The Outcomes and Quality of Pancreatic Islet Cells Isolated from Surgical Specimens for Research on Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
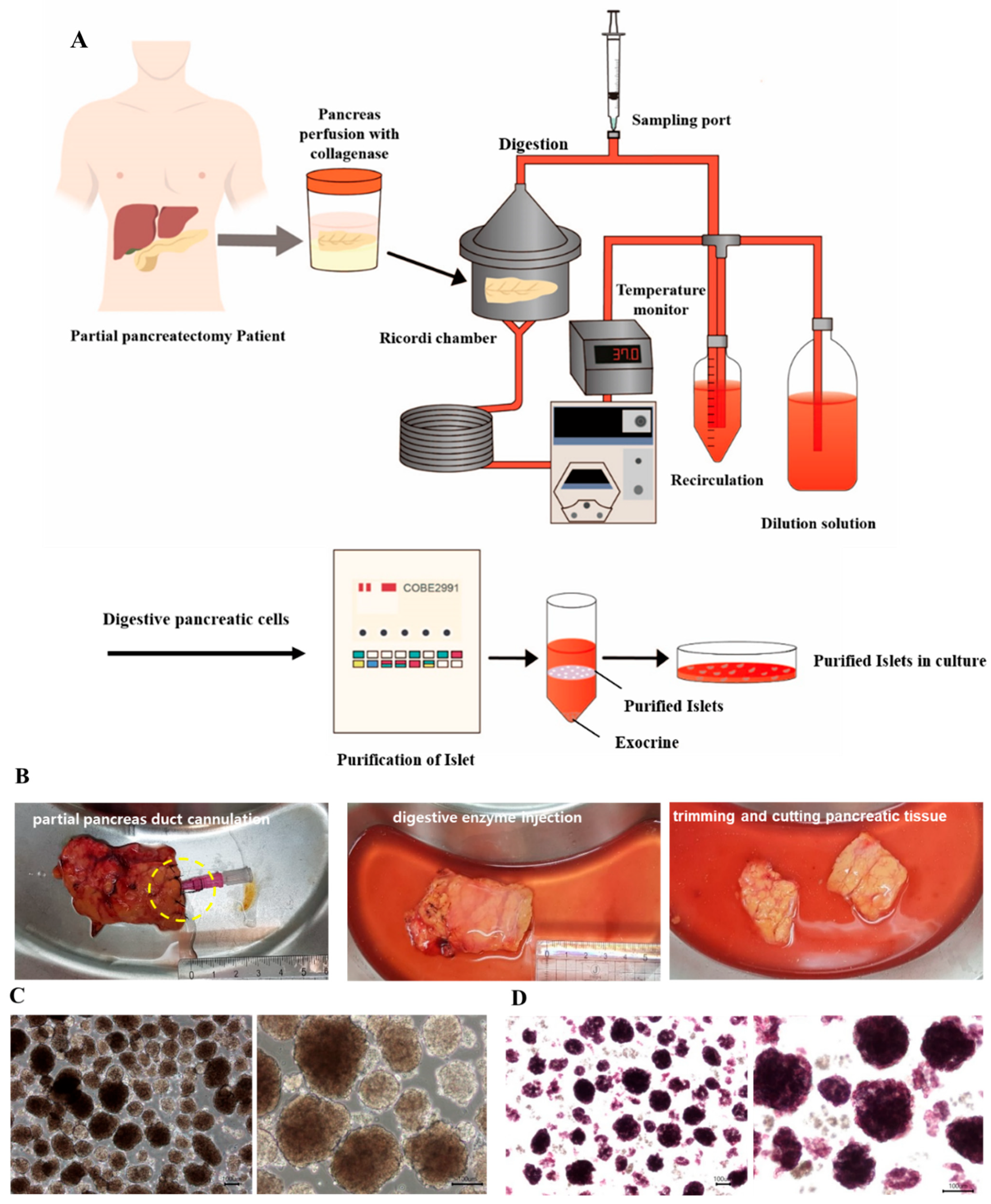
2.1. Pancreatic Resection and Harvest of Pancreatic Tissue
2.2. Islet Isolation
2.3. Islet Yield and Viability
2.4. Islet Culture
2.5. Glucose-Stimulated Insulin Secretion (GSIS) Assay
2.6. RNA and DNA Extraction
2.7. Immunostaining
2.8. Mitochondrial Respiratory Function Assessment
2.9. Cryopreservation and Thawing
2.10. Transplantation of Islets into Diabetic Mice
2.11. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.12. Statistical Analysis
3. Results
3.1. Donor Characteristics
3.2. Islet Yield from the Partially Resected Pancreas
3.3. Factor Analysis of Isolation Outcomes
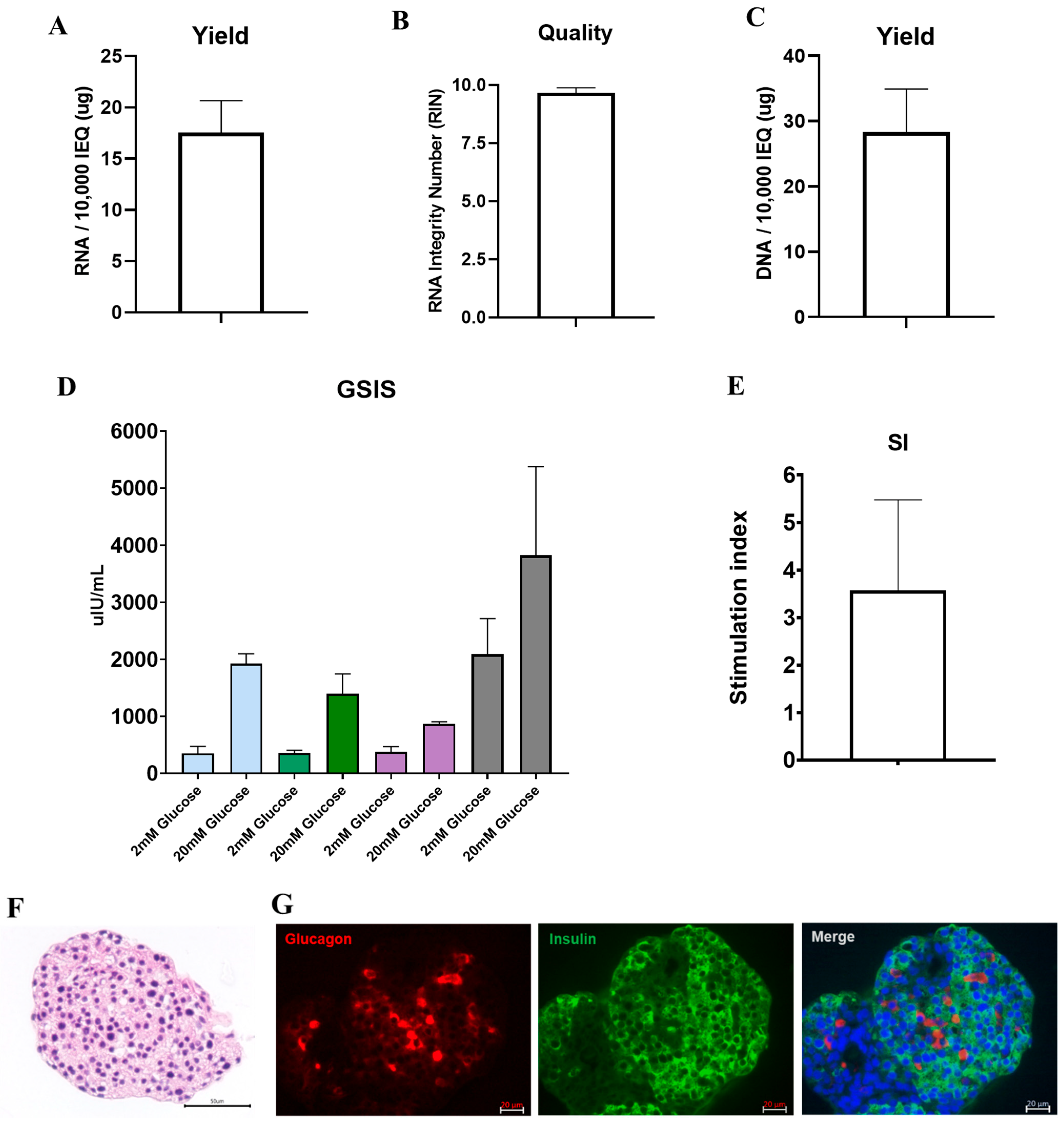
3.4. DNA and RNA Quality, Glucose Responsiveness, and Histology
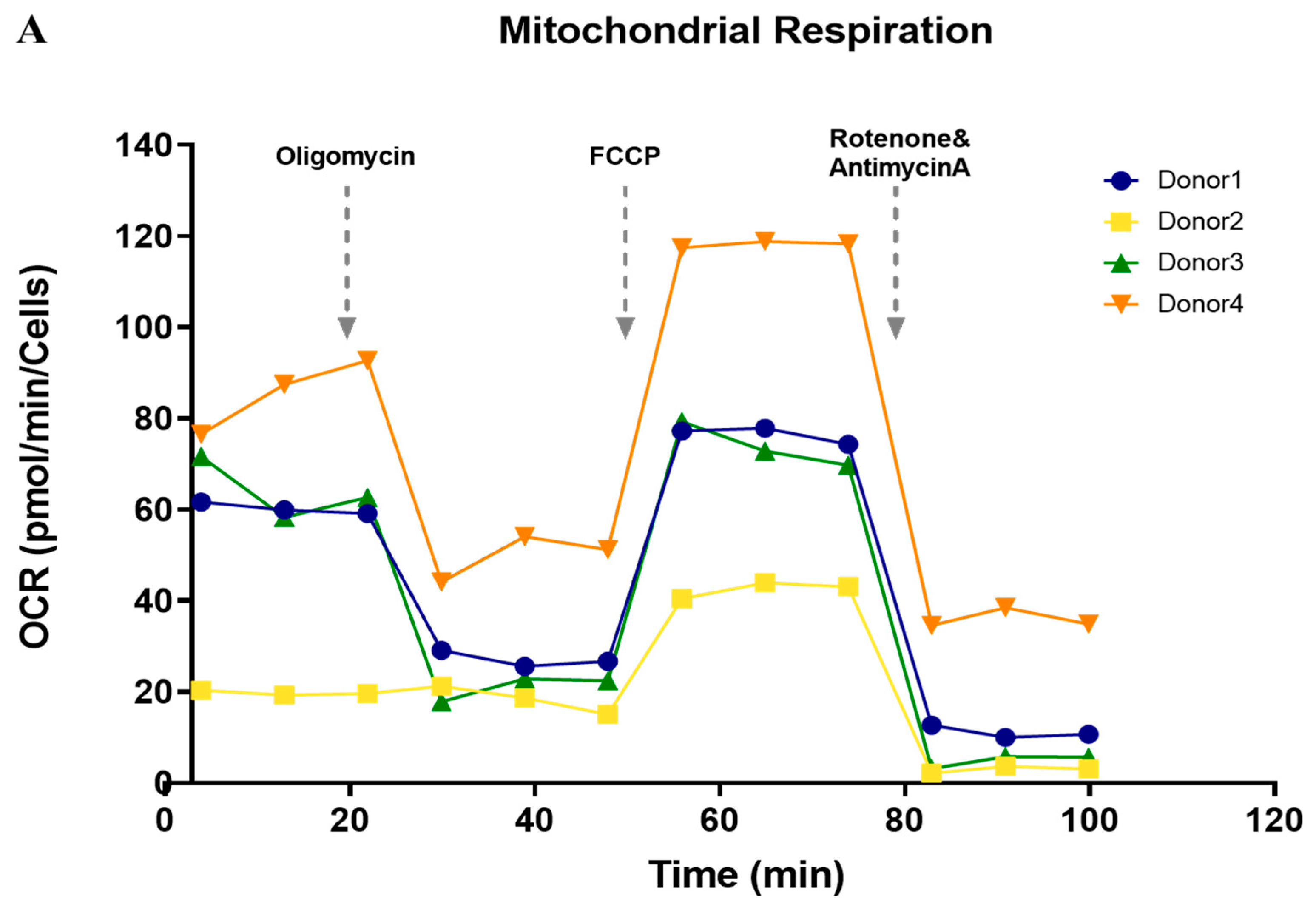
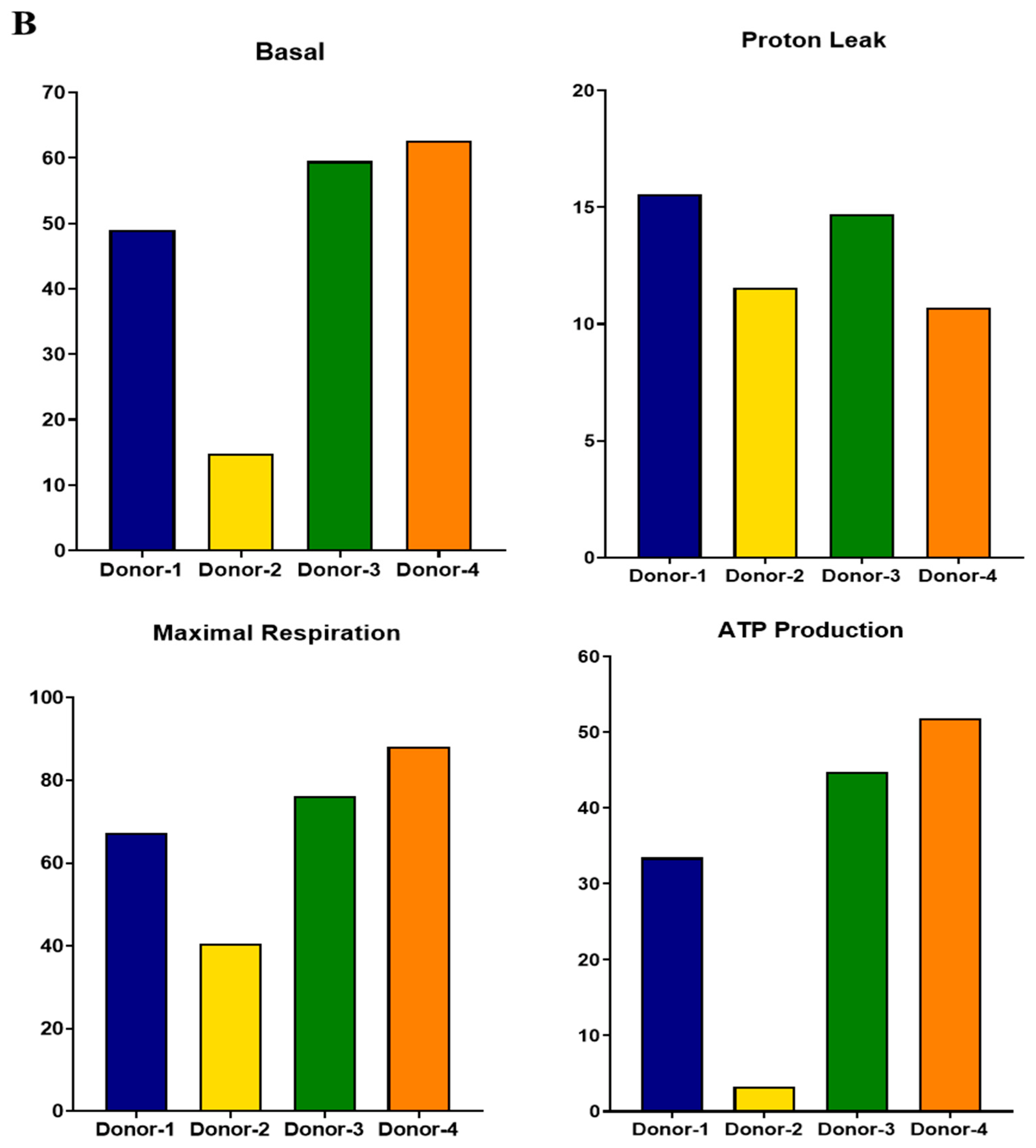
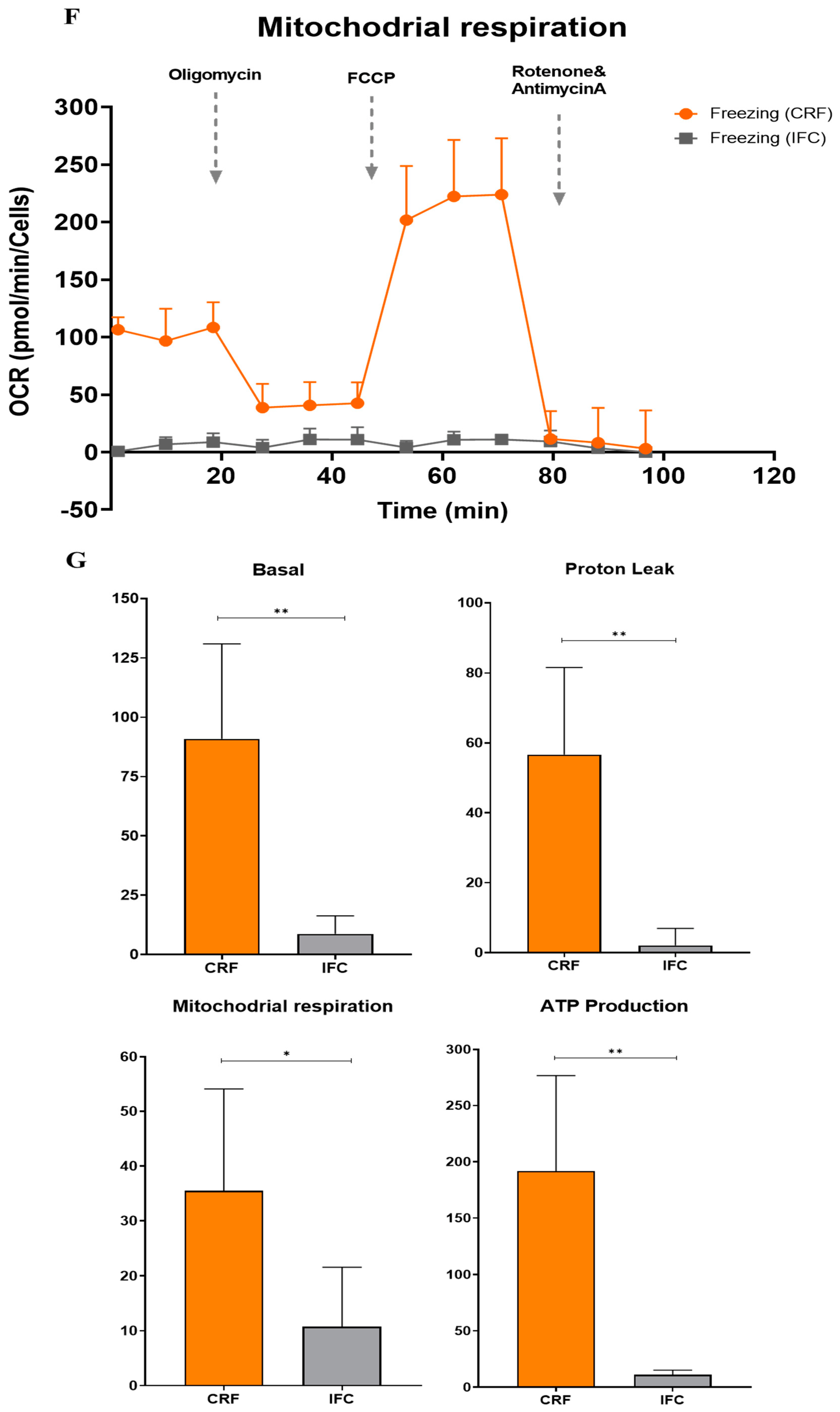
3.5. Functional Analysis of Mitochondrial Oxidation
3.6. Cryopreservation of Islet Cells Isolated from Partial Pancreas Tissue
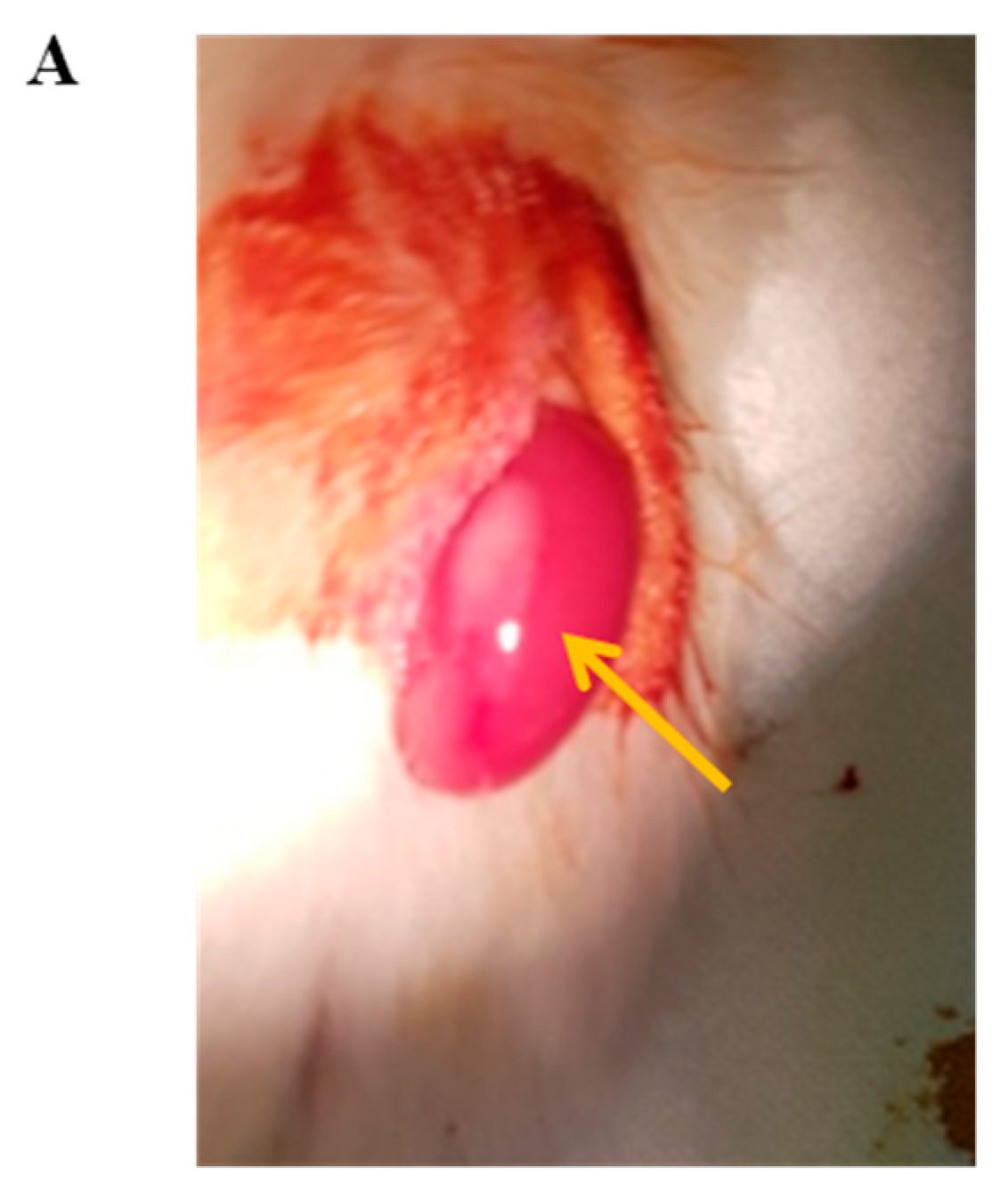
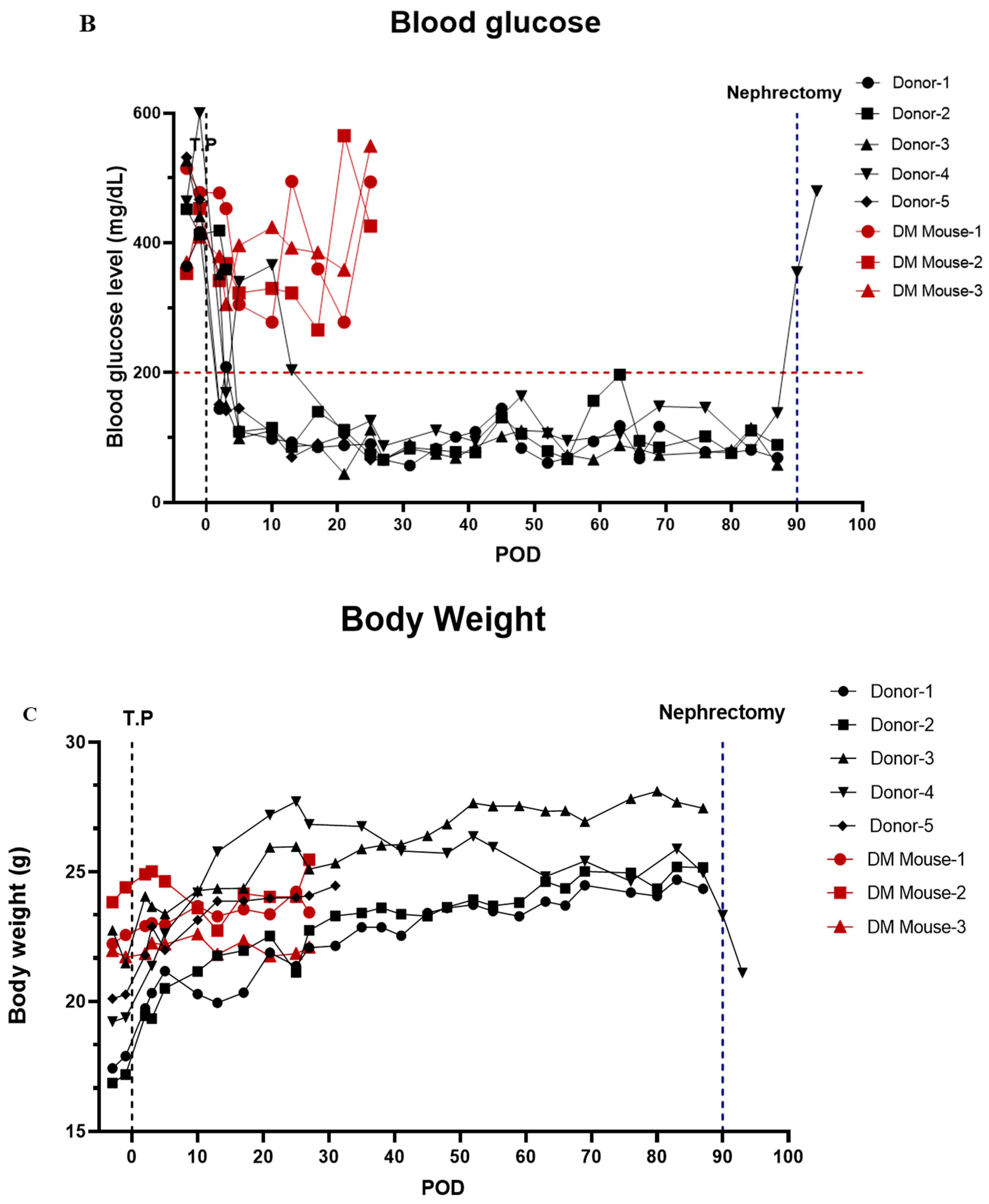
3.7. In Vivo Function of Islet Cells Isolated from the Partially Resected Pancreas
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shapiro, A.J.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet Transplantation in Seven Patients with Type 1 Diabetes Mellitus Using a Glucocorticoid-Free Immunosuppressive Regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Lyon, J.; Fox, J.E.M.; Spigelman, A.F.; Kim, R.; Smith, N.; O’Gorman, D.; Kin, T.; Shapiro, A.M.J.; Rajotte, R.V.; MacDonald, P. Research-Focused Isolation of Human Islets From Donors With and Without Diabetes at the Alberta Diabetes Institute IsletCore. Endocrinology 2015, 157, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakey, J.R.; Burridge, P.W.; Shapiro, A.M. Technical aspects of islet preparation and transplantation. Transpl. Int. 2003, 16, 613–632. [Google Scholar] [CrossRef] [PubMed]
- Bellin, M.D.; Balamurugan, A.N.; Pruett, T.L.; Sutherland, D.E.R. No Islets Left Behind: Islet Autotransplantation for Surgery-Induced Diabetes. Curr. Diabetes Rep. 2012, 12, 580–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaddis, J.; Danobeitia, J.S.; Niland, J.C.; Stiller, T.; Fernandez, L.A. Multicenter Analysis of Novel and Established Variables Associated with Successful Human Islet Isolation Outcomes. Am. J. Transplant. 2010, 10, 646–656. [Google Scholar] [CrossRef]
- Hilling, D.E.; Bouwman, E.; Terpstra, O.T.; de Mheen, P.M.-V. Effects of Donor-, Pancreas-, and Isolation-Related Variables on Human Islet Isolation Outcome: A Systematic Review. Cell Transplant. 2014, 23, 921–928. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Han, D.; Kang, C.; We, Y.; Back, J.; Kim, Y.; Kim, J.; Lim, D. Analysis on Donor and Isolation-Related Factors of Successful Isolation of Human Islet of Langerhans From Human Cadaveric Donors. Transplant. Proc. 2005, 37, 3402–3403. [Google Scholar] [CrossRef]
- Matsumoto, S.; Okitsu, T.; Iwanaga, Y.; Noguchi, H.; Nagata, H.; Yonekawa, Y.; Yamada, Y.; Nakai, Y.; Ueda, M.; Ishii, A.; et al. Insulin Independence of Unstable Diabetic Patient After Single Living Donor Islet Transplantation. Transplant. Proc. 2005, 37, 3427–3429. [Google Scholar] [CrossRef]
- Solimena, M.; Schulte, A.M.; Marselli, L.; Ehehalt, F.; Richter, D.; Kleeberg, M.; Mziaut, H.; Knoch, K.-P.; Parnis, J.; Bugliani, M.; et al. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia 2017, 61, 641–657. [Google Scholar] [CrossRef] [Green Version]
- Bötticher, G.; Sturm, D.; Ehehalt, F.; Knoch, K.P.; Kersting, S.; Grützmann, R.; Baretton, G.B.; Solimena, M.; Saeger, H.D.; Sturm, D. Isolation of Human Islets from Partially Pancreatectomized Patients. J. Vis. Exp. 2011, 53, e2962. [Google Scholar] [CrossRef]
- Ricordi, C.; Lacy, P.E.; Fink, E.H.; Olack, B.J.; Scharp, D.W. An automated method for the isolation of human pancreatic islets. Diabetes 1988, 37, 413–420. [Google Scholar] [CrossRef]
- Kang, E.; Wang, X.; Tippner-Hedges, R.; Ma, H.; Folmes, C.D.; Gutierrez, N.M.; Lee, Y.; Van Dyken, C.; Ahmed, R.; Li, Y.; et al. Age-related accumulation of somatic mitochondrial DNA mutations in adult-derived human iPSCs. Cell Stem Cell 2016, 18, 625–636. [Google Scholar] [CrossRef]
- Ma, H.; Folmes, C.D.; Wu, J.; Morey, R.; Mora-Castilla, S.; Ocampo, A.; Ma, L.; Poulton, J.; Wang, X.; Ahmed, R.J.N. Metabolic rescue in pluripotent cells from patients with mtDNA disease. Nature 2015, 524, 234–238. [Google Scholar] [CrossRef]
- Briones, R.M.; Miranda, J.M.; Mellado-Gil, J.M.; Castro, M.J.; Gonzalez-Molina, M.; Cuesta-Munoz, A.L.; Frutos, M. Differential analysis of donor characteristics for pancreas and islet transplantation. Transpl. Proc. 2006, 38, 2579–2581. [Google Scholar] [CrossRef]
- Matsumoto, S.; Zhang, G.; Qualley, S.; Clever, J.; Tombrello, Y.; Strong, D.M.; Reems, J.A. Analysis of donor factors affecting human islet isolation with current isolation protocol. Transpl. Proc. 2004, 36, 1034–1036. [Google Scholar] [CrossRef]
- Nano, R.; Clissi, B.; Melzi, R.; Calori, G.; Maffi, P.; Antonioli, B.; Bertuzzi, F. Islet isolation for allotransplantation: Variables associated with successful islet yield and graft function. Diabetologia 2005, 48, 906–912. [Google Scholar] [CrossRef] [Green Version]
- Ponte, G.M.; Pileggi, A.; Messinger, S.; Alejandro, A.; Ichii, H.; Baidal, D.A.; Alejandro, R. Toward maximizing the success rates of human islet isolation: Influence of donor and isolation factors. Cell Transpl. 2007, 16, 595–607. [Google Scholar] [CrossRef]
- Wang, Y.; Danielson, K.K.; Ropski, A.; Harvat, T.; Barbaro, B.; Paushter, D.; Oberholzer, J. Systematic analysis of donor and isolation factor’s impact on human islet yield and size distribution. Cell Transpl. 2013, 22, 2323–2333. [Google Scholar] [CrossRef] [Green Version]
- Butler, A.E.; Janson, J.; Bonner-Weir, S.; Ritzel, R.; Rizza, R.A.; Butler, P.C. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 2003, 52, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Sakuraba, H.; Mizukami, H.; Yagihashi, N.; Wada, R.; Hanyu, C.; Yagihashi, S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 2002, 45, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Deng, S.; Vatamaniuk, M.; Huang, X.; Doliba, N.; Lian, M.M.; Frank, A.; Markmann, J.F. Structural and functional abnormalities in the islets isolated from type 2 diabetic subjects. Diabetes 2004, 53, 624–632. [Google Scholar] [CrossRef] [Green Version]
- Hayek, A.; Guardian, C. Hormone release, islet yield, and transplantation of fetal and neonatal rat dorsal and ventral pancreatic islets. Diabetes 1986, 35, 1189–1192. [Google Scholar] [CrossRef]
- Elayat, A.A.; el-Naggar, M.M.; Tahir, M. An immunocytochemical and morphometric study of the rat pancreatic islets. J. Anat. 1995, 186 Pt 3, 629–637. [Google Scholar]
- Wang, X.; Misawa, R.; Zielinski, M.C.; Cowen, P.; Jo, J.; Periwal, V.; Hara, M. Regional differences in islet distribution in the human pancreas—Preferential beta-cell loss in the head region in patients with type 2 diabetes. PLoS ONE 2013, 8, e67454. [Google Scholar]
- Stunnenberg, H.G.; Abrignani, S.; Adams, D.; de Almeida, M.; Altucci, L.; Amin, V.; Patel, R. The International Human Epigenome Consortium: A Blueprint for Scientific Collaboration and Discovery. Cell Transpl. 2016, 167, 1145–1149. [Google Scholar]
- Rajotte, R.V.; Stewart, H.L.; Voss, W.A.G.; Shnitka, T.K.; Dossetor, J.B. Viability studies on frozen-thawed rat islets of Langerhans. Cryobiology 1977, 14, 116–120. [Google Scholar] [CrossRef]
- Wise, M.H.; Gordon, C.; Johnson, R.W. Intraportal autotransplantation of cryopreserved porcine islets of Langerhans. Cryobiology 1985, 22, 359–366. [Google Scholar] [CrossRef]
- Foreman, J.; Moriya, H.; Taylor, M.J. Effect of cooling rate and its interaction with pre-freeze and post-thaw tissue culture on the in vitro and in vivo function of cryopreserved pancreatic islets. Transpl. Int. 1993, 6, 191–200. [Google Scholar] [CrossRef]
- Evans, M.G.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Reversal of diabetes in dogs by transplantation of pure cryopreserved islets. Transplantation 1990, 50, 202–206. [Google Scholar] [CrossRef]
- Kneteman, N.M.; Alderson, D.; Scharp, D.W.; Lacy, P.E. Prolonged cryopreservation of purified human pancreatic islets. Diabetes 1989, 38 (Suppl. 1), 176–178. [Google Scholar] [CrossRef]
- Miyamoto, M.; Mullen, Y.; Stein, E.; Watanabe, Y.; Kenmochi, T.; Watt, P.C.; Brunicardi, F.C. Cryopreservation of human islets by a fully automated cryo-unit. Transpl. Proc. 1994, 26, 832. [Google Scholar]
- Rajotte, R.V.; Warnock, G.L.; Bruch, L.C.; Procyshyn, A.W. Transplantation of cryopreserved and fresh rat islets and canine pancreatic fragments: Comparison of cryopreservation protocols. Cryobiology 1983, 20, 169–184. [Google Scholar] [CrossRef]
- Rajotte, R.V.; Warnock, G.L.; Kneteman, N.N. Cryopreservation of insulin-producing tissue in rats and dogs. World J. Surg. 1984, 8, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Pereyra, L.H.; Gordon, D.A.; MacKenzie, G.H. Application of cryopreservation techniques to islet cell allotransplantation. Cryobiology 1983, 20, 205–210. [Google Scholar] [CrossRef]
- Rich, S.J.; Swift, S.; Thirdborough, S.M.; Rumford, G.; James, R.F.L.; London, N.J.M. Cryopreservation of rat islets of Langerhans: A comparison of two techniques. Cryobiology 1993, 30, 407–412. [Google Scholar] [CrossRef]


| Characteristic | Value |
|---|---|
| Donor (n) | 82 |
| Age (years) | 54.6 ± 15.1 |
| Sex | Female: 52.4%, Male: 47.6% |
| BMI (kg/m2) | 25.5 ± 8.6 |
| Disease for resection | NET 20%, IPMN 16.7%, SPN 10%, SPT 10%, MCN 10%, Other disease 33.7% |
| Diabetes in underlying disease | No: 57.8%, Yes: 42.2% |
| Specimen location in pancreas | Head: 26.8%, Body and Tail: 73.2% |
| Time from operation room to laboratory (min) | 31.1 ± 7.9 |
| Specimen size (g) | 23.4 ± 10.6 |
| Enzyme for digestion | Collagenase P: 43.9%, Liberase MTF/CT: 56.1% |
| Digestion time (min) | 10.5 ± 5.8 |
| Before purification islet IEQ | 124,006 ± 88,959 |
| After purification islet IEQ | 59,593 ± 56,651 |
| Purity (%, DTZ staining (+)) | 71.5 ± 20.9 |
| Outcome: IEQ before Purification per Tissue Weight (n = 82) † | Outcome: IEQ after Purification per Tissue Weight (n = 82) † | |||||||
|---|---|---|---|---|---|---|---|---|
| Multivariable | Multivariable | |||||||
| Mean | SD | SE | p-Value | Mean | SD | SE | p-Value | |
| Sex | ||||||||
| M | 4617.67 | 3461.01 | 2061.48 | 1525.37 | ||||
| F | 6306.61 | 4111.94 | 2990.06 | 3141.08 | ||||
| Age | ||||||||
| <40 | 6163.71 | 3138.68 | 2134.05 | 1750.32 | ||||
| ≥40 | 5356.61 | 4073.58 | 2657.84 | 2705.37 | ||||
| DM | ||||||||
| 0 | 6588.86 | 4205.94 | 3088.35 | 2848.05 | ||||
| 1 | 3712.82 | 2656.2 | 0.16319 | 0.0009 | 1655.95 | 1701.14 | 0.224 | 0.0046 |
| BMI | ||||||||
| <23 | 5690.83 | 4542.5 | 2776.7 | 3242.78 | ||||
| ≥23 | 5442 | 3576.69 | 2443.24 | 2142.74 | ||||
| Specimen location in pancreas | ||||||||
| Head | 3063.71 | 2589.58 | 1115.59 | 869.95 | ||||
| Body and tail | 6364.14 | 3982.25 | 0.18417 | 0.0096 | 3000.51 | 2773.16 | 0.251 | 0.0156 |
| Enzyme type | ||||||||
| Liberase | 4695.78 | 3550.12 | 2005.06 | 1815.83 | ||||
| Collagenase | 6582.13 | 4103.68 | 3264.03 | 3140.41 | ||||
| Digestion time | ||||||||
| <10 | 4038.22 | 3542.01 | 2413.42 | 2327.99 | ||||
| ≥10 | 6785.75 | 3789.76 | 0.15404 | <0.0001 | 2759.1 | 2718.19 | ||
| Before Freezing (IEQ) | After Freezing (IEQ) | Recovery Rate (%) | |
|---|---|---|---|
| Not frozen | 1500 | 1504 | 100.3 |
| Isopropanol-based freezing container (IFC) | 1500 | 1320 | 88 |
| Controlled-rate freezer (CRF) | 1500 | 1405 | 93.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.Y.; Kim, Y.H.; Lee, S.; Lee, Y.N.; Go, H.S.; Hwang, D.W.; Song, K.B.; Lee, J.H.; Lee, W.; So, S.; et al. The Outcomes and Quality of Pancreatic Islet Cells Isolated from Surgical Specimens for Research on Diabetes Mellitus. Cells 2022, 11, 2335. https://doi.org/10.3390/cells11152335
Oh JY, Kim YH, Lee S, Lee YN, Go HS, Hwang DW, Song KB, Lee JH, Lee W, So S, et al. The Outcomes and Quality of Pancreatic Islet Cells Isolated from Surgical Specimens for Research on Diabetes Mellitus. Cells. 2022; 11(15):2335. https://doi.org/10.3390/cells11152335
Chicago/Turabian StyleOh, Ju Yun, Yang Hee Kim, Song Lee, Yu Na Lee, Han Se Go, Dae Wook Hwang, Ki Byung Song, Jae Hoon Lee, Woohyung Lee, Seongjun So, and et al. 2022. "The Outcomes and Quality of Pancreatic Islet Cells Isolated from Surgical Specimens for Research on Diabetes Mellitus" Cells 11, no. 15: 2335. https://doi.org/10.3390/cells11152335
APA StyleOh, J. Y., Kim, Y. H., Lee, S., Lee, Y. N., Go, H. S., Hwang, D. W., Song, K. B., Lee, J. H., Lee, W., So, S., Kang, E., Jun, E., Shim, I. K., & Kim, S. C. (2022). The Outcomes and Quality of Pancreatic Islet Cells Isolated from Surgical Specimens for Research on Diabetes Mellitus. Cells, 11(15), 2335. https://doi.org/10.3390/cells11152335









