Smooth Muscle Myosin Localizes at the Leading Edge and Regulates the Redistribution of Actin-regulatory Proteins during Migration
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Wound Healing Assay
2.3. Immunoblot Analysis and Coimmunoprecipitation
2.4. Cell KD and Cell Transfection
2.5. Fluorescent Microscopy
2.6. Statistical Analysis
3. Results
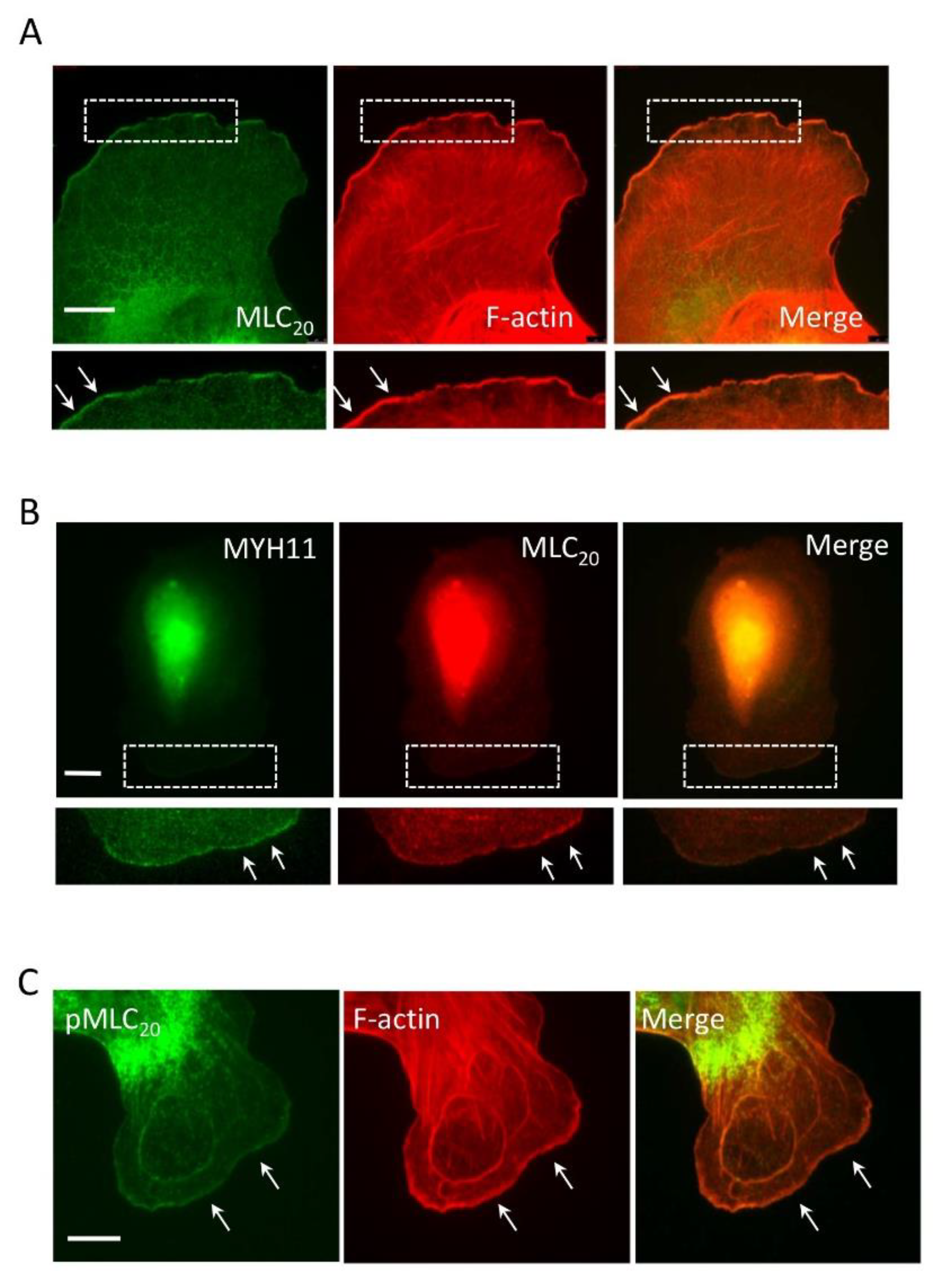
3.1. Smooth Muscle Myosin Localizes at the Leading Cell Edge
3.2. Knockdown of MLC20 and MYH11 Inhibits Smooth Muscle Cell Motility
3.3. KD of MLC20 Reduces Recruitment of c-Abl, Cortactin, Pfn-1, and Abi1 to the Cell Edge
3.4. KD of MYH11 Reduces Recruitment of c-Abl, Cortactin, Pfn-1, and Abi1 to the Cell Edge
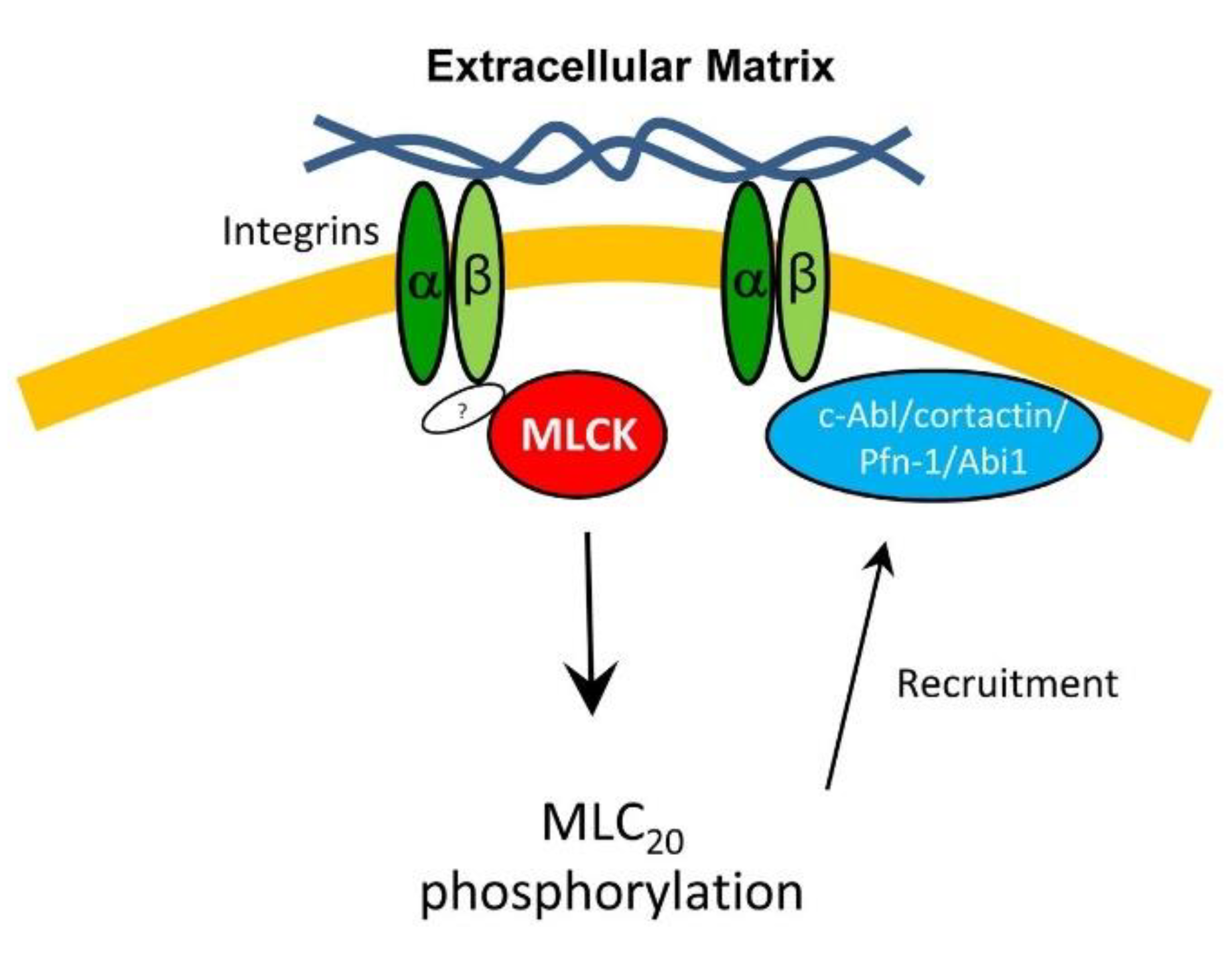
3.5. MLCK and Integrin β1 Colocalizes at the Leading Edge
3.6. Inhibition of MLC20 Phosphorylation Reduces the Recruitment of Actin-regulatory Proteins
3.7. Inhibition of Actin Polymerization Affects the Recruitment of Actin-regulatory Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abi1 | Abl interactor-1 |
| Arp2/3 | Actin Related Protein 2/3 complex |
| c-Abl | Abelson tyrosine kinase |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HASM | Human airway smooth muscle |
| KD | Knockdown |
| MLC20 | 20-kDa myosin light chain |
| MLCK | Myosin light chain kinase |
| N-WASP | Neuronal Wiskott–Aldrich Syndrome Protein |
| Pfn-1 | Profilin-1 |
| siRNA | Small interfering RNA |
| WAVE | WAS family member |
References
- Walsh, M.P. Vascular smooth muscle myosin light chain diphosphorylation: Mechanism, function, and pathological implications. IUBMB Life 2011, 63, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Huang, J.; He, W.; Zhu, M.; Kamm, K.E.; Stull, J.T. Signaling through myosin light chain kinase in smooth muscles. J. Biol. Chem. 2013, 288, 7596–7605. [Google Scholar] [CrossRef] [PubMed]
- Petrov, D.; Dahan, I.; Cohen-Kfir, E.; Ravid, S. Apkczeta affects directed cell migration through the regulation of myosin light chain phosphorylation. Cell Adhes. Migr. 2017, 11, 347–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freed, D.H.; Chilton, L.; Li, Y.; Dangerfield, A.L.; Raizman, J.E.; Rattan, S.G.; Visen, N.; Hryshko, L.V.; Dixon, I.M.C. Role of myosin light chain kinase in cardiotrophin-1-induced cardiac myofibroblast cell migration. Am. J. Physiol. Circ. Physiol. 2011, 301, H514–H522. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, Z.; He, Y.; Li, P.; Chen, Y.; Chen, X.; Jiang, Y.; Qin, X.; Li, S.; Li, T.; et al. Non-muscle myosin II isoforms orchestrate substrate stiffness sensing to promote cancer cell contractility and migration. Cancer Lett. 2021, 524, 245–258. [Google Scholar] [CrossRef]
- Pollard, T.D.; Cooper, J.A. Actin, a Central Player in Cell Shape and Movement. Science. 2009, 326, 1208–1212. [Google Scholar] [CrossRef]
- Gerthoffer, W.T. Migration of airway smooth muscle cells. Proc. Am. Thorac. Soc. 2008, 5, 97–105. [Google Scholar] [CrossRef]
- Cleary, R.A.; Wang, R.; Waqar, O.; Singer, H.A.; Tang, D.D. Role of c-Abl tyrosine kinase in smooth muscle cell migration. Am. J. Physiol. Physiol. 2014, 306, C753–C761. [Google Scholar] [CrossRef][Green Version]
- Tang, D.D.; Gerlach, B.D. The roles and regulation of the actin cytoskeleton, intermediate filaments and microtubules in smooth muscle cell migration. Respir. Res. 2017, 18, 1–12. [Google Scholar] [CrossRef]
- Tang, D.D. The Dynamic Actin Cytoskeleton in Smooth Muscle. 2018, 81, 1–38. Adv. Pharmacol. 2018, 81, 1–38. [Google Scholar] [CrossRef]
- Wang, T.; Cleary, R.A.; Wang, R.; Tang, D.D. Role of the Adapter Protein Abi1 in Actin-associated Signaling and Smooth Muscle Contraction. J. Biol. Chem. 2013, 288, 20713–20722. [Google Scholar] [CrossRef]
- Ring, C.; Ginsberg, M.H.; Haling, J.; Pendergast, A.M. Abl-interactor-1 (Abi1) has a role in cardiovascular and placental development and is a binding partner of the α4 integrin. Proc. Natl. Acad. Sci. USA 2010, 108, 149–154. [Google Scholar] [CrossRef]
- Innocenti, M.; Gerboth, S.; Rottner, K.; Lai, F.P.L.; Hertzog, M.; Stradal, T.; Frittoli, E.; Didry, D.; Polo, S.; Disanza, A.; et al. Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat. Cell Biol. 2005, 7, 969–976. [Google Scholar] [CrossRef]
- Dubielecka, P.M.; Ladwein, K.I.; Xiong, X.; Migeotte, I.; Chorzalska, A.; Anderson, K.V.; Sawicki, J.A.; Rottner, K.; Stradal, T.E.; Kotula, L. Essential role for Abi1 in embryonic survival and WAVE2 complex integrity. Proc. Natl. Acad. Sci. USA 2011, 108, 7022–7027. [Google Scholar] [CrossRef]
- Trivedi, D.V.; Nag, S.; Spudich, A.; Ruppel, K.M.; Spudich, J.A. The Myosin Family of Mechanoenzymes: From Mechanisms to Therapeutic Approaches. Annu. Rev. Biochem. 2020, 89, 667–693. [Google Scholar] [CrossRef]
- Chen, C.; Tao, T.; Wen, C.; He, W.-Q.; Qiao, Y.-N.; Gao, Y.-Q.; Chen, X.; Wang, P.; Chen, C.-P.; Zhao, W.; et al. Myosin Light Chain Kinase (MLCK) Regulates Cell Migration in a Myosin Regulatory Light Chain Phosphorylation-independent Mechanism. J. Biol. Chem. 2014, 289, 28478–28488. [Google Scholar] [CrossRef]
- Parkash, J.; Cimino, I.; Ferraris, N.; Casoni, F.; Wray, S.; Cappy, H.; Prevot, V.; Giacobini, P. Suppression of beta1-integrin in gonadotropin-releasing hormone cells disrupts migration and axonal extension resulting in severe reproductive alterations. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 16992–17002. [Google Scholar] [CrossRef]
- Wang, T.; Wang, R.; Cleary, R.A.; Gannon, O.J.; Tang, D.D. Recruitment of beta-catenin to n-cadherin is necessary for smooth muscle contraction. J. Biol. Chem. 2015, 290, 8913–8924. [Google Scholar] [CrossRef]
- Wang, R.; Cleary, R.A.; Wang, T.; Li, J.; Tang, D.D. The Association of Cortactin with Profilin-1 Is Critical for Smooth Muscle Contraction. J. Biol. Chem. 2014, 289, 14157–14169. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, G.; Wang, R.; Tang, D.D. Acetylation of Abelson interactor 1 at K416 regulates actin cytoskeleton and smooth muscle contraction. FASEB J. 2021, 35, e21811. [Google Scholar] [CrossRef]
- Li, Q.-F.; Spinelli, A.M.; Wang, R.; Anfinogenova, Y.; Singer, H.A.; Tang, D.D. Critical Role of Vimentin Phosphorylation at Ser-56 by p21-activated Kinase in Vimentin Cytoskeleton Signaling. J. Biol. Chem. 2006, 281, 34716–34724. [Google Scholar] [CrossRef]
- Liao, G.; Wang, R.; Rezey, A.C.; Gerlach, B.D.; Tang, D.D. Microrna mir-509 regulates erk1/2, the vimentin network, and focal adhesions by targeting plk1. Sci. Rep. 2018, 8, 12635. [Google Scholar] [CrossRef]
- Liao, G.; Panettieri, R.A.; Tang, D.D. Microrna-203 negatively regulates c-abl, erk1/2 phosphorylation, and proliferation in smooth muscle cells. Physiol. Rep. 2015, 3, e12541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rezey, A.C.; Wang, R.; Tang, D.D. Role and regulation of Abelson tyrosine kinase in Crk-associated substrate/profilin-1 interaction and airway smooth muscle contraction. Respir. Res. 2018, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, R.; Gannon, O.J.; Rezey, A.C.; Jiang, S.; Gerlach, B.D.; Liao, G.; Tang, D.D. Polo-like kinase 1 regulates vimentin phosphorylation at ser-56 and contraction in smooth muscle. J. Biol. Chem. 2016, 291, 23693–23703. [Google Scholar] [CrossRef] [PubMed]
- Rezey, A.C.; Gerlach, B.D.; Wang, R.; Liao, G.; Tang, D.D. Plk1 Mediates Paxillin Phosphorylation (Ser-272), Centrosome Maturation, and Airway Smooth Muscle Layer Thickening in Allergic Asthma. Sci. Rep. 2019, 9, 7555. [Google Scholar] [CrossRef]
- Wang, R.; Liao, G.; Wang, Y.; Tang, D.D. Distinctive roles of Abi1 in regulating actin-associated proteins during human smooth muscle cell migration. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Liao, G.; Wang, R.; Tang, D.D. Plk1 Regulates Caspase-9 Phosphorylation at Ser-196 and Apoptosis of Human Airway Smooth Muscle Cells. Am. J. Respir. Cell Mol. Biol. 2022, 66, 223–234. [Google Scholar] [CrossRef]
- Tang, D.D.; Zhang, W.; Gunst, S.J. The Adapter Protein CrkII Regulates Neuronal Wiskott-Aldrich Syndrome Protein, Actin Polymerization, and Tension Development during Contractile Stimulation of Smooth Muscle. J. Biol. Chem. 2005, 280, 23380–23389. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Y.; Liao, G.; Chen, B.; Panettieri, R.A.; Penn, R.B., Jr.; Tang, D.D. Abi1 mediates airway smooth muscle cell proliferation and airway remodeling via jak2/stat3 signaling. iScience 2022, 25, 103833. [Google Scholar] [CrossRef]
- Tang, D.D.; Bai, Y.; Gunst, S.J. Silencing of p21-activated kinase attenuates vimentin phosphorylation on Ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem. J. 2005, 388, 773–783. [Google Scholar] [CrossRef]
- Gerlach, B.D.; Tubbesing, K.; Liao, G.; Rezey, A.C.; Wang, R.; Barroso, M.; Tang, D.D. Phosphorylation of gmfgamma by c-abl coordinates lamellipodial and focal adhesion dynamics to regulate airway smooth muscle cell migration. Am. J. Respir. Cell Mol. Biol. 2019, 61, 219–231. [Google Scholar] [CrossRef]
- Gilbert, M.A.; Schultz-Rogers, L.; Rajagopalan, R.; Grochowski, C.M.; Wilkins, B.J.; Biswas, S.; Conlin, L.K.; Fiorino, K.N.; Dhamija, R.; Pack, M.A.; et al. Protein-elongating mutations in myh11 are implicated in a dominantly inherited smooth muscle dysmotility syndrome with severe esophageal, gastric, and intestinal disease. Hum. Mutat. 2020, 41, 973–982. [Google Scholar] [CrossRef]
- Ding, Z.; Lambrechts, A.; Parepally, M.; Roy, P. Silencing profilin-1 inhibits endothelial cell proliferation, migration and cord morphogenesis. J. Cell Sci. 2006, 119, 4127–4137. [Google Scholar] [CrossRef]
- Lapetina, S.; Mader, C.C.; Machida, K.; Mayer, B.J.; Koleske, A.J. Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J. Cell Biol. 2009, 185, 503–519. [Google Scholar] [CrossRef]
- Pinon, P.; Wehrle-Haller, B. Integrins: Versatile receptors controlling melanocyte adhesion, migration and proliferation. Pigment Cell Melanoma Res. 2010, 24, 282–294. [Google Scholar] [CrossRef]
- Balamatsias, D.; Kong, A.M.; Waters, J.E.; Sriratana, A.; Gurung, R.; Bailey, C.G.; Rasko, J.E.; Tiganis, T.; Macaulay, S.L.; Mitchell, C.A. Identification of P-Rex1 as a Novel Rac1-Guanine Nucleotide Exchange Factor (GEF) That Promotes Actin Remodeling and GLUT4 Protein Trafficking in Adipocytes. J. Biol. Chem. 2011, 286, 43229–43240. [Google Scholar] [CrossRef]
- Fujita, A.; Shishido, T.; Yuan, Y.; Inamoto, E.; Narumiya, S.; Watanabe, N. Imatinib Mesylate (STI571)-Induced Cell Edge Translocation of Kinase-Active and Kinase-Defective Abelson Kinase: Requirements of Myristoylation and src Homology 3 Domain. Mol. Pharmacol. 2008, 75, 75–84. [Google Scholar] [CrossRef]
- Zhang, W.; Gunst, S.J. Non-muscle (NM) myosin heavy chain phosphorylation regulates the formation of NM myosin filaments, adhesome assembly and smooth muscle contraction. J. Physiol. 2017, 595, 4279–4300. [Google Scholar] [CrossRef]
- Blanchoin, L.; Boujemaa-Paterski, R.; Sykes, C.; Plastino, J. Actin Dynamics, Architecture, and Mechanics in Cell Motility. Physiol. Rev. 2014, 94, 235–263. [Google Scholar] [CrossRef]
- Kampourakis, T.; Sun, Y.-B.; Irving, M. Myosin light chain phosphorylation enhances contraction of heart muscle via structural changes in both thick and thin filaments. Proc. Natl. Acad. Sci. USA 2016, 113, E3039–E3047. [Google Scholar] [CrossRef] [PubMed]
- Stull, J.T.; Kamm, K.E.; Krueger, J.K.; Lin, P.-J.; Luby-Phelps, K.; Zhi, G. 12 Ca2+/Calmodulin-dependent myosin light-chain kinases. Adv. Second Messenger Phosphoprot. Res. 1997, 31, 141–150. [Google Scholar] [CrossRef]
- Meng, W.; Takeichi, M. Adherens Junction: Molecular Architecture and Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a002899. [Google Scholar] [CrossRef]
- Totsukawa, G.; Yamakita, Y.; Yamashiro, S.; Hartshorne, D.J.; Sasaki, Y.; Matsumura, F. Distinct Roles of Rock (Rho-Kinase) and Mlck in Spatial Regulation of Mlc Phosphorylation for Assembly of Stress Fibers and Focal Adhesions in 3t3 Fibroblasts. J. Cell Biol. 2000, 150, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.G.N.; Verin, A.D.; Schaphorst, K.; Siddiqui, R.; Patterson, C.E.; Csortos, C.; Natarajan, V. Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60src. Am. J. Physiol. Cell. Mol. Physiol. 1999, 276, L989–L998. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Arbel, E.; Tang, D.D. Smooth Muscle Myosin Localizes at the Leading Edge and Regulates the Redistribution of Actin-regulatory Proteins during Migration. Cells 2022, 11, 2334. https://doi.org/10.3390/cells11152334
Wang R, Arbel E, Tang DD. Smooth Muscle Myosin Localizes at the Leading Edge and Regulates the Redistribution of Actin-regulatory Proteins during Migration. Cells. 2022; 11(15):2334. https://doi.org/10.3390/cells11152334
Chicago/Turabian StyleWang, Ruping, Eylon Arbel, and Dale D. Tang. 2022. "Smooth Muscle Myosin Localizes at the Leading Edge and Regulates the Redistribution of Actin-regulatory Proteins during Migration" Cells 11, no. 15: 2334. https://doi.org/10.3390/cells11152334
APA StyleWang, R., Arbel, E., & Tang, D. D. (2022). Smooth Muscle Myosin Localizes at the Leading Edge and Regulates the Redistribution of Actin-regulatory Proteins during Migration. Cells, 11(15), 2334. https://doi.org/10.3390/cells11152334






