Abrocitinib Attenuates Microglia-Mediated Neuroinflammation after Traumatic Brain Injury via Inhibiting the JAK1/STAT1/NF-κB Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Fluid Percussion Injury (FPI) Model
2.3. Bioinformatic Analysis
2.4. MRI
2.5. Hematoxylin and Eosin (H&E) Staining and Nissl Staining
2.6. Evans Blue Dye Extravasation
2.7. Cerebral Blood Flow
2.8. Modified Neurological Behavioral Tests
2.9. IHC Staining
2.10. TUNEL Staining
2.11. Cell Culture, LPS Model, Experimental Design, and Drug Administration
2.12. Immunofluorescence Staining
2.13. Routine Analysis of Blood
2.14. Enzyme-Linked Immunosorbent Assay (ELISA)
2.15. Western Blot
2.16. Statistical Analysis
3. Results
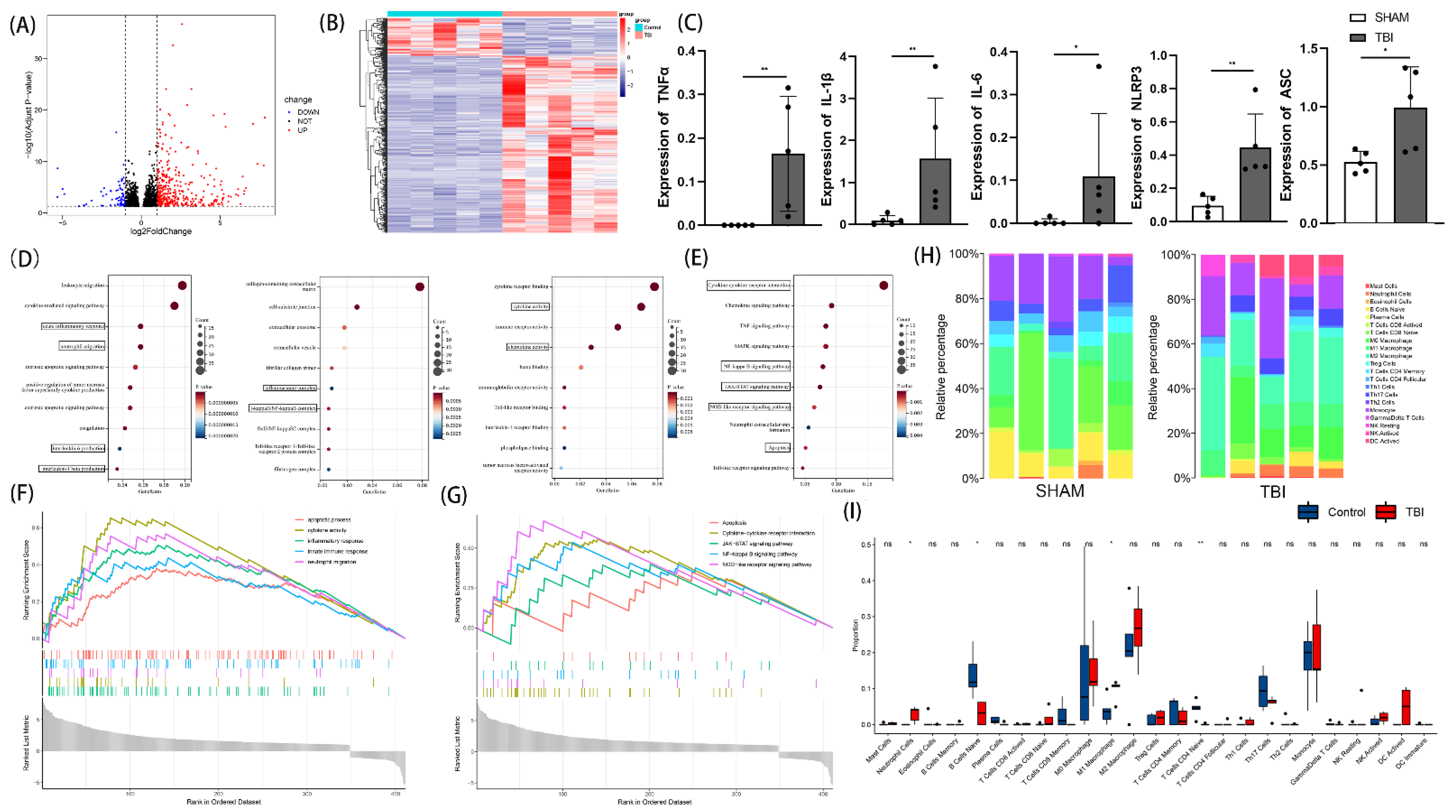
3.1. Sequence-to-Function Analysis via Next-Generation Sequencing (NGS)
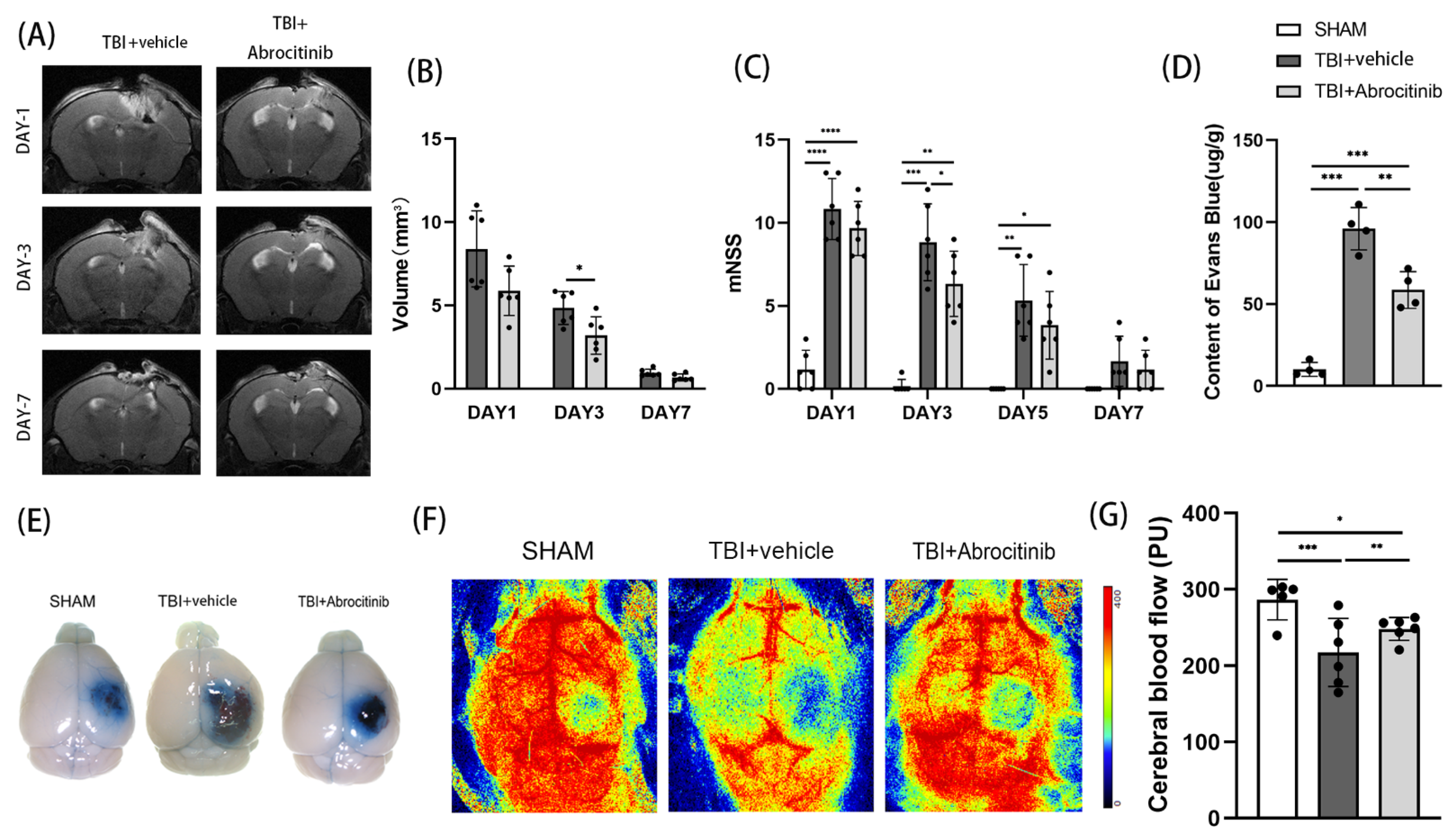
3.2. Abrocitinib Ameliorated Brain Injury and Improved Neurological Outcomes after TBI
3.3. Abrocitinib Promoted the Survival of Neurons and Reduced Apoptosis
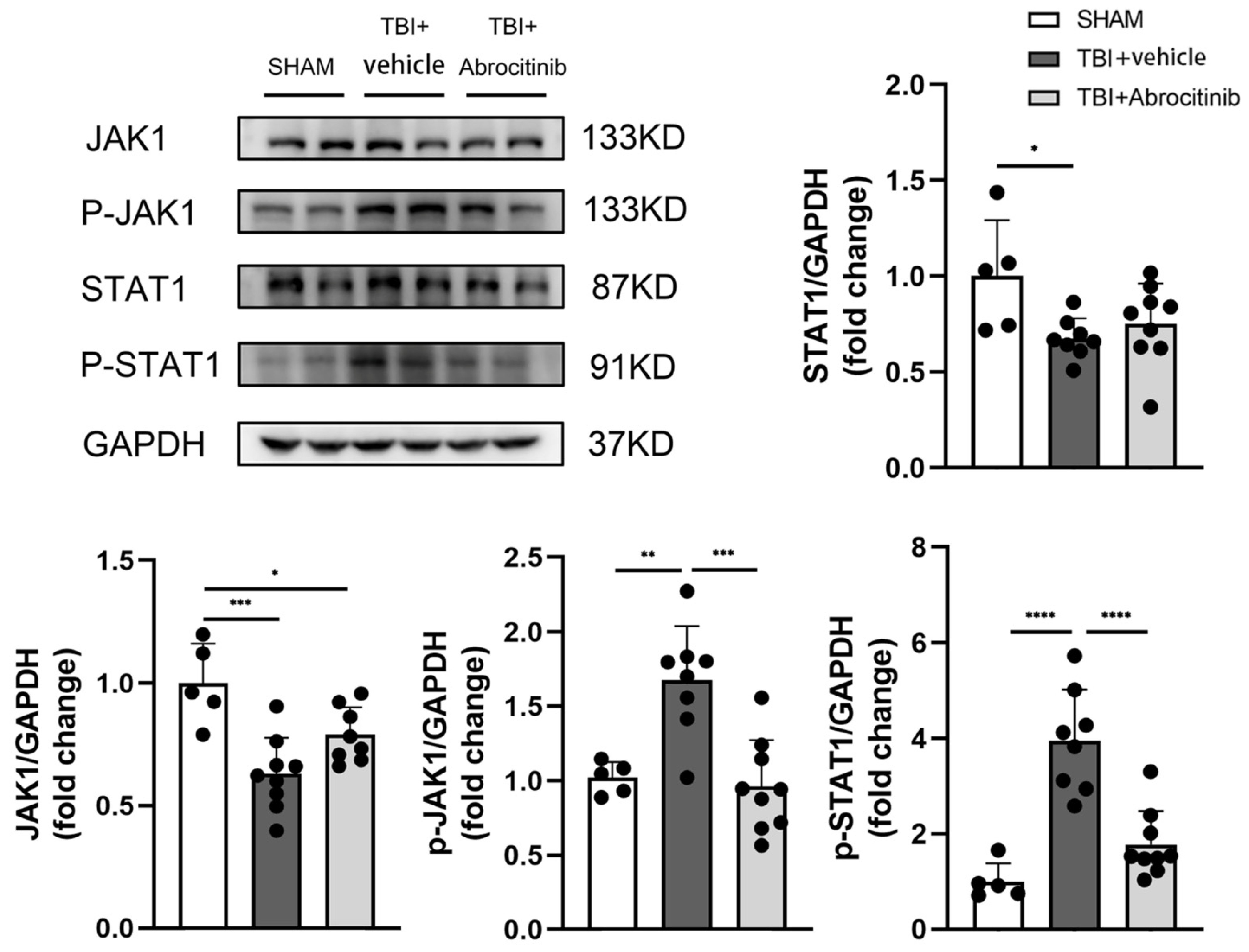
3.4. Abrocitinib Significantly Inhibited Activation of JAK1/STAT1
3.5. Abrocitinib Did Not Change Blood Counts after TBI
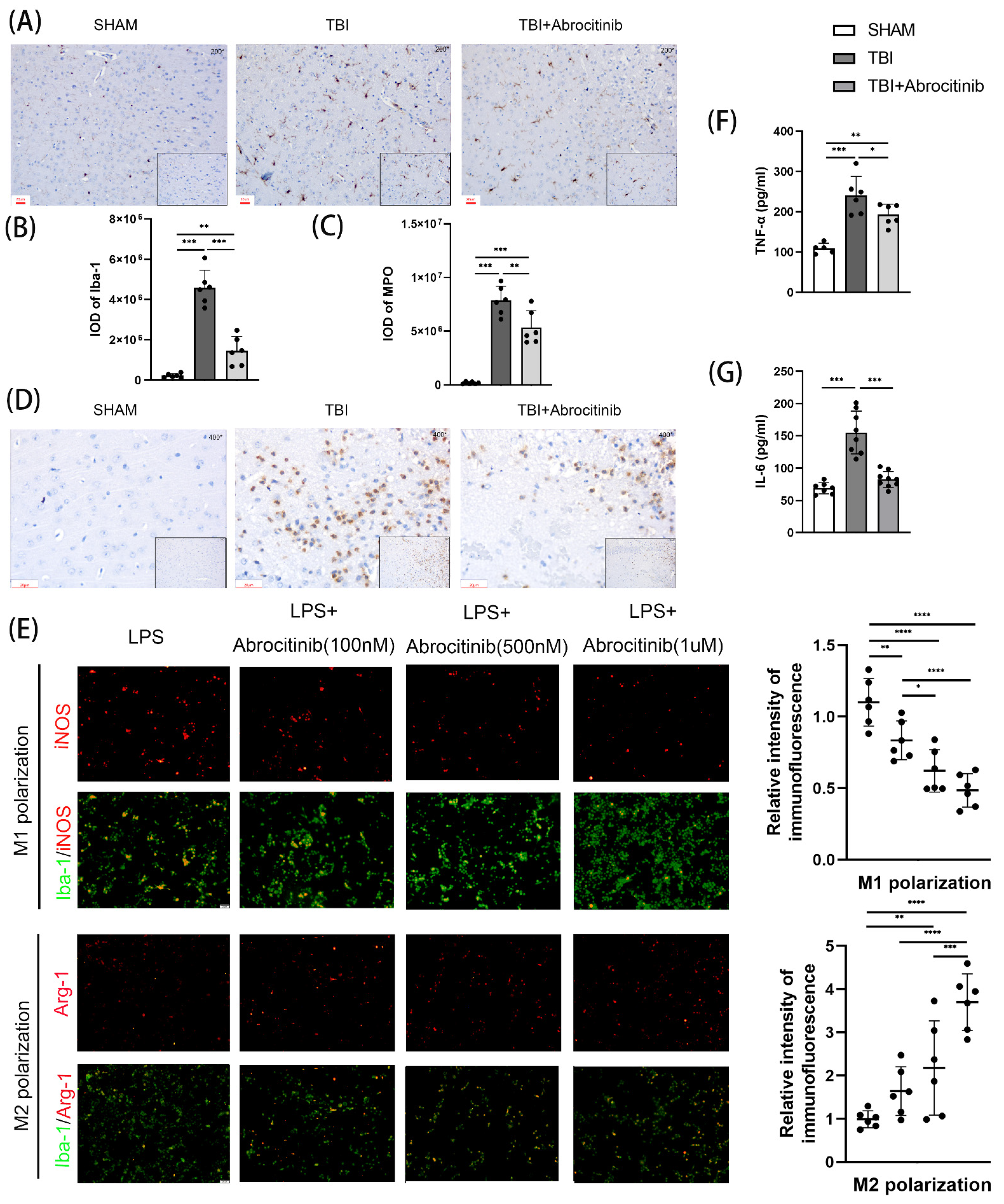
3.6. Abrocitinib Reduced the Infiltration of Inflammatory Cells and the Activation of Microglia, Inhibited M1 Polarization and Promoted M2 Polarization, and Further Decreased Pro-Inflammatory Cytokines
3.7. Abrocitinib Induced Significant Anti-Inflammatory Effects and Decreased Pyroptosis in Brain Tissue after TBI by Restraining the NF-κB Pathway
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I.; Demographics, I. Clinical Assessment Working Group of the, I. Interagency Initiative toward Common Data Elements for Research on Traumatic Brain, and H. Psychological, Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef]
- Mollayeva, T.; Mollayeva, S.; Colantonio, A. Traumatic brain injury: Sex, gender and intersecting vulnerabilities. Nat. Rev. Neurol. 2018, 14, 711–722. [Google Scholar] [CrossRef]
- Dinet, V.; Petry, K.G.; Badaut, J. Brain-Immune Interactions and Neuroinflammation After Traumatic Brain Injury. Front. Neurosci. 2019, 13, 1178. [Google Scholar] [CrossRef]
- Humble, S.S.; Wilson, L.D.; Wang, L.; Long, D.A.; Smith, M.A.; Siktberg, J.C.; Mirhoseini, M.F.; Bhatia, A.; Pruthi, S.; Day, M.A.; et al. Prognosis of diffuse axonal injury with traumatic brain injury. J. Trauma Acute Care Surg. 2018, 85, 155–159. [Google Scholar] [CrossRef]
- Cai, L.; Gong, Q.; Qi, L.; Xu, T.; Suo, Q.; Li, X.; Wang, W.; Jing, Y.; Yang, D.; Xu, Z.; et al. ACT001 attenuates microglia-mediated neuroinflammation after traumatic brain injury via inhibiting AKT/NFkappaB/NLRP3 pathway. Cell Commun. Signal 2022, 20, 56. [Google Scholar] [CrossRef]
- Capizzi, A.; Woo, J.; Verduzco-Gutierrez, M. Traumatic Brain Injury: An Overview of Epidemiology, Pathophysiology, and Medical Management. Med. Clin. N. Am. 2020, 104, 213–238. [Google Scholar] [CrossRef]
- Shively, S.B.; Priemer, D.S.; Stein, M.B.; Perl, D.P. Pathophysiology of Traumatic Brain Injury, Chronic Traumatic Encephalopathy, and Neuropsychiatric Clinical Expression. Psychiatr. Clin. N. Am. 2021, 44, 443–458. [Google Scholar] [CrossRef]
- Simon, D.W.; McGeachy, M.J.; Bayir, H.; Clark, R.S.B.; Loane, D.J.; Kochanek, P.M. The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 2017, 13, 572. [Google Scholar] [CrossRef]
- Loane, D.J.; Kumar, A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp. Neurol. 2016, 275 Pt 3, 316–327. [Google Scholar] [CrossRef]
- Donat, C.K.; Scott, G.; Gentleman, S.M.; Sastre, M. Microglial Activation in Traumatic Brain Injury. Front. Aging Neurosci. 2017, 9, 208. [Google Scholar] [CrossRef]
- Mishra, A.; Bandopadhyay, R.; Singh, P.K.; Mishra, P.S.; Sharma, N.; Khurana, N. Neuroinflammation in neurological disorders: Pharmacotherapeutic targets from bench to bedside. Metab. Brain Dis. 2021, 36, 1591–1626. [Google Scholar] [CrossRef] [PubMed]
- Owen, K.L.; Brockwell, N.K.; Parker, B.S. JAK-STAT Signaling: A Double-Edged Sword of Immune Regulation and Cancer Progression. Cancers 2019, 11, 2002. [Google Scholar] [CrossRef] [PubMed]
- Hebenstreit, D.; Horejs-Hoeck, J.; Duschl, A. JAK/STAT-dependent gene regulation by cytokines. Drug News Perspect. 2005, 18, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Stephanou, A.; Scarabelli, T.M.; Brar, B.K.; Nakanishi, Y.; Matsumura, M.; Knight, R.A.; Latchman, D.S. Induction of apoptosis and Fas receptor/Fas ligand expression by ischemia/reperfusion in cardiac myocytes requires serine 727 of the STAT-1 transcription factor but not tyrosine 701. J. Biol. Chem. 2001, 276, 28340–28347. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Nishiguchi, S. Importance of Kupffer cells in the development of acute liver injuries in mice. Int. J. Mol. Sci. 2014, 15, 7711–7730. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Gan, Z.S.; Wang, Q.Q.; Li, J.H.; Wang, X.L.; Wang, Y.Z.; Du, H.H. Iron Reduces M1 Macrophage Polarization in RAW264.7 Macrophages Associated with Inhibition of STAT1. Mediators Inflamm. 2017, 2017, 8570818. [Google Scholar] [CrossRef]
- Huang, X.P.; Ding, H.; Lu, J.D.; Tang, Y.H.; Deng, B.X.; Deng, C.Q. Effects of the Combination of the Main Active Components of Astragalus and Panax notoginseng on Inflammation and Apoptosis of Nerve Cell after Cerebral Ischemia-Reperfusion. Am. J. Chin. Med. 2015, 43, 1419–1438. [Google Scholar] [CrossRef]
- Rosa, J.M.; Farre-Alins, V.; Ortega, M.C.; Navarrete, M.; Lopez-Rodriguez, A.B.; Palomino-Antolin, A.; Fernandez-Lopez, E.; Vila-Del Sol, V.; Decouty, C.; Narros-Fernandez, P.; et al. TLR4 pathway impairs synaptic number and cerebrovascular functions through astrocyte activation following traumatic brain injury. Br. J. Pharmacol. 2021, 178, 3395–3413. [Google Scholar] [CrossRef]
- Han, J.W.; Shim, D.W.; Shin, W.Y.; Kim, M.K.; Shim, E.J.; Sun, X.; Koppula, S.; Kim, T.J.; Kang, T.B.; Lee, K.H. Juniperus rigida Sieb. extract inhibits inflammatory responses via attenuation of TRIF-dependent signaling and inflammasome activation. J. Ethnopharmacol. 2016, 190, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cai, W.; Zhao, Z.; Hilton, T.; Wang, M.; Yeon, J.; Liu, W.; Zhang, F.; Shi, F.D.; Wu, X.; et al. Lactadherin promotes microvesicle clearance to prevent coagulopathy and improves survival of severe TBI mice. Blood 2018, 131, 563–572. [Google Scholar] [CrossRef]
- Xu, X.; Wang, C.; Wu, Y.; Houck, K.; Hilton, T.; Zhou, A.; Wu, X.; Han, C.; Yang, M.; Yang, W.; et al. Conformation-dependent blockage of activated VWF improves outcomes of traumatic brain injury in mice. Blood 2021, 137, 544–555. [Google Scholar] [CrossRef]
- Li, T.; Wang, D.; Tian, Y.; Yu, H.; Wang, Y.; Quan, W.; Cui, W.; Zhou, L.; Chen, J.; Jiang, R.; et al. Effects of atorvastatin on the inflammation regulation and elimination of subdural hematoma in rats. J. Neurol. Sci. 2014, 341, 88–96. [Google Scholar] [CrossRef]
- Wu, H.; Wu, T.; Han, X.; Wan, J.; Jiang, C.; Chen, W.; Lu, H.; Yang, Q.; Wang, J. Cerebroprotection by the neuronal PGE2 receptor EP2 after intracerebral hemorrhage in middle-aged mice. J. Cereb. Blood Flow Metab. 2017, 37, 39–51. [Google Scholar] [CrossRef]
- Gu, J.; Bao, Y.; Chen, J.; Huang, C.; Zhang, X.; Jiang, R.; Liu, Q.; Liu, Y.; Xu, X.; Shi, W. The Expression of NP847 and Sox2 after TBI and Its Influence on NSCs. Front. Cell Neurosci. 2016, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Tasheva, S.; Soler, R.M. NF-kappaB signaling pathways: Role in nervous system physiology and pathology. Neuroscientist 2013, 19, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, X.; Wang, Y.; Zhou, C. Vitamin D Protects against Traumatic Brain Injury via Modulating TLR4/MyD88/NF-kappaB Pathway-Mediated Microglial Polarization and Neuroinflammation. Biomed. Res. Int. 2022, 2022, 3363036. [Google Scholar] [CrossRef]
- Villapol, S.; Byrnes, K.R.; Symes, A.J. Temporal dynamics of cerebral blood flow, cortical damage, apoptosis, astrocyte-vasculature interaction and astrogliosis in the pericontusional region after traumatic brain injury. Front. Neurol. 2014, 5, 82. [Google Scholar] [CrossRef]
- Carron, S.F.; Alwis, D.S.; Rajan, R. Traumatic Brain Injury and Neuronal Functionality Changes in Sensory Cortex. Front. Syst. Neurosci. 2016, 10, 47. [Google Scholar] [CrossRef]
- Stoica, B.A.; Faden, A.I. Cell death mechanisms and modulation in traumatic brain injury. Neurotherapeutics 2010, 7, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gao, W.; Cheng, S.; Yin, D.; Li, F.; Wu, Y.; Sun, D.; Zhou, S.; Wang, D.; Zhang, Y.; et al. Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J. Neuroinflamm. 2017, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Jassam, Y.N.; Izzy, S.; Whalen, M.; McGavern, D.B.; El Khoury, J. Neuroimmunology of Traumatic Brain Injury: Time for a Paradigm Shift. Neuron 2017, 95, 1246–1265. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Garden, G.A. Epigenetics and the modulation of neuroinflammation. Neurotherapeutics 2013, 10, 782–788. [Google Scholar] [CrossRef]
- Corps, K.N.; Roth, T.L.; McGavern, D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015, 72, 355–362. [Google Scholar] [CrossRef]
- Shi, H.; Wang, H.L.; Pu, H.J.; Shi, Y.J.; Zhang, J.; Zhang, W.T.; Wang, G.H.; Hu, X.M.; Leak, R.K.; Chen, J.; et al. Ethyl pyruvate protects against blood-brain barrier damage and improves long-term neurological outcomes in a rat model of traumatic brain injury. CNS Neurosci. Ther. 2015, 21, 374–384. [Google Scholar] [CrossRef]
- Lee, J.; Costantini, T.W.; D’Mello, R.; Eliceiri, B.P.; Coimbra, R.; Bansal, V. Altering leukocyte recruitment following traumatic brain injury with ghrelin therapy. J. Trauma Acute Care Surg. 2014, 77, 709–715. [Google Scholar] [CrossRef]
- Dixon, K.J. Pathophysiology of Traumatic Brain Injury. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 215–225. [Google Scholar] [CrossRef]
- Yeh, C.H.; Shih, H.C.; Hong, H.M.; Lee, S.S.; Yang, M.L.; Chen, C.J.; Kuan, Y.H. Protective effect of wogonin on proinflammatory cytokine generation via Jak1/3-STAT1/3 pathway in lipopolysaccharide stimulated BV2 microglial cells. Toxicol. Ind. Health 2015, 31, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.; Kershaw, N.J.; Babon, J.J. The molecular details of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018, 27, 1984–2009. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Cai, H.; Zhang, X.; Sun, J.; Feng, X.; Yuan, H.; Zhang, X.; Xiao, B.; Li, Q. An active marine halophenol derivative attenuates lipopolysaccharide-induced acute liver injury in mice by improving M2 macrophage-mediated therapy. Int. Immunopharmacol. 2021, 96, 107676. [Google Scholar] [CrossRef]
- Becher, B.; Spath, S.; Goverman, J. Cytokine networks in neuroinflammation. Nat. Rev. Immunol. 2017, 17, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Bu, G. Attenuating oxidized low density lipoprotein (ox-LDL)-induced macrophages damage via inhibiting C-type lectin domain family 2 (CLEC2) expression through janus kinase 1 (JAK1)/signal transducers and activators of transcription-1 (STAT1) pathway. Bioengineered 2022, 13, 6440–6449. [Google Scholar] [CrossRef]
- Luan, X.; Cong, Z.; Anastassiades, T.P.; Gao, Y. N-Butyrylated Hyaluronic Acid Achieves Anti-Inflammatory Effects In Vitro and in Adjuvant-Induced Immune Activation in Rats. Molecules 2022, 27, 3267. [Google Scholar] [CrossRef]
- Piaszyk-Borychowska, A.; Szeles, L.; Csermely, A.; Chiang, H.C.; Wesoly, J.; Lee, C.K.; Nagy, L.; Bluyssen, H.A.R. Signal Integration of IFN-I and IFN-II With TLR4 Involves Sequential Recruitment of STAT1-Complexes and NFkappaB to Enhance Pro-inflammatory Transcription. Front. Immunol. 2019, 10, 1253. [Google Scholar] [CrossRef]
- Platanitis, E.; Decker, T. Regulatory Networks Involving STATs, IRFs, and NFkappaB in Inflammation. Front. Immunol. 2018, 9, 2542. [Google Scholar] [CrossRef]
- Barbosa Lima, L.E.; Muxel, S.M.; Kinker, G.S.; Carvalho-Sousa, C.E.; da Silveira Cruz-Machado, S.; Markus, R.P.; Fernandes, P. STAT1-NFkappaB crosstalk triggered by interferon gamma regulates noradrenaline-induced pineal hormonal production. J. Pineal Res. 2019, 67, e12599. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Ridder, D.A.; Schwaninger, M. NF-kappaB signaling in cerebral ischemia. Neuroscience 2009, 158, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; McBride, D.W.; Zhang, J.H. Axl activation attenuates neuroinflammation by inhibiting the TLR/TRAF/NF-kappaB pathway after MCAO in rats. Neurobiol. Dis. 2018, 110, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.; Methe, H.; Kim, J.O.; Edelman, E.R. NF-kappaB activity in endothelial cells is modulated by cell substratum interactions and influences chemokine-mediated adhesion of natural killer cells. Cell Transplant. 2009, 18, 261–273. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]


| Sham | TBI + Vehicle | TBI + Abrocitinib | |
|---|---|---|---|
| Number of White Blood Cells (×109/L) | 2.41 ± 1.36 | 2.50 ± 1.28 | 2.81 ± 0.99 |
| Number of Neutrophils (×109/L) | 0.33 ± 0.09 | 0.58 ± 0.24 * | 0.57 ± 0.23 * |
| Number of Red Blood Cells (×1012/L) | 8.27 ± 0.53 | 8.22 ± 0.97 | 8.28 ± 0.49 |
| Number of Platelets (×109/L) | 659.14 ± 180.31 | 687.88 ± 118.02 | 716.71 ± 84.52 |
| Percentage of Neutrophils (%) | 12.77 ± 3.05 | 24.93 ± 9.44 ** | 20.45 ± 3.09 * |
| Hemoglobin (g/L) | 121.0 ± 5.88 | 125.25 ± 6.16 | 118.86 ± 6.47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Li, L.; Peng, R.; Hao, H.; Zhang, H.; Gao, Y.; Wang, C.; Li, F.; Liu, X.; Chen, F.; et al. Abrocitinib Attenuates Microglia-Mediated Neuroinflammation after Traumatic Brain Injury via Inhibiting the JAK1/STAT1/NF-κB Pathway. Cells 2022, 11, 3588. https://doi.org/10.3390/cells11223588
Li T, Li L, Peng R, Hao H, Zhang H, Gao Y, Wang C, Li F, Liu X, Chen F, et al. Abrocitinib Attenuates Microglia-Mediated Neuroinflammation after Traumatic Brain Injury via Inhibiting the JAK1/STAT1/NF-κB Pathway. Cells. 2022; 11(22):3588. https://doi.org/10.3390/cells11223588
Chicago/Turabian StyleLi, Tuo, Lei Li, Ruilong Peng, Hongying Hao, Hejun Zhang, Yalong Gao, Cong Wang, Fanjian Li, Xilei Liu, Fanglian Chen, and et al. 2022. "Abrocitinib Attenuates Microglia-Mediated Neuroinflammation after Traumatic Brain Injury via Inhibiting the JAK1/STAT1/NF-κB Pathway" Cells 11, no. 22: 3588. https://doi.org/10.3390/cells11223588
APA StyleLi, T., Li, L., Peng, R., Hao, H., Zhang, H., Gao, Y., Wang, C., Li, F., Liu, X., Chen, F., Zhang, S., & Zhang, J. (2022). Abrocitinib Attenuates Microglia-Mediated Neuroinflammation after Traumatic Brain Injury via Inhibiting the JAK1/STAT1/NF-κB Pathway. Cells, 11(22), 3588. https://doi.org/10.3390/cells11223588






