Abstract
The human immunodeficiency virus-1 (HIV) enters the brain shortly after infection, leading to long-term neurological complications in half of the HIV-infected population, even in the current anti-retroviral therapy (ART) era. Despite decades of research, no biomarkers can objectively measure and, more importantly, predict the onset of HIV-associated neurocognitive disorders. Several biomarkers have been proposed; however, most of them only reflect late events of neuronal damage. Our laboratory recently identified that ATP and PGE2, inflammatory molecules released through Pannexin-1 channels, are elevated in the serum of HIV-infected individuals compared to uninfected individuals and other inflammatory diseases. More importantly, high circulating ATP levels, but not PGE2, can predict a decline in cognition, suggesting that HIV-infected individuals have impaired ATP metabolism and associated signaling. We identified that Pannexin-1 channel opening contributes to the high serological ATP levels, and ATP in the circulation could be used as a biomarker of HIV-associated cognitive impairment. In addition, we believe that ATP is a major contributor to chronic inflammation in the HIV-infected population, even in the anti-retroviral era. Here, we discuss the mechanisms associated with Pannexin-1 channel opening within the circulation, as well as within the resident viral reservoirs, ATP dysregulation, and cognitive disease observed in the HIV-infected population.
1. Introduction
Human immunodeficiency virus type 1 (HIV), the causative agent for acquired immunodeficiency syndrome (AIDS), was identified four decades ago [1]. Despite the introduction of several successful anti-retroviral regimens to reduce systemic replication, HIV reservoirs seed early after infection, preventing viral eradication [2,3]. Upon anti-retroviral therapy (ART) interruption, the virus in latently infected cells rebounds, repopulating the individual with the virus in a few weeks [4,5,6,7]. Despite ART reducing systemic viral replication, chronic events such as residual viral protein expression and secretion are ongoing, inducing chronic neurotoxicity and associated cognitive decline [8,9,10].
The main objective of the ART regimens is to reduce viral replication and allow the body to reconstitute the immune system. An additional goal of ART is to target infected cells to enable them to present viral antigens to be recognized by the immune system and kill them [11,12]. However, most mechanisms of viral eradication require active replication, which is the basis for the current “shock and kill” approaches [13]. The “shock and kill” approach relies on the intrinsic toxicity of viral proteins in productively infected cells and a better recognition of the infected cells by the immune system, reducing the reservoir pool [14]. However, this approach has several issues, including the potential uncontrolled virus reactivation and associated immune compromise [15]. Moreover, most latently infected cells are not “uniform”; they cannot or do not respond to a single reactivator agent such as histone deacetylases or protein kinase C activators, suggesting different mechanisms of silencing and reactivation [16,17,18,19]. In addition, the reactivation mechanism depends on the cell type infected, including different cell populations, differentiation stage, proliferation, tissue compartmentalization, and cell lineage. Thus, all viral reservoirs need a specific mechanism of reactivation to be eliminated [13,20,21]. These issues complicate not only viral eradication, but also provide a unique viral evolution that can better adapt into particular immune and ART situations, increasing the flexibility of the virus to persist for extended periods [22]. Only recently, experiments in humans with interrupted ART indicate that viral rebound is diverse and cannot only be explained by the circulating virus, suggesting a significant contribution of multiple viral reservoirs to viral rebound and the viral repopulation of the body [23]. Another complication of the current cure strategies is the clear identity of all or most of the viral reservoirs in humans and animal models. Currently, the identification, quantification, and characterization of viral reservoirs are mostly focused on the blood, which probably does not represent the real viral reservoirs present in different tissues, including the brain [24,25]. The most thoroughly characterized component of the viral reservoirs is the nature of transcriptionally silent DNA proviruses integrated within memory CD4+ T lymphocytes [26,27]. However, several questions remain open to better understand these few latently infected cells, including the integration sites, mechanism of viral silencing, survival, reactivation, viral spread, and the host factors behind all of these mechanisms. In addition, some cell populations, such as central and stem cell memory T cells, could be cycling or exhibit self-renewal without activation or viral replication [28,29]. In addition, rare CD4+ T lymphocytes, such as effector memory T lymphocytes, have higher intact and inducible provirus frequencies, suggesting that particular cell populations can be better infected and reactivated [30]. However, our data in macrophage/microglia and astrocytes indicate that good infectivity and replication do not account for a good reservoir. For example, astrocytes have low infectivity and replication, but they have a highly efficient mechanism of viral transfer and amplification mediated by cell-to-cell contact not described in other reservoirs that spread infection by the soluble virus [31].
Additionally, in the context of HIV, these reservoirs are known to have dysregulation of hemichannels and gap junction proteins, such as Pannexin-1 (Panx-1) and Connexin 43, which promote the local secretion of inflammatory molecules such as ATP (adenosine-5’-triphosphate) and lipids [32,33]. The secretion or propagation of these inflammatory molecules through these hemichannels or gap junctions can lead to bystander apoptosis [34]. Some mechanisms, including viral transfer, are shown in elite and exceptional elite controllers, where ongoing viral replication still occurs at a low level, but the body is capable of maintaining immunity and suppressing viral replication even in the absence of ART [35]. In these populations, the majority of proviruses are in a silent state; however, like in astrocytes, HIV controllers are highly heterogeneous, and many silencing mechanisms have not yet been characterized, including survival, latency, and immune evasion in the absence of ART, and bystander damage.
In addition, recent publications indicated that some CD4+ T cell populations in chronic HIV-infected individuals under ART are anergic and/or exhausted with specific markers such as immune checkpoint receptors programmed death protein 1 (PD-1), cytotoxic lymphocyte-associated protein 4 (CTLA-4), and others [36,37]. These mechanisms have not been examined in other viral reservoirs such as myeloid or astrocytes; however, these cell types are “embedded” in the tissues and are extremely difficult to examine compared to the circulating viral reservoirs present in the blood.
Interestingly, even in HIV-infected individuals on effective suppressive ART, cells with active replication and viral protein translation have been described [9,10,38]. Despite the expression of viral mRNA and proteins as potential identifiers of infected cells, HIV-infected cells persist for years to decades, suggesting alternative survival mechanisms or a slow turnover to maintain a pool of reservoirs in each tissue. As current HIV curative treatments, such as “shock and kill”, cannot effectively reactivate latent HIV due to the diffuse and heterogeneous nature of viral reservoirs, we must treat HIV infection as a chronic disease. Thus, the early diagnosis or identification of associated comorbidities as well as the mechanisms of toxicity can provide interventional strategies to prevent and even revert some long-term consequences of chronic HIV infection. This review is intended to detail current topics related to HIV, including persistence within viral reservoirs, biomarkers of infection, and the newly identified host protein, Panx-1, and its function as a mediator of potential biomarkers of CNS damage in the current ART era.
2. Viral Reservoirs in the Brain
The brain and the peripheral nervous system are tissues colonized by myeloid and astrocytic viral reservoirs early after primary infection. The brain lacks T cells and other immune components such as immunoglobulins due to the selectivity of the blood–brain barrier (BBB) that excludes most immune components [39]. However, the brain is susceptible to several neurotropic viruses, including polyomavirus JC, Zika virus, dengue, Japanese encephalitis virus, West Nile, and HIV [40,41,42,43,44,45]. In the brain, the main infected cells correspond to microglia/macrophages and a small population of astrocytes [31,46]. However, the role of myeloid and glial cells has only recently been examined. In the ART era, a wide spectrum of neurological diseases termed HIV-associated neurocognitive disorders (HAND) have been detected in about half of the HIV-infected population [47]. The mechanisms maintaining high prevalence of HAND in the current ART era are unknown [48]. Instead, several groups propose that viral reservoirs in microglia/macrophages and astrocytes play a key role in chronic central nervous system (CNS) dysfunction. However, only recently have significant efforts occurred to identify and characterize the CNS viral reservoirs but have resulted in surprising and unique data sets compared to latently infected peripheral T cells [49,50,51,52,53,54,55]. Thus, viral reservoirs in the brain are different and unique.
2.1. Microglia/Macrophages
Microglia are the resident macrophages of the CNS and perivascular macrophages as the major phagocytes in the brain [56]. However, astrocytes can also have limited immune antigen presentation, but significantly contribute to inflammation [57]. In the pre-ART era, microglia/macrophages were the predominant cells involved in inflammation and neuronal/glial apoptosis [58]. These pre-ART conditions were recapitulated in felines and macaques using or analyzing infected macrophages [59,60]. Most CNS damage was associated with excessive viral replication, inflammation, and secretion of neurotoxic proteins, resulting in neuronal compromise and changes in cognition [61]. Further, the damage was amplified by the recruitment of uninfected cells into areas with HIV [62], including the transmigration of uninfected and HIV-infected monocytes into the CNS [63]. Both microglia and macrophages harbor the virus and resist apoptosis, serving as viral reservoirs. Our data indicate that a small population of latently infected microglia/macrophages survives the infection and silences the virus by a mechanism that prevents the proper formation of the apoptosome, preventing cell death [64]. We also identified that survived latently infected microglia/macrophages had a unique metabolic profile similar to the metabolic profile observed in glioblastoma stem cells [53]. Therefore, microglia and brain macrophages are critical targets of the HIV reservoirs and must be included in future cure strategy approaches.
2.2. Astrocytes
Astrocytes are the most abundant cell population in the brain, and they control key synaptic and immune functions during normal and pathological functions [65]. Astrocytes are diverse, heterogeneous, and have area-specific functions. Examples include tanycytes, radial, Bergmann glia, protoplasmic, fibrous, velate, marginal glia, and perivascular ependymal glia [66]. Astrocytes have been described to be targeted by HIV in vitro and in vivo for several groups; however, the number of infected astrocytes is low (around 5%) [32,67,68]. Astrocytes are negative for CD4, but express CC Motif Chemokine Receptor 5 (CCR5) and CXC Motif Chemokine Receptor 4 (CXCR4), suggesting some entry restrictions [69]. Only recently, the improvement of several techniques to identify and characterize viral reservoirs in blood and tissues confirmed that astrocytes had low numbers of infected cells but more than sufficient to repopulate an entire animal in the absence of other infected cells [67]. Our studies using human astrocytes identified that despite the low rate of infection and low/undetectable replication, HIV-infected astrocytes could generate aberrant intracellular toxic signals such as inositol triphosphate (IP3) and calcium but did not result in apoptosis due to the presence of the nef protein preventing IP3 receptor overactivation and the subsequent apoptosome formation [70]. More importantly, residual viral replication is silenced even in the absence of ART, suggesting that astrocytes, but not other cell types, had intrinsic silencing mechanisms. However, upon viral reactivating agents, the few infected astrocytes can efficiently transfer the infectious virions to macrophages and T lymphocytes without astrocyte death, supporting astrocytes as a different viral reservoir. In addition, we demonstrated that most of the survival and viral silencing/reactivation mechanisms were mediated by the loss of proper interactions among the endoplasmic reticulum, the Golgi apparatus, and the mitochondria, all essential components of the apoptotic process [34,70]. Therefore, HIV-infected astrocytes correspond to a versatile and different kind of viral reservoir than lymphoid and myeloid, suggesting that new mechanisms of survival, escape, and viral silencing are present in these cells.
3. Crusade to Find Reliable Biomarkers to Identify Chronic and Acute HIV-Mediated Damage
A critical role of viral reservoirs in the current ART era is chronic bystander damage described in several tissues, including the CNS [70,71,72,73,74,75]. However, the mechanisms of damage or identifying specific disease biomarkers in the current ART era are lacking. Overall, biomarkers reflect or predict the changes in pathological conditions. Even then, in 50% of HIV-infected individuals, the field fails to identify the mechanisms of chronic CNS disease in the HIV-infected population. Several biomarkers of HAND have been proposed over the years, including neurofilament light chain (NFL) [71], β-Amyloid1-42 [76,77], calcium-binding protein B [75,78], extracellular vesicles [75,79], and the Wnt-related proteins [80]. Other groups identified immune markers such as soluble CD14 [81], soluble CD163 [82,83,84], neopterin [85,86], Cathepsin B [87], kynurenine to tryptophan ratio [88,89,90,91], monocyte chemoattractant protein-1 (MCP-1 or CCL2) [92,93,94], tumor necrosis factor-alpha [8,84,95], interleukin-6 (IL-6) [94,95], interferon-γ-inducible protein (IP-10 or CXCL-10) [94,96], interleukin-8 (IL-8 or CXCL-8) [96,97], interferon alpha [90,98,99], intercellular adhesion molecule-5 [100], lipopolysaccharide (LPS) [81,101], brain-derived neurotrophic factor (BDNF) [102], and several growth factors [103]. Despite the long list of potential biomarkers of HIV CNS disease, most are associated with the late events of tissue destruction or significant immune activation and cannot detect early or chronic stages of CNS compromise.
Other approaches to identifying the early stages of CNS damage include non-invasive neuroimaging methods. Although these methods are not designed to identify biomarkers, they could provide essential information on brain behavior and structural and metabolic compromise. Moreover, the type of brain damage induced by HIV infection has been examined using multiple techniques, including postmortem tissues, cognitive assessment, and imaging technologies such as diffusion basis spectral imaging (DBSI). Most structural approaches consistently show a decrease in gray matter volumes and white matter microstructural abnormalities, called hyper-densities, compared to uninfected individuals [104,105]. The hyper-densities have been associated with inflammation and compromised blood vessels or mini “stroke” areas based on cellularity [106,107,108,109]. However, the nature and mechanism of the damage were unknown. Our group demonstrated that the serum adenosine triphosphate (ATP) and prostaglandin E2 (PGE2) levels were elevated in HIV patients relative to samples obtained from uninfected individuals [110]. However, only ATP was predictive of the early stages of cognitive decline in the HIV-infected population [110]. We identified that viral reservoirs, myeloid and astrocytic, can release ATP, contributing to the purinergic dysfunction [33,111,112]. We also identified that residual expression of gp120 and virus could activate Panx-1 and Connexin-43 hemichannels in several cell types, further increasing ATP release during chronic HIV infection. More importantly, high levels of ATP in the serum of HIV-infected individuals can be used as a biomarker of cognitive impairment and chronic infection.
4. HIV and Panx-1 Interactions
The Pannexin family of proteins was first identified due to their sequence homology with gap junction proteins in invertebrates, innexins [113]. Pannexins share structural topology despite the lack of sequence homology with connexins [114]. Panx-1 is the most extensively studied and characterized [115]. The other two Pannexin family proteins, Panx-2 and Panx-3, have a more selective, tissue-specific expression profile relative to Panx-1 [113]. Each of the three Pannexins has four transmembrane domains, conferring two extracellular loops, a cytoplasmic loop, and a cytoplasmic C-terminus that varies in length among them [116]. The N-terminus is the most conserved, but they have divergent sequences from the C-terminus [117]. Panx-1 was thought to form a hexameric channel complex, but recent publications suggested that the multimeric conformation of Panx-1 into pannexin channels is in a heptameric state [118,119,120]. Earlier work suggested that Panx-1 could form cell-to-cell gap junctions between cells, much like their connexin counterparts [121,122]. The evidence against this is the need for glycosylation on the extracellular loop for membrane trafficking, which is thought to preclude the dimerization of two channels from forming intercellular gap junctions [123,124]. Functional analysis by scrape-loading also demonstrates the lack of gap junction formation between Panx-1s of neighboring cells [123]. However, when the N-linked glycosylation site is altered, preventing this post-translational modification, structural biology data suggests gap junction formation is possible [125]. One recent article argued that gap junction-forming innexins contain N-glycosylation sites at their extracellular loops and could form gap junction types of channels [126]. Using TC620 cells, an oligodendroglioma cell line, dye and electrical cell coupling were shown; the cell-to-cell coupling was reduced when Panx-1 was either knocked down by siRNA or inhibited by an inhibitory peptide [126]. However, Panx-1′s capacity to form gap junctions is still an open question.
Functionally, Panx-1 allows the movement of molecules up to 1 kDa across the plasma membrane [127]. The channels have affinity for anionic substrates, as demonstrated by single-channel patch-clamp electrophysiology [128]. They are in a constitutively closed state, but several stimuli are associated with the opening of the channel, which allows the efflux of ATP, prostaglandins, and several small molecules [115]. One of the first prominent roles identified was the release of ATP by Panx-1 as a “find-me” signal, which was the signaling event for early apoptotic activity [129]. The ATP secreted by a cell could then act as a chemoattractant for phagocytes to clear cellular debris of the apoptosed cell [130]. This constitutively open state of Panx-1 is associated with a caspase-3 or caspase-7 cleavage event but is also seen in Panx-1 truncation at the putative cleavage site [129]. When various C-terminal truncations were made, varying activities of Panx-1 were demonstrated, suggesting that the C-terminal tail plays an autoinhibitory function [131]. A study involving the autoinhibitory domain of Panx-1 also demonstrated that the interaction between this domain and the Panx-1 pore is not necessarily specific, as some C-terminal scramble mutants showed similar activity to the wildtype [132].
The induction of Panx-1 opening has several different mechanisms, such as changes in ion concentrations, mechanical stimulation, or post-translational modification [115]. The opening of Panx-1 was shown to occur upon potassium ion treatment, particularly when extracellular K+ concentrations reached 50–60 mM [133]. A publication showed that an increase in intracellular calcium ions within oocytes could also induce the opening of Panx-1 [134]. However, this activity was not seen within mammalian cells [135]. This was examined by administering varying concentrations of calcium ions, the alteration or depletion of intracellular calcium, and treatment with a phospholipase C inhibitor using patch-clamping [135]. Regarding mechanical stimulation, suction-based assays showed stretch activation of Panx-1 channels within oocytes [136]. Later studies within mammalian cells demonstrated that the mechanical induction of Panx-1 opening is inhibited by adenosine and cyclic adenosine monophosphate (cAMP) analogs [137]. Within the same article, this group also showed that T302 and S328, putative protein kinase A (PKA) phosphorylation sites, are essential for the stretch activation of Panx-1 due to the phosphomimetic mutants showing reduced activation from the same stimulus [137]. Work using rat astrocytes derived from the optic nerve also demonstrated the release of ATP through Panx-1 using mechanical means and in response to hypotonic swelling [138].
As mentioned above, apoptosis-dependent Panx-1 activation occurs by the cleavage of the C-terminal tail [129]. Concatemeric Panx-1 studies showed the stepwise activation of Panx-1 by the subsequent cleavage of the C-terminal tails by electrophysiology [118]. Ablation of the caspase cleavage site using a D376A/D379A mutant, termed PANX1-CR, showed the suppression of ATP release and Panx-1 C-terminal cleavage [129,139]. A single-cell dynamics model using Förster resonance energy transfer biosensors demonstrated that caspase-3 activity and decreased intracellular ATP level temporally coincided during apoptosis [139]. The glycosylation state also plays a role in the trafficking of Panx-1 to the plasma membrane as tunicamycin treatment, which prevents global protein glycosylation, showed a reduction in the membrane-localized Panx-1 [123]. Interestingly, a glycosylation-devoid mutant, N254Q, can still form functional channels in mammalian cells [123]. However, a similar study with zebrafish Panx-1 demonstrated reduced function but corroborated the reduced membrane localization in mammalian cells [123,140].
Other post-translational modifications, such as S-nitrosylation and phosphorylation, are known to modulate Panx-1 activity [141]. The addition of a nitric oxide (NO) donor, sodium nitroprusside, showed the inhibition of Panx-1 by a cyclic guanosine monophosphate (cGMP) and protein kinase G-dependent mechanism [142]. When S206, the predicted protein kinase G recognition site, was mutated to alanine, the inhibition of Panx-1 by sodium nitroprusside was reduced [142]. Treatment by other NO donors such as S-nitrosoglutathione or diethylamine NONOate also showed inhibitory effects on Panx-1 activity in Panx-1-transfected HEK293T cells [143]. The mutagenesis of C40 and C346, the proposed S-nitrosylation sites, reduced the inhibition of Panx-1 by NO, which was argued to be independent of cGMP signaling [143]. These articles suggested NO has an inhibitory effect on Panx-1 within HEK293 cells, albeit through different mechanisms; another article presented data within hippocampal neurons, which suggested that NO enhanced Panx-1 opening during oxygen–glucose deprivation [144]. In terms of HIV, NO has shown antiviral and proviral effects on viral replication [145]. Regardless of the effect of NO on HIV replication, multiple studies have shown it to be elevated within the serum of HIV-infected patients relative to uninfected or cART-adherent patients [146]. Overall, the data on HIV are poorly known, but Panx-1 channels are mostly in a closed state in uninfected cells, but upon primary infection or during chronic infection, the channel becomes open, enabling ATP to concentrate in the serum of HIV-infected individuals (Figure 1). Future studies will examine the mechanisms described in this section in HIV-infected cells.
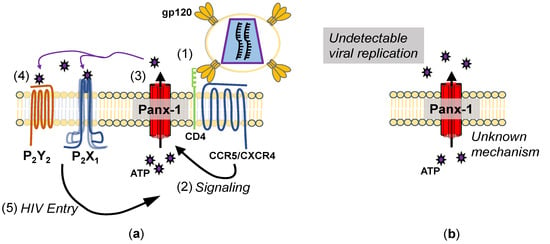
Figure 1.
Mechanisms of Panx-1 opening during HIV infection. (a) Mechanism of Panx-1 opening during acute infection. (1) Upon binding to CD4 and CCR5 (or CXCR4), (2) signaling through this binding leads to Panx-1 becoming open, (3) releasing ATP. (4) The ATP released then binds to purinergic receptors, (5) allowing HIV entry. Inhibition of Panx-1 ATP release or P2 receptors inhibits HIV entry. (b) Mechanisms of Panx-1 opening during chronic infection. Peripheral blood mononuclear cells (PBMCs) isolated from HIV-infected patients show Panx-1 in a spontaneously open state, despite a lack of viral replication, but the mechanism is unknown.
5. Panx-1 and Purinergic Signaling Axis in HIV Infection
ATP has many functions inside and outside of cells. It operates as the main “energy currency” within cells, a substrate for nucleic acid synthesis, and a secondary messenger [147]. Extracellular ATP, secreted by cells by either vesicular secretion or through channels such as Panx-1, plays a role in signaling inflammation as a neurotransmitter and platelet activation [147]. Once released, ATP is a strong inflammatory molecule quickly degraded by soluble or membrane-associated ATPases such as ectonucleotide triphosphate diphosphohydrolase 1 (CD39) [148]. CD39 catabolizes its substrate into AMP upon binding to ATP or ADP, which can subsequently be catabolized into adenosine by CD73 [149]. Adenosine deaminase, either soluble or CD26-associated, converts the adenosine into inosine [150]. ATP and subsequent metabolites function through P1 or P2 purinergic receptors [151] (Figure 2). The four subtypes of P1 receptors, A1, A2A, A2B, and A3, selectively bind to adenosine [151]. P2 receptors, which are activated by both purines and pyrimidines, include ionotropic P2X (P2X1–7) and metabotropic P2Y (P2Y1,2,4,6,11,12,13,14) receptors [151].
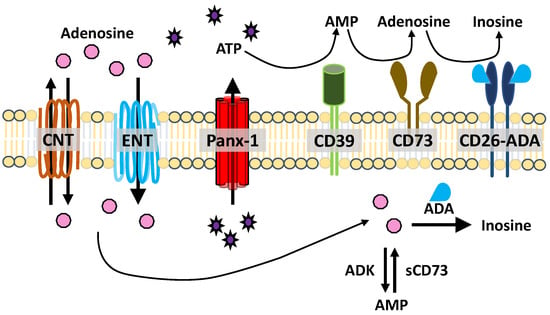
Figure 2.
ATP release and metabolism. ATP is released through hemichannels, such as Panx-1. ATP (and ADP) are degraded into AMP by CD39. AMP is catabolized into adenosine by CD73. Adenosine is deaminated into inosine by adenosine deaminase (ADA), which is associated with CD26. Concentrative and equilibrated nucleoside transporters (CNT, ENT) facilitate nucleoside uptake and transport. Intracellular adenosine can be deaminated by ADA or converted to AMP by soluble adenosine kinase (ADK). Intracellular AMP is converted to adenosine by soluble CD73 (sCD73).
In non-pathological conditions, the role of Panx-1 in the release of ATP is demonstrated upon the stimulation of various membrane receptors [152]. The release of ATP through Panx-1 contributes to T cell activation in an autocrine fashion [153]. This Panx-1-dependent release of ATP also occurs in response to the binding of chemokines to T cells to induce cellular polarization and a migratory phenotype [154]. Similarly, when HIV binds to a target cell through receptor/co-receptor and gp120 interactions, Panx-1 briefly opens, releasing ATP [155,156]. The ATP released then activates purinergic receptors, P2X1 and P2Y2, leading to HIV fusion and subsequent entry [157]. Signaling through P2Y2 in response to HIV infection showed an increase in Pyk2 Y402 phosphorylation, similar to work within cell lines that demonstrated the binding of gp120 to CCR5 [158]. Other work in macrophages demonstrated that the NLR family pyrin domain-containing 3 (NLRP3) activation impairs HIV entry by inhibiting F-actin remodeling [159]. In general, inhibiting F-actin remodeling with reagents such as latrunculin reduces viral infectivity and HIV entry [159]. The activation of P2Y2 during HIV entry stimulates the recruitment of an E3 ubiquitin ligase to promote the degradation of NLRP3, allowing a more permissive state for HIV entry [159]. Canonically, NLRP3 inflammasome activation is associated with Panx-1 release of ATP and functional coupling with P2X4 and P2X7 [160]. Signaling through ionotropic receptors leads to the cleavage and activation of caspase-1, which in turn cleaves pro-IL-1β into mature IL-1β [161]. For HIV infection, the downregulation of NLRP3 is advantageous for HIV, not only for entry as mentioned above, but also due to the reduction of HIV replication in the presence of recombinant IL-1β [162]. The binding of CCR5 to gp120 activated Gαq to propagate a Rac-1-mediated actin cytoskeleton remodeling for fusion [158]. A dissenting article suggested that both inhibition of CD39 by polyoxotungstate-1 (POM-1) and the treatment of high levels of ATP during or before the infection of macrophages inhibited HIV infection [163]. However, some polyoxometalates have shown inhibitory effects on reverse transcriptase (RT) in vitro, so some inhibitory effects on HIV replication are likely due to the direct inhibition of RT [164].
Additionally, the treatment with high levels of ATP could also induce the internalization of Panx-1 within cell lines [165]. Further, the knockdown of either Panx-1 or P2Y2 showed a reduction in HIV infection and replication, suggesting that releasing ATP and transducing signals through P2Y2 are important for viral replication [155]. Lastly, the release of ATP and subsequent signaling requirements for HIV entry are evident when apyrase, which depletes extracellular ATP, is present during HIV infection in vitro [155].
Our data in simian immunodeficiency virus (SIV)-infected macaques indicate that blocking Panx-1 channel opening after SIV infection prevents immune compromise, leukocyte differentiation induced by the virus, transmigration into the CNS, and loss of complex synapses [33,110]. These data indicate that despite the complex animal model, mimetic peptides to Panx-1 can be used to prevent CNS damage and improve immune response. These data are outstanding because targeting these channels can provide an additional treatment to prevent immune and CNS damage and open several therapeutic avenues using purinergic/adenosine drugs to prevent and even reverse the damage produced by viral reservoirs in the current ART era.
Not only is Panx-1 required for HIV entry, but replication as well. At 48 to 72 hours post-HIV infection, Panx-1 becomes open in primary PBMCs and T cells, as demonstrated by dye uptake [156]. This is distinct from the entry-mediated opening or cytokine binding-like (such as SDF-1) as the opening of Panx-1 in these latter timepoints is prolonged [156]. This prolonged opening of Panx-1, similar to the entry-mediated opening, is also required for HIV replication, as the pharmacological inhibition or knockdown of Panx-1 after the infection has been established abolishes HIV replication [156]. Similar to entry, after infection is established, the pharmacological inhibition of P2X1, P2X7, and P2Y1 purinergic receptors reduces HIV replication within human macrophages [157]. Some evidence supports the purinergic receptor antagonist, oxidized ATP [146], which can suppress reverse transcriptase in vitro using either virus-free enzymes or a cell-free system with virus particles [166]. Another purinergic receptor antagonist, suramin, has also shown inhibitory effects on RT in vitro [167]. These effects and the ones referred to in the above paragraph regarding POM-1 are all off-target effects of the pharmacological purinergic receptor inhibitors that influence HIV [168]. Therefore, phenotypes seen by treatment with these inhibitors must be analyzed carefully and be conducted in tandem with siRNA knockdowns to ensure the phenotype is recapitulated.
6. Panx-1 and Purinergic Signaling Axis in NeuroHIV
Panx-1 has many roles within the CNS, such as neuronal development, neurite formation, dendritic spine development, and synaptic plasticity [169]. Knockout models of Panx-1 and pharmacological inhibition demonstrate aberrations in neuronal cell maintenance and neurite formation [170]. Aside from Panx-1’s role in normal physiological function and development, it has demonstrated multiple roles in pathological conditions [112]. A recent publication demonstrated that ATP within serum is a potential biomarker of neurocognitive impairment in HIV patients [110]. ATP plays many roles within serum, but, in excess, can be detrimental to anatomical barriers [171]. Mouse models have shown that, through purinergic receptors, ATP can induce vascular inflammation and promote atherosclerosis, as evidenced by atherosclerotic lesions among the endothelia [172]. The deficiency of P2X4 within an atherosclerotic mouse model showed reduced cytokine production, decreased leukocyte invasion within the endothelium, and reduced endothelial expression of adhesion molecules [173]. Multiple in vitro models have recapitulated ATP-induced blood–brain barrier [67] permeability, including one demonstrating P2X7 receptor dependence [110]. This mechanism of purinergic signaling-induced blood–brain barrier permeability showed that the downstream signaling of the P2X7 receptor, including the expression of IL-1β and subsequent metalloprotease expression (MMP), induced the tight junction disruption of endothelial cells [174]. This cascade is characterized by Panx-1 coupling with P2X7 to induce IL-1β release within macrophages and inflammasome activation in neuronal cells [175]. Multiple in vivo and in vitro models have shown MMP-induced BBB dysfunction by tight junction protein degradation [176].
Adenosine and P1 agonists have also demonstrated detrimental effects on the BBB [177]. Adenosine receptor agonists are known to disrupt the BBB [178]. In vitro, BBB co-culture assays using adenosine receptor agonists show an increase in endothelial cell permeability to both 10 kDa. Dextran also increased the transmigration of T lymphocytes [179]. In addition, the adenosine receptor agonist NECA (1-(6-amino-9H-purin-9-yl)-1-deoxy-N-ethyl-β-d-ribofuranuronamide) induced an increase in BBB permeability in a mouse model [180]. In the same study, Lexiscan, an agonist to adenosine receptor A2A, demonstrated increased BBB permeability [180]. Another study showing the treatment of Lexiscan on either an endothelial cell line or primary human endothelial cells shows the reduction of P-glycoprotein expression and function, as well as an increase in the expression of MMP-9 [181]. P-glycoprotein, a drug efflux pump expressed by endothelial cells of the BBB, can have both a proviral and antiviral activity [182]. The expression of P-glycoprotein in HIV-infected cells was increased to the benefit of HIV, as the accumulation of a nucleoside reverse transcriptase inhibitor was reduced relative to uninfected cells [183]. At the BBB, the lack of P-glycoprotein demonstrated the reduced capacity to eliminate drugs and other toxic metabolites from the brain [184]. Although this may alleviate the decreased delivery of ART into the brain, the function of P-glycoprotein is to be a sentinel to eliminate or prevent the accumulation of toxic substances within the brain [182]. These data only denote the early understanding of the stages of signaling and related toxicity mediated by viral reservoirs, Panx-1 channels, ATP, and purinergic receptors. The data also indicate that the chronic nature of HIV in the current ART era is different from other diseases and requires a significant effort to prevent the associated damage observed in at least 50% of the HIV-infected population.
7. Conclusions and Future Studies
Currently, the status of NeuroHIV in the ART era requires a significant re-evaluation of several mechanisms described early on in the AIDS pandemic, including the identification of viral reservoirs and their diversity, mechanisms of survival, latency, reactivation, and bystander toxicity. However, more important are the host proteins involved in these mechanisms that are probably different from those described for the typical HIV life cycle. Our data identified that Panx-1, a large ionic channel, mediates the release of ATP and inflammatory lipids by a mechanism of infection, but can be replication-independent. Circulating levels of ATP can be used as a biomarker of cognitive impairment in the HIV-infected population, and we believe that ATP contributes to BBB dysfunction. However, we believe other ionic channels are also involved in entry, infection, and replication, and the generation and stability of viral reservoirs. Thus, understanding the interactions between viral and host components could provide new avenues of treatment to prevent and even reverse CNS damage in the HIV-infected population.
8. Patents
The described work resulted in the US patent 17/476,910.
Author Contributions
All authors participated equally in the preparation of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Institute of Mental Health grants MH096625 and MH128082, the National Institute of Neurological Disorders and Stroke, NS105584, and the UTMB Sealy Institute for Vaccine Sciences and the UTMB Institute for Human Infections & Immunity (to E.A.E.).
Acknowledgments
We want to thank National NeuroAIDS Tissue Consortium (NNTC) for collecting and providing all human samples for our research. The NNTC is made possible through funding from the NIMH and NINDS by the following grants: Manhattan HIV Brain Bank (MHBB): U24MH100931; Texas NeuroAIDS Research Center (TNRC): U24MH100930; National Neurological AIDS Bank (NNAB): U24MH100929; California NeuroAIDS Tissue Network (CNTN): U24MH100928; and Data Coordinating Center (DCC): U24MH100925.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barré-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vézinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-Lymphotropic Retrovirus from a Patient at Risk for Acquired Immune Deficiency Syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Finzi, D.; Blankson, J.N.; Siliciano, J.D.; Margolick, J.B.; Chadwick, K.; Pierson, T.C.; Smith, K.; Lisziewicz, J.; Lori, F.; Flexner, C.; et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 1999, 5, 512–517. [Google Scholar] [CrossRef]
- Valcour, V.; Chalermchai, T.; Sailasuta, N.; Marovich, M.; Lerdlum, S.; Suttichom, D.; Suwanwela, N.C.; Jagodzinski, L.L.; Michael, N.L.; Spudich, S.; et al. Central Nervous System Viral Invasion and Inflammation During Acute HIV Infection. J. Infect. Dis. 2012, 206, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.T.; Bhat, N.; Yoder, C.; Chun, T.-W.; Metcalf, J.A.; Dewar, R.; Natarajan, V.; Lempicki, R.A.; Adelsberger, J.W.; Miller, K.D.; et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. USA 1999, 96, 15109–15114. [Google Scholar] [CrossRef] [PubMed]
- Joos, B.; Fischer, M.; Kuster, H.; Pillai, S.K.; Wong, J.K.; Böni, J.; Hirschel, B.; Weber, R.; Trkola, A.; Günthard, H.F.; et al. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc. Natl. Acad. Sci. USA 2008, 105, 16725–16730. [Google Scholar] [CrossRef]
- Hamlyn, E.; Ewings, F.M.; Porter, K.; Cooper, D.A.; Tambussi, G.; Schechter, M.; Pedersen, C.; Okulicz, J.F.; McClure, M.; Babiker, A.; et al. Plasma HIV Viral Rebound following Protocol-Indicated Cessation of ART Commenced in Primary and Chronic HIV Infection. PLoS ONE 2012, 7, e43754. [Google Scholar] [CrossRef]
- Ho, Y.-C.; Shan, L.; Hosmane, N.N.; Wang, J.; Laskey, S.B.; Rosenbloom, D.I.; Lai, J.; Blankson, J.N.; Siliciano, J.D.; Siliciano, R.F. Replication-Competent Noninduced Proviruses in the Latent Reservoir Increase Barrier to HIV-1 Cure. Cell 2013, 155, 540–551. [Google Scholar] [CrossRef]
- Rychert, J.; Strick, D.; Bazner, S.; Robinson, J.; Rosenberg, E. Detection of HIV gp120 in plasma during early HIV infection is associated with increased proinflammatory and immu-noregulatory cytokines. AIDS Res. Hum. Retrovir. 2010, 26, 1139–1145. [Google Scholar] [CrossRef]
- Ferdin, J.; Goričar, K.; Dolžan, V.; Plemenitaš, A.; Martin, J.N.; Peterlin, B.M.; Deeks, S.G.; Lenassi, M. Viral protein Nef is detected in plasma of half of HIV-infected adults with undetectable plasma HIV RNA. PLoS ONE 2018, 13, e0191613. [Google Scholar] [CrossRef]
- Imamichi, H.; Smith, M.; Adelsberger, J.W.; Izumi, T.; Scrimieri, F.; Sherman, B.T.; Rehm, C.A.; Imamichi, T.; Pau, A.; Catalfamo, M.; et al. Defective HIV-1 proviruses produce viral proteins. Proc. Natl. Acad. Sci. USA 2020, 117, 3704–3710. [Google Scholar] [CrossRef]
- Kim, Y.; Anderson, J.L.; Lewin, S.R. Getting the “Kill” into “Shock and Kill”: Strategies to Eliminate Latent HIV. Cell Host Microbe 2018, 23, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Nixon, C.C.; Mavigner, M.; Sampey, G.C.; Brooks, A.D.; Spagnuolo, R.A.; Irlbeck, D.M.; Mattingly, C.; Ho, P.T.; Schoof, N.; Cammon, C.G.; et al. Systemic HIV and SIV latency reversal via non-canonical NF-κB signalling in vivo. Nature 2020, 578, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Abner, E.; Jordan, A. HIV “shock and kill” therapy: In need of revision. Antivir. Res. 2019, 166, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Doitsh, G.; Greene, W.C. Dissecting How CD4 T Cells Are Lost During HIV Infection. Cell Host Microbe 2016, 19, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; De Crignis, E.; Rokx, C.; Verbon, A.; van Gelder, T.; Mahmoudi, T.; Katsikis, P.D.; Mueller, Y.M. T cell toxicity of HIV latency reversing agents. Pharmacol. Res. 2018, 139, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Archin, N.M.; Liberty, A.L.; Kashuba, A.D.; Choudhary, S.K.; Kuruc, J.D.; Crooks, A.M.; Parker, D.C.; Anderson, E.M.; Kearney, M.F.; Strain, M.C.; et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 2012, 487, 482–485. [Google Scholar] [CrossRef]
- Jiang, G.; Mendes, E.A.; Kaiser, P.; Sankaran-Walters, S.; Tang, Y.; Weber, M.G.; Melcher, G.P.; Thompson, G.R., III; Tanuri, A.; Pianowski, L.F.; et al. Reactivation of HIV latency by a newly modified Ingenol derivative via protein kinase Cδ-NF-κB signaling. AIDS 2014, 28, 1555–1566. [Google Scholar] [CrossRef]
- Jiang, G.; Dandekar, S. Targeting NF-κB signaling with protein kinase C agonists as an emerging strategy for combating HIV latency. AIDS Res. Hum. Retrovir. 2015, 31, 4–12. [Google Scholar] [CrossRef]
- Matsuda, K.; Islam, S.; Takada, T.; Tsuchiya, K.; Tan, B.J.Y.; Hattori, S.-I.; Katsuya, H.; Kitagawa, K.; Kim, K.S.; Matsuo, M.; et al. A widely distributed HIV-1 provirus elimination assay to evaluate latency-reversing agents in vitro. Cell Rep. Methods 2021, 1, 100122. [Google Scholar] [CrossRef]
- Chun, T.-W.; Engel, D.; Mizell, S.B.; Ehler, L.A.; Fauci, A.S. Induction of HIV-1 Replication in Latently Infected CD4+ T Cells Using a Combination of Cytokines. J. Exp. Med. 1998, 188, 83–91. [Google Scholar] [CrossRef]
- Graziano, F.; Aimola, G.; Forlani, G.; Turrini, F.; Accolla, R.S.; Vicenzi, E.; Poli, G. Reversible Human Immunodeficiency Virus Type-1 Latency in Primary Human Monocyte-Derived Macrophages Induced by Sustained M1 Polarization. Sci. Rep. 2018, 8, 14249. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, K.C.; Agopian, K.A.; Mukerji, J.; Gabuzda, D. Evidence for Adaptive Evolution at the Divergence Between Lymphoid and Brain HIV-1nefGenes. AIDS Res. Hum. Retroviruses 2010, 26, 495–500. [Google Scholar] [CrossRef] [PubMed]
- De Scheerder, M.-A.; Vrancken, B.; Dellicour, S.; Schlub, T.; Lee, E.; Shao, W.; Rutsaert, S.; Verhofstede, C.; Kerre, T.; Malfait, T.; et al. HIV Rebound Is Predominantly Fueled by Genetically Identical Viral Expansions from Diverse Reservoirs. Cell Host Microbe 2019, 26, 347–358.e7. [Google Scholar] [CrossRef] [PubMed]
- Churchill, M.J.; Deeks, S.G.; Margolis, D.M.; Siliciano, R.F.; Swanstrom, R. HIV reservoirs: What, where and how to target them. Nat. Rev. Microbiol. 2016, 14, 55–60. [Google Scholar] [CrossRef]
- Busman-Sahay, K.; Starke, C.E.; Nekorchuk, M.D.; Estes, J.D. Eliminating HIV reservoirs for a cure: The issue is in the tissue. Curr. Opin. HIV AIDS 2021, 16, 200–208. [Google Scholar] [CrossRef]
- Kwon, K.J.; Timmons, A.E.; Sengupta, S.; Simonetti, F.R.; Zhang, H.; Hoh, R.; Deeks, S.G.; Siliciano, J.D.; Siliciano, R.F. Different human resting memory CD4+ T cell subsets show similar low inducibility of latent HIV-1 proviruses. Sci. Transl. Med. 2020, 12, eaax6795. [Google Scholar] [CrossRef]
- Hosmane, N.N.; Kwon, K.J.; Bruner, K.M.; Capoferri, A.A.; Beg, S.; Rosenbloom, D.I.; Keele, B.F.; Ho, Y.-C.; Siliciano, J.D.; Siliciano, R.F. Proliferation of latently infected CD4+ T cells carrying replication-competent HIV-1: Potential role in latent reservoir dynamics. J. Exp. Med. 2017, 214, 959–972. [Google Scholar] [CrossRef]
- Graef, P.; Buchholz, V.R.; Stemberger, C.; Flossdorf, M.; Henkel, L.; Schiemann, M.; Drexler, I.; Höfer, T.; Riddell, S.R.; Busch, D.H. Serial Transfer of Single-Cell-Derived Immunocompetence Reveals Stemness of CD8+ Central Memory T Cells. Immunity 2014, 41, 116–126. [Google Scholar] [CrossRef]
- Smith-Raska, M.R.; Arenzana, T.L.; D’Cruz, L.M.; Khodadadi-Jamayran, A.; Tsirigos, A.; Goldrath, A.W.; Reizis, B. The Transcription Factor Zfx Regulates Peripheral T Cell Self-Renewal and Proliferation. Front. Immunol. 2018, 9, 1482. [Google Scholar] [CrossRef]
- Kulpa, D.A.; Talla, A.; Brehm, J.H.; Ribeiro, S.P.; Yuan, S.; Bebin-Blackwell, A.-G.; Miller, M.; Barnard, R.; Deeks, S.; Hazuda, D.; et al. Differentiation into an Effector Memory Phenotype Potentiates HIV-1 Latency Reversal in CD4+ T Cells. J. Virol. 2019, 93, e00969-19. [Google Scholar] [CrossRef]
- Valdebenito, S.; Castellano, P.; Ajasin, D.; Eugenin, E.A. Astrocytes are HIV reservoirs in the brain: A cell type with poor HIV infectivity and replication but efficient cell-to-cell viral transfer. J. Neurochem. 2021, 158, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Eugenin, E.A.; Clements, J.E.; Zink, M.C.; Berman, J.W. Human Immunodeficiency Virus Infection of Human Astrocytes Disrupts Blood-Brain Barrier Integrity by a Gap Junction-Dependent Mechanism. J. Neurosci. 2011, 31, 9456–9465. [Google Scholar] [CrossRef]
- Gorska, A.M.; Donoso, M.; Valdebenito, S.; Prideaux, B.; Queen, S.; Scemes, E.; Clements, J.; Eugenin, E. Human immunodeficiency virus-1/simian immunodeficiency virus infection induces opening of pannexin-1 channels resulting in neuronal synaptic compromise: A novel therapeutic opportunity to prevent NeuroHIV. J. Neurochem. 2021, 158, 500–521. [Google Scholar] [CrossRef] [PubMed]
- Eugenin, E.A.; Berman, J.W. Cytochrome c dysregulation induced by HIV infection of astrocytes results in bystander apoptosis of uninfected astrocytes by an IP3 and calcium-dependent mechanism. J. Neurochem. 2013, 127, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Woldemeskel, B.A.; Kwaa, A.K.; Blankson, J.N. Viral reservoirs in elite controllers of HIV-1 infection: Implications for HIV cure strategies. eBioMedicine 2020, 62, 103118. [Google Scholar] [CrossRef]
- Fenwick, C.; Joo, V.; Jacquier, P.; Noto, A.; Banga, R.; Perreau, M.; Pantaleo, G. T-cell exhaustion in HIV infection. Immunol. Rev. 2019, 292, 149–163. [Google Scholar] [CrossRef]
- Chen, H.; Moussa, M.; Catalfamo, M. The Role of Immunomodulatory Receptors in the Pathogenesis of HIV Infection: A Therapeutic Opportunity for HIV Cure? Front. Immunol. 2020, 11, 1223. [Google Scholar] [CrossRef]
- Barat, C.; Proust, A.; Deshiere, A.; Leboeuf, M.; Drouin, J.; Tremblay, M.J. Astrocytes sustain long-term productive HIV-1 infection without establishment of reactivable viral latency. Glia 2018, 66, 1363–1381. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Retallack, H.; Di Lullo, E.; Arias, C.; Knopp, K.A.; Laurie, M.T.; Sandoval-Espinosa, C.; Mancia Leon, W.R.; Krencik, R.; Ullian, E.M.; Spatazza, J.; et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl. Acad. Sci. USA 2016, 113, 14408–14413. [Google Scholar] [CrossRef]
- Li, G.-H.; Ning, Z.-J.; Liu, Y.-M.; Li, X.-H. Neurological Manifestations of Dengue Infection. Front. Cell. Infect. Microbiol. 2017, 7, 449. [Google Scholar] [CrossRef] [PubMed]
- Wollebo, H.S.; White, M.K.; Gordon, J.; Berger, J.R.; Khalili, K. Persistence and pathogenesis of the neurotropic polyomavirus JC. Ann. Neurol. 2015, 77, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, L.; Lannes, N. Review of Emerging Japanese Encephalitis Virus: New Aspects and Concepts about Entry into the Brain and Inter-Cellular Spreading. Pathogens 2019, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Koraka, P.; Osterhaus, A.; Martina, B. West Nile Virus: Immunity and Pathogenesis. Viruses 2011, 3, 811–828. [Google Scholar] [CrossRef]
- Ash, M.; Al-Harthi, L.; Schneider, J. HIV in the Brain: Identifying Viral Reservoirs and Addressing the Challenges of an HIV Cure. Vaccines 2021, 9, 867. [Google Scholar] [CrossRef]
- Borrajo López, A.; Penedo, M.A.; Rivera-Baltanas, T.; Pérez-Rodríguez, D.; Alonso-Crespo, D.; Fernández-Pereira, C.; Olivares, J.M.; Agís-Balboa, R.C. Microglia: The Real Foe in HIV-1-Associated Neurocognitive Disorders? Biomedicines 2021, 9, 925. [Google Scholar] [CrossRef]
- Wei, J.; Hou, J.; Su, B.; Jiang, T.; Guo, C.; Wang, W.; Zhang, Y.; Chang, B.; Wu, H.; Zhang, T. The Prevalence of Frascati-Criteria-Based HIV-Associated Neurocognitive Disorder (HAND) in HIV-Infected Adults: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 581346. [Google Scholar] [CrossRef]
- Saylor, D.; Dickens, A.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-associated neurocognitive disorder—pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016, 12, 234–248. [Google Scholar] [CrossRef]
- Chen, M.; Li, M.; Budai, M.M.; Rice, A.P.; Kimata, J.T.; Mohan, M.; Wang, J. Clearance of HIV-1 or SIV reservoirs by promotion of apoptosis and inhibition of autophagy: Targeting intracellular molecules in cure-directed strategies. J. Leukoc. Biol. 2022. [Google Scholar] [CrossRef]
- Giron, L.B.; Papasavvas, E.; Yin, X.; Goldman, A.R.; Tang, H.-Y.; Palmer, C.S.; Landay, A.L.; Li, J.Z.; Koethe, J.R.; Mounzer, K.; et al. Phospholipid Metabolism Is Associated with Time to HIV Rebound upon Treatment Interruption. mBio 2021, 12, e03444-20. [Google Scholar] [CrossRef]
- Whyte-Allman, S.-K.; Bendayan, R. HIV-1 Sanctuary Sites—the Role of Membrane-Associated Drug Transporters and Drug Metabolic Enzymes. AAPS J. 2020, 22, 118. [Google Scholar] [CrossRef] [PubMed]
- Gorska, A.M.; Eugenin, E.A. The Glutamate System as a Crucial Regulator of CNS Toxicity and Survival of HIV Reservoirs. Front. Cell. Infect. Microbiol. 2020, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Castellano, P.; Prevedel, L.; Valdebenito, S.; Eugenin, E.A. HIV infection and latency induce a unique metabolic signature in human macrophages. Sci. Rep. 2019, 9, 3941. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.; Palchaudhuri, R.; Albargy, H.; Abdel-Mohsen, M.; Crowe, S.M. Exploiting immune cell metabolic machinery for functional HIV cure and the prevention of inflammaging. F1000Research 2018, 7, 125. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Kaminski, R.; Deshmane, S.; Langford, D.; Khalili, K.; Amini, S.; Datta, P.K. Role of Hexokinase-1 in the survival of HIV-1-infected macrophages. Cell Cycle 2015, 14, 980–989. [Google Scholar] [CrossRef]
- Bennett, M.L.; Bennett, F. The influence of environment and origin on brain resident macrophages and implications for therapy. Nat. Neurosci. 2019, 23, 157–166. [Google Scholar] [CrossRef]
- Dong, Y.; Benveniste, E.N. Immune function of astrocytes. Glia 2001, 36, 180–190. [Google Scholar] [CrossRef]
- Xu, Y.; Kulkosky, J.; Acheampong, E.; Nunnari, G.; Sullivan, J.; Pomerantz, R.J. HIV-1-mediated apoptosis of neuronal cells: Proximal molecular mechanisms of HIV-1-induced encephalopathy. Proc. Natl. Acad. Sci. USA 2004, 101, 7070–7075. [Google Scholar] [CrossRef]
- Hein, A.; Martin, J.P.; Dörries, R. Early pathological changes in the central nervous system of acutely fe-line-immunodeficiency-virus-infected cats. Virology 2005, 343, 162–170. [Google Scholar] [CrossRef]
- González, R.G.; Cheng, L.L.; Westmoreland, S.V.; Sakaie, K.E.; Becerra, L.R.; Lee, P.L.; Masliah, E.; Lackner, A.A. Early brain injury in the SIV–macaque model of AIDS. AIDS 2000, 14, 2841–2849. [Google Scholar] [CrossRef]
- Irollo, E.; Luchetta, J.; Ho, C.; Nash, B.; Meucci, O. Mechanisms of neuronal dysfunction in HIV-associated neurocognitive disorders. Exp. 2021, 78, 4283–4303. [Google Scholar] [CrossRef] [PubMed]
- Kramer-Hämmerle, S.; Rothenaigner, I.; Wolff, H.; Bell, J.E.; Brack-Werner, R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005, 111, 194–213. [Google Scholar] [CrossRef] [PubMed]
- León-Rivera, R.; Veenstra, M.; Donoso, M.; Tell, E.; Eugenin, E.A.; Morgello, S.; Berman, J.W. Central Nervous System (CNS) Viral Seeding by Mature Monocytes and Potential Therapies to Reduce CNS Viral Reservoirs in the cART Era. mBio 2021, 12, e03633-20. [Google Scholar] [CrossRef]
- Castellano, P.; Prevedel, L.; Eugenin, E.A. HIV-infected macrophages and microglia that survive acute infection become viral res-ervoirs by a mechanism involving Bim. Sci. Rep. 2017, 7, 12866. [Google Scholar] [CrossRef]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and Functions in Brain Pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Duran, R.C.D.; Wang, C.-Y.; Zheng, H.; Deneen, B.; Wu, J.Q. Brain Region-Specific Gene Signatures Revealed by Distinct Astrocyte Subpopulations Unveil Links to Glioma and Neurodegenerative Diseases. Eneuro 2019, 6. [Google Scholar] [CrossRef]
- Lutgen, V.; Narasipura, S.D.; Barbian, H.J.; Richards, M.; Wallace, J.; Razmpour, R.; Buzhdygan, T.; Ramirez, S.; Prevedel, L.; Eugenin, E.A.; et al. HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLoS Pathog. 2020, 16, e1008381. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-H.; Maric, D.; Major, E.O.; Nath, A. Productive HIV infection in astrocytes can be established via a nonclassical mechanism. AIDS 2020, 34, 963–978. [Google Scholar] [CrossRef]
- Xu, X.; Wicki-Stordeur, L.E.; Sanchez-Arias, J.; Liu, M.; Weaver, M.S.; Choi, C.S.W.; Swayne, L.A. Probenecid Disrupts a Novel Pannexin 1-Collapsin Response Mediator Protein 2 Interaction and Increases Microtubule Stability. Front. Cell. Neurosci. 2018, 12, 124. [Google Scholar] [CrossRef]
- Malik, S.; Valdebenito, S.; D’Amico, D.; Prideaux, B.; Eugenin, E.A. HIV infection of astrocytes compromises inter-organelle interactions and inositol phosphate metabolism: A potential mechanism of bystander damage and viral reservoir survival. Prog. Neurobiol. 2021, 206, 102157. [Google Scholar] [CrossRef]
- Abdulle, S.; Mellgren, A.; Brew, B.J.; Cinque, P.; Hagberg, L.; Price, R.W.; Rosengren, L.; Gisslén, M. CSF neurofilament protein (NFL)—a marker of active HIV-related neurodegeneration. J. Neurol. 2007, 254, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Krut, J.J.; Mellberg, T.; Price, R.W.; Hagberg, L.; Fuchs, D.; Rosengren, L.; Nilsson, S.; Zetterberg, H.; Gisslén, M. Biomarker Evidence of Axonal Injury in Neuroasymptomatic HIV-1 Patients. PLoS ONE 2014, 9, e88591. [Google Scholar] [CrossRef]
- Gisslén, M.; Price, R.W.; Andreasson, U.; Norgren, N.; Nilsson, S.; Hagberg, L.; Fuchs, D.; Spudich, S.; Blennow, K.; Zetterberg, H. Plasma Concentration of the Neurofilament Light Protein (NFL) is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine 2016, 3, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Blennow, K.; Hagberg, L.; Nilsson, S.; Price, R.W.; Schouten, J.; Spudich, S.; Underwood, J.; Zetterberg, H.; Gisslén, M. Neurofilament light chain protein as a marker of neuronal injury: Review of its use in HIV-1 infection and reference values for HIV-negative controls. Expert Rev. Mol. Diagn. 2017, 17, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Guha, D.; Mukerji, S.S.; Chettimada, S.; Misra, V.; Lorenz, D.R.; Morgello, S.; Gabuzda, D. Cerebrospinal fluid extracellular vesicles and neurofilament light protein as biomarkers of central nervous system injury in HIV-infected patients on antiretroviral therapy. AIDS 2019, 33, 615–625. [Google Scholar] [CrossRef]
- de Almeida, S.M.; Ribeiro, C.E.; Rotta, I.; Piovesan, M.; Tang, B.; Vaida, F.; Raboni, S.M.; Letendre, S.; Potter, M.; Batistela Fernandes, M.S.; et al. Biomarkers of neuronal injury and amyloid metabolism in the cerebrospinal fluid of patients infected with HIV-1 subtypes B and C. J. Neurovirol. 2018, 24, 28–40. [Google Scholar] [CrossRef]
- Howdle, G.C.; Quidé, Y.; Kassem, M.S.; Johnson, K.; Rae, C.D.; Brew, B.J.; Cysique, L.A. Brain amyloid in virally suppressed HIV-associated neurocognitive disorder. Neurol.-Neuroimmunol. Neuroinflammation 2020, 7, e739. [Google Scholar] [CrossRef]
- Du Pasquier, R.A.R.; Jilek, S.; Kalubi, M.; Yerly, S.; Fux, C.A.; Gutmann, C.; Cusini, A.; Günthard, H.; Cavassini, M.; Vernazza, P.L. Marked increase of the astrocytic marker S100B in the cerebrospinal fluid of HIV-infected patients on LPV/r-monotherapy. AIDS 2013, 27, 203–210. [Google Scholar] [CrossRef]
- Urbanelli, L.; Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Porcellati, S.; Emiliani, C. The Role of Extracellular Vesicles in Viral Infection and Transmission. Vaccines 2019, 7, 102. [Google Scholar] [CrossRef]
- Al-Harthi, L. Interplay between Wnt/beta-catenin signaling and HIV: Virologic and biologic consequences in the CNS. J. Neuroimmune Pharmacol. 2012, 7, 731–739. [Google Scholar] [CrossRef]
- Lyons, J.L.; Uno, H.; Ancuta, P.; Kamat, A.; Moore, D.J.; Singer, E.J.; Morgello, S.; Gabuzda, D. Plasma sCD14 Is a Biomarker Associated with Impaired Neurocognitive Test Performance in Attention and Learning Domains in HIV Infection. JAIDS J. Acquir. Immune Defic. Syndr. 2011, 57, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Burdo, T.H.; Weiffenbach, A.; Woods, S.P.; Letendre, S.; Ellis, R.; Williams, K.C. Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 2013, 27, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Santiago, J.; Schrier, R.D.; de Oliveira, M.F.; Gianella, S.; Var, S.R.; Day, T.R.C.; Ramirez-Gaona, M.; Suben, J.D.; Murrell, B.; Massanella, M.; et al. Cell-free mitochondrial DNA in CSF is associated with early viral rebound, inflammation, and severity of neurocognitive deficits in HIV infection. J. NeuroVirology 2015, 22, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Jumare, J.; Akolo, C.; Ndembi, N.; Bwala, S.; Alabi, P.; Okwuasaba, K.; Adebiyi, R.; Umlauf, A.; Cherner, M.; Abimiku, A.; et al. Elevated Plasma Levels of sCD14 and MCP-1 Are Associated with HIV Associated Neurocognitive Disorders Among Antiretroviral-Naive Individuals in Nigeria. JAIDS J. Acquir. Immune Defic. Syndr. 2020, 84, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Valcour, V.G.; Ananworanich, J.; Agsalda, M.; Sailasuta, N.; Chalermchai, T.; Schuetz, A.; Shikuma, C.; Liang, C.Y.; Jirajariyavej, S.; Sithinamsuwan, P.; et al. HIV DNA reservoir increases risk for cognitive disorders in cART-naive patients. PLoS ONE 2013, 8, e70164. [Google Scholar] [CrossRef]
- Farhadian, S.; Patel, P.; Spudich, S. Neurological Complications of HIV Infection. Curr. Infect. Dis. Rep. 2017, 19, 50. [Google Scholar] [CrossRef]
- Cantres-Rosario, Y.; Plaud-Valentín, M.; Gerena, Y.; Skolasky, R.; Wojna, V.; Meléndez, L.M. Cathepsin B and cystatin B in HIV-seropositive women are associated with infection and HIV-1-associated neurocognitive disorders. AIDS 2013, 27, 347–356. [Google Scholar] [CrossRef]
- Adu-Gyamfi, C.G.; Snyman, T.; Makhathini, L.; Otwombe, K.; Darboe, F.; Penn-Nicholson, A.; Fisher, M.; Savulescu, D.; Hoffmann, C.; Chaisson, R.; et al. Diagnostic accuracy of plasma kynurenine/tryptophan ratio, measured by enzyme-linked immunosorbent assay, for pulmonary tuberculosis. Int. J. Infect. Dis. 2020, 99, 441–448. [Google Scholar] [CrossRef]
- Gelpi, M.; Hartling, H.J.; Ueland, P.M.; Ullum, H.; Trøseid, M.; Nielsen, S.D. Tryptophan catabolism and immune activation in primary and chronic HIV infection. BMC Infect. Dis. 2017, 17, 349. [Google Scholar] [CrossRef]
- Corano Scheri, G.; Fard, S.N.; Schietroma, I.; Mastrangelo, A.; Pinacchio, C.; Giustini, N.; Serafino, S.; De Girolamo, G.; Cavallari, E.N.; Statzu, M.; et al. Modulation of Tryptophan/Serotonin Pathway by Probiotic Supplementation in Human Immunodeficiency Vi-rus-Positive Patients: Preliminary Results of a New Study Approach. Int. J. Tryptophan Res. 2017, 10, 1178646917710668. [Google Scholar] [CrossRef]
- Adu-Gyamfi, C.; Savulescu, D.; Mikhathani, L.; Otwombe, K.; Salazar-Austin, N.; Chaisson, R.; Martinson, N.; George, J.; Suchard, M. Plasma Kynurenine-to-Tryptophan Ratio, a Highly Sensitive Blood-Based Diagnostic Tool for Tuberculosis in Pregnant Women Living with Human Immunodeficiency Virus (HIV). Clin. Infect. Dis. 2021, 73, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Eugenin, E.A.; Osiecki, K.; Lopez, L.; Goldstein, H.; Calderon, T.M.; Berman, J.W. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: A potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 2006, 26, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.; Eugenin, E.A.; Calderon, T.M.; Berman, J.W. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J. Leukoc. Biol. 2012, 91, 401–415. [Google Scholar] [CrossRef]
- Burlacu, R.; Umlauf, A.; Marcotte, T.D.; Soontornniyomkij, B.; Diaconu, C.C.; Bulacu-Talnariu, A.; Temereanca, A.; Ruta, S.M.; Letendre, S.; Ene, L.; et al. Plasma CXCL10 correlates with HAND in HIV-infected women. J. Neurovirol. 2019, 26, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.F.; Chaillon, A.; Nakazawa, M.; Vargas, M.; Letendre, S.L.; Strain, M.C.; Ellis, R.J.; Morris, S.; Little, S.J.; Smith, D.; et al. Early Antiretroviral Therapy Is Associated with Lower HIV DNA Molecular Diversity and Lower Inflammation in Cerebrospinal Fluid but Does Not Prevent the Establishment of Compartmentalized HIV DNA Populations. PLoS Pathog. 2017, 13, e1006112. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, A.; Qiao, L.; Sheng, B.; Xu, M.; Li, W.; Chen, D. The Relationship of CSF and Plasma Cytokine Levels in HIV Infected Patients with Neurocognitive Impairment. BioMed Res. Int. 2015, 2015, 506872. [Google Scholar] [CrossRef]
- Ozturk, T.; Kollhoff, A.; Anderson, A.M.; Howell, J.C.; Loring, D.W.; Waldrop-Valverde, D.; Franklin, D.; Letendre, S.; Tyor, W.R.; Hu, W.T. Linked CSF reduction of phosphorylated tau and IL-8 in HIV associated neurocognitive disorder. Sci. Rep. 2019, 9, 8733. [Google Scholar] [CrossRef]
- Cassol, E.; Misra, V.; Morgello, S.; Gabuzda, D. Applications and limitations of inflammatory biomarkers for studies on neurocognitive impairment in HIV infection. J. Neuroimmune Pharmacol. 2013, 8, 1087–1097. [Google Scholar] [CrossRef]
- Anderson, A.M.; Lennox, J.L.; Mulligan, M.M.; Loring, D.; Zetterberg, H.; Blennow, K.; Kessing, C.; Koneru, R.; Easley, K.; Tyor, W.R. Cerebrospinal fluid interferon alpha levels correlate with neurocognitive impairment in ambulatory HIV-Infected individuals. J. Neurovirol. 2016, 23, 106–112. [Google Scholar] [CrossRef]
- Yuan, L.; Wei, F.; Zhang, X.; Guo, X.; Lu, X.; Su, B.; Zhang, T.; Wu, H.; Chen, D. Intercellular Adhesion Molecular-5 as Marker in HIV Associated Neurocognitive Disorder. Aging Dis. 2017, 8, 250–256. [Google Scholar] [CrossRef][Green Version]
- Vassallo, M.; Dunais, B.; Durant, J.; Carsenti-Dellamonica, H.; Harvey-Langton, A.; Cottalorda, J.; Ticchioni, M.; Laffon, M.; Lebrun-Frenay, C.; Dellamonica, P.; et al. Relevance of lipopolysaccharide levels in HIV-associated neurocognitive impairment: The Neuradapt study. J. Neurovirol. 2013, 19, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Abassi, M.; Morawski, B.M.; Nakigozi, G.; Nakasujja, N.; Kong, X.; Meya, D.B.; Robertson, K.; Gray, R.; Wawer, M.J.; Sacktor, N.; et al. Cerebrospinal fluid biomarkers and HIV-associated neurocognitive disorders in HIV-infected individuals in Rakai, Uganda. J. Neurovirol. 2016, 23, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Kallianpur, A.R.; the CHARTER Study Group; Gittleman, H.; Letendre, S.; Ellis, R.; Barnholtz-Sloan, J.S.; Bush, W.S.; Heaton, R.; Samuels, D.C.; Franklin, D.R.; et al. Cerebrospinal Fluid Ceruloplasmin, Haptoglobin, and Vascular Endothelial Growth Factor Are Associated with Neurocognitive Impairment in Adults with HIV Infection. Mol. Neurobiol. 2018, 56, 3808–3818. [Google Scholar] [CrossRef] [PubMed]
- Underwood, J.; Cole, J.H.; Caan, M.W.; De Francesco, D.; Leech, R.; Van Zoest, R.A.; Su, T.; Geurtsen, G.; Schmand, B.A.; Portegies, P.; et al. Gray and White Matter Abnormalities in Treated Human Immunodeficiency Virus Disease and Their Relationship to Cognitive Function. Clin. Infect. Dis. 2017, 65, 422–432. [Google Scholar] [CrossRef]
- Van Zoest, R.A.; Underwood, J.; De Francesco, D.; Sabin, C.A.; Cole, J.H.; Wit, F.W.; Caan, M.W.; Kootstra, N.A.; Fuchs, D.; Zetterberg, H.; et al. Structural Brain Abnormalities in Successfully Treated HIV Infection: Associations with Disease and Cerebrospinal Fluid Biomarkers. J. Infect. Dis. 2017, 217, 69–81. [Google Scholar] [CrossRef]
- Su, T.; Wit, F.W.; Caan, M.W.; Schouten, J.; Prins, M.; Geurtsen, G.; Cole, J.; Sharp, D.J.; Richard, E.; Reneman, L.; et al. White matter hyperintensities in relation to cognition in HIV-infected men with sustained suppressed viral load on combination antiretroviral therapy. AIDS 2016, 30, 2329–2339. [Google Scholar] [CrossRef]
- Eggers, C.; For the German Association of Neuro-AIDS und Neuro-Infectiology (DGNANI); Arendt, G.; Hahn, K.; Husstedt, I.W.; Maschke, M.; Neuen-Jacob, E.; Obermann, M.; Rosenkranz, T.; Schielke, E.; et al. HIV-1-associated neurocognitive disorder: Epidemiology, pathogenesis, diagnosis, and treatment. J. Neurol. 2017, 264, 1715–1727. [Google Scholar] [CrossRef]
- Strain, J.F.; Burdo, T.H.; Song, S.-K.; Sun, P.; El-Ghazzawy, O.; Nelson, B.; Westerhaus, E.; Baker, L.; Vaida, F.; Ances, B.M. Diffusion Basis Spectral Imaging Detects Ongoing Brain Inflammation in Virologically Well-Controlled HIV+ Patients. JAIDS J. Acquir. Immune Defic. Syndr. 2017, 76, 423–430. [Google Scholar] [CrossRef]
- Sanford, R.; Strain, J.; Dadar, M.; Maranzano, J.; Bonnet, A.; Mayo, N.E.; Scott, S.C.; Fellows, L.K.; Ances, B.M.; Collins, D.L. HIV infection and cerebral small vessel disease are independently associated with brain atrophy and cognitive impairment. AIDS 2019, 33, 1197–1205. [Google Scholar] [CrossRef]
- Velasquez, S.; Prevedel, L.; Valdebenito, S.; Gorska, A.M.; Golovko, M.; Khan, N.; Geiger, J.; Eugenin, E.A. Circulating levels of ATP is a biomarker of HIV cognitive impairment. eBioMedicine 2019, 51, 102503. [Google Scholar] [CrossRef]
- Gajardo-Gómez, R.; Santibañez, C.A.; Labra, V.C.; Gómez, G.I.; Eugenin, E.A.; Orellana, J.A. HIV gp120 Protein Increases the Function of Connexin 43 Hemichannels and Pannexin-1 Channels in As-trocytes: Repercussions on Astroglial Function. Int. J. Mol. Sci. 2020, 21, 2503. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Dalal, M.; Contreras, J. Pannexin-1 Channels as Mediators of Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 5189. [Google Scholar] [CrossRef]
- Baranova, A.; Ivanov, D.; Petrash, N.; Pestova, A.; Skoblov, M.; Kelmanson, I.; Shagin, D.; Nazarenko, S.; Geraymovych, E.; Litvin, O.; et al. The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 2004, 83, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Scemes, E.; Suadicani, S.O.; Dahl, G.; Spray, D.C. Connexin and pannexin mediated cell–cell communication. Neuron Glia Biol. 2007, 3, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Whyte-Fagundes, P.; Zoidl, G. Mechanisms of pannexin1 channel gating and regulation. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2018, 1860, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Gehi, R.; Laird, D.W. The biochemistry and function of pannexin channels. Biochim. Biophys. Acta BBA Biomembr. 2013, 1828, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Bhalla, R.; Nag, K.; Laird, D.W. Glycosylation Regulates Pannexin Intermixing and Cellular Localization. Mol. Biol. Cell 2009, 20, 4313–4323. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; Jin, X.; Medina, C.B.; Leonhardt, S.A.; Kiessling, V.; Bennett, B.C.; Shu, S.; Tamm, L.K.; Yeager, M.; Ravichandran, K.; et al. A quantized mechanism for activation of pannexin channels. Nat. Commun. 2017, 8, 14324. [Google Scholar] [CrossRef]
- Michalski, K.; Syrjanen, J.L.; Henze, E.; Kumpf, J.; Furukawa, H.; Kawate, T. The Cryo-EM structure of pannexin 1 reveals unique motifs for ion selection and inhibition. Elife 2020, 9. [Google Scholar] [CrossRef]
- Qu, R.; Dong, L.; Zhang, J.; Yu, X.; Wang, L.; Zhu, S. Cryo-EM structure of human heptameric Pannexin 1 channel. Cell Res. 2020, 30, 446–448. [Google Scholar] [CrossRef]
- Abeele, F.V.; Bidaux, G.; Gordienko, D.; Beck, B.; Panchin, Y.; Baranova, A.; Ivanov, D.; Skryma, R.; Prevarskaya, N. Functional implications of calcium permeability of the channel formed by pannexin 1. J. Cell Biol. 2006, 174, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Sahu, G.; Sukumaran, S.; Bera, A.K. Pannexins form gap junctions with electrophysiological and pharmacological properties distinct from connexins. Sci. Rep. 2014, 4, 4955. [Google Scholar] [CrossRef] [PubMed]
- Boassa, D.; Ambrosi, C.; Qiu, F.; Dahl, G.; Gaietta, G.; Sosinsky, G. Pannexin1 Channels Contain a Glycosylation Site That Targets the Hexamer to the Plasma Membrane. J. Biol. Chem. 2007, 282, 31733–31743. [Google Scholar] [CrossRef] [PubMed]
- Sosinsky, G.E.; Boassa, D.; Dermietzel, R.; Duffy, H.S.; Laird, D.W.; MacVicar, B.A.; Naus, C.C.; Penuela, S.; Scemes, E.; Spray, D.C.; et al. Pannexin channels are not gap junction hemichannels. Channels 2011, 5, 193–197. [Google Scholar] [CrossRef]
- Ruan, Z.; Orozco, I.J.; Du, J.; Lü, W. Structures of human pannexin 1 reveal ion pathways and mechanism of gating. Nature 2020, 584, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Prado, N.; Soto, P.A.; López, X.; Choi, E.J.; Marquez-Miranda, V.; Rojas, M.; Duarte, Y.; Lee, J.; González-Nilo, F.D.; Sáez, J.C. Endogenous pannexin1 channels form functional intercellular cell-cell channels with characteristic volt-age-dependent properties. Proc. Natl. Acad. Sci. USA 2022, 119, e2202104119. [Google Scholar] [CrossRef]
- Sandilos, J.K.; Bayliss, D.A. Physiological mechanisms for the modulation of pannexin 1 channel activity. J. Physiol. 2012, 590, 6257–6266. [Google Scholar] [CrossRef]
- Ma, W.; Compan, V.; Zheng, W.; Martin, E.; North, R.A.; Verkhratsky, A.; Surprenant, A. Pannexin 1 forms an anion-selective channel. Pflügers Arch.-Eur. J. Physiol. 2012, 463, 585–592. [Google Scholar] [CrossRef]
- Chekeni, F.B.; Elliott, M.R.; Sandilos, J.K.; Walk, S.F.; Kinchen, J.M.; Lazarowski, E.R.; Armstrong, A.J.; Penuela, S.; Laird, D.W.; Salvesen, G.S.; et al. Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 2010, 467, 863–867. [Google Scholar] [CrossRef]
- Ravichandran, K.S. Find-me and eat-me signals in apoptotic cell clearance: Progress and conundrums. J. Exp. Med. 2010, 207, 1807–1817. [Google Scholar] [CrossRef]
- Sandilos, J.K.; Chiu, Y.-H.; Chekeni, F.B.; Armstrong, A.J.; Walk, S.F.; Ravichandran, K.S.; Bayliss, D.A. Pannexin 1, an ATP Release Channel, Is Activated by Caspase Cleavage of Its Pore-associated C-terminal Autoinhibitory Region. J. Biol. Chem. 2012, 287, 11303–11311. [Google Scholar] [CrossRef] [PubMed]
- Dourado, M.; Wong, E.; Hackos, D.H. Pannexin-1 Is Blocked by Its C-Terminus through a Delocalized Non-Specific Interaction Surface. PLoS ONE 2014, 9, e99596. [Google Scholar] [CrossRef] [PubMed]
- Silverman, W.R.; Vaccari, J.P.D.R.; Locovei, S.; Qiu, F.; Carlsson, S.K.; Scemes, E.; Keane, R.W.; Dahl, G. The Pannexin 1 Channel Activates the Inflammasome in Neurons and Astrocytes. J. Biol. Chem. 2009, 284, 18143–18151. [Google Scholar] [CrossRef] [PubMed]
- Locovei, S.; Wang, J.; Dahl, G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2005, 580, 239–244. [Google Scholar] [CrossRef]
- Ma, W.; Hui, H.; Pelegrin, P.; Surprenant, A. Pharmacological Characterization of Pannexin-1 Currents Expressed in Mammalian Cells. J. Pharmacol. Exp. Ther. 2008, 328, 409–418. [Google Scholar] [CrossRef]
- Bao, L.; Locovei, S.; Dahl, G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004, 572, 65–68. [Google Scholar] [CrossRef]
- López, X.; Escamilla, R.; Fernández, P.; Duarte, Y.; González-Nilo, F.; Palacios-Prado, N.; Martinez, A.D.; Sáez, J.C. Stretch-Induced Activation of Pannexin 1 Channels Can Be Prevented by PKA-Dependent Phosphorylation. Int. J. Mol. Sci. 2020, 21, 9180. [Google Scholar] [CrossRef]
- Beckel, J.M.; Argall, A.J.; Lim, J.C.; Xia, J.; Lu, W.; Coffey, E.E.; Macarak, E.J.; Shahidullah, M.; Delamere, N.; Zode, G.S.; et al. Mechanosensitive release of adenosine 5′-triphosphate through pannexin channels and mechanosensitive upregulation of pannexin channels in optic nerve head astrocytes: A mechanism for purinergic involvement in chronic strain. Glia 2014, 62, 1486–1501. [Google Scholar] [CrossRef]
- Imamura, H.; Sakamoto, S.; Yoshida, T.; Matsui, Y.; Penuela, S.; Laird, D.W.; Mizukami, S.; Kikuchi, K.; Kakizuka, A. Single-cell dynamics of pannexin-1-facilitated programmed ATP loss during apoptosis. Elife 2020, 9. [Google Scholar] [CrossRef]
- Prochnow, N.; Hoffmann, S.; Vroman, R.; Klooster, J.; Bunse, S.; Kamermans, M.; Dermietzel, R.; Zoidl, G. Pannexin1 in the outer retina of the zebrafish, Danio rerio. Neuroscience 2009, 162, 1039–1054. [Google Scholar] [CrossRef]
- Boyce, A.K.; Epp, A.L.; Nagarajan, A.; Swayne, L.A. Transcriptional and post-translational regulation of pannexins. Biochim. et Biophys. Acta (BBA)-Biomembr. 2018, 1860, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Poornima, V.; Vallabhaneni, S.; Mukhopadhyay, M.; Bera, A.K. Nitric oxide inhibits the pannexin 1 channel through a cGMP-PKG dependent pathway. Nitric Oxide 2015, 47, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lohman, A.W.; Weaver, J.L.; Billaud, M.; Sandilos, J.K.; Griffiths, R.; Straub, A.C.; Penuela, S.; Leitinger, N.; Laird, D.W.; Bayliss, D.A.; et al. S-Nitrosylation Inhibits Pannexin 1 Channel Function. J. Biol. Chem. 2012, 287, 39602–39612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Deng, T.; Sun, Y.; Liu, K.; Yang, Y.; Zheng, X. Role for nitric oxide in permeability of hippocampal neuronal hemichannels during oxygen glucose deprivation. J. Neurosci. Res. 2008, 86, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Torre, D.; Pugliese, A.; Speranza, F. Role of nitric oxide in HIV-1 infection: Friend or foe? Lancet Infect Dis. 2002, 2, 273–280. [Google Scholar] [CrossRef]
- Groeneveld, P.H.P.; Kroon, F.P.; Nibbering, P.H.; Bruisten, S.M.; Van Swieten, P.; Van Furth, R. Increased Production of Nitric Oxide Correlates with Viral Load and Activation of Mononuclear Phagocytes in HIV-infected Patients. Scand. J. Infect. Dis. 1996, 28, 341–345. [Google Scholar] [CrossRef]
- Dunn, J.; Grider, M. Physiology, Adenosine Triphosphate; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lohman, A.W.; Billaud, M.; Isakson, B.E. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc. Res. 2012, 95, 269–280. [Google Scholar] [CrossRef]
- Goueli, S.A.; Hsiao, K. Monitoring and characterizing soluble and membrane-bound ectonucleotidases CD73 and CD39. PLoS ONE 2019, 14, e0220094. [Google Scholar] [CrossRef]
- Hashikawa, T.; Hooker, S.W.; Maj, J.G.; Knott-Craig, C.J.; Takedachi, M.; Murakami, S.; Thompson, L.F. Regulation of adenosine receptor engagement by ecto-adenosine deaminase. FASEB J. 2003, 18, 131–133. [Google Scholar] [CrossRef]
- Burnstock, G. Purine and purinergic receptors. Brain Neurosci. Adv. 2018, 2, 2398212818817494. [Google Scholar] [CrossRef]
- Praetorius, H.A.; Leipziger, J. ATP release from non-excitable cells. Purinergic Signal. 2009, 5, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Schenk, U.; Westendorf, A.M.; Radaelli, E.; Casati, A.; Ferro, M.; Fumagalli, M.; Verderio, C.; Buer, J.; Scanziani, E.; Grassi, F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signal. 2008, 1, ra6. [Google Scholar] [CrossRef] [PubMed]
- Velasquez, S.; Malik, S.; Lutz, S.; Scemes, E.; Eugenin, E.A. Pannexin1 Channels Are Required for Chemokine-Mediated Migration of CD4+T Lymphocytes: Role in Inflammation and Experimental Autoimmune Encephalomyelitis. J. Immunol. 2016, 196, 4338–4347. [Google Scholar] [CrossRef] [PubMed]
- Séror, C.; Melki, M.-T.; Subra, F.; Raza, S.Q.; Bras, M.; Saïdi, H.; Nardacci, R.; Voisin, L.; Paoletti, A.; Law, F.; et al. Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J. Exp. Med. 2011, 208, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Orellana, J.A.; Velasquez, S.; Williams, D.W.; Sáez, J.C.; Berman, J.W.; Eugenin, E.A. Pannexin1 hemichannels are critical for HIV infection of human primary CD4+ T lymphocytes. J. Leukoc. Biol. 2013, 94, 399–407. [Google Scholar] [CrossRef]
- Hazleton, J.E.; Berman, J.W.; Eugenin, E.A. Purinergic Receptors Are Required for HIV-1 Infection of Primary Human Macrophages. J. Immunol. 2012, 188, 4488–4495. [Google Scholar] [CrossRef]
- Harmon, B.; Ratner, L. Induction of the Galpha(q) signaling cascade by the human immunodeficiency virus envelope is required for virus entry. J. Virol. 2008, 82, 9191–9205. [Google Scholar] [CrossRef]
- Paoletti, A.; Allouch, A.; Caillet, M.; Saïdi, H.; Subra, F.; Nardacci, R.; Wu, Q.; Muradova, Z.; Voisin, L.; Raza, S.Q.; et al. HIV-1 Envelope Overcomes NLRP3-Mediated Inhibition of F-Actin Polymerization for Viral Entry. Cell Rep. 2019, 28, 3381–3394.e7. [Google Scholar] [CrossRef]
- Hung, S.-C.; Choi, C.H.; Said-Sadier, N.; Johnson, L.; Atanasova, K.; Sellami, H.; Yilmaz, O.; Ojcius, D.M. P2X4 Assembles with P2X7 and Pannexin-1 in Gingival Epithelial Cells and Modulates ATP-induced Reactive Oxygen Species Production and Inflammasome Activation. PLoS ONE 2013, 8, e70210. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Mazziotta, C.; Lanzillotti, C.; Stefani, C.; Badiale, G.; Campione, G.; Martini, F.; Tognon, M. The Role of Purinergic P2X7 Receptor in Inflammation and Cancer: Novel Molecular Insights and Clinical Applica-tions. Cancers 2022, 14, 1116. [Google Scholar] [CrossRef]
- Wang, X.; Mbondji-Wonje, C.; Zhao, J.; Hewlett, I. IL-1β and IL-18 inhibition of HIV-1 replication in Jurkat cells and PBMCs. Biochem. Biophys. Res. Commun. 2016, 473, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Schachter, J.; Delgado, K.V.; Barreto-De-Souza, V.; Bou-Habib, D.C.; Persechini, P.M.; Meyer-Fernandes, J.R. Inhibition of ecto-ATPase activities impairs HIV-1 infection of macrophages. Immunobiology 2015, 220, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.S.; Jones, C.J.; Mahmood, N.; Evans, I.G.; Goff, M.; Cooper, R.; Hay, A.J. Anti-(human immunodeficiency virus) activity of polyoxotungstates and their inhibition of human immunodeficiency virus reverse transcriptase. Biochem. J. 1995, 307, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Boyce, A.K.; Kim, M.S.; Wicki-Stordeur, L.E.; Swayne, L.A. ATP stimulates pannexin 1 internalization to endosomal compartments. Biochem. J. 2015, 470, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Schachter, J.; Valadao, A.L.C.; Aguiar, R.S.; Barreto-De-Souza, V.; Rossi, A.D.; Arantes, P.R.; Verli, H.; Quintana, P.G.; Heise, N.; Tanuri, A.; et al. 2′,3′-Dialdehyde of ATP, ADP, and adenosine inhibit HIV-1 reverse transcriptase and HIV-1 replication. Curr. HIV Res. 2014, 12, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, K.D.; Hunsmann, G.; Hartmann, H.; Nickel, P. Inhibition of Human Immunodeficiency Virus Type I Reverse Transcriptase by Suramin-related Compounds. J. Gen. Virol. 1987, 68, 2183–2192. [Google Scholar] [CrossRef]
- Freeman, T.L.; Swartz, T.H. Purinergic Receptors: Elucidating the Role of these Immune Mediators in HIV-1 Fusion. Viruses 2020, 12, 290. [Google Scholar] [CrossRef]
- Sanchez-Arias, J.C.; van der Slagt, E.; Vecchiarelli, H.A.; Candlish, R.C.; York, N.; Young, P.A.; Shevtsova, O.; Juma, A.; Tremblay, M.; Swayne, L.A. Purinergic signaling in nervous system health and disease: Focus on pannexin 1. Pharmacol. Ther. 2021, 225, 107840. [Google Scholar] [CrossRef]
- Wicki-Stordeur, L.E.; Sanchez-Arias, J.C.; Dhaliwal, J.; Carmona-Wagner, E.O.; Shestopalov, V.I.; Lagace, D.C.; Swayne, L.A. Pannexin 1 Differentially Affects Neural Precursor Cell Maintenance in the Ventricular Zone and Peri-Infarct Cortex. J. Neurosci. 2016, 36, 1203–1210. [Google Scholar] [CrossRef]
- Aslam, M.; Gündüz, D.; Troidl, C.; Heger, J.; Hamm, C.; Schulz, R. Purinergic Regulation of Endothelial Barrier Function. Int. J. Mol. Sci. 2021, 22, 1207. [Google Scholar] [CrossRef]
- Stachon, P.; Geis, S.; Peikert, A.; Heidenreich, A.; Michel, N.A.; Ünal, F.; Hoppe, N.; Dufner, B.; Schulte, L.; Marchini, T.; et al. Extracellular ATP Induces Vascular Inflammation and Atherosclerosis via Purinergic Receptor Y2 in Mice. Arter. Thromb. Vasc. Biol. 2016, 36, 1577–1586. [Google Scholar] [CrossRef] [PubMed]
- Peikert, A.; König, S.; Suchanek, D.; Rofa, K.; Schäfer, I.; Dimanski, D.; Karnbrock, L.; Bulatova, K.; Engelmann, J.; Hoppe, N.; et al. P2X4 deficiency reduces atherosclerosis and plaque inflammation in mice. Sci Rep. 2022, 12, 2801. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhao, K.; Zhang, X.; Zhang, J.; Xu, B. ATP Induces Disruption of Tight Junction Proteins via IL-1 Beta-Dependent MMP-9 Activation of Human Blood-Brain Barrier In Vitro. Neural Plast. 2016, 2016, 8928530. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, P.; Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix Metalloproteinase-Mediated Disruption of Tight Junction Proteins in Cerebral Vessels is Reversed by Synthetic Matrix Metalloproteinase Inhibitor in Focal Ischemia in Rat. J. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Bynoe, M.S.; Viret, C.; Yan, A.; Kim, D.-G. Adenosine receptor signaling: A key to opening the blood–brain door. Fluids Barriers CNS 2015, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.; Anders, N.M.; Mangraviti, A.; Wanjiku, T.M.; Sankey, E.W.; Liu, A.; Brem, H.; Tyler, B.; Rudek, M.A.; Grossman, S.A. The effect of regadenoson-induced transient disruption of the blood–brain barrier on temozolomide delivery to normal rat brain. J. Neuro-Oncol. 2015, 126, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-G.; Bynoe, M.S. A2A Adenosine Receptor Regulates the Human Blood-Brain Barrier Permeability. Mol. Neurobiol. 2014, 52, 664–678. [Google Scholar] [CrossRef]
- Carman, A.; Mills, J.H.; Krenz, A.; Kim, D.-G.; Bynoe, M.S. Adenosine Receptor Signaling Modulates Permeability of the Blood-Brain Barrier. J. Neurosci. 2011, 31, 13272–13280. [Google Scholar] [CrossRef]
- Kim, D.-G.; Bynoe, M.S. A2A adenosine receptor modulates drug efflux transporter P-glycoprotein at the blood-brain barrier. J. Clin. Investig. 2016, 126, 1717–1733. [Google Scholar] [CrossRef]
- Sankatsing, S.U.C.; Beijnen, J.H.; Schinkel, A.H.; Lange, J.M.A.; Prins, J.M. P Glycoprotein in Human Immunodeficiency Virus Type 1 Infection and Therapy. Antimicrob. Agents Chemother. 2004, 48, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Gollapudi, S.; Gupta, S. Human immunodeficiency virus I-induced expression of P-glycoprotein. Biochem. Biophys. Res. Commun. 1990, 171, 1002–1007. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Wagenaar, E.; Van Deemter, L.; Mol, C.; Borst, P. Absence of the mdr1a P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J. Clin. Investig. 1995, 96, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).