Single-Molecule and Vesicle Trafficking Analysis of Ubiquitination Involved in the Activity of Ammonium Transporter AMT1;3 in Arbidopsis under High Ammonium Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Construct
2.2. Growth Conditions
2.3. Western Blot Analysis
2.4. Confocal Laser Scanning Microscopy and Image Analysis
2.5. VA-TIRF Microscopy and Single-Particle Fluorescence Image
2.6. Fluorescence-Correlation Spectroscopy Analysis
2.7. Heterologous Expression of AMT1;3-EGFP in Yeast
3. Results
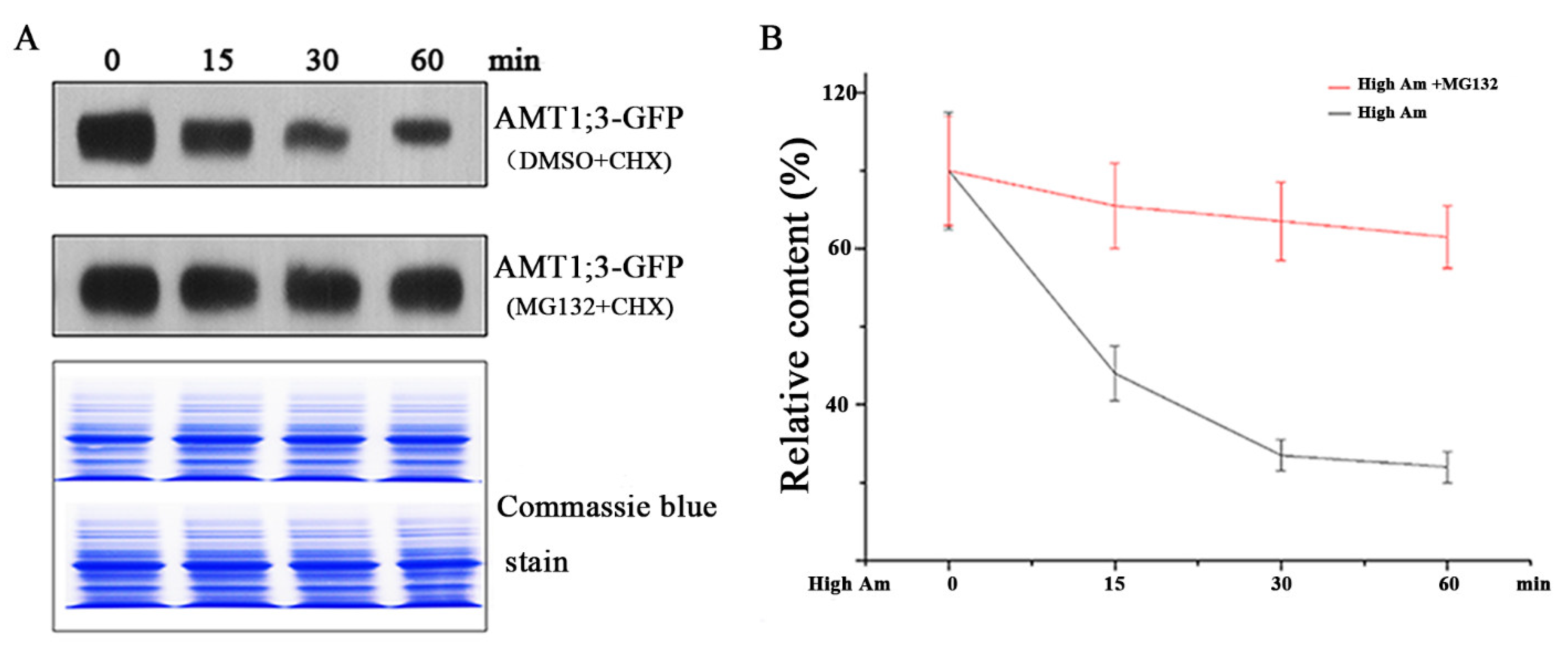
3.1. Ubiquitination Is Correlated with the Degradation of AMT1;3 under High Ammonium Stress
3.2. Ubiquitination Affects the Dynamics of AMT1;3 under High Ammonium Stress
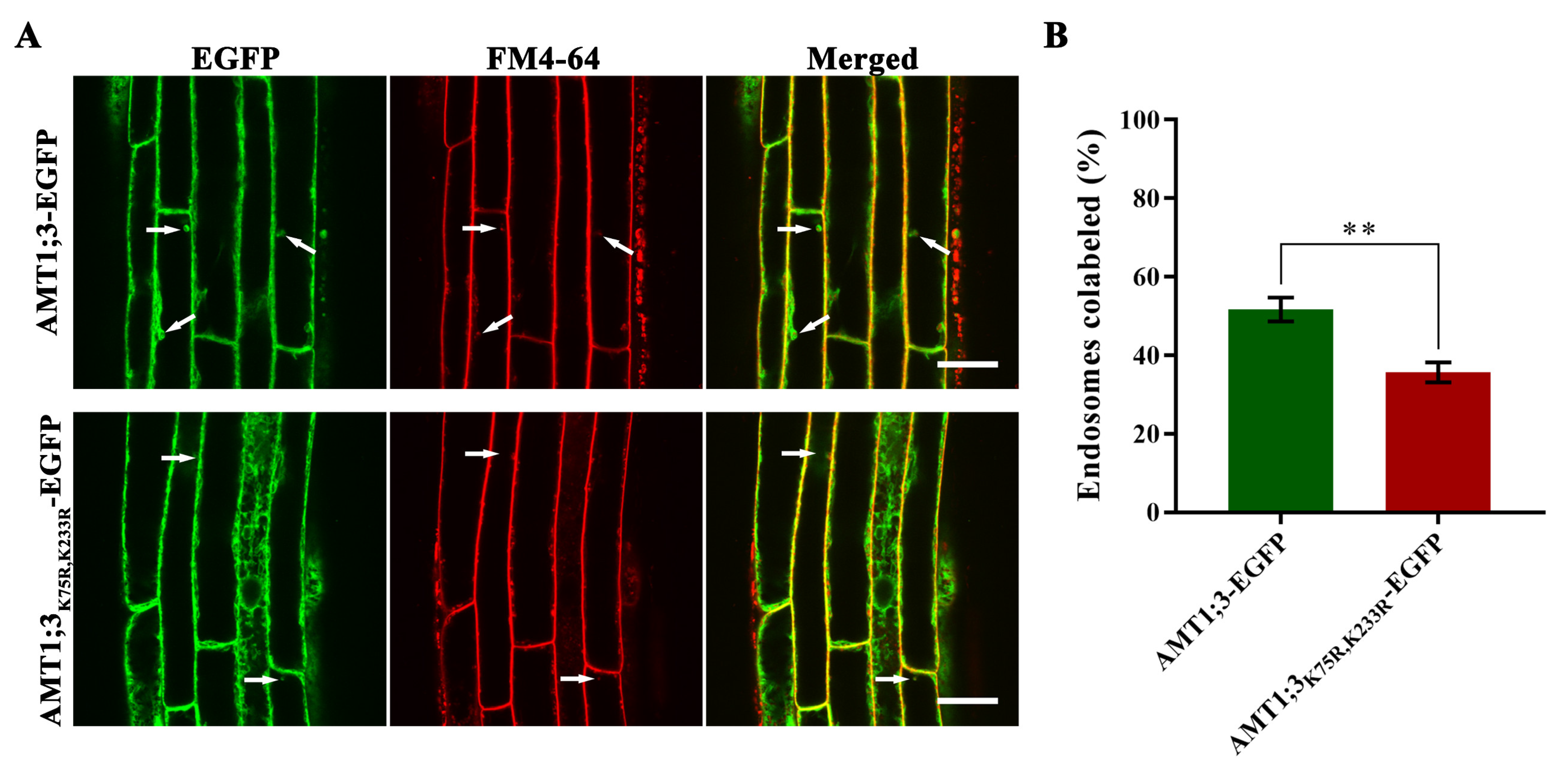
3.3. Loss of AMT1;3 Ubiquitination Impairs Endocytosis under High Ammonium Stress
3.4. Ubiquitination Affects Vesicular Transport of the AMT1;3 Protein under High Ammonium Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miflin, B.J.; Lea, P.J. The pathway of nitrogen assimilation in plants. Phytochemistry 1976, 15, 873–885. [Google Scholar] [CrossRef]
- Näsholm, T.; Ekblad, A.; Nordin, A.; Giesler, R.; Högberg, M.; Högberg, P. Boreal forest plants take up organic nitrogen. Nature 1998, 392, 914–916. [Google Scholar] [CrossRef]
- Crawford, N.M.; Forde, B.G. Molecular and developmental biology of inorganic nitrogen nutrition. Arab. Book 2002, 1, e0011. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef]
- Martin, M.H.; Marschner, H. Mineral nutrition of higher plants. J. Ecol. 1988, 76, 1250. [Google Scholar] [CrossRef]
- Von Wirén, N.; Merrick, M. Regulation and function of ammonium carriers in bacteria, fungi, and plants. Top. Curr. Genet. 2004, 9, 95–120. [Google Scholar]
- Sonia, G.; Laurence, L.; Alain, G.; Olaf, N.; Wolf, B.F.; Nicolaus, V.W. Three functional transporters for constitutive, diurnally regulated, and starvation-induced uptake of ammonium into Arabidopsis roots. Plant Cell 1999, 11, 937–947. [Google Scholar]
- Yuan, L.; Loque, D.; Kojima, S.; Rauch, S.; Ishiyama, K.; Inoue, E.; Takahashi, H.; von Wirén, N. The organization of High-Affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-Type transporters. Plant Cell 2007, 19, 2636–2652. [Google Scholar] [CrossRef]
- Loqué, D.; Yuan, L.; Kojima, S.; Gojon, A.; Wirth, J.; Gazzarrini, S.; Ishiyama, K.; Takahashi, H.; Von Wirén, N. Additive contribution of AMT1;1 and AMT1;3 to high-affinity ammonium uptake across the plasma membrane of nitrogen-deficient Arabidopsis roots. Plant J. 2010, 48, 522–534. [Google Scholar] [CrossRef]
- Wang, Q.L.; Zhao, Y.Y.; Luo, W.X.; Li, R.L.; He, Q.H.; Fang, X.H.; Michele, R.D.; Ast, C.; Wirén, N.V.; Lin, J.X. Single-particle analysis reveals shutoff control of the Arabidopsis ammonium transporter AMT1;3 by clustering and internalization. Proc. Natl. Acad. Sci. USA 2013, 110, 13204–13209. [Google Scholar] [CrossRef]
- Yuan, L.; Gu, R.; Xuan, Y.; Smith-Valle, E.; Loqué, D.; Frommer, W.B.; von Wirén, N. Allosteric regulation of transport activity by heterotrimerization of Arabidopsis ammonium transporter complexes in vivo. Plant Cell 2013, 25, 974–984. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Huarancca Reyes, T.; Uemura, T.; Baral, A.; Fujimaki, A.; Luo, Y.; Morita, Y.; Saeki, Y.; Maekawa, S.; Yasuda, S.; et al. The TGN/EE SNARE protein SYP61 and the ubiquitin ligase ATL31 cooperatively regulate plant responses to carbon/nitrogen conditions in Arabidopsis. Plant Cell 2022, 29, 34. [Google Scholar] [CrossRef]
- Eugene, O.; David, A.; Michael, R. Principles of Ubiquitin-Dependent Signaling. Annu. Rev. Cell Dev. Biol. 2018, 34, 137–162. [Google Scholar]
- Barberon, M.; Zelazny, E.; Robert, S.; Conéjéro, G.; Curie, C.; Friml, J.; Vert, G. Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc. Natl. Acad. Sci. USA 2011, 108, E450–E458. [Google Scholar] [CrossRef]
- Dubeaux, G.; Neveu, J.; Zelazny, E.; Vert, G. Metal sensing by the IRT1 transporter-receptor orchestrates its own degradation and plant metal nutrition. Mol. Cell 2018, 69, 953–964. [Google Scholar] [CrossRef]
- Kasai, K.; Takano, J.; Miwa, K.; Toyoda, A.; Fujiwara, T. High boron-induced ubiquitination regulates vacuolar sorting of the BOR1 borate transporter in Arabidopsis thaliana. J. Biol. Chem. 2011, 286, 6175–6183. [Google Scholar] [CrossRef]
- Yoshinari, A.; Hosokawa, T.; Beier, M.P.; Oshima, K.; Ogino, Y.; Hori, C.; Takasuka, T.E.; Fukao, Y.; Fujiwara, T.; Takano, J. Transport-coupled ubiquitination of the borate transporter BOR1 for its boron-dependent degradation. Plant Cell 2021, 33, 420–438. [Google Scholar] [CrossRef]
- Korbei, B.; Moulinier-Anzola, J.; De-Araujo, L.; Lucyshyn, D.; Retzer, K.; Khan, M.A.; Luschnig, C. Arabidopsis TOL proteins act as gatekeepers for vacuolar sorting of PIN2 plasma membrane protein. Curr. Biol. 2013, 23, 2500–2505. [Google Scholar] [CrossRef]
- Cao, Y.Y.; He, Q.Z.H.; Qi, Z.X.; Zhang, Y.; Lu, L.; Xue, J.Y.; Li, R.L.; Lin, J.X. Dynamics and endocytosis of flot1 in Arabidopsis require CPI1 function. Int. J. Mol. Sci. 2020, 21, 1552. [Google Scholar] [CrossRef]
- Yu, M.; Liu, H.J.; Dong, Z.Y.; Xiao, J.W.; Su, B.D.; Fan, L.S.; Komis, G.; Samaj, J.; Lin, J.X.; Li, R.L. The dynamics and endocytosis of Flot1 protein in response to flg22 in Arabidopsis. J. Physiol. 2017, 215, 73–84. [Google Scholar] [CrossRef]
- Cui, Y.N.; Li, X.J.; Yu, M.; Li, R.L.; Fan, L.S.; Zhu, Y.F.; Lin, J.X. Sterols regulate endocytic pathways during flg22-induced defense responses in Arabidopsis. Development 2018, 145, 19. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.R.; Vega-Sánchez, M.E.; Zhu, T.; Wang, G.L. Ubiquitination-mediated protein degradation and modification: An emerging theme in plant-microbe interactions. Cell Res. 2006, 16, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, A.; Moriya, K.; Sonoda, Y.; Ikeda, A.; Von Wirén, N.; Hayakawa, T.; Yamaguchi, J.; Yamaya, T. Constitutive expression of a novel-type ammonium transporter OsAMT2 in rice plants. Plant Cell Physiol. 2003, 44, 206–211. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, X.; Zhang, N.; Feng, X.; Huang, Y.; Zeng, Q.; Wu, J.; Zhang, J.; Qi, Y. Genome-wide identification and transcriptional analysis of ammonium transporters in Saccharum. Genomics 2021, 113, 1671–1680. [Google Scholar] [CrossRef]
- Huang, L.; Li, J.; Zhang, B.; Hao, Y.; Ma, F. Genome-wide identification and expression analysis of AMT gene family in Apple (Malus domestica Borkh.). Horticulturae 2022, 8, 457. [Google Scholar] [CrossRef]
- Wu, X.; Liu, T.; Zhang, Y.; Duan, F.; Neuhäuser, B.; Ludewig, U.; Schulze, W.X.; Yuan, L. Ammonium and nitrate regulate NH4+ uptake activity of Arabidopsis ammonium transporter AtAMT1;3 via phosphorylation at multiple C-terminal sites. J. Exp. Bot. 2019, 70, 4919–4930. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Riezman, H. Proteasome-Independent functions of ubiquitin in endocytosis and signaling. Science 2007, 315, 201–205. [Google Scholar] [CrossRef]
- Isono, E.; Kalinowska, K. ESCRT-dependent degradation of ubiquitylated plasma membrane proteins in plants. Curr. Opin. Plant Biol. 2017, 40, 49–55. [Google Scholar] [CrossRef]
- Lindy, A.; René, B.; Nenad, M.; Tomasz, P.; Justyna, W.; Jeanette, C.M.; Tobias, S.; Jirí, F.; Christian, L. Intracellular trafficking and proteolysis of the Arabidopsis auxin efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 2006, 8, 249–256. [Google Scholar]
- Vera, G.; Thomas, S.; Heidrun, H.; Sophia, M.; Tobias, M.; Thomas, B.; Marta, D.T.; John, W.M.; Silke, R. Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr. Biol. 2008, 18, 1824–1832. [Google Scholar]
- Hyun, K.L.; Seok, K.C.; Ora, S.; Xu, Z.Y.; Inhwan, H.; Woo, T.K. Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell 2009, 21, 622–641. [Google Scholar]
- Jentsch, T.J. Discovery of CLC transport proteins: Cloning, structure, function and pathophysiology. J. Physiol. 2015, 593, 4091–4109. [Google Scholar] [CrossRef]
- Haglund, K.; Sigismund, S.; Polo, S.; Szymkiewicz, I.; Di Fiore, P.P.; Dikic, I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 2003, 5, 461–466. [Google Scholar] [CrossRef]
- Guo, X.; Shen, S.; Song, S.; He, S.; Cui, Y.; Xing, G.; Wang, J.; Yin, Y.; Fan, L.; He, F.; et al. The E3 ligase Smurf1 regulates Wolfram syndrome protein stability at the endoplasmic reticulum. J. Biol. Chem. 2011, 286, 18037–18047. [Google Scholar] [CrossRef]
- Nakatsu, F.; Sakuma, M.; Matsuo, Y.; Arase, H.; Yamasaki, S.; Nakamura, N.; Saito, T.; Ohno, H. A Di-leucine signal in the ubiquitin moiety. Possible involvement in ubiquitination mediated endocytosis. J. Biol. Chem. 2000, 275, 26213–26219. [Google Scholar] [CrossRef]
- Terrell, J.; Shih, S.; Dunn, R.; Hicke, L. A function for monoubiquitination in the internalization of a G protein-coupled receptor. Mol. Cell 1998, 1, 193–202. [Google Scholar] [CrossRef]
- Ma, X.; Claus, L.A.; Leslie, M.E.; Tao, K.; Wu, Z.; Liu, J.; Yu, X.; Li, B.; Zhou, J.; Savatin, D.V.; et al. Ligand-induced monoubiquitination of BIK1 regulates plant immunity. Nature 2020, 581, 199–203. [Google Scholar] [CrossRef]
- Bonifacino, J.S.; Traub, L.M. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 2003, 72, 395–447. [Google Scholar] [CrossRef]
- Martins, S.; Dohmann, E.; Cayrel, A.; Johnson, A.; Fischer, W.; Pojer, F.; Satiat-Jeunemaître, B.; Jaillais, Y.; Chory, J.; Geldner, N.; et al. Internalization and vacuolar targeting of the brassinosteroid hormone receptor BRI1 are regulated by ubiquitination. Nat. Commun. 2015, 6, 6151. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Cao, Y.; Ge, Y.; Xu, J.; Li, R.; Yang, M.; Chen, Y.; Wu, D.; Xiao, J.; Li, R. Single-Molecule and Vesicle Trafficking Analysis of Ubiquitination Involved in the Activity of Ammonium Transporter AMT1;3 in Arbidopsis under High Ammonium Stress. Cells 2022, 11, 3651. https://doi.org/10.3390/cells11223651
Zhao R, Cao Y, Ge Y, Xu J, Li R, Yang M, Chen Y, Wu D, Xiao J, Li R. Single-Molecule and Vesicle Trafficking Analysis of Ubiquitination Involved in the Activity of Ammonium Transporter AMT1;3 in Arbidopsis under High Ammonium Stress. Cells. 2022; 11(22):3651. https://doi.org/10.3390/cells11223651
Chicago/Turabian StyleZhao, Ran, Yangyang Cao, Yanrui Ge, Jing Xu, Ruofan Li, Mei Yang, Yingying Chen, Dingjie Wu, Jianwei Xiao, and Ruili Li. 2022. "Single-Molecule and Vesicle Trafficking Analysis of Ubiquitination Involved in the Activity of Ammonium Transporter AMT1;3 in Arbidopsis under High Ammonium Stress" Cells 11, no. 22: 3651. https://doi.org/10.3390/cells11223651
APA StyleZhao, R., Cao, Y., Ge, Y., Xu, J., Li, R., Yang, M., Chen, Y., Wu, D., Xiao, J., & Li, R. (2022). Single-Molecule and Vesicle Trafficking Analysis of Ubiquitination Involved in the Activity of Ammonium Transporter AMT1;3 in Arbidopsis under High Ammonium Stress. Cells, 11(22), 3651. https://doi.org/10.3390/cells11223651





