Abstract
Constitutive heterochromatin represents a significant fraction of eukaryotic genomes (10% in Arabidopsis, 20% in humans, 30% in D. melanogaster, and up to 85% in certain nematodes) and shares similar genetic and molecular properties in animal and plant species. Studies conducted over the last few years on D. melanogaster and other organisms led to the discovery of several functions associated with constitutive heterochromatin. This made it possible to revise the concept that this ubiquitous genomic territory is incompatible with gene expression. The aim of this review is to focus the attention on a group of protein-coding genes resident in D. melanogaster constitutive of heterochromatin, which are implicated in different steps of cell division.
1. Introduction
“But there’s no such thing as the unknown, only things temporarily hidden, temporarily not understood.”Captain James T. Kirk, from movie: Star Trek Beyond
The term heterochromatin was originally defined cytologically by Heitz in 1928 [1] as chromosomal regions that appear deeply stained at the prophase and retain a compact state throughout all stages of the mitotic cell cycle, as opposed to euchromatin, which undergoes decondensation and condensation cycles. Later on, heterochromatin was further categorized into facultative and constitutive [2]. Facultative heterochromatin corresponds to euchromatic portions of the genomes (chromosome regions, entire chromosomes, or even whole chromosome sets), which undergo silencing during development [3,4,5]. By contrast, constitutive heterochromatin occurs primarily in large blocks made up of several DNA megabases that include centromeric or telomeric regions, is enriched in repetitive sequences compared to euchromatin, and shows the same cytological and molecular characteristics on both homologous chromosomes [6].
Constitutive heterochromatin is a ubiquitous and quantitatively significant component of eukaryotic genomes (10% in Arabidopsis, 20% in humans, 30% in D. melanogaster, and up to 90% in certain nematodes). A number of characteristic properties have historically been assigned to constitutive heterochromatin in nearly all animal and plant species, which are antithetical compared to those of euchromatin [6]: (i) strongly reduced level of meiotic recombination; (ii) low gene density; (iii) mosaic inactivation of the expression of euchromatic genes when moved nearby, a phenomenon termed position effect variegation (PEV); (iv) late replication during the S phase; (v) transcriptional inactivity; (vi) enrichment in highly repetitive satellite DNA and transposable elements; and (vii) the presence of silent epigenetic marks (mainly H3K9 methylation). Together, these properties have led to the view that constitutive heterochromatin is a “genomic desert” made up of junk DNA. However, studies conducted over the last few years have contributed to revising the concept of constitutive heterochromatin, and the notion that this ubiquitous genomic component is incompatible with gene expression no longer seems to be a general rule [6].
Sequencing and annotation of the genome of D. melanogaster combined with high-resolution cytogenetic analyses have greatly facilitated studies aimed at characterizing the organization and function of constitutive heterochromatin [6,7,8,9,10,11,12,13,14,15]. It emerged that this model organism contains a minimum of 230 protein-coding genes [9] mapping to constitutive heterochromatin, whose borders were defined by cytogenomic and epigenomic approaches [6]. Thus, the gene number in constitutive heterochromatin of D. melanogaster is significantly greater than that originally defined by a classical genetic analysis [16,17]. This result can be explained by assuming that most genes escaped mutational analysis because they are nonessential or, alternatively, that some loci with complex complementation behaviors indeed contain several vital genes, as in the case of l(2)41Ae [13].
Intriguingly, the expression of these genes is compromised if they are moved away from the pericentromeric regions by chromosome rearrangements [6,17]. Thus, they can “live and work” properly within a genomic environment with silencing properties, a conclusion that represents a kind of paradox. A combination of negative and active histone modification marks, together with the contribution of key epigenetic regulators such as the HP1 protein [18,19], may be crucial players in the regulation of gene expression in constitutive heterochromatin [6,20]. However, these aspects have been extensively reviewed elsewhere and will not reexamined in detail here.
The genomic size of D. melanogaster heterochromatic genes is, on average, up to ten time larger than that of euchromatic ones, due to the presence of large transposable element-rich introns, and together, they account for a significant fraction (at least 40%) of the entire constitutive heterochromatin [6]. Thus, this peculiar genome component is not that gene-poor as previously believed and, in spite of its ability to induce silencing, can be quite dynamic.
According to the 5.1 release of the D. melanogaster heterochromatin, the gene ontology (GO) analysis showed that heterochromatic and euchromatic genes encode similar categories of functions [9]. However, some classes of functions appear to be overrepresented in constitutive heterochromatin, relative to euchromatin. It is the case of the 35-fold enrichment for putative membrane cation transporter domains or for DNA or protein-binding domains [9].
Here, we will focus our attention on a group of single-copy protein-coding genes resident in constitutive heterochromatin (Table 1) with experimentally validated or putative functions implicated in the proper execution of cell division. The functions of these genes were selected according to the biological role described in the scientific literature or according to the associated Gene Ontology terms in FlyBase.

Table 1.
List of the examined heterochromatic genes with their functions and cytogenetic and genomic locations. In this review, CG17493 and CG17528 were named CentrinB and Dmel-doublecortin, respectively (names not reported in FlyBase). Map positions as described in the HDGP project and FlyBase. Hsap = human orthologs; Ortho map = chromosome map of human orthologs; n.d. = not detected; n.a. = not allowed.
In eukaryotes, a failure of mitosis and cytokinesis results in aneuploid or polyploid cells that promote tumorigenic transformation [21,22]. Thus, the study of genes controlling mechanisms underlying different steps of cell division can contribute to both cancer and human developmental diseases.
Finally, it is worth noting that the heterochromatic genes studied here are active during different tissues and developmental stages of D. melanogaster, and their average expression levels are comparable to those of euchromatic cell cycle genes (Figure 1).
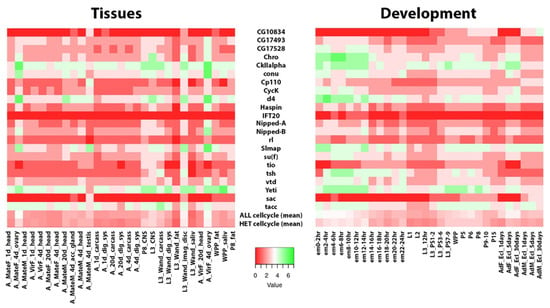
Figure 1.
Heatmaps showing expression profiles of the examined single-copy coding genes. Developmental stages (left panel) and tissues expression (right panel). Shades of color from red to green indicate the expression bin classification from 1 (no/extremely low expression) to 7 (very high expression). Developmental stages and tissues expression data were obtained from FlyBase. Tissues (heads, ovaries, testis, carcasses, digestive system, CNS, fat, imaginal discs, and salivary glands) were obtained from different developmental stages, different timing, or different physiological conditions, as indicated (em: embryos; A: adults; L1–L3: larvae 1st–3rd instar; WPP: pupae early stage; P1–P15: late pupae; F/M: females/males; Mate/Vir: Mated/Virgin). “ALL cell cycle:” mean expression of 745 genes whose products are involved in cell division and for which expression data are available from ModEncode and obtained from FlyBase (textual search query “cell division”). “HET cell cycle”: mean expression of 22 heterochromatic genes discussed in this review.
2. Functions Related to Chromosome/Chromatin Organization and Gene Expression
2.1. Yeti and Nipped-A Genes Encode Two Subunits of the dTip60 Chromatin Remodeling Complex
2.1.1. Yeti
Mutations in this gene are recessive lethal, and affect individual viability and proper chromosome organization in both mitosis and meiosis [6,8,16,23,24,25].
The YETI protein was originally identified as a kinesin-binding protein and was later found to be a component of the D. melanogaster Tip60 (dTip60) chromatin remodeling complex [23,24,25]. The dTip60 complex is made up of 14 other core subunits (BAP55, dGAS41, dPontin, dReptin, Nipped-A, e(Pc), dYL1, dDMAP1, Act87B, dMrg15, dMrgBP, dTRA1, dIng3, and dEaf6) and is required for the replacement of acetylated phospho-H2A.V by unmodified H2A.V via Domino (Dom) ATPase [26,27,28,29,30].
A role of the YETI protein in cell cycle control during both mitosis and meiosis was also suggested [23,31,32]. Notably, YETI was found to undergo relocation from interphase chromatin to the midbody and play a direct extra-chromatin role in the control of cytokinesis in D. melanogaster S2 cells [33].
The human ortholog of the Yeti gene is the Craniofacial Development Protein 1 gene, CFDP1 (OMIM number 608108), which maps to chromosome 16 in 16q22.2-q22.3. As for the YETI protein, the CFDP1 protein is a subunit of the human SRCAP chromatin remodeling complex [34,35], evolutionary related to the dTip60, and functions beyond chromatin remodeling, being required for the proper execution of cell division in HeLa cells [33]. Interestingly, the chicken CFDP1, also called CENP-29, has been reported to be associated with kinetochores [36]. In a human proteomic study, CFDP1 was found to interact with Ewing sarcoma related protein (EWSR1) whose mutations leads to Ewing’s sarcoma, a type of cancer that forms in bone or soft tissue [37].
According to the role of YETI in meiosis [23], CFDP1 has been found to physically interact with TALDO1 [38,39,40], a hallmark of human and murine spermatogenesis [41,42], and with HIST1H2BA, a testis/sperm-specific member of the histone H2B family [43]. Taken together, the above-mentioned studies suggest that YETI, CFDP1 and their family of orthologs are multifaceted proteins that play essential roles for proper execution of cell division [24,25,33], in addition to their canonical functions in chromatin remodeling.
2.1.2. Nipped-A (Nip-A)
Mutations in this gene are also recessive lethal [44] and RNAi depletion of the Nip-A protein causes early larval lethality [45]. The Nip-A gene encodes the Tra1/TRRAP protein, a conserved subunit of both dTip60 and SAGA chromatin remodeling complexes [36,37,45]. In yeast and humans, the Nip-A ortholog interacts with transcriptional activators to recruit Tip60 and SAGA complexes [46,47,48].
2.2. Nipped-B and Verthandi Encode Two Subunits of the Cohesin Complex
2.2.1. Nipped-B (Nip-B)
Mutations in this gene are recessive lethal and affect sister chromatid cohesion [49,50,51,52]. In accord with the lethal phenotype, the Nip-B protein was found to interact with the Cohesin complex that is required for sister chromatid cohesion and chromosome segregation in different organisms [50,51,52,53,54,55,56]. The Cohesin complex consists of a heterodimer of the Smc1 and Smc3 (structural maintenance of chromosome) cohesin, and of other proteins [57,58]. According to the current cohesion models, this complex forms a ring-like structure in which the cohesins encircle the two sister chromatids and is required but not sufficient for sister chromatid cohesion [57,58,59,60].
The human ortholog of Drosophila Nip-B is NIPBL, whose heterozygous mutations account for about 60% of the cases of Cornelia de Lange syndrome (CdLS), a genetic disorder with multiple developmental abnormalities [61]. NIPBL, interacting with Mau-2 (Scc2) cohesion, forms the Kollerin complex, an evolutionary conserved complex crucial for chromatin loading of the Cohesin complex [52,54]. Mau-2 mutations can also cause CdLS [62]. As for D. melanogaster Nip-B, depletion of NIPBL and of the vertebrate homologs in vivo or in cultured cells also cause chromatid cohesion defects [55,63,64]. A functional cooperation between NIPBL and the bromodomain-Containing Protein 4 (BRD4) in regulating gene expression at the promoter level has been highlighted [65]. Notably, mutations of BRD4 were also described to cause a CdLS-like phenotype [66].
2.2.2. Verthandi (vtd)
It was identified by genetic analyses and mapped to the pericentromeric heterochromatin o chromosome 3 [67,68,69]. Ten vtd alleles have been originally found, some in screens aimed to identify recessive lethal mutations mapping to 3L heterochromatin while others were in additional screens either for dominant suppressors of a dominant gain-of-function allele of hedgehog or for of dominant suppressors of Polycomb (Pc) mutations [67,68].
Later on, in the process of annotating the D. melanogaster heterochromatin genome sequence, vtd was found to encode the RAD21 protein, a subunit of the Cohesin complex [70]. In accordance, vtd mutations disrupt sister chromatid cohesion and chromosome segregation [70]. The vtd protein was detected in pericentric heterochromatic regions [71,72], in polytene chromosome interbands [73], and along the synaptonemal complex [74]. Mutations of the human ortholog of vtd, RAD21L1 (RAD21-like 1) cause a mild Cornelia de Lange syndrome phenotype [75,76].
2.3. Other Genes Involved in Chromatin Organization
2.3.1. Teashirt (tsh)
Loss of function alleles of tsh were found to be recessive lethal at the stage of third instar larvae and caused metaphase arrest in mitosis a phenotype was also observed after RNAi mediated depletion in S2 Drosophila culture cells [77]. These results suggested that the tsh gene exerts a positive control on cell proliferation. The tsh product is a homeotic protein that play a role in chromatin organization and transcription modulation acting as both repressor and activator [78].
The tsh gene shares common activities with the nearby located tiptop (tio) gene, whose protein product is also involved in chromatin organization and gene expression control. Notably, there is a molecular cross-talk between tsh and tio proteins, which repress each other’s expression [79].
The human orthologs of tsh, TSHZ1, TSHZ2, and TSHZ3 (teashirt zinc finger homeobox), may act as transcriptional repressors during developmental processes [80,81].
2.3.2. D4
It encodes a noncatalytic subunit of the BRG1/BRM-Associated Factor (BAF) chromatin-remodeling complex [82,83], which plays a role in the epigenetic regulation of transcription through the identification of histone modifications [84]. Indeed, pull-down experiments have shown that the d4 protein only interacts with BAF-specific complex members [83]. Heterozygous mutations of DPF2, the human ortholog of Drosophila d4, are responsible for eight unrelated cases of Coffin-Siris syndrome-7, which includes several developmental alterations [85].
3. Functions Related to Mitotic Apparatus/Microtubule Binding
3.1. Mitotic Genes Also Implicated in Ciliogenesis
Despite the restricted cilia expression in Drosophila, ciliary proteins could play essential roles in cell division control, as suggested by the experimental evidence on the following four genes.
3.1.1. Centriolar Coiled Coil Protein 110 (CP110)
Studies carried out in C. elegans and mammals have shown that the CP110 protein is implicated in several aspect of cell division control: centriole duplication and length, mitotic spindle assembly, cytokinesis, genome stability, and suppression of ciliogenesis [86,87,88,89]. The D. melanogaster CP110 protein localizes to the distal end of both mother and daughter centrioles, where it “caps” the centriole [90]. In another study, D. melanogaster mutant flies lacking CP110 were viable and fertile with no obvious defects in cell division, centriole duplication, or cilia formation [87]. However, in cells lacking CP110, the centrioles were 10% longer than those in WT cells, while, in cells overexpressing CP110, they were 20% shorter [87]. Based on these results, the authors suggested that, in contrast to mammals, D. melanogaster CP110 may play only a minor role in regulating centriole length [87].
3.1.2. CentrinB
It encodes a protein orthologous to human Centrin-1 (CETN1) and Centrin-2 (CETN2) proteins. CETN1 is specifically expressed in ciliated cells, while CETN2 is expressed in all epithelial proliferation cells [91]. Centrins are small calcium-binding proteins that are ubiquitous centrosome components and regulate microtubule organizing center (MTOC) duplication [92,93]. This evidence is suggestive for an involvement of the CentrinB protein in centriole duplication during mitotic cell cycle. However, in a screen of 17,759 RNAi lines for searching genes involved in muscle morphogenesis, depletion of CentrinB did not significantly affect the viability or other phenotypic traits [94]. Thus, additional targeted experiments are needed to elucidate the function of CG17493 protein and to test its possible involvement in centriole duplication/function.
3.1.3. Intraflagellar Transport 20 (IFT20)
It was identified by comparative genomic analyses to search genes involved in cilia biogenesis and function [95,96]. However, no functional data from both forward and reverse genetics analyses are thus far available. Some information comes from the mouse and human orthologs, mIFT20 and hIFT20, respectively. The mIFT20 plays roles in controlling the Wnt signaling and cell proliferation and is required for proper positioning of the centrosome in nondividing cells and correct orientation of the mitotic spindle in mouse kidney cells [97]. Moreover, conditional ablation of the mIFT20 gene in adult mouse results in loss of primary cilia and Shh signaling in the hippocampal stem cell population and consequently in a reduced numbers of proliferating amplifying progenitors [98].
Recent studies indicate that mutations of hIFT20 are associated with numerous system-related diseases, such as those of the nervous and respiratory systems [99]. The hIFT20 protein moves back and forth between the Golgi body and ciliated microtubules and regulates the length of primary cilia [100,101,102]. It also promotes the organization of Golgi-associated MTs and reorientation of the Golgi toward the direction of invasion in colorectal cancer (CRC) cells, probably by regulating the growth dynamics [103].
3.1.4. Sterile Affecting Ciliogenesis (sac)
Mutations of this gene affect D. melanogaster spermatogenesis and results in male sterility, a phenotype associated with aberrant cytokinesis, immotile flagella, and altered localization of subcellular structures. The sac gene encodes a component of the flagellar axoneme [104]. These observations are suggestive for a role of sac in ciliogenesis and cytokinesis during spermatogenesis.
3.2. Other Genes Related to Mitotic Apparatus
3.2.1. CG10834
The protein encoded by this gene belongs to the LC7/roadblock dynein light chain (LC) family of D. melanogaster [105]. Members of this family show two human orthologs: Dynein Light Chain Roadblock Type 1 (DYNLRB1) and Type 2 (DYNLRB2). DYNLRB1 was first identified in D. melanogaster during a genetic screen, in which roadblock mutants (i.e., roblz) exhibited mitotic defects [106,107]. The CG10834 protein is predicted to enable dynein intermediate chain-binding activity and to be active in centrosome. It may be also involved in microtubule-based movement and participate to the cytoplasmic dynein complex [108].
3.2.2. Sarcolemma Associated Protein (Slmap)
It encodes a subunit of the evolutionary conserved Striatin-interacting Phosphatases and Kinases, STRIPAK, a complex of Drosophila. This complex was found to be involved in numerous cellular and developmental processes [109,110,111,112]. A possible role of the SLMAP protein in cell division derives from studies carried out in mouse, where a novel isoform of SLMAP was found to be a centrosomes component and its overexpression caused lethality, whereas its loss affected cell cycle progression [113]. Interestingly, the human SLMAP was found to be one of the causative genes of Brugada syndrome, a cardiac channelopathy [114,115].
3.2.3. CG17528 (to Be Named Dmel-doublecortin)
The function of this gene still needs to be elucidated due to the lack of functional studies in the literature. Our preliminary experiments using the GAL4-UAS system suggest that in vivo the RNAi depletion of CG17528 results in a reduction of individual viability, suggesting that this gene is essential for fly development (Prozzillo Y., Bizzochi G., and Messina G., unpublished).
Additional information comes from bioinformatic analyses. Three orthologs of the CG17528 gene are found in humans: DCLK1, DCLK2, and DCX. The corresponding encoded proteins, DCLK1, DCLK2, and DCX, are members of the Microtubule-Associated Proteins (MAPs) family [116,117,118] and show a significant sequence/domain conservation with the CG17528 protein (Figure 2A). When considering the entire aminoacid sequence, DCLK1 and DCLK2 show about 45 and 42% sequence similarity with CG17528, respectively, and also share three conserved functional domains showing significant levels of identity (Figure 2): two N-terminal doublecortin (DCX) domains with microtubule binding activity [116] and a C-terminal domain (STK) with protein kinase activity. The DCX protein, in addition to the N-terminal doublecortin domains, carries a Ser/Pro-rich region, which interacts with several protein kinases but lacks the C-terminal STK domain. The evolutionary conservation of the doublecortin domains showed by the protein product of the CG17528 gene, strongly suggests that it encodes for a doublecortin-like protein; thus, in the absence of a specific name, we decided to call it D. melanogaster doublecortin (D. mel-doublecortin).
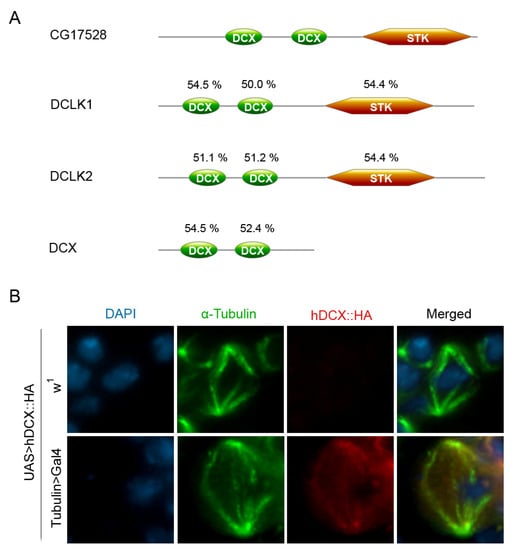
Figure 2.
The CG17528 (d-doublecortin) protein. (A) Sequence conservation of CG17528 with its human orthologs. Schematic representation of specific functional domains showing identity levels. Pairwise sequence alignment and protein domain analyses were performed using EMBOSS Needle (www.ebi.ac.uk/Tools/psa/emboss_needle/ (accessed on 4 August 2022)) and PROSITE (www.expasy.org/resources/prosite (accessed on 28 July 2022)), respectively. (B) Expression and localization of a HA-tagged human DCX fusion protein in larval brain cells of D. melanogaster. Squashes preparation stained with DAPI (blue), anti-α-tubulin (green), and anti-HA (red). After expression with the Tubulin>GAL4 driver, the HA signals were found at both the spindle poles and fibers. The signals were absent in the control flies carrying the HA-tagged human DCX fusion gene, in absence of the driver.
The DCLK1 and DCX genes are co-expressed in migrating neurons, suggesting that they may act cooperatively to regulate microtubule dynamics in migrating neurons [117]. Interestingly, the DCX protein physically interacts with the microtubule cytoskeleton and its localization overlaps with that of microtubules in cultured cortical neurons [117].
Notably, the DCX gene, which map to the X chromosome, is the causative gene of the X-linked lissencephaly 1 and subcortical band heterotopia [119]. The pathological mutations map in DCX protein domains and impair its binding to microtubules leading to a failure of neuroblasts migration from the proliferative ventricular zone toward the pial surface.
Using the GAL4-UAS system, we expressed a HA-tagged human DCX fusion protein in neural ganglia of D. melanogaster third instar larvae and found that it colocalizes with α-tubulin at the mitotic spindle (Figure 2B, Prozzillo Y., Bizzochi G., and Messina G., unpublished). The results of these experiments could be also of importance for studies aimed at identifying evolutionary conserved DCX interactors in the mitotic apparatus.
3.2.4. Chromator (Chro)
The Chro protein, localizes to polytene interbands and to the spindle and the centrosomes during mitosis. It was originally identified in yeast two hybrid screening as an interactor of the putative spindle matrix component, Skeletor. Its role in spindle function and chromosome segregation has been confirmed by RNAi-mediated knockdown in S2 cells [120,121]. The Chro protein localization to polytene interbands is also suggestive for a role in maintaining chromatin structure during interphase. This peculiar localization is due to the interaction with the interband-specific zinc-finger protein Z4 [122]. An involvement in chromatin organization was also supported by experiments showing that the lack of Chro protein leads to disorganization and misalignment of band/interband regions resulting in coiling and folding of the polytene chromosomes [121]. More recently, ectopic tethering of the Chro protein to intercalary heterochromatin causes local chromatin decondensation, formation of novel DNase I hypersensitive sites, and recruitment of several “open chromatin” marks, while retaining late-replicating behavior, similarly to the wild-type untargeted region [123]. Thus, Chro, like YETI, appears to be a multifaceted protein. No human orthologs of Chromator have been described since this gene was found to be invertebrate-specific.
3.2.5. Transforming Acidic Coiled-Coil Protein (tacc)
It encodes a centrosomal protein that helps to stabilize microtubules [124]. The tacc gene is essential for proper spindle function in early D. melanogaster embryo. The TACC protein seems to influence microtubules indirectly, primarily through its interaction with the product of the mini spindles (msps) gene [125]. The TACC protein is phosphorylated by the Aurora A kinase and this modification activates its ability to stabilize microtubules [126]. In humans, gene fusions of TACC1/TACC3 orthologs with FGFR1 were associated to gliosarcomas and giant cells glioblastomas [127].
4. Functions Related to Kinase Activity and of Cell Cycle Regulation
4.1. Suppressor of Forked Gene (su(f))
Temperature-sensitive mutations of this gene display an increased number of metaphases with overcondensed chromosomes and asymmetric or reduced mitotic spindles in. larval brain and in imaginal discs suggesting a role in cell proliferation. The Su(f) protein is a homolog of the 77-K subunit of human cleavage stimulation factor required for cleavage of pre-mRNAs. In D. melanogaster, the Su(f) protein accumulates in mitotically active cells during different developmental stages [128] and is required for proliferation of both somatic and germ cells [129].
Mutations of the S. pombe ortholog Rna14 exhibit defects in cell cycle progression with high level of septation, and the double mutant of rna14-11 and bub1 knockout exhibits high degree of chromosome mis-segregation [130].
The potential role of the su(f) protein in cell cycle progression could be indirect, as suggested by its role in processing the 3′ end of mRNA required for progression through metaphase [130].
4.2. Cyclin K (CycK)
It encodes a cyclin-homologous subunit that forms a complex with the transcriptional kinase encoded by Cdk12 [131]. This complex phosphorylates the carboxy terminal domain of the large subunit of RNA polymerase II and contributes to pre-mRNA processing, transcription, and chromatin structure. Human CycK is a 70-kDa protein with a C-terminal proline-rich region [132,133]. It associates with Cdk12 and Cdk13 in two separate complexes, playing roles in cell cycle regulation as other cyclin-dependent kinases (CDKs) [134].
4.3. Rolled (rl)
It was one of the first genes associated with pericentric heterochromatin by genetic analyses [135] and later was cytogenetically mapped to the region h41 of the deep heterochromatin of the right arm of chromosome 2 [16].
The rl gene encodes a mitogen activated protein (MAP) kinase, the Drosophila ortholog of human mitogen-activated protein kinase 3 (MAPK3), a core component of the RAS/MAPK pathway [136]. Null mutations of the rl gene are recessive lethal at early larval stages [16]. In addition, they result in a reduced mitotic index in the larval central nervous system, consistent with an interphase block to cell cycle progression, associated with a low frequency of cells showing chromosome over-condensation in mitosis and abnormal anaphase figures [136]. Moreover, loss-of-function mutations of rl impair the ability to arrest in mitosis in the presence of the microtubule-destabilizing drug colchicine and enhance the mutant phenotype of abnormal spindle (asp) gene, while rl gain-of-function mutations suppress the asp phenotype [137]. The asp gene encodes a microtubule-binding protein that associates with the spindle [138], and asp mutations result in abnormal arrays of spindle microtubules in both meiosis and mitosis [139,140,141]. Furthermore, the somatic activation of rolled downstream of EGFR is required to synchronize the mitotic divisions and regulate the transition to meiosis [142].
A central role for rolled in the proper targeting of axons has been suggested based on observations that rolled MAP kinase loss affects the axonal organization in both Drosophila and zebrafish [143,144].
4.4. Haspin
The Haspin protein is a serine/threonine-protein kinase a highly conserved kinase in eukaryotes [143,144,145]. Most of the findings on D. melanogaster Haspin gene came from the work of Fresan et al. [127]. They found that the Haspin protein phosphorylates histone H3T3 and is involved in sister chromatid cohesion during mitosis. The loss of Haspin causes a decrease in adult longevity and fertility in flies, while, at the cellular level, it affects the nuclear size and morphology and compromises the insulator activity in enhancer-blocking assays. In accord, Haspin mutations are suppressor of position–effect variegation. In conclusion, the Haspin protein may play roles in both genome organization of interphase cells and in chromatin regulation in D. melanogaster [146].
In humans, in addition to histone H3T3 phosphorylation and chromatid cohesion [147], the Haspin protein is involved in the proper recruitment of the Chromosomal Passenger Complex (CPC) at the centromeric chromatin to activate Aurora B, thus allowing kinetochore–microtubule attachments. The detection of the Haspin protein and its mRNA in murine male germ cells was suggestive for a role of this kinase in cell cycle regulation of haploid cells [145,148,149]. When ectopically expressed in HEK-293 cells, Haspin localizes to the nucleus, shows DNA-binding capacity, and led to reduced cell proliferation. It was therefore suggested that Haspin could play a negative control of cell-cycle and differentiation of haploid germ cells [148].
4.5. Conundrum (conu)
Genetic analyses have shown that conu is not required for viability [150]. It encodes a Rho GTPase activating protein (RhoGAP) that negatively regulates Rho1 activity at the cell cortex via interaction with the product of Moesin (Moe), an ezrin, radixin, and moesin (ERM) protein [150]. Consistent with its sequence similarity to other RhoGAP proteins, the conu protein has GAP activity for Rho1 in vitro and negatively regulates Rho1 in vivo and promotes cell proliferation in Drosophila epithelial tissues [150].
The mammalian ortholog of conu is ARHGAP18. The ARHGAP18 protein has a GAP activity for RhoA, the human homologue of Drosophila Rho1, and is required in cell shape, spreading, and migration control [151]. ARHGAP18 overexpression suppress cell proliferation, migration, invasion, and tumor growth in gastric cancer [152].
4.6. Casein Kinase-II Alpha (CkIIα)
It encodes a protein of the casein kinases family defined by their preferential utilization of caseins proteins as substrates. The alpha chain contains the catalytic site. The involvement of CKIIa in cell progression was originally suggested by RNAi studies in S2 cells [153,154]. Later, the critical role of CKIIa in the cell cycle was shown by Ducat et al. [155] in an effort to identify novel proteins important for microtubule assembly in mitosis.
More recently, microarray and flow cytometry-based approaches identified CKIIa in transcriptional networks controlling the cell cycle [156].
Finally, it has been recently suggested that the physical interaction of CKIIa with the ribosomal protein RPL22 [157] may be relevant in the regulation of transposon activity in D. melanogaster [158,159], implying a role for CKIIa in the stability of the genome during the cell cycle progression. CKIIa activity has been linked to behavioral disorders since it regulates slgA, the homolog of human PRODH, in the brain, suggesting its involvement in the generation of the phenotypes observed in Drosophila model for neuropsychiatric disorders [160].
5. Concluding Remarks
Constitutive heterochromatin is an ubiquitous and quantitatively significant component of eukaryotic genomes but it has been regarded for a long time merely as a “genomic desert” of functions or “graveyard” for dead transposable elements.
Recently, the “dogma” of silent heterochromatin has been revisited, providing a new interpretation of D. melanogaster constitutive heterochromatin in functional terms [6]. In particular, in this model organism, due to the great progress achieved by genetic and genomic analyses, hundreds of transcriptionally active genes have been identified in the constitutive heterochromatin [6,7,8,9,10,11,12,13,14,15]. However, despite this, the function of most genes has yet to be elucidated, and other genes may still remain undisclosed due to the gaps in the assembly of the Drosophila heterochromatin genome sequence.
Here, we have focused our attention on heterochromatic protein-coding genes involved in different steps of cell division, including chromatin/chromosome organization, mitotic apparatus, and cell cycle regulation. Cell division is a fundamental event common to most lifeforms. Thus, we think that presenting an overview of these genes will be also useful for a wide range of researchers who are interested in elucidating the molecular pathways and mechanisms underlying proper execution of cell division and its dysfunctions, which is relevant to both basic and applied research.
Interestingly, the heterochromatic genes under analysis are expressed during different developmental stages and are evolutionary conserved, with most human orthologs involved in genetic diseases. It is indeed already known that 75% of human genes involved in genetic disease have a functional ortholog in D. melanogaster [161,162,163].
It follows that studying the genes described here in Drosophila or in other animal models will also help to better characterize the corresponding human disease-causing genes, their protein products, and corresponding interaction networks.
The results of different studies showed that the present-day heterochromatin genes of D. melanogaster arose through an evolutionary repositioning of ancestral gene clusters located in the euchromatin of progenitor species [164]. Interestingly, the human orthologs of the D. melanogaster genes studied here are found in euchromatin (Table 1), and this appears to be a general rule. Thus, it is conceivable that during genome evolution these genes maintained similar functions, being properly expressed independently of their genomic locations, albeit some differences may exist in the regulation pattern during development and differentiation. This can be an interesting aspect to be investigated in future studies on Drosophila species.
In conclusion, multiple complementary approaches and experimental efforts are required to get a more complete view on the coding genes harbored by D. melanogaster constitutive heterochromatin and to elucidate their roles and regulatory requirements.
Author Contributions
Writing—review and editing, G.M., Y.P., G.B., R.M.M., and P.D. Supervision, P.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the University of Bari, Progetti di Ricerca di Ateneo #00869718Ricat (R.M.M.) and Sapienza University of Rome, Progetti di Ricerca di Ateneo #RM120172B851A176 (P.D.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heitz, E. Das Heterochromatin der Moose; Bornträger: Stuttgart, Germany, 1928. [Google Scholar]
- Brown, S.W. Heterochromatin. Science 1966, 151, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Elgin, S.C.; Reuter, G. Position-effect variegation, heterochromatin formation, and gene silencing in Drosophila. Cold Spring Harb. Perspect. Biol. 2013, 5, a017780. [Google Scholar] [CrossRef] [PubMed]
- Robert Finestra, T.; Gribnau, J. X chromosome inactivation: Silencing, topology and reactivation. Curr. Opin. Cell Biol. 2017, 46, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Nur, U. Heterochromatization and euchromatization of whole genomes in scale insects (Coccoidea: Homoptera). Development 1990, 108, 29–34. [Google Scholar] [CrossRef]
- Marsano, R.M.; Giordano, E.; Messina, G.; Dimitri, P. A New Portrait of Constitutive Heterochromatin: Lessons from Drosophila melanogaster. Trends Genet. 2019, 35, 615–631. [Google Scholar] [CrossRef]
- Hoskins, R.A.; Smith, C.D.; Carlson, J.W.; Carvalho, A.B.; Halpern, A.; Kaminker, J.S.; Kennedy, C.; Mungall, C.J.; Sullivan, B.A.; Sutton, G.G.; et al. Heterochromatic sequences in a Drosophila whole-genome shotgun assembly. Genome Biol. 2002, 3, research0085.1. [Google Scholar] [CrossRef]
- Corradini, N.; Rossi, F.; Verni, F.; Dimitri, P. FISH analysis of Drosophila melanogaster heterochromatin using BACs and P elements. Chromosoma 2003, 112, 26–37. [Google Scholar] [CrossRef]
- Smith, C.D.; Shu, S.; Mungall, C.J.; Karpen, G.H. The Release 5.1 annotation of Drosophila melanogaster heterochromatin. Science 2007, 316, 1586–1591. [Google Scholar] [CrossRef]
- Rossi, F.; Moschetti, R.; Caizzi, R.; Corradini, N.; Dimitri, P. Cytogenetic and molecular characterization of heterochromatin gene models in Drosophila melanogaster. Genetics 2007, 175, 595–607. [Google Scholar] [CrossRef][Green Version]
- Hoskins, R.A.; Carlson, J.W.; Kennedy, C.; Acevedo, D.; Evans-Holm, M.; Frise, E.; Wan, K.H.; Park, S.; Mendez-Lago, M.; Rossi, F.; et al. Sequence finishing and mapping of Drosophila melanogaster heterochromatin. Science 2007, 316, 1625–1628. [Google Scholar] [CrossRef]
- Dimitri, P.; Caizzi, R.; Giordano, E.; Carmela Accardo, M.; Lattanzi, G.; Biamonti, G. Constitutive heterochromatin: A surprising variety of expressed sequences. Chromosoma 2009, 118, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, A.B.; Alm, C.; Cealiac, I.; Sinclair, D.A.; Honda, B.M.; Rossi, F.; Dimitri, P.; Hilliker, A.J. Essential loci in centromeric heterochromatin of Drosophila melanogaster. I: The right arm of chromosome 2. Genetics 2010, 185, 479–495. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, R.A.; Carlson, J.W.; Wan, K.H.; Park, S.; Mendez, I.; Galle, S.E.; Booth, B.W.; Pfeiffer, B.D.; George, R.A.; Svirskas, R.; et al. The Release 6 reference sequence of the Drosophila melanogaster genome. Genome Res. 2015, 25, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Syrzycka, M.; Hallson, G.; Fitzpatrick, K.A.; Kim, I.; Cotsworth, S.; Hollebakken, R.E.; Simonetto, K.; Yang, L.; Luongo, S.; Beja, K.; et al. Genetic and Molecular Analysis of Essential Genes in Centromeric Heterochromatin of the Left Arm of Chromosome 3 in Drosophila melanogaster. G3 Genes Genomes Genet. 2019, 9, 1581–1595. [Google Scholar] [CrossRef]
- Gatti, M.; Pimpinelli, S. Functional elements in Drosophila melanogaster heterochromatin. Annu. Rev. Genet. 1992, 26, 239–275. [Google Scholar] [CrossRef]
- Dimitri, P. Cytogenetic analysis of the second chromosome heterochromatin of Drosophila melanogaster. Genetics 1991, 127, 553–564. [Google Scholar] [CrossRef]
- Fanti, L.; Pimpinelli, S. HP1: A functionally multifaceted protein. Curr. Opin. Genet. Dev. 2008, 18, 169–174. [Google Scholar] [CrossRef]
- Wallrath, L.L.; Rodriguez-Tirado, F.; Geyer, P.K. Shining Light on the Dark Side of the Genome. Cells 2022, 11, 330. [Google Scholar] [CrossRef]
- Saha, P.; Sowpati, D.T.; Soujanya, M.; Srivastava, I.; Mishra, R.K. Interplay of pericentromeric genome organization and chromatin landscape regulates the expression of Drosophila melanogaster heterochromatic genes. Epigenetics Chromatin 2020, 13, 41. [Google Scholar] [CrossRef]
- Lens, S.M.A.; Medema, R.H. Cytokinesis defects and cancer. Nat. Rev. Cancer 2019, 19, 32–45. [Google Scholar] [CrossRef]
- Sivakumar, S.; Gorbsky, G.J. Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat. Rev. Mol. Cell Biol. 2015, 16, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Cenci, G.; Belloni, G.; Dimitri, P. 1(2)41Aa, a heterochromatic gene of Drosophila melanogaster, is required for mitotic and meiotic chromosome condensation. Genet. Res. 2003, 81, 15–24. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Messina, G.; Damia, E.; Fanti, L.; Atterrato, M.T.; Celauro, E.; Mariotti, F.R.; Accardo, M.C.; Walther, M.; Verni, F.; Picchioni, D.; et al. Yeti, an essential Drosophila melanogaster gene, encodes a protein required for chromatin organization. J. Cell Sci. 2014, 127, 2577–2588. [Google Scholar] [CrossRef] [PubMed]
- Prozzillo, Y.; Delle Monache, F.; Ferreri, D.; Cuticone, S.; Dimitri, P.; Messina, G. The True Story of Yeti, the “Abominable” Heterochromatic Gene of Drosophila melanogaster. Front. Physiol. 2019, 10, 1093. [Google Scholar] [CrossRef]
- Kusch, T.; Florens, L.; Macdonald, W.H.; Swanson, S.K.; Glaser, R.L.; Yates, J.R., 3rd; Abmayr, S.M.; Washburn, M.P.; Workman, J.L. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science 2004, 306, 2084–2087. [Google Scholar] [CrossRef]
- March-Diaz, R.; Reyes, J.C. The beauty of being a variant: H2A.Z and the SWR1 complex in plants. Mol. Plant 2009, 2, 565–577. [Google Scholar] [CrossRef]
- Messina, G.; Celauro, E.; Atterrato, M.T.; Giordano, E.; Iwashita, S.; Dimitri, P. The Bucentaur (BCNT) protein family: A long-neglected class of essential proteins required for chromatin/chromosome organization and function. Chromosoma 2015, 124, 153–162. [Google Scholar] [CrossRef]
- Messina, G.; Atterrato, M.T.; Dimitri, P. When chromatin organisation floats astray: The Srcap gene and Floating-Harbor syndrome. J. Med. Genet. 2016, 53, 793–797. [Google Scholar] [CrossRef]
- Messina, G.; Prozzillo, Y.; Delle Monache, F.; Santopietro, M.V.; Atterrato, M.T.; Dimitri, P. The ATPase SRCAP is associated with the mitotic apparatus, uncovering novel molecular aspects of Floating-Harbor syndrome. BMC Biol. 2021, 19, 184. [Google Scholar] [CrossRef]
- Billmann, M.; Horn, T.; Fischer, B.; Sandmann, T.; Huber, W.; Boutros, M. A genetic interaction map of cell cycle regulators. Mol. Biol. Cell 2016, 27, 1397–1407. [Google Scholar] [CrossRef]
- Messina, G.; Atterrato, M.T.; Fanti, L.; Giordano, E.; Dimitri, P. Expression of human Cfdp1 gene in Drosophila reveals new insights into the function of the evolutionarily conserved BCNT protein family. Sci. Rep. 2016, 6, 25511. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Prozzillo, Y.; Monache, F.D.; Santopietro, M.V.; Dimitri, P. Evolutionary conserved relocation of chromatin remodeling complexes to the mitotic apparatus. BMC Biol. 2022, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Havugimana, P.C.; Hart, G.T.; Nepusz, T.; Yang, H.; Turinsky, A.L.; Li, Z.; Wang, P.I.; Boutz, D.R.; Fong, V.; Phanse, S.; et al. A census of human soluble protein complexes. Cell 2012, 150, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Messina, G.; Atterrato, M.T.; Prozzillo, Y.; Piacentini, L.; Losada, A.; Dimitri, P. The human Cranio Facial Development Protein 1 (Cfdp1) gene encodes a protein required for the maintenance of higher-order chromatin organization. Sci. Rep. 2017, 7, 45022. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Bukowski-Wills, J.C.; Sanchez-Pulido, L.; Alves Fde, L.; Wood, L.; Chen, Z.A.; Platani, M.; Fischer, L.; Hudson, D.F.; Ponting, C.P.; et al. The protein composition of mitotic chromosomes determined using multiclassifier combinatorial proteomics. Cell 2010, 142, 810–821. [Google Scholar] [CrossRef]
- Bode-Lesniewska, B.; Fritz, C.; Exner, G.U.; Wagner, U.; Fuchs, B. EWSR1-NFATC2 and FUS-NFATC2 Gene Fusion-Associated Mesenchymal Tumors: Clinicopathologic Correlation and Literature Review. Sarcoma 2019, 2019, 9386390. [Google Scholar] [CrossRef]
- Perl, A.; Qian, Y.; Chohan, K.R.; Shirley, C.R.; Amidon, W.; Banerjee, S.; Middleton, F.A.; Conkrite, K.L.; Barcza, M.; Gonchoroff, N.; et al. Transaldolase is essential for maintenance of the mitochondrial transmembrane potential and fertility of spermatozoa. Proc. Natl. Acad. Sci. USA 2006, 103, 14813–14818. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Ting, L.; Bruckner, R.J.; Gebreab, F.; Gygi, M.P.; Szpyt, J.; Tam, S.; Zarraga, G.; Colby, G.; Baltier, K.; et al. The BioPlex Network: A Systematic Exploration of the Human Interactome. Cell 2015, 162, 425–440. [Google Scholar] [CrossRef]
- Huttlin, E.L.; Bruckner, R.J.; Paulo, J.A.; Cannon, J.R.; Ting, L.; Baltier, K.; Colby, G.; Gebreab, F.; Gygi, M.P.; Parzen, H.; et al. Architecture of the human interactome defines protein communities and disease networks. Nature 2017, 545, 505–509. [Google Scholar] [CrossRef]
- Berkovits, B.D.; Wolgemuth, D.J. The role of the double bromodomain-containing BET genes during mammalian spermatogenesis. Curr. Top. Dev. Biol. 2013, 102, 293–326. [Google Scholar] [CrossRef]
- Green, C.D.; Ma, Q.; Manske, G.L.; Shami, A.N.; Zheng, X.; Marini, S.; Moritz, L.; Sultan, C.; Gurczynski, S.J.; Moore, B.B.; et al. A Comprehensive Roadmap of Murine Spermatogenesis Defined by Single-Cell RNA-Seq. Dev. Cell 2018, 46, 651–667.e10. [Google Scholar] [CrossRef] [PubMed]
- Zalensky, A.O.; Siino, J.S.; Gineitis, A.A.; Zalenskaya, I.A.; Tomilin, N.V.; Yau, P.; Bradbury, E.M. Human testis/sperm-specific histone H2B (hTSH2B). Molecular cloning and characterization. J. Biol. Chem. 2002, 277, 43474–43480. [Google Scholar] [CrossRef] [PubMed]
- Gause, M.; Eissenberg, J.C.; Macrae, A.F.; Dorsett, M.; Misulovin, Z.; Dorsett, D. Nipped-A, the Tra1/TRRAP subunit of the Drosophila SAGA and Tip60 complexes, has multiple roles in Notch signaling during wing development. Mol. Cell. Biol. 2006, 26, 2347–2359. [Google Scholar] [CrossRef] [PubMed]
- Prozzillo, Y.; Cuticone, S.; Ferreri, D.; Fattorini, G.; Messina, G.; Dimitri, P. In Vivo Silencing of Genes Coding for dTip60 Chromatin Remodeling Complex Subunits Affects Polytene Chromosome Organization and Proper Development in Drosophila melanogaster. Int. J. Mol. Sci. 2021, 22, 4525. [Google Scholar] [CrossRef] [PubMed]
- Grant, P.A.; Schieltz, D.; Pray-Grant, M.G.; Yates, J.R., 3rd; Workman, J.L. The ATM-related cofactor Tra1 is a component of the purified SAGA complex. Mol. Cell 1998, 2, 863–867. [Google Scholar] [CrossRef]
- Brown, C.E.; Howe, L.; Sousa, K.; Alley, S.C.; Carrozza, M.J.; Tan, S.; Workman, J.L. Recruitment of HAT complexes by direct activator interactions with the ATM-related Tra1 subunit. Science 2001, 292, 2333–2337. [Google Scholar] [CrossRef]
- Carrozza, M.J.; Utley, R.T.; Workman, J.L.; Cote, J. The diverse functions of histone acetyltransferase complexes. Trends Genet. 2003, 19, 321–329. [Google Scholar] [CrossRef]
- Rollins, R.A.; Morcillo, P.; Dorsett, D. Nipped-B, a Drosophila homologue of chromosomal adherins, participates in activation by remote enhancers in the cut and Ultrabithorax genes. Genetics 1999, 152, 577–593. [Google Scholar] [CrossRef]
- Seitan, V.C.; Banks, P.; Laval, S.; Majid, N.A.; Dorsett, D.; Rana, A.; Smith, J.; Bateman, A.; Krpic, S.; Hostert, A.; et al. Metazoan Scc4 homologs link sister chromatid cohesion to cell and axon migration guidance. PLoS Biol. 2006, 4, e242. [Google Scholar] [CrossRef]
- Takahashi, T.S.; Yiu, P.; Chou, M.F.; Gygi, S.; Walter, J.C. Recruitment of Xenopus Scc2 and cohesin to chromatin requires the pre-replication complex. Nat. Cell Biol. 2004, 6, 991–996. [Google Scholar] [CrossRef]
- Watrin, E.; Schleiffer, A.; Tanaka, K.; Eisenhaber, F.; Nasmyth, K.; Peters, J.M. Human Scc4 is required for cohesin binding to chromatin, sister-chromatid cohesion, and mitotic progression. Curr. Biol. 2006, 16, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Gruber, S.; Tanaka, K.; Haering, C.H.; Mechtler, K.; Nasmyth, K. ATP hydrolysis is required for cohesin’s association with chromosomes. Curr. Biol. 2003, 13, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Ciosk, R.; Shirayama, M.; Shevchenko, A.; Tanaka, T.; Toth, A.; Shevchenko, A.; Nasmyth, K. Cohesin’s binding to chromosomes depends on a separate complex consisting of Scc2 and Scc4 proteins. Mol. Cell 2000, 5, 243–254. [Google Scholar] [CrossRef]
- Gillespie, P.J.; Hirano, T. Scc2 couples replication licensing to sister chromatid cohesion in Xenopus egg extracts. Curr. Biol. 2004, 14, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, T.; Nagao, K.; Kawasaki, Y.; Furuya, K.; Murakami, A.; Morishita, J.; Yuasa, T.; Sutani, T.; Kearsey, S.E.; Uhlmann, F.; et al. Characterization of fission yeast cohesin: Essential anaphase proteolysis of Rad21 phosphorylated in the S phase. Genes Dev. 2000, 14, 2757–2770. [Google Scholar] [CrossRef]
- Hirano, T. At the heart of the chromosome: SMC proteins in action. Nat. Rev. Mol. Cell. Biol. 2006, 7, 311–322. [Google Scholar] [CrossRef]
- Nasmyth, K.; Haering, C.H. The structure and function of SMC and kleisin complexes. Annu. Rev. Biochem. 2005, 74, 595–648. [Google Scholar] [CrossRef]
- Huang, C.E.; Milutinovich, M.; Koshland, D. Rings, bracelet or snaps: Fashionable alternatives for Smc complexes. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2005, 360, 537–542. [Google Scholar] [CrossRef]
- Gause, M.; Webber, H.A.; Misulovin, Z.; Haller, G.; Rollins, R.A.; Eissenberg, J.C.; Bickel, S.E.; Dorsett, D. Functional links between Drosophila Nipped-B and cohesin in somatic and meiotic cells. Chromosoma 2008, 117, 51–66. [Google Scholar] [CrossRef]
- Krantz, I.D.; McCallum, J.; DeScipio, C.; Kaur, M.; Gillis, L.A.; Yaeger, D.; Jukofsky, L.; Wasserman, N.; Bottani, A.; Morris, C.A.; et al. Cornelia de Lange syndrome is caused by mutations in NIPBL, the human homolog of Drosophila melanogaster Nipped-B. Nat. Genet. 2004, 36, 631–635. [Google Scholar] [CrossRef]
- Parenti, I.; Diab, F.; Gil, S.R.; Mulugeta, E.; Casa, V.; Berutti, R.; Brouwer, R.W.W.; Dupe, V.; Eckhold, J.; Graf, E.; et al. MAU2 and NIPBL Variants Impair the Heterodimerization of the Cohesin Loader Subunits and Cause Cornelia de Lange Syndrome. Cell Rep. 2020, 31, 107647. [Google Scholar] [CrossRef]
- Rollins, R.A.; Korom, M.; Aulner, N.; Martens, A.; Dorsett, D. Drosophila nipped-B protein supports sister chromatid cohesion and opposes the stromalin/Scc3 cohesion factor to facilitate long-range activation of the cut gene. Mol. Cell. Biol. 2004, 24, 3100–3111. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; DeScipio, C.; McCallum, J.; Yaeger, D.; Devoto, M.; Jackson, L.G.; Spinner, N.B.; Krantz, I.D. Precocious sister chromatid separation (PSCS) in Cornelia de Lange syndrome. Am. J. Med. Genet. A 2005, 138, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Luna-Pelaez, N.; March-Diaz, R.; Ceballos-Chavez, M.; Guerrero-Martinez, J.A.; Grazioli, P.; Garcia-Gutierrez, P.; Vaccari, T.; Massa, V.; Reyes, J.C.; Garcia-Dominguez, M. The Cornelia de Lange Syndrome-associated factor NIPBL interacts with BRD4 ET domain for transcription control of a common set of genes. Cell Death Dis. 2019, 10, 548. [Google Scholar] [CrossRef] [PubMed]
- Olley, G.; Ansari, M.; Bengani, H.; Grimes, G.R.; Rhodes, J.; von Kriegsheim, A.; Blatnik, A.; Stewart, F.J.; Wakeling, E.; Carroll, N.; et al. BRD4 interacts with NIPBL and BRD4 is mutated in a Cornelia de Lange-like syndrome. Nat. Genet. 2018, 50, 329–332. [Google Scholar] [CrossRef]
- Schulze, S.; Sinclair, D.A.; Silva, E.; Fitzpatrick, K.A.; Singh, M.; Lloyd, V.K.; Morin, K.A.; Kim, J.; Holm, D.G.; Kennison, J.A.; et al. Essential genes in proximal 3L heterochromatin of Drosophila melanogaster. Mol. Genet. Genom. 2001, 264, 782–789. [Google Scholar] [CrossRef]
- Fitzpatrick, K.A.; Sinclair, D.A.; Schulze, S.R.; Syrzycka, M.; Honda, B.M. A genetic and molecular profile of third chromosome centric heterochromatin in Drosophila melanogaster. Genome 2005, 48, 571–584. [Google Scholar] [CrossRef]
- Koryakov, D.E.; Zhimulev, I.F.; Dimitri, P. Cytogenetic analysis of the third chromosome heterochromatin of Drosophila melanogaster. Genetics 2002, 160, 509–517. [Google Scholar] [CrossRef]
- Hallson, G.; Syrzycka, M.; Beck, S.A.; Kennison, J.A.; Dorsett, D.; Page, S.L.; Hunter, S.M.; Keall, R.; Warren, W.D.; Brock, H.W.; et al. The Drosophila cohesin subunit Rad21 is a trithorax group (trxG) protein. Proc. Natl. Acad. Sci. USA 2008, 105, 12405–12410. [Google Scholar] [CrossRef]
- Oliveira, R.A.; Kotadia, S.; Tavares, A.; Mirkovic, M.; Bowlin, K.; Eichinger, C.S.; Nasmyth, K.; Sullivan, W. Centromere-independent accumulation of cohesin at ectopic heterochromatin sites induces chromosome stretching during anaphase. PLoS Biol. 2014, 12, e1001962. [Google Scholar] [CrossRef]
- Valdeolmillos, A.; Rufas, J.S.; Suja, J.A.; Vass, S.; Heck, M.M.; Martinez, A.C.; Barbero, J.L. Drosophila cohesins DSA1 and Drad21 persist and colocalize along the centromeric heterochromatin during mitosis. Biol. Cell 2004, 96, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Pauli, A.; Althoff, F.; Oliveira, R.A.; Heidmann, S.; Schuldiner, O.; Lehner, C.F.; Dickson, B.J.; Nasmyth, K. Cell-type-specific TEV protease cleavage reveals cohesin functions in Drosophila neurons. Dev. Cell 2008, 14, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Urban, E.; Nagarkar-Jaiswal, S.; Lehner, C.F.; Heidmann, S.K. The cohesin subunit Rad21 is required for synaptonemal complex maintenance, but not sister chromatid cohesion, during Drosophila female meiosis. PLoS Genet. 2014, 10, e1004540. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, M.A.; Bando, M.; Nakato, R.; Watrin, E.; Itoh, T.; Minamino, M.; Saitoh, K.; Komata, M.; Katou, Y.; Clark, D.; et al. HDAC8 mutations in Cornelia de Lange syndrome affect the cohesin acetylation cycle. Nature 2012, 489, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, M.A.; Wilde, J.J.; Albrecht, M.; Dickinson, E.; Tennstedt, S.; Braunholz, D.; Monnich, M.; Yan, Y.; Xu, W.; Gil-Rodriguez, M.C.; et al. RAD21 mutations cause a human cohesinopathy. Am. J. Hum. Genet. 2012, 90, 1014–1027. [Google Scholar] [CrossRef]
- Pimentel, S.A.B.J. Analysis of Teashirt Mutants Affecting Cell Proliferation in Drosophila Melanogaster; University of Lisboa: Lisboa, Portogal, 2010. [Google Scholar]
- Alexandre, E.; Graba, Y.; Fasano, L.; Gallet, A.; Perrin, L.; De Zulueta, P.; Pradel, J.; Kerridge, S.; Jacq, B. The Drosophila teashirt homeotic protein is a DNA-binding protein and modulo, a HOM-C regulated modifier of variegation, is a likely candidate for being a direct target gene. Mech. Dev. 1996, 59, 191–204. [Google Scholar] [CrossRef]
- Datta, R.R.; Lurye, J.M.; Kumar, J.P. Restriction of ectopic eye formation by Drosophila teashirt and tiptop to the developing antenna. Dev. Dyn. 2009, 238, 2202–2210. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Akram, A.; Katsel, P.; Haroutunian, V.; Schmeidler, J.; Beecham, G.; Haines, J.L.; Pericak-Vance, M.A.; Buxbaum, J.D. FE65 binds Teashirt, inhibiting expression of the primate-specific caspase-4. PLoS ONE 2009, 4, e5071. [Google Scholar] [CrossRef]
- Consortium, T.U. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2020, 49, D480–D489. [Google Scholar] [CrossRef]
- Mashtalir, N.; D’Avino, A.R.; Michel, B.C.; Luo, J.; Pan, J.; Otto, J.E.; Zullow, H.J.; McKenzie, Z.M.; Kubiak, R.L.; St Pierre, R.; et al. Modular Organization and Assembly of SWI/SNF Family Chromatin Remodeling Complexes. Cell 2018, 175, 1272–1288.e1220. [Google Scholar] [CrossRef]
- Barish, S.; Barakat, T.S.; Michel, B.C.; Mashtalir, N.; Phillips, J.B.; Valencia, A.M.; Ugur, B.; Wegner, J.; Scott, T.M.; Bostwick, B.; et al. BICRA, a SWI/SNF Complex Member, Is Associated with BAF-Disorder Related Phenotypes in Humans and Model Organisms. Am. J. Hum. Genet. 2020, 107, 1096–1112. [Google Scholar] [CrossRef] [PubMed]
- Clapier, C.R.; Cairns, B.R. The biology of chromatin remodeling complexes. Annu. Rev. Biochem. 2009, 78, 273–304. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, G.; Vergarajauregui, S.; Endele, S.; Popp, B.; Buttner, C.; Ekici, A.B.; Gerard, M.; Bramswig, N.C.; Albrecht, B.; Clayton-Smith, J.; et al. Mutations in the BAF-Complex Subunit DPF2 Are Associated with Coffin-Siris Syndrome. Am. J. Hum. Genet. 2018, 102, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Spektor, A.; Tsang, W.Y.; Khoo, D.; Dynlacht, B.D. Cep97 and CP110 suppress a cilia assembly program. Cell 2007, 130, 678–690. [Google Scholar] [CrossRef]
- Franz, A.; Roque, H.; Saurya, S.; Dobbelaere, J.; Raff, J.W. CP110 exhibits novel regulatory activities during centriole assembly in Drosophila. J. Cell Biol. 2013, 203, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Hodges, M.E.; Scheumann, N.; Wickstead, B.; Langdale, J.A.; Gull, K. Reconstructing the evolutionary history of the centriole from protein components. J. Cell Sci. 2010, 123, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Indjeian, V.B.; McManus, M.; Wang, L.; Dynlacht, B.D. CP110, a cell cycle-dependent CDK substrate, regulates centrosome duplication in human cells. Dev. Cell 2002, 3, 339–350. [Google Scholar] [CrossRef]
- Delgehyr, N.; Rangone, H.; Fu, J.; Mao, G.; Tom, B.; Riparbelli, M.G.; Callaini, G.; Glover, D.M. Klp10A, a microtubule-depolymerizing kinesin-13, cooperates with CP110 to control Drosophila centriole length. Curr. Biol. 2012, 22, 502–509. [Google Scholar] [CrossRef]
- Sawant, D.B.; Majumder, S.; Perkins, J.L.; Yang, C.H.; Eyers, P.A.; Fisk, H.A. Centrin 3 is an inhibitor of centrosomal Mps1 and antagonizes centrin 2 function. Mol. Biol. Cell 2015, 26, 3741–3753. [Google Scholar] [CrossRef]
- Salisbury, J.L. Centrin, centrosomes, and mitotic spindle poles. Curr. Opin. Cell Biol. 1995, 7, 39–45. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, X.; Yang, B. Calcium-induced human centrin 1 self-assembly and double-regulating the binding with peptide R18-Sfi1p. Int. J. Biol. Macromol. 2019, 128, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Schnorrer, F.; Schonbauer, C.; Langer, C.C.; Dietzl, G.; Novatchkova, M.; Schernhuber, K.; Fellner, M.; Azaryan, A.; Radolf, M.; Stark, A.; et al. Systematic genetic analysis of muscle morphogenesis and function in Drosophila. Nature 2010, 464, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Avidor-Reiss, T.; Maer, A.M.; Koundakjian, E.; Polyanovsky, A.; Keil, T.; Subramaniam, S.; Zuker, C.S. Decoding cilia function: Defining specialized genes required for compartmentalized cilia biogenesis. Cell 2004, 117, 527–539. [Google Scholar] [CrossRef]
- Laurencon, A.; Dubruille, R.; Efimenko, E.; Grenier, G.; Bissett, R.; Cortier, E.; Rolland, V.; Swoboda, P.; Durand, B. Identification of novel regulatory factor X (RFX) target genes by comparative genomics in Drosophila species. Genome Biol. 2007, 8, R195. [Google Scholar] [CrossRef]
- Jonassen, J.A.; San Agustin, J.; Follit, J.A.; Pazour, G.J. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J. Cell Biol. 2008, 183, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Amador-Arjona, A.; Elliott, J.; Miller, A.; Ginbey, A.; Pazour, G.J.; Enikolopov, G.; Roberts, A.J.; Terskikh, A.V. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: Implications for learning and memory. J. Neurosci. 2011, 31, 9933–9944. [Google Scholar] [CrossRef]
- Zhou, M.H.; Lin, Y.; Zhang, Z.G. Intraflagellar transport 20: New target for the treatment of ciliopathies. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118641. [Google Scholar] [CrossRef]
- Follit, J.A.; Tuft, R.A.; Fogarty, K.E.; Pazour, G.J. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol. Biol. Cell 2006, 17, 3781–3792. [Google Scholar] [CrossRef]
- Follit, J.A.; San Agustin, J.T.; Xu, F.; Jonassen, J.A.; Samtani, R.; Lo, C.W.; Pazour, G.J. The Golgin GMAP210/TRIP11 anchors IFT20 to the Golgi complex. PLoS Genet. 2008, 4, e1000315. [Google Scholar] [CrossRef]
- Stoetzel, C.; Bar, S.; De Craene, J.O.; Scheidecker, S.; Etard, C.; Chicher, J.; Reck, J.R.; Perrault, I.; Geoffroy, V.; Chennen, K.; et al. A mutation in VPS15 (PIK3R4) causes a ciliopathy and affects IFT20 release from the cis-Golgi. Nat. Commun. 2016, 7, 13586. [Google Scholar] [CrossRef]
- Aoki, T.; Nishita, M.; Sonoda, J.; Ikeda, T.; Kakeji, Y.; Minami, Y. Intraflagellar transport 20 promotes collective cancer cell invasion by regulating polarized organization of Golgi-associated microtubules. Cancer Sci. 2019, 110, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Bauerly, E.; Akiyama, T.; Staber, C.; Yi, K.; Gibson, M.C. Impact of cilia-related genes on mitochondrial dynamics during Drosophila spermatogenesis. Dev. Biol. 2022, 482, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Zur Lage, P.; Newton, F.G.; Jarman, A.P. Survey of the Ciliary Motility Machinery of Drosophila Sperm and Ciliated Mechanosensory Neurons Reveals Unexpected Cell-Type Specific Variations: A Model for Motile Ciliopathies. Front. Genet. 2019, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.B.; Patel-King, R.S.; Benashski, S.E.; McCaffery, J.M.; Goldstein, L.S.; King, S.M. Drosophila roadblock and Chlamydomonas LC7: A conserved family of dynein-associated proteins involved in axonal transport, flagellar motility, and mitosis. J. Cell Biol. 1999, 146, 165–180. [Google Scholar] [CrossRef][Green Version]
- Terenzio, M.; Di Pizio, A.; Rishal, I.; Marvaldi, L.; Di Matteo, P.; Kawaguchi, R.; Coppola, G.; Schiavo, G.; Fisher, E.M.C.; Fainzilber, M. DYNLRB1 is essential for dynein mediated transport and neuronal survival. Neurobiol. Dis. 2020, 140, 104816. [Google Scholar] [CrossRef]
- Alliance of Genome Resources, C. Alliance of Genome Resources Portal: Unified model organism research platform. Nucleic Acids Res. 2020, 48, D650–D658. [Google Scholar] [CrossRef]
- Goudreault, M.; D’Ambrosio, L.M.; Kean, M.J.; Mullin, M.J.; Larsen, B.G.; Sanchez, A.; Chaudhry, S.; Chen, G.I.; Sicheri, F.; Nesvizhskii, A.I.; et al. A PP2A phosphatase high density interaction network identifies a novel striatin-interacting phosphatase and kinase complex linked to the cerebral cavernous malformation 3 (CCM3) protein. Mol. Cell. Proteom. 2009, 8, 157–171. [Google Scholar] [CrossRef]
- Byers, J.T.; Guzzo, R.M.; Salih, M.; Tuana, B.S. Hydrophobic profiles of the tail anchors in SLMAP dictate subcellular targeting. BMC Cell. Biol. 2009, 10, 48. [Google Scholar] [CrossRef]
- Frost, A.; Elgort, M.G.; Brandman, O.; Ives, C.; Collins, S.R.; Miller-Vedam, L.; Weibezahn, J.; Hein, M.Y.; Poser, I.; Mann, M.; et al. Functional repurposing revealed by comparing S. pombe and S. cerevisiae genetic interactions. Cell 2012, 149, 1339–1352. [Google Scholar] [CrossRef]
- Nordzieke, S.; Zobel, T.; Franzel, B.; Wolters, D.A.; Kuck, U.; Teichert, I. A fungal sarcolemmal membrane-associated protein (SLMAP) homolog plays a fundamental role in development and localizes to the nuclear envelope, endoplasmic reticulum, and mitochondria. Eukaryot. Cell 2015, 14, 345–358. [Google Scholar] [CrossRef]
- Guzzo, R.M.; Sevinc, S.; Salih, M.; Tuana, B.S. A novel isoform of sarcolemmal membrane-associated protein (SLMAP) is a component of the microtubule organizing centre. J. Cell Sci. 2004, 117, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Takahashi, N.; Ohno, S.; Sakurada, H.; Nakamura, K.; On, Y.K.; Park, J.E.; Makiyama, T.; Horie, M.; Arimura, T.; et al. Novel SCN3B mutation associated with brugada syndrome affects intracellular trafficking and function of Nav1.5. Circ. J. 2013, 77, 959–967. [Google Scholar] [CrossRef]
- Ishikawa, T.; Sato, A.; Marcou, C.A.; Tester, D.J.; Ackerman, M.J.; Crotti, L.; Schwartz, P.J.; On, Y.K.; Park, J.E.; Nakamura, K.; et al. A novel disease gene for Brugada syndrome: Sarcolemmal membrane-associated protein gene mutations impair intracellular trafficking of hNav1.5. Circ. Arrhythmia Electrophysiol. 2012, 5, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, J.G.; Lin, P.T.; Flanagan, L.A.; Walsh, C.A. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 1999, 23, 257–271. [Google Scholar] [CrossRef]
- Lin, P.T.; Gleeson, J.G.; Corbo, J.C.; Flanagan, L.; Walsh, C.A. DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization. J. Neurosci. 2000, 20, 9152–9161. [Google Scholar] [CrossRef]
- Taylor, K.R.; Holzer, A.K.; Bazan, J.F.; Walsh, C.A.; Gleeson, J.G. Patient mutations in doublecortin define a repeated tubulin-binding domain. J. Biol. Chem. 2000, 275, 34442–34450. [Google Scholar] [CrossRef]
- Des Portes, V.; Pinard, J.M.; Billuart, P.; Vinet, M.C.; Koulakoff, A.; Carrie, A.; Gelot, A.; Dupuis, E.; Motte, J.; Berwald-Netter, Y.; et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell 1998, 92, 51–61. [Google Scholar] [CrossRef]
- Rath, U.; Wang, D.; Ding, Y.; Xu, Y.Z.; Qi, H.; Blacketer, M.J.; Girton, J.; Johansen, J.; Johansen, K.M. Chromator, a novel and essential chromodomain protein interacts directly with the putative spindle matrix protein skeletor. J. Cell. Biochem. 2004, 93, 1033–1047. [Google Scholar] [CrossRef]
- Rath, U.; Ding, Y.; Deng, H.; Qi, H.; Bao, X.; Zhang, W.; Girton, J.; Johansen, J.; Johansen, K.M. The chromodomain protein, Chromator, interacts with JIL-1 kinase and regulates the structure of Drosophila polytene chromosomes. J. Cell Sci. 2006, 119, 2332–2341. [Google Scholar] [CrossRef]
- Eggert, H.; Gortchakov, A.; Saumweber, H. Identification of the Drosophila interband-specific protein Z4 as a DNA-binding zinc-finger protein determining chromosomal structure. J. Cell Sci. 2004, 117, 4253–4264. [Google Scholar] [CrossRef]
- Pokholkova, G.V.; Demakov, S.A.; Andreenkov, O.V.; Andreenkova, N.G.; Volkova, E.I.; Belyaeva, E.S.; Zhimulev, I.F. Tethering of CHROMATOR and dCTCF proteins results in decompaction of condensed bands in the Drosophila melanogaster polytene chromosomes but does not affect their transcription and replication timing. PLoS ONE 2018, 13, e0192634. [Google Scholar] [CrossRef] [PubMed]
- Gergely, F.; Kidd, D.; Jeffers, K.; Wakefield, J.G.; Raff, J.W. D-TACC: A novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J. 2000, 19, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Guruharsha, K.G.; Rual, J.F.; Zhai, B.; Mintseris, J.; Vaidya, P.; Vaidya, N.; Beekman, C.; Wong, C.; Rhee, D.Y.; Cenaj, O.; et al. A protein complex network of Drosophila melanogaster. Cell 2011, 147, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Galletta, B.J.; Fagerstrom, C.J.; Schoborg, T.A.; McLamarrah, T.A.; Ryniawec, J.M.; Buster, D.W.; Slep, K.C.; Rogers, G.C.; Rusan, N.M. A centrosome interactome provides insight into organelle assembly and reveals a non-duplication role for Plk4. Nat. Commun. 2016, 7, 12476. [Google Scholar] [CrossRef]
- Xu, T.; Wang, H.; Huang, X.; Li, W.; Huang, Q.; Yan, Y.; Chen, J. Gene Fusion in Malignant Glioma: An Emerging Target for Next-Generation Personalized Treatment. Transl. Oncol. 2018, 11, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Audibert, A.; Juge, F.; Simonelig, M. The suppressor of forked protein of Drosophila, a homologue of the human 77K protein required for mRNA 3’-end formation, accumulates in mitotically-active cells. Mech. Dev. 1998, 72, 53–63. [Google Scholar] [CrossRef]
- Audibert, A.; Simonelig, M. Autoregulation at the level of mRNA 3’ end formation of the suppressor of forked gene of Drosophila melanogaster is conserved in Drosophila virilis. Proc. Natl. Acad. Sci. USA 1998, 95, 14302–14307. [Google Scholar] [CrossRef]
- Sonkar, A.; Yadav, S.; Ahmed, S. Cleavage and polyadenylation factor, Rna14 is an essential protein required for the maintenance of genomic integrity in fission yeast Schizosaccharomyces pombe. Biochim. Biophys. Acta 2016, 1863, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, B.; Liu, P.; Phatnani, H.P.; Fuda, N.J.; Cooper, J.J.; Price, D.H.; Adelman, K.; Lis, J.T.; Greenleaf, A.L. CDK12 is a transcription elongation-associated CTD kinase, the metazoan ortholog of yeast Ctk1. Genes Dev. 2010, 24, 2303–2316. [Google Scholar] [CrossRef]
- Edwards, M.C.; Wong, C.; Elledge, S.J. Human cyclin K, a novel RNA polymerase II-associated cyclin possessing both carboxy-terminal domain kinase and Cdk-activating kinase activity. Mol. Cell. Biol. 1998, 18, 4291–4300. [Google Scholar] [CrossRef]
- Blazek, D.; Kohoutek, J.; Bartholomeeusen, K.; Johansen, E.; Hulinkova, P.; Luo, Z.; Cimermancic, P.; Ule, J.; Peterlin, B.M. The Cyclin K/Cdk12 complex maintains genomic stability via regulation of expression of DNA damage response genes. Genes Dev. 2011, 25, 2158–2172. [Google Scholar] [CrossRef] [PubMed]
- Kohoutek, J.; Blazek, D. Cyclin K goes with Cdk12 and Cdk13. Cell Div. 2012, 7, 12. [Google Scholar] [CrossRef]
- Hilliker, A.J. Genetic analysis of the centromeric heterochromatin of chromosome 2 of Drosophila melanogaster: Deficiency mapping of EMS-induced lethal complementation groups. Genetics 1976, 83, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Biggs, W.H., 3rd; Zipursky, S.L. Primary structure, expression, and signal-dependent tyrosine phosphorylation of a Drosophila homolog of extracellular signal-regulated kinase. Proc. Natl. Acad. Sci. USA 1992, 89, 6295–6299. [Google Scholar] [CrossRef]
- Nishida, Y.; Inoue, Y.H.; Tsuda, L.; Adachi-Yamada, T.; Lim, Y.M.; Hata, M.; Ha, H.Y.; Sugiyama, S. The Raf/MAP kinase cascade in cell cycle regulation and differentiation in Drosophila. Cell Struct. Funct. 1996, 21, 437–444. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saunders, R.D.; Avides, M.C.; Howard, T.; Gonzalez, C.; Glover, D.M. The Drosophila gene abnormal spindle encodes a novel microtubule-associated protein that associates with the polar regions of the mitotic spindle. J. Cell Biol. 1997, 137, 881–890. [Google Scholar] [CrossRef]
- Ripoll, P.; Pimpinelli, S.; Valdivia, M.M.; Avila, J. A cell division mutant of Drosophila with a functionally abnormal spindle. Cell 1985, 41, 907–912. [Google Scholar] [CrossRef]
- Gonzalez, C.; Saunders, R.D.; Casal, J.; Molina, I.; Carmena, M.; Ripoll, P.; Glover, D.M. Mutations at the asp locus of Drosophila lead to multiple free centrosomes in syncytial embryos, but restrict centrosome duplication in larval neuroblasts. J. Cell Sci. 1990, 96 Pt 4, 605–616. [Google Scholar] [CrossRef]
- Casal, J.; Gonzalez, C.; Wandosell, F.; Avila, J.; Ripoll, P. Abnormal meiotic spindles cause a cascade of defects during spermatogenesis in asp males of Drosophila. Development 1990, 108, 251–260. [Google Scholar] [CrossRef]
- Gupta, S.; Varshney, B.; Chatterjee, S.; Ray, K. Somatic ERK activation during transit amplification is essential for maintaining the synchrony of germline divisions in Drosophila testis. Open Biol. 2018, 8, 180033. [Google Scholar] [CrossRef]
- Blaker-Lee, A.; Gupta, S.; McCammon, J.M.; De Rienzo, G.; Sive, H. Zebrafish homologs of genes within 16p11.2, a genomic region associated with brain disorders, are active during brain development, and include two deletion dosage sensor genes. Dis. Model. Mech. 2012, 5, 834–851. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Park, H.R.; Lee, J.H. MAPK3 at the Autism-Linked Human 16p11.2 Locus Influences Precise Synaptic Target Selection at Drosophila Larval Neuromuscular Junctions. Mol. Cells 2017, 40, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.M. Haspin-like proteins: A new family of evolutionarily conserved putative eukaryotic protein kinases. Protein Sci. 2001, 10, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Fresan, U.; Rodriguez-Sanchez, M.A.; Reina, O.; Corces, V.G.; Espinas, M.L. Haspin kinase modulates nuclear architecture and Polycomb-dependent gene silencing. PLoS Genet. 2020, 16, e1008962. [Google Scholar] [CrossRef]
- Dai, J.; Sullivan, B.A.; Higgins, J.M. Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Dev. Cell 2006, 11, 741–750. [Google Scholar] [CrossRef]
- Tanaka, H.; Yoshimura, Y.; Nozaki, M.; Yomogida, K.; Tsuchida, J.; Tosaka, Y.; Habu, T.; Nakanishi, T.; Okada, M.; Nojima, H.; et al. Identification and characterization of a haploid germ cell-specific nuclear protein kinase (Haspin) in spermatid nuclei and its effects on somatic cells. J. Biol. Chem. 1999, 274, 17049–17057. [Google Scholar] [CrossRef]
- Tanaka, H.; Iguchi, N.; Nakamura, Y.; Kohroki, J.; de Carvalho, C.E.; Nishimune, Y. Cloning and characterization of human haspin gene encoding haploid germ cell-specific nuclear protein kinase. Mol. Hum. Reprod. 2001, 7, 211–218. [Google Scholar] [CrossRef]
- Neisch, A.L.; Formstecher, E.; Fehon, R.G. Conundrum, an ARHGAP18 orthologue, regulates RhoA and proliferation through interactions with Moesin. Mol. Biol. Cell 2013, 24, 1420–1433. [Google Scholar] [CrossRef]
- Maeda, M.; Hasegawa, H.; Hyodo, T.; Ito, S.; Asano, E.; Yuang, H.; Funasaka, K.; Shimokata, K.; Hasegawa, Y.; Hamaguchi, M.; et al. ARHGAP18, a GTPase-activating protein for RhoA, controls cell shape, spreading, and motility. Mol. Biol. Cell 2011, 22, 3840–3852. [Google Scholar] [CrossRef]
- Li, Y.; Ji, S.; Fu, L.; Jiang, T.; Wu, D.; Meng, F. Over-expression of ARHGAP18 suppressed cell proliferation, migration, invasion, and tumor growth in gastric cancer by restraining over-activation of MAPK signaling pathways. Onco Targets Ther. 2018, 11, 279–290. [Google Scholar] [CrossRef]
- Bettencourt-Dias, M.; Giet, R.; Sinka, R.; Mazumdar, A.; Lock, W.G.; Balloux, F.; Zafiropoulos, P.J.; Yamaguchi, S.; Winter, S.; Carthew, R.W.; et al. Genome-wide survey of protein kinases required for cell cycle progression. Nature 2004, 432, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Goshima, G.; Wollman, R.; Goodwin, S.S.; Zhang, N.; Scholey, J.M.; Vale, R.D.; Stuurman, N. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 2007, 316, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Ducat, D.; Kawaguchi, S.; Liu, H.; Yates, J.R., 3rd; Zheng, Y. Regulation of microtubule assembly and organization in mitosis by the AAA+ ATPase Pontin. Mol. Biol. Cell 2008, 19, 3097–3110. [Google Scholar] [CrossRef] [PubMed]
- Bonke, M.; Turunen, M.; Sokolova, M.; Vaharautio, A.; Kivioja, T.; Taipale, M.; Bjorklund, M.; Taipale, J. Transcriptional networks controlling the cell cycle. G3 Genes Genomes Genet. 2013, 3, 75–90. [Google Scholar] [CrossRef]
- Zhao, W.; Bidwai, A.P.; Glover, C.V. Interaction of casein kinase II with ribosomal protein L22 of Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2002, 298, 60–66. [Google Scholar] [CrossRef]
- Berloco, M.F.; Minervini, C.F.; Moschetti, R.; Palazzo, A.; Viggiano, L.; Marsano, R.M. Evidence of the Physical Interaction between Rpl22 and the Transposable Element Doc5, a Heterochromatic Transposon of Drosophila melanogaster. Genes 2021, 12, 1997. [Google Scholar] [CrossRef]
- Minervini, C.F.; Berloco, M.F.; Marsano, R.M.; Viggiano, L. The Ribosomal Protein RpL22 Interacts In Vitro with 5’-UTR Sequences Found in Some Drosophila melanogaster Transposons. Genes 2022, 13, 305. [Google Scholar] [CrossRef]
- Zwarts, L.; Vulsteke, V.; Buhl, E.; Hodge, J.J.L.; Callaerts, P. SlgA, encoded by the homolog of the human schizophrenia-associated gene PRODH, acts in clock neurons to regulate Drosophila aggression. Dis. Model. Mech. 2017, 10, 705–716. [Google Scholar] [CrossRef]
- Reiter, L.T.; Potocki, L.; Chien, S.; Gribskov, M.; Bier, E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001, 11, 1114–1125. [Google Scholar] [CrossRef]
- Chow, C.Y.; Reiter, L.T. Etiology of Human Genetic Disease on the Fly. Trends Genet. 2017, 33, 391–398. [Google Scholar] [CrossRef]
- Link, N.; Bellen, H.J. Using Drosophila to drive the diagnosis and understand the mechanisms of rare human diseases. Development 2020, 147, dev191411. [Google Scholar] [CrossRef] [PubMed]
- Caizzi, R.; Moschetti, R.; Piacentini, L.; Fanti, L.; Marsano, R.M.; Dimitri, P. Comparative Genomic Analyses Provide New Insights into the Evolutionary Dynamics of Heterochromatin in Drosophila. PLoS Genet. 2016, 12, e1006212. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).