cells (a knock-in model), ependymal cells were primarily unresponsive following SCI leading to the conclusion that ependymal cells are not NSCs in the mammalian spinal cord [31]. Whether the discrepancy between these studies can be entirely explained by the mouse model is not clear. Other considerations include the fact that ependymal cells can be further categorized based on ciliation patterns (uni-, bi-, and multi-ciliated) and there is a general lack of knowledge about the homology of these subpopulations in terms of gene and protein expression [114,115,116,117]. As a result, the stemness of ependymal cells continues to be an area of controversy in the literature.
If there are lessons to be learned from regenerative-competent species, they would support the potential “stemness” of at least a subpopulation of ependymal cells. Ependymo-radial glia (ERGs) are present in all species capable of spontaneous spinal cord regeneration and are defined based on their ciliation and location in the PVZ [37,55]. Structurally, ERGs have a long radial process that contacts the pial surface of the cord, similar to radial glia in developing mammals [36,41,118]. As with mammalian NSCs, ERGs express Nestin, Sox2, and GFAP [33,34,119]. Following injury, ERG activation (proliferation, migration, and differentiation) drives regeneration of the spinal cord and the re-establishment of regionally specific domains [33,34,36,37]. To date, comparative studies examining the homology of ERGs to distinct mammalian NSC populations remain unexplored. However, similarities in the morphology and protein expression of mammalian NSCs and ERGs highlight the idea that the lack of regenerative-competence in mammals may be related to a less-permissive niche.
4.2. Tanycytes—A Population of Glial Progenitors?
A sub-population of ependymal cells known as tanycytes are present in the spinal cord niche. Tanycytes are identified by their expression of Nestin, GFAP, Sox2 and Pax6 [114]. These cells have an apical process that reaches the cerebrospinal fluid (CSF) and a basal process that contacts the blood vessels [29,108,114,120]. Functionally, tanycytes filter molecules such as proteins and enzymes from the blood that normally cannot pass through the blood-spinal cord-barrier, and pass them into the CSF via transcellular transport [114]. Tanycytes have recently been proposed as a subpopulation of NSCs due to their ability to form self-renewing colonies following in vitro isolation. However, tanycyte derived colonies were not multipotent in vitro and were restricted in their differentiation to glial progeny (astrocytes, and oligodendrocytes) suggesting they are glial progenitors rather than bona fide NSCs [114].
4.3. Cerebrospinal Fluid (CSF) Contacting Cells: Immature Neurons, NSCs or Both?
Alongside ependymal cells, CSF contacting neurons (CSFcNs) are found within the PVZ [121]. CSFcNs can be subdivided into morphologically and functionally distinct Type I and Type II phenotypes. Type I cells express GABA, somatostatin and glutamate receptors and can fire action potentials [122,123]. Morphologically, CSFcNs have a bulb-like ending that project into the central canal and ventro-laterally oriented processes that project between cells [123,124]. Conversely, Type II CSFcNs have a flattened cell body and have thin lateral processes that can be directed dorsally, ventrally or laterally and do not show any active neuronal properties [123,125]. Common to both types of CSFcNs is the expression of PDK2L1 (TRPP2, a cation channel that localized to primary cilia) [126], along with canonical markers of neurons such as DCX, PSA-NCAM, and Nkx6.1 [121,127,128]. The function of Type II CSFcNs is thought to be related to sensing the composition and direction of flow of the CSF [121]. The ability of CSFcNs to regulate endogenous NSCs is not known. However, studies have shown that GABA release from CSFcNs inhibits NSC proliferation during development and in the hippocampus [129,130]. GABA could serve a similar function in the mature spinal cord.
Most interesting, very recent work has identified CFScNs as potential stem cell population in the adult spinal cord [60]. CSFcNs isolated via cell sorting for the pan-CSFcN marker PKD2L1 [124], specifically from the cervical spinal cord, were reported to generate multipotent colonies in the presence of growth factors used to isolate dNSCs (EGF and FGF2) [60]. Moreover, the CSFcN-derived colonies expressed dNSC markers (Sox2, GFAP and Nestin) and could be passaged multiple times [60]. This compelling stem cell behavior is unpredicted from cells that express a mature neuronal phenotype. While currently limited to in vitro characterization, important next steps to examine this population in vivo will determine whether CSFcNs are a novel subpopulation of spinal cord NSCs.
4.4. Endothelial Cells—Paracrine Modulators of NSCs
Endothelial cells that comprise blood vessels influence NSC kinetics including self-renewal and progenitor cell fate, through the release of paracrine factors including vascular endothelial growth factor (VEGF) and amyloid precursor protein (APP) [131,132,133]. Loss of function studies using interference RNA to reduce VEGF expression led to decreased Notch and Pten signaling and a concomitant reduction in NSCs and progeny resulting from reduced proliferation [132,134]. Additionally, using a transgenic conditional knockout mouse (TiE2-Cre:APP-floxed) to deplete APP production specifically from endothelial cells in the neurovascular niche, resulted in increased NSC proliferation in vivo. These findings highlight the role of endothelial cell-derived factors in regulating NSC activity [134].
4.5. The Extracellular Matrix: A Regulator of NSC Function
A number of extracellular matrix (ECM) proteins such as tenascin-C (TN-C), laminin, and chondroitin sulfate proteoglycans (CSPGs) can regulate NSC behavior in stem cell niches. TN-C is glycoprotein expressed by developing astrocytes in mammals that inhibits the proliferation of both NSCs and their progeny. This subclass of the tenascin family contains fibronectin and fibrinogen elements that, when knocked out in transgenic mouse models, lead to increased proliferation of NSC progeny as a result of increased FGF2 signaling [135,136]. Laminin, a prominent ECM protein found in basement membranes, has the opposite effect leading to enhanced NSC self-renewal and concomitant reduction in progenitor cell differentiation via the laminin receptor integrin α6β1 found on NSCs [137,138]. Other ECM molecules include CSPGs which are expressed by oligodendrocytes and secreted into the ECM. These have been shown to reduce the proliferation of NSCs in vitro and in vivo and this inhibition is lost when CSPGs are degraded through the application of the enzyme chondroitinase [104]. Hence, the impact of the ECM on NSCs and their progeny is clear and has important implications for neural repair as the ECM is markedly changed in disease and injury states.
5. Regulating Neural Precursors to Enhance Neurorepair
Improving structural repair and functional recovery following SCI in mammals remains an elusive challenge in the field of regenerative medicine. We hypothesize that improved outcomes will result from enhancing and modulating the proliferation, migration, and differentiation of endogenous NSCs and their progeny, as well as altering the niche to support, rather than inhibit, neurorepair (Figure 3). There are several promising approaches to improve the response of endogenous NSCs and alter the niche that surrounds them. Here, we describe parenchymal cell activity that impacts NSCs following injury. The promise of pharmacological agents to promote repair and the development of novel therapeutics, specifically the application of electrical stimulation, to mobilize NSCs following SCI.
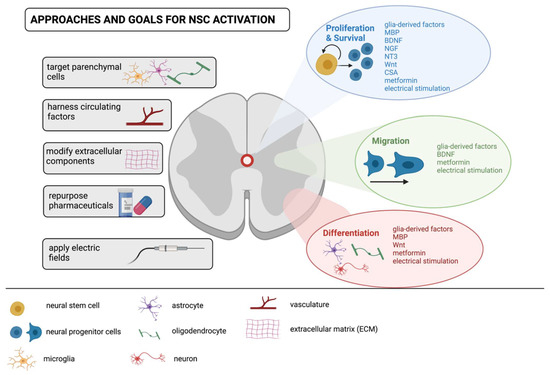
Figure 3.
Approaches, goals and targets for modulating endogenous NSCs following spinal cord injury. Towards the goal of enhancing NSC activation to promote self-repair of the injured spinal cord, approaches encompass targeting the response of parenchymal cells to release NSC modulating factors (e.g., glia-derived factors, myelin basic protein (MBP), brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), neurotrophin-3 (NT-3)); modifying extracellular matrix components to regulate cell adhesion (for example),and/or administering pharmaceuticals (e.g., metformin and cyclosporin A (CsA)) that modify cell behavior through proliferation, survival, migration and differentiation. Novel therapeutics such as applied electric fields can also impact NSC behavior and is another approach that shows promise and warrants further investigation.
5.1. Microglia, Astrocytes and Oligodendrocytes: Parenchymal “Influencers” on NSCs
Microglia, the immune cells of the CNS, are first responders following injury. Under homeostatic conditions microglia have a ramified morphology and sample the microenvironment for any perturbations in the spinal cord [139,140,141]. Following SCI, microglia and infiltrating macrophages, hereafter referred to as “microglia”, retract their processes and adopt an ameboid morphology in response to cytokines released from damaged cells in the parenchyma, such as interleukin 1ß (IL-1ß), tumor necrosis factor α (TNFα, interferon-γ (IFN-γ), adenosine triphosphate (ATP), and nitric oxide (NO) [140]. Activated microglia are proliferative cells that themselves release pro-inflammatory cytokines such as IL-1ß, IL-12, TNFα, and nitric oxide synthase (iNOS), which exacerbate the neurotoxicity [140,142,143]. Microglia can also be “alternatively” activated and release anti-inflammatory factors such as IL-10, transforming growth factor ß (TGFβ), and cluster differentiation 206 (CD206), which are neuroprotective [139,144]. Interestingly, microglia state is now well established as a continuum, rather than static states [145], hence modification of this population serves as possible avenue for altering NSCs. Irrespective of the activation state (pro- or anti-inflammatory), studies have shown that NSC behavior is modified in the presence of microglia. Using in vitro assays with microglia conditioned media, NSCs were less proliferative in the presence of pro-inflammatory microglia and generated more astrocytes relative to anti-inflammatory conditions. Interestingly, the migratory behavior of NPCs was increased in the Boyden chamber assay in the presence of anti-inflammatory microglia conditioned media [146,147]. Subsequent in vivo analyses revealed that the fate of NSC progeny is linked to microglia activation state whereby infusion of pro-inflammatory microglia-conditioned media drives a reduction in proliferation and neurogenesis, whereas anti-inflammatory microglia-conditioned media enhances NSC-derived neurogenesis and increases proliferation [146,147].
Extracellular-released vesicles such as exosomes provide a unique type of inter-cellular communication consisting of proteins, lipids, and nucleic acids. In the nervous system, microglia-derived exosomes (MDEs) contribute to neuron-glial crosstalk under homeostatic conditions as well as is in injury/diseased states [148,149]. Following injury, MDEs release factors including microRNAs (miRNAs) enriched in pro-inflammatory and neurotrophic signals. For example, MiR-155 MDE release has been shown to enhance pro-inflammatory microglia whereas MiR-21 can promote anti-inflammatory microglia [150,151,152]. Considering the ability of MDEs to release miRNAs, they may have a role in altering NSCs kinetics [149,152]. For example, MiR-21 has been shown to regulate EGFR and FGFR mediated signaling during neural development and could serve as a novel tool for modulating EGF- and FGF-responsive dNSC behavior.
In mammals, recent studies have revealed that activated microglia play an important role in limiting the injury following SCI [11,153]. Indeed, depletion of microglia after SCI disrupts glial scar formation and leads to reduced neuronal and oligodendrocyte survival. At the same time, it is well established that an extended pro-inflammatory state following injury can lead to increased impairments [140,142,154] Interestingly, in regeneration-competent species, pro-inflammatory cytokines in the early stages following injury are necessary for the success of NSC-mediated spinal cord repair. This was demonstrated using cytokine-specific zebrafish knock-out models whereby the loss of TNF-α and IL-1β impaired spinal cord regeneration. This study also revealed a notably shorter pro-inflammatory phase following injury in zebrafish [155]. These data support the hypothesis that shortened pro-inflammatory states that rapidly transition to anti-inflammatory states, are necessary for NSC-mediated spinal cord regeneration [155,156]. Hence, studies aimed at modulating the balance (both extent and duration) of microglia activation in mammals and examining the outcome on NSC activation are important next steps.
Alongside microglia, astrocytes are prominent effectors of the microenvironment [16,157]. Under homeostatic conditions, they provide support and protection to neurons [158] and after SCI, astrocytes are the principal cell type that contributes to the formation of the glial scar [16,19,25,159]. Astrocytes hypertrophy, proliferate, and migrate to the site of injury where they release pro and anti-inflammatory cytokines, chemokines and trophic factors [160,161,162]. There is considerable evidence demonstrating that reducing glial scar formation leads to worse structural and behavioral outcomes following SCI [11,32,163]. However, this is in contrast to what has been shown in regeneration competent species following SCI where there is a complete lack of glial scar formation [41,164,165,166]. To date, the mechanisms that underlie this scar-free healing and regeneration remain largely unexplored, but uncovering key molecules or astrocyte sub-populations present in species capable of spontaneous CNS repair would provide important insight into potential avenues to support NSC-mediated repair through glial scar modification. One hypothesis is that the proportion of neurotoxic (A1) and neuroprotective (A2) astrocytes [142,167] differs in species with varying regenerative capacity following SCI. Future studies exploring these differences in astrocytes and their effect on endogenous NSCs would be valuable to advance the field.
In mammals, it is known is that astrocytes influence the activity of endogenous NSCs, in the presence or absence of injury [168,169,170,171]. Within the spinal cord under homeostatic conditions, astrocytes release bone morphogenic protein (BMP) antagonists (i.e., Noggin) and differential screening-selected gene aberrative in neuroblastoma (DAN) to effectively limit the proliferation of NSCs and the differentiation of NSC progeny [100,172]. In contrast, specific to the brain, the secretion of BMPs promotes proliferation and differentiation of NSCs, leading to constitutive neurogenesis [173,174,175]. Further differences in astrocytes along the neuroaxis likely play a role regulating NSC behavior, with or without injury. For example, glutamate transporter 1 (GLT1) is responsible for taking up excess glutamate and protecting from excitotoxicity and is expressed 10-fold less in spinal cord astrocytes compared to those in the brain [176] Spinal cord astrocytes express higher levels of GFAP, as well as IL-6 and its signaling partner STAT3, which play a role in the injury response [177]. Further, regional distribution of the guidance molecule Sem3a is found in the spinal cord, with the highest concentrations ventrally, potentially impacting NSC behavior differentially within the PVZ [178].
Distinct gene profiles of astrocytes from neurogenic and non-neurogenic regions of the CNS have been identified along with a number of astrocyte-derived factors that inhibit NSC-derived neurogenesis (such as insulin-like growth factor-6, decorin and enkaphalin) [179]. Specific to the spinal cord, in vitro studies have used lipopolysaccharide (LPS) treatment to induce astrocyte activation and collected activated astrocyte-conditioned media (ACM) for NSC co-cultures [180,181]. These studies revealed significant increases in NSC proliferation and survival mediated by IL-6 in the ACM, as well as upregulation of the neurogenic transcription factors Mash1, Hes5, and Neurog1 [180]. With a sound knowledge of the astrocyte heterogeneity and astrocyte derived factors that regulate NSC behavior, both the activity of NSCs and their subsequent progeny could be regulated to promote neural regeneration.
Oligodendrocytes are the myelinating cells in the CNS. While the presence of mature oligodendrocytes has not been demonstrated to directly affect NSC behavior, exposure to oligodendrocyte-associated proteins released following injury/disease has been shown to have profound effects on their behavior [48,182] Following SCI, oligodendrocytes undergo rapid cell death which persists for up to three months post-injury [183]. One of the most abundant proteins comprising mature is myelin basic protein (MBP). Normally cytoplasmic is released into the microenvironment following SCI [184]. Both in vitro and in vivo studies have shown that MBP is inhibitory to NSC proliferation and NSC-derived oligodendrogenesis [48,182]. Most interesting, the observed inhibition is not a direct effect of MBP on NSCs, but instead is due to the indirect effect of MBP on spinal cord PVZ cells, which then release an unidentified factor that acts on NSCs [46,176]. Equally intriguing, this MBP-mediated inhibition of NSC activation and oligodendrogenesis is specific to PVZ cells from the spinal cord niche. The brain NSC niche does not release inhibitory factors as a result of MBP exposure [176]. With regard to NSC activation as a strategy to promote neural repair following SCI, sequestering released MBP or modulating its interaction with the niche may be an important future direction.
5.2. Bloodborne and Extracellular Factors
Cells within the PVZ are in direct contact with blood vessels [114] and numerous studies have revealed the impact of bloodborne factors on NPC behavior [185,186,187]. To date, most studies have focused on NSCs in the brain. However, these studies are useful for establishing key targets that could be applied, or reduced, to regulate NSCs in the spinal cord. Studies utilizing heterochronic parabiosis have shown that the infusion of TGF-β family member GDF11, a factor present in young blood, was sufficient to increase neurogenesis on aged mice [187]. Conversely, bloodborne factors corticosterone and chemokine CCL11 inhibit SVZ-derived neurogenesis [188]. Within the spinal cord, erythropoietin has been shown to enhance neurogenesis and oligodendrogenesis from NSCs isolated in vitro (although it’s efficacy and whether it has direct effects on NSCs in vivo following SCI is not clearly established) [189]. In addition, circulating growth hormones, as well as prolactin, regulate the proliferation and migration of endogenous NPCs in the brain [190,191,192].
Neurotrophic factor expression is altered following SCI [193,194,195]. While a multitude of neurotrophic factors have been shown to alter outcomes [193], we focus on those that have been shown to regulate NSC behaviors. Brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and neurotrophin-3 (NT-3) are all upregulated following SCI and have all been shown to increase proliferation of endogenous NSCs in vitro [196,197,198]. Using in vitro assays, BDNF was shown to promote angiogenesis which drives NSC proliferation through the neurovascular niche [199]. The NSC neurovascular niche is distinct from non-neurogenic regions of the CNS and is characterized by interconnected, linear blood vessels that provide support for neuroblast migration as well as the delivery of bloodborne factors [200,201] The delivery of BDNF via transplanted NPCs genetically modified to over-express BDNF increased NSC survival (as compared to non-BDNF expressing NPCs), improved integration of NSC-derived progeny within neural circuits and promoted functional recovery [199,202]. The delivery of NGF increases NPC proliferation and survival and enhances the activity of injury induced neurotrophic factors such as NT-3 [203,204,205]. Infusing growth factors that are known to activate dNSCs, such as EGF and FGF2, also enhances NSC proliferation following SCI and promotes functional recovery [114,206,207]. Notably, the efficacy of NSC activating factors in promoting functional recovery has not been clearly demonstrated for the majority of factors.
Another approach for expanding the population of rare NSCs is through enhanced symmetric division. This would enable exponential expansion of the size of the NPC pool available for recruitment. Wnt proteins have been shown to play a role in symmetric cell division in several stem cell populations including hematopoietic, intestinal, and neural stem cells [208,209,210,211,212]. In the spinal cord, enhancing Wnt/ß-catenin signaling in NSCs via Wnt3a application both in vitro and in vivo, increased neuronal differentiation at the expense of astrocyte differentiation [213,214]. The pleiotropic effects of Wnt signaling in the CNS make it a promising factor to enhance neural repair via endogenous NPCs.
For all exogenous factor application strategies, defining the optimal timing, delivery method and location for delivery remain significant challenges for treating SCI. Most studies have applied exogenous factors in close proximity to the lesion and in the acute and subacute phases (i.e., within two weeks) following SCI [193]. Delivery of exogenous factors has been accomplished through direct injection, sustained release mini-pumps, transplantation of genetically modified cells, viral transduction directly into the parenchyma, as well as hydrogel or polymer-based delivery approaches. Each has demonstrated some degree of promise including tissue sparing, axonal outgrowth and/or functional recovery [193,215,216,217] but comparisons across studies are challenging due to the variations in experimental paradigms. Studies exploring the long-term impact of NSC activation are limited and could impact health outcomes [193,218] For instance, NSCs that are constitutively recruited into cycle post-injury may enter senescence, and senescent cells can negatively impact tissue health [219,220].
As with all interventions related to promoting neural repair, lessons from regeneration competent species may provide insight into establishing which neurotrophic factors, when and for how long, the exogenous factor should be delivered for optimal outcomes. For example, across teleost fish and urodeles, Wnt, EGF, FGF, BDNF, and NGF signaling have all been shown to play a role in the success of spontaneous, NSC-mediated, CNS regeneration [221,222,223,224,225]. In urodeles, EGF and FGF have been shown to be required for NSC proliferation and migration during spinal cord repair [226]. Studies exploiting the naturally occurring dynamics of these factors in spinal cord repair remains a useful, but under-utilized, resource when tasked with enhancing NSC-mediated repair.
5.3. The Pleiotropic Power of Repurposed Pharmaceuticals
The application of repurposed pharmacological agents is a particularly attractive approach for enhancing the response of endogenous NSCs, as it relates to their minimal invasiveness and the strong safety profile for drugs already in clinical use [95,227,228,229,230]. Examples repurposed drugs that have demonstrated effects directly on NPCs and/or niche cells that regulate their behavior include cyclosporin A (CsA) and metformin. CsA is a commonly used immunosuppressive drug to treat autoimmune disorders and to prevent graft rejection following organ transplant which has direct effects on NSCs [231,232]. Using both in vitro and in vivo assays, CsA prevented apoptotic cell death of NPCs through a calcineurin-independent inhibition of mitochondrial permeability pore formation. CsA expands the size of the NSC pool in both the brain and spinal cord [232,233]. At clinically relevant doses delivered systemically or intracranially through an intraventricular osmotic minipump or via a hydrogel composite, CsA was sufficient to promote functional recovery in two models of stroke (motor and cognitive) and in both rats and mice [234,235,236,237]. To date, its potential to enhance outcomes following SCI remains unexplored. Given CsA’s immunomodulatory properties, delivery at early times and for several days following SCI would have the dual role of dampening the immune response (mimicking what is observed in regeneration-competent species) and activating endogenous NSCs, providing a promising therapeutic strategy. Of note, CsA is commonly used in stem cell-based transplant studies to decrease graft rejection. Given the successful outcomes of many cell transplant-based interventions, it would be of interest to discern whether CsA’s effects on endogenous NPCs is also contributing the success of the therapeutic strategy.
Another repurposed drug that has shown considerable therapeutic promise for treating the injured CNS is the anti-diabetic drug metformin. Metformin has pleiotropic effects in the CNS. In NPCs, it stimulates adenosine monophosphate (AMP) kinase activity which activates the atypical protein kinase C (aPKC)-CBP pathway leading to increased proliferation and survival of NPCs through FOXO3 and Tap73 activity [228,229,238,239,240]. Furthermore, metformin has anti-inflammatory effects through AMPK-dependent and independent inhibition of NF-κB [241,242,243]. Metformin has also been shown to enhance glycolysis and promote angiogenesis [241,244,245,246].
Following brain injury, metformin leads to improved functional outcomes in pre-clinical animal models (stroke, traumatic brain injury and cranial radiation) and has led to promising results in children with acquired brain injury [98,99,240,247,248]. The positive outcomes are correlated with increased survival/proliferation of endogenous NSCs, NPC migration to sites of injury and enhanced neuronal and oligodendroglia differentiation [98,228,229], as well as with indirect effects through modification of the NSC niche by decreasing microglial activation following injury [247,249]. Metformin has demonstrated age and sex-dependent effects on brain NSCs—expanding the size of the NSC pool in adult females, but not adult males [248]. Additionally, metformin has been shown to influence OPCs following demyelinating injury, enhancing both their proliferation and differentiation into mature oligodendrocytes [250]. Indeed, CNS remyelination from OPCs after SCI has been attributed to resident spinal cord OPCS. There is some indication that newly formed OPC-derived Schwann cells may also play a role in remyelination [251]. Most relevant to SCI repair, recent work has shown that metformin delivery in the acute phase following SCI leads to improved functional outcomes in both males and females [239]. The pleiotropic effects of metformin make it challenging to discern the cell-based mechanism (s) that underlie the success of the treatment However, the strong correlation with improved functional outcomes and NPC activation suggest that endogenous NPCs, directly or through niche modulation, are playing a role in the positive outcomes.
5.4. Regulating Neural Stem Cell Behaviour with Electric Fields
Beyond pharmacological approaches, electrical stimulation has demonstrated promising evidence as a novel approach to activate NSCs towards the goal of facilitating neural repair. The significant role of electric fields (EF) as an environmental cue in development [252,253] and wound healing [254,255] shed light on its potential therapeutic application in neural regeneration. With an optimized stimulation paradigm, electrical stimulation following SCI could help to augment the intrinsic reparative response mediated by NSCs, enhancing their migration, and driving their differentiation into neurons and oligodendrocytes. In vitro and in vivo studies reveal that NSCs along the neuroaxis are electrosensitive cells, and their behavior can be regulated in terms of their kinetics, migration, and differentiation, by the presence of an EF [256,257,258]. Specifically, EFs have been shown to induce changes restricted to undifferentiated NSCs in a dose-dependent manner [257]. When stimulated, NSCs from both the brain and spinal cord undergo rapid, directed migration in vitro [257], and this has been elegantly demonstrated with spinal cord NSCs in organotypic slice cultures [259]. The EF application effectively guides NSCs to lesion sites where they can contribute to cell replacement, immunomodulation, and remodeling of damaged tissue [260]. Electric field application impacts a number of molecular and cellular signaling pathways including upregulation of phosphatidylinositol-3-kinase (PI3K) [259], Rac1/Tiam1/Pak-1 signaling pathway [261], and altering calcium influx dependent cell kinetics [262,263]. Applied EFs are potent enough to efficiently divert NSCs from their default migratory pathways [264], supporting their utility in directing NSCs towards the lesion site to aid in neural repair. Proliferation kinetics and differentiation of NSC progeny is also impacted by applied EFs which have demonstrated pro-survival effects on NSCs and enhance differentiation of progeny into neurons and oligodendrocytes [258] Recent studies have discovered the potential of EF in ameliorating neuroinflammation by shifting microglial states from pro-inflammatory to the anti-inflammatory phenotype at lesion sites in the CNS in vivo [265,266]. Taken together, EFs have the potential to enhance directed migration and increase the survival of NSCs post-injury through both direct effects and niche-mediated alterations making EF application a feasible and customizable approach to improve functional recovery following SCI. With optimized parameters, the ability of EFs to regulate NSC kinetic behavior affords a novel and exciting possibility to repair the injured spinal cord by harnessing the full potential of endogenous NSCs. More recently, clinically-focused therapies aimed to improve outcomes following SCI are investigating the application of epidural electric stimulation. To date, this has been proven to be a promising rehabilitation strategy when used in conjunction with physiotherapy [267,268]. The underlying cell-based mechanism is not well understood and studies investigating whether epidural stimulation alters endogenous NSCs following SCI would provide important insight regarding the mechanism by which motor function is restored.
6. Future Directions
Endogenous NSCs are promising cell target following SCI as a means to replace lost cells and potentially restore neural circuitry. It is unlikely that there is a single-bullet approach that will lead to SCI repair. Hence, combinatorial strategies that incorporate NSC activation and rehabilitation (for example) warrant further study. A comprehensive understanding of the NSC pool phenotypes, and factors that regulate their behavior, combined with a sound knowledge of which cells are most relevant for neural repair and improved function, are at the forefront of developing novel therapeutics. To this end, the field must continue to examine the contribution of endogenous NSCs to the regenerative process through foundational experiments such as lineage-tracking using viral vector and transgenic mice models. Novel approaches including NSC-subpopulation-specific ablation on neural repair will provide insight into the cell-based mechanisms that underlie cell replacement and/or functional recovery. One of the aspects of NPC-mediated repair that must be considered is the injury model, as maintaining the periventricular NSC niche is imperative for the proposed endogenous repair strategies. In considering different models of injury, the fact that dNSCs and pNSCs, specifically, are able to migrate within the parenchyma to get to the site of the lesion, suggests that neural repair mediated by NSCs “from afar” may still be a feasible approach. Beyond this, studies that explore the window of efficacy for NPC activation and sex and age-dependent factors that influence outcomes are also critical for realizing self-repair of the injured spinal cord.
7. Conclusions
The inherent capacity for NSC activation in mammals is promising in terms of harnessing the potential of NSCs for repair of the injured spinal cord. We propose that a comprehensive understanding of resident NSCs characteristics, their lineage relationship, and the factors that regulate their behavior will enhance our ability to unlock the regenerative potential of the mammalian spinal cord. While the complexity of the pathophysiology that follows SCI remains a challenge, it is also a relatively widespread feat that across the animal kingdom, many groups, including teleost fish, urodeles, and some lizards are capable of both perfect and imperfect structural repair of the spinal cord which results in functional recovery. What remains a challenge in the field is establishing whether improved structural and functional outcomes are mediated by endogenous NSC activation. Indeed, while correlations between NSC activation and improved outcomes have been demonstrated, it is unclear whether NSCs are necessary and/or sufficient for the beneficial results. Studies using lineage tracking and NSC ablation models are critical to answer this important question. Further, future studies should employ and exploit the regenerative expertise of these species to establish key targets, NSC sub-populations, and environmental factors that are required for successful spinal cord repair. Future goals include the identification of spinal cord NSC subpopulations and using lineage tracking to identify their contribution to neural repair following interventions that directly, or indirectly through the niche, enhance their activation in the injured spinal cord. Considerations of timing, degree of injury, as well as age and sex will undoubtedly impact structural and functional outcomes following SCI and are critical aspects to consider to ultimately realize endogenous NSC activation strategies to treat SCI survivors.
Author Contributions
Conceptualization, E.A.B.G., N.L. and C.M.M.; writing—original draft, review and editing, E.A.B.G., N.L., K.S.K.L. and C.M.M.; figures, E.A.B.G., N.L. and C.M.M.; project administration, E.A.B.G.; supervision, C.M.M. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by the Canadian Institute of Health Research (CIHR studentship, N.L.) and the Wings for Life Foundation (Operating Grant, C.M.M. and E.A.B.G.).
Acknowledgments
The authors would like to thank members of the Morshead lab for their valuable discussion during the preparation of this manuscript. All figures were created with BioRender.com (https://help.biorender.com/en/articles/3619405-how-do-i-cite-biorender).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kwon, B.K.; Tetzlaff, W.; Grauer, J.N.; Beiner, J.; Vaccaro, A.R. Pathophysiology and Pharmacologic Treatment of Acute Spinal Cord Injury. Spine J. 2004, 4, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Sekhon, L.H.S.; Fehlings, M.G. Epidemiology, Demographics, and Pathophysiology of Acute Spinal Cord Injury. Spine 2001, 26, S2–S12. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Velumian, A.A.; Fehlings, M.G. The Role of Excitotoxicity in Secondary Mechanisms of Spinal Cord Injury: A Review with an Emphasis on the Implications for White Matter Degeneration. J. Neurotrauma 2004, 21, 754–774. [Google Scholar] [CrossRef] [PubMed]
- Brockie, S.; Hong, J.; Fehlings, M.G. The Role of Microglia in Modulating Neuroinflammation after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 9706. [Google Scholar] [CrossRef]
- Oyinbo, C.A. Secondary Injury Mechanisms in Traumatic Spinal Cord Injury: A Nugget of This Multiply Cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar]
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef]
- Pan, W.; Kastin, A.J. Cytokine Transport across the Injured Blood-Spinal Cord Barrier. Curr. Pharm. Des. 2008, 14, 1620–1624. [Google Scholar] [CrossRef][Green Version]
- Rezvan, M.; Meknatkhah, S.; Hassannejad, Z.; Sharif-Alhoseini, M.; Zadegan, S.A.; Shokraneh, F.; Vaccaro, A.R.; Lu, Y.; Rahimi-Movaghar, V. Time-Dependent Microglia and Macrophages Response after Traumatic Spinal Cord Injury in Rat: A Systematic Review. Injury 2020, 51, 2390–2401. [Google Scholar] [CrossRef]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.-E.; Vernoux, N.; Tremblay, M.-È.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia Are an Essential Component of the Neuroprotective Scar That Forms after Spinal Cord Injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.J.; Popovich, P.G. Inflammation and Its Role in Neuroprotection, Axonal Regeneration and Functional Recovery after Spinal Cord Injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Pukos, N.; Goodus, M.T.; Sahinkaya, F.R.; McTigue, D.M. Myelin Status and Oligodendrocyte Lineage Cells over Time after Spinal Cord Injury: What Do We Know and What Still Needs to Be Unwrapped? Glia 2019, 67, 2178–2202. [Google Scholar] [CrossRef]
- Tripathi, R.; McTigue, D.M. Prominent Oligodendrocyte Genesis along the Border of Spinal Contusion Lesions. Glia 2007, 55, 698–711. [Google Scholar] [CrossRef]
- Lytle, J.M.; Chittajallu, R.; Wrathall, J.R.; Gallo, V. NG2 Cell Response in the CNP-EGFP Mouse after Contusive Spinal Cord Injury. Glia 2009, 57, 270–285. [Google Scholar] [CrossRef]
- Okada, S.; Hara, M.; Kobayakawa, K.; Matsumoto, Y.; Nakashima, Y. Astrocyte Reactivity and Astrogliosis after Spinal Cord Injury. Neurosci. Res. 2018, 126, 39–43. [Google Scholar] [CrossRef]
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Hackett, A.R.; Lee, J.K. Understanding the NG2 Glial Scar after Spinal Cord Injury. Front. Neurol. 2016, 7, 199. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Not Everything Is Scary about a Glial Scar. Nature 2016, 532, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Jiang, M.; Li, H. Functional Requirement of Dicer1 and MiR-17-5p in Reactive Astrocyte Proliferation after Spinal Cord Injury in the Mouse. Glia 2014, 62, 2044–2060. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Kanner, S.; Zacs, M.; Frisca, F.; Pinto, A.R.; Currie, P.D.; Pinkas-Kramarski, R. Rapamycin Increases Neuronal Survival, Reduces Inflammation and Astrocyte Proliferation after Spinal Cord Injury. Mol. Cell Neurosci. 2015, 68, 82–91. [Google Scholar] [CrossRef]
- Okada, S.; Nakamura, M.; Katoh, H.; Miyao, T.; Shimazaki, T.; Ishii, K.; Yamane, J.; Yoshimura, A.; Iwamoto, Y.; Toyama, Y.; et al. Conditional Ablation of Stat3 or Socs3 Discloses a Dual Role for Reactive Astrocytes after Spinal Cord Injury. Nat. Med. 2006, 12, 829–834. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Silver, J. The Glial Scar Is More than Just Astrocytes. Exp. Neurol. 2016, 286, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Lukovic, D.; Stojkovic, M.; Moreno-Manzano, V.; Jendelova, P.; Sykova, E.; Bhattacharya, S.S.; Erceg, S. Concise Review: Reactive Astrocytes and Stem Cells in Spinal Cord Injury: Good Guys or Bad Guys? Stem Cells 2015, 33, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte Scar Formation Aids Central Nervous System Axon Regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Chen, C.Z.; Neumann, B.; Förster, S.; Franklin, R.J.M. Schwann Cell Remyelination of the Central Nervous System: Why Does It Happen and What Are the Benefits? Open Biol. 2021, 11, 200352. [Google Scholar] [CrossRef]
- Meletis, K.; Barnabé-Heider, F.; Carlén, M.; Evergren, E.; Tomilin, N.; Shupliakov, O.; Frisén, J. Spinal Cord Injury Reveals Multilineage Differentiation of Ependymal Cells. PLoS Biol. 2008, 6, e182. [Google Scholar] [CrossRef]
- Barnabé-Heider, F.; Göritz, C.; Sabelström, H.; Takebayashi, H.; Pfrieger, F.W.; Meletis, K.; Frisén, J. Origin of New Glial Cells in Intact and Injured Adult Spinal Cord. Cell Stem. Cell 2010, 7, 470–482. [Google Scholar] [CrossRef]
- Ren, Y.; Ao, Y.; O’Shea, T.M.; Burda, J.E.; Bernstein, A.M.; Brumm, A.J.; Muthusamy, N.; Ghashghaei, H.T.; Carmichael, S.T.; Cheng, L.; et al. Ependymal Cell Contribution to Scar Formation after Spinal Cord Injury Is Minimal, Local and Dependent on Direct Ependymal Injury. Sci. Rep. 2017, 7, 41122. [Google Scholar] [CrossRef] [PubMed]
- Sabelström, H.; Stenudd, M.; Réu, P.; Dias, D.O.; Elfineh, M.; Zdunek, S.; Damberg, P.; Göritz, C.; Frisén, J. Resident Neural Stem Cells Restrict Tissue Damage and Neuronal Loss after Spinal Cord Injury in Mice. Science 2013, 342, 637–640. [Google Scholar] [CrossRef]
- Becker, C.G.; Becker, T. Adult Zebrafish as a Model for Successful Central Nervous System Regeneration. Restor. Neurol. Neurosci. 2008, 26, 71–80. [Google Scholar]
- Gilbert, E.A.B.; Vickaryous, M.K. Neural Stem/Progenitor Cells Are Activated during Tail Regeneration in the Leopard Gecko (Eublepharis Macularius). J. Comp. Neurol. 2018, 526, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Mchedlishvili, L.; Mazurov, V.; Grassme, K.S.; Goehler, K.; Robl, B.; Tazaki, A.; Roensch, K.; Duemmler, A.; Tanaka, E.M. Reconstitution of the Central and Peripheral Nervous System during Salamander Tail Regeneration. Proc. Natl. Acad. Sci. USA 2012, 109, E2258–E2266. [Google Scholar] [CrossRef] [PubMed]
- Mchedlishvili, L.; Epperlein, H.H.; Telzerow, A.; Tanaka, E.M. A Clonal Analysis of Neural Progenitors during Axolotl Spinal Cord Regeneration Reveals Evidence for Both Spatially Restricted and Multipotent Progenitors. Development 2007, 134, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.G.; Becker, T. Neuronal Regeneration from Ependymo-Radial Glial Cells: Cook, Little Pot, Cook! Dev. Cell 2015, 32, 516–527. [Google Scholar] [CrossRef]
- Harel, N.Y.; Strittmatter, S.M. Can Regenerating Axons Recapitulate Developmental Guidance during Recovery from Spinal Cord Injury? Nat. Rev. Neurosci. 2006, 7, 603–616. [Google Scholar] [CrossRef]
- Ferretti, P.; Zhang, F.; O’Neill, P. Changes in Spinal Cord Regenerative Ability through Phylogenesis and Development: Lessons to Be Learnt. Dev. Dyn. 2003, 226, 245–256. [Google Scholar] [CrossRef]
- Becker, C.G.; Corral, R.D.D. Neural Development and Regeneration: It’s All in Your Spinal Cord. Development 2015, 142, 811–816. [Google Scholar] [CrossRef]
- Tanaka, E.M.; Ferretti, P. Considering the Evolution of Regeneration in the Central Nervous System. Nat. Rev. Neurosci. 2009, 10, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Tazaki, A.; Tanaka, E.M.; Fei, J.-F. Salamander Spinal Cord Regeneration: The Ultimate Positive Control in Vertebrate Spinal Cord Regeneration. Dev. Biol. 2017, 432, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.B.; Morshead, C. Stem Cell Heterogeneity and Regenerative Competence: The Enormous Potential of Rare Cells. Neural. Regen Res. 2021, 16, 285. [Google Scholar] [CrossRef] [PubMed]
- Morshead, C.M.; Reynolds, B.A.; Craig, C.G.; McBurney, M.W.; Staines, W.A.; Morassutti, D.; Weiss, S.; van der Kooy, D. Neural Stem Cells in the Adult Mammalian Forebrain: A Relatively Quiescent Subpopulation of Subependymal Cells. Neuron 1994, 13, 1071–1082. [Google Scholar] [CrossRef]
- Reynolds, B.; Weiss, S. Generation of Neurons and Astrocytes from Isolated Cells of the Adult Mammalian Central Nervous System. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef]
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS Stem Cells Are Present in the Adult Mammalian Spinal Cord and Ventricular Neuroaxis. J. Neurosci. 1996, 16, 7599–7609. [Google Scholar] [CrossRef]
- Barnabé-Heider, F.; Frisén, J. Stem Cells for Spinal Cord Repair. Cell Stem. Cell 2008, 3, 16–24. [Google Scholar] [CrossRef]
- Xu, W.; Sachewsky, N.; Azimi, A.; Hung, M.; Gappasov, A.; Morshead, C.M. Myelin Basic Protein Regulates Primitive and Definitive Neural Stem Cell Proliferation from the Adult Spinal Cord. Stem. Cells 2017, 35, 485–496. [Google Scholar] [CrossRef]
- Weiss, S.; Reynolds, B.A.; Vescovi, A.L.; Morshead, C.; Craig, C.G.; van der Kooy, D. Is There a Neural Stem Cell in the Mammalian Forebrain? Trends Neurosci. 1996, 19, 387–393. [Google Scholar] [CrossRef]
- Coles-Takabe, B.L.K.; Brain, I.; Purpura, K.A.; Karpowicz, P.; Zandstra, P.W.; Morshead, C.M.; van der Kooy, D. Don’t Look: Growing Clonal versus Nonclonal Neural Stem Cell Colonies. Stem. Cells 2008, 26, 2938–2944. [Google Scholar] [CrossRef]
- Johansson, C.B.; Momma, S.; Clarke, D.L.; Risling, M.; Lendahl, U.; Frisén, J. Identification of a Neural Stem Cell in the Adult Mammalian Central Nervous System. Cell 1999, 96, 25–34. [Google Scholar] [CrossRef]
- Horner, P.J.; Power, A.E.; Kempermann, G.; Kuhn, H.G.; Palmer, T.D.; Winkler, J.; Thal, L.J.; Gage, F.H. Proliferation and Differentiation of Progenitor Cells throughout the Intact Adult Rat Spinal Cord. J. Neurosci. 2000, 20, 2218–2228. [Google Scholar] [CrossRef]
- Shihabuddin, L.S.; Horner, P.J.; Ray, J.; Gage, F.H. Adult Spinal Cord Stem Cells Generate Neurons after Transplantation in the Adult Dentate Gyrus. J. Neurosci. 2000, 20, 8727–8735. [Google Scholar] [CrossRef] [PubMed]
- Temple, S. The Development of Neural Stem Cells. Nature 2001, 414, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.G.; Becker, T.; Hugnot, J.-P. The Spinal Ependymal Zone as a Source of Endogenous Repair Cells across Vertebrates. Prog. Neurobiol. 2018, 170, 67–80. [Google Scholar] [CrossRef]
- Horky, L.L.; Galimi, F.; Gage, F.H.; Horner, P.J. Fate of Endogenous Stem/Progenitor Cells Following Spinal Cord Injury. J. Comp. Neurol. 2006, 498, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.A.; Weiss, S. Clonal and Population Analyses Demonstrate That an EGF-Responsive Mammalian Embryonic CNS Precursor Is a Stem Cell. Dev. Biol. 1996, 175, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ghazale, H.; Ripoll, C.; Leventoux, N.; Jacob, L.; Azar, S.; Mamaeva, D.; Glasson, Y.; Calvo, C.-F.; Thomas, J.-L.; Meneceur, S.; et al. RNA Profiling of the Human and Mouse Spinal Cord Stem Cell Niches Reveals an Embryonic-like Regionalization with MSX1+ Roof-Plate-Derived Cells. Stem. Cell Rep. 2019, 12, 1159–1177. [Google Scholar] [CrossRef]
- Chevreau, R.; Ghazale, H.; Ripoll, C.; Chalfouh, C.; Delarue, Q.; Hemonnot-Girard, A.L.; Mamaeva, D.; Hirbec, H.; Rothhut, B.; Wahane, S.; et al. RNA Profiling of Mouse Ependymal Cells after Spinal Cord Injury Identifies the Oncostatin Pathway as a Potential Key Regulator of Spinal Cord Stem Cell Fate. Cells 2021, 10, 3332. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, Y.; Zhang, H.; Chen, L.; Cao, L.; Yang, L.; Wang, C.; Pan, Y.; Tang, Q.; Tan, W.; et al. The Neural Stem Cell Properties of PKD2L1+ Cerebrospinal Fluid-Contacting Neurons in Vitro. Front. Cell Neurosci. 2021, 15, 630882. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Plane, J.M.; Jiang, P.; Zhou, C.J.; Deng, W. Concise Review: Quiescent and Active States of Endogenous Adult Neural Stem Cells: Identification and Characterization. Stem. Cells 2011, 29, 907–912. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G. Activity Dependency and Aging in the Regulation of Adult Neurogenesis. Csh. Perspect. Biol. 2015, 7, a018929. [Google Scholar] [CrossRef]
- Fabel, K.; Kempermann, G. Physical Activity and the Regulation of Neurogenesis in the Adult and Aging Brain. Neuromol. Med. 2008, 10, 59–66. [Google Scholar] [CrossRef]
- Redmond, S.A.; Figueres-Oñate, M.; Obernier, K.; Nascimento, M.A.; Parraguez, J.I.; López-Mascaraque, L.; Fuentealba, L.C.; Alvarez-Buylla, A. Development of Ependymal and Postnatal Neural Stem Cells and Their Origin from a Common Embryonic Progenitor. Cell Rep. 2019, 27, 429–441.e3. [Google Scholar] [CrossRef]
- Furutachi, S.; Miya, H.; Watanabe, T.; Kawai, H.; Yamasaki, N.; Harada, Y.; Imayoshi, I.; Nelson, M.; Nakayama, K.I.; Hirabayashi, Y.; et al. Slowly Dividing Neural Progenitors Are an Embryonic Origin of Adult Neural Stem Cells. Nat. Neurosci. 2015, 18, 657–665. [Google Scholar] [CrossRef]
- Marqués-Torrejón, M.Á.; Williams, C.A.C.; Southgate, B.; Alfazema, N.; Clements, M.P.; Garcia-Diaz, C.; Blin, C.; Arranz-Emparan, N.; Fraser, J.; Gammoh, N.; et al. LRIG1 Is a Gatekeeper to Exit from Quiescence in Adult Neural Stem Cells. Nat. Commun. 2021, 12, 2594. [Google Scholar] [CrossRef] [PubMed]
- Codega, P.; Silva-Vargas, V.; Paul, A.; Maldonado-Soto, A.R.; Deleo, A.M.; Pastrana, E.; Doetsch, F. Prospective Identification and Purification of Quiescent Adult Neural Stem Cells from Their in Vivo Niche. Neuron 2014, 82, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Imura, T.; Nakano, I.; Kornblum, H.I.; Sofroniew, M.V. Phenotypic and Functional Heterogeneity of GFAP-expressing Cells in Vitro: Differential Expression of LeX/CD15 by GFAP-expressing Multipotent Neural Stem Cells and Non-neurogenic Astrocytes. Glia 2006, 53, 277–293. [Google Scholar] [CrossRef]
- Ellis, P.; Fagan, B.M.; Magness, S.T.; Hutton, S.; Taranova, O.; Hayashi, S.; McMahon, A.; Rao, M.; Pevny, L. SOX2, a Persistent Marker for Multipotential Neural Stem Cells Derived from Embryonic Stem Cells, the Embryo or the Adult. Dev. Neurosci. 2004, 26, 148–165. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Z. Employing Endogenous NSCs to Promote Recovery of Spinal Cord Injury. Stem. Cells Int. 2019, 2019, 1958631. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, N.; Xu, W.; Morshead, C.M. A Neurosphere Assay to Evaluate Endogenous Neural Stem Cell Activation in a Mouse Model of Minimal Spinal Cord Injury. J. Vis. Exp. 2018, 139, e57727. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Xue, X.; Nie, H.; Wu, X.; Sun, M.; Qiao, L.; Li, X.; Xu, B.; Xiao, Z.; Zhao, Y.; et al. Single-Cell RNA Sequencing Reveals Nestin+ Active Neural Stem Cells Outside the Central Canal after Spinal Cord Injury. Sci. China Life Sci. 2021, 65, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Stenudd, M.; Sabelström, H.; Frisén, J. Role of Endogenous Neural Stem Cells in Spinal Cord Injury and Repair. JAMA Neurol. 2015, 72, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Mothe, A.J.; Tator, C.H. Proliferation, Migration, and Differentiation of Endogenous Ependymal Region Stem/Progenitor Cells Following Minimal Spinal Cord Injury in the Adult Rat. Neuroscience 2005, 131, 177–187. [Google Scholar] [CrossRef]
- Hitoshi, S.; Seaberg, R.M.; Koscik, C.; Alexson, T.; Kusunoki, S.; Kanazawa, I.; Tsuji, S.; van der Kooy, D. Primitive Neural Stem Cells from the Mammalian Epiblast Differentiate to Definitive Neural Stem Cells under the Control of Notch Signaling. Genes Dev. 2004, 18, 1806–1811. [Google Scholar] [CrossRef]
- Sachewsky, N.; Xu, W.; Fuehrmann, T.; Kooy, D.V.D.; Morshead, C.M. Lineage Tracing Reveals the Hierarchical Relationship between Neural Stem Cell Populations in the Mouse Forebrain. Sci Rep. 2019, 9, 17730. [Google Scholar] [CrossRef]
- Sachewsky, N.; Leeder, R.; Xu, W.; Rose, K.L.; Yu, F.; van der Kooy, D.; Morshead, C.M. Primitive Neural Stem Cells in the Adult Mammalian Brain Give Rise to GFAP-Expressing Neural Stem Cells. Stem. Cell Rep. 2014, 2, 810–824. [Google Scholar] [CrossRef]
- Reeve, R.L.; Yammine, S.Z.; Morshead, C.M.; van der Kooy, D. Quiescent Oct4+ Neural Stem Cells (NSCs) Repopulate Ablated Glial Fibrillary Acidic Protein+ NSCs in the Adult Mouse Brain. Stem. Cells 2017, 35, 2071–2082. [Google Scholar] [CrossRef]
- Reeve, R.L.; Yammine, S.Z.; DeVeale, B.; van der Kooy, D. Targeted Activation of Primitive Neural Stem Cells in the Mouse Brain. Eur. J. Neurosci. 2016, 43, 1474–1485. [Google Scholar] [CrossRef]
- Llorens-Bobadilla, E.; Zhao, S.; Baser, A.; Saiz-Castro, G.; Zwadlo, K.; Martin-Villalba, A. Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells That Become Activated upon Brain Injury. Cell Stem. Cell 2015, 17, 329–340. [Google Scholar] [CrossRef]
- Beck, C.W.; Christen, B.; Slack, J.M.W. Molecular Pathways Needed for Regeneration of Spinal Cord and Muscle in a Vertebrate. Dev. Cell 2003, 5, 429–439. [Google Scholar] [CrossRef]
- Gengatharan, A.; Malvaut, S.; Marymonchyk, A.; Ghareghani, M.; Snapyan, M.; Fischer-Sternjak, J.; Ninkovic, J.; Götz, M.; Saghatelyan, A. Adult Neural Stem Cell Activation in Mice Is Regulated by the Day/Night Cycle and Intracellular Calcium Dynamics. Cell 2021, 184, 709–722.e13. [Google Scholar] [CrossRef] [PubMed]
- Linnarsson, S. Sequencing Single Cells Reveals Sequential Stem Cell States. Cell Stem. Cell 2015, 17, 251–252. [Google Scholar] [CrossRef] [PubMed]
- Urbán, N.; Blomfield, I.M.; Guillemot, F. Quiescence of Adult Mammalian Neural Stem Cells: A Highly Regulated Rest. Neuron 2019, 104, 834–848. [Google Scholar] [CrossRef]
- Mira, H.; Andreu, Z.; Suh, H.; Lie, D.C.; Jessberger, S.; Consiglio, A.; Emeterio, J.S.; Hortigüela, R.; Marqués-Torrejón, M.Á.; Nakashima, K.; et al. Signaling through BMPR-IA Regulates Quiescence and Long-Term Activity of Neural Stem Cells in the Adult Hippocampus. Cell Stem. Cell 2010, 7, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Clevers, H. Coexistence of Quiescent and Active Adult Stem Cells in Mammals. Science 2010, 327, 542–545. [Google Scholar] [CrossRef]
- Piccin, D.; Yu, F.; Morshead, C.M. Notch Signaling Imparts and Preserves Neural Stem Characteristics in the Adult Brain. Stem. Cells Dev. 2013, 22, 1541–1550. [Google Scholar] [CrossRef]
- Morshead, C.; van der Kooy, D. Postmitotic Death Is the Fate of Constitutively Proliferating Cells in the Subependymal Layer of the Adult Mouse Brain. J. Neurosci. 1992, 12, 249–256. [Google Scholar] [CrossRef]
- Morizur, L.; Chicheportiche, A.; Gauthier, L.R.; Daynac, M.; Boussin, F.D.; Mouthon, M.-A. Distinct Molecular Signatures of Quiescent and Activated Adult Neural Stem Cells Reveal Specific Interactions with Their Microenvironment. Stem. Cell Rep. 2018, 11, 565–577. [Google Scholar] [CrossRef]
- Suh, H.; Deng, W.; Gage, F.H. Signaling in Adult Neurogenesis. Annu Rev. Cell Dev. Bi 2009, 25, 253–275. [Google Scholar] [CrossRef]
- Llorens-Bobadilla, E.; Chell, J.M.; Merre, P.L.; Wu, Y.; Zamboni, M.; Bergenstråhle, J.; Stenudd, M.; Sopova, E.; Lundeberg, J.; Shupliakov, O.; et al. A Latent Lineage Potential in Resident Neural Stem Cells Enables Spinal Cord Repair. Science 2020, 370, eabb8795. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.Y.; Huang, J.; Wang, H. Waking up Quiescent Neural Stem Cells: Molecular Mechanisms and Implications in Neurodevelopmental Disorders. PLoS Genet. 2020, 16, e1008653. [Google Scholar] [CrossRef] [PubMed]
- Christie, K.J.; Turnley, A.M. Regulation of Endogenous Neural Stem/Progenitor Cells for Neural Repair—Factors That Promote Neurogenesis and Gliogenesis in the Normal and Damaged Brain. Front. Cell Neurosci. 2013, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, G.; Duart-Abadia, P.; Jordán-Pla, A.; Domingo-Muelas, A.; Blasco-Chamarro, L.; Ferrón, S.R.; Morante-Redolat, J.M.; Fariñas, I. Adult Neural Stem Cells Are Alerted by Systemic Inflammation through TNF-α Receptor Signaling. Cell Stem. Cell 2021, 28, 285–299.e9. [Google Scholar] [CrossRef]
- Miller, F.D.; Kaplan, D.R. Mobilizing Endogenous Stem Cells for Repair and Regeneration: Are We There Yet? Cell Stem. Cell 2012, 10, 650–652. [Google Scholar] [CrossRef]
- Daynac, M.; Chicheportiche, A.; Pineda, J.R.; Gauthier, L.R.; Boussin, F.D.; Mouthon, M.-A. Quiescent Neural Stem Cells Exit Dormancy upon Alteration of GABAAR Signaling Following Radiation Damage. Stem. Cell Res. 2013, 11, 516–528. [Google Scholar] [CrossRef]
- Wang, L.; Gu, S.; Gan, J.; Tian, Y.; Zhang, F.; Zhao, H.; Lei, D. Neural Stem Cells Overexpressing Nerve Growth Factor Improve Functional Recovery in Rats Following Spinal Cord Injury via Modulating Microenvironment and Enhancing Endogenous Neurogenesis. Front. Cell Neurosci. 2021, 15, 773375. [Google Scholar] [CrossRef]
- Dadwal, P.; Mahmud, N.; Sinai, L.; Azimi, A.; Fatt, M.; Wondisford, F.E.; Miller, F.D.; Morshead, C.M. Activating Endogenous Neural Precursor Cells Using Metformin Leads to Neural Repair and Functional Recovery in a Model of Childhood Brain Injury. Stem. Cell Rep. 2015, 5, 166–173. [Google Scholar] [CrossRef]
- Ayoub, R.; Ruddy, R.M.; Cox, E.; Oyefiade, A.; Derkach, D.; Laughlin, S.; Ades-aron, B.; Shirzadi, Z.; Fieremans, E.; MacIntosh, B.J.; et al. Assessment of Cognitive and Neural Recovery in Survivors of Pediatric Brain Tumors in a Pilot Clinical Trial Using Metformin. Nat. Med. 2020, 26, 1285–1294. [Google Scholar] [CrossRef]
- Hugnot, J.-P.; Franzen, R. The Spinal Cord Ependymal Region: A Stem Cell Niche in the Caudal Central Nervous System. Front. Biosci. (Landmark Ed.) 2011, 16, 1044–1059. [Google Scholar] [CrossRef]
- Marichal, N.; Reali, C.; Trujillo-Cenóz, O.; Russo, R.E. Spinal Cord Stem Cells In Their Microenvironment: The Ependyma as a Stem Cell Niche. Adv. Exp. Med. Biol. 2017, 1041, 55–79. [Google Scholar] [CrossRef]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the Stem Cell Niche for Tissue Regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, J.P.; Silva, W.N.; Costa, A.C.; Picoli, C.C.; Bitencourt, F.C.O.; Coimbra-Campos, L.M.C.; Resende, R.R.; Magno, L.A.V.; Romano-Silva, M.A.; Mintz, A.; et al. Neural Stem Cell Niche Heterogeneity. Semin. Cell Dev. Biol. 2019, 95, 42–53. [Google Scholar] [CrossRef]
- Kazanis, I.; ffrench-Constant, C. Extracellular Matrix and the Neural Stem Cell Niche. Dev. Neurobiol. 2011, 71, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, R.M.; Morshead, C.M. Home Sweet Home: The Neural Stem Cell Niche throughout Development and after Injury. Cell Tissue Res. 2017, 371, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, J. Bridging the Gap with Functional Collagen Scaffolds: Tuning Endogenous Neural Stem Cells for Severe Spinal Cord Injury Repair. Biomater Sci.-UK 2017, 6, 265–271. [Google Scholar] [CrossRef]
- Suzuki, H.; Ahuja, C.S.; Salewski, R.P.; Li, L.; Satkunendrarajah, K.; Nagoshi, N.; Shibata, S.; Fehlings, M.G. Neural Stem Cell Mediated Recovery Is Enhanced by Chondroitinase ABC Pretreatment in Chronic Cervical Spinal Cord Injury. PLoS ONE 2017, 12, e0182339. [Google Scholar] [CrossRef]
- Hamilton, L.K.; Truong, M.K.V.; Bednarczyk, M.R.; Aumont, A.; Fernandes, K.J.L. Cellular Organization of the Central Canal Ependymal Zone, a Niche of Latent Neural Stem Cells in the Adult Mammalian Spinal Cord. Neuroscience 2009, 164, 1044–1056. [Google Scholar] [CrossRef]
- Muthusamy, N.; Brumm, A.; Zhang, X.; Carmichael, S.T.; Ghashghaei, H.T. Foxj1 Expressing Ependymal Cells Do Not Contribute New Cells to Sites of Injury or Stroke in the Mouse Forebrain. Sci. Rep.-UK 2018, 8, 1766. [Google Scholar] [CrossRef]
- Abdi, K.; Lai, C.-H.; Paez-Gonzalez, P.; Lay, M.; Pyun, J.; Kuo, C.T. Uncovering Inherent Cellular Plasticity of Multiciliated Ependyma Leading to Ventricular Wall Transformation and Hydrocephalus. Nat. Commun. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Shah, P.T.; Stratton, J.A.; Stykel, M.G.; Abbasi, S.; Sharma, S.; Mayr, K.A.; Koblinger, K.; Whelan, P.J.; Biernaskie, J. Single-Cell Transcriptomics and Fate Mapping of Ependymal Cells Reveals an Absence of Neural Stem Cell Function. Cell 2018, 173, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Worthington, W.C., Jr.; Cathcart, R.S., III. Ependymal Cilia: Distribution and Activity in the Adult Human Brain. Science 1963, 139, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Manzano, V. Ependymal Cells in the Spinal Cord as Neuronal Progenitors. Curr. Opin. Pharm. 2020, 50, 82–87. [Google Scholar] [CrossRef]
- Furube, E.; Ishii, H.; Nambu, Y.; Kurganov, E.; Nagaoka, S.; Morita, M.; Miyata, S. Neural Stem Cell Phenotype of Tanycyte-like Ependymal Cells in the Circumventricular Organs and Central Canal of Adult Mouse Brain. Sci. Rep.-UK 2020, 10, 2826. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Cervello, C.; Soriano-Navarro, M.; Mirzadeh, Z.; Alvarez-Buylla, A.; Garcia-Verdugo, J.M. Biciliated Ependymal Cell Proliferation Contributes to Spinal Cord Growth. J. Comp. Neurol. 2012, 520, 3528–3552. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, Z.; Han, Y.-G.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cilia Organize Ependymal Planar Polarity. J. Neurosci. 2010, 30, 2600–2610. [Google Scholar] [CrossRef]
- Alfaro-Cervello, C.; Cebrian-Silla, A.; Soriano-Navarro, M.; Garcia-Tarraga, P.; Matías-Guiu, J.; Gomez-Pinedo, U.; Aguilar, P.M.; Alvarez-Buylla, A.; Luquin, M.-R.; Garcia-Verdugo, J.M. The Adult Macaque Spinal Cord Central Canal Zone Contains Proliferative Cells and Closely Resembles the Human. J. Comp. Neurol. 2014, 522, 1800–1817. [Google Scholar] [CrossRef]
- Malatesta, P.; Götz, M. Radial Glia—From Boring Cables to Stem Cell Stars. Development 2013, 140, 483–486. [Google Scholar] [CrossRef]
- Fei, J.-F.; Schuez, M.; Tazaki, A.; Taniguchi, Y.; Roensch, K.; Tanaka, E.M. CRISPR-Mediated Genomic Deletion of Sox2 in the Axolotl Shows a Requirement in Spinal Cord Neural Stem Cell Amplification during Tail Regeneration. Stem. Cell Rep. 2014, 3, 444–459. [Google Scholar] [CrossRef]
- Bruni, J.E. Ependymal Development, Proliferation, and Functions: A Review. Microsc. Res. Tech. 1998, 41, 2–13. [Google Scholar] [CrossRef]
- Petracca, Y.L.; Sartoretti, M.M.; Bella, D.J.D.; Marin-Burgin, A.; Carcagno, A.L.; Schinder, A.F.; Lanuza, G.M. The Late and Dual Origin of Cerebrospinal Fluid-Contacting Neurons in the Mouse Spinal Cord. Development 2016, 143, 880–891. [Google Scholar] [CrossRef]
- Christenson, J.; Alford, S.; Grillner, S.; Hökfelt, T. Co-Localized GABA and Somatostatin Use Different Ionic Mechanisms to Hyperpolarize Target Neurons in the Lamprey Spinal Cord. Neurosci. Lett. 1991, 134, 93–97. [Google Scholar] [CrossRef]
- Jalalvand, E.; Robertson, B.; Wallén, P.; Hill, R.H.; Grillner, S. Laterally Projecting Cerebrospinal Fluid-Contacting Cells in the Lamprey Spinal Cord Are of Two Distinct Types. J. Comp. Neurol. 2014, 522, 1753–1768. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.E.; Fernández, A.; Reali, C.; Radmilovich, M.; Trujillo-Cenóz, O. Functional and Molecular Clues Reveal Precursor-like Cells and Immature Neurones in the Turtle Spinal Cord. J. Physiol. 2004, 560, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Jalalvand, E.; Robertson, B.; Wallén, P.; Grillner, S. Ciliated Neurons Lining the Central Canal Sense Both Fluid Movement and PH through ASIC3. Nat. Commun. 2016, 7, 10002. [Google Scholar] [CrossRef]
- Busch, T.; Köttgen, M.; Hofherr, A. TRPP2 Ion Channels: Critical Regulators of Organ Morphogenesis in Health and Disease. Cell Calcium. 2017, 66, 25–32. [Google Scholar] [CrossRef]
- Kútna, V.; Ševc, J.; Gombalová, Z.; Matiašová, A.; Daxnerová, Z. Enigmatic Cerebrospinal Fluid-Contacting Neurons Arise Even after the Termination of Neurogenesis in the Rat Spinal Cord during Embryonic Development and Retain Their Immature-like Characteristics until Adulthood. Acta Histochem. 2014, 116, 278–285. [Google Scholar] [CrossRef]
- Orts-Del’Immagine, A.; Trouslard, J.; Airault, C.; Hugnot, J.-P.; Cordier, B.; Doan, T.; Kastner, A.; Wanaverbecq, N. Postnatal Maturation of Mouse Medullo-Spinal Cerebrospinal Fluid-Contacting Neurons. Neuroscience 2017, 343, 39–54. [Google Scholar] [CrossRef]
- Pontes, A.; Zhang, Y.; Hu, W. Novel Functions of GABA Signaling in Adult Neurogenesis. Front. Biol. 2013, 8, 496–507. [Google Scholar] [CrossRef]
- Giachino, C.; Barz, M.; Tchorz, J.S.; Tome, M.; Gassmann, M.; Bischofberger, J.; Bettler, B.; Taylor, V. GABA Suppresses Neurogenesis in the Adult Hippocampus through GABAB Receptors. J. Cell Sci. 2014, 127, e1. [Google Scholar] [CrossRef]
- Azevedo, P.O.; Lousado, L.; Paiva, A.E.; Andreotti, J.P.; Santos, G.S.P.; Sena, I.F.G.; Prazeres, P.H.D.M.; Filev, R.; Mintz, A.; Birbrair, A. Endothelial Cells Maintain Neural Stem Cells Quiescent in Their Niche. Neuroscience 2017, 363, 62–65. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Ma, D.; Yang, Y. Endothelial Cells Promote Neural Stem Cell Proliferation and Differentiation Associated with VEGF Activated Notch and Pten Signaling. Dev. Dynam 2010, 239, 2345–2353. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, Z.; Li, X.; Zhao, Y.; Wu, X.; Han, J.; Chen, B.; Li, J.; Fan, C.; Xu, B.; et al. Vascular Endothelial Growth Factor Activates Neural Stem Cells through Epidermal Growth Factor Receptor Signal after Spinal Cord Injury. Cns. Neurosci. 2019, 25, 375–385. [Google Scholar] [CrossRef]
- Sato, Y.; Uchida, Y.; Hu, J.; Young-Pearse, T.L.; Niikura, T.; Mukouyama, Y. Soluble APP Functions as a Vascular Niche Signal That Controls Adult Neural Stem Cell Number. Development 2017, 144, 2730–2736. [Google Scholar] [CrossRef]
- Tucker, R.; Drabikowski, K.; Hess, J.; Ferralli, J.; Chiquet-Ehrismann, R.; Adams, J. Phylogenetic Analysis of the Tenascin Gene Family: Evidence of Origin Early in the Chordate Lineage. BMC Evol. Biol. 2006, 6, 60. [Google Scholar] [CrossRef]
- Karus, M.; Denecke, B.; ffrench-Constant, C.; Wiese, S.; Faissner, A. The Extracellular Matrix Molecule Tenascin C Modulates Expression Levels and Territories of Key Patterning Genes during Spinal Cord Astrocyte Specification. Development 2011, 138, 5321–5331. [Google Scholar] [CrossRef]
- Imbeault, S.; Gauvin, L.G.; Toeg, H.D.; Pettit, A.; Sorbara, C.D.; Migahed, L.; DesRoches, R.; Menzies, A.S.; Nishii, K.; Paul, D.L.; et al. The Extracellular Matrix Controls Gap Junction Protein Expression and Function in Postnatal Hippocampal Neural Progenitor Cells. BMC Neurosci. 2009, 10, 13. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Y.; Kokovay, E.; Lin, G.; Chuang, S.-M.; Goderie, S.K.; Roysam, B.; Temple, S. Adult SVZ Stem Cells Lie in a Vascular Niche: A Quantitative Analysis of Niche Cell-Cell Interactions. Cell Stem. Cell 2008, 3, 289–300. [Google Scholar] [CrossRef]
- Carvalho-Paulo, D.; Neto, J.B.T.; Filho, C.S.; de Oliveira, T.C.G.; de Sousa, A.A.; dos Reis, R.R.; dos Santos, Z.A.; de Lima, C.M.; de Oliveira, M.A.; Said, N.M.; et al. Microglial Morphology Across Distantly Related Species: Phylogenetic, Environmental and Age Influences on Microglia Reactivity and Surveillance States. Front. Immunol. 2021, 12, 683026. [Google Scholar] [CrossRef]
- David, S.; Kroner, A. Repertoire of Microglial and Macrophage Responses after Spinal Cord Injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef]
- Akhmetzyanova, E.; Kletenkov, K.; Mukhamedshina, Y.; Rizvanov, A. Different Approaches to Modulation of Microglia Phenotypes after Spinal Cord Injury. Front. Syst. Neurosci. 2019, 13, 37. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Sato, K. Effects of Microglia on Neurogenesis. Glia 2015, 63, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Shechter, R.; Schwartz, M. Harnessing Monocyte-derived Macrophages to Control Central Nervous System Pathologies: No Longer ‘If’ but ‘How’. J. Pathol. 2013, 229, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Town, T.; Nikolic, V.; Tan, J. The Microglial “Activation” Continuum: From Innate to Adaptive Responses. J. Neuroinflamm. 2005, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Vay, S.U.; Flitsch, L.J.; Rabenstein, M.; Rogall, R.; Blaschke, S.; Kleinhaus, J.; Reinert, N.; Bach, A.; Fink, G.R.; Schroeter, M.; et al. The Plasticity of Primary Microglia and Their Multifaceted Effects on Endogenous Neural Stem Cells in Vitro and in Vivo. J. Neuroinflamm. 2018, 15, 226. [Google Scholar] [CrossRef]
- Osman, A.M.; Rodhe, J.; Shen, X.; Dominguez, C.A.; Joseph, B.; Blomgren, K. The Secretome of Microglia Regulate Neural Stem Cell Function. Neuroscience 2019, 405, 92–102. [Google Scholar] [CrossRef]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-Derived Microparticles Mediate Neuroinflammation after Traumatic Brain Injury. J. Neuroinflamm. 2017, 14, 47. [Google Scholar] [CrossRef]
- Hou, B.-R.; Jiang, C.; Wang, Z.-N.; Ren, H.-J. Exosome-Mediated Crosstalk between Microglia and Neural Stem Cells in the Repair of Brain Injury. Neural. Regen Res. 2019, 15, 1023–1024. [Google Scholar] [CrossRef]
- Butovsky, O.; Jedrychowski, M.P.; Cialic, R.; Krasemann, S.; Murugaiyan, G.; Fanek, Z.; Greco, D.J.; Wu, P.M.; Doykan, C.E.; Kiner, O.; et al. Targeting MiR-155 Restores Abnormal Microglia and Attenuates Disease in SOD1 Mice. Ann. Neurol. 2015, 77, 75–99. [Google Scholar] [CrossRef]
- Huang, S.; Ge, X.; Yu, J.; Han, Z.; Yin, Z.; Li, Y.; Chen, F.; Wang, H.; Zhang, J.; Lei, P. Increased MiR-124-3p in Microglial Exosomes Following Traumatic Brain Injury Inhibits Neuronal Inflammation and Contributes to Neurite Outgrowth via Their Transfer into Neurons. Faseb J. 2018, 32, 512–528. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.E.; Parikshak, N.N.; Belgard, T.G.; Geschwind, D.H. Genome-Wide, Integrative Analysis Implicates MicroRNA Dysregulation in Autism Spectrum Disorder. Nat. Neurosci. 2016, 19, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, X.; Kawaguchi, R.; Zhang, Y.; Wang, Q.; Monavarfeshani, A.; Yang, Z.; Chen, B.; Shi, Z.; Meng, H.; et al. Microglia-Organized Scar-Free Spinal Cord Repair in Neonatal Mice. Nature 2020, 587, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Nunan, R.; Sivasathiaseelan, H.; Khan, D.; Zaben, M.; Gray, W. Microglial VPAC1R Mediates a Novel Mechanism of Neuroimmune-Modulation of Hippocampal Precursor Cells via IL-4 Release. Glia 2014, 62, 1313–1327. [Google Scholar] [CrossRef]
- Tsarouchas, T.M.; Wehner, D.; Cavone, L.; Munir, T.; Keatinge, M.; Lambertus, M.; Underhill, A.; Barrett, T.; Kassapis, E.; Ogryzko, N.; et al. Dynamic Control of Proinflammatory Cytokines Il-1β and Tnf-α by Macrophages in Zebrafish Spinal Cord Regeneration. Nat. Commun. 2018, 9, 4670. [Google Scholar] [CrossRef]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of Two Distinct Macrophage Subsets with Divergent Effects Causing Either Neurotoxicity or Regeneration in the Injured Mouse Spinal Cord. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef]
- Ling, E.; Leblond, C.P. Investigation of Glial Cells in Semithin Sections. II. Variation with Age in the Numbers of the Various Glial Cell Types in Rat Cortex and Corpus Callosum. J. Comp. Neurol. 1973, 149, 73–81. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Wanner, I.B.; Anderson, M.A.; Song, B.; Levine, J.; Fernandez, A.; Gray-Thompson, Z.; Ao, Y.; Sofroniew, M.V. Glial Scar Borders Are Formed by Newly Proliferated, Elongated Astrocytes That Interact to Corral Inflammatory and Fibrotic Cells via STAT3-Dependent Mechanisms after Spinal Cord Injury. J. Neurosci. 2013, 33, 12870–12886. [Google Scholar] [CrossRef]
- Ridet, J.L.; Privat, A.; Malhotra, S.K.; Gage, F.H. Reactive Astrocytes: Cellular and Molecular Cues to Biological Function. Trends Neurosci. 1997, 20, 570–577. [Google Scholar] [CrossRef]
- Lau, L.T.; Yu, A.C.-H. Astrocytes Produce and Release Interleukin-1, Interleukin-6, Tumor Necrosis Factor Alpha and Interferon-Gamma Following Traumatic and Metabolic Injury. J. Neurotraum. 2001, 18, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Pöyhönen, S.; Er, S.; Domanskyi, A.; Airavaara, M. Effects of Neurotrophic Factors in Glial Cells in the Central Nervous System: Expression and Properties in Neurodegeneration and Injury. Front. Physiol. 2019, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive Astrocytes Protect Tissue and Preserve Function after Spinal Cord Injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Tica, J.; Didangelos, A. Comparative Transcriptomics of Rat and Axolotl After Spinal Cord Injury Dissects Differences and Similarities in Inflammatory and Matrix Remodeling Gene Expression Patterns. Front. Neurosci.-Switz 2018, 12, 808. [Google Scholar] [CrossRef]
- Antos, C.L.; Tanaka, E.M. Vertebrates That Regenerate As Models for Guiding Stem Cells. In The Cell Biology of Stem Cells; Springer: Boston, MA, USA, 2010; Volume 695, pp. 184–214. ISBN 978-1-4419-7036-7. [Google Scholar]
- Zambusi, A.; Ninkovic, J. Regeneration of the Central Nervous System-Principles from Brain Regeneration in Adult Zebrafish. World J. Stem Cells 2020, 12, 8–24. [Google Scholar] [CrossRef]
- Miller, S.J. Astrocyte Heterogeneity in the Adult Central Nervous System. Front. Cell Neurosci. 2018, 12, 401. [Google Scholar] [CrossRef]
- Emsley, J.G.; Arlotta, P.; Macklis, J.D. Star-Cross’d Neurons: Astroglial Effects on Neural Repair in the Adult Mammalian CNS. Trends Neurosci. 2004, 27, 238–240. [Google Scholar] [CrossRef]
- Horner, P.J.; Palmer, T.D. New Roles for Astrocytes: The Nightlife of an ‘Astrocyte’. La Vida Loca! Trends Neurosci. 2003, 26, 597–603. [Google Scholar] [CrossRef]
- Song, H.; Stevens, C.F.; Gage, F.H. Astroglia Induce Neurogenesis from Adult Neural Stem Cells. Nature 2002, 417, 39–44. [Google Scholar] [CrossRef]
- Faijerson, J.; Tinsley, R.B.; Apricó, K.; Thorsell, A.; Nodin, C.; Nilsson, M.; Blomstrand, F.; Eriksson, P.S. Reactive Astrogliosis Induces Astrocytic Differentiation of Adult Neural Stem/Progenitor Cells in Vitro. J. Neurosci. Res. 2006, 84, 1415–1424. [Google Scholar] [CrossRef]
- Lim, D.A.; Tramontin, A.D.; Trevejo, J.M.; Herrera, D.G.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Noggin Antagonizes BMP Signaling to Create a Niche for Adult Neurogenesis. Neuron 2000, 28, 713–726. [Google Scholar] [CrossRef]
- Jovanovic, V.M.; Salti, A.; Tilleman, H.; Zega, K.; Jukic, M.M.; Zou, H.; Friedel, R.H.; Prakash, N.; Blaess, S.; Edenhofer, F.; et al. BMP/SMAD Pathway Promotes Neurogenesis of Midbrain Dopaminergic Neurons In Vivo and in Human Induced Pluripotent and Neural Stem Cells. J. Neurosci. 2018, 38, 1662–1676. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, W.; Yang, P. Current States of Endogenous Stem Cells in Adult Spinal Cord. J. Neurosci. Res. 2015, 93, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, J.; Zhou, L.; Pollard, S.M.; Smith, A. Interplay between FGF2 and BMP Controls the Self-Renewal, Dormancy and Differentiation of Rat Neural Stem Cells. J. Cell Sci. 2011, 124, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Regan, M.R.; Huang, Y.H.; Kim, Y.S.; Dykes-Hoberg, M.I.; Jin, L.; Watkins, A.M.; Bergles, D.E.; Rothstein, J.D. Variations in Promoter Activity Reveal a Differential Expression and Physiology of Glutamate Transporters by Glia in the Developing and Mature CNS. J. Neurosci. 2007, 27, 6607–6619. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Walters, G.; Paulsen, A.R.; Scarisbrick, I.A. Astrocyte Heterogeneity across the Brain and Spinal Cord Occurs Developmentally, in Adulthood and in Response to Demyelination. PLoS ONE 2017, 12, e0180697. [Google Scholar] [CrossRef]
- Molofsky, A.V.; Kelley, K.W.; Tsai, H.-H.; Redmond, S.A.; Chang, S.M.; Madireddy, L.; Chan, J.R.; Baranzini, S.E.; Ullian, E.M.; Rowitch, D.H. Astrocyte-Encoded Positional Cues Maintain Sensorimotor Circuit Integrity. Nature 2014, 509, 189–194. [Google Scholar] [CrossRef]
- Barkho, B.Z.; Song, H.; Aimone, J.B.; Smrt, R.D.; Kuwabara, T.; Nakashima, K.; Gage, F.H.; Zhao, X. Identification of Astrocyte-Expressed Factors That Modulate Neural Stem/Progenitor Cell Differentiation. Stem. Cells Dev. 2006, 15, 407–421. [Google Scholar] [CrossRef]
- Wang, F.; Hao, H.; Zhao, S.; Zhang, Y.; Liu, Q.; Liu, H.; Liu, S.; Yuan, Q.; Bing, L.; Ling, E.-A.; et al. Roles of Activated Astrocyte in Neural Stem Cell Proliferation and Differentiation. Stem. Cell Res. 2011, 7, 41–53. [Google Scholar] [CrossRef]
- Wang, F.-W.; Jia, D.-Y.; Du, Z.-H.; Fu, J.; Zhao, S.-D.; Liu, S.-M.; Zhang, Y.-M.; Ling, E.-A.; Hao, A.-J. Roles of Activated Astrocytes in Bone Marrow Stromal Cell Proliferation and Differentiation. Neuroscience 2009, 160, 319–329. [Google Scholar] [CrossRef]
- Lakshman, N.; Bourget, C.; Siu, R.; Bamm, V.V.; Xu, W.; Harauz, G.; Morshead, C.M. Niche-dependent Inhibition of Neural Stem Cell Proliferation and Oligodendrogenesis Is Mediated by the Presence of Myelin Basic Protein. Stem. Cells 2021, 39, 776–786. [Google Scholar] [CrossRef]
- Almad, A.; Sahinkaya, F.R.; McTigue, D.M. Oligodendrocyte Fate after Spinal Cord Injury. Neurotherapeutics 2011, 8, 262–273. [Google Scholar] [CrossRef]
- Lamers, K.J.; de Reus, H.P.; Jongen, P.J. Myelin Basic Protein in CSF as Indicator of Disease Activity in Multiple Sclerosis. Mult. Scler. 1998, 4, 124–126. [Google Scholar] [CrossRef]
- Karakatsani, A.; Shah, B.; de Almodovar, C.R. Blood Vessels as Regulators of Neural Stem Cell Properties. Front. Mol. Neurosci. 2019, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Androutsellis-Theotokis, A.; Rueger, M.A.; Park, D.M.; Boyd, J.D.; Padmanabhan, R.; Campanati, L.; Stewart, C.V.; LeFranc, Y.; Plenz, D.; Walbridge, S.; et al. Angiogenic Factors Stimulate Growth of Adult Neural Stem Cells. PLoS ONE 2010, 5, e9414. [Google Scholar] [CrossRef][Green Version]
- Katsimpardi, L.; Litterman, N.K.; Schein, P.A.; Miller, C.M.; Loffredo, F.S.; Wojtkiewicz, G.R.; Chen, J.W.; Lee, R.T.; Wagers, A.J.; Rubin, L.L. Vascular and Neurogenic Rejuvenation of the Aging Mouse Brain by Young Systemic Factors. Science 2014, 344, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The Ageing Systemic Milieu Negatively Regulates Neurogenesis and Cognitive Function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef]
- Zhang, H.; Fang, X.; Huang, D.; Luo, Q.; Zheng, M.; Wang, K.; Cao, L.; Yin, Z. Erythropoietin Signaling Increases Neurogenesis and Oligodendrogenesis of Endogenous Neural Stem Cells Following Spinal Cord Injury Both in Vivo and in Vitro. Mol. Med. Rep. 2018, 17, 264–272. [Google Scholar] [CrossRef]
- Pathipati, P.; Gorba, T.; Scheepens, A.; Goffin, V.; Sun, Y.; Fraser, M. Growth Hormone and Prolactin Regulate Human Neural Stem Cell Regenerative Activity. Neuroscience 2011, 190, 409–427. [Google Scholar] [CrossRef]
- Kolb, B.; Morshead, C.; Gonzalez, C.; Kim, M.; Gregg, C.; Shingo, T.; Weiss, S. Growth Factor-Stimulated Generation of New Cortical Tissue and Functional Recovery after Stroke Damage to the Motor Cortex of Rats. J. Cereb. Blood Flow Metab. 2007, 27, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Shingo, T.; Gregg, C.; Enwere, E.; Fujikawa, H.; Hassam, R.; Geary, C.; Cross, J.C.; Weiss, S. Pregnancy-Stimulated Neurogenesis in the Adult Female Forebrain Mediated by Prolactin. Science 2003, 299, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.R.; Lovett, S.J.; Majda, B.T.; Yoon, J.H.; Wheeler, L.P.G.; Hodgetts, S.I. Neurotrophic Factors for Spinal Cord Repair: Which, Where, How and When to Apply, and for What Period of Time? Brain Res. 2015, 1619, 36–71. [Google Scholar] [CrossRef]
- Hodgetts, S.I.; Harvey, A.R. Neurotrophic Factors Used to Treat Spinal Cord Injury. Vitam. Horm. 2016, 104, 405–457. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; O’Shea, T.M.; Burda, J.E.; Ao, Y.; Barlatey, S.L.; Bernstein, A.M.; Kim, J.H.; James, N.D.; Rogers, A.; Kato, B.; et al. Required Growth Facilitators Propel Axon Regeneration across Complete Spinal Cord Injury. Nature 2018, 561, 396–400. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Cai, Q.; Shen, Y.-Y.; Cai, X.-Y.; Lei, H.-Y. Combined Use of NGF/BDNF/BFGF Promotes Proliferation and Differentiation of Neural Stem Cells in Vitro. Int. J. Dev. Neurosci. 2014, 38, 74–78. [Google Scholar] [CrossRef] [PubMed]
- McTigue, D.M.; Horner, P.J.; Stokes, B.T.; Gage, F.H. Neurotrophin-3 and Brain-Derived Neurotrophic Factor Induce Oligodendrocyte Proliferation and Myelination of Regenerating Axons in the Contused Adult Rat Spinal Cord. J. Neurosci. 1998, 18, 5354–5365. [Google Scholar] [CrossRef]
- Schnell, L.; Schneider, R.; Kolbeck, R.; Barde, Y.-A.; Schwab, M.E. Neurotrophin-3 Enhances Sprouting of Corticospinal Tract during Development and after Adult Spinal Cord Lesion. Nature 1994, 367, 170–173. [Google Scholar] [CrossRef]
- Li, Q.; Ford, M.C.; Lavik, E.B.; Madri, J.A. Modeling the Neurovascular Niche: VEGF- and BDNF-mediated Cross-talk between Neural Stem Cells and Endothelial Cells: An in Vitro Study. J. Neurosci. Res. 2006, 84, 1656–1668. [Google Scholar] [CrossRef]
- Tavazoie, M.; der Veken, L.V.; Silva-Vargas, V.; Louissaint, M.; Colonna, L.; Zaidi, B.; Garcia-Verdugo, J.M.; Doetsch, F. A Specialized Vascular Niche for Adult Neural Stem Cells. Cell Stem. Cell 2008, 3, 279–288. [Google Scholar] [CrossRef]
- Sun, G.J.; Zhou, Y.; Stadel, R.P.; Moss, J.; Yong, J.H.A.; Ito, S.; Kawasaki, N.K.; Phan, A.T.; Oh, J.H.; Modak, N.; et al. Tangential Migration of Neuronal Precursors of Glutamatergic Neurons in the Adult Mammalian Brain. PNAS 2015, 112, 9484–9489. [Google Scholar] [CrossRef]
- Chang, D.-J.; Cho, H.-Y.; Hwang, S.; Lee, N.; Choi, C.; Lee, H.; Hong, K.S.; Oh, S.-H.; Kim, H.S.; Shin, D.A.; et al. Therapeutic Effect of BDNF-Overexpressing Human Neural Stem Cells (F3.BDNF) in a Contusion Model of Spinal Cord Injury in Rats. Int. J. Mol. Sci. 2021, 22, 6970. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.-L.; Zou, Y.; Shi, Y.; Zhang, P.; Zhang, R.-P.; Dai, X.-J.; Liu, B.; Wang, T.-H. Tree Shrew Neural Stem Cell Transplantation Promotes Functional Recovery of Tree Shrews with a Hemi-Sectioned Spinal Cord Injury by Upregulating Nerve Growth Factor Expression. Int. J. Mol. Med. 2018, 41, 3267–3277. [Google Scholar] [CrossRef]
- Yang, Z.; Duan, H.; Mo, L.; Qiao, H.; Li, X. The Effect of the Dosage of NT-3/Chitosan Carriers on the Proliferation and Differentiation of Neural Stem Cells. Biomaterials 2010, 31, 4846–4854. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xiang, Z.; Ying, Y.; Huang, Z.; Tu, Y.; Chen, M.; Ye, J.; Dou, H.; Sheng, S.; Li, X.; et al. Nerve Growth Factor (NGF) with Hypoxia Response Elements Loaded by Adeno-Associated Virus (AAV) Combined with Neural Stem Cells Improve the Spinal Cord Injury Recovery. Cell Death Discov. 2021, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Tator, C.H. Intrathecal Administration of Epidermal Growth Factor and Fibroblast Growth Factor 2 Promotes Ependymal Proliferation and Functional Recovery after Spinal Cord Injury in Adult Rats. J. Neurotraum. 2002, 19, 223–238. [Google Scholar] [CrossRef]
- Martens, D.J.; Seaberg, R.M.; Kooy, D.V.D. In Vivo Infusions of Exogenous Growth Factors into the Fourth Ventricle of the Adult Mouse Brain Increase the Proliferation of Neural Progenitors around the Fourth Ventricle and the Central Canal of the Spinal Cord. Eur. J. Neurosci. 2002, 16, 1045–1057. [Google Scholar] [CrossRef]
- Mah, A.T.; Yan, K.S.; Kuo, C.J. Wnt Pathway Regulation of Intestinal Stem Cells. J. Physiol. 2016, 594, 4837–4847. [Google Scholar] [CrossRef]
- Famili, F.; Perez, L.G.; Naber, B.A.; Noordermeer, J.N.; Fradkin, L.G.; Staal, F.J. The Non-Canonical Wnt Receptor Ryk Regulates Hematopoietic Stem Cell Repopulation in Part by Controlling Proliferation and Apoptosis. Cell Death Dis. 2016, 7, e2479. [Google Scholar] [CrossRef]
- Ortega, F.; Gascón, S.; Masserdotti, G.; Deshpande, A.; Simon, C.; Fischer, J.; Dimou, L.; Lie, D.C.; Schroeder, T.; Berninger, B. Oligodendrogliogenic and Neurogenic Adult Subependymal Zone Neural Stem Cells Constitute Distinct Lineages and Exhibit Differential Responsiveness to Wnt Signalling. Nat. Cell Biol. 2013, 15, 602–613. [Google Scholar] [CrossRef]
- Piccin, D.; Morshead, C.M. Wnt Signaling Regulates Symmetry of Division of Neural Stem Cells in the Adult Brain and in Response to Injury. Stem. Cells 2011, 29, 528–538. [Google Scholar] [CrossRef]
- Reya, T.; Duncan, A.W.; Ailles, L.; Domen, J.; Scherer, D.C.; Willert, K.; Hintz, L.; Nusse, R.; Weissman, I.L. A Role for Wnt Signalling in Self-Renewal of Haematopoietic Stem Cells. Nature 2003, 423, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-S.; Zhang, H.; Wang, W.; Hua, X.-Y.; Hu, Y.; Zhang, S.-Q.; Li, G.-W. Wnt-3a Protein Promote Neuronal Differentiation of Neural Stem Cells Derived from Adult Mouse Spinal Cord. Neurol. Res. 2013, 29, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, C.; Xiao, Z.; Zhao, Y.; Zhang, H.; Sun, J.; Zhuang, Y.; Wu, X.; Shi, J.; Chen, Y.; et al. A Collagen Microchannel Scaffold Carrying Paclitaxel-Liposomes Induces Neuronal Differentiation of Neural Stem Cells through Wnt/β-Catenin Signaling for Spinal Cord Injury Repair. Biomaterials 2018, 183, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Perale, G.; Rossi, F.; Sundstrom, E.; Bacchiega, S.; Masi, M.; Forloni, G.; Veglianese, P. Hydrogels in Spinal Cord Injury Repair Strategies. ACS Chem. Neurosci. 2011, 2, 336–345. [Google Scholar] [CrossRef]
- Shultz, R.B.; Zhong, Y. Hydrogel-Based Local Drug Delivery Strategies for Spinal Cord Repair. Neural. Regen Res. 2020, 16, 247–253. [Google Scholar] [CrossRef]
- Hlavac, N.; Kasper, M.; Schmidt, C.E. Progress toward Finding the Perfect Match: Hydrogels for Treatment of Central Nervous System Injury. Mater. Today Adv. 2020, 6, 100039. [Google Scholar] [CrossRef]
- Tom, V.J.; Sandrow-Feinberg, H.R.; Miller, K.; Domitrovich, C.; Bouyer, J.; Zhukareva, V.; Klaw, M.C.; Lemay, M.A.; Houlé, J.D. Exogenous BDNF Enhances the Integration of Chronically Injured Axons That Regenerate through a Peripheral Nerve Grafted into a Chondroitinase-Treated Spinal Cord Injury Site. Exp. Neurol. 2013, 239, 91–100. [Google Scholar] [CrossRef]
- Hayflick, L. Human Cells and Aging. Sci. Am. 1968, 218, 32–37. [Google Scholar] [CrossRef]
- Chan, M.; Yuan, H.; Soifer, I.; Maile, T.M.; Wang, R.Y.; Ireland, A.; O’Brien, J.; Goudeau, J.; Chan, L.; Vijay, T.; et al. Revisiting the Hayflick Limit: Insights from an Integrated Analysis of Changing Transcripts, Proteins, Metabolites and Chromatin. Biorxiv 2021. [Google Scholar] [CrossRef]
- Albors, A.R.; Tazaki, A.; Rost, F.; Nowoshilow, S.; Chara, O.; Tanaka, E.M. Planar Cell Polarity-Mediated Induction of Neural Stem Cell Expansion during Axolotl Spinal Cord Regeneration. Elife 2015, 4, e10230. [Google Scholar] [CrossRef]
- Chernoff, E.A.G.; Stocum, D.L.; Nye, H.L.D.; Cameron, J.A. Urodele Spinal Cord Regeneration and Related Processes. Dev. Dyn. 2003, 226, 295–307. [Google Scholar] [CrossRef]
- Lucini, C.; D’Angelo, L.; Cacialli, P.; Palladino, A.; de Girolamo, P. BDNF, Brain, and Regeneration: Insights from Zebrafish. Int. J. Mol. Sci. 2018, 19, 3155. [Google Scholar] [CrossRef] [PubMed]
- Tonge, D.A.; Leclere, P.G. Directed Axonal Growth towards Axolotl Limb Blastemas in Vitro. Neuroscience 2000, 100, 201–211. [Google Scholar] [CrossRef]
- Wehner, D.; Tsarouchas, T.M.; Michael, A.; Haase, C.; Weidinger, G.; Reimer, M.M.; Becker, T.; Becker, C.G. Wnt Signaling Controls Pro-Regenerative Collagen XII in Functional Spinal Cord Regeneration in Zebrafish. Nat. Commun. 2017, 8, 126. [Google Scholar] [CrossRef]
- O’Hara, C.M.; Chernoff, E.A.G. Growth Factor Modulation of Injury-Reactive Ependymal Cell Proliferation and Migration. Tissue Cell 1994, 26, 599–611. [Google Scholar] [CrossRef]
- Wagers, A.J. The Stem Cell Niche in Regenerative Medicine. Cell Stem. Cell 2012, 10, 362–369. [Google Scholar] [CrossRef]
- Wang, J.; Gallagher, D.; DeVito, L.M.; Cancino, G.I.; Tsui, D.; He, L.; Keller, G.M.; Frankland, P.W.; Kaplan, D.R.; Miller, F.D. Metformin Activates an Atypical PKC-CBP Pathway to Promote Neurogenesis and Enhance Spatial Memory Formation. Cell Stem. Cell 2012, 11, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Fatt, M.; Hsu, K.; He, L.; Wondisford, F.; Miller, F.D.; Kaplan, D.R.; Wang, J. Metformin Acts on Two Different Molecular Pathways to Enhance Adult Neural Precursor Proliferation/Self-Renewal and Differentiation. Stem. Cell Rep. 2015, 5, 988–995. [Google Scholar] [CrossRef]
- Erlandsson, A.; Morshead, C.M. Exploiting the Properties of Adult Stem Cells for the Treatment of Disease. Curr. Opin. Mol. 2006, 8, 331–337. [Google Scholar]
- Tedesco, D.; Haragsim, L. Cyclosporine: A Review. J. Transplant. 2012, 2012, 230386. [Google Scholar] [CrossRef]
- Hunt, J.; Cheng, A.; Hoyles, A.; Jervis, E.; Morshead, C.M. Cyclosporin A Has Direct Effects on Adult Neural Precursor Cells. J. Neurosci. 2010, 30, 2888–2896. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.; Morshead, C. Cyclosporin A Enhances Cell Survival in Neural Precursor Populations in the Adult Central Nervous System. Mol. Cell. Pharmacol. 2010, 3, 2888–2896. [Google Scholar] [CrossRef]
- Erlandsson, A.; Lin, C.-H.A.; Yu, F.; Morshead, C.M. Immunosuppression Promotes Endogenous Neural Stem and Progenitor Cell Migration and Tissue Regeneration after Ischemic Injury. Exp. Neurol. 2011, 230, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Sachewsky, N.; Hunt, J.; Cooke, M.J.; Azimi, A.; Zarin, T.; Miu, C.; Shoichet, M.S.; Morshead, C.M. Cyclosporin A Enhances Neural Precursor Cell Survival in Mice through a Calcineurin-Independent Pathway. Dis. Model. Mech. 2014, 7, 953–961. [Google Scholar] [CrossRef]
- Tuladhar, A.; Morshead, C.M.; Shoichet, M.S. Circumventing the Blood-Brain Barrier: Local Delivery of Cyclosporin A Stimulates Stem Cells in Stroke-Injured Rat Brain. J. Control. Release 2015, 215, 1–11. [Google Scholar] [CrossRef]
- Nusrat, L.; Livingston-Thomas, J.M.; Raguthevan, V.; Adams, K.; Vonderwalde, I.; Corbett, D.; Morshead, C.M. Cyclosporin A-Mediated Activation of Endogenous Neural Precursor Cells Promotes Cognitive Recovery in a Mouse Model of Stroke. Front. Aging Neurosci. 2018, 10, 93. [Google Scholar] [CrossRef]
- Potts, M.B.; Lim, D.A. An Old Drug for New Ideas: Metformin Promotes Adult Neurogenesis and Spatial Memory Formation. Cell Stem. Cell 2012, 11, 5–6. [Google Scholar] [CrossRef]
- Gilbert, E.; Kehtari, T.; Morshead, C. Metformin Activates Resident Neural Stem and Progenitor Cells, Reduces Inflammation and Improves Functional Recovery Following Spinal Cord Injury. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Tao, L.; Li, D.; Liu, H.; Jiang, F.; Xu, Y.; Cao, Y.; Gao, R.; Chen, G. Neuroprotective Effects of Metformin on Traumatic Brain Injury in Rats Associated with NF-ΚB and MAPK Signaling Pathway. Brain Res. Bull. 2018, 140, 154–161. [Google Scholar] [CrossRef]
- Łabuzek, K.; Liber, S.; Gabryel, B.; Okopień, B. Metformin Has Adenosine-Monophosphate Activated Protein Kinase (AMPK)-Independent Effects on LPS-Stimulated Rat Primary Microglial Cultures. Pharm. Rep. 2010, 62, 827–848. [Google Scholar] [CrossRef]
- DiBona, V.L.; Shah, M.K.; Krause, K.J.; Zhu, W.; Voglewede, M.M.; Smith, D.M.; Crockett, D.P.; Zhang, H. Metformin Reduces Neuroinflammation and Improves Cognitive Functions after Traumatic Brain Injury. Neurosci. Res. 2021, 172, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y. Metformin and Inflammation: Its Potential Beyond Glucose-Lowering Effect. Endocr. Metab. Immune Disord. Targets 2015, 15, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin Inhibits Cytokine-Induced Nuclear Factor KappaB Activation via AMP-Activated Protein Kinase Activation in Vascular Endothelial Cells. Hypertens Dallas Tex 1979 2006, 47, 1183–1188. [Google Scholar]
- Jin, Q.; Cheng, J.; Liu, Y.; Wu, J.; Wang, X.; Wei, S.; Zhou, X.; Qin, Z.; Jia, J.; Zhen, X. Improvement of Functional Recovery by Chronic Metformin Treatment Is Associated with Enhanced Alternative Activation of Microglia/Macrophages and Increased Angiogenesis and Neurogenesis Following Experimental Stroke. Brain Behav. Immun. 2014, 40, 131–142. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, G.; Zhang, Z.; Wang, Y.; Yang, G.-Y. Metformin Promotes Focal Angiogenesis and Neurogenesis in Mice Following Middle Cerebral Artery Occlusion. Neurosci. Lett. 2014, 579, 46–51. [Google Scholar] [CrossRef]
- Livingston, J.M.; Syeda, T.; Christie, T.; Gilbert, E.A.B.; Morshead, C.M. Subacute Metformin Treatment Reduces Inflammation and Improves Functional Outcome Following Neonatal Hypoxia Ischemia. Brain Behav. Immun.—Health 2020, 7, 100119. [Google Scholar] [CrossRef]
- Ruddy, R.M.; Adams, K.V.; Morshead, C.M. Age- and Sex-Dependent Effects of Metformin on Neural Precursor Cells and Cognitive Recovery in a Model of Neonatal Stroke. Sci. Adv. 2019, 5, eaax1912. [Google Scholar] [CrossRef]
- Derkach, D.; Kehtari, T.; Renaud, M.; Heidari, M.; Lakshman, N.; Morshead, C.M. Metformin Pretreatment Rescues Olfactory Memory Associated with Subependymal Zone Neurogenesis in a Juvenile Model of Cranial Irradiation. Cell Rep. Med. 2021, 2, 100231. [Google Scholar] [CrossRef]
- Kosaraju, J.; Seegobin, M.; Gouveia, A.; Syal, C.; Sarma, S.N.; Lu, K.J.; Ilin, J.; He, L.; Wondisford, F.E.; Lagace, D.; et al. Metformin Promotes CNS Remyelination and Improves Social Interaction Following Focal Demyelination through CBP Ser436 Phosphorylation. Exp. Neurol. 2020, 334, 113454. [Google Scholar] [CrossRef]
- Ma, D.; Wang, B.; Zawadzka, M.; Gonzalez, G.; Wu, Z.; Yu, B.; Rawlins, E.L.; Franklin, R.J.M.; Zhao, C. A Subpopulation of Foxj1-Expressing, Nonmyelinating Schwann Cells of the Peripheral Nervous System Contribute to Schwann Cell Remyelination in the Central Nervous System. J. Neurosci. 2018, 38, 9228–9239. [Google Scholar] [CrossRef]
- Adams, D.S.; Robinson, K.R.; Fukumoto, T.; Yuan, S.; Albertson, R.C.; Yelick, P.; Kuo, L.; McSweeney, M.; Levin, M. Early, H+-V-ATPase-Dependent Proton Flux Is Necessary for Consistent Left-Right Patterning of Non-Mammalian Vertebrates. Development 2006, 133, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, N.; Dotta, B.T. Electromagnetic Fields as Structure-Function Zeitgebers in Biological Systems: Environmental Orchestrations of Morphogenesis and Consciousness. Front. Integr. Neurosci. 2014, 8, 84. [Google Scholar] [CrossRef] [PubMed]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling Cell Behavior Electrically: Current Views and Future Potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef] [PubMed]
- Nuccitelli, R.; Nuccitelli, P.; Ramlatchan, S.; Sanger, R.; Smith, P.J.S. Imaging the Electric Field Associated with Mouse and Human Skin Wounds. Wound Repair Regen 2008, 16, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Ariza, C.A.; Fleury, A.T.; Tormos, C.J.; Petruk, V.; Chawla, S.; Oh, J.; Sakaguchi, D.S.; Mallapragada, S.K. The Influence of Electric Fields on Hippocampal Neural Progenitor Cells. Stem. Cell Rev. Rep. 2010, 6, 585–600. [Google Scholar] [CrossRef]
- Babona-Pilipos, R.; Droujinine, I.A.; Popovic, M.R.; Morshead, C.M. Adult Subependymal Neural Precursors, but Not Differentiated Cells, Undergo Rapid Cathodal Migration in the Presence of Direct Current Electric Fields. PLoS ONE 2011, 6, e23808. [Google Scholar] [CrossRef][Green Version]
- Sefton, E.; Iwasa, S.N.; Morrison, T.; Naguib, H.E.; Popovic, M.R.; Morshead, C.M. Electric Field Application In Vivo Regulates Neural Precursor Cell Behavior in the Adult Mammalian Forebrain. ENeuro 2020, 7, ENEURO.0273-20.2020. [Google Scholar] [CrossRef]
- Meng, X.; Li, W.; Young, F.; Gao, R.; Chalmers, L.; Zhao, M.; Song, B. Electric Field-Controlled Directed Migration of Neural Progenitor Cells in 2D and 3D Environments. J. Vis. Exp. Jove 2012, 60, e3453. [Google Scholar] [CrossRef]
- Williamson, M.R.; Jones, T.A.; Drew, M.R. Functions of Subventricular Zone Neural Precursor Cells in Stroke Recovery. Behav. Brain Res. 2019, 376, 112209. [Google Scholar] [CrossRef]
- Jeong, S.H.; Jun, S.B.; Song, J.K.; Kim, S.J. Activity-Dependent Neuronal Cell Migration Induced by Electrical Stimulation. Med. Biol. Eng. Comput. 2009, 47, 93–99. [Google Scholar] [CrossRef]
- Babona-Pilipos, R.; Liu, N.; Pritchard-Oh, A.; Mok, A.; Badawi, D.; Popovic, M.R.; Morshead, C.M. Calcium Influx Differentially Regulates Migration Velocity and Directedness in Response to Electric Field Application. Exp. Cell Res. 2018, 368, 202–214. [Google Scholar] [CrossRef]
- Nakajima, K.; Zhu, K.; Sun, Y.-H.; Hegyi, B.; Zeng, Q.; Murphy, C.J.; Small, J.V.; Chen-Izu, Y.; Izumiya, Y.; Penninger, J.M.; et al. KCNJ15/Kir4.2 Couples with Polyamines to Sense Weak Extracellular Electric Fields in Galvanotaxis. Nat. Commun. 2015, 6, 8532. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, S.N.; Rashidi, A.; Sefton, E.; Liu, N.X.; Popovic, M.R.; Morshead, C.M. Charge-Balanced Electrical Stimulation Can Modulate Neural Precursor Cell Migration in the Presence of Endogenous Electric Fields in Mouse Brains. ENeuro 2019, 6, ENEURO.0382-19.2019. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lyon, J.G.; Alvarado-Velez, M.; Betancur, M.I.; Mokarram, N.; Shin, J.H.; Bellamkonda, R.V. Enriching Neural Stem Cell and Pro-Healing Glial Phenotypes with Electrical Stimulation after Traumatic Brain Injury in Male Rats. Biorxiv 2020. [Google Scholar] [CrossRef]
- Ayanwuyi, L.; Tokarska, N.; McLean, N.A.; Johnston, J.M.; Verge, V.M.K. Brief Electrical Nerve Stimulation Enhances Intrinsic Repair Capacity of the Focally Demyelinated Central Nervous System. Neural. Regen Res. 2021, 17, 1042–1050. [Google Scholar] [CrossRef]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Straaten, M.G.V.; Drubach, D.I.; et al. Neuromodulation of Lumbosacral Spinal Networks Enables Independent Stepping after Complete Paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).