Abstract
Pancreatic cancer (PC) is the fourth leading cause of cancer-related mortality with limited diagnostic and therapeutic options. Although immunotherapy has shown promise in the treatment of several cancers, its role in pancreatic cancer is rather limited. Several studies have focused on determining the role of the tumor microenvironment with cancer-cell-intrinsic events and tumor-infiltrating immune cellular properties. However, in the past decade, there has been emerging research aimed at delineating the role of the host microbiome, including the metabolites from microbes and host responses, on pancreatic tumorigenesis. Importantly, there is emerging evidence suggesting the beneficial role of a gut microbiome transplant to improve immunotherapeutic outcomes in cancer patients. In this review, we summarize the recent understanding of the role of the microbiome in pancreatic cancer progression, along with its clinical diagnostic and therapeutic implications.
1. Introduction
Pancreatic cancer (PC) is the fourth leading cause of cancer-related deaths in the USA [1]. An estimated 62,210 new cases of PC are predicted to be diagnosed in 2022. While surgical resection remains the only curative therapy, only 10–20% of patients have resectable tumors at diagnosis [2]. Unfortunately, most of the patients at the time of diagnosis are at an advanced stage and with distant metastases, thus, limiting treatment options [3]. Pancreatic ductal adenocarcinoma (PDAC) accounts for more than 90% of all PCs. Despite continued efforts, the 5-year overall survival rate of PDAC patients remains at approximately 10% for all stages combined and deteriorates to 3% for those with distant metastasis [4]. Under extremely rare early detection instances, when the primary tumor is confined to the pancreas and is below 2 cm in diameter, the 5-year survival rate dramatically increases to 46% [5]. Hence, early screening, prevention and novel therapeutic approaches are needed for PC management.
The number of bacteria inhabiting the human body is estimated to be in the similar range as the total number of cells in the human body [6]. With advancements in sequencing technology, an in-depth examination and characterization of the genetic make-up of the microbiota (referred to as the microbiome) has become affordable. The human microbiome plays an essential role in maintaining body homeostasis, and an imbalance of the microbiota, a state known as dysbiosis, can contribute to the pathogenesis of many diseases [7]. In general, while healthy microbiota (eubiosis) contribute to a healthy immune system, a microbial dysbiosis is recognized to contribute to carcinogenesis and poor treatment outcomes [8]. Pathogenic microbes linked to human infections have also been identified as etiologic agents for 15%–20% of global cancer cases [9]. In this communication, we review the current understanding of the impact of the microbiome on PC tumorigenesis, along with its potential impact on therapeutic outcomes.
2. Pancreatic Microbiome
In general, while the pancreas was thought to be out of the reach of the gut microbiota, several studies have found the existence of bacteria in normal pancreatic tissue and pancreatic tumor samples. However, the challenge remains on defining the ‘normal’ microbiota for the pancreas. Various studies have found that while Bacteroidetes was found in pancreatic tissue from healthy samples, pancreatic cancer samples showed higher levels of three microbial taxa, namely, Proteobacteria, Enterobacteriaceae, and Pseudomonadaceae, of which Bacteroidetes and Firmicutes were the most common [10,11,12]. Along with bacteria, fungal colonization (mycobiome) with Malassezia species was enriched in PDAC samples [13]. Along with pancreatic localization, a differential gut and oral microbiota profile has been shown to correlate with pancreatic cancer prevalence. There was a significantly lower diversity (alpha diversity) of gut microbiota and mycobiota in pancreatic cancer patients, and were enriched with Bacteroidetes, Klebsiella and Ascomycota, along with lower concentrations of Firmicutes and Proteobacteria [14,15,16,17]. In contrast, an analysis of the oral microbiome demonstrated that pancreatic cancer patients demonstrated a higher diversity of microbiota with Haemophilus, Porphyromonas, Leptotrichia and Fusobacteria compared to healthy adults [18]. In another study, the presence of oral pathogens, P. gingivalis and Aggregatibacter actinomycetemcomitans, was associated with a higher risk of pancreatic cancer, while Fusobacteria was associated with a decreased risk [19]. The localization of various bacterial species with reported implications on PC are noted in Table 1.

Table 1.
Microbial taxa with propensity to cause pancreatic cancer.
In a study by Geller et al., 76% of the 113 PDAC tissue samples detected bacterial DNA, while only 15% (p < 0.005) of normal pancreas tissue had bacterial localization [29]. Murine studies with fluorescence-tagged micro/mycobiota demonstrated that within half-an-hour of oral administration, the tagged E. faecalis, E coli and Saccharomyces cerevisiae were found in mouse pancreatic tissue, suggesting that bacterial colonization to the pancreas can happen through the gastrointestinal route, possibly through the pancreatic duct [11,13]. However, in stark contrast, in another murine study, orally administered Campylobacter jejuni did not colonize in pancreatic tissue even after 8 weeks [30]. Interestingly, in an antibiotic-treated mouse model, non-invasive Salmonella was carried from the gut lumen to the mesenteric lymph nodes following antibiotic treatment with CX3CR1+ monocyte/dendritic cells [31]. The reasons for this opposing evidence need further study to determine if pancreatic colonization is microbiota-specific or host-specific. The differences in study sizes, designs, sampling methods and primers used for 16S rRNA amplification make interpretation and generalization difficult. Moreover, the decrease in gut microbial diversity in cancer patients compared to healthy individuals, along with the differences in oro-intestinal microbiome between pancreatitis and pancreatic cancer, makes the conclusions on cancer-specific microbiome difficult [32,33,34,35].
3. Role of Microbiome in Pancreatic Carcinogenesis
Several molecular mechanisms have been suggested to mediate microbial dysbiosis and pancreatic cancer. An innate-immune and pro-inflammatory signaling molecule, such as toll-like receptor (TLR)-2/4, was considered to play a role in P. gingivalis-mediated pancreatic carcinogenesis [36]. A metabolic influencing signaling factor, Akt, was also shown to play a role in dysbiosis-mediated carcinogenesis [37]. A genetically engineered murine tumor model (KrasG12D/PTENlox/+) demonstrated that antibiotic treatment decreased the incidence of cancer [30]. Germ-free studies have shown that a lack of microbial growth was associated with increased anti-tumor M1 macrophage differentiation, tumor infiltration with cytotoxic CD8+ and CD4+ T cells, decreased myeloid-derived suppressor cells (MDSCs) and enhanced anti-tumor effects of PD-1 monoclonal antibody therapy [11,38]. Along these lines, another important observation was that prior antibiotic exposure, but not concurrent antibiotic use, can negatively impact the clinical efficacy of immunotherapy in some non-PDAC tumors [39]. These apparently contradictory literature evidences indicate the need for further molecular and functional microbial studies in PC [40,41].
3.1. Alterations of Microbial Metabolites
Microbial dysbiosis reduces the thickness of the mucus layer and, thus, decreases the host antimicrobial defense. This would also cause a decreased release of short-chain fatty acids (SCFAs) and gut peptides such as glucagon-like peptide-1 (GLP-1) and peptide YY [42]. The microbial fermentation of fiber produces SCFAs, which interact with host G-protein-coupled receptors, resulting in reduced chronic inflammation and the suppression of carcinogenesis [43,44]. The SCFAs have also shown to suppress histone deacetylase and induce apoptosis, leading to the inhibition of cell proliferation and metastasis [45,46]. However, in contrast, SCFAs have also shown to be correlated with the expansion of immunosuppressive pro-cancer regulatory CD4+T cells (Treg) and a resistance to anti-cancer immune-checkpoint inhibitor therapy [47]. This apparently conflicting role of SCFAs, with direct anti-tumor effects on cancer cells and pro-tumor immunosuppressive immune cells in the tumor microenvironment, only points out the need for a more thorough study to determine the role of microbial dysbiosis-mediated SCFA production on carcinogenesis.
The reduced thickness of the gut mucous membrane would cause the absorption of the released bacterial inflammatory metabolites, such as lipopolysaccharides (LPS) and lipoteichoic acid (LTA), into the host’s circulation. These bacterial metabolites, which are specifically released from Gram-positive bacteria, induce an inflammatory activation of TLR-mediated innate immune responses, along with the pro-inflammatory activation of macrophages, CD4+ and CD8+ T adaptive immune cells. Murine studies have demonstrated that these inflammatory events mediated by infection of Enterococcus faecalis could lead to chronic pancreatitis, an important predisposing factor for pancreatic carcinogenesis [48]. The 7α-dehydroxylating bacteria metabolize host cholic acid from bile to deoxycholic acid (DCA), which is associated with DNA damage and genome instability, potentially inducing PC tumorigenesis [49,50,51]. Further, DCA is known to upregulate the epidermal growth factor receptor (EGFR), mitogen-activated protein kinase (MAPK) and STAT3 oncogenic-signaling pathways [52]. Studies by Halimi et al. have shown that the isolation of the pancreatic microbiome obtained from pancreatic cystic lesions associated with invasive cancer demonstrated the presence of gamma-Proteobacteria and Bacilli. Interestingly, the ex vivo co-culture of this isolated pancreatic microbiota with an immortalized primary healthy human pancreatic cell line (hTERT-HPNE cells), early pancreatic adenocarcinoma cell line (Capan-2 cells) and late pancreatic adenocarcinoma cell line (AsPC-1 cells), demonstrated that the bacteria were able to enter all three cell lines and induce DNA double-strand damage. This ex vivo damage of pancreatic cells by the isolated pancreatic microbiota was inhibited by a co-treatment using the antibiotic gentamycin, suggesting a direct DNA-damaging impact of pancreatic microbiota with possible oncogenesis [53]. Similarly, butyrate (an SCFA) is considered to interfere with epigenetic modifications and transcriptional gene regulation [54]. Certain bacterial toxins, such as colibactin and cyclomodulins (specifically Bacteroides fragilis toxins), are known to trigger double-stranded DNA damage and interfere with cell cycle repair, leading to carcinogenesis [55,56].
3.2. Microbiota-Mediated Immunoregulation
Host immune cells interplay with gut microbes and maintain a symbiotic relationship to maintain human health [57]. Compositions of the microbiota are influenced by the immune system. Conversely, gut microbiota also plays an indispensable role in the maturation and continued education of the host immune system [58]. This delicate homeostatic balance, when disrupted, could lead to abnormal immune responses and tumorigenesis [59,60].
A limited number of germline-encoded pattern recognition receptors (PRRs) exist in the innate immune system. These receptors recognize the ‘non-host-self’ nature of microorganisms’ genetic material and proteins, often known as pathogen-associated molecular patterns. In addition to detecting conserved microbe-associated molecular patterns, TLRs can also be activated by inflammation or damage-associated molecular patterns (DAMPs). As downstream targets of these pattern-recognition molecules, the NF-κB and MAPK signaling pathways are activated, which initiate cytokine production and the further recruitment of pro-inflammatory entities that are ultimately involved in the development of cancer [61]. Importantly, one example of protection against pancreatic carcinogenesis is the blockade of the activation of TLR4/7 to inhibit its interactions with the STAT3, Notch, NF-κB and MAPK pathways. This effect has been successfully tested in systemic lupus erythematosus [62]. Similarly, several studies have focused on another PRR, the nucleotide-binding oligomerization domain (Nod)-like receptors (NLRs). These receptors (such as NLRP1, NLRP3 and NLRP4) recognize microbial signals to activate the caspase-1 inflammasome complex and induce the secretion of interleukin (IL)-1β and IL-18 [63]. IL-1β expression in a pancreatic tumor microenvironment is associated with treatment resistance and poor prognosis. The microbial dysbiosis could induce TLR4-NLRP3 signaling, resulting in an enhanced secretion of IL-1β by pancreatic tumor cells [64]. This secreted IL-1β, through the activation of pancreatic stellate cells, could induce mesenchymal fibrosis, which is shown to cause chemo/immuno-therapeutic resistance [64,65]. Additionally, NLRs participate in bacterial clearance by inducing the activation of NF-κB, P38/MAP kinase and interferon signaling, regulating autophagy-associated protein expressions and promoting autophagosome formation (such as NOD1 and NOD2) [66,67]. The intestinal microbiota also plays a critical role in the maturation and continued education of the host immune system [58], provides protection against pathogen overgrowth [68] and influences host cell proliferation [69] and vascularization [70].
Along with the modulation of the innate immune responses mentioned above, the pancreatic microbiome also promotes pro-cancerous adaptive immune responses. Using preclinical pancreatic cancer models, Pushalkar et al. demonstrated that the passive transfer of bacteria obtained from pancreatic ductal carcinoma hosts into normal mice induced oncogenesis. Further, the ablation of the pancreatic microbiome was associated with a reduction in the frequency of tumor-infiltrating immune cells with immunosuppressive tumorigenic myeloid-derived suppressor cells (MDSCs), along with an increase in anti-tumor M1 macrophage, Th1/CD4+T cells and CD8+T cells in the pancreatic tumor microenvironment. Additionally, the ablation of the microbiome caused an enhanced expression of PD1 on tumor-infiltrating CD4+ and CD8+ T cells, thus, enhancing the immunotherapeutic efficacy of anti-PD1 monoclonal antibodies (mAbs) [11]. Along similar lines, studies by Riquelme et al. demonstrated that long-term survivors (LTSs) of human pancreatic ductal adenocarcinoma had a higher microbial diversity than short-term survivors (STSs). This was also associated with a higher frequency of anti-tumor cytotoxic granzyme+CD8+T cells [71]. An overview of the microbiome-mediated modulation of innate and adaptive immune responses leading to potential carcinogenesis is shown in Figure 1.
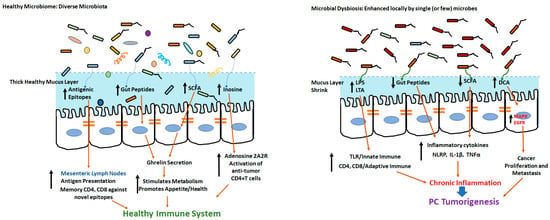
Figure 1.
In the presence of healthy microbiome with diverse microbes, the gut epithelium has thick intact mucus membrane and promotes immune health through diverse antigenic epitopes and development of novel memory CD4 and CD8+T cells. Microbial dysbiosis and colonization of single (or a few) harmful microbes could lead to ablation of mucous membrane and release of deleterious metabolites leading to tumorigenesis.
4. Secondary Impact of Lifestyle Co-Morbidities on Microbiome-Modulated Carcinogenesis
Gut microbiota is also known to be impacted by lifestyle and metabolic co-morbidities, such as a high-fat/low-fiber diet, obesity, type 2 diabetes, smoking and alcohol consumption. Dysbiosis-mediated obesity could increase the risk of cancer due to the generation of pro-carcinogenic microbial metabolites, the modulation of gut peptides and pro-inflammatory tumorigenic changes [72,73]. Chronic pancreatitis is a strong risk factor for PC [74], presenting as a localized or systemic inflammation. Non-alcoholic fatty pancreatic disease was identified as a novel clinical obesity-related disease that increases pancreatic fatty degeneration and may progress to chronic inflammation and PC [75]. Bacterial overgrowth in the small intestine, as evidenced by a duodenal fluid analysis, was observed in 92% of chronic pancreatitis patients [76]. In contrast, a meta-analysis by El Kurdi et al. demonstrated that small intestinal bacterial overgrowth accounts for only 38% of chronic pancreatitis complications [77]. Similarly, in type 2 diabetic patients, an increase in Lactobacillus species along with a decrease in SCFA (butyrate)-producing Roseburia intestinalis and Faecalibacterium prausnitzii was observed [78]. These data warrant further study to understand the interplay between lifestyle-associated cancer co-morbidities and microbiota towards pancreatic carcinogenesis.
5. Microbiome-Specific Biomarkers
Unfortunately, there are only limited options for the clinical usage of a viable and effective diagnostic/prognostic biomarker for PC. Despite low sensitivity and specificity, the carbohydrate antigen 19–9 (CA19–9) continues to be the only available biomarker utilized in PC [79]. As several studies have shown an association between specific bacterial species and pancreatic cancer, an important area of research would be the identification of a novel microbial biomarker for pancreatic cancer. In a study with 283 PDAC tumor samples, Fusobacterium species were detected in 8.8% of the tumor samples and demonstrated a significant positive correlation along with a decreased cancer-survival rate, independent of other clinical and molecular features [21]. Similar evidence was found in a study using a duodenal fluid analysis, wherein Fusobacterium was correlated with short-term survivors (STSs < 5 years) of PDAC [17]. Another study by Riquelme et al. showed that microbial diversity, as a composite biomarker, was higher in long-term survivors (LTSs > 5 years). Further, the LTS cohort demonstrated microbial signatures enriched with Pseudoxanthomonas, Streptomyces, Saccharopolyspora and Bacillus clausii [71]. Farrell et al. used a qPCR-based technique to show that, compared to healthy controls, the saliva of PC patients demonstrated an increase of 31 bacterial species and a reduction of 25 bacterial species [80]. In another study by Sun et al. wherein the researchers analyzed the saliva samples of patients with PC (n = 10), benign pancreatic disease (BPD) (n = 17) and healthy controls (n = 10) using 16S rDNA high-throughput sequencing, they demonstrated high concentrations of Fusobacterium periodonticum and low concentrations of Neisseria mucosa as specific risk factors for PC, thus, indicating their role as potential microbial diagnostic markers of PC [81]. More large cohort studies are needed to determine a PC-specific microbial biomarker.
6. Microbiome in Cancer Therapy
Studies have shown that microbiota can significantly alter the efficacy of chemotherapy. For example, PDAC tumors enriched with Proteobacteria-γ (including K. pneumoniae) induced gemcitabine resistance due to the presence of a long isoform of the enzyme cytidine deaminase (CDDL) in the bacteria [29,82]. Interestingly, ciprofloxacin abrogated this microbiota-induced gemcitabine resistance [29]. Similarly, in pre-clinical models, gut microbiota has shown to enhance the side-effects of irinotecan-based chemotherapeutic treatment in PDAC [83,84]. An enzyme produced by mycoplasma, pyrimidine nucleoside phosphorylase, mediates the phosphorylation of uridine, 2′-deoxyuridine and thymidine, which can reduce the nucleoside-based chemotherapeutic anti-cancer outcomes [85]. In mice inoculated with Porphyromonas gingivalis, treatment with probiotics inhibited the expression and phosphorylation of SMAD3, thus, probably playing a role in the inhibition of mitosis and cell proliferation [86]. A xenograft tumor model treated with oral Lactobacillus casei induced the activation of p53-mediated apoptosis and increased the efficacy of 5-FU and cisplatin, resulting in beneficial anti-tumor outcomes [87]. However, the exact molecular basis of the probiotic-mediated anti-tumor chemotherapeutic efficacy is still unclear.
Gut microbiota is considered to enhance the antigenic repertoire and immune health, along with increasing anti-tumor responses through the inflammatory activation of tumor-infiltrating immune cells [88,89]. However, a chemical released from Bacteroides fragilis, polysaccharide A, induced the differentiation of naïve CD4+T cells to immunosuppressive pro-tumor regulatory CD4+T cells (Treg) [90]. Immunotherapy with checkpoint inhibitors (ICIs) has been a profound failure in PDAC [91]. While an immunosuppressive environment is seen in all solid tumors, the specific reasons behind PDAC having a distinctly poor response to ICI-based therapeutic approaches are unclear [92,93,94]. There is limited molecular understanding of the expression pattern of checkpoint inhibitors (PD1, PDL1 and CTLA4) in a PDAC tumor microenvironment. An antibiotic-treatment-mediated upregulation of PD1 expression in PDAC tumor-infiltrating immune cells resulting in positive outcomes following anti-PD1-based ICI therapy was reported in pre-clinical models [11]. In a melanoma study, the microbial composition in melanoma patients was found to be different between immunotherapy responders and non-responders. In this study, Bifidobacterium was associated with a better response to ICIs [10,95]. The bacterial species, Bifidobacterium pseudolongum, Lactobacillus johnsonii and Olsenella species have demonstrated enhanced efficacy of ICIs in four different murine cancer models through the production of inosine, which through interaction with adenosine A2A receptor induced anti-tumor CD4+T cell activation [96].
A clinical study using a co-treatment of pembrolizumab (NCT03637803) with lyophilized bacteria showed limited initial efficacy in otherwise ICI-refractory metastatic non-small cell lung cancer, renal cancer and PDAC. There is another ongoing clinical trial to evaluate the combinatorial benefit of probiotics with vancomycin and nivolumab (NCT03785210) in patients with refractory hepatocellular carcinoma and pancreatic cancers. Interestingly, two phase I clinical trials have shown that in resistant metastatic melanoma, fecal microbiota transplantation (FMT) can improve the response to ICI [97,98]. In an antibiotic-treated PC murine model, FMT caused a bacterial colonization of pancreatic tumors along with an infiltration with cytotoxic CD8+T cells [71]. Similar studies that combine FMT and immunotherapy are underway in gastrointestinal cancers, prostate cancer, non-small cell lung cancer and mesothelioma (NCT04130763, NCT04729322, NCT04521075, NCT04116775, NCT04056026, NCT03819296 and NCT04163289). In addition, the co-administration of probiotics and/or a high-fiber diet with ICI is being rigorously evaluated in renal, breast cancer, non-small cell lung and colorectal cancers (NCT03829111, NCT03775850 and NCT04909034). There is growing evidence that specific dietary changes can alter the intestinal microbiota [99], which can be associated with an altered response to immunotherapy. Despite these studies, ICI therapies in the current forms have yet to demonstrate activity in pancreatic cancer, and would likely limit the applicability of FMT to enhance ICI in this disease. Various ongoing clinical trials studying the impact (and/or characterization) of the microbiome in PC are listed in Table 2.

Table 2.
Clinical trials to study the microbiota associated with pancreatic cancer.
The application of genetically engineered microbiota in cancer therapy is an area of intense research interest. In bacteria-based cancer therapy, Salmonella, a facultative strain that is well-studied with a fully sequenced genome, is considered specifically attractive due to its tumor localization capability and natural toxicity [100]. Tan et al. showed that a single dose of attenuated Salmonella typhimurium that was bioengineered to express cytolysin A markedly inhibited the growth of murine PC xeno- and orthografts. This was accompanied by the destruction of stromal cells in PC, along with enhanced infiltration with anti-tumor immune cells [101]. Similar pre-clinical studies with S. typhimurium bioengineered to express collagenase and hyaluronidase demonstrated reduced collagen fibers in PC, resulting in a reduced tumor burden and proliferation [102,103]. Attenuated Listeria monocytogenes is another bacterium extensively studied in cancer therapy, due to its ability to efficiently activate TLR-mediated innate immune responses and antigen presentation, resulting in CD8+T-cell-mediated anti-tumor responses [104,105,106]. Bioengineered Listeria monocytogenes designed to express mesothelin, a PC-associated tumor antigen, induced the efficient activation of cytotoxic CD8+T cell responses, inducing PC tumor regression [107,108]. Unfortunately, a human trial based on this approach was disappointing, as it was not better than standard conventional chemotherapy [109,110,111]. However, future studies combining standard chemo-/immunotherapy with engineered microbiota could provide better outcomes.
7. Conclusions
The role of microbiota on carcinogenesis is an emerging area of research. Several, apparently contradictory, observations have only pointed to a need for more thorough and standardized future studies to analyze this problem, which include robust sample collections, study size and defining the normal microbiota among various demographics and geographical distributions. This understanding could lead to future applications of microbiome-based diagnostic/prognostic biomarkers and therapeutic interventions with probiotics, FMT and/or antibiotic co-treatment. Further, more detailed molecular studies are needed to uncover the functional impact of the microbiome in pancreatic cancer. Such immunological and biochemical studies would provide deeper insights into microbiome-mediated pancreatic carcinogenesis to enable the development of novel therapies to reduce the mortality and quality of life of pancreatic cancer patients.
Author Contributions
S.A., L.V.Y., V.T. and M.M. were involved in writing and proof-reading this article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- American Cancer Society. Cancer Facts & Figures 2022. Atlanta Am. Cancer Soc. 2022. [Google Scholar]
- Andersson, R.; Haglund, C.; Seppanen, H.; Ansari, D. Pancreatic cancer—The past, the present, and the future. Scand. J. Gastroenterol. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kindler, H.L. A Glimmer of Hope for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2463–2464. [Google Scholar] [CrossRef] [PubMed]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.E.; Prendergast, C.; Lowy, A.M. Borderline resectable pancreatic cancer: Definitions and management. World J. Gastroenterol. 2014, 20, 10740–10751. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, D.; Leung, R.K.; Guan, W.; Au, W.W. Involvement of gut microbiome in human health and disease: Brief overview, knowledge gaps and research opportunities. Gut Pathog. 2018, 10, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmink, B.A.; Khan, M.A.W.; Hermann, A.; Gopalakrishnan, V.; Wargo, J.A. The microbiome, cancer, and cancer therapy. Nat. Med. 2019, 25, 377. [Google Scholar] [CrossRef]
- Vandeven, N.; Nghiem, P. Pathogen-driven cancers and emerging immune therapeutic strategies. Cancer Immunol. Res. 2014, 2, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Pushalkar, S.; Hundeyin, M.; Daley, D.; Zambirinis, C.P.; Kurz, E.; Mishra, A.; Mohan, N.; Aykut, B.; Usyk, M.; Torres, L.E.; et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018, 8, 403–416. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.Y.; Kim, T.B.; Kim, J.; Choi, H.W.; Kim, E.J.; Yoo, H.J.; Lee, S.; Jun, H.R.; Yoo, W.; Kim, S.; et al. Diversity in the Extracellular Vesicle-Derived Microbiome of Tissues According to Tumor Progression in Pancreatic Cancer. Cancers 2020, 12, 2346. [Google Scholar] [CrossRef] [PubMed]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.B.; Aveson, V.; Firek, B.; Yeh, A.; Brooks, B.; Brower-Sinning, R.; Steve, J.; Banfield, J.F.; Zureikat, A.; Hogg, M.; et al. Disturbances of the Perioperative Microbiome Across Multiple Body Sites in Patients Undergoing Pancreaticoduodenectomy. Pancreas 2017, 46, 260–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Jiang, J.; Xie, H.; Li, A.; Lu, H.; Xu, S.; Zhou, L.; Zhang, H.; Cui, G.; Chen, X.; et al. Gut microbial profile analysis by MiSeq sequencing of pancreatic carcinoma patients in China. Oncotarget 2017, 8, 95176–95191. [Google Scholar] [CrossRef] [Green Version]
- Kartal, E.; Schmidt, T.S.B.; Molina-Montes, E.; Rodriguez-Perales, S.; Wirbel, J.; Maistrenko, O.M.; Akanni, W.A.; Alashkar Alhamwe, B.; Alves, R.J.; Carrato, A.; et al. A faecal microbiota signature with high specificity for pancreatic cancer. Gut 2022, 71, 1359–1372. [Google Scholar] [CrossRef] [PubMed]
- Kohi, S.; Macgregor-Das, A.; Dbouk, M.; Yoshida, T.; Chuidian, M.; Abe, T.; Borges, M.; Lennon, A.M.; Shin, E.J.; Canto, M.I.; et al. Alterations in the Duodenal Fluid Microbiome of Patients with Pancreatic Cancer. Clin. Gastroenterol. Hepatol. 2022, 20, e196–e227. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ren, Z.; Li, A.; Li, J.; Xu, S.; Zhang, H.; Jiang, J.; Yang, J.; Luo, Q.; Zhou, K.; et al. Tongue coating microbiome data distinguish patients with pancreatic head cancer from healthy controls. J. Oral. Microbiol. 2019, 11, 1563409. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Alekseyenko, A.V.; Wu, J.; Peters, B.A.; Jacobs, E.J.; Gapstur, S.M.; Purdue, M.P.; Abnet, C.C.; Stolzenberg-Solomon, R.; Miller, G.; et al. Human oral microbiome and prospective risk for pancreatic cancer: A population-based nested case-control study. Gut 2018, 67, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Wei, A.L.; Li, M.; Li, G.Q.; Wang, X.; Hu, W.M.; Li, Z.L.; Yuan, J.; Liu, H.Y.; Zhou, L.L.; Li, K.; et al. Oral microbiome and pancreatic cancer. World J. Gastroenterol. 2020, 26, 7679–7692. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhashi, K.; Nosho, K.; Sukawa, Y.; Matsunaga, Y.; Ito, M.; Kurihara, H.; Kanno, S.; Igarashi, H.; Naito, T.; Adachi, Y.; et al. Association of Fusobacterium species in pancreatic cancer tissues with molecular features and prognosis. Oncotarget 2015, 6, 7209–7220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Castillo, E.; Meier, R.; Chung, M.; Koestler, D.C.; Chen, T.; Paster, B.J.; Charpentier, K.P.; Kelsey, K.T.; Izard, J.; Michaud, D.S. The Microbiomes of Pancreatic and Duodenum Tissue Overlap and Are Highly Subject Specific but Differ between Pancreatic Cancer and Noncancer Subjects. Cancer Epidemiol. Biomark. Prev. 2019, 28, 370–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkharaan, H.; Lu, L.; Gabarrini, G.; Halimi, A.; Ateeb, Z.; Sobkowiak, M.J.; Davanian, H.; Fernandez Moro, C.; Jansson, L.; Del Chiaro, M.; et al. Circulating and Salivary Antibodies to Fusobacterium nucleatum Are Associated with Cystic Pancreatic Neoplasm Malignancy. Front. Immunol. 2020, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Michaud, D.S.; Izard, J.; Wilhelm-Benartzi, C.S.; You, D.H.; Grote, V.A.; Tjonneland, A.; Dahm, C.C.; Overvad, K.; Jenab, M.; Fedirko, V.; et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut 2013, 62, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, W.; Wu, J. Helicobacter pylori infection and pancreatic cancer risk: A meta-analysis. J. Cancer Res. Ther. 2016, 12, 229–232. [Google Scholar]
- Trikudanathan, G.; Philip, A.; Dasanu, C.A.; Baker, W.L. Association between Helicobacter pylori infection and pancreatic cancer. A cumulative meta-analysis. JOP J. Pancreas 2011, 12, 26–31. [Google Scholar]
- Schulte, A.; Pandeya, N.; Fawcett, J.; Fritschi, L.; Risch, H.A.; Webb, P.M.; Whiteman, D.C.; Neale, R.E. Association between Helicobacter pylori and pancreatic cancer risk: A meta-analysis. Cancer Causes Control 2015, 26, 1027. [Google Scholar] [CrossRef]
- Chakladar, J.; Kuo, S.Z.; Castaneda, G.; Li, W.T.; Gnanasekar, A.; Yu, M.A.; Chang, E.Y.; Wang, X.Q.; Ongkeko, W.M. The Pancreatic Microbiome is Associated with Carcinogenesis and Worse Prognosis in Males and Smokers. Cancers 2020, 12, 2672. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.M.; Gharaibeh, R.Z.; Gauthier, J.; Beveridge, M.; Pope, J.L.; Guijarro, M.V.; Yu, Q.; He, Z.; Ohland, C.; Newsome, R.; et al. Intestinal microbiota enhances pancreatic carcinogenesis in preclinical models. Carcinogenesis 2018, 39, 1068–1078. [Google Scholar] [CrossRef]
- Diehl, G.E.; Longman, R.S.; Zhang, J.X.; Breart, B.; Galan, C.; Cuesta, A.; Schwab, S.R.; Littman, D.R. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature 2013, 494, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Aldars-Garcia, L.; Chaparro, M.; Gisbert, J.P. Systematic Review: The Gut Microbiome and Its Potential Clinical Application in Inflammatory Bowel Disease. Microorganisms 2021, 9, 977. [Google Scholar] [CrossRef] [PubMed]
- Alamri, A. Diversity of Microbial Signatures in Asthmatic Airways. Int. J. Gen. Med. 2021, 14, 1367–1378. [Google Scholar] [CrossRef]
- Hrncir, T.; Hrncirova, L.; Kverka, M.; Hromadka, R.; Machova, V.; Trckova, E.; Kostovcikova, K.; Kralickova, P.; Krejsek, J.; Tlaskalova-Hogenova, H. Gut Microbiota and NAFLD: Pathogenetic Mechanisms, Microbiota Signatures, and Therapeutic Interventions. Microorganisms 2021, 9, 957. [Google Scholar] [CrossRef]
- Kunovsky, L.; Dite, P.; Jabandziev, P.; Dolina, J.; Vaculova, J.; Blaho, M.; Bojkova, M.; Dvorackova, J.; Uvirova, M.; Kala, Z.; et al. Helicobacter pylori infection and other bacteria in pancreatic cancer and autoimmune pancreatitis. World J. Gastrointest. Oncol. 2021, 13, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Binder Gallimidi, A.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnanasekaran, J.; Binder Gallimidi, A.; Saba, E.; Pandi, K.; Eli Berchoer, L.; Hermano, E.; Angabo, S.; Makkawi, H.A.; Khashan, A.; Daoud, A.; et al. Intracellular Porphyromonas gingivalis Promotes the Tumorigenic Behavior of Pancreatic Carcinoma Cells. Cancers 2020, 12, 2331. [Google Scholar] [CrossRef] [PubMed]
- Sethi, V.; Kurtom, S.; Tarique, M.; Lavania, S.; Malchiodi, Z.; Hellmund, L.; Zhang, L.; Sharma, U.; Giri, B.; Garg, B.; et al. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology 2018, 155, 33–37.e6. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of Prior Antibiotic Treatment with Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients with Cancer. JAMA Oncol. 2019, 5, 1774. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, T.; Richard, C.; Elkrief, A.; Hosomi, Y.; Benlaifaoui, M.; Mimpen, I.; Terrisse, S.; Derosa, L.; Zitvogel, L.; Routy, B.; et al. The Gut Microbiome Associates with Immune Checkpoint Inhibition Outcomes in Patients with Advanced Non-Small Cell Lung Cancer. Cancer Immunol. Res. 2020, 8, 1243–1250. [Google Scholar] [CrossRef]
- Jin, Y.; Dong, H.; Xia, L.; Yang, Y.; Zhu, Y.; Shen, Y.; Zheng, H.; Yao, C.; Wang, Y.; Lu, S. The Diversity of Gut Microbiome is Associated with Favorable Responses to Anti-Programmed Death 1 Immunotherapy in Chinese Patients with NSCLC. J. Thorac. Oncol. 2019, 14, 1378. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Jordan, B.F. Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Sivaprakasam, S.; Gurav, A.; Paschall, A.V.; Coe, G.L.; Chaudhary, K.; Cai, Y.; Kolhe, R.; Martin, P.; Browning, D.; Huang, L.; et al. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis 2016, 5, e238. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Kwon, O.; Ryu, T.Y.; Jung, C.R.; Kim, J.; Min, J.K.; Kim, D.S.; Son, M.Y.; Cho, H.S. Propionate of a microbiota metabolite induces cell apoptosis and cell cycle arrest in lung cancer. Mol. Med. Rep. 2019, 20, 1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohara, T.; Mori, T. Antiproliferative Effects of Short-chain Fatty Acids on Human Colorectal Cancer Cells via Gene Expression Inhibition. Anticancer Res. 2019, 39, 4659–4666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutzac, C.; Jouniaux, J.M.; Paci, A.; Schmidt, J.; Mallardo, D.; Seck, A.; Asvatourian, V.; Cassard, L.; Saulnier, P.; Lacroix, L.; et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 2020, 11, 2168. [Google Scholar] [CrossRef]
- Maekawa, T.; Fukaya, R.; Takamatsu, S.; Itoyama, S.; Fukuoka, T.; Yamada, M.; Hata, T.; Nagaoka, S.; Kawamoto, K.; Eguchi, H.; et al. Possible involvement of Enterococcus infection in the pathogenesis of chronic pancreatitis and cancer. Biochem. Biophys. Res. Commun. 2018, 506, 962–969. [Google Scholar] [CrossRef]
- Cao, H.; Xu, M.; Dong, W.; Deng, B.; Wang, S.; Zhang, Y.; Wang, S.; Luo, S.; Wang, W.; Qi, Y.; et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int. J. Cancer 2017, 140, 2545–2556. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Wang, S.; Dong, W.; Liu, L.; Xu, M.; Wang, Y.; Liu, T.; Zhang, Y.; Wang, B.; Cao, H. Interplay between bile acids and the gut microbiota promotes intestinal carcinogenesis. Mol. Carcinog. 2019, 58, 1155–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagathihalli, N.S.; Beesetty, Y.; Lee, W.; Washington, M.K.; Chen, X.; Lockhart, A.C.; Merchant, N.B. Novel mechanistic insights into ectodomain shedding of EGFR Ligands Amphiregulin and TGF-alpha: Impact on gastrointestinal cancers driven by secondary bile acids. Cancer Res. 2014, 74, 2062–2072. [Google Scholar] [CrossRef] [Green Version]
- Halimi, A.; Gabarrini, G.; Sobkowiak, M.J.; Ateeb, Z.; Davanian, H.; Gaiser, R.A.; Arnelo, U.; Valente, R.; Wong, A.Y.W.; Moro, C.F.; et al. Isolation of pancreatic microbiota from cystic precursors of pancreatic cancer with intracellular growth and DNA damaging properties. Gut Microbes 2021, 13, 1983101. [Google Scholar] [CrossRef]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Cougnoux, A.; Dalmasso, G.; Martinez, R.; Buc, E.; Delmas, J.; Gibold, L.; Sauvanet, P.; Darcha, C.; Dechelotte, P.; Bonnet, M.; et al. Bacterial genotoxin colibactin promotes colon tumour growth by inducing a senescence-associated secretory phenotype. Gut 2014, 63, 1932–1942. [Google Scholar] [CrossRef]
- Nougayrede, J.P.; Taieb, F.; De Rycke, J.; Oswald, E. Cyclomodulins: Bacterial effectors that modulate the eukaryotic cell cycle. Trends Microbiol. 2005, 13, 103–110. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667. [Google Scholar] [CrossRef]
- Fulde, M.; Hornef, M.W. Maturation of the enteric mucosal innate immune system during the postnatal period. Immunol. Rev. 2014, 260, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800. [Google Scholar] [CrossRef] [Green Version]
- Rakoff-Nahoum, S.; Medzhitov, R. Toll-like receptors and cancer. Nat. Rev. Cancer 2009, 9, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ochi, A.; Graffeo, C.S.; Zambirinis, C.P.; Rehman, A.; Hackman, M.; Fallon, N.; Barilla, R.M.; Henning, J.R.; Jamal, M.; Rao, R.; et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J. Clin. Investig. 2012, 122, 4118–4129. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Mayor, A.; Tschopp, J. The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 2009, 27, 229–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Shapiro, B.; Vucic, E.A.; Vogt, S.; Bar-Sagi, D. Tumor Cell-Derived IL1beta Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res. 2020, 80, 1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016, 6, 852. [Google Scholar] [CrossRef] [Green Version]
- Moreira, L.O.; Zamboni, D.S. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, T.; Hovingh, E.S.; Foerster, E.G.; Abdel-Nour, M.; Philpott, D.J.; Girardin, S.E. NOD1 and NOD2 in inflammation, immunity and disease. Arch. Biochem. Biophys. 2019, 670, 69–81. [Google Scholar] [CrossRef]
- Kamada, N.; Chen, G.Y.; Inohara, N.; Nunez, G. Control of pathogens and pathobionts by the gut microbiota. Nat. Immunol. 2013, 14, 685–690. [Google Scholar] [CrossRef]
- Ijssennagger, N.; Belzer, C.; Hooiveld, G.J.; Dekker, J.; van Mil, S.W.; Muller, M.; Kleerebezem, M.; van der Meer, R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc. Natl. Acad. Sci. USA 2015, 112, 10038–10043. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, C.; Bergentall, M.; Greiner, T.U.; Schaffner, F.; Ostergren-Lunden, G.; Petersen, L.C.; Ruf, W.; Backhed, F. Tissue factor and PAR1 promote microbiota-induced intestinal vascular remodelling. Nature 2012, 483, 627–631. [Google Scholar] [CrossRef] [Green Version]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; San Lucas, A.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.J.; Prabhu, K.S.; Vijay-Kumar, M. The microbiome and obesity-an established risk for certain types of cancer. Cancer J. 2014, 20, 176. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Morris, J.S.; Liu, J.; Hassan, M.M.; Day, R.S.; Bondy, M.L.; Abbruzzese, J.L. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. Jama 2009, 301, 2553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkegard, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef] [Green Version]
- Tomita, Y.; Azuma, K.; Nonaka, Y.; Kamada, Y.; Tomoeda, M.; Kishida, M.; Tanemura, M.; Miyoshi, E. Pancreatic fatty degeneration and fibrosis as predisposing factors for the development of pancreatic ductal adenocarcinoma. Pancreas 2014, 43, 1032–1041. [Google Scholar] [CrossRef]
- Kumar, K.; Ghoshal, U.C.; Srivastava, D.; Misra, A.; Mohindra, S. Small intestinal bacterial overgrowth is common both among patients with alcoholic and idiopathic chronic pancreatitis. Pancreatology 2014, 14, 280–283. [Google Scholar] [CrossRef]
- El Kurdi, B.; Babar, S.; El Iskandarani, M.; Bataineh, A.; Lerch, M.M.; Young, M.; Singh, V.P. Factors That Affect Prevalence of Small Intestinal Bacterial Overgrowth in Chronic Pancreatitis: A Systematic Review, Meta-Analysis, and Meta-Regression. Clin. Transl. Gastroenterol. 2019, 10, e00072. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Scara, S.; Bottoni, P.; Scatena, R. CA 19-9: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 247–260. [Google Scholar]
- Farrell, J.J.; Zhang, L.; Zhou, H.; Chia, D.; Elashoff, D.; Akin, D.; Paster, B.J.; Joshipura, K.; Wong, D.T. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 2012, 61, 582–588. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, X.; Zhou, Y.; Wang, J.; Ma, R.; Ren, X.; Wang, H.; Zou, L. Characterization of Oral Microbiome and Exploration of Potential Biomarkers in Patients with Pancreatic Cancer. Biomed. Res. Int. 2020, 2020, 4712498. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.; Hank, T.; Qadan, M.; Ciprani, D.; Michelakos, T.; Niess, H.; Heiliger, C.; Ilmer, M.; D’Haese, J.G.; Ferrone, C.R.; et al. Influence of Klebsiella pneumoniae and quinolone treatment on prognosis in patients with pancreatic cancer. Br. J. Surg. 2021, 108, 709. [Google Scholar] [PubMed]
- Mathijssen, R.H.; van Alphen, R.J.; Verweij, J.; Loos, W.J.; Nooter, K.; Stoter, G.; Sparreboom, A. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11). Clin. Cancer Res. 2001, 7, 2182. [Google Scholar]
- Stringer, A.M.; Gibson, R.J.; Logan, R.M.; Bowen, J.M.; Yeoh, A.S.; Keefe, D.M. Faecal microflora and beta-glucuronidase expression are altered in an irinotecan-induced diarrhea model in rats. Cancer Biol. Ther. 2008, 7, 1919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vande Voorde, J.; Sabuncuoglu, S.; Noppen, S.; Hofer, A.; Ranjbarian, F.; Fieuws, S.; Balzarini, J.; Liekens, S. Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine. J. Biol. Chem. 2014, 289, 13054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.M.; Hsu, L.J.; Lee, H.L.; Lin, C.P.; Huang, S.W.; Lai, C.J.; Lin, C.W.; Chen, W.T.; Chen, Y.J.; Lin, Y.C.; et al. Lactobacillus Attenuate the Progression of Pancreatic Cancer Promoted by Porphyromonas Gingivalis in K-ras(G12D) Transgenic Mice. Cancers 2020, 12, 3522. [Google Scholar] [CrossRef]
- Kita, A.; Fujiya, M.; Konishi, H.; Tanaka, H.; Kashima, S.; Iwama, T.; Ijiri, M.; Murakami, Y.; Takauji, S.; Goto, T.; et al. Probioticderived ferrichrome inhibits the growth of refractory pancreatic cancer cells. Int. J. Oncol. 2020, 57, 721–732. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef] [Green Version]
- Balachandran, V.P.; Beatty, G.L.; Dougan, S.K. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 2019, 156, 2056–2072. [Google Scholar] [CrossRef]
- Upadhrasta, S.; Zheng, L. Strategies in Developing Immunotherapy for Pancreatic Cancer: Recognizing and Correcting Multiple Immune “defects” in the Tumor Microenvironment. J. Clin. Med. 2019, 8, 1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feig, C.; Jones, J.O.; Kraman, M.; Wells, R.J.; Deonarine, A.; Chan, D.S.; Connell, C.M.; Roberts, E.W.; Zhao, Q.; Caballero, O.L.; et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 20212–20217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Byrne, K.T.; Yan, F.; Yamazoe, T.; Chen, Z.; Baslan, T.; Richman, L.P.; Lin, J.H.; Sun, Y.H.; Rech, A.J.; et al. Tumor Cell-Intrinsic Factors Underlie Heterogeneity of Immune Cell Infiltration and Response to Immunotherapy. Immunity 2018, 49, 178–193.e7. [Google Scholar] [CrossRef] [Green Version]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- O’Keefe, S.J.; Li, J.V.; Lahti, L.; Ou, J.; Carbonero, F.; Mohammed, K.; Posma, J.M.; Kinross, J.; Wahl, E.; Ruder, E.; et al. Fat, fibre and cancer risk in African Americans and rural Africans. Nat. Commun. 2015, 6, 6342. [Google Scholar] [CrossRef] [Green Version]
- Leschner, S.; Weiss, S. Salmonella-allies in the fight against cancer. J. Mol. Med. 2010, 88, 763–773. [Google Scholar] [CrossRef]
- Tan, W.; Duong, M.T.; Zuo, C.; Qin, Y.; Zhang, Y.; Guo, Y.; Hong, Y.; Zheng, J.H.; Min, J.J. Targeting of pancreatic cancer cells and stromal cells using engineered oncolytic Salmonella typhimurium. Mol. Ther. 2022, 30, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Ebelt, N.D.; Zamloot, V.; Zuniga, E.; Passi, K.B.; Sobocinski, L.J.; Young, C.A.; Blazar, B.R.; Manuel, E.R. Collagenase-Expressing Salmonella Targets Major Collagens in Pancreatic Cancer Leading to Reductions in Immunosuppressive Subsets and Tumor Growth. Cancers 2021, 13, 3565. [Google Scholar] [CrossRef]
- Ebelt, N.D.; Zuniga, E.; Passi, K.B.; Sobocinski, L.J.; Manuel, E.R. Hyaluronidase-Expressing Salmonella Effectively Targets Tumor-Associated Hyaluronic Acid in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2020, 19, 706–716. [Google Scholar] [CrossRef] [Green Version]
- Chavez-Arroyo, A.; Portnoy, D.A. Why is Listeria monocytogenes such a potent inducer of CD8+ T-cells? Cell Microbiol. 2020, 22, e13175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flickinger, J.C., Jr.; Rodeck, U.; Snook, A.E. Listeria monocytogenes as a Vector for Cancer Immunotherapy: Current Understanding and Progress. Vaccines 2018, 6, 48. [Google Scholar] [CrossRef] [Green Version]
- Salas-Benito, D.; Melero, I.; Ponz-Sarvise, M. Vaccination for Pancreatic Ductal Adenocarcinoma: A Hard Nut to Crack. Clin. Cancer Res. 2019, 25, 5435–5437. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.M.; Blair, A.B.; Lauer, P.; Foley, K.; Che, X.; Soares, K.; Xia, T.; Muth, S.T.; Kleponis, J.; Armstrong, T.D.; et al. Anti-pancreatic tumor efficacy of a Listeria-based, Annexin A2-targeting immunotherapy in combination with anti-PD-1 antibodies. J. Immunother. Cancer 2019, 7, 132. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Lira, V.; Hudson, T.E.; Lemmens, E.E.; Hanson, W.G.; Flores, R.; Barajas, G.; Katibah, G.E.; Desbien, A.L.; Lauer, P.; et al. Recombinant Listeria promotes tumor rejection by CD8(+) T cell-dependent remodeling of the tumor microenvironment. Proc. Natl. Acad. Sci. USA 2018, 115, 8179. [Google Scholar] [CrossRef] [Green Version]
- Brockstedt, D.G.; Giedlin, M.A.; Leong, M.L.; Bahjat, K.S.; Gao, Y.; Luckett, W.; Liu, W.; Cook, D.N.; Portnoy, D.A.; Dubensky, T.W., Jr. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc. Natl. Acad. Sci. USA 2004, 101, 13832–13837. [Google Scholar] [CrossRef] [Green Version]
- Le, D.T.; Picozzi, V.J.; Ko, A.H.; Wainberg, Z.A.; Kindler, H.; Wang-Gillam, A.; Oberstein, P.; Morse, M.A.; Zeh, H.J., 3rd; Weekes, C.; et al. Results from a Phase IIb, Randomized, Multicenter Study of GVAX Pancreas and CRS-207 Compared with Chemotherapy in Adults with Previously Treated Metastatic Pancreatic Adenocarcinoma (ECLIPSE Study). Clin. Cancer Res. 2019, 25, 5493–5502. [Google Scholar] [CrossRef]
- Le, D.T.; Wang-Gillam, A.; Picozzi, V.; Greten, T.F.; Crocenzi, T.; Springett, G.; Morse, M.; Zeh, H.; Cohen, D.; Fine, R.L.; et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 2015, 33, 1325–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).